Published online Apr 7, 2025. doi: 10.3748/wjg.v31.i13.100566
Revised: December 27, 2024
Accepted: March 11, 2025
Published online: April 7, 2025
Processing time: 224 Days and 0.9 Hours
Liver hepatocellular carcinoma (LIHC) is a highly aggressive cancer with poor prognosis due to its complex tumor microenvironment (TME) and immune eva
To explore how the SOCS2 affects Treg activity in LIHC and its impact on tumor growth and metastasis.
LIHC transcriptome data from The Cancer Genome Atlas database were analyzed using Gene Set Enrichment Analysis, Estimation of Stromal and Immune Cells in Malignant Tumors Using Expression Data, and Cell-Type Identification by Esti
SOCS2 overexpression inhibited Treg cell activity, reducing LIHC cell migration and invasion while increasing apoptosis. In vivo, SOCS2 suppressed tumor growth and metastasis, confirming its therapeutic potential.
SOCS2 modulates CD4+ T function in the TME, contributing to LIHC progression. Targeting SOCS2 presents a potential therapeutic strategy for treating LIHC.
Core Tip: This study provides novel insights into the role of the suppressor of cytokine signaling 2 (SOCS2) in inhibiting liver hepatocellular carcinoma (LIHC) growth and metastasis by modulating regulatory T-cell (Treg) activity. Through comprehensive bioinformatics analysis and both in vitro and in vivo experiments, we demonstrated that SOCS2 overexpression suppresses Treg cell activity, enhances cancer cell apoptosis, and reduces tumor migration and invasion. These findings highlight SOCS2 as a potential therapeutic target for improving LIHC prognosis by modulating immune responses in the tumor microenvironment.
- Citation: Lan X, Zhang H, Chen ZY, Wang J, Zhang SC, Li Q, Ke JY, Wei W, Huang R, Tang X, Chen SP, Huang TT, Zhou YW. Suppressor of cytokine signaling 2 modulates regulatory T cell activity to suppress liver hepatocellular carcinoma growth and metastasis. World J Gastroenterol 2025; 31(13): 100566
- URL: https://www.wjgnet.com/1007-9327/full/v31/i13/100566.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i13.100566
Liver hepatocellular carcinoma (LIHC) is one of the most common and deadly malignancies globally, ranking high in both incidence and mortality among all cancers[1-3]. The high prevalence of LIHC is associated with various factors, including chronic viral hepatitis infections (such as hepatitis B and C viruses), prolonged alcohol abuse, non-alcoholic fatty liver disease, and exposure to aflatoxins[4-6]. Despite recent advancements in early detection and treatment of LIHC, such as liver transplantation, radiofrequency ablation, and targeted therapeutic agents like sorafenib, the overall prognosis of LIHC remains poor[7-9]. This is primarily attributed to the high heterogeneity and complex tumor microenvironment (TME) of LIHC, which collectively impede complete tumor eradication, leading to a high propensity for recurrence and metastasis[10-12].
Within the TME, regulatory T cells (Tregs) play a crucial role as immune-suppressive cells that inhibit anti-tumor immune responses through various mechanisms, thus facilitating tumor growth and metastasis[13-15]. Tregs regulate the host's immune response by secreting suppressive cytokines [such as interleukin (IL)-10 and Transforming growth factor β (TGF-β)] and directly interacting with effector T cells, enabling tumor cells to evade immune surveillance[16]. Studies have indicated a close association between high infiltration of Tregs and poor prognosis in various cancer types, inclu
Suppressor of cytokine signaling 2 (SOCS2) is a protein that negatively regulates cytokine signaling and plays a critical role in various immune and inflammatory responses[23]. SOCS2 mainly inhibits the Janus kinase/signal transducer and activator of the transcription (JAK/STAT) signaling pathway, negatively modulating the action of cytokines and im
Recent advances in bioinformatics analysis and high-throughput sequencing technologies have provided new means to elucidate the intricate molecular mechanisms within the TME[29,30]. Analyzing the LIHC transcriptome data from The Cancer Genome Atlas (TCGA) database can yield detailed insights into Treg cell infiltration, gene expression patterns, and their association with prognosis[31]. Gene Set Enrichment Analysis (GSEA) can uncover significant differences in specific gene sets among different groups, thereby offering insights into immune and metabolic pathways[32,33]. Additionally, utilizing ESTIMATE and Cibersort methods allows for the assessment of immune cell infiltration in the TME, while Weighted Gene Co-expression Network Analysis (WGCNA) aids in exploring the relationship between Treg cell infiltration and gene expression patterns. By combining in vitro and in vivo experiments, such as Transwell migration assays, wound healing experiments, and Enzyme-linked immunosorbent assay (ELISA) assays, the specific role of SOCS2 in regulating Treg cell function and tumor progression can be further validated[34,35].
The aim of this study is to investigate the impact of SOCS2 on the cytotoxicity of Treg cells in LIHC and its potential mechanisms on tumor growth and metastasis through a comprehensive approach integrating bioinformatics analysis and in vitro and in vivo experiments. Specifically, we analyzed the LIHC transcriptome data from the TCGA database and utilized methods such as GSEA, ESTIMATE, Cibersort, and WGCNA to extensively explore the role of Treg cells in the LIHC microenvironment and their relationship with SOCS2. Subsequently, through in vitro experiments, we validated the effect of SOCS2 on Treg cell activity and observed its regulatory role in the migration, invasion, and apoptosis of LIHC cells. Finally, using an in vivo mouse model, we assessed the potential role of SOCS2 in suppressing liver cancer growth and metastasis. The study findings help unveil the critical role of SOCS2 in the immune microenvironment of LIHC, providing a novel potential therapeutic strategy to improve the prognosis of LIHC patients.
Data from TCGA database (https://cancergenome.nih.gov/) was downloaded for TCGA-LIHC study, comprising mRNA-seq expression profiles and corresponding clinical information, encompassing a total of 373 tumor tissues and 50 normal tissues. In the cohort, patients with a follow-up time of 0 days, as well as those missing survival status or survival time information, were excluded. Additionally, duplicate patients were merged, resulting in a final cohort of 367 patients for subsequent prognostic analysis. From the ICGC database (https://dcc.icgc.org/), a liver cancer dataset containing 240 tumor samples and 202 normal samples was downloaded. Additionally, the GEO database (https://www.ncbi.nlm.nih.gov/gds) was used to obtain the liver cancer datasets GSE89377 and GSE76427, with GSE89377 including 35 tumor sam
Genes with differential expression (DEGs) were identified using the limma package in the R programming language, based on the criteria of |logFC| > 1 and a P value < 0.05.
For GSEA, the GSEA software (version 3.0) was obtained from the GSEA website. The c7.immunesigdb.v7.4.symbols.gmt subset was downloaded from the Molecular Signatures Database (http://www.gsea-msigdb.org/gsea/downloads.jsp) to assess relevant pathways and molecular mechanisms. Gene expression profiles and phenotype groupings were used, with the minimum gene set size set to 5, the maximum gene set size to 5000, and 1000 permutations performed. Statistical significance was determined when the P value was < 0.05 and the False Discovery Rate (FDR) was < 0.25.
Additionally, the c2.cp.kegg.v7.4.symbols.gmt subset was downloaded to evaluate relevant pathways and mecha
The CIBERSORT algorithm, implemented in R software with the "e1071" and "preprocessCore" packages, was used to evaluate 22 immune-infiltrating cell types based on the expression profile. Statistical analysis and visualization were performed using R version 4.2.1, employing the ggplot2 package.
The expression levels of ABCA8, APCDD1, and SOCS2, and their associations with the 22 immune cell types, were calculated and visualized using the ggpubr and ggExtra packages in R. Immune infiltration scores, stromal scores, and estimate scores were computed using the Estimate package based on predefined markers. Visualization and statistical analysis were also conducted in R version 4.2.1 using ggplot2.
Gene expression profiles were used to compute the Median Absolute Deviation (MAD) for each gene, and the bottom 50% of genes with the smallest MAD values were excluded. Outlier genes and samples were removed using the goodSamplesGenes method from the WGCNA package in R.
A scale-free co-expression network was constructed, with the minimum module size for the gene dendrogram set to 40, a sensitivity of 3, and module merging performed when the dissimilarity was less than 0.25. Ultimately, 35 co-expression modules were identified, with the grey module representing genes that could not be assigned to any specific module.
Based on Treg cell infiltration status, LIHC patients were stratified into high infiltration (Treg-High) and low infiltration (Treg-Low) groups. The correlation between modules and infiltration groups was analyzed using the Pearson correlation test (P < 0.05). The most significant module associated with Treg infiltration-related genes was selected for further analysis.
Univariate Cox regression analysis was performed to identify independent prognostic genes significantly associated with overall survival (OS) in patients (P < 0.05). These independent prognostic genes were subsequently included in the least absolute shrinkage and selection operator (LASSO) Cox regression analysis to establish a risk model. In this model, LASSO penalized the model parameters using the L1 norm to prevent overfitting. The algorithm controlled model complexity by introducing an L1-norm penalty, with the parameter λ regulating the penalty's strength. As λ increased, the penalization of linear models with more variables became stronger. This feature enabled LASSO to shrink parameter values to zero, creating a sparse parameter space ideal for feature selection.
The expression values of candidate genes were used to plot receiver operating characteristic (ROC) curves in both training and validation datasets with the pROC package in R. This approach evaluated the accuracy of disease status prediction based on gene expression.
The risk score (RS) for each patient was calculated by combining the expression levels of 29 genes with their corresponding LASSO coefficients. The formula for calculating the RS was as follows: RS = -0.019ABCA8 + 0.673ANP32C - 0.010APCDD1 + 0.0007CCL25 + 0.0034DKK4 + 0.0125FADS6 + 0.1134FAM166A - 0.5117GULOP - 0.0482HOMER2 + 0.0145IFI44 L + 0.0206IL17D + 0.03676 LPAL2 + 0.5272MPO - 0.0014NOTUM + 0.0736NRAP + 0.0197NXPE3 - 0.0041PXDC1 + 0.00580PXMP2 + 0.0477RSPO4 + 0.0187SELP - 0.0020SLC22A11 - 0.0341SOCS2 - 0.0169SRD5A1 + 0.3830SRGAP2D + 1.9640TEX38 + 0.1091TIAM1 + 0.9052UBE2Q2 L - 0.0320UBXN8 - 9.00227 × ZSCAN5C.
Based on the calculated RS, the samples were divided into high-risk and low-risk groups for further prognosis analysis. Survival differences between the two groups were analyzed using the survfit function in the R package survival, with significance evaluated using the log-rank test. Significant variations in prognosis were observed between the groups.
Kaplan-Meier (KM) curves and ROC analyses were conducted on genes identified by the LASSO model. Prognostic features were constructed by identifying genes with superior prognostic and diagnostic performance. The RS for each patient was determined using the risk scoring formula, defined as: Where n represents the number of genes in the prognostic features, λi denotes the LASSO coefficient for gene i, and Expi signifies the expression value of gene i.
Patients were classified into high-risk or low-risk groups according to the optimal RS cutoff value. The prognostic significance and diagnostic capability of the RS were evaluated using KM curves and ROC analysis. Univariate and multivariate Cox regression analyses were then conducted to confirm the independent prognostic value of the RS under various clinical features.
Independent prognostic features were incorporated into the development of line plots (nomograms). The predictive accuracy of the nomograms for survival was assessed using the concordance index, comparing actual and predicted survival rates. Calibration curves were generated to visualize the agreement between predicted and observed outcomes. Prognostic decision curve analysis (DCA) was performed using the "stdca.R" package in R to evaluate the net clinical benefit.
Proportionate hazard assumption testing and survival regression fitting were carried out using Cox regression with the survival package in R. The results were visualized using the survminer and ggplot2 packages. Within the TCGA-LIHC dataset, the Wilcoxon rank-sum test was applied to determine the differential expression of specific genes between normal and tumor groups. The data were then visualized using the ggplot2 package for clarity.
Peripheral blood mononuclear cells (PBMCs) from human donors were obtained from Hefei All Things Biotechnology Co., Ltd. (Delf-10731). Naïve T cells were isolated from PBMCs using the CD4+ Naïve T Cell Isolation Kit (Catalog No. 11331D, Thermo Fisher). Dynabeads™ were mixed with the PBMC sample in a culture tube for incubation. Using a magnetic separator, bead-bound cells were separated from unbound cells. The positively isolated cells were washed, and DETACHaBEAD™ reagent was added to release the cells. Pure CD4+ naïve T cells without beads were collected.
The isolation of human Tregs from PBMCs was performed using a Treg isolation kit (11363D, Thermo Fisher). Initially, CD4+ T cells were enriched by negative selection with a mixture of monoclonal antibodies targeting human CD4 (MHCD0405, Thermo Fisher) and mouse IgG antibodies against CD14 (13-0149-82, Thermo Fisher), CD16a (11-0168-42, Thermo Fisher), CD56 (MA5-11563, Thermo Fisher), CD123 (306002, BioLegend), CD36 (11-0369-42, Thermo Fisher), CD8 (MA5-13473, Thermo Fisher), CD19 (12-0199-42, Thermo Fisher), and glycophorin A (17-9987-42, Thermo Fisher), in combination with anti-mouse IgG antibodies (11042, Thermo Fisher) conjugated to superparamagnetic polystyrene beads. Subsequently, CD4+CD25high Tregs were purified from the enriched CD4 T cells using magnetic beads coated with anti-CD25 antibodies (11157D, Thermo Fisher). Finally, magnetic beads were removed from the isolated Tregs using DETACHaBEAD® buffer.
CD4+ naïve T cells were cultured on plates coated with anti-CD3 (10 μg/mL, MAB100, R&D Systems) and anti-CD28 (5 μg/mL, MAB3421, R&D Systems) antibodies. RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) and IL-2 (100 U/mL) was used to maintain cell proliferation. Differentiation protocols included the following: Th1 Differentiation: IL-12 (10 ng/mL) and anti-IL-4 antibody (10 μg/mL) were added to the culture medium, and cells were incubated for 5 days to promote interferon (IFN)-γ expression and Th1 differentiation. Th17 Differentiation: IL-6 (20 ng/mL), TGF-β (2.5 ng/mL), IL-23 (10 ng/mL, R&D Systems), and anti-IFN-γ antibody (10 μg/mL, MAB285, R&D Systems) were added to the culture medium, and cells were incubated for 5 days to promote IL-17 expression and Th17 differentiation. Treg Differentiation: TGF-β (5 ng/mL) and IL-2 (20 U/mL) were added to the culture medium, and cells were incubated for 5 days to induce Foxp3 expression and promote Treg differentiation. Recombinant human cytokines, including IL-1β (579402), IL-2 (589102), IL-4 (574004), IL-6 (570802), IL-23 (574102), and TGF-β (580709), were purchased from BioLegend Human Tregs were cultured in RPMI-1640 medium supplemented with IL-2 and TGF-β. Cells were appropriately stimulated using anti-CD3 and anti-CD28 antibodies to promote proliferation while maintaining optimal cell density. The culture conditions were maintained at 37 °C in 5% CO2. Medium changes were performed every two days, and cell morphology was monitored under a microscope. The cells were cultured for 7-10 days.
The Treg cells were collected using cold PBS and then resuspended in 1 × PBS buffer containing 1% bovine serum albumin. Subsequently, cell staining was performed on ice using the following antibodies: CD4 PE (345769, BD), CD25 FITC (11-0257-42, Sigma-Aldrich), LAP FITC (mouse, 141413, BioLegend), LAP FITC (human, 300009, BioLegend), GARP PE (mouse, 142903, BioLegend), and GARP PE (human, 352503, BioLegend) for a staining duration of 30 minutes. Flow cytometry analysis was then conducted using a flow cytometer instrument (C500, Beckman, United States), and data analysis was performed using FlowJo software.
Naïve T cells or Treg cells in the logarithmic growth phase were collected and overexpressed with SOCS2 through lentivirus infection. The cells were divided into two groups: Treg+oe-NC group (infected with empty lentivirus) and Treg+oe-SOCS2 group (infected with lentivirus overexpressing SOCS2). The lentivirus for overexpression was constructed and provided by Shanghai Genecore Biotechnology Co., Ltd. The infection procedure was as follows: Treg cells in the logarithmic growth phase were suspended at a concentration of 5 × 104 cells/mL, seeded in a 6-well plate with 2 mL per well, and incubated at 37 °C overnight. Subsequently, each well was infected with overexpressing recombinant lentivirus at a final concentration of 1 × 108 TU/mL, followed by further experiments after 48 hours.
Huh-7 cells (SCSP-526, ATCC) were cultured in RPMI-1640 medium containing 10% fetal bovine serum (12483020, Gibco), 100 units/mL penicillin, and 100 μg/mL streptomycin (C0222, Beyotime).
For the co-culture experiment, a 24-well plate with inner wells containing pores of 0.4 μm (FTW070, Beyotime) was used to physically separate CD4 T cells and Huh-7 cells (HB-8065, ATCC). Huh-7 cells (2 × 105 cells per well) were grown in 1.5 mL of RPMI-1640 medium with 10% fetal bovine serum in the outer wells. Subsequently, isolated human CD4 T cells (4 × 105 cells per well) were added to the inner wells containing RPMI-1640 medium supplemented with IL-2. The CD4 T cells were moderately activated with anti-CD3/CD28 antibodies, and the cell density was maintained to promote proliferation. The cells were cultured at 37 °C in a 5% CO2 atmosphere for 7-10 days. During this period, the medium was replaced every two days, and cell morphology was monitored via microscopy. Afterward, CD4 T cells and Huh-7 cells were collected, washed with PBS, and prepared for subsequent experiments.
The cell grouping is as follows: Control group (inner wells without any cells), CD4+T group (with added CD4 T cells), oe-NC-CD4 T group (with CD4 T cells infected with empty overexpressing lentivirus), and oe-SOCS2-CD4 T group (with CD4 T cells infected with overexpressing SOCS2 Lentivirus).
The invasive potential of Huh-7 cells was evaluated using a Transwell assay. The Transwell chambers (353097; BD Biosciences) were pre-coated with a Matrigel (356234; BD Bioscience) basement membrane, and a polycarbonate membrane separated the upper and lower chambers. Both chambers were filled with RPMI-1640 medium. Huh-7 cells, treated through co-culture, were harvested and reseeded in the upper chamber at a density of 2 × 105 cells per well. The chambers were incubated in a 5% CO2, 37 °C humidified incubator for 24 hours. After 48 hours, the non-migrated cells in the upper chamber were removed with a cotton swab. The cells on the underside of the membrane were stained with 0.05% crystal violet (C0121, Beyotime) and observed under an inverted microscope.
Following a 24-hour co-culture of cells, a wound healing experiment was conducted. Huh-7 cells (1 × 105 cells per well) were seeded in a 24-well plate to achieve a confluence of 60%-70%. A plastic pipette tip was used to create a wound on the cell monolayer, which was then washed twice with PBS buffer. Subsequently, the monolayer of cells was incubated in serum-free RPMI-1640 medium at 37 °C for 24 hours. Finally, five random fields at the edge of the wound were observed under an inverted microscope.
Huh-7 cells were collected by centrifugation after treatment. The cells were centrifuged at 1000 × g in pre-cooled PBS for 5 minutes, and the supernatant was discarded. This step was repeated twice. Subsequently, the cells were resuspended in 500 μL pre-cooled PBS and stained with the dual dye reagent V-FITC/propidium iodide for apoptosis analysis using flow cytometry (CytoFLEX, Beckman Coulter, Brea, CA, United States). Finally, data analysis was performed using FlowJo software.
LDH activity was measured using the LDH Cytotoxicity Assay Kit (C0016, Beyotime) following the manufacturer's protocol. Briefly, 50 µL of conditioned media (CM) was collected from each sample. The cells were lysed at 37 °C for 45 minutes using 10 µL of lysis buffer provided in the assay kit, and then 50 µL of lysed cells was collected from each sample. LDH levels were measured in both the CM and lysed samples. Absorbance was measured at 490 nm and 680 nm using the Epoch Microplate Spectrophotometer (Bio-Tek, Winooski, VT, United States).
Cell toxicity was assessed using CCK-8 (C0037, Beyotime). Huh-7 cells, treated with co-culture, were seeded in a 96-well plate at a density of 4 × 103 cells per well with 100 μL of medium. After 24 hours of incubation, 10 μL of CCK-8 solution was added to each well, followed by further incubation for 120 minutes before measuring the optical density at 450 value.
The culture supernatant was centrifuged at 1500 × g for 15 minutes. Following the instructions provided in the ELISA antibody manual, IL-10 (human, E-HSEL-H0005, Elabscience) and IFN-γ (human, E-EL-H0108, Elabscience) were added to each well with 100 μL of dilution buffer, 100 μL of sample, and 100 μL of standard. After an incubation period of 90 minutes at 37 °C, the wells were then incubated with 100 μL of biotinylated detection antibody at 37 °C for 1 hour; subsequently, the samples were washed three times with a cleaning agent for 2 minutes each time. Following this, 100 μL of horseradish peroxidase conjugate was added to each well and incubated at 37 °C for 30 minutes. After five washes, a substrate reagent was added and incubated in the dark at 37 °C for 15 minutes to stop the reaction. The absorbance was measured at 450 nm using the Epoch microplate spectrophotometer (Bio-Tek, Winooski, VT, United States). Each sample was run in triplicate wells.
Balb/c nude mice [BALB/c-Tg(D011.10)10 Loh/J were acquired from Shanghai Slike Experimental Animal Co., Ltd.] to establish a subcutaneous transplantation liver cancer mouse model. The experimental groups were as follows: The control group (injected with 1 × 106 Huh-7 cells in 100 μL), the Treg group (injected with 1 × 106 Huh-7 cells in 100 μL and 5 × 105 Treg cells in 100 μL), the oe-NC-Treg group (injected with 1 × 106 Huh-7 cells in 100 μL and 5 × 105 oe-NC-Treg cells in 100 μL), and the oe-SOCS2-Treg group (injected with 1 × 106 Huh-7 cells in 100 μL and 5 × 105 oe-SOCS2-Treg cells in 100 μL). Tumor volume was measured weekly following cell injection on the right side of the mice. On day 35, the mice were euthanized, and tumor samples were collected for further analysis[36].
Flow cytometry analysis of Treg, Th1, and Th17 cells in mouse spleen and lymph node tissues was performed as follows: First, tumor tissues were excised from mice, minced, and digested with Collagenase IV and DNase I at 37 °C to prepare a single-cell suspension, which was filtered through a 70 µm cell strainer. If necessary, red blood cells were removed using a red blood cell lysis buffer. Subsequently, 106 cells were incubated with surface marker antibodies, including FITC anti-mouse CD4 (Catalog No. 100405, BioLegend) and PE anti-mouse CD25 (Catalog No. A26508, ABclonal) for Treg cells; FITC anti-mouse CD4 and APC anti-mouse IFN-γ (Catalog No. 554413, BD Bioscience) for Th1 cells; and FITC anti-mouse CD4 and PE anti-mouse IL-17A (Catalog No. 559502, BD Bioscience) for Th17 cells. Incubation was conducted on ice, protected from light, for 30 minutes. For intracellular markers such as FoxP3, IFN-γ, and IL-17A, cells were fixed and permeabilized before staining with corresponding fluorescent antibodies. Finally, data were acquired using a BD FACSCanto™ II flow cytometer (BD, United States) and analyzed with FlowJo software to determine the proportion and functional characteristics of cell subsets[37].
Following the euthanasia of the mice, tumor samples were collected and fixed in a 4% formaldehyde solution. The skin was embedded in paraffin, sectioned into 4 μm thick slices, and stained with Hematoxylin and eosin (H&E) staining reagent (Product No. C0105M, Beyotime) for one minute. Subsequently, the slices were rinsed in running water until clear. The tissue was then counterstained in an eosin solution for 15 seconds. After counterstaining, the tissue sections were immediately transferred to 95% ethanol, followed by dehydration through 100% ethanol and xylene. Prior to imaging, the slides were fixed with neutral balsam mounting medium (Product No. C1795, Sigma) and air-dried. Finally, imaging was conducted using a microscope.
Following the euthanasia of the mice, tumor samples were collected and fixed in a 4% formaldehyde solution (Product No. P0147A, Beyotime). The skin was embedded in paraffin and sectioned into 4 μm thick slices, and antigen retrieval was performed by heating the slices in a microwave with EDTA solution (Product No. P0085, Beyotime). Subsequently, the slices were treated with 3% hydrogen peroxide and blocked using goat serum (Product No. C0265, Beyotime). The primary antibody, FOXP3 (98377, CST), was applied to the slices and incubated overnight at 4 °C. Following this, the slices were incubated with biotinylated IgG secondary antibody and then with avidin-biotin-peroxidase complex for 20 minutes. Finally, signal detection was carried out using a DAB reagent (Product No. P0202, Beyotime). Scanning and analysis were performed using the Pannoramic Midi scanner (3DHISTECH).
The TUNEL assay kit (C1088, Biyuntian) was utilized to assess apoptosis in tumor cells. In brief, after deparaffinization of tumor sections, proteinase K (ST533, Beyotime) was employed to remove proteins from the cell nuclei. Subsequently, tissue slices were immersed in 3% hydrogen peroxide for 10 minutes, incubated with terminal deoxynucleotidyl transferase enzyme (EP0161, Thermo Scientific) for 1 hour at 37 °C, followed by incubation with chain-specific avidin-FITC (S3762, Merck) for 30 minutes at 37 °C. Lastly, apoptosis in tumor tissues was examined using a confocal microscope (LMS710, ZEISS).
BALB/c nude mice were utilized for the tail vein injection experiment to assess the metastatic capacity of LIHC cells in vivo. The experiment was divided into the following groups: The control group (injected with 1 × 106 Huh-7 cells in 100 μL), Tregs group (injected with 1 × 106 Huh-7 cells in 100 μL and 5 × 105 Treg cells in 100 μL), oe-NC-Treg group (injected with 1 × 106 Huh-7 cells in 100 μL and 5 × 105 oe-NC-Treg cells in 100 μL), and oe-SOCS2-Treg group (injected with 1 × 106 Huh-7 cells in 100 μL and 5 × 105 oe-SOCS2-Treg cells in 100 μL). The cells were injected into the tail vein of the nude mice. After 8 weeks, the mice's lungs were extracted and embedded in paraffin for H&E staining, and the number of lung nodules was calculated[38].
The data presented in this study are the means ± SD of at least three independent experimental trials. A two-sample independent t-test was employed to compare the two groups; for comparisons involving three or more groups, a one-way analysis of variance (ANOVA) was conducted. Following a significant result in the ANOVA, Tukey's Honestly Significant Difference post-hoc test was used to further compare differences between individual groups. In cases where data did not follow a normal distribution or exhibited unequal variances, the Mann-Whitney U test or Kruskal-Wallis H test was applied. Statistical analyses were performed using GraphPad Prism 9 (GraphPad Software, Inc.) and the R programming language. A significance level of 0.05 was set, and a two-tailed P value < 0.05 was considered statistically significant.
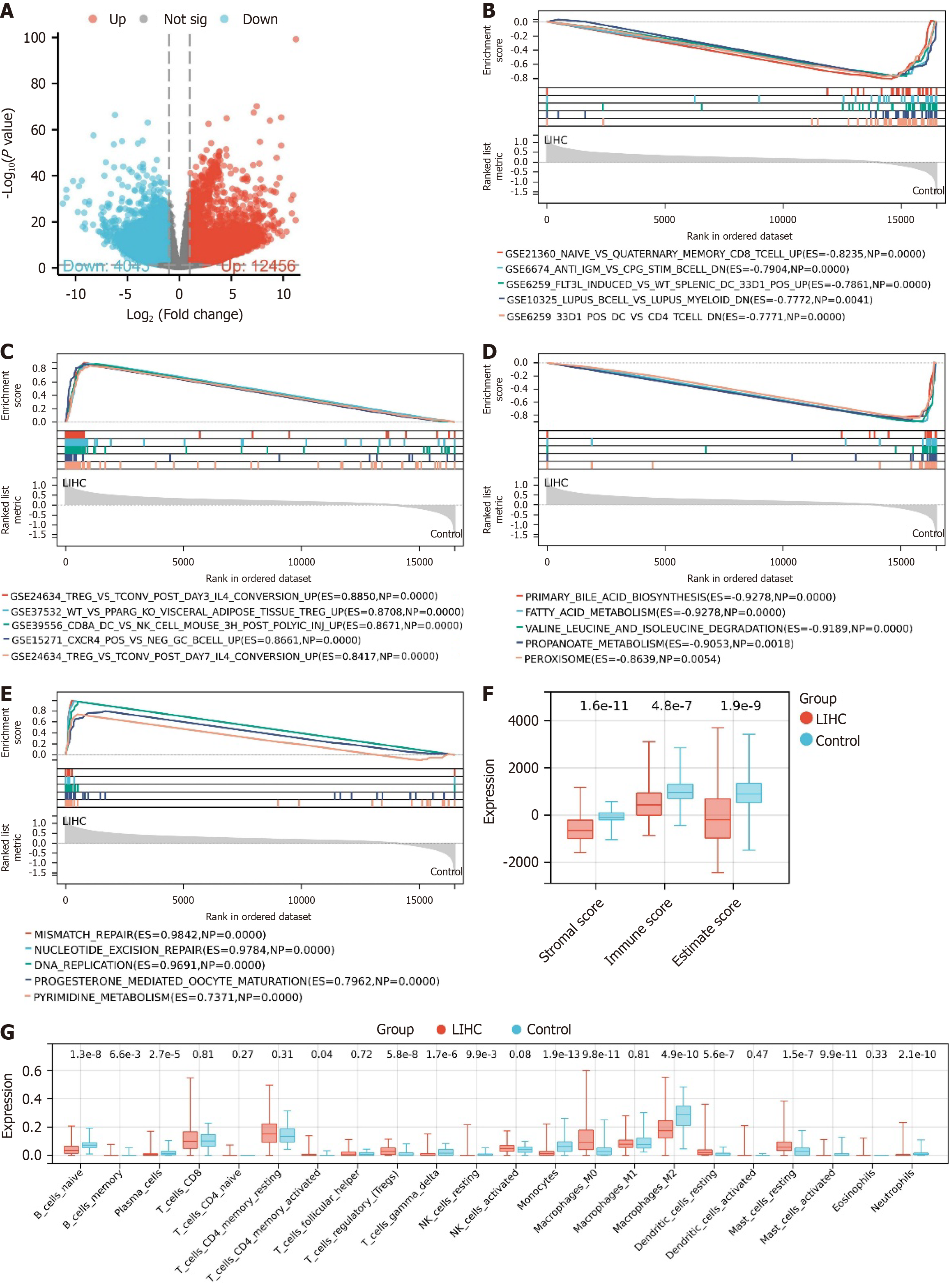
Transcriptome sequencing data for LIHC, comprising 373 tumor tissues and 50 normal tissues, were downloaded from the TCGA database. Differential analysis was conducted using the "LIMMA" package, identifying 164991 significantly DEGs, including 4043 downregulated genes and 12456 upregulated genes (Figure 1A). Initially, GSEA enrichment analysis using the ImmuneSigDB gene set revealed associations between the Control group and CD8+ T cells, B cells, and CD4+ T cells (Figure 1B). Conversely, in the LIHC group, enrichment analysis indicated a higher prevalence of signaling pathways associated with Treg, as well as connections to CD8α+ dendritic cells, NK cells, and B cells (Figure 1C).
Subsequently, employing the KEGG gene set for GSEA enrichment analysis revealed that the Control group was predominantly enriched in primary bile acid biosynthesis, fatty acid metabolism, degradation of isoleucine, leucine, and valine, propionate metabolism, and peroxisomes (Figure 1D). In contrast, the LIHC group exhibited significant enrich
Using the ESTIMATE method for immune infiltration analysis, it was observed that in comparison to the control group, the LIHC group showed notably elevated stromal score, immune score, and ESTIMATE score. This suggests a significant increase in the infiltration levels of stromal and immune cells in LIHC samples compared to the control group, indicating significant alterations in the immune and stromal components within the TME (Figure 1F).
Furthermore, CIBERSORT immune infiltration analysis results demonstrated significant variations in the infiltration levels of various immune cell types in the LIHC group. Specifically, the expression levels of naive B cells, CD8+ T cells, Treg cells, M0 and M1 macrophages, and activated dendritic cells were significantly upregulated, while the expression levels of memory B cells, resting NK cells, and neutrophils were significantly downregulated (Figure 1G).
Combining the results of GSEA and immune infiltration analysis, we identified a significant association between Treg cells and samples from the LIHC group. Studies have indicated that tumor-infiltrating lymphocytes (TIL) often suppress the activity of T effector cells, potentially leading to cancer progression. Tregs inhibit self-reactive T cells and anti-tumor immune responses[39]. Within TIL, Tregs are abundant and highly activated[40], believed to be a primary mechanism of tumor-induced immune suppression[41]. Therefore, this study focuses on Treg cells.
Collectively, the results indicate significant gene expression changes and reshaping of the immune microenvironment in LIHC, particularly the significant upregulation of Treg cells, revealing the complex immune regulatory mechanisms involved in tumor progression.
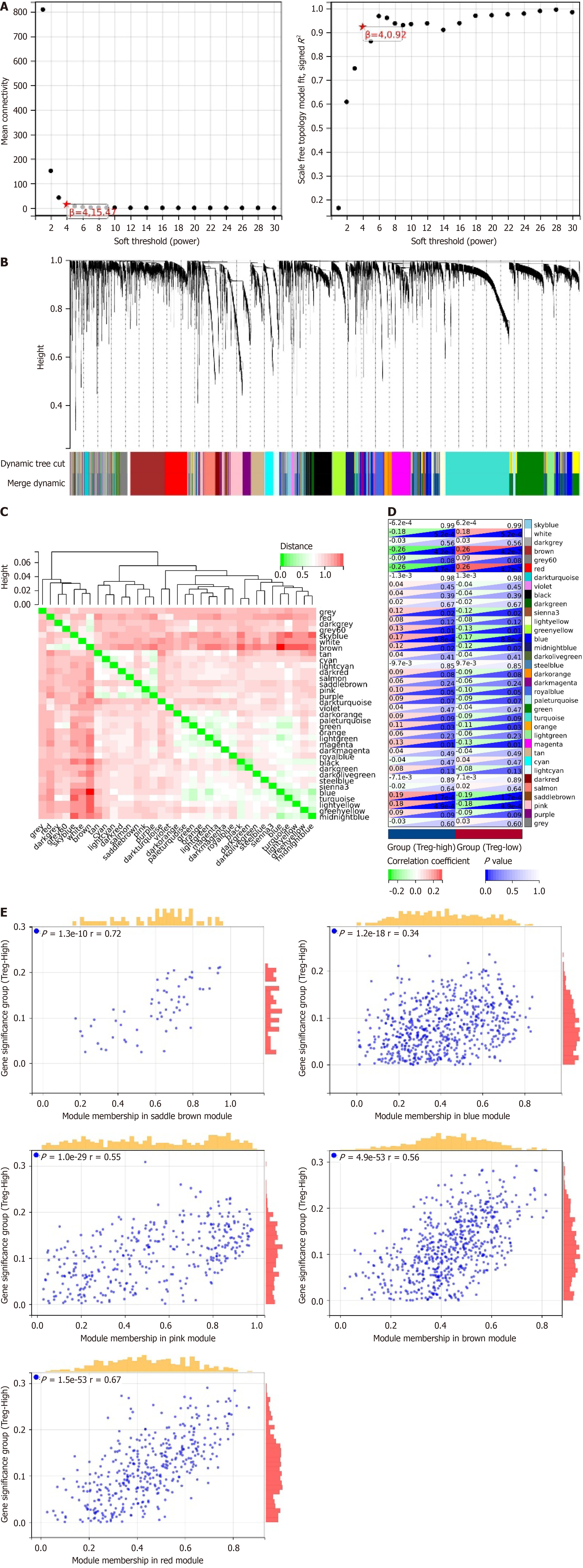
Our study revealed a significant increase in Treg cells within tumors of the LIHC group. To further investigate the underlying molecular mechanisms, we performed WGCNA using a selection of genes from the tumor group among the 16491 DEGs mentioned earlier. Setting the soft threshold parameter β to 4 to satisfy scale-free network properties, with a minimum module size of 40 and a module merge threshold of 0.25, a total of 35 modules were identified (Figure 2A), as depicted in the dendrogram in Figure 2B. Figure 2C illustrates the interrelations among these 35 modules.
By categorizing the Treg cell numbers into a high infiltration group (Treg-High) and a low infiltration group (Treg-Low), we further analyzed the significantly correlated gene modules. Notably, the red module showed a significant negative correlation with Treg-High (correlation = -0.26, P = 4.7e-7), as did the brown module (correlation = -0.26, P = 4.7e-7). Conversely, the blue module exhibited a significant positive correlation with Treg-High (correlation = 0.17, P = 8.8e-4), along with the saddlebrown module (correlation = 0.19, P = 1.7e-4), and the pink module (correlation = 0.18, P = 4.9e-4; Figure 2D and E).
Based on the above results, we selected the genes from these 5 modules as significantly correlated with the infiltration of Treg cells, totaling 2102 genes. Through the WGCNA method, we successfully identified gene modules closely associated with Treg cell infiltration, revealing the potential molecular mechanisms underlying Treg cell infiltration in LIHC.
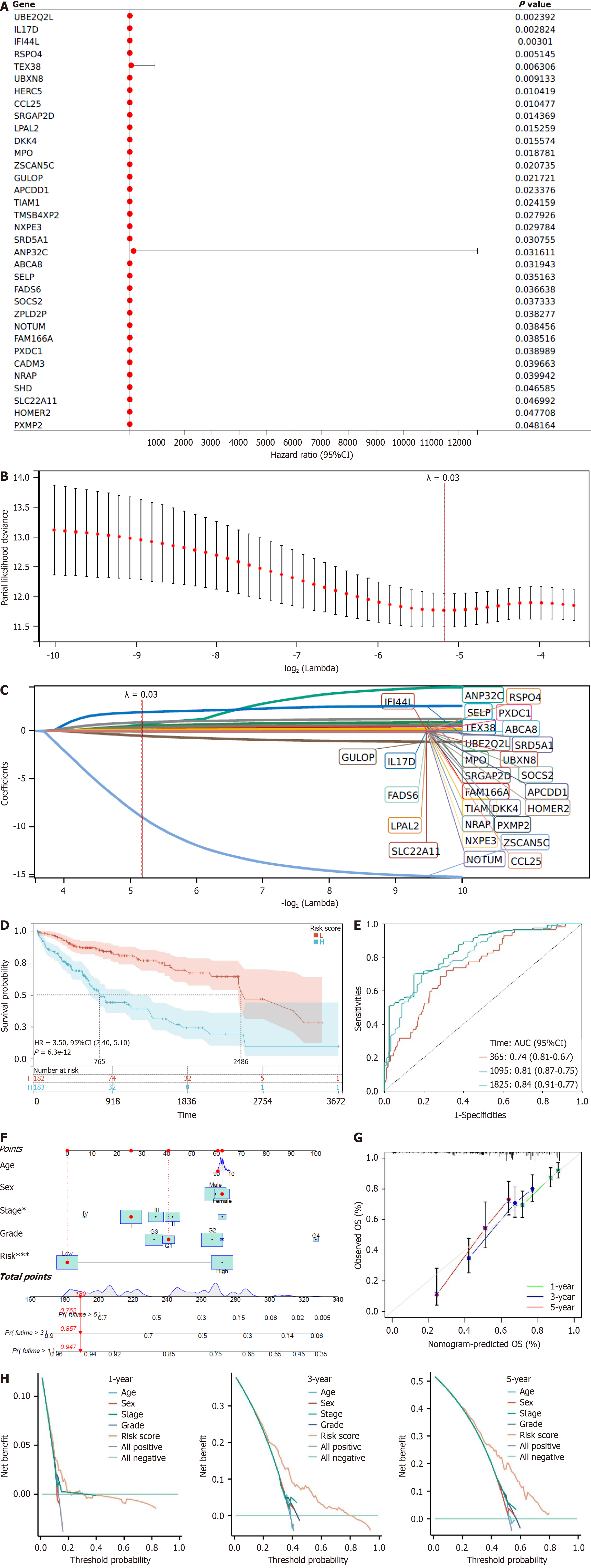
The analysis identified a total of 2102 DEGs significantly associated with Treg cell infiltration, which reduced to 1173 genes after filtering out non-coding RNA. Subsequent single-factor COX regression analysis identified 34 DEGs significantly correlated with OS rate (P < 0.05; Figure 3A). Further LASSO COX analysis with a set Lambda value of 0.03 integrated survival time, survival status, and gene expression data, resulting in the refinement of independent prognostic genes to 29 (Figure 3B and C, Supplementary Table 1).
The RS of patients was calculated by linearly combining the expression levels of these 29 genes with their corresponding LASSO coefficients[42]. Using the median RS, the TCGA-LIHC cohort was further divided into the high-risk group (n = 182) and low-risk group (n = 185), with patients having unknown survival status or survival time of 0 being excluded. KM curves were employed to compare the prognosis differences between the two groups, demonstrating a significant divergence (P < 0.001), with markedly lower survival rates in the high-risk group compared to the low-risk group (Figure 3D). Furthermore, ROC analysis at 1, 3, and 5 years revealed AUCs of 0.74, 0.81, and 0.84, respectively, for survival prediction (Figure 3E). In conclusion, higher RSs correspond to poorer prognosis and lower survival rates for patients.
Furthermore, we constructed nomograms incorporating stage, grade, age, and risk group to accurately predict the 1-year, 3-year, and 5-year survival of LIHC patients. A higher total score indicates lower survival rates (Figure 3F). Calibration curves demonstrated a high consistency between the predicted OS rates and the actual observed survival rates at 1, 3, and 5 years, indicating the high accuracy of prognostic gene markers (Figure 3G). DCA revealed that the gene nomograms exhibit strong clinical prognostic capabilities. The net benefit results indicated that the combined model outperformed the single-factor model, suggesting that the nomograms can assist clinicians in assessing patients' prog
We identified key genes significantly associated with Treg cell infiltration and OS in LIHC patients and selected 29 independent prognostic genes through WGCNA and LASSO Cox analysis.
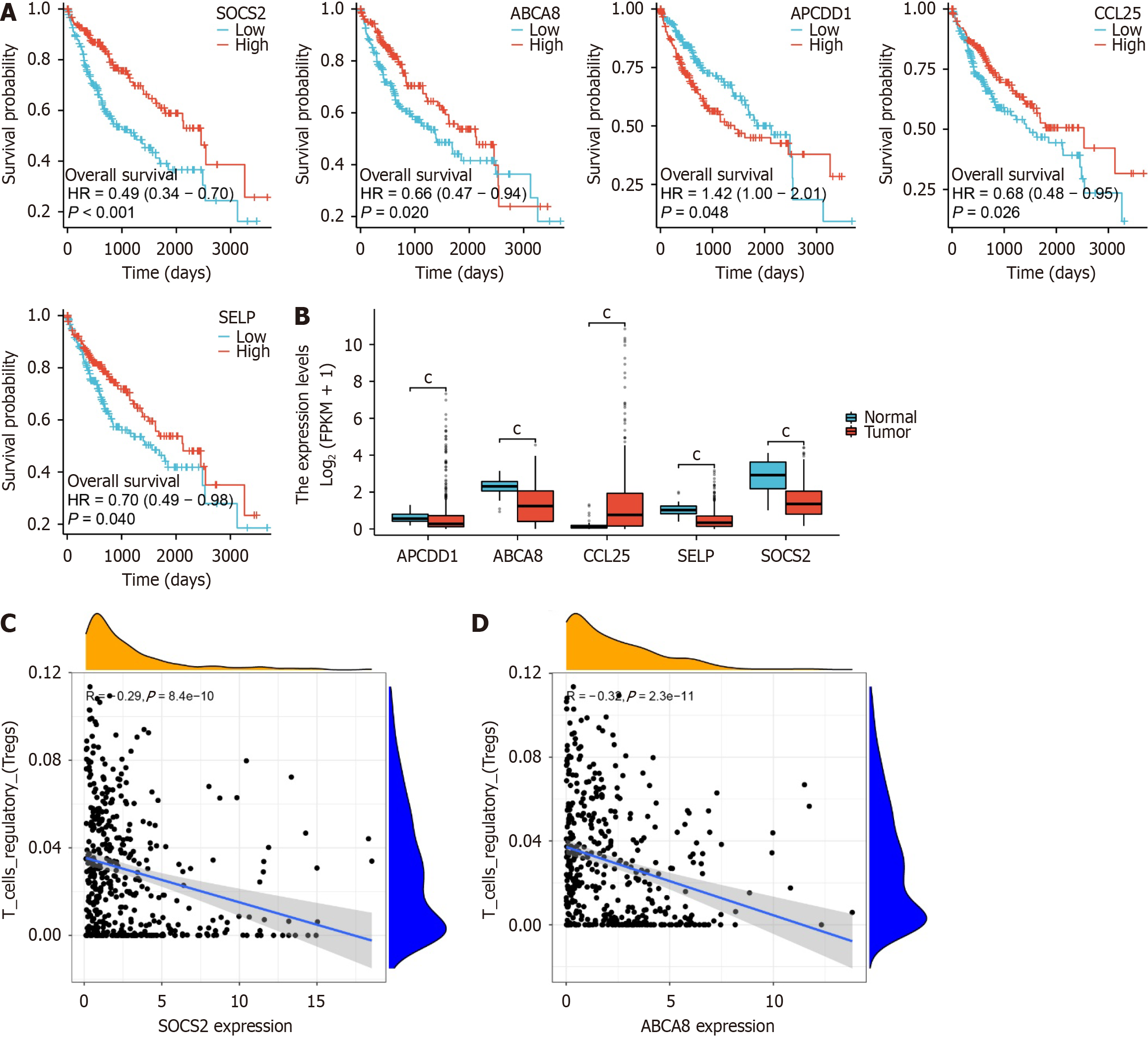
Through the aforementioned analysis, we identified 29 DEGs that significantly influence the prognosis of LIHC. Subsequently, survival analysis was conducted individually for these 29 genes in the TCGA-LIHC dataset. The results indicated that low expression of ABCA8, CCL25, SELP, and SOCS2 was associated with poorer survival outcomes, whereas the low expression of APCDD1 was correlated with longer survival time (Figure 4A). The remaining 24 genes did not exhibit significant differences (Supplementary Figure 1).
Furthermore, the box plots illustrate the expression profiles of these five genes. It was observed that APCDD1 had significantly lower expression in the tumor group, whereas CCL25 displayed significantly higher expression in the tumor group, which contradicted the survival analysis results (Figure 4B). Consequently, we have excluded APCDD1 and CCL25 from further consideration.
Subsequently, we analyzed the correlation of ABCA8, SELP, and SOCS2 with immune cell infiltration and found no correlation between SELP and Treg cells. However, both ABCA8 (R = -0.32, P = 2.3e-11) and SOCS2 (R = -0.29, P = 8.4e-10) showed significant negative correlations with Treg cells (Figure 4C and D, Supplementary Figures 2-4).
In addition, comprehensive survival curve analysis revealed that SOCS2 exhibited higher significance (P < 0.001) compared to ABCA8 (P = 0.02; Figure 4A). Studies have indicated that SOCS2 can promote ferroptosis and radio-sensitization in LIHC[26] and inhibit the progression of LIHC[43]. Furthermore, in mice lacking SOCS2, there was a significant increase in Treg cell numbers[44]. Differential expression analysis of liver cancer datasets from the ICGC and GEO databases also revealed that SOCS2 was significantly downregulated in tumor tissues (Supplementary Figure 5). Based on this evidence, we preliminarily suggest a certain correlation between the lack of SOCS2 and the progression of LIHC.
Through machine learning, we successfully identified 29 genes significantly associated with LIHC prognosis. These genes, validated through multiple steps, have demonstrated robust prognostic predictive ability. Integrating survival analysis and immune infiltration analysis, we ultimately identified SOCS2 as a key gene significantly associated with Treg cells in LIHC.
Based on bioinformatics analysis, significant gene expression changes and immune microenvironment remodeling were observed in LIHC, particularly with a marked increase in Treg cells, suggesting that elevated Treg cells may suppress the functions of other effector CD4 T cells. To investigate the impact of CD4 T cells on liver cancer, CD4 T cells were co-cultured with Huh-7 cells.
The Transwell assay results demonstrated that the invasive ability of Huh-7 cells in the CD4 T cell group was significantly reduced compared to the Control group (Supplementary Figure 6A). Wound healing assay results showed that the migration ability of Huh-7 cells in the CD4 T co-culture group was weaker than that in the Control group (Supplementary Figure 6B). Apoptosis detection revealed that the apoptosis rate of Huh-7 cells in the CD4 T group was significantly higher compared to the Control group after co-culture treatment (Supplementary Figure 6C).
Cell apoptosis leads to the release of LDH into the extracellular environment, and LDH levels can be used to infer the degree of apoptosis[45]. Measurement of LDH release in Huh-7 cells after co-culture treatment indicated a significant increase in LDH release in the CD4 T group compared to the Control group (Supplementary Figure 6D). CCK-8 assay results showed that the viability of Huh-7 cells in the CD4 T group decreased after co-culture treatment compared to the Control group (Supplementary Figure 6E). In summary, these findings indicate that CD4 T cells have the ability to inhibit the growth of liver cancer cells.
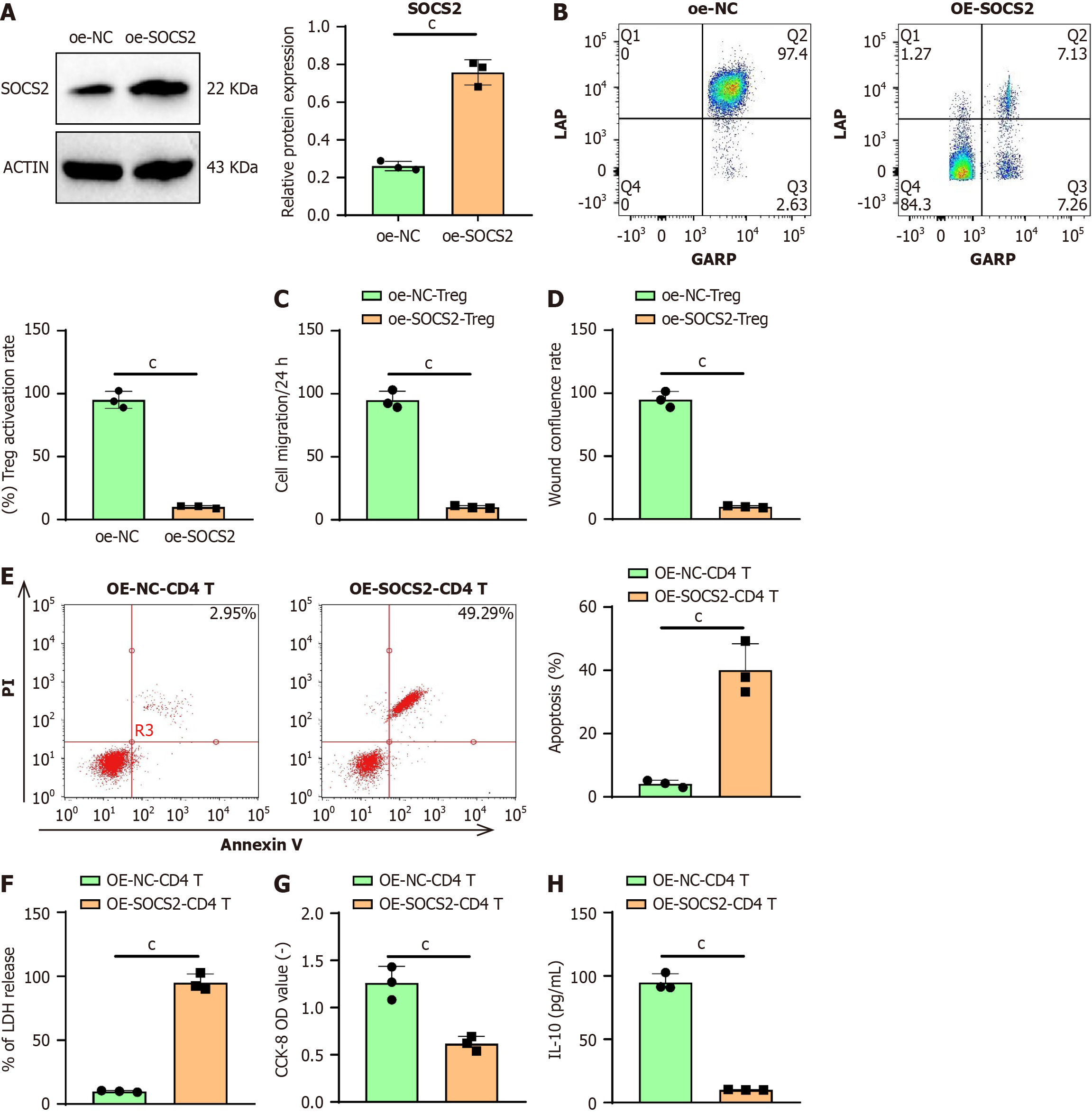
Based on the results of bioinformatics analysis, we further explored the impact of SOCS2 on the antitumor functions of CD4 T cells. Given the negative correlation between SOCS2 and Treg cells, we performed overexpression experiments of SOCS2 in CD4 T cells. Western blot analysis revealed that SOCS2 protein levels were significantly elevated in CD4 T cells in the OE-SOCS2 group compared to the OE-NC group (Figure 5A). GARP and LAP are major markers of Treg cell activation[46]. Flow cytometry showed a reduction in the number of activated Treg cells within CD4 T cells in the OE-SOCS2 group compared to the OE-NC group (Figure 5B).
The results of the Transwell assay indicated that the invasive ability of Huh-7 cells was significantly reduced in the oe-SOCS2-CD4 T group compared to the oe-NC-CD4 T group (Figure 5C). Similarly, wound healing assay results demon
The apoptosis detection results indicate that in co-cultured Huh-7 cells, the apoptosis rate in the oe-SOCS2-CD4 T group was significantly higher compared to the oe-NC-CD4 T group (Figure 5E). The LDH release assay results show that in co-cultured Huh-7 cells, the LDH release level in the oe-SOCS2-CD4 T group increased relative to the oe-NC-CD4 T group (Figure 5F). CCK-8 assay results demonstrate that in co-cultured Huh-7 cells, cell viability in the oe-SOCS2-Treg group was lower than in the oe-NC-Treg group (Figure 5G). ELISA analysis reveals that in co-cultured Huh-7 cells, the expression level of IL-10 in the oe-SOCS2-CD4 T group decreased compared to the oe-NC-CD4 T group (Figure 5H).
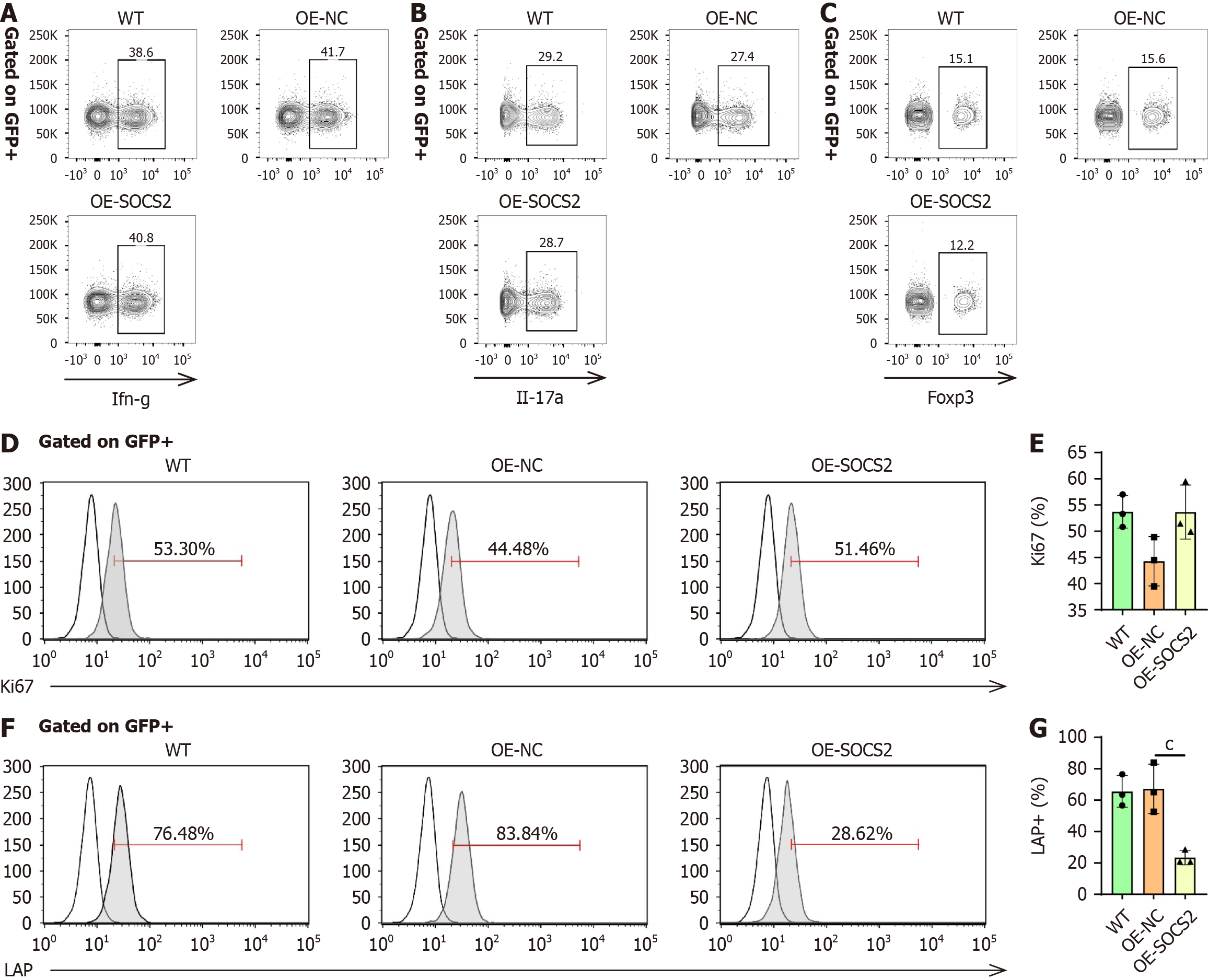
To further elucidate the effects of SOCS2 on Treg cell function, we isolated CD4 naïve T cells and transduced them with SOCS2 Lentivirus. The differentiation of CD4 naïve T cells into Th1, Th17, and Treg cells was evaluated. The results showed that SOCS2 overexpression did not affect the differentiation of CD4 naïve T cells into Th1, Th17, or Treg cells (Figure 6A-C).
Next, Treg cells were isolated from human PBMCs. Following purification, the proportion of Treg cells reached 97% (Supplementary Figure 7). Lentiviral transduction was used to overexpress SOCS2 in Treg cells, and its effects on Treg cell function were examined. The results showed that SOCS2 overexpression did not affect Ki67 expression, indicating no impact on Treg cell proliferation (Figure 6D and E). Interestingly, LAP expression in Treg cells was significantly reduced after SOCS2 overexpression (Figure 6F and G), suggesting that SOCS2 directly inhibits Treg cell activity.
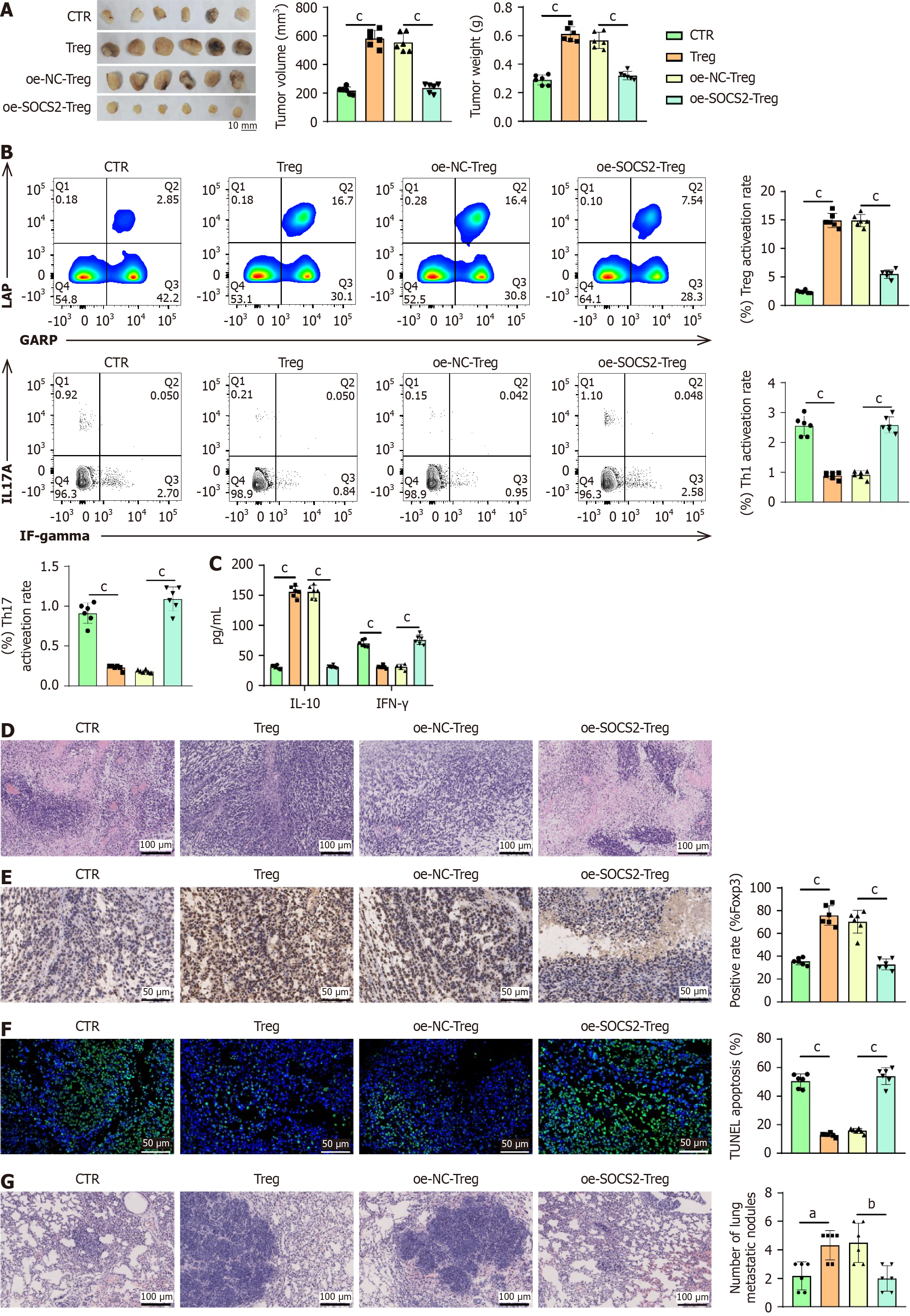
To further validate the impact of SOCS2 on Treg cells and LIHC cells, we established a subcutaneous transplantation mouse model of liver cancer. Analysis of the tumor tissues from the mice revealed that the tumor size and weight were greater in the Treg group compared to the control group, whereas the tumor size decreased and weight reduced in the oe-SOCS2-Treg group compared to the oe-NC-Treg group (Figure 7A).
Flow cytometry analysis demonstrated that the activation rate of Treg cells was higher in the Treg group compared to the control group, whereas the activation rate decreased in the oe-SOCS2-Treg group compared to the oe-NC-Treg group (Figure 7B). Meanwhile, the proportions of Th1 and Th17 cells were significantly reduced in the Treg group compared to the CTR group. In contrast, the activation rates of Th1 and Th17 cells were significantly increased in the oe-SOCS2-Treg group compared to the oe-NC-Treg group (Figure 7B).
Using ELISA to detect inflammatory factors, the results indicated that in comparison to the control group, the Treg group showed an increase in the expression of the anti-inflammatory factor IL-10 and a decrease in the pro-inflammatory factor IFN-γ. Conversely, when compared to the oe-NC-Treg group, the oe-SOCS2-Treg group exhibited a decrease in IL-10 expression and an increase in IFN-γ expression (Figure 7C).
Analysis of tumor tissues through H&E staining revealed that the tumor tissues in the Treg group were densely arranged compared to the control group, while the tumor tissues in the oe-SOCS2-Treg group showed a more loosely arranged structure when compared to the oe-NC-Treg group (Figure 7D).
In immunohistochemical analysis of tumor tissues, it was observed that compared to the control group, the expression of the Treg key marker FOXP3 was upregulated in the Treg group. Conversely, the oe-SOCS2-Treg group demonstrated a decrease in FOXP3 expression compared to the oe-NC-Treg group (Figure 7E).
Finally, utilizing TUNEL staining to assess apoptosis in tumor tissues, the results revealed that compared to the control group, the Treg group exhibited a reduction in cell apoptosis, while the oe-SOCS2-Treg group showed an increase in cell apoptosis relative to the oe-NC-Treg group (Figure 7F).
To further investigate the impact of SOCS2 on Treg cells in LIHC metastasis, analysis of lung tissues from mice using H&E staining demonstrated an increase in the number of metastatic foci in the Treg group compared to the control group. Conversely, a decrease in the number of metastatic foci was observed in the oe-SOCS2-Treg group when compared to the oe-NC-Treg group (Figure 7G). These findings suggest that SOCS2 Limits the growth and metastasis of LIHC by inhibiting the activity of Treg cells.
This study revealed the crucial role of SOCS2 in LIHC through bioinformatics analysis and in vitro and in vivo experiments. It was found that SOCS2 can suppress the immune reactions in the TME by regulating the function of Tregs, thereby impacting the growth and metastasis of LIHC. This finding is consistent with the dual roles of SOCS2 observed in other types of cancer, but the specific mechanism in LIHC remains unclear[26,34,47]. Our study, for the first time, elucidated the significant role of SOCS2 in LIHC by influencing Treg cells, providing novel insights into understanding the role of SOCS2 in LIHC.
Previous studies have shown that Treg cells play a crucial immunosuppressive role in various tumors, and their high infiltration is often associated with poor prognosis[48-50]. Through the analysis of LIHC data in the TCGA database, this study further confirmed the role of Treg cells in promoting tumor growth and metastasis in LIHC. Our results align with existing literature, emphasizing the significance of Treg cells as a potential therapeutic target in LIHC. Additionally, KM curve analysis revealed a significantly lower survival rate in the high-risk group compared to the low-risk group, closely linked to high Treg cell infiltration, further supporting the pivotal role of Treg cells in the progression of LIHC[51].
This study utilized bioinformatics methods such as GSEA, ESTIMATE, and CIBERSORT to reveal that SOCS2 may affect the progression of LIHC by regulating the activity of Treg cells in the TME. SOCS2, a member of the SOCS protein family, primarily regulates cytokine signaling by inhibiting the JAK/STAT signaling pathway[52]. This negative feedback mechanism has been extensively studied for its role in immune regulation and cancer. Growing evidence suggests that modulating SOCS2 expression could be a novel approach to antitumor therapy. For instance, in studies on gastric cancer and non-small cell lung cancer, SOCS2 was shown to regulate cell proliferation and apoptosis via the JAK/STAT pathway[52,53]. Our findings are consistent with some previous studies reporting the mechanism of SOCS2 regulating immune responses through the JAK/STAT signaling pathway[54]. In vitro experiments further confirmed the impact of SOCS2 on the activity of Treg cells and its regulation of migration, invasion, and apoptosis of LIHC cells, providing additional insight into the mechanisms by which SOCS2 functions in LIHC. These results suggest that SOCS2 may serve as a key regulatory factor in exerting its anti-tumor effect by inhibiting the functionality of Treg cells. However, research on specific SOCS2 inhibitors or activators remains limited and primarily focuses on indirect regulatory pathways[52,55] or other SOCS family members, such as SOCS1[56].
In addition to SOCS2, other immune regulatory factors, such as PD-1, CTLA-4, etc., also play a crucial role in the im
This study was conducted in vitro and in vivo experiments to validate the impact of SOCS2 on Treg cells and LIHC cells. The results of in vitro experiments demonstrated that overexpression of SOCS2 can inhibit the activity of Treg cells, thereby limiting the migration, invasion, and increasing apoptosis of LIHC cells. Subsequent in vivo experiments using a mouse model further confirmed the role of SOCS2 in inhibiting liver cancer growth and metastasis. These findings, consistent with the role of SOCS2 in other cancers from previous studies, support the potential of SOCS2 as a therapeutic target in LIHC. Of particular note, TUNEL staining revealed that the overexpression of SOCS2 significantly increased the apoptotic rate of cells in tumor tissues, providing direct evidence for the potential of SOCS2 as a therapeutic target.
In comparison to previous studies, this study is the first to systematically elucidate the mechanism by which SOCS2 regulates Treg cells in LIHC. Previous research has primarily focused on the role of SOCS2 in other types of cancer and its modulation through the JAK/STAT signaling pathway, whereas this study expands the scope of investigation on SOCS2 in LIHC by examining its specific regulatory effects on Treg cells[25]. This discovery fills a gap in the research on SOCS2 in LIHC and provides valuable insights for future studies in this area. Additionally, our findings suggest that SOCS2 is not merely a simple tumor suppressor gene; its mechanisms of action may vary across different types of tumors, war
Despite demonstrating the inhibitory effects of SOCS2 on tumor progression in vivo models, these models cannot fully replicate the complexity of human LIHC. Therefore, further validation is required for translational applications of these findings. Future research should prioritize the use of larger-scale human clinical samples to validate the efficacy and mechanisms of SOCS2. Multicenter large-scale clinical datasets could provide more representative tumor patient data, enhancing the applicability of the conclusions[57]. Additionally, prospective clinical trials are necessary to evaluate the specific role of SOCS2 in LIHC patients and to further understand its feasibility as a potential therapeutic target.
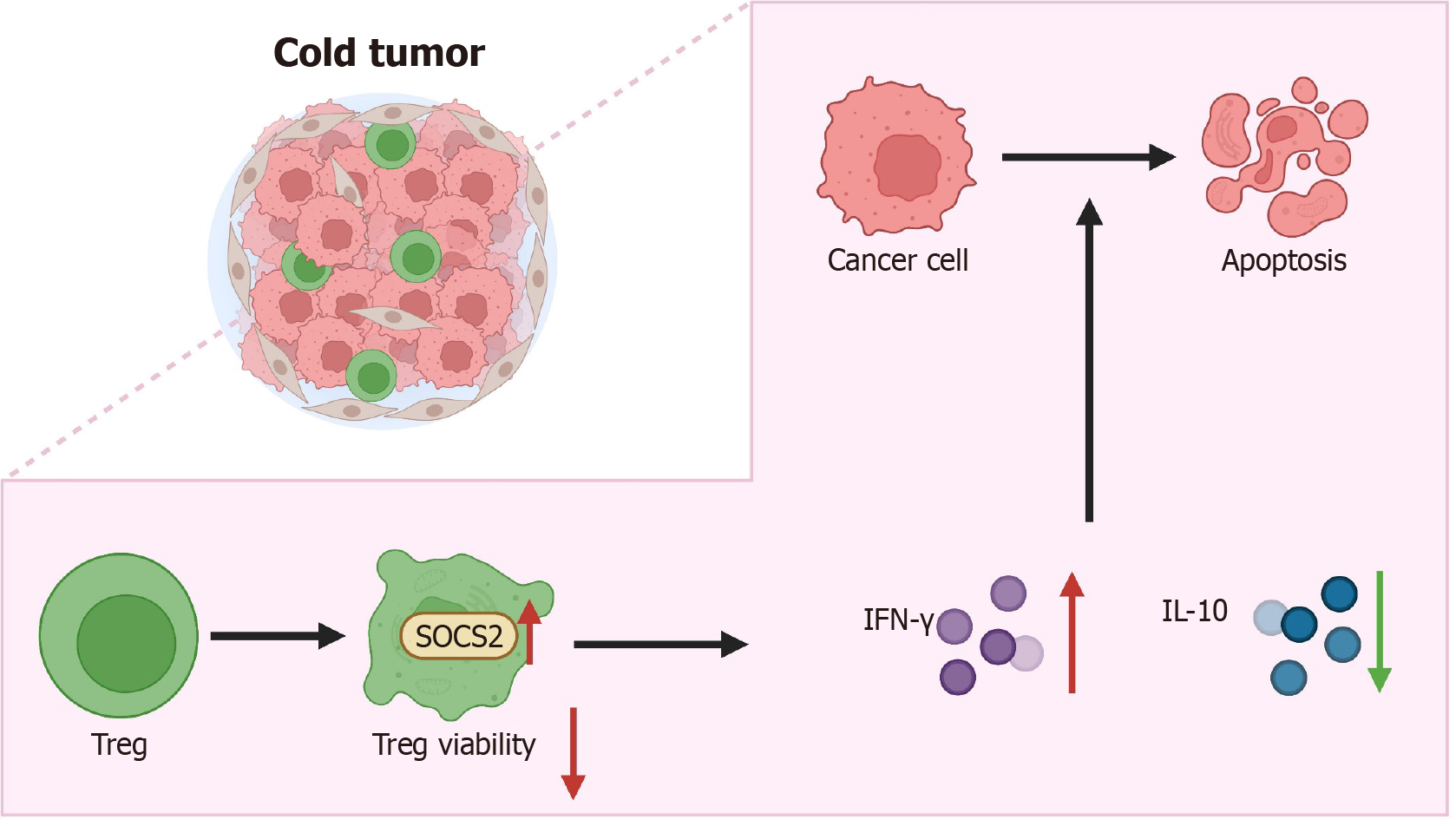
In conclusion, this study has revealed the pivotal role of SOCS2 in regulating the functionality of Treg cells in the TME. By inhibiting the activity of Treg cells, SOCS2 significantly restricts the growth and metastasis of LIHC (Figure 8). These findings not only offer new insights into the potential of SOCS2 as a therapeutic target for LIHC but also lay a theoretical foundation for future cancer immunotherapy strategies. This study has revealed that SOCS2 inhibits the immune reactions in the TME, promoting tumor growth and metastasis in LIHC by regulating the functionality of Treg cells. This discovery holds significant scientific value and provides a new perspective for understanding the immune evasion mechanisms in LIHC. Moreover, the research results indicate that SOCS2 could serve as a potential therapeutic target for LIHC, improving patient prognosis by modulating the activity of Treg cells. This lays the theoretical groundwork for developing novel immunotherapy strategies with significant clinical application potential, especially in combination with other immune checkpoint inhibitors, which may substantially enhance treatment effectiveness. Despite systematically revealing the role of SOCS2 in LIHC through bioinformatics analysis and in vivo and in vitro experiments, this study has several limitations. First, while the in vitro and in vivo results support the tumor-suppressive effects of SOCS2, these models cannot fully replicate the complexity of human LIHC. Thus, the clinical applicability of these laboratory findings needs to be validated using larger-scale clinical samples and multicenter datasets to improve the generalizability and robustness of the conclusions. Second, this study primarily focused on the regulatory role of SOCS2 in Treg cells, while its potential functions in other immune cell types remain unexplored, which may limit the comprehensive understanding of its immunoregulatory roles. Additionally, SOCS2 may exhibit differential mechanisms across various tumor types, warranting further investigation in a broader range of cancers. Future studies should aim to elucidate the detailed molecular mechanisms of SOCS2 in LIHC and evaluate its potential for clinical applications. Specifically, larger-scale clinical samples and multicenter studies are needed to validate the efficacy and safety of SOCS2 as a therapeutic target for LIHC. Further exploration of SOCS2 interactions with other immune regulators, particularly its relationships with tumor-associated macrophages or NK cells, could provide a basis for developing multi-target combination therapies. Advanced techniques such as gene editing and single-cell sequencing could be employed to investigate SOCS2's roles in different immune cell types, uncovering its comprehensive mechanisms within the LIHC immune microenvironment and providing more precise therapeutic targets for personalized treatment. Additionally, prospective clinical trials should be conducted to evaluate the feasibility of SOCS2 as a therapeutic target in LIHC patients, facilitating its translation into clinical applications. Through these efforts, we hope to develop more effective therapeutic options and improve survival outcomes for LIHC patients.
| 1. | Tapper EB, Parikh ND. Diagnosis and Management of Cirrhosis and Its Complications: A Review. JAMA. 2023;329:1589-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 152] [Article Influence: 76.0] [Reference Citation Analysis (33)] |
| 2. | Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, Laversanne M, McGlynn KA, Soerjomataram I. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598-1606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 1072] [Article Influence: 357.3] [Reference Citation Analysis (0)] |
| 3. | Sarcognato S, Sacchi D, Fassan M, Fabris L, Cadamuro M, Zanus G, Cataldo I, Capelli P, Baciorri F, Cacciatore M, Guido M. Cholangiocarcinoma. Pathologica. 2021;113:158-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 103] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 4. | Rizzo GEM, Cabibbo G, Craxì A. Hepatitis B Virus-Associated Hepatocellular Carcinoma. Viruses. 2022;14:986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 107] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 5. | Petruzziello A. Epidemiology of Hepatitis B Virus (HBV) and Hepatitis C Virus (HCV) Related Hepatocellular Carcinoma. Open Virol J. 2018;12:26-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 163] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 6. | Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156:477-491.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 1219] [Article Influence: 203.2] [Reference Citation Analysis (1)] |
| 7. | Chidambaranathan-Reghupaty S, Fisher PB, Sarkar D. Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. Adv Cancer Res. 2021;149:1-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 558] [Article Influence: 111.6] [Reference Citation Analysis (0)] |
| 8. | Chakraborty E, Sarkar D. Emerging Therapies for Hepatocellular Carcinoma (HCC). Cancers (Basel). 2022;14:2798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 204] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 9. | Su T, Huang M, Liao J, Lin S, Yu P, Yang J, Cai Y, Zhu S, Xu L, Peng Z, Peng S, Chen S, Kuang M. Insufficient Radiofrequency Ablation Promotes Hepatocellular Carcinoma Metastasis Through N6-Methyladenosine mRNA Methylation-Dependent Mechanism. Hepatology. 2021;74:1339-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 10. | Cheng K, Cai N, Zhu J, Yang X, Liang H, Zhang W. Tumor-associated macrophages in liver cancer: From mechanisms to therapy. Cancer Commun (Lond). 2022;42:1112-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 211] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 11. | Sun Y, Wu P, Zhang Z, Wang Z, Zhou K, Song M, Ji Y, Zang F, Lou L, Rao K, Wang P, Gu Y, Gu J, Lu B, Chen L, Pan X, Zhao X, Peng L, Liu D, Chen X, Wu K, Lin P, Wu L, Su Y, Du M, Hou Y, Yang X, Qiu S, Shi Y, Sun H, Zhou J, Huang X, Peng DH, Zhang L, Fan J. Integrated multi-omics profiling to dissect the spatiotemporal evolution of metastatic hepatocellular carcinoma. Cancer Cell. 2024;42:135-156.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 12. | Wang Z, Wang Y, Gao P, Ding J. Immune checkpoint inhibitor resistance in hepatocellular carcinoma. Cancer Lett. 2023;555:216038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 85] [Reference Citation Analysis (0)] |
| 13. | Tay C, Tanaka A, Sakaguchi S. Tumor-infiltrating regulatory T cells as targets of cancer immunotherapy. Cancer Cell. 2023;41:450-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 264] [Reference Citation Analysis (0)] |
| 14. | Hu C, Qiao W, Li X, Ning ZK, Liu J, Dalangood S, Li H, Yu X, Zong Z, Wen Z, Gui J. Tumor-secreted FGF21 acts as an immune suppressor by rewiring cholesterol metabolism of CD8(+)T cells. Cell Metab. 2024;36:630-647.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 35] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 15. | Hezaveh K, Shinde RS, Klötgen A, Halaby MJ, Lamorte S, Ciudad MT, Quevedo R, Neufeld L, Liu ZQ, Jin R, Grünwald BT, Foerster EG, Chaharlangi D, Guo M, Makhijani P, Zhang X, Pugh TJ, Pinto DM, Co IL, McGuigan AP, Jang GH, Khokha R, Ohashi PS, O'Kane GM, Gallinger S, Navarre WW, Maughan H, Philpott DJ, Brooks DG, McGaha TL. Tryptophan-derived microbial metabolites activate the aryl hydrocarbon receptor in tumor-associated macrophages to suppress anti-tumor immunity. Immunity. 2022;55:324-340.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 363] [Article Influence: 121.0] [Reference Citation Analysis (0)] |
| 16. | Kuan R, Agrawal DK, Thankam FG. Treg cells in atherosclerosis. Mol Biol Rep. 2021;48:4897-4910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 17. | Osna NA, Rasineni K, Ganesan M, Donohue TM Jr, Kharbanda KK. Pathogenesis of Alcohol-Associated Liver Disease. J Clin Exp Hepatol. 2022;12:1492-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 18. | Yi C, Chen L, Lin Z, Liu L, Shao W, Zhang R, Lin J, Zhang J, Zhu W, Jia H, Qin L, Lu L, Chen J. Lenvatinib Targets FGF Receptor 4 to Enhance Antitumor Immune Response of Anti-Programmed Cell Death-1 in HCC. Hepatology. 2021;74:2544-2560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 214] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 19. | Gao Y, You M, Fu J, Tian M, Zhong X, Du C, Hong Z, Zhu Z, Liu J, Markowitz GJ, Wang FS, Yang P. Intratumoral stem-like CCR4+ regulatory T cells orchestrate the immunosuppressive microenvironment in HCC associated with hepatitis B. J Hepatol. 2022;76:148-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 100] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 20. | Hu Z, Chen G, Zhao Y, Gao H, Li L, Yin Y, Jiang J, Wang L, Mang Y, Gao Y, Zhang S, Ran J, Li L. Exosome-derived circCCAR1 promotes CD8 + T-cell dysfunction and anti-PD1 resistance in hepatocellular carcinoma. Mol Cancer. 2023;22:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 189] [Reference Citation Analysis (0)] |
| 21. | Lee YH, Chuah S, Nguyen PHD, Lim CJ, Lai HLH, Wasser M, Chua C, Lim TKH, Leow WQ, Loh TJ, Wan WK, Pang YH, Soon G, Cheow PC, Kam JH, Iyer S, Kow A, Bonney GK, Chan CY, Chung A, Goh BKP, Zhai W, Chow PKH, Albani S, Liu H, Chew V. IFNγ(-)IL-17(+) CD8 T cells contribute to immunosuppression and tumor progression in human hepatocellular carcinoma. Cancer Lett. 2023;552:215977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 22. | Wang H, Zhang H, Wang Y, Brown ZJ, Xia Y, Huang Z, Shen C, Hu Z, Beane J, Ansa-Addo EA, Huang H, Tian D, Tsung A. Regulatory T-cell and neutrophil extracellular trap interaction contributes to carcinogenesis in non-alcoholic steatohepatitis. J Hepatol. 2021;75:1271-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 245] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 23. | Liu W, Tao YH, Lu CP, Zhang L, Chen J, Lin ZH. Transcriptomic analysis of skin immunity genes in the Chinese spiny frog (Quasipaa spinosa) after Proteus mirabilis infection. Comp Biochem Physiol Part D Genomics Proteomics. 2024;49:101172. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Wu L, Huang G, Hong H, Xu X, Lu X, Li J. MiR-452-5p facilitates retinoblastoma cell growth and invasion via the SOCS3/JAK2/STAT3 pathway. J Biochem Mol Toxicol. 2023;37:e23501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 25. | He M, Cai Y, Yuan Z, Zhang L, Lu H. Upregulation of SOCS2 causes mitochondrial dysfunction and promotes ferroptosis in pancreatic cancer cells. Acta Biochim Pol. 2023;70:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Chen Q, Zheng W, Guan J, Liu H, Dan Y, Zhu L, Song Y, Zhou Y, Zhao X, Zhang Y, Bai Y, Pan Y, Zhang J, Shao C. SOCS2-enhanced ubiquitination of SLC7A11 promotes ferroptosis and radiosensitization in hepatocellular carcinoma. Cell Death Differ. 2023;30:137-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 188] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 27. | Xian X, Cai LL, Li Y, Wang RC, Xu YH, Chen YJ, Xie YH, Zhu XL, Li YF. Neuron secrete exosomes containing miR-9-5p to promote polarization of M1 microglia in depression. J Nanobiotechnology. 2022;20:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 91] [Reference Citation Analysis (0)] |
| 28. | Cheng C, Wang P, Yang Y, Du X, Xia H, Liu J, Lu L, Wu H, Liu Q. Smoking-Induced M2-TAMs, via circEML4 in EVs, Promote the Progression of NSCLC through ALKBH5-Regulated m6A Modification of SOCS2 in NSCLC Cells. Adv Sci (Weinh). 2023;10:e2300953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 29. | Micheel J, Safrastyan A, Wollny D. Advances in Non-Coding RNA Sequencing. Noncoding RNA. 2021;7:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 30. | Erkes A, Grove RP, Žarković M, Krautwurst S, Koebnik R, Morgan RD, Wilson GG, Hölzer M, Marz M, Boch J, Grau J. Assembling highly repetitive Xanthomonas TALomes using Oxford Nanopore sequencing. BMC Genomics. 2023;24:151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 31. | Xu BH, Jiang JH, Luo T, Jiang ZJ, Liu XY, Li LQ. Signature of prognostic epithelial-mesenchymal transition related long noncoding RNAs (ERLs) in hepatocellular carcinoma. Medicine (Baltimore). 2021;100:e26762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Wang Q, Gu Y, Chen J, Liu X, Xie C, Wang X. Bioinformatics gene analysis for potential biomarkers and therapeutic targets of Parkinson's disease based on neutrophil extracellular traps. Front Aging Neurosci. 2024;16:1388226. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 33. | Carreras J. Artificial Intelligence Analysis of Ulcerative Colitis Using an Autoimmune Discovery Transcriptomic Panel. Healthcare (Basel). 2022;10:1476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Zhang Z, Wang S, Zhu Z, Nie B. Identification of potential feature genes in non-alcoholic fatty liver disease using bioinformatics analysis and machine learning strategies. Comput Biol Med. 2023;157:106724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 35. | Bai Q, Liu H, Guo H, Lin H, Song X, Jin Y, Liu Y, Guo H, Liang S, Song R, Wang J, Qu Z, Guo H, Jiang H, Liu L, Yang H. Identification of Hub Genes Associated With Development and Microenvironment of Hepatocellular Carcinoma by Weighted Gene Co-expression Network Analysis and Differential Gene Expression Analysis. Front Genet. 2020;11:615308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 36. | Zhang D, Zhan D, Zhang R, Sun Y, Duan C, Yang J, Wei J, Li X, Lu Y, Lai X. Treg-derived TGF-β1 dampens cGAS-STING signaling to downregulate the expression of class I MHC complex in multiple myeloma. Sci Rep. 2024;14:11593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 37. | Shan W, Zhang W, Xue F, Ma Y, Dong L, Wang T, Zheng Y, Feng D, Chang M, Yuan G, Wang X. Schistosoma japonicum peptide SJMHE1 inhibits acute and chronic colitis induced by dextran sulfate sodium in mice. Parasit Vectors. 2021;14:455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Dou C, Xu Q, Liu J, Wang Y, Zhou Z, Yao W, Jiang K, Cheng J, Zhang C, Tu K. SHMT1 inhibits the metastasis of HCC by repressing NOX1-mediated ROS production. J Exp Clin Cancer Res. 2019;38:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 39. | Sfanos KS, Bruno TC, Maris CH, Xu L, Thoburn CJ, DeMarzo AM, Meeker AK, Isaacs WB, Drake CG. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14:3254-3261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 354] [Cited by in RCA: 350] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 40. | Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 1360] [Article Influence: 151.1] [Reference Citation Analysis (0)] |
| 41. | Moreno Ayala MA, Li Z, DuPage M. Treg programming and therapeutic reprogramming in cancer. Immunology. 2019;157:198-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 42. | Yue T, Chen S, Zhu J, Guo S, Huang Z, Wang P, Zuo S, Liu Y. The aging-related risk signature in colorectal cancer. Aging (Albany NY). 2021;13:7330-7349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 43. | Cabrera-Galván JJ, Araujo E, de Mirecki-Garrido M, Pérez-Rodríguez D, Guerra B, Aranda-Tavío H, Guerra-Rodríguez M, Brito-Casillas Y, Melián C, Martínez-Martín MS, Fernández-Pérez L, Recio C. SOCS2 protects against chemical-induced hepatocellular carcinoma progression by modulating inflammation and cell proliferation in the liver. Biomed Pharmacother. 2023;157:114060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 44. | Val CH, de Oliveira MC, Lacerda DR, Barroso A, Batista NV, Menezes-Garcia Z, de Assis DRR, Cramer AT, Brant F, Teixeira MM, Glória Souza D, Ferreira AM, Machado FS. SOCS2 modulates adipose tissue inflammation and expansion in mice. J Nutr Biochem. 2020;76:108304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 45. | Lekshmi A, Varadarajan SN, Lupitha SS, Indira D, Mathew KA, Chandrasekharan Nair A, Nair M, Prasad T, Sekar H, Gopalakrishnan AK, Murali A, Santhoshkumar TR. A quantitative real-time approach for discriminating apoptosis and necrosis. Cell Death Discov. 2017;3:16101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 46. | Abd Al Samid M, Chaudhary B, Khaled YS, Ammori BJ, Elkord E. Combining FoxP3 and Helios with GARP/LAP markers can identify expanded Treg subsets in cancer patients. Oncotarget. 2016;7:14083-14094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 47. | Zhang Q, Wei T, Yan L, Zhu S, Jin W, Bai Y, Zeng Y, Zhang X, Yin Z, Yang J, Zhang W, Wu M, Zhang Y, Liu L. Hypoxia-Responsive lncRNA AC115619 Encodes a Micropeptide That Suppresses m6A Modifications and Hepatocellular Carcinoma Progression. Cancer Res. 2023;83:2496-2512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 48. | Ma H, Kang Z, Foo TK, Shen Z, Xia B. Disrupted BRCA1-PALB2 interaction induces tumor immunosuppression and T-lymphocyte infiltration in HCC through cGAS-STING pathway. Hepatology. 2023;77:33-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 49. | Liu Z, Zhang Z, Zhang Y, Zhou W, Zhang X, Peng C, Ji T, Zou X, Zhang Z, Ren Z. Spatial transcriptomics reveals that metabolic characteristics define the tumor immunosuppression microenvironment via iCAF transformation in oral squamous cell carcinoma. Int J Oral Sci. 2024;16:9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 50. | Li Z, Deng Y, Sun H, Tan C, Li H, Mo F, Wang Y, Li J, Zhou Z, Sun M. Redox modulation with a perfluorocarbon nanoparticle to reverse Treg-mediated immunosuppression and enhance anti-tumor immunity. J Control Release. 2023;358:579-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 51. | Granito A, Muratori L, Lalanne C, Quarneti C, Ferri S, Guidi M, Lenzi M, Muratori P. Hepatocellular carcinoma in viral and autoimmune liver diseases: Role of CD4+ CD25+ Foxp3+ regulatory T cells in the immune microenvironment. World J Gastroenterol. 2021;27:2994-3009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 122] [Cited by in RCA: 131] [Article Influence: 32.8] [Reference Citation Analysis (1)] |
| 52. | Tong JL, Wang LL, Ling XF, Wang MX, Cao W, Liu YY. MiR-875 can regulate the proliferation and apoptosis of non-small cell lung cancer cells via targeting SOCS2. Eur Rev Med Pharmacol Sci. 2019;23:5235-5241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 53. | Guo W, Li W, Yuan L, Mei X, Hu W. MicroRNA-106a-3p Induces Apatinib Resistance and Activates Janus-Activated Kinase 2 (JAK2)/Signal Transducer and Activator of Transcription 3 (STAT3) by Targeting the SOCS System in Gastric Cancer. Med Sci Monit. 2019;25:10122-10128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Wang F, Wang X, Li J, Lv P, Han M, Li L, Chen Z, Dong L, Wang N, Gu Y. CircNOL10 suppresses breast cancer progression by sponging miR-767-5p to regulate SOCS2/JAK/STAT signaling. J Biomed Sci. 2021;28:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 55. | Zhang R, Huo CH. Long Noncoding RNA SOCS2-AS Promotes Leukemogenesis in FLT3-ITD+ Acute Myeloid Leukemia Through miRNA-221. Onco Targets Ther. 2020;13:2925-2934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 56. | Doti N, Scognamiglio PL, Madonna S, Scarponi C, Ruvo M, Perretta G, Albanesi C, Marasco D. New mimetic peptides of the kinase-inhibitory region (KIR) of SOCS1 through focused peptide libraries. Biochem J. 2012;443:231-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 57. | Luo P, Yin P, Hua R, Tan Y, Li Z, Qiu G, Yin Z, Xie X, Wang X, Chen W, Zhou L, Wang X, Li Y, Chen H, Gao L, Lu X, Wu T, Wang H, Niu J, Xu G. A Large-scale, multicenter serum metabolite biomarker identification study for the early detection of hepatocellular carcinoma. Hepatology. 2018;67:662-675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 284] [Article Influence: 40.6] [Reference Citation Analysis (0)] |