Published online Apr 7, 2025. doi: 10.3748/wjg.v31.i13.104370
Revised: February 24, 2025
Accepted: March 10, 2025
Published online: April 7, 2025
Processing time: 103 Days and 5.2 Hours
Early detection of esophageal squamous neoplasms (ESN) is essential for impro
To develop a pCLE computer-aided diagnostic system for ESN and assess its diagnostic performance and assistant efficiency for nonexpert endoscopists.
The intelligent confocal laser endomicroscopy (iCLE) system consists of image recognition (based on inception-ResNet V2), video diagnosis, and quality judg
A total of 25056 images from 2803 patients were selected for iCLE training and validation. Another 2442 images from 226 patients were used for testing. iCLE achieved a high accuracy of 98.3%, sensitivity of 95.3% and specificity of 98.8% for diagnosing ESN images. A total of 2581 patients underwent upper gastrointestinal pCLE examination and were prospectively screened; 54 patients with suspected ESN were enrolled. Overall, 187 videos from 67 lesions were assessed by iCLE, three nonexpert and three expert endoscopists. iCLE achieved a high accuracy, sensitivity and specificity of 90.9%, 92.0%, and 90.2%, respectively. Compared to experts, iCLE showed signi
iCLE system demonstrated high diagnostic performance for ESN. It can assist nonexpert endoscopists in improving the diagnostic efficiency of pCLE for ESN and has the potential for reducing unnecessary biopsies.
Core Tip: The optical diagnosis of esophageal squamous neoplasms (ESN) is challenging. Probe-based confocal laser endomicroscopy (pCLE) enables the optical biopsy of ESN; however, its application is affected by the difficulty of image interpretation. This study developed the first pCLE computer-aided diagnostic system (intelligent confocal laser endomicroscopy) for ESN, enabling real-time diagnosis of pCLE videos. Intelligent confocal laser endomicroscopy was evaluated in a prospective study and compared with endoscopists with different expertise levels. It demonstrated higher sensitivity than both nonexpert and expert endoscopists. Additionally, it assists nonexperts in significantly improving diagnostic accuracy and sensitivity. This system has the potential to assist endoscopists in the application of pCLE and reduce unnecessary biopsies.
- Citation: Ma T, Liu GQ, Guo J, Ji R, Shao XJ, Li YQ, Li Z, Zuo XL. Artificial intelligence-aided optical biopsy improves the diagnosis of esophageal squamous neoplasm. World J Gastroenterol 2025; 31(13): 104370
- URL: https://www.wjgnet.com/1007-9327/full/v31/i13/104370.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i13.104370
Esophageal cancer is a major global health issue, ranking as the 11th most commonly diagnosed cancer and 7th leading cause of cancer-related mortality worldwide[1]. Epidemiological studies have shown a high disease burden, with more than 85% of global esophageal cancer cases attributed to squamous cell carcinomas (SCC), especially in central and eastern Asia. Nearly half of all cases worldwide occur in China[2,3]. Early detection and treatment of esophageal squa
Endoscopy, in conjunction with biopsy sampling, is the primary diagnostic method for ESN. The identification of ESN using conventional white-light endoscopy (WLE) is challenging owing to their subtle appearance, leading to a low diag
pCLE enables real-time, in vivo histological observation of the gastrointestinal mucosa at the cellular and microvascular levels. This approach aims to overcome the inherent limitations of traditional endoscopic sampling techniques using optical biopsy combined with targeted biopsy[6]. The mini-probe of the pCLE can be inserted into the working channel of other endoscopes, such as a high-definition endoscope with NBI, allowing microscopic imaging via direct mucosal contact. Previous studies have demonstrated that pCLE is highly effective for the diagnosis of ESN, with both high sensitivity (94%-95.7%) and specificity (90%)[4,6-8]. Notably, pCLE can accurately distinguish benign from malignant Lugol-unstained esophageal lesions in patients with head and neck cancers[7]. In addition, pCLE can be efficiently used in combination with IEE to reduce the number of biopsy specimens required for diagnosis[8]. However, the interpretation of pCLE images requires a substantial knowledge of pathology and professional training, which limits its application and promotion[9].
Despite the variety of endoscopic equipment available, a proportion of lesions remain misdiagnosed owing to varia
In this study, our team developed a pCLE computer-aided diagnostic system, termed intelligent confocal laser endomicroscopy (iCLE), for the diagnosis of ESN based on pCLE. This study aimed to compare the diagnostic perfor
This prospective diagnostic study was conducted at the Qilu Hospital of Shandong University (Jinan, Shandong Province, China). The iCLE system was developed using deep-learning networks. pCLE images and videos were retrospectively collected from the pCLE database of the Qilu Hospital for the development, validation, and image testing of the iCLE system. A prospective study was conducted to evaluate the diagnostic performance of iCLE for pCLE videos and to compare it with that of endoscopists with varying levels of expertise. In addition, the assistant efficiency of iCLE for non-expert endoscopists was also assessed.
This study was approved by the Ethics Committee of Qilu Hospital of Shandong University and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients. The study was registered at https://clinicaltrials.gov/ (No. NCT04136236).
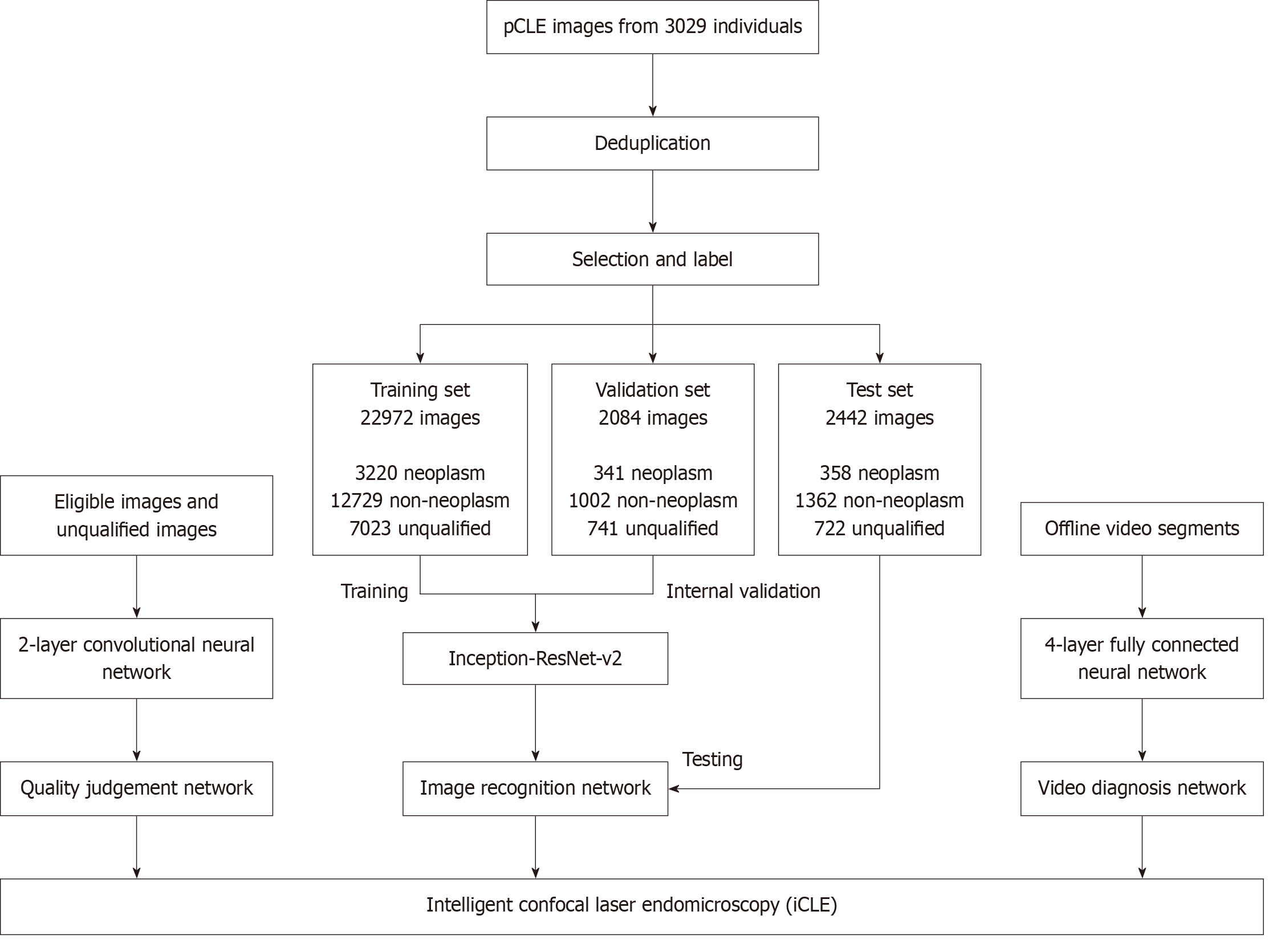
pCLE images and videos obtained between April 2014 and May 2020 were retrospectively collected for the development of the iCLE system. Data were obtained from consecutive patients who underwent pCLE examinations. Videos of the esophagus were reviewed and extracted into single-frame images. A deduplication software was developed to eliminate highly similar frames by comparing the Hamming distances between them. A workflow diagram depicting this process is shown in Supplementary Figure 1. This approach ensures that the selected images from the same lesion are repre
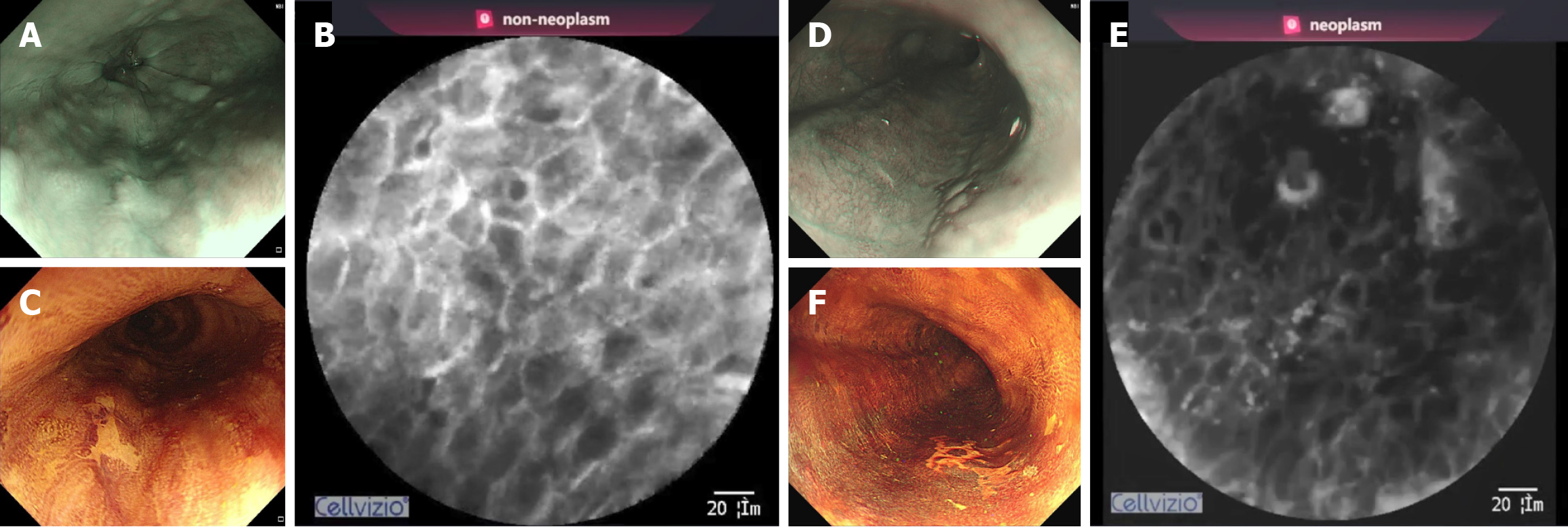
The diagnostic criteria for pCLE were based on the surface maturation scoring (SMS) system[8]. This system evaluates the features of surface maturation in the esophageal squamous epithelium, including the existence of halos, gradients, polarity, and the compass effect. The absence of all of these features (SMS score = 0) was considered a neoplasm. All images and videos were reviewed and marked by two experienced endoscopists, each with experience in over 100 pCLE procedures, and verified by another senior expert endoscopist. Histological diagnosis served as the gold standard for labeling. If the diagnosis of one image or video was discrepant, the three endoscopists discussed the findings to reach a consensus; if consensus was unattainable, the image or video was discarded.
The images from the different patients were then partitioned into three distinct sets: Training, validation, and test sets. This approach effectively prevented data leakage by ensuring that images from the same patient were contained exclusively within a single dataset. The labeled pCLE images underwent further preprocessing, including image cropping, resizing, and whitening, to eliminate interference factors and enhance image features. Subsequently, the images were converted into grayscale images with a size (299 × 299 × 1) and input into the model.
The iCLE system was constructed using neural networks and included three submodules: Image recognition network (Supplementary Figure 2), video diagnosis network, and quality judgment network. The performance of the various deep neural networks, including VGG, GoogleLeNet, RestNet, and inception-ResNet V2 were tested for pCLE image recognition efficiency. Among these, inception-ResNet V2 demonstrated the best performance, and was therefore selected as the final model of the image recognition network. The model was trained and validated using the TensorFlow 1.9.0 framework. During training, 32 images were randomly selected from the training set (batch size = 32) for each training iteration. After each epoch, the model was validated using the validation set. The training was stopped if the validation accuracy did not improve over 20 consecutive epochs, with the maximum number of epochs set to 200. Training was performed using gradient descent, with the Adam optimizer employed to optimize the network parameters. The initial learning rate was set to 0.001 and the learning rate adjustment strategy was based on Adam’s dynamic learning rate mechanism. This network used a pCLE image as the input and generated a three-dimensional feature vector. The feature vector was then processed using Softmax and converted into probabilities for the three diagnostic categories. Subse
The diagnostic capability of iCLE was first evaluated using still pCLE images. Images from the test set were analyzed using a computer equipped with the AI. Then a prospective study was conducted.
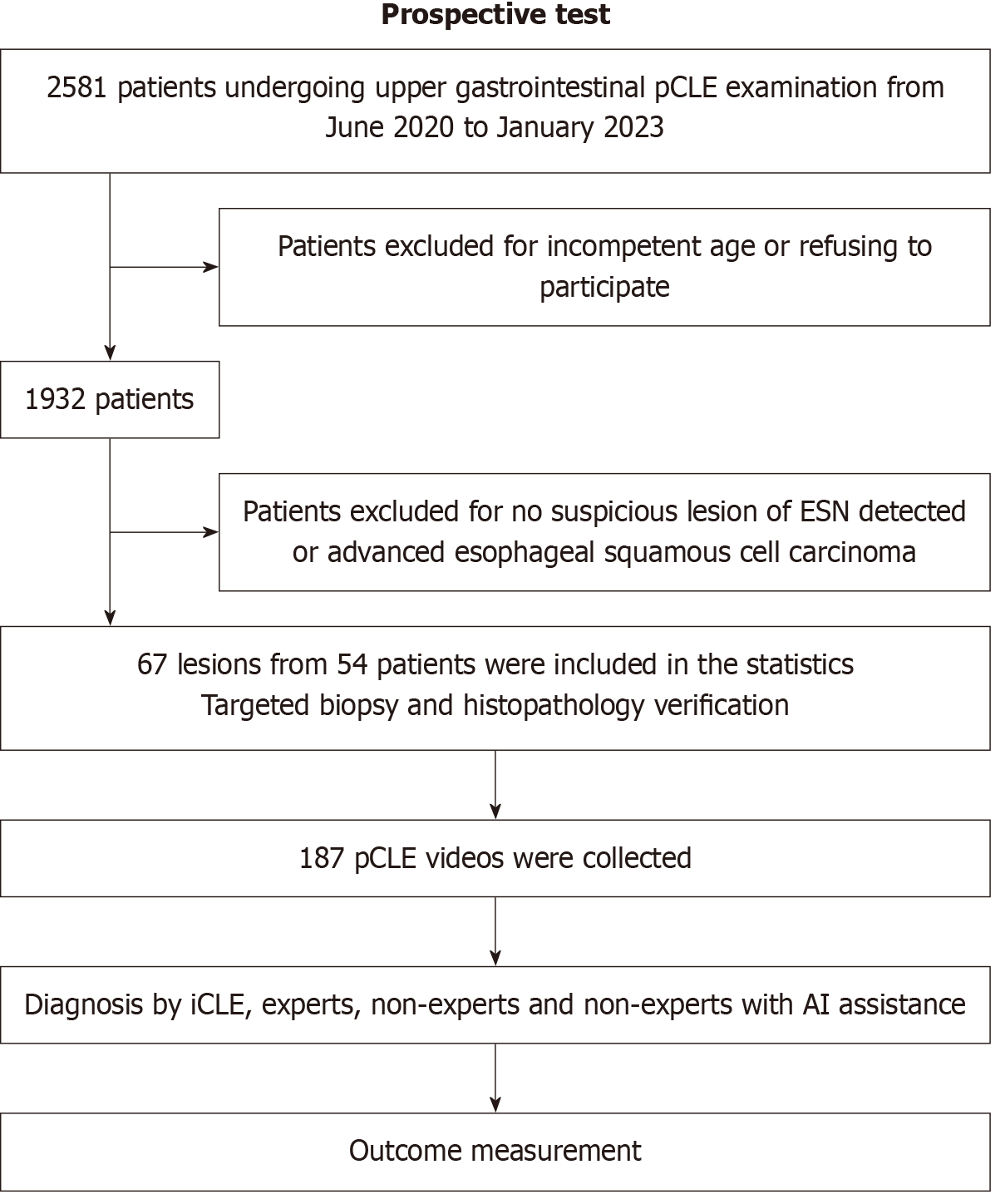
From June 2020 to January 2023, patients who underwent upper gastrointestinal pCLE at the Qilu Hospital of Shandong University for screening, surveillance, or evaluation of gastrointestinal symptoms were included in the study. Patients aged 18-80 years who provided informed consent were included in the study. Exclusion criteria included advanced esophageal SSC or esophageal stenosis, the absence of suspicious ESN lesions on WLE or IEE, known allergy to fluore
The sample size was calculated using PASS15 (NCSS, LLC, Kaysville, MD, United States). We assumed that the sensitivity of non-expert endoscopists for pCLE diagnosis of ESN was 80% and would improve to 90% with iCLE assistance. A sample size of 137 was required to achieve 90% statistical power to detect a difference, using a two-sided exact test with a significance level (alpha) of 0.05. If the dropout rate was 20%, then at least 172 videos should be recognized.
Expert endoscopists, each with experience in more than 100 pCLE examinations, conducted all endoscopic procedures. These were performed using either a GIF-HQ290 endoscope with an Evis Lucera Elite CV-290 system (Olympus, Tokyo, Japan) or an EG29-i10 endoscope with an EPK-i7000 image processor (Pentax, Tokyo, Japan). A Cellvizio probe (Cellvizio, Paris, France) was used to perform the pCLE. Following routine preparation for anesthetic gastroscopy, the patients underwent upper gastrointestinal endoscopy under general anesthesia and electrocardiographic monitoring. The esophagus was observed using both WLE and IEE, and suspicious lesions were recorded along with their macroscopic characteristics, location, and size. All the IEE observations were performed without magnification. Suspicious esophageal lesions were examined using pCLE for further differentiation. Fluorescein sodium (0.02 mL/kg, 10%) was administered intravenously and the pCLE probe was inserted through the working channel of the endoscope. Multipoint scanning was performed from the periphery to the center of the lesion, and the number of observation points was determined based on the lesion size. At each observation point, the probe remained in place until a recognizable, stable video was obtained. Endomicroscopic videos of different lesions were stored separately for further analysis. Finally, targeted biopsies were obtained from the positions with the most histological abnormalities identified using pCLE. All the specimens were sent for histopathological examination and evaluated by experienced pathologists. Diagnosis was based on the Vienna classification[15].
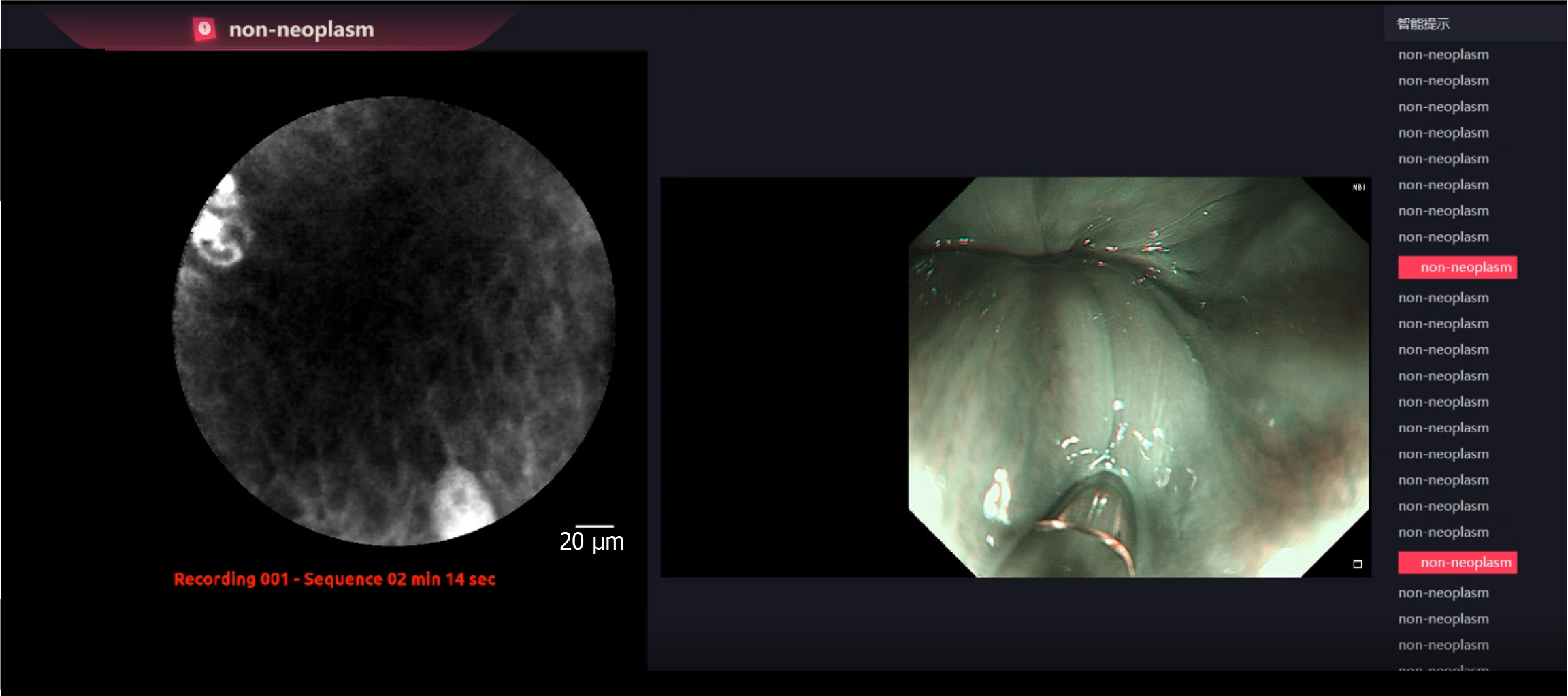
After examination, the pCLE videos were divided into recognizable video segments (containing sufficient pCLE images for diagnosis) and anonymized. For large lesions, pCLE videos were split into multiple non-overlapping video segments. The iCLE and endoscopists (three experts and three non-experts) classified each video as either neoplasm or non-neoplasm. The pCLE videos were displayed on a computer in a predetermined order for each endoscopist to make a diagnosis, whereas the iCLE processed the videos on another computer. Endoscopists were blinded to the endoscopic and pathological diagnoses. Endoscopists with experience in at least 100 pCLE procedures were defined as experts; non-expert endoscopists were those who could perform routine gastroscopy and had learned about pCLE, but had performed fewer than 100 procedures. After a 2-week washout period, non-expert endoscopists examined the videos again, which presented the AI diagnoses. Histological diagnosis was considered the gold standard. The workflow for evaluating the iCLE system is shown in Figure 4. Information on the endoscopists involved in the diagnosis is shown in Supplementary Table 1.
The primary outcome measure was the diagnostic performance of iCLE in a prospective study, assessed using diagnostic accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for ESN classification. The secondary outcome measure was the comparison of the diagnostic performances of iCLE, expert, and non-expert endoscopists, as well as the evaluation of the assistance provided by iCLE to non-expert endoscopists.
McNemar’s test was used to compare the diagnostic performances of iCLE, endoscopists with different expertise levels, and AI-assisted non-experts. Statistical significance was defined as a two-sided P value < 0.05. All statistical analyses were performed using the SPSS version 25 software (IBM Corp., Armonk, NY, United States). All authors have access to the study data and have reviewed and approved the final manuscript.
A total of 3029 patient pCLE images and videos from Qilu Hospital of Shandong University were retrospectively collected. After the selection and deduplication process, a total of 22972 images (3220 neoplasm, 12729 non-neoplasm, and 7023 unqualified images) from 2564 patients were allocated to the training set. Additionally, 2084 images (341 neoplasm, 1002 non-neoplasm, and 741 unqualified images) from 239 patients were assigned to the validation set. Another 2442 images (358 neoplasm, 1362 non-neoplasm, and 722 unqualified images) from 226 patients were used as the test set. A total of 85 video segments were collected to train the video recognition ability. Between June 2020 and January 2023, 2581 patients underwent upper gastrointestinal pCLE examination and were prospectively screened; of these, 54 patients with suspected ESN were enrolled according to the inclusion and exclusion criteria. Eventually, 67 lesions from 54 patients were detected, and 187 videos were used for video testing. No adverse events were reported. The baseline characteristics of the patients and their lesions are shown in Table 1.
| Baseline characteristics | n |
| Patients | 54 |
| Male | 39 (72.2) |
| Female | 15 (27.8) |
| Age, mean (range), years | 59.3 (38-77) |
| Lesions | 67 |
| Lesion size, mean, cm | 1.26 |
| Location | |
| Upper esophagus | 5 |
| Middle esophagus | 35 |
| Lower esophagus | 27 |
| Macroscopic type | |
| I | 3 |
| 0-IIa | 3 |
| 0-IIb | 58 |
| 0-IIa + IIc | 3 |
| Histological diagnosis | |
| Esophageal squamous cell carcinoma | 8 |
| HGIN | 9 |
| LGIN | 8 |
| Inflammatory/hyperplastic lesions | 42 |
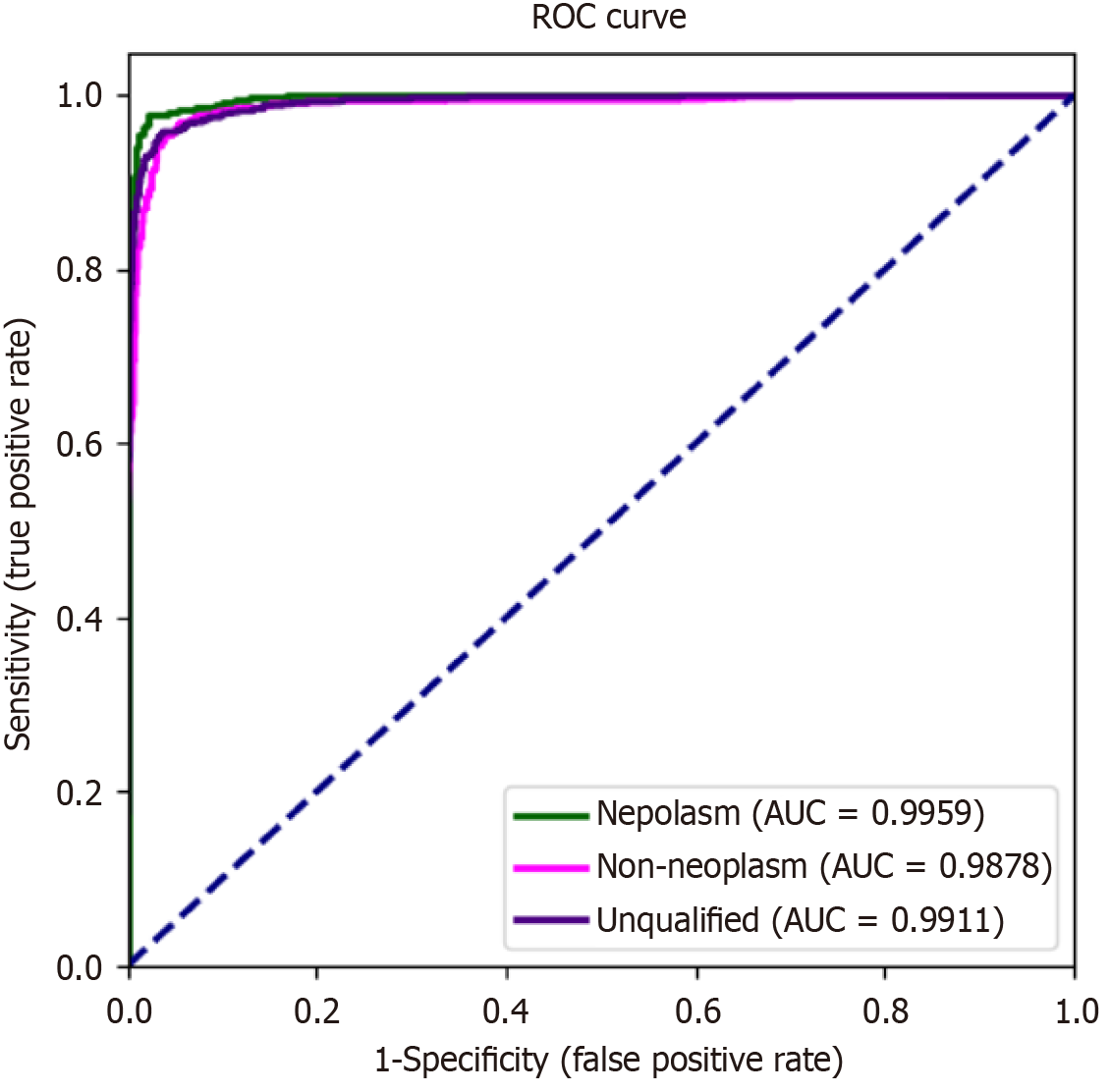
The diagnostic performance of iCLE was satisfactory. iCLE achieved a high accuracy of 98.3% (95% confidence interval, 97.8%-98.8%), sensitivity of 95.3% (93.0%-97.5%), specificity of 98.8% (98.4%-99.3%), PPV of 93.4% (90.9%-96.0%), and NPV of 99.2% (98.8%-99.6%) for diagnosing ESN images. The receiver operating characteristic curve (Figure 5) showed a high area under the curve (0.9878-0.9959). The decision curve analysis curve is shown in Supplementary Figure 3. Table 2 shows the diagnostic performance of the pCLE videos. The diagnostic measurements of iCLE and experts were signi
| Parameter | iCLE, % (95%CI) | Expert, % (95%CI) | NE, % (95%CI) | NE with iCLE, % (95%CI) | P value | Expert vs NE | iCLE vs NE | iCLE vs expert | NE with iCLE vs NE | NE with iCLE vs expert |
| Accuracy | 90.9 (88.5-93.3) | 88.2 (85.6-90.9) | 83.6 (80.5-86.7) | 88.6 (86.0-91.2) | 0.026a | < 0.001a | 0.143 | 0.016a | 0.863 | |
| Sensitivity | 92.0 (88.4-95.6) | 80.4 (75.2-85.7) | 76.0 (70.4-81.6) | 89.8 (85.8-93.8) | 0.254 | < 0.001a | < 0.001a | < 0.001a | 0.005a | |
| Specificity | 90.2 (87.0-93.4) | 93.5 (90.8-96.1) | 88.7 (85.3-92.1) | 87.8 (84.3-91.3) | 0.031a | 0.532 | 0.121 | 0.718 | 0.012a | |
| PPV | 86.3 (81.9-90.6) | 89.2 (84.8-93.5) | 81.8 (76.5-87.1) | 83.1 (78.4-87.9) | 0.035a | 0.199 | 0.354 | 0.718 | 0.068 | |
| NPV | 94.4 (91.9-96.9) | 87.7 (84.3-91.1) | 84.7 (80.9-88.4) | 92.8 (89.9-95.6) | 0.238 | < 0.001a | 0.003a | 0.001a | 0.028a |
In this study, we developed a computer-aided pCLE diagnostic system for ESN diagnosis and evaluated its effectiveness using images and prospective video validation. iCLE achieved high diagnostic performance in both tests and was superior to expert and non-expert endoscopists in video diagnosis. AI assistance significantly improved the diagnostic efficiency of non-expert endoscopists and achieved higher sensitivity than experts. To the best of our knowledge, iCLE is the first AI program for pCLE that enables the in vivo histological evaluation of esophageal squamous epithelial lesions.
The LCE and IEE are the most commonly used techniques for detecting ESN. LCE has high sensitivity but is limited by the low specificity and discomfort associated with the procedure. IEE has higher specificity and similar sensitivity compared to LCE and can be combined with other techniques for optical diagnosis. Our previous study demonstrated that IEE combined with pCLE is an effective diagnostic strategy[8]. pCLE provides real-time histological assessments by visualizing intraepillary capillary and squamous epithelial cells. While IEE identifies suspicious lesions as red flags, pCLE differentiates and guides targeted biopsies, which can improve both detection rates and diagnostic accuracy of ESN. However, ESN diagnosis is based on the SMS system and requires endoscopists to acquire histopathological knowledge and experience. The skills and expertise of endoscopists vary considerably, and interobserver agreement for pCLE diagnosis between non-expert and specialist endoscopists is variable. It is difficult for non-expert endoscopists to achieve sufficient diagnostic performance. As shown in this study, the diagnostic accuracy, specificity, and PPV of non-expert endoscopists were significantly lower than those of the expert endoscopists. The subjective nature of pCLE diagnosis by non-expert endoscopists compromises the reliability of optical biopsies. Therefore, the development of computer-based algorithms for image interpretation is urgently required to improve CLE technology.
Although several effective AI systems have been developed to assist in the detection of ESN using WLE, IEE, or ME-IEE, iCLE represents the first AI system designed for pCLE-based diagnosis of ESN. The iCLE system achieved superior diagnostic performance compared to endoscopists with different expertise levels and demonstrated significantly higher sensitivity and NPV than both non-expert and expert endoscopists. Endoscopists were more cautious of diagnosing neoplastic lesions without the presentation of WLE and IEE images. Additionally, some LGIN lesions were diagnosed as inflammatory lesions by the endoscopists, leading to a lower sensitivity among them. The experts had the highest speci
Accurate biopsy acquisition is essential for determining the appropriate treatment or follow-up strategies. Non-targeted biopsies may result in the incorrect assessment and management of neoplastic lesions, potentially affecting patient prognosis. In routine examinations, although LCE or IEE are used to detect esophageal lesions, many biopsies are performed to distinguish between benign and malignant lesions, especially in cases of multicentric dysplasia, which is characterized by multiple Lugol-voiding lesions (LVLs) observed on LCE. Identifying the most severe lesions in patients with LVLs remains challenging. Generally, endoscopists tend to perform multiple biopsies to ensure histopathological accuracy. However, redundant biopsies increase the cost to patients and the workload of pathologists. In addition, it may lead to submucosal fibrosis of the lesion, complicating ESD and increasing the risk of muscle injury. As demonstrated by the Asian consensus for the diagnosis of upper gastrointestinal neoplasms, biopsy can be avoided if the endoscopic diagnosis achieves high accuracy for benign lesions and a high NPV for neoplasms[16]. iCLE can reduce the difficulty of pCLE analysis and provide objective referential diagnoses for non-expert endoscopists. Considering the high NPV of iCLE (94.4%), biopsies may not be required for non-neoplastic lesions diagnosed using AI, unless endoscopists have concerns. Targeted biopsies should be performed at sites where the AI indicates a neoplasm. Therefore, iCLE has the potential to improve biopsy accuracy and prevent misdiagnoses. It can also reduce the number of biopsies required for diagnosis and the associated costs of histopathological examinations, thereby improving the cost-effectiveness of gastroscopy. Considering the high sensitivity of iCLE, it may be helpful for determining the lateral margins for ESD, particularly in patients with LVLs.
This study has several strengths. First, the sample size of the training set was sufficiently large, encompassing data from 2564 patients. This extensive dataset allowed the AI to comprehensively learn the characteristics of pCLE images, guaranteeing its generalizability. Second, we developed a specialized software to facilitate the deduplication of pCLE images. Images in the test set collected from the same lesion may be highly similar, leading to overfitting of the neural network. Additionally, as it is difficult for humans to objectively assess the degree of resemblance between images, all images collected were selected using the deduplication software and highly similar images were discarded, which decreased selection bias and made the study more objective and convincing. Third, the iCLE was trained to recognize unqualified images and videos. The pCLE examinations may produce unqualified images, particularly when performed by non-expert endoscopists, as rapid movements during image acquisition create artifacts. These unqualified images may lead to misdiagnosis. We specifically designed a neural network in the iCLE to identify unqualified images, trained with a variety of such images and video segments. iCLE does not provide a diagnosis when recognizing unqualified images, which decreases their impact on the diagnosis.
This study has some limitations. Firstly, the diagnoses of the pCLE operators in this prospective study were not included in the comparison. As the operators had access to the WLE and IEE characteristics of the lesions, their diagnosis based on pCLE may have been inevitably affected, potentially leading to an overestimation of diagnostic performance. Second, the patients enrolled consisted of a general outpatient population rather than a high-risk population for ESN; as a result, limited lesions were detected despite the large number of patients undergoing the examination. Third, all the pCLE procedures were performed by experienced endoscopists. Although iCLE can identify unqualified images and videos, its diagnostic efficacy should be further assessed when general endoscopists perform pCLE. Finally, this was a single-center study; therefore, iCLE should be further evaluated in a multicenter trial.
We developed the first pCLE computer-aided diagnostic system for ESN. iCLE can achieve high-level diagnostic per
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 8001] [Article Influence: 8001.0] [Reference Citation Analysis (2)] |
| 2. | Abnet CC, Arnold M, Wei WQ. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology. 2018;154:360-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 1154] [Article Influence: 164.9] [Reference Citation Analysis (1)] |
| 3. | Morgan E, Soerjomataram I, Rumgay H, Coleman HG, Thrift AP, Vignat J, Laversanne M, Ferlay J, Arnold M. The Global Landscape of Esophageal Squamous Cell Carcinoma and Esophageal Adenocarcinoma Incidence and Mortality in 2020 and Projections to 2040: New Estimates From GLOBOCAN 2020. Gastroenterology. 2022;163:649-658.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 548] [Article Influence: 182.7] [Reference Citation Analysis (0)] |
| 4. | di Pietro M, Canto MI, Fitzgerald RC. Endoscopic Management of Early Adenocarcinoma and Squamous Cell Carcinoma of the Esophagus: Screening, Diagnosis, and Therapy. Gastroenterology. 2018;154:421-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 173] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 5. | Muto M, Minashi K, Yano T, Saito Y, Oda I, Nonaka S, Omori T, Sugiura H, Goda K, Kaise M, Inoue H, Ishikawa H, Ochiai A, Shimoda T, Watanabe H, Tajiri H, Saito D. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol. 2010;28:1566-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 523] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 6. | Wang KK, Carr-Locke DL, Singh SK, Neumann H, Bertani H, Galmiche JP, Arsenescu RI, Caillol F, Chang KJ, Chaussade S, Coron E, Costamagna G, Dlugosz A, Ian Gan S, Giovannini M, Gress FG, Haluszka O, Ho KY, Kahaleh M, Konda VJ, Prat F, Shah RJ, Sharma P, Slivka A, Wolfsen HC, Zfass A. Use of probe-based confocal laser endomicroscopy (pCLE) in gastrointestinal applications. A consensus report based on clinical evidence. United European Gastroenterol J. 2015;3:230-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Safatle-Ribeiro AV, Baba ER, Faraj SF, Rios JT, de Lima MS, Martins BC, Geiger SN, Pennacchi C, Gusman C, Kawaguti FS, Uemura RS, de Melo ES, Ribeiro U Jr, Maluf-Filho F. Diagnostic accuracy of probe-based confocal laser endomicroscopy in Lugol-unstained esophageal superficial lesions of patients with head and neck cancer. Gastrointest Endosc. 2017;85:1195-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Guo J, Li CQ, Li M, Zuo XL, Yu T, Liu JW, Liu J, Kou GJ, Li YQ. Diagnostic value of probe-based confocal laser endomicroscopy and high-definition virtual chromoendoscopy in early esophageal squamous neoplasia. Gastrointest Endosc. 2015;81:1346-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | ASGE Technology Committee. Confocal laser endomicroscopy. Gastrointest Endosc. 2014;80:928-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 122] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 10. | Januszewicz W, Witczak K, Wieszczy P, Socha M, Turkot MH, Wojciechowska U, Didkowska J, Kaminski MF, Regula J. Prevalence and risk factors of upper gastrointestinal cancers missed during endoscopy: a nationwide registry-based study. Endoscopy. 2022;54:653-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 11. | Yuan XL, Liu W, Lin YX, Deng QY, Gao YP, Wan L, Zhang B, Zhang T, Zhang WH, Bi XG, Yang GD, Zhu BH, Zhang F, Qin XB, Pan F, Zeng XH, Chaudhry H, Pang MY, Yang J, Zhang JY, Hu B. Effect of an artificial intelligence-assisted system on endoscopic diagnosis of superficial oesophageal squamous cell carcinoma and precancerous lesions: a multicentre, tandem, double-blind, randomised controlled trial. Lancet Gastroenterol Hepatol. 2024;9:34-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 12. | Li SW, Zhang LH, Cai Y, Zhou XB, Fu XY, Song YQ, Xu SW, Tang SP, Luo RQ, Huang Q, Yan LL, He SQ, Zhang Y, Wang J, Ge SQ, Gu BB, Peng JB, Wang Y, Fang LN, Wu WD, Ye WG, Zhu M, Luo DH, Jin XX, Yang HD, Zhou JJ, Wang ZZ, Wu JF, Qin QQ, Lu YD, Wang F, Chen YH, Chen X, Xu SJ, Tung TH, Luo CW, Ye LP, Yu HG, Mao XL. Deep learning assists detection of esophageal cancer and precursor lesions in a prospective, randomized controlled study. Sci Transl Med. 2024;16:eadk5395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Reference Citation Analysis (0)] |
| 13. | Zhang L, Luo R, Tang D, Zhang J, Su Y, Mao X, Ye L, Yao L, Zhou W, Zhou J, Lu Z, Zhang M, Xu Y, Deng Y, Huang X, He C, Xiao Y, Wang J, Wu L, Li J, Zou X, Yu H. Human-Like Artificial Intelligent System for Predicting Invasion Depth of Esophageal Squamous Cell Carcinoma Using Magnifying Narrow-Band Imaging Endoscopy: A Retrospective Multicenter Study. Clin Transl Gastroenterol. 2023;14:e00606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Yuan XL, Liu W, Liu Y, Zeng XH, Mou Y, Wu CC, Ye LS, Zhang YH, He L, Feng J, Zhang WH, Wang J, Chen X, Hu YX, Zhang KH, Hu B. Artificial intelligence for diagnosing microvessels of precancerous lesions and superficial esophageal squamous cell carcinomas: a multicenter study. Surg Endosc. 2022;36:8651-8662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut. 2002;51:130-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 498] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 16. | Chiu PWY, Uedo N, Singh R, Gotoda T, Ng EKW, Yao K, Ang TL, Ho SH, Kikuchi D, Yao F, Pittayanon R, Goda K, Lau JYW, Tajiri H, Inoue H. An Asian consensus on standards of diagnostic upper endoscopy for neoplasia. Gut. 2019;68:186-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |