Published online Mar 28, 2025. doi: 10.3748/wjg.v31.i12.102007
Revised: January 23, 2025
Accepted: February 26, 2025
Published online: March 28, 2025
Processing time: 171 Days and 18.1 Hours
Liver transplantation (LT) is recognized as an effective approach that offers survival benefits for patients with acute-on-chronic liver failure (ACLF). How
To depict a comprehensive postoperative picture of patients with ACLF of vary
Systematic searches in Web of Science, EMBASE, PubMed, and Cochrane data
A total of 17 studies involving 28025 participants were included. Patients with ACLF-1 and ACLF-2 have favorable survival within one year, with survival rates reaching 87% [95% confidence interval (CI): 84%-91%] and 86% (95%CI: 81%-91%), respectively. Despite the relatively lower survival (73%, 95%CI: 66%-80%) and higher incidence of infection (48%, 95%CI: 29%-67%) observed in ACLF-3 patients, their survival exceeds that of those who do not undergo LT. Moreover, post-transplant survival was highest in North America across all ACLF grades.
LT can provide survival advantages for ACLF patients. To optimize the utilization of scarce donor organs and improve prognosis, comprehensive preoperative health evaluations are essential, especially for ACLF-3 patients.
Core Tip: Given the challenges associated with liver transplantation (LT) in the treatment of acute-on-chronic liver failure (ACLF), it is crucial to evaluate patient prognosis across different ACLF grades following LT. This meta-analysis revealed that ACLF-1 and ACLF-2 patients achieve favorable survival outcomes following LT. Although ACLF-3 patients exhibit relatively lower survival rates and higher infection rates, LT remains a promising option to improve their prognosis. To optimize the use of limited donor organs, further refinement of organ allocation and scoring systems is imperative. A comprehensive assessment of pre-transplant health status, concurrent organ failure, and donor characteristics should be performed before LT.
- Citation: Li ZX, Zeng JH, Zhong HL, Peng B. Liver transplantation improves prognosis across all grades of acute-on-chronic liver failure patients: A systematic review and meta-analysis. World J Gastroenterol 2025; 31(12): 102007
- URL: https://www.wjgnet.com/1007-9327/full/v31/i12/102007.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i12.102007
Acute-on-chronic liver failure (ACLF), a life-threatening syndrome characterized by acute decompensation in patients with chronic liver disease or liver cirrhosis, presents high short-term mortality and imparts a substantial global burden[1]. In the current guidelines, the primary therapeutic approach for ACLF involves accurate management of precipitating events, treatment of sepsis, administration of organ support, and consideration of liver transplantation (LT) in selected patients[2,3].
Notably, LT has been demonstrated to provide survival benefits to ACLF patients[4]. However, the absence of definite selection criteria poses challenges for timely treatment. A subset of patients with ACLF may have relatively lower severity scores according to the model for end-stage liver disease (MELD) or MELD-Na, despite the presence of severe conditions. Therefore, these patients may encounter challenges in securing organ prioritization, notwithstanding the potential for achieving a favorable prognosis after LT. Conversely, some critically ill patients with ACLF may manifest a severe clinical profile characterized by multiple organ failure, leading to undesirable utilization of a scarce donor pool when LT is conducted within this subgroup. This dual challenge of restricted donor availability and uncertain prognosis highlights the need for strict screening of LT recipients among patients with ACLF across distinct severity grades.
Currently, several single-center cohort studies have reported the post-transplant survival conditions of patients with ACLF with different severity grades. Herein, we conducted a meta-analysis of these studies to depict a comprehensive post-LT profile for patients with ACLF of varying severity. Our aim is to provide insights that will contribute to enhancing organ allocation strategies and updating LT selection criteria.
This meta-analysis was registered with PROSPERO (CRD42024562657) and reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses[5].
Two independent researchers (Li ZX and Zeng JH) conducted a systematic literature search through four online databases, including Web of Science, EMBASE, PubMed, and Cochrane, from database inception until December 26, 2023. There were no restrictions on language or date of publication. The key words and medical subject heading terms used for each database included “liver transplantation” and “acute on chronic liver failure”. Literature search details are explained in Supplementary Table 1.
We first reviewed the title and abstract of the retrieved studies. Studies that did not report the post-transplant outcomes of patients with ACLF were excluded. The full texts of those studies that were relevant were next evaluated for inclusion eligibility. The following criteria were used to select studies: (1) Studies adopted case-control or cohort study designs; (2) Studies included adults with ACLF; and (3) Studies defined ACLF and its grades via the European Association for the Study of the Liver-Chronic Liver Failure (EASL-CLIF) criteria[6]. The primary outcome was patient survival within one year. The secondary outcome was any complication following LT. If multiple articles provided data from the same data source, we included the one with the largest sample size. In addition, we did not include case reports, conference abstracts, editorials, commentaries, or reviews. Studies including multiorgan transplantation were also excluded. Conflicts during study selection were resolved by group discussion. The reasons for full-text articles being excluded are provided in Supplementary Table 2.
The following information for each study was extracted by two researchers (Li ZX and Peng B): First author’s last name, year of publication, geographical region, study period, sample size for each ACLF grade, mean age, percentage of male participants, laboratory data (total bilirubin concentration, serum creatinine concentration, and international normalized ratio), percentage of specific organ failure (liver, kidney, brain, coagulation, circulation, and lung) at time of LT, predictive scores (MELD and CLIF-C ACLF score) at LT time, donor characteristics [cold ischemia time (CIT) and warm ischemia time (WIT)], and postoperative outcomes stratified by ACLF grade. The Newcastle-Ottawa quality assessment scale (NOS) was utilized by two researchers (Zeng JH and Zhong HL) to assess the quality of the included studies[7]. Studies with more than seven points were considered high quality.
Extracted data from the included studies were used to evaluate survival rates at different time points and incidence rates of complications after LT. Additionally, the region-specific one-year survival rates were also pooled. To analyze the consistency of evidence, the I2 statistic was used to estimate the heterogeneity between studies. I2 > 50% or P value < 0.10 indicates substantial heterogeneity and the random effect model was employed; otherwise, the fixed effect model (also referred as common effect model) was used to pool the estimates. Leave-one-out sensitivity analyses were conducted to validate the stability of the results. Publication bias was assessed via Egger’s test and visual inspection of funnel plots, with P value < 0.05 indicating statistical significance. When publication bias was suspected, the trim-and-fill method was further employed. All the statistical analyses were performed with the “meta” and “forestplot” packages of R software (version 4.2.0). All presented P value were two-sided, and statistical significance was set at the 5% level.
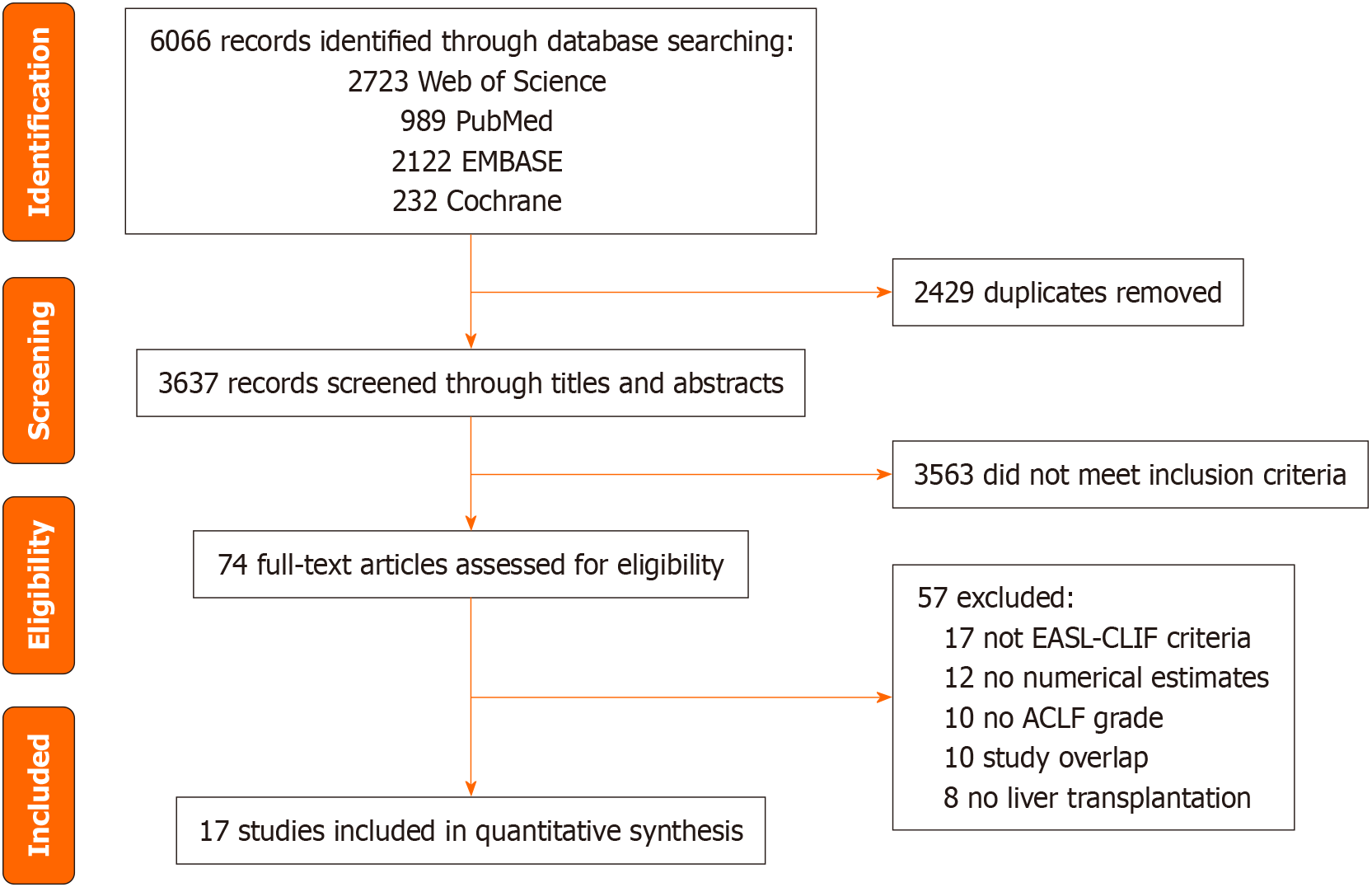
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram is shown in Figure 1. In our initial literature search, we identified 6066 items including 2723 from Web of Science, 2122 from EMBASE, 989 from PubMed, and 232 from the Cochrane Library. After removing duplicates, selecting studies based on inclusion criteria, and performing a full-text screening, we ultimately identified 17 studies across Asia, Europe, North America, and South America for meta-analysis[8-24] (Table 1). All these studies were published from 2016 to 2023, with sample sizes ranging from the range of 60 to 25777. NOS scores ranged from 7 to 9.
| Ref. | Country | Study period | No. | NOS score | ||
| ACLF-1 | ACLF-2 | ACLF-3 | ||||
| Agbim et al[8], 2020 | United States | 2006-2013 | 50 | 32 | 19 | 8 |
| Artru et al[9], 2017 | France | 2008-2014 | 119 | 145 | 73 | 9 |
| Artzner et al[10], 2020 | France | 2007-2017 | 0 | 0 | 152 | 9 |
| Belli et al[11], 2021 | Europe | 2018-2019 | 58 | 78 | 98 | 7 |
| Benítez et al[12], 2023 | Chile | 2013-2020 | 27 | 23 | 25 | 9 |
| Bhatti et al[13], 2018 | Pakistan | 2012-2016 | 43 | 15 | 2 | 8 |
| Cervantes-Alvarez et al[14], 2022 | Mexico | 2015-2019 | 40 | 33 | 22 | 8 |
| Levesque et al[15], 2017 | France | 2008-2013 | 68 | 42 | 30 | 9 |
| Marciano et al[16], 2019 | Argentina | 2010-2016 | 34 | 18 | 8 | 8 |
| Qian et al[17], 20221 | China | 2015-2017 | 76 | 59 | 38 | 9 |
| Singh et al[18], 2024 | India | 2017-2021 | 11 | 38 | 54 | 8 |
| Sundaram et al[19], 2020 | United States | 2004-2018 | 8757 | 9039 | 7981 | 9 |
| Sundaram et al[20], 20221 | United States and Canada | 2018-2019 | 61 | 74 | 77 | 8 |
| Sundaram et al[21], 20231 | United States and Canada | 2018-2019 | 61 | 74 | 77 | 8 |
| Xia et al[22], 20221 | China | 2015-2021 | 18 | 97 | 47 | 9 |
| Yang et al[23], 2022 | China | 2012-2019 | 5 | 91 | 36 | 9 |
| Zhu et al[24], 2023 | China | 2018-2020 | 75 | 64 | 73 | 8 |
A total of 28025 patients diagnosed with ACLF were enrolled in this study. The distribution across ACLF grades revealed that 33.7% of the patients were classified as ACLF-1, 35.1% as ACLF-2, and 31.2% as ACLF-3. The mean age ranged from 45.9 to 60.0 years. Body mass index (BMI) data from four studies indicated that the median BMI of patients ranged from 25.5 to 29.6 kg/m2[8,13,15,18]. The etiology of chronic liver disease or cirrhosis was reported in all 17 studies. Among these, eight studies identified alcohol-related liver disease as the primary etiology for ACLF[8-11,15,18,20,21], whereas three studies highlighted hepatitis C virus infection[13,16,19]. Notably, all four studies conducted in China reported hepatitis B virus infection as the primary cause of ACLF, with a prevalence ranging from 61.7% to 84.1%[17,22-24]. Additionally, six studies reported the prevalence of hepatocellular carcinoma among patients with ACLF at LT, ranging from 2.9% to 15.1%[10,12,16,18,20,21].
In terms of distinct grades, patients with ACLF-3 presented elevated total bilirubin levels (23.59 mg/dL), serum creatinine levels (1.54 mg/dL), and international normalized ratio (3.21). Furthermore, patients with ACLF-3 had the highest MELD and CLIF-C ACLF scores at the time of LT (MELD score: 26.74 for ACLF-1, 33.85 for ACLF-2, and 39.97 for ACLF-3; CLIF-C ACLF score: 41.54 for ACLF-1, 48.85 for ACLF-2, and 61.37 for ACLF-3) (Supplementary Table 3). For donor characteristics stratified by the ACLF grade of recipients, six studies reported the CIT and WIT[9-11,15,18,20]. Additionally, Singh et al[18] provided information on graft weight, the graft-to-recipient weight ratio, and graft steatosis.
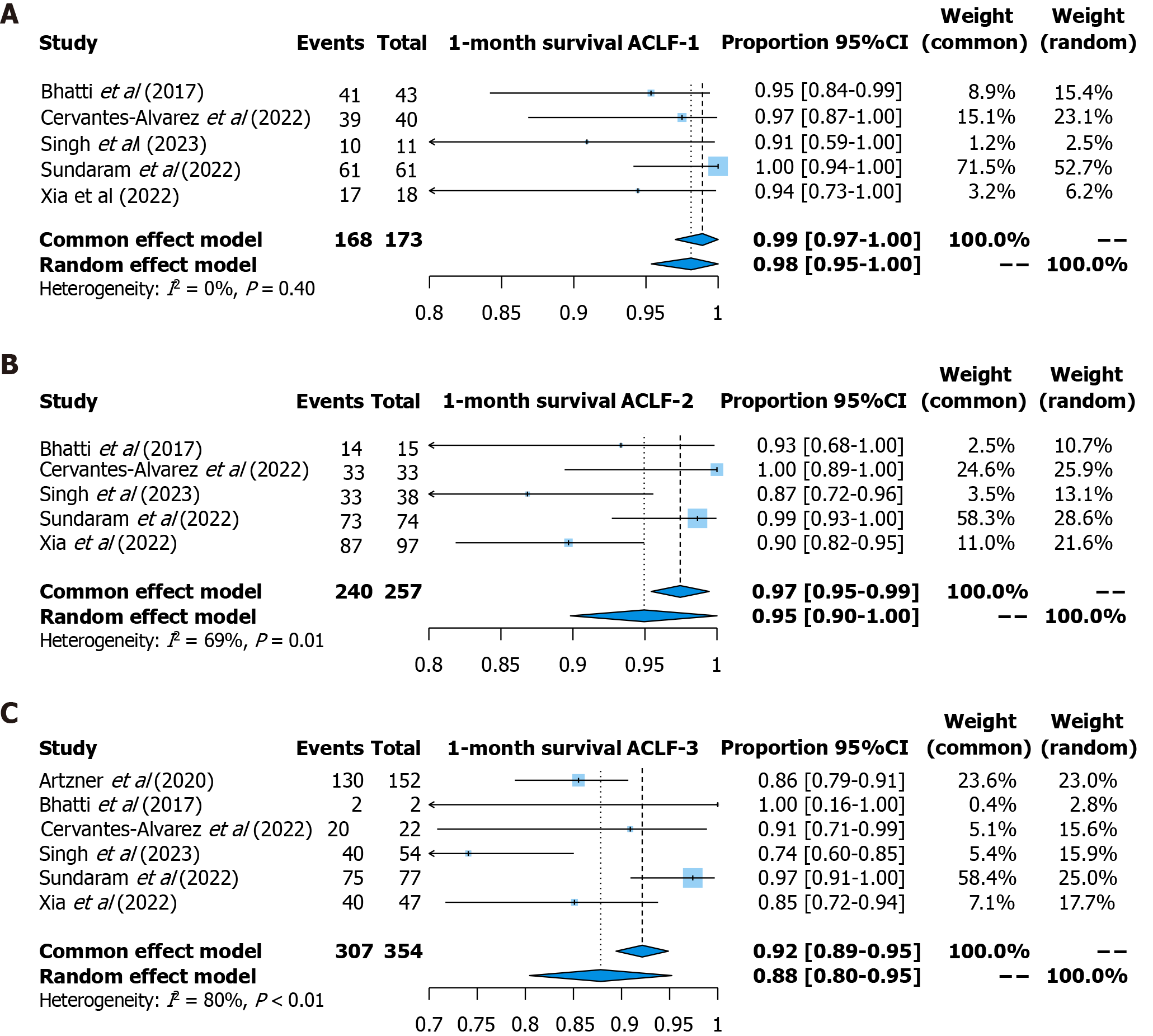
One-month survival: One-month survival was reported in six studies (173 patients with ACLF-1, 257 patients with ACLF-2, and 354 patients with ACLF-3). The pooled survival rate was 99% [95% confidence interval (CI): 97%-100%, fixed effects model, I2 = 0] for patients with ACLF-1, 95% (95%CI: 90%-100%, random effects model, I2 = 69%) for patients with ACLF-2, and 88% (95%CI: 80%-95%, random effects model, I2 = 80%) for patients with ACLF-3 (Figure 2).
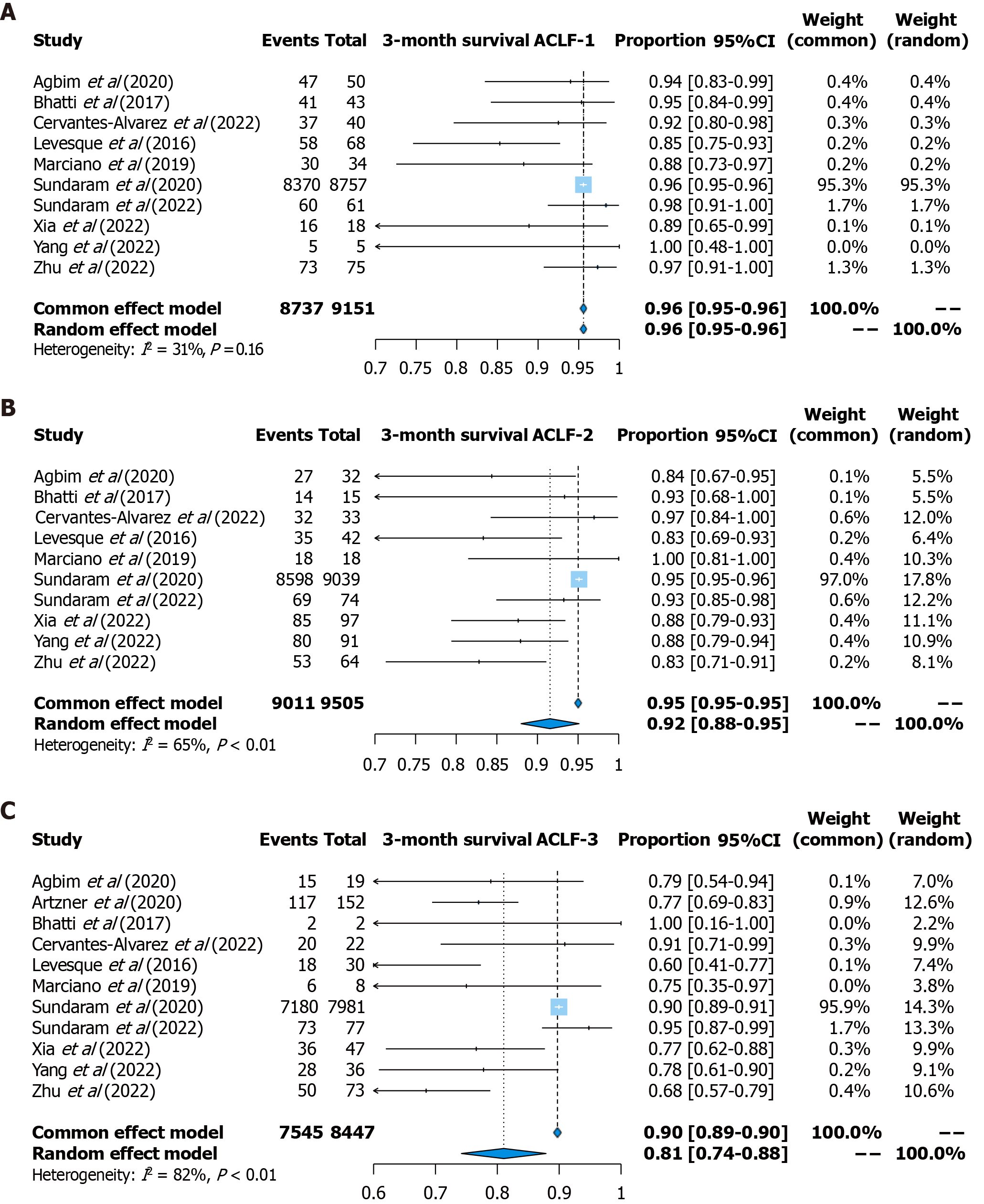
Three-month survival: Three-month survival was reported in 11 studies (9151 patients with ACLF-1, 9505 patients with ACLF-2, and 8447 patients with ACLF-3). The pooled survival rate was 96% (95%CI: 95%-96%, fixed effects model, I2 = 31%) for patients with ACLF-1, 92% (95%CI: 88%-95%, random effects model, I2 = 65%) for patients with ACLF-2, and 81% (95%CI: 74%-88%, random effects model, I2 = 82%) for patients with ACLF-3 (Figure 3).
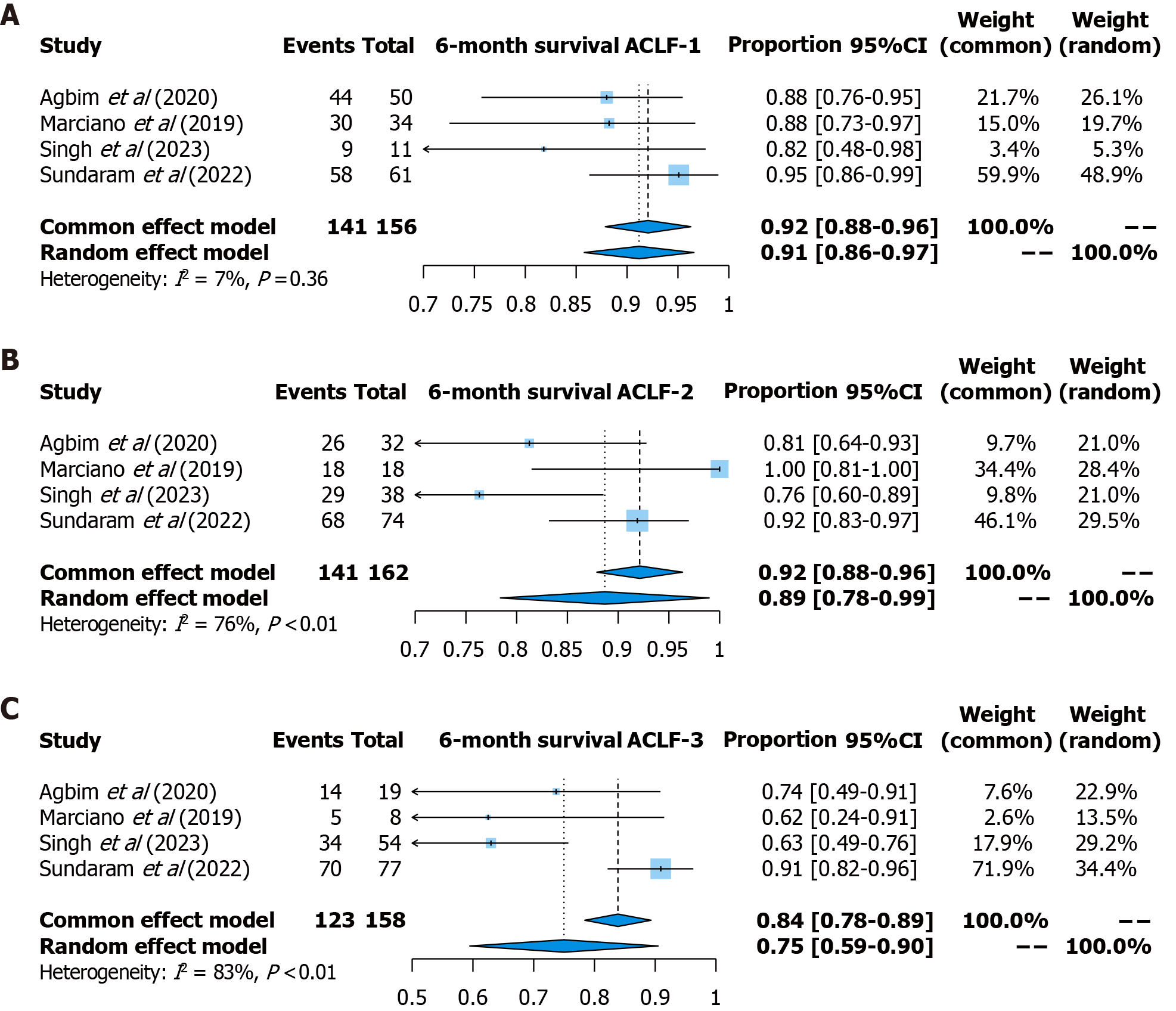
Six-month survival: Six-month survival was reported in four studies (156 patients with ACLF-1, 162 patients with ACLF-2, and 158 patients with ACLF-3). The pooled survival rate was 92% (95%CI: 88%-96%, fixed effects model, I2 = 7%) for patients with ACLF-1, 89% (95%CI: 78%-99%, random effects model, I2 = 76%) for patients with ACLF-2, and 75% (95%CI: 59%-90%, random effects model, I2 = 83%) for patients with ACLF-3 (Figure 4).
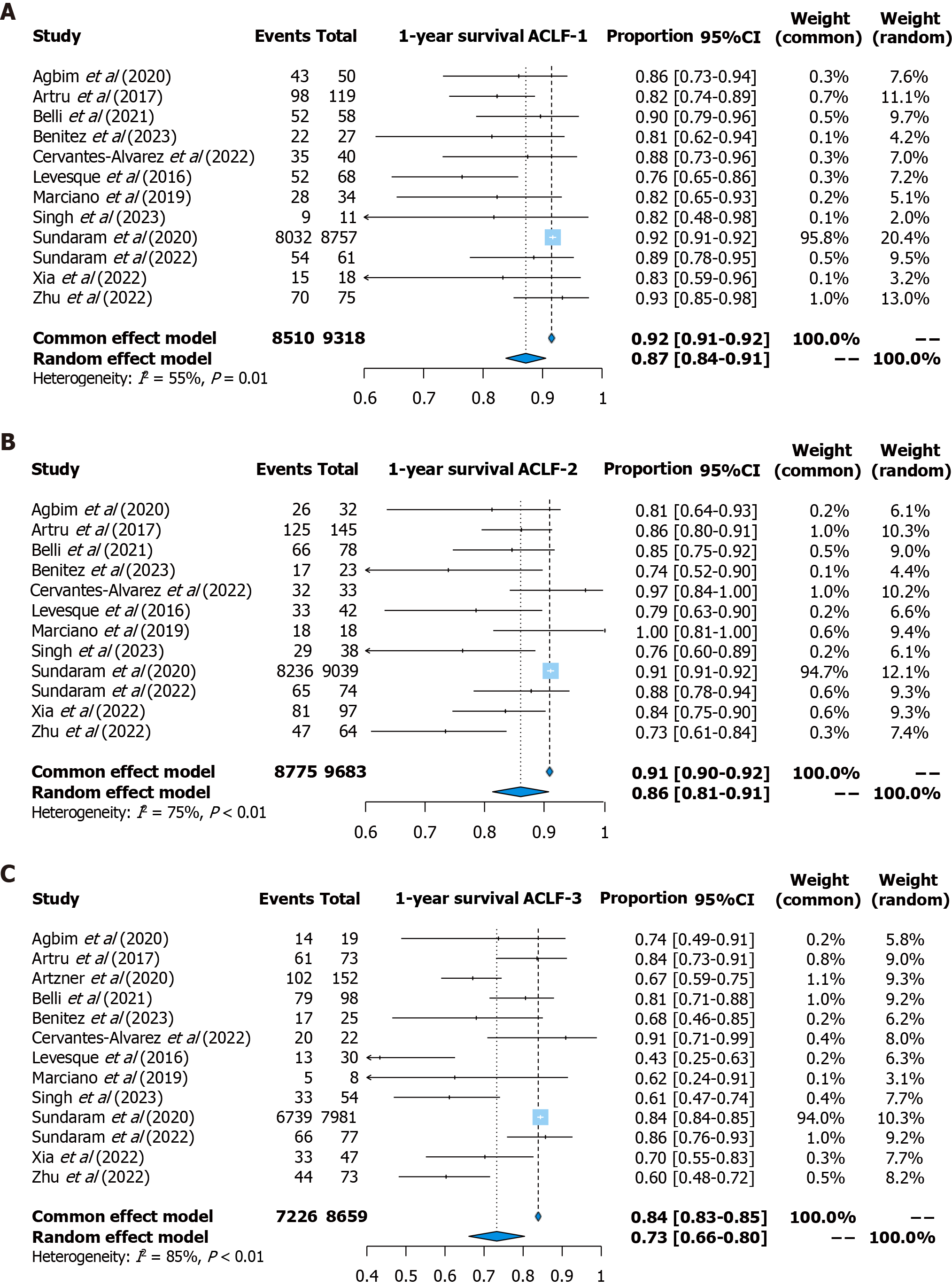
One-year survival: One-year survival was reported in 13 studies (9318 patients with ACLF-1, 9683 patients with ACLF-2, and 8659 patients with ACLF-3). The pooled survival rate was 87% (95%CI: 84%-91%, random effects model, I2 = 55%) for patients with ACLF-1, 86% (95%CI: 81%-91%, random effects model, I2 = 75%) for patients with ACLF-2, and 73% (95%CI: 66%-80%, random effects model, I2 = 85%) for patients with ACLF-3 (Figure 5).
In addition, the region-specific survival rate was further calculated (Supplementary Table 4). Notably, the one-year survival rate in North America was the highest across all ACLF grades, with a pooled rate of 92% (95%CI: 91%-92%) for patients with ACLF-1, 91% (95%CI: 87%-95%) for patients with ACLF-2, and 84% (95%CI: 84%-85%) for patients with ACLF-3. In Asia, the survival rate for patients with ACLF-1 reached 92% (95%CI: 87%-97%), whereas for patients with ACLF-3, it was lower at 63% (95%CI: 56%-71%).
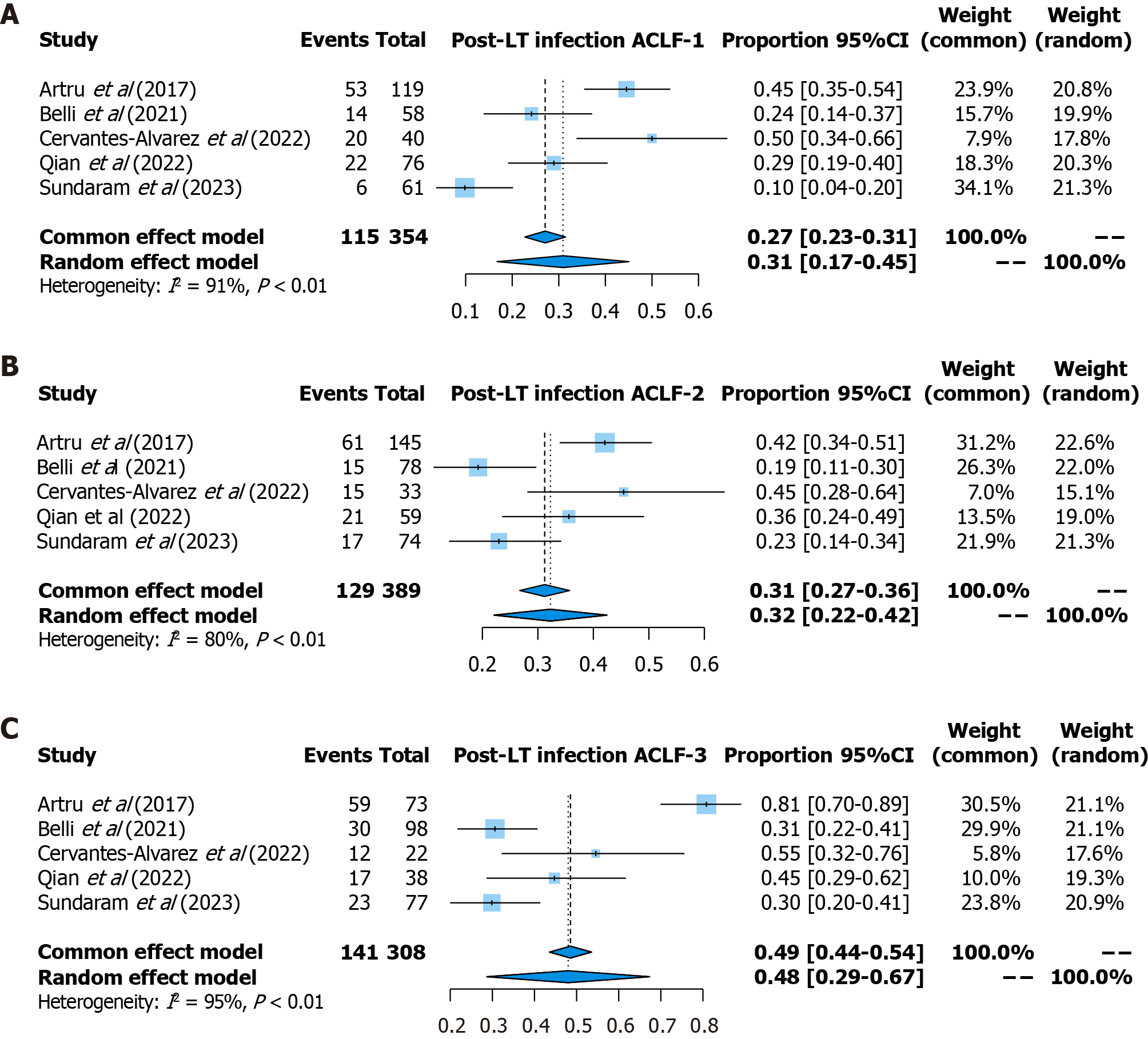
Post-transplant complications: For post-LT complications, the incidence of infection was reported in 5 studies (354 patients with ACLF-1, 389 patients with ACLF-2, and 308 patients with ACLF-3). The pooled prevalence of infection was 31% (95%CI: 17%-45%, random effects model, I2 = 91%) for patients with ACLF-1, 32% (95%CI: 22%-42%, random effects model, I2 = 80%) for patients with ACLF-2, and 48% (95%CI: 29%-67%, random effects model, I2 = 95%) for patients with ACLF-3 (Figure 6). Notably, Qian et al[17] reported the characteristics of isolated microorganisms and their infection site, highlighting carbapenem-resistant gram-negative organisms as the most common pathogens involved. In addition, three studies reported the incidence for biliary complications[9,18,21] and two studies reported the incidence of neurological complications[9,21].
All sensitivity analyses demonstrated the robustness of our findings, as each study was omitted in turn from our meta-analyses. Publication bias was observed regarding the outcomes for one-month survival and one-year survival among patients with ACLF-1, as well as three-month survival and one-year survival among patients with ACLF-3. Subsequently, corrected measures were obtained via the trim-and-fill method. Detailed information on leave-one-out sensitivity analysis, funnel plots, and outcomes rectified through trim-and-fill method are shown in Supplementary Tables 5-8 and Supplementary Figures 1-5.
This meta-analysis identified 17 studies involving 28025 patients with ACLF who underwent LT, with the following findings: (1) Patients with ACLF-1 and ACLF-2 have a favorable survival within one year; (2) Despite the relatively lower survival and higher incidence of infectious complications observed in patients with ACLF-3 than in those with ACLF-1 and ACLF-2, their survival exceeds that of those who do not experience LT; and (3) There are significant regional differences concerning post-transplant outcomes.
A previous meta-analysis has highlighted the survival benefits of LT by comparing post-transplant outcomes among patients receiving LT for ACLF, those receiving LT for other indications, and patients with ACLF not selected for LT[4]. This study further delves into the survival advantage of LT, focusing on post-LT outcomes across different grades of ACLF. Our aim is to provide a comprehensive perspective on post-LT outcomes, highlighting the survival benefits conferred by LT for patients with varied severities of ACLF and addressing certain controversies concerning LT selection for patients with ACLF. We appraised the robustness of this meta-analysis through an extensive literature search, meta-regression, and evaluation for publication bias. Moreover, our meta-analysis exclusively included studies attaining a minimum of seven stars according to the NOS, indicating that our findings are based on high-quality literature and possess high-level reliability.
Patients with ACLF often demonstrate a significant risk of short-term mortality during hospitalization, with a 28-day transplant-free mortality estimated at approximately 18.2% for patients ultimately classified as ACLF-1, 41.7% for those ultimately classified as ACLF-2, and 91.8% for those ultimately classified as ACLF-3[25]. However, effective therapeutic approaches for patients with ACLF, in addition to general intensive care interventions, are lacking. In many cases, LT is the only life-saving therapeutic option[26].
For ACLF-1 and ACLF-2 patients, they have notably favorable survival rates and relatively lower incidence of infection, suggesting a potential benefit in recommending early LT to improve patient prognosis. Notably, it is essential to acknowledge the rapid progression of ACLF, which can potentially transition from ACLF-1 to ACLF-3 within a short period, underscoring the critical importance of timely LT. However, the severity of the clinical conditions and risk of short-term death in these patients may be underestimated by the MELD and MELD-Na score-based organ allocation systems. This issue arises from the distinct pathophysiologic characteristics of ACLF compared with other forms of decompensated cirrhosis. The pathogenesis of ACLF involves severe systemic inflammation and subsequent processes such as cytokine storms, further leading to portal hypertension and multiple organ failure[27]. However, MELD and MELD-Na lack parameters to assess the extrahepatic nonrenal organ failure and systemic inflammation[28]; this partially explains why patients included in Zhu et al’s study presented lower MELD scores than patients in other included studies did, with three-month and one-year survival rates of only 68.5% and 60.3%, respectively, due to the higher prevalence of respiratory failure (52.1%) and cerebral failure (84.9%) at the time of LT[24]. Moreover, patients with the same MELD score may vary in their suitability for LT procedures, with some opting for LT and others considering supportive treatment only. The decision of whether to proceed with LT depends on various factors, such as the presence or absence of frailty, previous abdominal surgery, concurrent organ failure, and portal hypertension[2].
Given the circumstances above, several new allocation systems have been established and implemented. For example, the recommendations of the Spanish Society of Liver Transplantation advocate the utilization of the EASL-CLIF criteria rather than the MELD score to assess patient prognosis and increase transplant priority for patients with ACLF-2 and ACLF-3[29]. Additionally, the ACLF transplantation tier has been developed in the United Kingdom. Eligibility for expedited transplantation includes liver cirrhosis, severe liver failure, other organ failures requiring organ support in the intensive care unit, and a 28-day mortality risk greater than 50%[29]. In the future, updating organ allocation systems targeting patients with ACLF deserves increased attention in the decision-making process regarding which patients should prioritize LT.
Our study also demonstrated that LT could provide a survival advantage for patients with ACLF-3. The survival rates at one month, three months, six months, and one year after LT were 88%, 81%, 75%, and 73%, respectively. Nevertheless, several critical issues exist for these patients. According to an analysis of the United Network for Organ Sharing database, 14-day waitlist mortality in patients with ACLF-3 was higher than that in acute liver failure patients, independent of the MELD-Na score, highlighting the severity of ACLF-3 and the importance of early organ allocation to improve prognosis[30]. However, not all ACLF-3 patients achieve favorable outcomes after LT. Sundaram et al[31] reported that patients who experienced recovery from ACLF-3 at listing to ACLF 0-2 at LT tended to have a better probability of one-year survival. Conversely, factors such as advanced age, the need for mechanical ventilation, severe infections, or active gastrointestinal bleeding may be associated with futile LT[15,32,33]. Recent efforts have attempted to determine patients for whom LT might be futile. For example, Artzner et al[10] established the transplantation for ACLF-3 model to distinguish high-risk and low-risk groups among patients with ACLF-3, which incorporates four objective variables: Age ≥ 53 years, pre-LT arterial lactate level ≥ 4 mmol/L, mechanical ventilation with PaO2/FiO2 ≤ 200 mmHg, and pre-LT leukocyte count ≤ 10 G/L. Hernaez et al[34] developed the Sundaram ACLF-LT-Mortality score, which incorporates factors such as age, use of inotropes, presence of respiratory failure, diabetes mellitus, and BMI. This score can effectively predict mortality within one year after LT in patients with severe ACLF.
Notably, the different conditions of concurrent extrahepatic organ failure at the time of LT also affect survival outcomes. Xia et al[22] identified respiratory failure [hazard ratio (HR) = 3.516, 95%CI: 1.420-8.706] and cerebral failure (HR = 3.183, 95%CI: 1.338-7.572) as significant risk factors for post-LT death. Additionally, the number of organ failures was also an independent predictor of post-LT outcomes[32,35]. Notably, the CLIF-C ACLF scores do not incorporate weighted assessments for six specific types of organ failure. Moreover, differences exist in the definition and assessment of organ failure across various diagnostic criteria and scoring systems. For example, unlike the EASL-CLIF criteria, the Asian Pacific Association for the Study of the Liver criteria do not include respiratory or circulation failure[36]. The North American Consortium for Study of End-stage Liver Disease only evaluates four types of organ failure, including shock, West-Haven hepatic encephalopathy grades 3-4, renal failure requiring dialysis, and respiratory failure requiring mechanical ventilation, without considering liver-specific and coagulation parameters[37]. Large-scale, prospective, multicenter, cohort studies are further needed to conduct specific evaluations of different organs.
In addition to the recipient condition, the donor status also plays a crucial role in predicting patient prognosis. Among the included studies, six evaluated donor conditions such as CIT, WIT, graft weight, and graft steatosis. Notably, the potential adverse effects of using marginal donor livers, particularly those with a donor risk index ≥ 1.7, have been identified as risk factors for post-LT mortality[33]. Consequently, differences in donor selection for patients with ACLF-2 and ACLF-3 may lead to heterogeneity in survival estimates. Future scoring systems should consider donor conditions to comprehensively evaluate the prognosis of patients with ACLF who are receiving LT, adjust allocation choices, and prevent organ wasting.
With respect to post-LT complications, infections, particularly bacterial infections, are highly prevalent. Upon the initial presentation of ACLF, infection occurs in up to 40% of these patients[2]. In some Western countries, bacterial infection, including spontaneous bacterial peritonitis, urinary-tract infections, and pneumonia, is considered the predominant precipitating event[38,39]. This study demonstrated that infections also represent the primary complication following LT, especially among patients with ACLF-3. The pathogenesis of infections among patients with ACLF is associated with various factors, including decreased immune function, impaired intestinal barrier function, and shifts in microbiota composition[40,41]. Moreover, patients with ACLF exhibit an elevated risk for multidrug-resistant bacterial infections and fungal infections, suggesting that the selection of postoperative anti-infective therapy warrants inclusion in clinical guidelines[38].
However, this study has potential limitations. First, some of the estimates provided in our study identified significant heterogeneity. In subgroup analyses stratified by region, we observed that one-year survival among patients with ACLF-3 reached 84% in North America, whereas in Asia and South America, it was only 63% and 67%, respectively. Given the diverse etiologies of chronic underlying liver conditions, variations in organ allocation systems, and surgical techniques across different regions, further research is needed to explore the source of heterogeneity. Second, we restricted our inclusion criteria to studies that identified ACLF and its grades based on EASL-CLIF. While this approach helps mitigate bias stemming from differing definitions of ACLF grades due to diverse diagnostic criteria, it also limits the inclusion of literature from regions utilizing alternative diagnostic standards. Third, we lacked access to relevant data in Africa and Australia, necessitating more research to determine regional disparities in ACLF survival outcomes. Finally, publication bias was likely present because of insufficient data availability.
In summary, we provide novel insights into the post-LT survival outcomes among ACLF patients with distinct severity grades. Given the ongoing debates regarding the selection of LT in ACLF management, our findings are timely and contribute to a better understanding of the survival advantages associated with LT. For patients with ACLF-1 and ACLF-2, LT should be actively considered as a treatment option, regardless of the potentially low MELD score. Additionally, concerning patients with ACLF-3, LT may also offer survival benefits to a subset of patients; however, the decision of whether to proceed with LT should be based on a comprehensive evaluation of preoperative patients’ status, concurrent organ failure, and the quality of donor organs.
This meta-analysis highlights the survival benefit conferred by LT to patients with ACLF, particularly those categorized as ACLF-1 or ACLF-2. To promote effective organ allocation and prevent organ waste, future research should comprehensively explore the impact of preoperative patient conditions and donor characteristics on post-LT survival outcomes among patients with ACLF of varying severity grades. Furthermore, there is an urgent need for the development of a scoring system targeted at patients with ACLF for organ allocation.
| 1. | Arroyo V, Moreau R, Jalan R. Acute-on-Chronic Liver Failure. N Engl J Med. 2020;382:2137-2145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 431] [Article Influence: 86.2] [Reference Citation Analysis (2)] |
| 2. | Bajaj JS, O'Leary JG, Lai JC, Wong F, Long MD, Wong RJ, Kamath PS. Acute-on-Chronic Liver Failure Clinical Guidelines. Am J Gastroenterol. 2022;117:225-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 118] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 3. | Yao J, Lei YG, Yi HM, Yang Y. Clinical strategies to improve the survival rate of liver recipients with acute-on-chronic liver failure. Hepatobiliary Pancreat Dis Int. 2023;22:41-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Abdallah MA, Waleed M, Bell MG, Nelson M, Wong R, Sundaram V, Singal AK. Systematic review with meta-analysis: liver transplant provides survival benefit in patients with acute on chronic liver failure. Aliment Pharmacol Ther. 2020;52:222-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 5. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6253] [Cited by in RCA: 7640] [Article Influence: 477.5] [Reference Citation Analysis (1)] |
| 6. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2171] [Article Influence: 180.9] [Reference Citation Analysis (5)] |
| 7. | Luchini C, Veronese N, Nottegar A, Shin JI, Gentile G, Granziol U, Soysal P, Alexinschi O, Smith L, Solmi M. Assessing the quality of studies in meta-research: Review/guidelines on the most important quality assessment tools. Pharm Stat. 2021;20:185-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 109] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 8. | Agbim U, Sharma A, Maliakkal B, Karri S, Yazawa M, Goldkamp W, Podila PSB, Vanatta JM, Gonzalez H, Molnar MZ, Nair SP, Eason JD, Satapathy SK. Outcomes of Liver Transplant Recipients With Acute-on-Chronic Liver Failure Based on EASL-CLIF Consortium Definition: A Single-center Study. Transplant Direct. 2020;6:e544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Artru F, Louvet A, Ruiz I, Levesque E, Labreuche J, Ursic-Bedoya J, Lassailly G, Dharancy S, Boleslawski E, Lebuffe G, Kipnis E, Ichai P, Coilly A, De Martin E, Antonini TM, Vibert E, Jaber S, Herrerro A, Samuel D, Duhamel A, Pageaux GP, Mathurin P, Saliba F. Liver transplantation in the most severely ill cirrhotic patients: A multicenter study in acute-on-chronic liver failure grade 3. J Hepatol. 2017;67:708-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 287] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 10. | Artzner T, Michard B, Weiss E, Barbier L, Noorah Z, Merle JC, Paugam-Burtz C, Francoz C, Durand F, Soubrane O, Pirani T, Theocharidou E, O'Grady J, Bernal W, Heaton N, Salamé E, Bucur P, Barraud H, Lefebvre F, Serfaty L, Besch C, Bachellier P, Schneider F, Levesque E, Faitot F. Liver transplantation for critically ill cirrhotic patients: Stratifying utility based on pretransplant factors. Am J Transplant. 2020;20:2437-2448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 11. | Belli LS, Duvoux C, Artzner T, Bernal W, Conti S, Cortesi PA, Sacleux SC, Pageaux GP, Radenne S, Trebicka J, Fernandez J, Perricone G, Piano S, Nadalin S, Morelli MC, Martini S, Polak WG, Zieniewicz K, Toso C, Berenguer M, Iegri C, Invernizzi F, Volpes R, Karam V, Adam R, Faitot F, Rabinovich L, Saliba F, Meunier L, Lesurtel M, Uschner FE, Fondevila C, Michard B, Coilly A, Meszaros M, Poinsot D, Schnitzbauer A, De Carlis LG, Fumagalli R, Angeli P, Arroyo V, Jalan R; ELITA/EF-CLIF working group. Liver transplantation for patients with acute-on-chronic liver failure (ACLF) in Europe: Results of the ELITA/EF-CLIF collaborative study (ECLIS). J Hepatol. 2021;75:610-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 122] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 12. | Benítez C, Arnold J, Cambindo V, Schoenfeldt F, Cancino A, Ibáñez S, Grandy C, Hunfan P, González J, Guerra C, Godoy E, Araneda V, Mollo C, Poniachik J, Urzúa A, Cattaneo M, Roblero JP, Oppenheimer I, Pizarro V. Effect of acute on chronic liver failure over post-transplant survival. Ann Hepatol. 2023;28:101128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 13. | Bhatti ABH, Dar FS, Butt MO, Sahaab E, Salih M, Shah NH, Khan NY, Zia HH, Khan EU, Khan NA. Living Donor Liver Transplantation for Acute on Chronic Liver Failure Based on EASL-CLIF Diagnostic Criteria. J Clin Exp Hepatol. 2018;8:136-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Cervantes-Alvarez E, Vilatoba M, Limon-de la Rosa N, Mendez-Guerrero O, Kershenobich D, Torre A, Navarro-Alvarez N. Liver transplantation is beneficial regardless of cirrhosis stage or acute-on-chronic liver failure grade: A single-center experience. World J Gastroenterol. 2022;28:5881-5892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Levesque E, Winter A, Noorah Z, Daurès JP, Landais P, Feray C, Azoulay D. Impact of acute-on-chronic liver failure on 90-day mortality following a first liver transplantation. Liver Int. 2017;37:684-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 16. | Marciano S, Mauro E, Giunta D, Torres MC, Diaz JM, Bermudez C, Gutierrez-Acevedo MN, Narvaez A, Ortíz J, Dirchwolf M, Pollarsky F, Rojas-Saunero LP, Gadano A. Impact of acute-on-chronic liver failure on post-transplant survival and on kidney outcomes. Eur J Gastroenterol Hepatol. 2019;31:1157-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Qian YB, Chen F, Hang HL, Shen C, Han LZ, Deng YX, Xia L, Zhang JJ, Xia Q. Risk factors and outcomes of early infection in liver transplant recipients with acute-on-chronic liver failure. J Dig Dis. 2022;23:642-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 18. | Singh SA, Pampaniya H, Mehtani R, Jadaun SS, Kumar M, Khurana S, Das DJ, Gupta S, Saigal S. Living donor liver transplant in acute on chronic liver failure grade 3: Who not to transplant. Dig Liver Dis. 2024;56:152-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Sundaram V, Mahmud N, Perricone G, Katarey D, Wong RJ, Karvellas CJ, Fortune BE, Rahimi RS, Maddur H, Jou JH, Kriss M, Stein LL, Lee M, Jalan R; Multi-Organ Dysfunction, Evaluation for Liver Transplantation (MODEL) Consortium. Longterm Outcomes of Patients Undergoing Liver Transplantation for Acute-on-Chronic Liver Failure. Liver Transpl. 2020;26:1594-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 20. | Sundaram V, Patel S, Shetty K, Lindenmeyer CC, Rahimi RS, Flocco G, Al-Attar A, Karvellas CJ, Challa S, Maddur H, Jou JH, Kriss M, Stein LL, Xiao AH, Vyhmeister RH, Green EW, Campbell B, Cranford W, Mahmud N, Fortune BE; Multi‐Organ Dysfunction and Evaluation for Liver Transplantation (MODEL) Consortium. Risk Factors for Posttransplantation Mortality in Recipients With Grade 3 Acute-on-Chronic Liver Failure: Analysis of a North American Consortium. Liver Transpl. 2022;28:1078-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Sundaram V, Lindenmeyer CC, Shetty K, Rahimi RS, Al-Attar A, Flocco G, Fortune BE, Gong C, Challa S, Maddur H, Jou JH, Kriss M, Stein LL, Xiao AH, Vyhmeister RH, Green EW, Campbell B, Piscitello AJ, Cranford W, Levitsky J, Karvellas CJ; Multi-Organ Dysfunction and Evaluation for Liver Transplantation (MODEL) Consortium. Patients With Acute-on-Chronic Liver Failure Have Greater Healthcare Resource Utilization After Liver Transplantation. Clin Gastroenterol Hepatol. 2023;21:704-712.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 22. | Xia L, Qiao ZY, Zhang ZJ, Lv ZC, Tong H, Tong Y, Wu HX, Chen XS, Sun HY, Zhang JJ, Thasler WE, Feng H, Xia Q. Transplantation for EASL-CLIF and APASL acute-on-chronic liver failure (ACLF) patients: The TEA cohort to evaluate long-term post-Transplant outcomes. EClinicalMedicine. 2022;49:101476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 23. | Yang M, Peng B, Zhuang Q, Li J, Liu H, Cheng K, Ming Y. Models to predict the short-term survival of acute-on-chronic liver failure patients following liver transplantation. BMC Gastroenterol. 2022;22:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Zhu CX, Yang L, Zhao H, Zhang Y, Tu S, Guo J, Yan D, Hu CX, Lu HF, Xu KJ, Huang JR, Li LJ. Impact of cirrhosis-related complications on posttransplant survival in patients with acute-on-chronic liver failure. Hepatobiliary Pancreat Dis Int. 2023;22:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 25. | Gustot T, Fernandez J, Garcia E, Morando F, Caraceni P, Alessandria C, Laleman W, Trebicka J, Elkrief L, Hopf C, Solís-Munoz P, Saliba F, Zeuzem S, Albillos A, Benten D, Montero-Alvarez JL, Chivas MT, Concepción M, Córdoba J, McCormick A, Stauber R, Vogel W, de Gottardi A, Welzel TM, Domenicali M, Risso A, Wendon J, Deulofeu C, Angeli P, Durand F, Pavesi M, Gerbes A, Jalan R, Moreau R, Ginés P, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL-CLIF Consortium. Clinical Course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology. 2015;62:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 481] [Article Influence: 48.1] [Reference Citation Analysis (1)] |
| 26. | Artru F, Trovato F, Morrison M, Bernal W, McPhail M. Liver transplantation for acute-on-chronic liver failure. Lancet Gastroenterol Hepatol. 2024;9:564-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 27. | Sarin SK, Choudhury A. Acute-on-chronic liver failure: terminology, mechanisms and management. Nat Rev Gastroenterol Hepatol. 2016;13:131-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 258] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 28. | Trebicka J, Sundaram V, Moreau R, Jalan R, Arroyo V. Liver Transplantation for Acute-on-Chronic Liver Failure: Science or Fiction? Liver Transpl. 2020;26:906-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 29. | Jalan R, Gustot T, Fernandez J, Bernal W. 'Equity' and 'Justice' for patients with acute-on chronic liver failure: A call to action. J Hepatol. 2021;75:1228-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 30. | Sundaram V, Shah P, Wong RJ, Karvellas CJ, Fortune BE, Mahmud N, Kuo A, Jalan R. Patients With Acute on Chronic Liver Failure Grade 3 Have Greater 14-Day Waitlist Mortality Than Status-1a Patients. Hepatology. 2019;70:334-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 31. | Sundaram V, Kogachi S, Wong RJ, Karvellas CJ, Fortune BE, Mahmud N, Levitsky J, Rahimi RS, Jalan R. Effect of the clinical course of acute-on-chronic liver failure prior to liver transplantation on post-transplant survival. J Hepatol. 2020;72:481-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 32. | Sacleux SC, Ichaï P, Coilly A, Boudon M, Lemaitre E, Sobesky R, De Martin E, Cailliez V, Kounis I, Poli E, Ordan MA, Pascale A, Duclos-Vallée JC, Feray C, Azoulay D, Vibert E, Cherqui D, Adam R, Samuel D, Saliba F. Liver transplant selection criteria and outcomes in critically ill patients with ACLF. JHEP Rep. 2024;6:100929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Sundaram V, Jalan R, Wu T, Volk ML, Asrani SK, Klein AS, Wong RJ. Factors Associated with Survival of Patients With Severe Acute-On-Chronic Liver Failure Before and After Liver Transplantation. Gastroenterology. 2019;156:1381-1391.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 249] [Article Influence: 41.5] [Reference Citation Analysis (1)] |
| 34. | Hernaez R, Karvellas CJ, Liu Y, Sacleux SC, Khemichian S, Stein LL, Shetty K, Lindenmeyer CC, Boike JR, Simonetto DA, Rahimi RS, Jalal PK, Izzy M, Kriss MS, Im GY, Lin MV, Jou JH, Fortune BE, Cholankeril G, Kuo A, Mahmud N, Kanwal F, Saliba F, Sundaram V, Artzner T, Jalan R; Multi-Organ Dysfunction and Evaluation for Liver Transplantation (MODEL) Consortium. The novel SALT-M score predicts 1-year post-transplant mortality in patients with severe acute-on-chronic liver failure. J Hepatol. 2023;79:717-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 35. | Thuluvath PJ, Thuluvath AJ, Hanish S, Savva Y. Liver transplantation in patients with multiple organ failures: Feasibility and outcomes. J Hepatol. 2018;69:1047-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 154] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 36. | Sarin SK, Choudhury A, Sharma MK, Maiwall R, Al Mahtab M, Rahman S, Saigal S, Saraf N, Soin AS, Devarbhavi H, Kim DJ, Dhiman RK, Duseja A, Taneja S, Eapen CE, Goel A, Ning Q, Chen T, Ma K, Duan Z, Yu C, Treeprasertsuk S, Hamid SS, Butt AS, Jafri W, Shukla A, Saraswat V, Tan SS, Sood A, Midha V, Goyal O, Ghazinyan H, Arora A, Hu J, Sahu M, Rao PN, Lee GH, Lim SG, Lesmana LA, Lesmana CR, Shah S, Prasad VGM, Payawal DA, Abbas Z, Dokmeci AK, Sollano JD, Carpio G, Shresta A, Lau GK, Fazal Karim M, Shiha G, Gani R, Kalista KF, Yuen MF, Alam S, Khanna R, Sood V, Lal BB, Pamecha V, Jindal A, Rajan V, Arora V, Yokosuka O, Niriella MA, Li H, Qi X, Tanaka A, Mochida S, Chaudhuri DR, Gane E, Win KM, Chen WT, Rela M, Kapoor D, Rastogi A, Kale P, Rastogi A, Sharma CB, Bajpai M, Singh V, Premkumar M, Maharashi S, Olithselvan A, Philips CA, Srivastava A, Yachha SK, Wani ZA, Thapa BR, Saraya A, Shalimar, Kumar A, Wadhawan M, Gupta S, Madan K, Sakhuja P, Vij V, Sharma BC, Garg H, Garg V, Kalal C, Anand L, Vyas T, Mathur RP, Kumar G, Jain P, Pasupuleti SSR, Chawla YK, Chowdhury A, Alam S, Song DS, Yang JM, Yoon EL; APASL ACLF Research Consortium (AARC) for APASL ACLF working Party. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13:353-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 614] [Cited by in RCA: 590] [Article Influence: 98.3] [Reference Citation Analysis (0)] |
| 37. | Bajaj JS, O'Leary JG, Reddy KR, Wong F, Biggins SW, Patton H, Fallon MB, Garcia-Tsao G, Maliakkal B, Malik R, Subramanian RM, Thacker LR, Kamath PS; North American Consortium For The Study Of End-Stage Liver Disease (NACSELD). Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology. 2014;60:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 436] [Article Influence: 39.6] [Reference Citation Analysis (1)] |
| 38. | Fernández J, Prado V, Trebicka J, Amoros A, Gustot T, Wiest R, Deulofeu C, Garcia E, Acevedo J, Fuhrmann V, Durand F, Sánchez C, Papp M, Caraceni P, Vargas V, Bañares R, Piano S, Janicko M, Albillos A, Alessandria C, Soriano G, Welzel TM, Laleman W, Gerbes A, De Gottardi A, Merli M, Coenraad M, Saliba F, Pavesi M, Jalan R, Ginès P, Angeli P, Arroyo V; European Foundation for the Study of Chronic Liver Failure (EF-Clif). Multidrug-resistant bacterial infections in patients with decompensated cirrhosis and with acute-on-chronic liver failure in Europe. J Hepatol. 2019;70:398-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 248] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 39. | Mücke MM, Rumyantseva T, Mücke VT, Schwarzkopf K, Joshi S, Kempf VAJ, Welsch C, Zeuzem S, Lange CM. Bacterial infection-triggered acute-on-chronic liver failure is associated with increased mortality. Liver Int. 2018;38:645-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 40. | Bonnel AR, Bunchorntavakul C, Reddy KR. Immune dysfunction and infections in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:727-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 281] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 41. |
Schierwagen R, Gu W, Brieger A, Brüne B, Ciesek S, Đikić I, Dimmeler S, Geisslinger G, Greten FR, Hermann E, Hildt E, Kempf VAJ, Klein S, Koch I, Mühl H, Müller V, Peiffer KH, Kestner RI, Piiper A, Rohde G, Scholich K, Schulz MH, Storf H, Toptan T, Vasa-Nicotera M, Vehreschild MJGT, Weigert A, Wild PJ, Zeuzem S, Engelmann C; |