Published online Mar 21, 2025. doi: 10.3748/wjg.v31.i11.98974
Revised: December 10, 2024
Accepted: February 17, 2025
Published online: March 21, 2025
Processing time: 245 Days and 23 Hours
Esophageal hypersensitivity is an important cause of refractory gastroesophageal reflux disease, in which patients do not respond to standard acid-suppressive therapy and suffer from continuous noncardiac chest pain and regurgitation. The N-methyl-D-aspartate receptor (NMDAR) may play a crucial role in the deve
To investigate the role of the NMDAR2B/protein kinase A (PKA)/cAMP-response element binding protein (CREB) signaling pathway in the development of esophageal neuropathic pain associated with gastroesophageal reflux disease (GERD).
Thirty-six 6-week-old specific pathogen free rats were randomly assigned to six groups: the control, model, model + NMDAR agonist, model + NMDAR anta
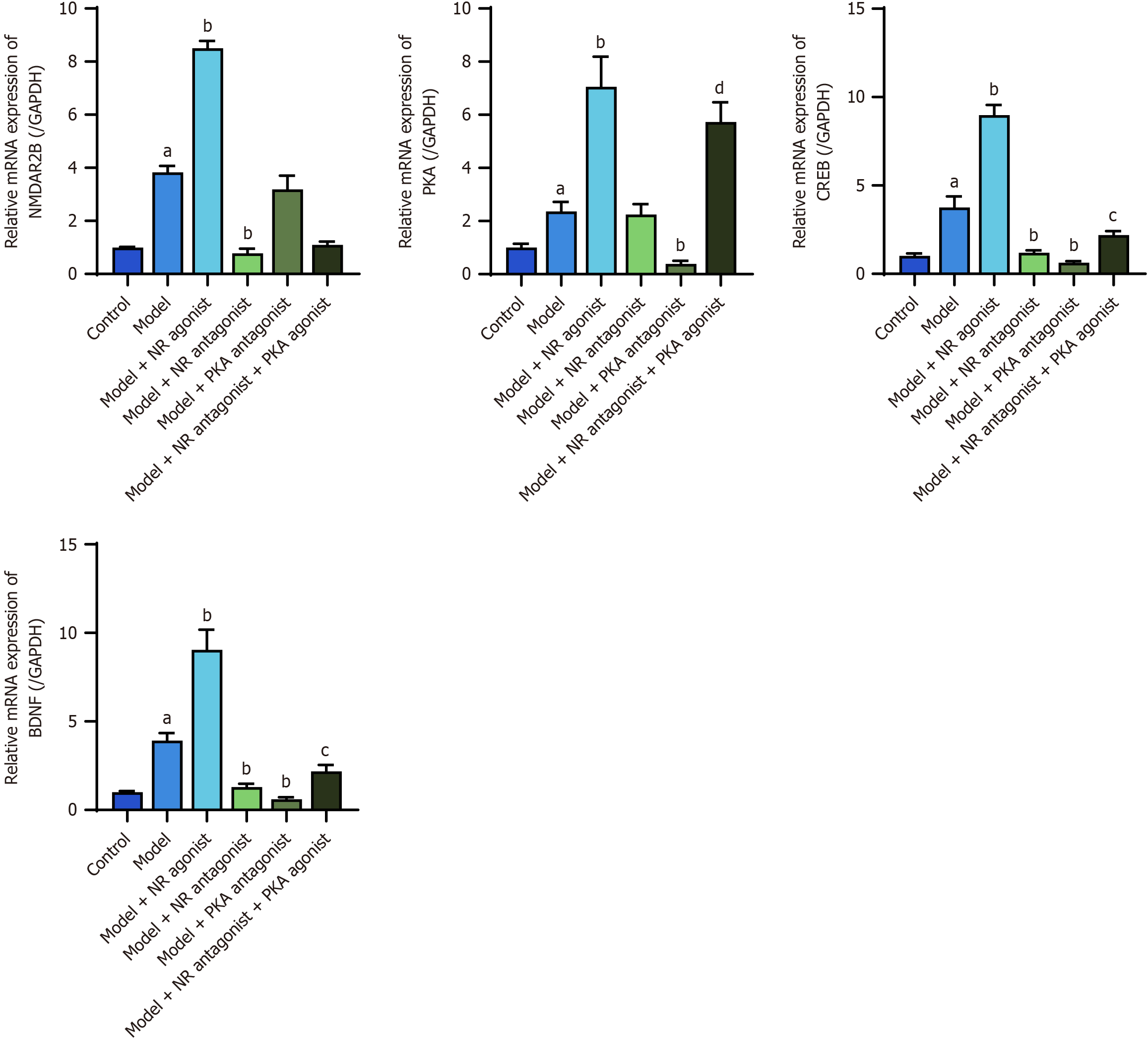
Compared with the control group, the model group presented significantly increased expression levels of the NMDAR2B, PKA, CREB, BDNF, substance P, and calcitonin gene-related peptide proteins and mRNAs in the cingulate gyrus, dorsal thalamus, spinal dorsal horn, and lower esophagus (P < 0.05). Compared with the model group, the model + NMDAR agonist group exhibited even higher expression levels of these proteins and mRNAs
The NMDAR2B signaling pathway plays a critical role in the development of neuropathic pain in GERD through the PKA/CREB/BDNF pathway.
Core Tip: Esophageal hypersensitivity is a major contributor to refractory gastroesophageal reflux disease (GERD), meaning that minor distension and physiological acid reflux can cause significant discomfort. Given that N-methyl-D-aspartate receptors (NMDARs) may play a crucial role in visceral hypersensitivity in functional gastrointestinal disorders, this study aimed to investigate whether NMDAR2B contributes to esophageal neuropathic pain. Immunohistochemistry, Western blot, RT-PCR and ELISA were used to measure the corresponding protein and mRNA expression levels in the cingulate gyrus, dorsal thalamus, spinal dorsal horn, esophageal tissues and serum of a rat model of esophageal hypersensitivity. Our findings suggest that the NMDAR2B/ protein kinase A/cAMP-response element binding protein signaling pathway contributes to esophageal neuropathic pain in GERD patients.
- Citation: Wang Y, Li GW, Zhu SL, Xu TT, Qin YW, Cheng CQ, Zheng QW, He C, Zhou BD, Fang SQ. NMDAR2B/PKA/CREB signaling pathway contributes to esophageal neuropathic pain in gastroesophageal reflux disease. World J Gastroenterol 2025; 31(11): 98974
- URL: https://www.wjgnet.com/1007-9327/full/v31/i11/98974.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i11.98974
Gastroesophageal reflux disease (GERD) is a common digestive disorder that is characterized mainly by reflux and heartburn. However, approximately 30%-40% of patients do not respond to standard acid-suppressive therapy, leading to refractory GERD (rGERD)[1,2]. According to the latest international guidelines[3,4], esophageal hypersensitivity plays a significant role in the refractory nature of this condition, although the precise mechanisms are still not well understood.
At the spinal segment, afferent nerves conveying peripheral visceral sensations terminate in the posterior horn of the spinal cord. Once activated, the sensory neurons in the posterior horn maintain a prolonged activation state. This persistent activation can result in a phenomenon known as "wind up", characterized by enduring pain memory even after the cessation of peripheral stimuli, due to neurosensitization in the posterior horn[5]. It is currently believed that the mechanism of spinal sensitization for this visceral sensation involves the activation of N-methyl-D-aspartate (NMDA) receptor in the posterior horn of the spinal cord[6].
Animal studies on irritable bowel syndrome, a functional gastrointestinal disorder affecting the lower digestive tract, have shown that the activation of the NMDA receptor (NMDAR)2B subunit can induce the expression of brain-derived neurotrophic factor (BDNF)[7,8], which plays a crucial role in the development of visceral hypersensitivity. However, the specific mechanisms of visceral hypersensitivity in upper digestive tract diseases remain poorly understood.
Our group has previously demonstrated through animal experiments that the NMDAR subunit is activated in the dorsal horn of the spinal cord in rats with esophageal hypersensitivity[9]. Therefore, this study aimed to investigate whether NMDAR2B regulates esophageal sensation via the protein kinase A (PKA)/cAMP-response element binding protein (CREB)-BDNF signaling pathway in GERD rats. By elucidating the mechanisms underlying esophageal visceral hypersensitivity, we hope to identify novel therapeutic targets for the treatment of rGERD.
Experimental animals: Thirty-six healthy male Sprague-Dawley (SD) rats, aged 6 weeks and weighing 200 ± 20 g, were used for this experiment. The rats were obtained from the Shanghai Laboratory Animal Quality Supervision and Testing Center. The animals were housed under controlled conditions: a temperature of 20 ± 2 °C, humidity of 50% ± 10%, and a 12-hour light/dark cycle. Sterilized bedding was provided, and the animals had ad libitum access to standard feed and water. Following a one-week acclimatization period, the experiments began.
Experimental antibodies and reagents: The experimental antibodies and reagents used are listed in the supplementary materials (Supplementary Table 1).
Major experimental instruments: The major experimental instruments used are detailed in the supplementary materials (Supplementary Table 2).
Animal model preparation: A rat model of GERD with esophageal visceral hypersensitivity was prepared using a combination of intraperitoneal sensitization with ovalbumin and localized esophageal stimulation, as described previously[10,11].
Modeling procedure: Healthy adult male SD rats were used for this experiment. On the first day, each rat received an intraperitoneal injection of 1.5 mL of a mixture containing 100 mg of ovalbumin and 200 mg of aluminum hydroxide adjuvant. Fourteen days after the initial sensitization, the rats were fasted for 12 hours before being subjected to the subsequent procedures under anesthesia induced by an intraperitoneal injection of 2% sodium pentobarbital (1 mL/kg).
Esophageal balloon dilation: The rats were placed in a supine position and immobilized on the experimental table. Using a mouth opener, the pharynx was fully exposed, and a PTCA balloon catheter was inserted orally and positioned approximately 8 cm from the incisors. A medical balloon inflation pressure pump was used to gradually inflate the balloon to a diameter of 3 mm, ensuring a steady increase in intraballoon pressure.
Esophageal acid perfusion: A drainage tube was inserted at the gastric cardia through incisions in the abdominal and gastric walls to collect the infused esophageal fluid. The anesthetized rats' heads were elevated by 20-30°. A single-lumen perfusion tube (inner diameter 0.50 mm, outer diameter 0.80 mm) was placed orally into the esophagus, with the catheter tip positioned 2-3 cm above the esophagogastric junction and secured in place. The other end of the tube was connected to a continuous perfusion pump. A 0.1 N hydrochloric acid solution, maintained at 37 °C, was perfused at a rate of 10 mL/hour for a total of 50 minutes.
Animal grouping and treatment: Thirty-six SD rats were randomly divided into six groups, each containing six rats: control, model, model + NMDAR agonist (NR agonist), model + NMDAR antagonist (NR antagonist), model + PKA antagonist, and model + NMDAR antagonist + PKA agonist (NR antagonist + PKA agonist). (1) Model + NMDAR agonist group: Rats received an NMDAR agonist (75 mg/kg, intraperitoneally) 30 minutes before anesthesia was induced by an intraperitoneal injection of 2% sodium pentobarbital (1 mL/kg); (2) Model + NMDAR antagonist group: Rats received an NMDAR antagonist (1 mg/kg, intraperitoneally) 30 minutes before anesthesia; (3) Model + PKA antagonist group: Rats received a PKA antagonist (2 mg/kg, intraperitoneally) 30 minutes before anesthesia; (4) Model + NMDAR antagonist + PKA agonist group: Rats received a PKA agonist (10 mg/kg, subcutaneously) 60 minutes before anesthesia and an NMDAR antagonist (1 mg/kg, intraperitoneally) 30 minutes before anesthesia; and (5) Control and model groups: Rats received the same volume of saline.
Sample collection: Blood collection from the aorta: Under deep anesthesia, a thoracotomy was performed to expose the heart. Blood (0.8-1.2 mL) was drawn from the left ventricle. The samples were left to stand for 30 minutes and then centrifuged at 3000 rpm for 15 minutes. The resulting serum was collected and stored at -20 °C.
Pathological tissue collection: Following blood collection, a cannula was inserted into the ascending aorta via the left ventricle. The circulatory system was perfused with 200 mL of 0.9% saline solution to clear the blood, followed by 500 mL of 4% paraformaldehyde solution (pH 7.4) at 4 °C for 1 hour. Once the limbs and spine became rigid, the spinal cord (T1-T6) and whole brain were collected, fixed in paraformaldehyde, and embedded in paraffin.
Frozen tissue collection: The lower esophagus, brain, and spinal cord were harvested, rinsed with 4 °C saline, dried with filter paper, placed in RNase-free EP tubes, and stored at -80 °C.
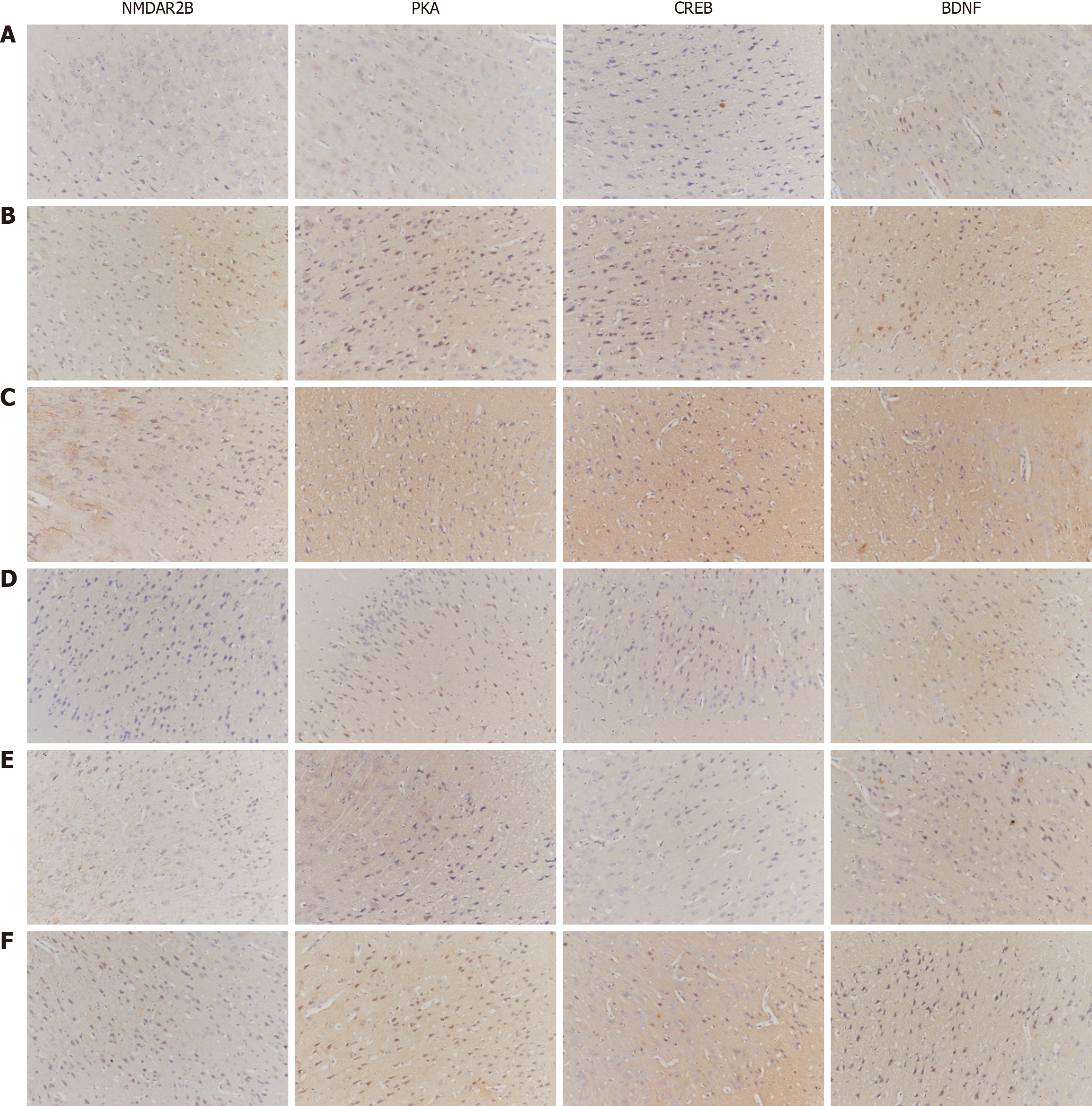
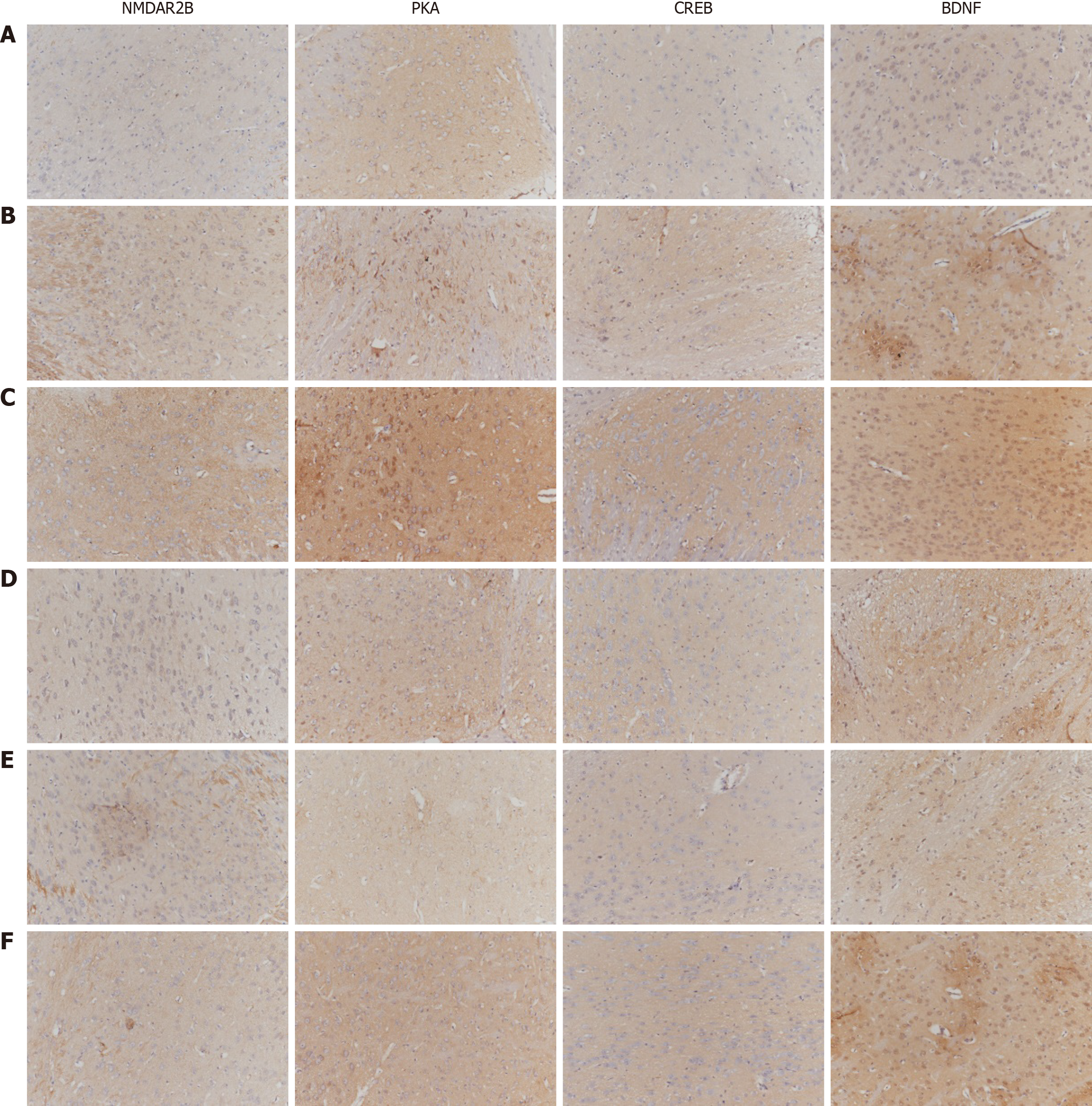
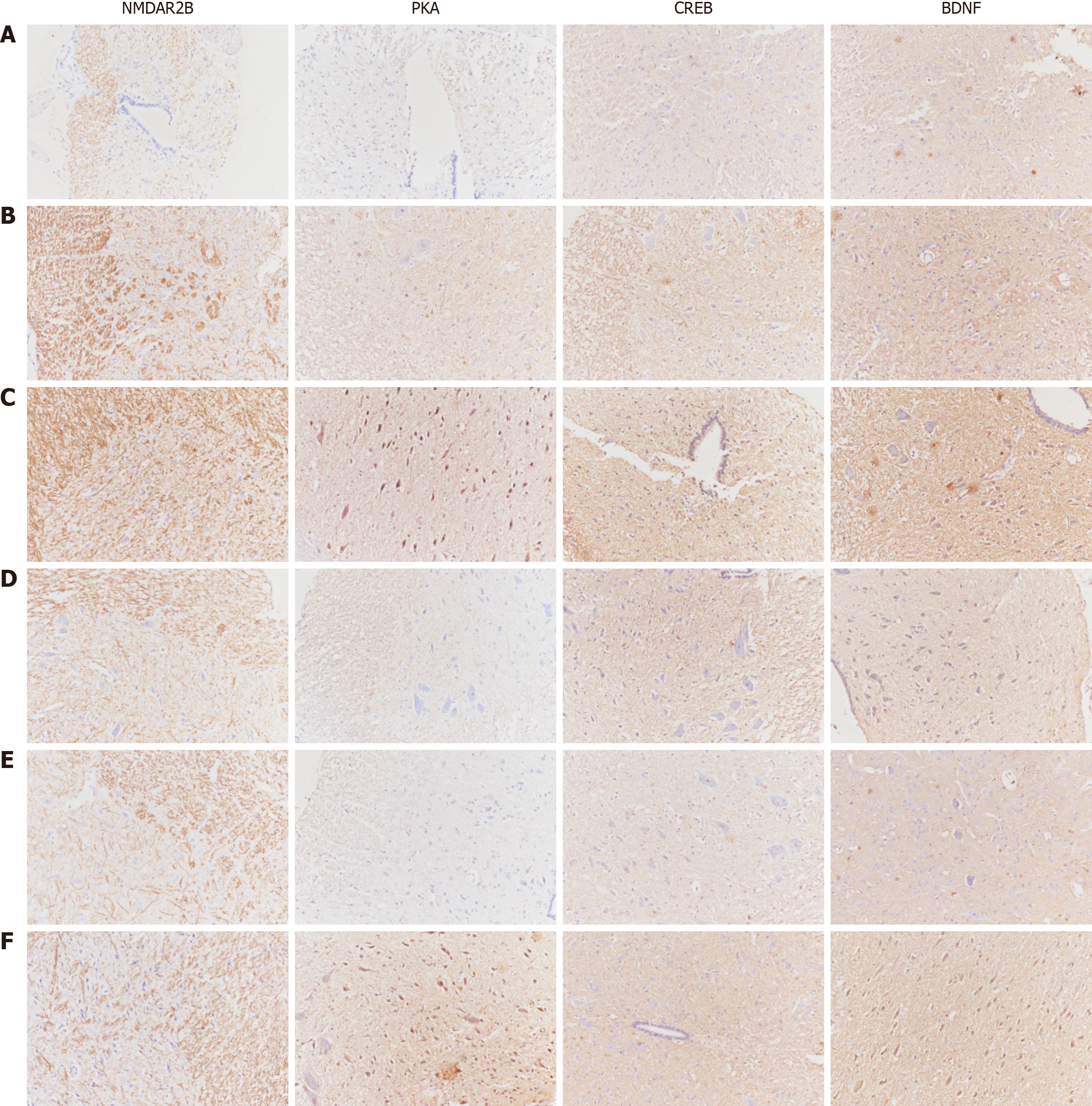
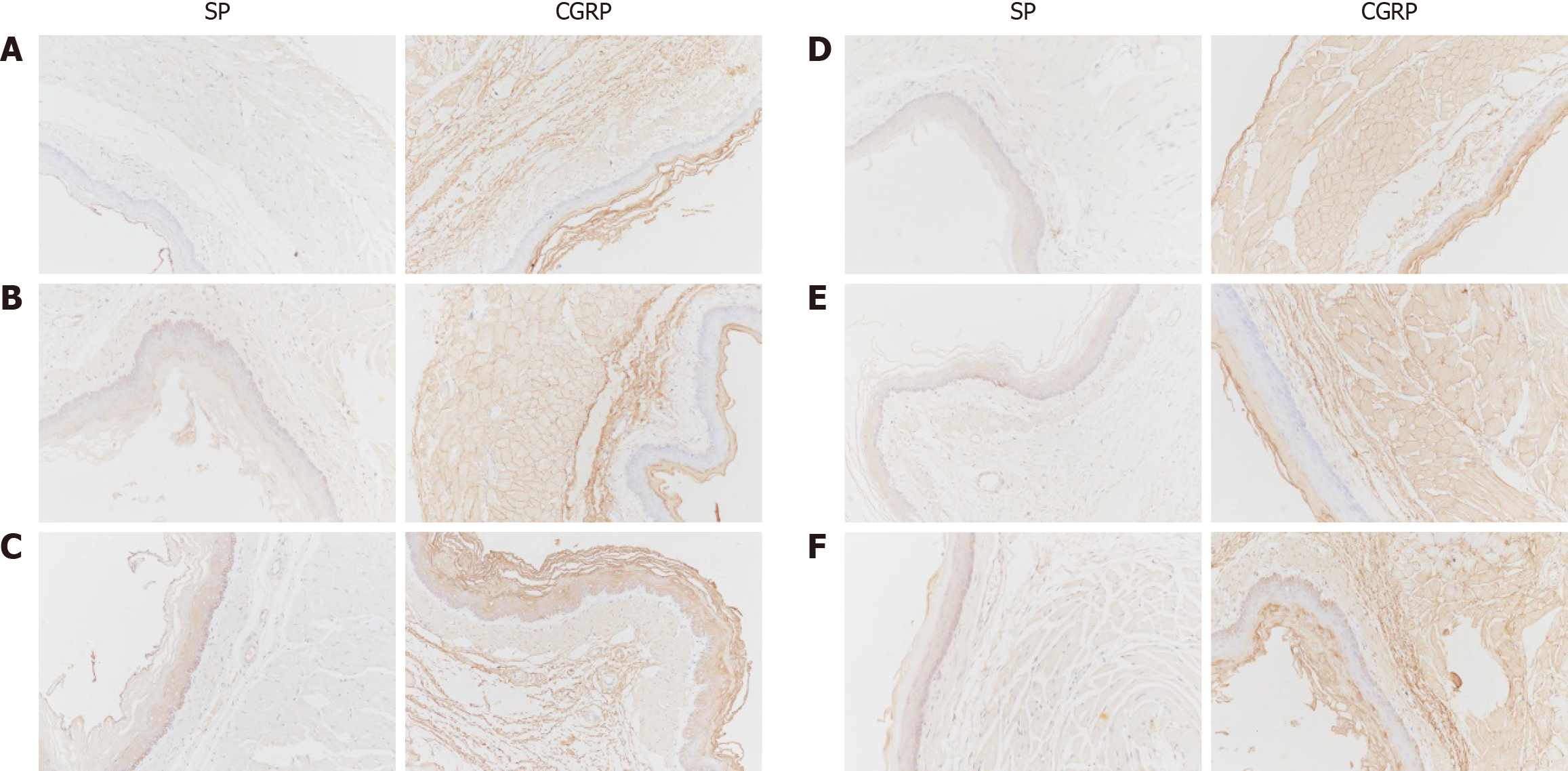
Observation indicators: The quantitative analysis of NMDA pathway-related gene and protein expression involves several techniques. Immunohistochemistry was used to semiquantitatively analyze the expression of NMDAR2B, PKA, CREB, and BDNF in the cingulate cortex, dorsal thalamus, and spinal cord dorsal horn. Additionally, substance P (SP) and calcitonin gene-related peptide (CGRP) expression in the lower esophageal mucosa was examined using immunohistochemistry. The expression of related proteins in 5 fields of vision at random was observed by the high-magnification microscopy. The average optical density (AOD), i.e., integrated optical density/area, of the positive reaction site of each field was calculated. The average value of the AOD in 5 fields represented the quantity of antigen. The larger the value was, the more the antigen was expressed. For quantitative protein analysis, Western blotting was conducted to measure the protein expression of NMDAR2B, PKA, CREB, and BDNF in the aforementioned brain regions, as well as SP and CGRP expression in the lower esophageal mucosa.
Furthermore, RT-PCR was used to measure the mRNA expression of NMDAR2B, PKA, CREB, and BDNF in the cingulate cortex, dorsal thalamus, and spinal cord, along with SP and CGRP mRNA expression in the lower esophageal mucosa. The sequences of primers used for RT-PCR are provided in Table 1. Experimental procedure: After the extraction of total RNA, DNA elimination (reaction conditions: 37 °C, 30 minutes; Hold, add 1 μL of EDTA; 65 °C, 10 minutes), reverse transcription (reaction conditions: 37 °C, 60 minutes; 85 °C, 5 minutes; 4 °C, 5 minutes; storage at -20 °C), real-time PCR amplification (reaction conditions: 95 °C, 10 minutes; 95 °C, 15 seconds; 60 °C, 45 seconds) and 40 cycles, melting curves: 95 °C, 15 seconds; 60 °C, 1 minute; 95 °C, 15 seconds; 60 °C, 15 seconds) were performed in turn.
| mRNA | Primers | Product length (bp) |
| NMDAR2B | Primer F 5’ AGCTCAGCGACCTGTATG 3’ | 204 |
| Primer R 5’ ACTCCCTCCTCGATTTGG 3’ | ||
| PKA | Primer F 5' TCCTTTGGGCGAGTGATG 3' | 283 |
| Primer R 5' CGTAGAAACGGGCGTGGG 3' | ||
| CREB | Primer F 5' CAGGGAGGAGCAATACAG 3' | 148 |
| Primer R 5' GCACTAGAATCTGCTGTCC 3' | ||
| BDNF | Primer F 5' TGGATGAGGACCAGAAGG 3' | 115 |
| Primer R 5' AGAAAGAGCAGAGGAGGC 3' | ||
| GAPDH | Primer F 5' GGAGTCTACTGGCGTCTTCAC 3' | 237 |
| Primer R 5' ATGAGCCCTTCCACGATGC 3' |
Serum BDNF levels were measured using ELISA. To each well, 40 µL of the sample was added, followed by the addition of 10 µL of biotin-labeled BDNF antibody. The mixture was then incubated at 37 °C for 30 minutes. Sub
Behavioral scoring involved the description and scoring of SD rats' responses to acid stimulation and balloon distension of the lower esophagus as follows: 1 point for no obvious response, 2 points for rapid breathing, 3 points for groaning, 4 points for mild struggle, and 5 points for severe struggle.
Statistical analysis was conducted using SPSS 20.0 software. Normally distributed data are presented as the mean ± SD, whereas data not adhering to a normal distribution are expressed as the median (interquartile range), denoted as M (P25, P75). For normally distributed data with homogeneous variance, one-way ANOVA followed by the least significant difference test was used. In cases where a normal distribution or homogeneity of variance assumption was violated, the Kruskal-Wallis test was used. A significance level of P < 0.05 was considered statistically significant.
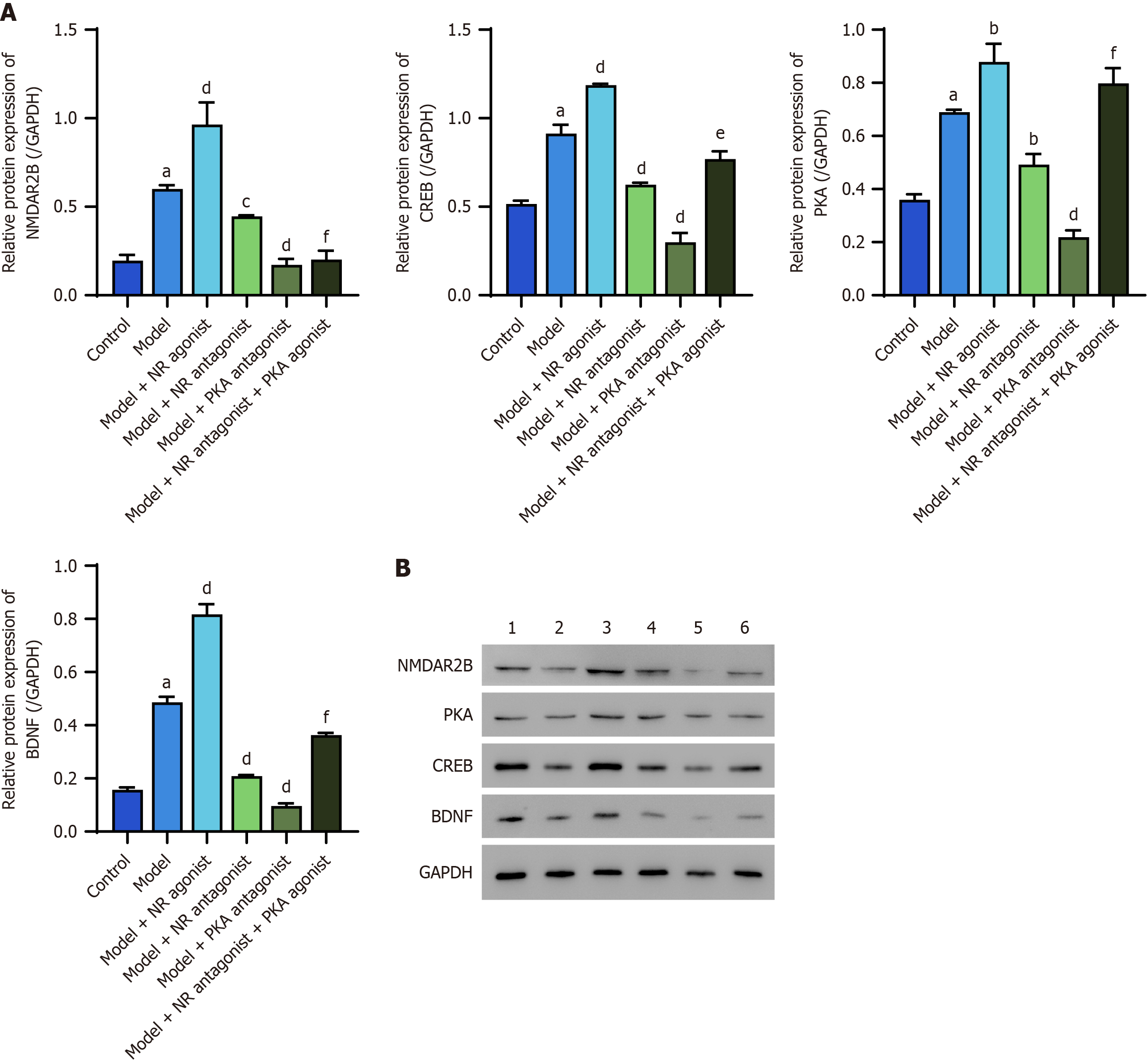
The protein expression levels of NMDAR2B, PKA, CREB, and BDNF in the cingulate cortex of model rats were significantly greater than those in the control group (P < 0.05). Additionally, the model + NR agonist group exhibited significantly greater expression of these proteins than did the model group (P < 0.05). Conversely, the model + NR antagonist group and the model + PKA antagonist group presented significantly lower expression levels of these proteins than did the model group (P < 0.05). Notably, the model + NR antagonist + PKA agonist group presented significantly higher expression levels of these proteins than did the model + NR antagonist group, except for NMDAR2B (Figure 1).
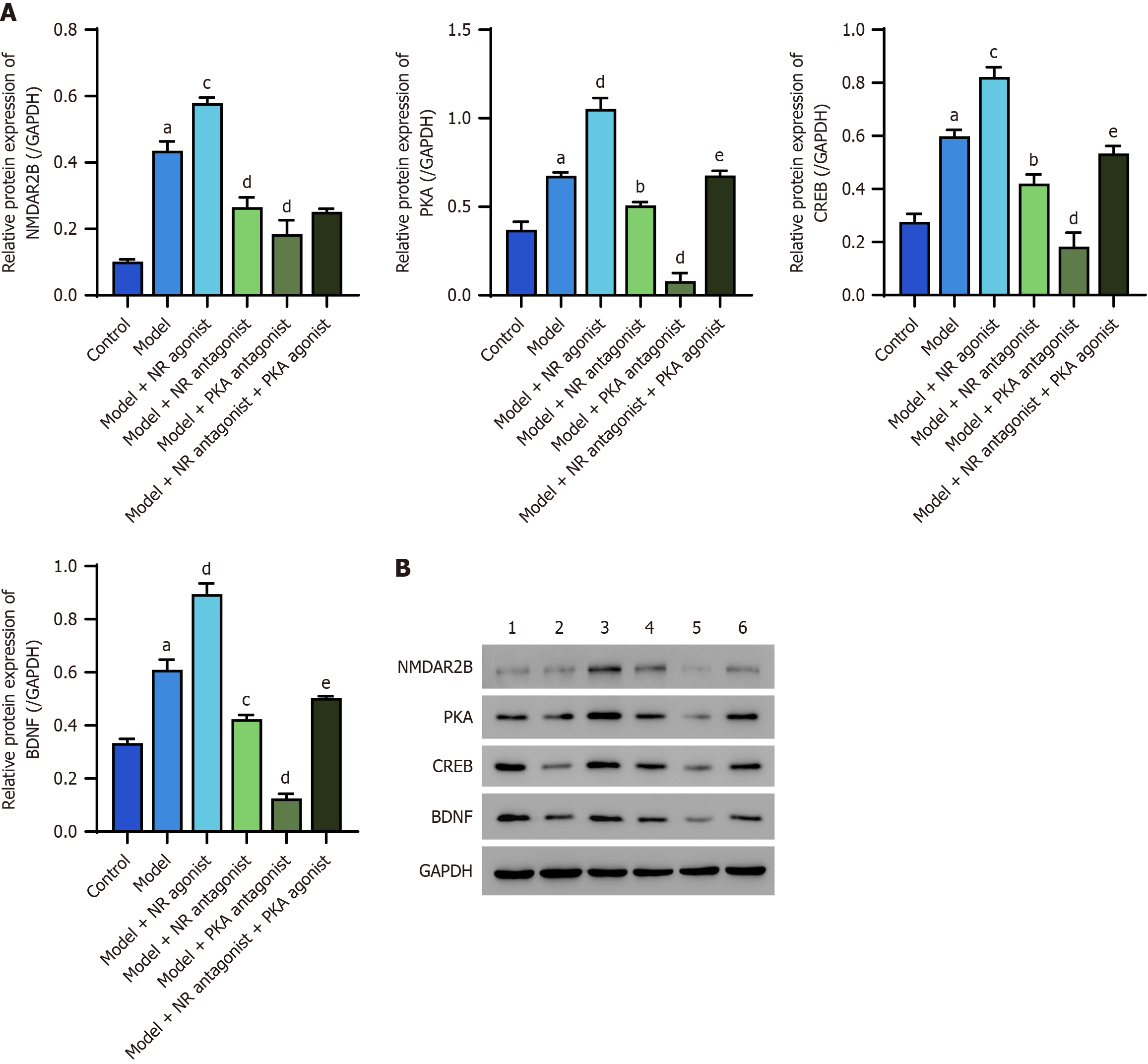
The protein expression levels of NMDAR2B, PKA, CREB, and BDNF in the dorsal thalamus of model rats were significantly greater than those in the control group (P < 0.05). The model + NR agonist group also presented significantly greater expression of these proteins compared to the model group (P < 0.05). In contrast, the model + NR antagonist group and the model + PKA antagonist group presented significantly lower expression levels of these proteins than did the model group (P < 0.05). The model + NR antagonist + PKA agonist group also presented significantly greater expression levels of all these proteins except for NMDAR2B than did the model + NR antagonist group (Figure 2).
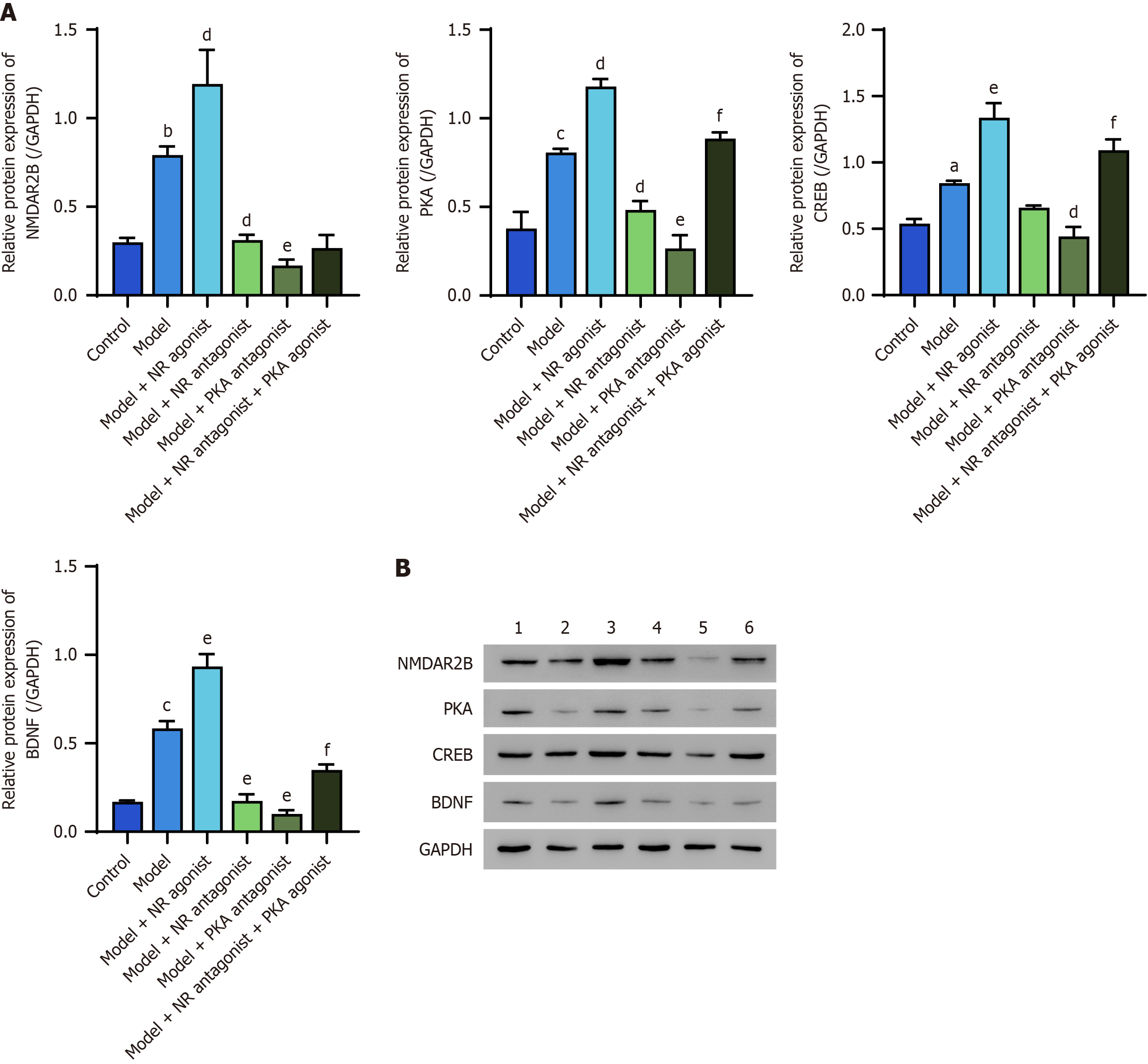
The protein expression levels of NMDAR2B, PKA, CREB, and BDNF in the posterior horn of the spinal cord of model rats were significantly greater than those in the control group (P < 0.05). Furthermore, the model + NR agonist group exhibited significantly greater expression of these proteins than did the model group (P < 0.05). In contrast, the model + NR antagonist group and the model + PKA antagonist group presented significantly lower expression levels than did the model group (P < 0.05). The model + NR antagonist + PKA agonist group presented significantly higher expression levels (except for NMDAR2B) compared to the model + NR antagonist group (Figure 3).
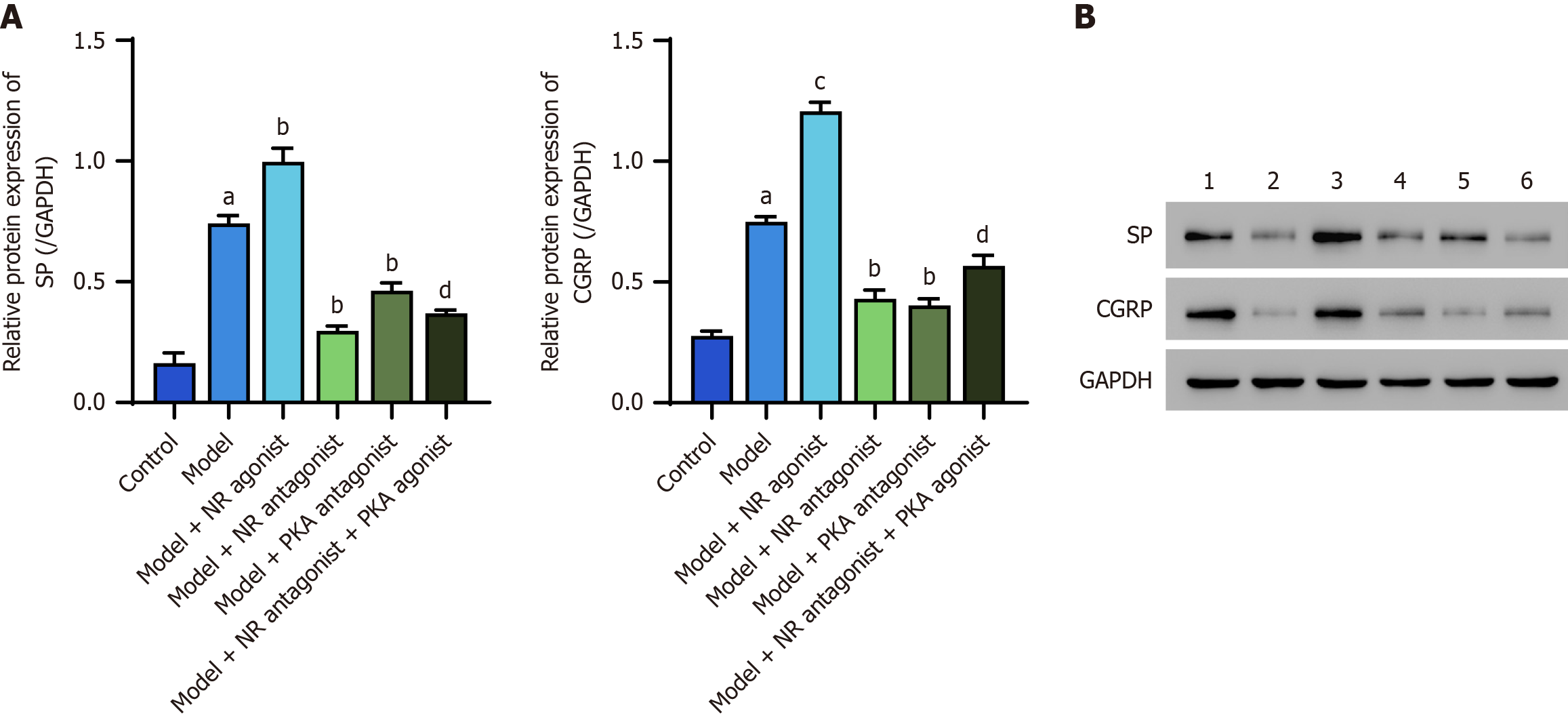
The protein expression levels of SP and CGRP in the lower esophageal mucosa of model rats were significantly greater than those in the control group (P < 0.05). The model + NR agonist group presented significantly greater expression of these proteins than did the model group (P < 0.05). Conversely, the model + NR antagonist group and the model + PKA antagonist group exhibited significantly lower expression levels than did the model group (P < 0.05). The model + NR antagonist + PKA agonist group presented significantly higher expression levels compared to the model + NR antagonist group (Figure 4).
Western blot analysis, in which GAPDH was used as an internal control, revealed that the protein expression levels of NMDAR2B, PKA, CREB, and BDNF in the cingulate cortex of model rats were significantly greater than those in the control group (P < 0.05). The model + NR agonist group presented significantly greater expression of these proteins than did the model group (P < 0.05). NMAR2B, PKA, CREB, and BDNF proteins in both the model + NR antagonist group and the model + PKA antagonist group were significantly downregulated compared with those in the model group (P < 0.05). The expression of related proteins (with the exception of NMDAR2B) in the model + NR antagonist + PKA agonist group was significantly greater than that in the model + NR antagonist group (P < 0.05; Figure 5).
Western blot analysis, in which GAPDH was used as a control, revealed significantly increased expression of the NMDAR2B, PKA, CREB, and BDNF proteins in the dorsal thalamus of model rats compared with those in the control group (P < 0.05). The model + NR agonist group presented a significantly greater expression of these proteins compared to the model group (P < 0.05). Conversely, the model + NR antagonist group and the model + PKA antagonist group presented significantly lower expression levels than did the model group (P < 0.05). Notably, the model + NR antagonist + PKA agonist group exhibited significantly greater expression of these proteins (with the exception of NMDAR2B) than did the model + NR antagonist group (P < 0.05; Figure 6).
Western blot analysis, in which GAPDH was used as a control, revealed significantly elevated protein levels of NMDAR2B, PKA, CREB, and BDNF in the posterior horn of the spinal cord in model rats compared with those in the control group (P < 0.05). The model + NR agonist group presented significantly higher levels of these proteins compared to the model group (P < 0.05). Conversely, the protein levels of NMDAR2B, PKA, and BDNF in the model + NR antagonist group and the protein levels of NMDAR2B, PKA, CREB, and BDNF in the model + PKA antagonist group were significantly lower than those in the model group (P < 0.05). Furthermore, the model + NR antagonist + PKA agonist group presented significantly higher protein levels (with the exception of NMDAR2B) than did the model + NR antagonist group (P < 0.05; Figure 7).
Western blot analysis, in which GAPDH was used as a control, revealed significantly increased levels of the SP and CGRP proteins in the lower esophageal mucosa of model rats compared with those in the control group (P < 0.05). The model + NR agonist group exhibited significantly greater levels of these proteins compared to the model group (P < 0.05). Conversely, the model + NR antagonist group and the model + PKA antagonist group presented significantly lower protein levels than did the model group (P < 0.05). Additionally, the model + NR antagonist + PKA agonist group exhibited significantly higher SP and CGRP protein levels than did the model + NR antagonist group (P < 0.05; Figure 8).
The RT-PCR results revealed significantly elevated expression of NMDAR2B, PKA, CREB, and BDNF mRNAs in the cingulate cortex of model rats compared with the control group (P < 0.05). The model + NR agonist group presented significantly greater levels of these mRNAs compared to the model group (P < 0.05). NMDAR2B, CREB, and BDNF mRNAs in the model + NR antagonist group and PKA, CREB, and BDNF mRNAs in the model + PKA antagonist group were significantly downregulated compared with those in the model group (P < 0.05). The model + NR antagonist + PKA agonist group exhibited significantly greater levels of PKA, CREB, and BDNF mRNAs compared to the model + NR antagonist group (P < 0.05; Figure 9).
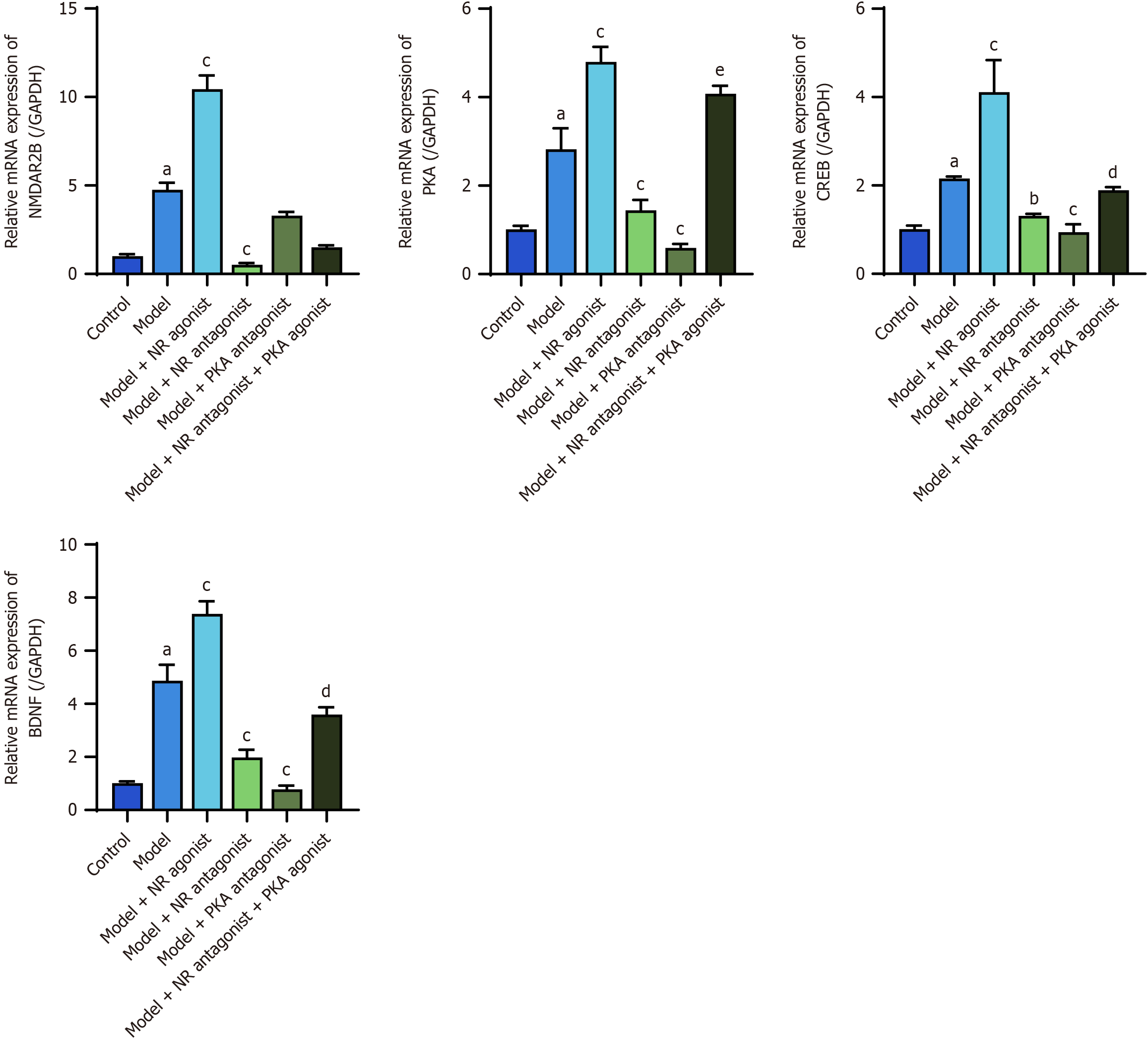
The RT-PCR results revealed significantly increased expression of the NMDAR2B, PKA, CREB, and BDNF mRNAs in the thalamus of model rats compared to the control group (P < 0.05). The model + NR agonist group exhibited significantly greater levels of these mRNAs compared to the model group (P < 0.05). NMDAR2B, PKA, CREB, and BDNF mRNAs in the model + NR antagonist group and PKA, CREB, and BDNF mRNAs in the model + PKA antagonist group were significantly downregulated compared with those in the model group (P < 0.05). The model + NR antagonist + PKA agonist group presented significantly greater levels of PKA, CREB, and BDNF mRNAs compared to the model + NR antagonist group (P < 0.05; Figure 10).
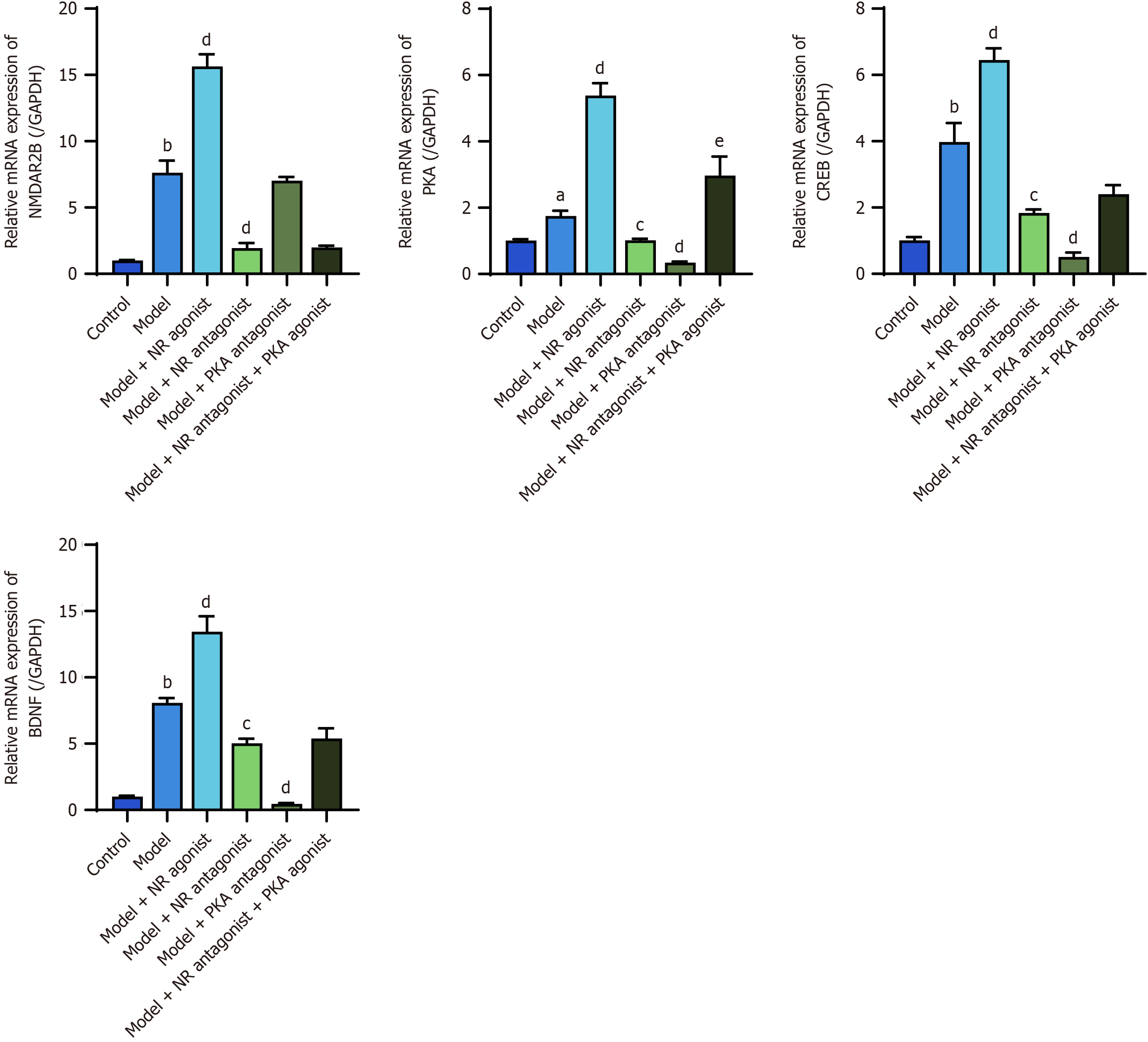
The RT-PCR results revealed significantly greater expression of the NMDAR2B, PKA, CREB, and BDNF mRNAs in the posterior horn of the spinal cord in model rats than in control rats (P < 0.05). The model + NR agonist group exhibited significantly greater levels of these mRNAs compared to the model group (P < 0.05). NMDAR2B, PKA, CREB, and BDNF mRNAs in the model + NR antagonist group and PKA, CREB, and BDNF mRNAs in the model + PKA antagonist group were significantly downregulated compared with those in the model group (P < 0.05). The model + NR antagonist + PKA agonist group presented significantly greater levels of PKA mRNAs compared to the model + NR antagonist group (P < 0.05; Figure 11).
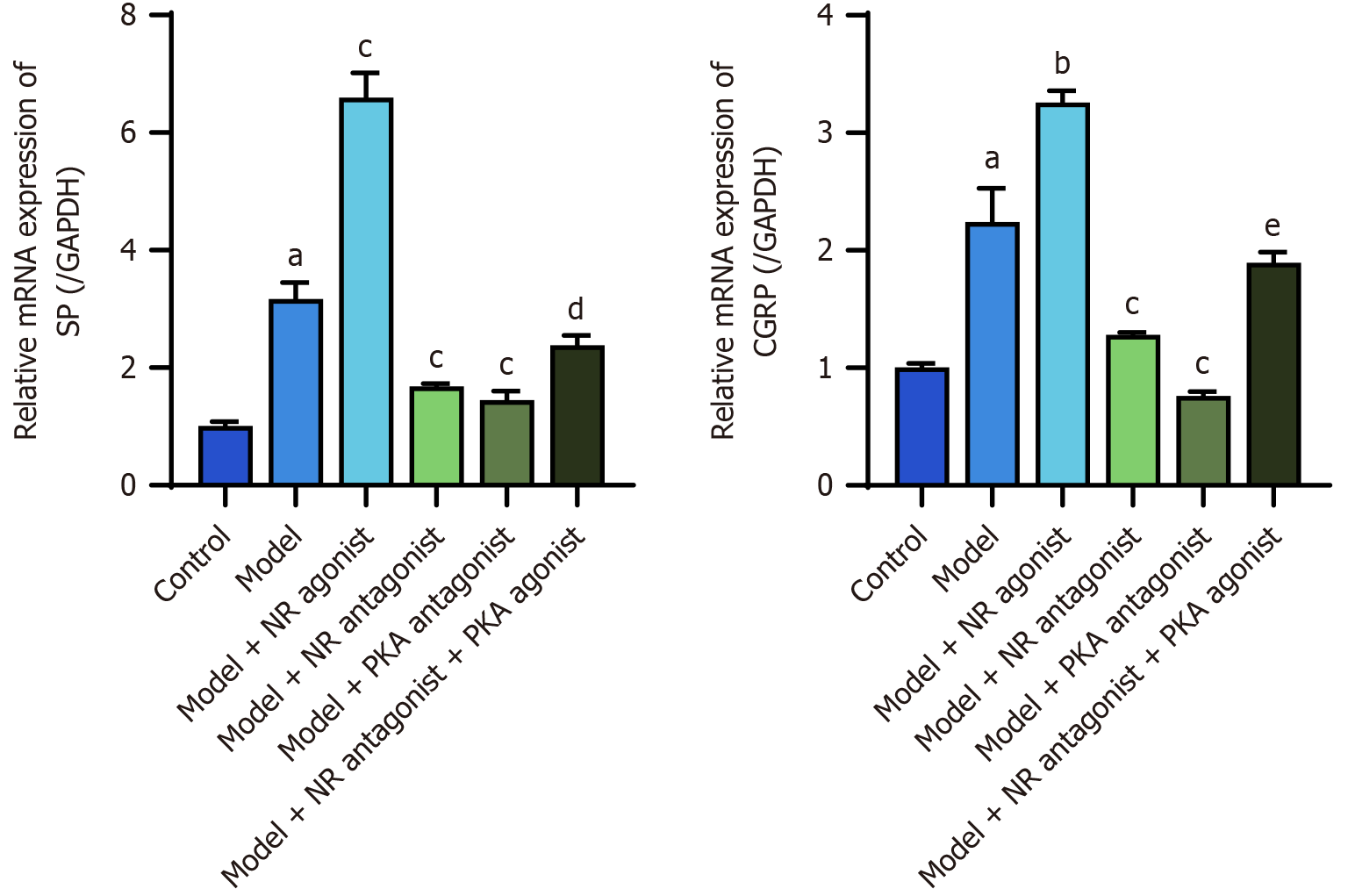
The RT-PCR results revealed significantly increased expression of the SP and CGRP mRNAs in the lower esophageal mucosa of model rats compared with the control group (P < 0.05). The model + NR agonist group exhibited significantly greater levels of these mRNAs compared to the model group (P < 0.05). The model + NR antagonist group and the model + PKA antagonist group presented significantly lower levels of these mRNAs than did the model group (P < 0.05). Additionally, the model + NR antagonist + PKA agonist group exhibited significantly greater levels of SP and CGRP mRNAs than did the model + NR antagonist group (P < 0.05; Figure 12).
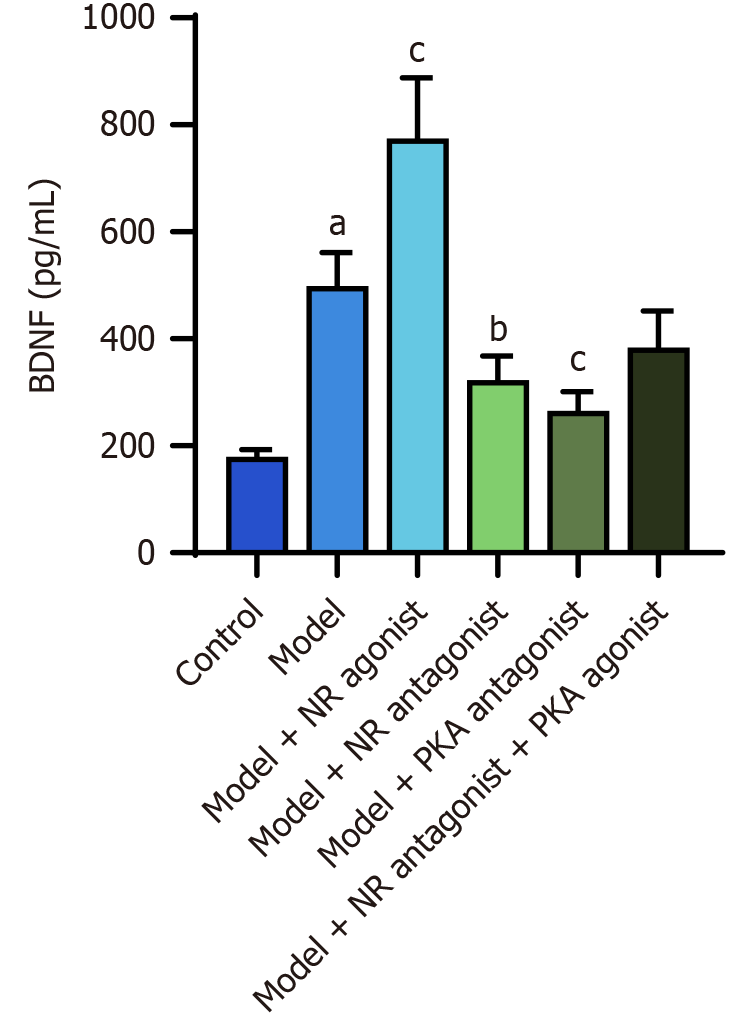
The ELISA results revealed that the serum BDNF level in the model group was significantly greater than that in the control group (P < 0.05). The model + NR agonist group exhibited higher serum BDNF level compared to the model group (P < 0.05). The serum BDNF levels in the model + NR antagonist group and the model + PKA antagonist group were significantly lower than that in the model group (P < 0.05; Table 2, Figure 13).
The behavioral scores for balloon distension and acid infusion were significantly greater in the model group than in the control group (P < 0.05). The behavioral scores of the model + NR agonist group, the model + NR antagonist group and the model + PKA antagonist group were significantly different from those of the model group (P < 0.05). Additionally, the behavioral scores of the model + NR antagonist + PKA agonist group were significantly greater than those of the model + NR antagonist group (P < 0.05; Table 3).
| Groups | Balloon distension | Acid infusion |
| Control | 1.00 (1.00, 2.00) | 2.00 ± 0.89 |
| Model | 3.00 ± 0.89a | 3.00 ± 0.89a |
| Model + NR agonist | 4.17 ± 0.75b | 4.17 ± 0.75b |
| Model + NR antagonist | 1.83 ± 0.75b | 2.00 ± 0.63b |
| Model + PKA antagonist | 1.83 ± 0.75b | 2.00 ± 0.89b |
| Model + NR antagonist + PKA agonist | 3.50 (2.75, 4.00)c | 3.50 (2.00, 4.00)c |
GERD is characterized by the backflow of stomach contents into the esophagus, causing discomfort and potential complications[12,13]. In 2013, the American Gastroenterological Association expanded the definition of GERD to include extraesophageal manifestations, covering a spectrum of symptoms, end-organ effects, and complications affecting the esophagus, mouth (including the throat), and lung[14]. Consequently, GERD presents with both typical symptoms such as reflux and heartburn, and atypical symptoms including chest pain, upper abdominal burning, bloating, belching, asthma, chronic cough, pharyngitis, and dental erosion, necessitating a multidisciplinary approach.
Globally, the prevalence of GERD ranges from 8% to 33%[15,16], with higher rates observed in Europe and North America than in Asia. A recent meta-analysis of 70 studies reported an overall GERD prevalence of 8.7% in mainland China. Over the past two decades, the prevalence of GERD in China has increased from 6.0% to 10.6%, with higher rates in the western and eastern regions than in the central region, influenced by regional variations in diet and lifestyle habits[17]. Although the prevalence of GERD in China is lower than that in Western countries, the increasing trend has garnered significant attention from researchers, particularly regarding rGERD, where symptoms persist after 8 weeks of treatment with double-dose proton pump inhibitors[18]. Refractory cases constitute approximately 30%-40% of the GERD population[1,2], presenting a substantial clinical challenge.
Esophageal hypersensitivity is a major contributor to rGERD and is characterized by increased sensitivity to chemical and mechanical stimuli within the esophagus. This heightened sensitivity means that minor distension and physiological acid reflux can cause significant discomfort, such as acid reflux and heartburn, severely impacting patients' quality of life. In the visceral sensory transmission pathway, impulses are transmitted from sensory nerve endings to the dorsal horn of the spinal cord via the dorsal root ganglia. The dorsal horn directly regulates pain feedback and receives descending regulatory signals from higher neural centers, making it a crucial hub for pain signal processing[19].
NMDAR, an ionotropic glutamate receptor, is extensively expressed in both the nervous system and peripheral tissues, including gastrointestinal mucosal epithelial cells[20,21]. Although NMDARs are distributed throughout the brain, the highest density is in the hippocampal CA1 region, with moderate levels in the CA3 region and dentate gyrus; the areas with the highest density in the cerebral cortex are the prefrontal cortex, anterior cingulate gyrus, and piriform cortex; in addition, there is high expression in the striatum, thalamus, and cerebellar granule cell layers. There is a reciprocal fiber connection between the anterior thalamic nucleus and the posterior cingulate cortex, and glutamate neurotransmitters play important roles in these two regions[22]. NMDARs also play a crucial role in visceral nociception, and its expression in the dorsal horn of the spinal cord influences visceral pain transmission[6]. Functional NMDARs consist of two NR1 subunits and two NR2 subunits, with the NR2 subunits playing a key role in visceral hypersensitivity, particularly in the dorsal horn[23]. There are four isoforms of NR2 (NR2A, NR2B, NR2C, and NR2D), each with distinct functions[24,25].
BDNF is an alkalinous protein synthesized in the nervous system. Its molecular monomer is a polypeptide composed of more than 100 amino acid residues and is composed mainly of beta-folding and random N-level structures. BDNF can regulate synaptic plasticity, change the morphology of neurons in the brain, promote the growth of dendrites and axons, and is abundant in areas such as the hippocampus, cingulate gyrus, thalamus, and spinal cord. Its role in nerve conduction has been a hot research topic in recent years. In neonatal models, visceral pain hypersensitivity is associated with the overexpression of BDNF in the dorsal horn[26]. In the mature nervous system, BDNF is closely linked to sensory hypersensitivity, enhancing the release of excitatory neurotransmitters and participating in the formation of long-term synaptic plasticity. It is also associated with regulating factors of sensation and motility such as SP and CGRP. Ab
In patients with irritable bowel syndrome, NMDAR2B induces intestinal hypersensitivity by promoting BDNF expression in the intestinal mucosa, an effect that can be blocked by NR2B-specific antagonists[29]. Tian et al[30] reported that NMDAR activation could regulate the expression of BDNF genes via CREB/CREB-binding protein in synapses, affecting sensory input and synaptic plasticity. These observations indicate that activation of the PKA pathway can increase the phosphorylation levels of kinases to induce downstream BDNF expression by regulating CREB expression and transcriptional activity, whereas blocking NMDAR can lead to downregulation of BDNF mRNA levels[31]. Researchers subsequently reported that BDNF also had a reverse effect on NMDARs, promoting glutamate synaptic transmission by regulating NMDAR phosphorylation, increasing NMDAR expression and activity, and increasing postsynaptic NMDAR enrichment, thereby regulating synaptic plasticity in a manner dependent on its activity[32]. We hypothesized that BDNF and NMDAR regulated each other in the central nervous system (spinal cord and brain), maintaining the normal response of the body to mechanical and chemical stimuli. Disruption of the dynamic balance between the two may lead to sensitization at the central level.
This study aimed to verify the existence of the NMDAR2B-PKA-CREB-BDNF pathway in a rat model of esophageal hypersensitivity and to determine whether PKA is an important kinase downstream of NMDAR2B. Our preliminary experiments confirmed that NMDAR was activated in the rat model, so comparisons among the 6 groups were performed: comparisons among the model + NR agonist, model + NR antagonist and model groups were used to clarify whether PKA and downstream gene expression were affected by NMDAR activation/antagonism; comparisons between the model + PKA antagonist and model groups were used to determine whether the expression of downstream genes was inhibited after PKA antagonism; and comparisons between the model + NR antagonist + PKA agonist and model + NR antagonist groups were used to reveal, after NMDAR antagonism following PKA activation, whether downstream gene expression was reactivated.
Our study demonstrated that in rats with esophageal hypersensitivity, the expression of NMDAR2B-PKA-CREB-BDNF pathway-related proteins and mRNAs was significantly greater than that in the control group. Further activation or inhibition of the NMDAR2B receptor resulted in corresponding increases or decreases in the expression of downstream PKA, CREB, and BDNF proteins and mRNAs, accompanied by changes in the peripheral esophageal sensitizers SP and CGRP. The behavioral scores for esophageal acid infusion and balloon distension in rats also correspondingly increased or decreased. Although the results of this study elucidate the effect of NMDAR2B on esophageal neuropathic pain, the corresponding relationship between the PKA-CREB-BDNF pathway and esophageal hypersensitivity has not been shown. It is unclear whether there are other pathways (such as the MAPK/ERK pathway) in addition to the PKA-CREB pathway that jointly intervene and interact with each other in the formation of visceral hypersensitivity. Moreover, in addition to the posterior horn of the spinal cord, the cingulate gyrus and the dorsal thalamus, the hippocampus and striatum, which are parts of the limbic system, may collectively participate in the central nervous system sensitization process, which was not assessed in this study. The functional NMDAR is composed of the NR1 and NR2 subunits. While not explored in this study, the relationship between the two and the regularity of their distribution will be the starting point for our next study.
In conclusion, these findings suggest that NMDAR2B plays a significant role in the development of neuropathic pain in GERD, which is mediated through the PKA-CREB-BDNF pathway. This result identifies a reliable molecular target for the clinical treatment of esophageal hypersensitivity and offers new therapeutic strategies and insights for the management of rGERD.
| 1. | El-Serag H, Becher A, Jones R. Systematic review: persistent reflux symptoms on proton pump inhibitor therapy in primary care and community studies. Aliment Pharmacol Ther. 2010;32:720-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 208] [Article Influence: 13.9] [Reference Citation Analysis (39)] |
| 2. | Roman S, Mion F. Refractory GERD, beyond proton pump inhibitors. Curr Opin Pharmacol. 2018;43:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Gyawali CP, Yadlapati R, Fass R, Katzka D, Pandolfino J, Savarino E, Sifrim D, Spechler S, Zerbib F, Fox MR, Bhatia S, de Bortoli N, Cho YK, Cisternas D, Chen CL, Cock C, Hani A, Remes Troche JM, Xiao Y, Vaezi MF, Roman S. Updates to the modern diagnosis of GERD: Lyon consensus 2.0. Gut. 2024;73:361-371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 149] [Article Influence: 149.0] [Reference Citation Analysis (0)] |
| 4. | Drossman DA, Hasler WL. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology. 2016;150:1257-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 993] [Article Influence: 110.3] [Reference Citation Analysis (0)] |
| 5. | Taheriyan F, Teshnehlab M, Gharibzadeh S. Presenting a Neuroid model of wind-up based on dynamic synapse. J Theor Biol. 2019;465:45-50. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Suciu A, Popa SL, Dumitrascu DL. Upper Gastrointestinal Sensitization And Symptom Generation. J Med Life. 2019;12:316-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Fukuchi M, Tabuchi A, Kuwana Y, Watanabe S, Inoue M, Takasaki I, Izumi H, Tanaka A, Inoue R, Mori H, Komatsu H, Takemori H, Okuno H, Bito H, Tsuda M. Neuromodulatory effect of Gαs- or Gαq-coupled G-protein-coupled receptor on NMDA receptor selectively activates the NMDA receptor/Ca2+/calcineurin/cAMP response element-binding protein-regulated transcriptional coactivator 1 pathway to effectively induce brain-derived neurotrophic factor expression in neurons. J Neurosci. 2015;35:5606-5624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Park H, Popescu A, Poo MM. Essential role of presynaptic NMDA receptors in activity-dependent BDNF secretion and corticostriatal LTP. Neuron. 2014;84:1009-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 9. | Wang Y, Zhou B, Fang S, Zhu S, Xu T, Dilikumaer M, Li G. Dynorphin participates in interaction between depression and non-erosive reflux disease. Esophagus. 2023;20:158-169. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Yang M, Chen DF, Fang DC. [Establish and evaluation of the rat model of esophageal visceral hypersensitivity]. Jujieshoushuxue Zazhi. 2008;17:77-79. |
| 11. | Jia BY, Xie CE, Wang ZB, Pei WJ, Li XH, Shi L, Liu JL, Han YF, Tan X, Ding PH, Sun ZM, Yuan WJ, Li JX. The effect of Heweijiangni-decoction on esophageal morphology in a rat model of OVA-induced visceral hypersensitivity followed by acid exposure. Cell Mol Biol (Noisy-le-grand). 2019;65:73-78. [PubMed] |
| 12. | Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900-1920; quiz 1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2368] [Cited by in RCA: 2432] [Article Influence: 128.0] [Reference Citation Analysis (2)] |
| 13. | Bajbouj M. [Diagnosis and therapy of atypical reflux symptoms when PPI therapy fails]. HNO. 2012;60:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308-328; quiz 329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1136] [Cited by in RCA: 1103] [Article Influence: 91.9] [Reference Citation Analysis (0)] |
| 15. | Gyawali CP, Kahrilas PJ, Savarino E, Zerbib F, Mion F, Smout AJPM, Vaezi M, Sifrim D, Fox MR, Vela MF, Tutuian R, Tack J, Bredenoord AJ, Pandolfino J, Roman S. Modern diagnosis of GERD: the Lyon Consensus. Gut. 2018;67:1351-1362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 672] [Cited by in RCA: 921] [Article Influence: 131.6] [Reference Citation Analysis (0)] |
| 16. | El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63:871-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 1251] [Article Influence: 113.7] [Reference Citation Analysis (2)] |
| 17. | Lu TL, Li SR, Zhang JM, Chen CW. Meta-analysis on the epidemiology of gastroesophageal reflux disease in China. World J Gastroenterol. 2022;28:6410-6420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (2)] |
| 18. | Chinese Society of Gastroenterology; Chinese Medical Association. [Chinese expert consensus of gastroesophageal reflux disease in 2020]. Zhonghua Xiaohua Zazhi. 2020;40:649-663. [DOI] [Full Text] |
| 19. | Shi HX, Dong YL, Xu AL, Yang JQ, Yan NJ, Yang Y, Wei W. [Effect of kidney warming and spleen strengthening on NMDA receptor and its phosphorylation expression in dorsal root ganglion of diarrhoeal irritable bowel syndrome Rats]. Shijie Zhongxiyijiehe Zazhi. 2021;16:2220-2225. [DOI] [Full Text] |
| 20. | Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2914] [Cited by in RCA: 2708] [Article Influence: 180.5] [Reference Citation Analysis (0)] |
| 21. | Seo JH, Fox JG, Peek RM Jr, Hagen SJ. N-methyl D-aspartate channels link ammonia and epithelial cell death mechanisms in Helicobacter pylori Infection. Gastroenterology. 2011;141:2064-2075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Manikonda PK, Rajendra P, Devendranath D, Gunasekaran B, Channakeshava, Aradhya RS, Sashidhar RB, Subramanyam C. Influence of extremely low frequency magnetic fields on Ca2+ signaling and NMDA receptor functions in rat hippocampus. Neurosci Lett. 2007;413:145-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Fan J, Wu X, Cao Z, Chen S, Owyang C, Li Y. Up-regulation of anterior cingulate cortex NR2B receptors contributes to visceral pain responses in rats. Gastroenterology. 2009;136:1732-1740.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14:383-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1443] [Cited by in RCA: 1525] [Article Influence: 127.1] [Reference Citation Analysis (0)] |
| 25. | Ferguson HJ, Wragg JW, Ward S, Heath VL, Ismail T, Bicknell R. Glutamate dependent NMDA receptor 2D is a novel angiogenic tumour endothelial marker in colorectal cancer. Oncotarget. 2016;7:20440-20454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Wu B, Xu C, Huang HH. [Expression profiles of brain-derived neurotrophic factor in the spinal dorsal horn of young rats with visceral hypersensitivity]. Zhongguo Dangdai Erke Zazhi. 2016;18:277-281. |
| 27. | Wang P, Du C, Chen FX, Li CQ, Yu YB, Han T, Akhtar S, Zuo XL, Tan XD, Li YQ. BDNF contributes to IBS-like colonic hypersensitivity via activating the enteroglia-nerve unit. Sci Rep. 2016;6:20320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (3)] |
| 28. | Salio C, Ferrini F. BDNF and GDNF expression in discrete populations of nociceptors. Ann Anat. 2016;207:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Zhang WX, Xu LD, Zhang MM, Guo CG, Zuo XL. [NR2B contributes to visceral hypersensitivity in irritable bowel syndrome via mBDNF]. Shandong Daxue Xuebao (Yixueban). 2017;55:17-22. [DOI] [Full Text] |
| 30. | Tian F, Hu XZ, Wu X, Jiang H, Pan H, Marini AM, Lipsky RH. Dynamic chromatin remodeling events in hippocampal neurons are associated with NMDA receptor-mediated activation of Bdnf gene promoter 1. J Neurochem. 2009;109:1375-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Tian F, Marini AM, Lipsky RH. NMDA receptor activation induces differential epigenetic modification of Bdnf promoters in hippocampal neurons. Amino Acids. 2010;38:1067-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Li S, Cai J, Feng ZB, Jin ZR, Liu BH, Zhao HY, Jing HB, Wei TJ, Yang GN, Liu LY, Cui YJ, Xing GG. BDNF Contributes to Spinal Long-Term Potentiation and Mechanical Hypersensitivity Via Fyn-Mediated Phosphorylation of NMDA Receptor GluN2B Subunit at Tyrosine 1472 in Rats Following Spinal Nerve Ligation. Neurochem Res. 2017;42:2712-2729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |