Published online Mar 21, 2025. doi: 10.3748/wjg.v31.i11.103507
Revised: December 26, 2024
Accepted: February 24, 2025
Published online: March 21, 2025
Processing time: 112 Days and 6.6 Hours
The intestinal flora (IF) has been linked to risks of non-communicable diseases, especially various cancers, stroke, and Alzheimer’s disease. However, many uncertainties of these associations during different stages of growth, deve
To explore the associations of the human IF with disease risks during different stages of growth, development, and aging to achieve more accurate and con
Cohort, cross-sectional, case-control, and Mendelian randomization studies published in the PubMed and Web of Science databases until December 31, 2023 were systematically reviewed to clarify the associations of the IF at the genus level with the risks of various non-communicable diseases, which were grouped in accordance with the 10th revision of the International Classification of Diseases.
In total, 57 studies were included to quantitatively examine the influence of the IF on the risks of 30 non-communicable diseases during different stages of growth, development, and aging. Population studies and Mendelian randomization studies confirmed positive associations of the abundances of Bifidobacterium and Ruminococcus with multiple sclerosis.
These findings contribute to a deeper understanding of the roles of the IF and provide novel evidence for effective strategies for the prevention and treatment of non-communicable diseases. In the future, it will be necessary to explore a greater variety of research techniques to uncover the specific mechanisms by which gut microbiota trigger diseases and conduct in-depth studies on the temporal relationship between microbiota alterations and diseases, so as to clarify the causal relationship more accurately.
Core Tip: The present study revealed significant associations between intestinal flora and numerous chronic non-communicable diseases, including cancer, cardiovascular disorders, and neurological conditions, along with the growth, development, and aging processes of the human body. These findings provide valuable insights into the pathogenic mechanisms of specific bacterial genera on the host, facilitating enhanced disease prevention and control strategies within the population and offering potential treatment options for related disorders.
- Citation: Jiang GH, Li HY, Xie LJ, Fan JY, Li SY, Yu WQ, Xu YT, He ML, Jiang Y, Bai X, Zhou J, Wang X. Changes in Intestinal flora is associated with chronic diseases. World J Gastroenterol 2025; 31(11): 103507
- URL: https://www.wjgnet.com/1007-9327/full/v31/i11/103507.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i11.103507
The human intestinal flora (IF) is composed of a large and complex community of microorganisms and related metabolic products in the gastrointestinal tract, as well as various potential genetic factors. An estimated 10 trillion individual bacteria, archaea, fungi, viruses, and parasites reside in the intestinal mucosa, which has an average surface area of about 200-300 m2[1]. Bacteria are relatively the most suitable microorganisms for growth in the intestinal microenvironment, accounting for up to 90% of the IF, among which Firmicutes, Bacteroidetes, Actinobacteria, Clostridium, Proteobacteria, Verrucobacteria, and Cyanobacteria are typically the seven most abundant genera[2]. The bacterial components of the IF, often classified as “beneficial”, “harmful”, or “neutral”, influence digestion, absorption, immune maturation, vitamin synthesis, development, aging, tumor susceptibility, blood regulation, and maintenance of physiological dynamics[3-5]. Disruption to the homeostasis of the IF has been linked to the onset of various diseases[6,7].
Epidemiological studies have suggested that the IF is closely associated with the occurrence and progression of various cancers[8,9], cardiovascular diseases[10,11], cerebrovascular disorders[12,13], and mental and psychological conditions[14,15]. However, the conclusions of previous studies are inconsistent owing to disparities in design, sample size, and population stratification[16-19]. Therefore, the aim of this systematic review and meta-analysis was to clarify the role of the IF in the risk of common non-communicable diseases during different stages of growth, development, and aging to provide effective strategies to maintain health.
Relevant articles were retrieved from the PubMed and Web of Science databases published until December 31, 2023 to investigate the associations of the IF and the development of various diseases at different stages of birth, physical development, and aging using the terms “microbiome” OR “gut microbiome” OR “microbial community” OR “gastrointestinal microbiome” OR “gut flora” OR “gastrointestinal microbiota” OR “microflora” OR “gastric microbiome” OR “intestinal microbiota” OR “intestinal flora” AND “odds ratio” OR “hazard risk” OR “hazard ratio”.
This analysis was limited to cohort, cross-sectional, case-control, and Mendelian randomization studies designed to evaluate the correlation of the IF with the risk of chronic non-communicable diseases at different stages of birth, physical development, and aging, and provided the odds ratio (OR) or hazard ratio (HR) with the 95% confidence interval (CI), or supplied relevant data to calculate the 95%CI. For multiple reports of the same or overlapping participants, the study with the largest sample size was included.
Cochran’s Q test was employed to evaluate across-study heterogeneity and the I2 value was calculated to assess the degree of variance due to heterogeneity. The DerSimonian and Laird random effects model was used if heterogeneity was evident (Q test P value < 0.05); otherwise, the fixed effects model was applied. The pooled effect size and 95%CI were calculated using Comprehensive Meta-Analysis software (version 2.0; https://meta-analysis.com/). A forest plot was generated using R software (version 3.4.1; https://cran-archive.r-project.org/bin/windows/base/old/3.4.1/).
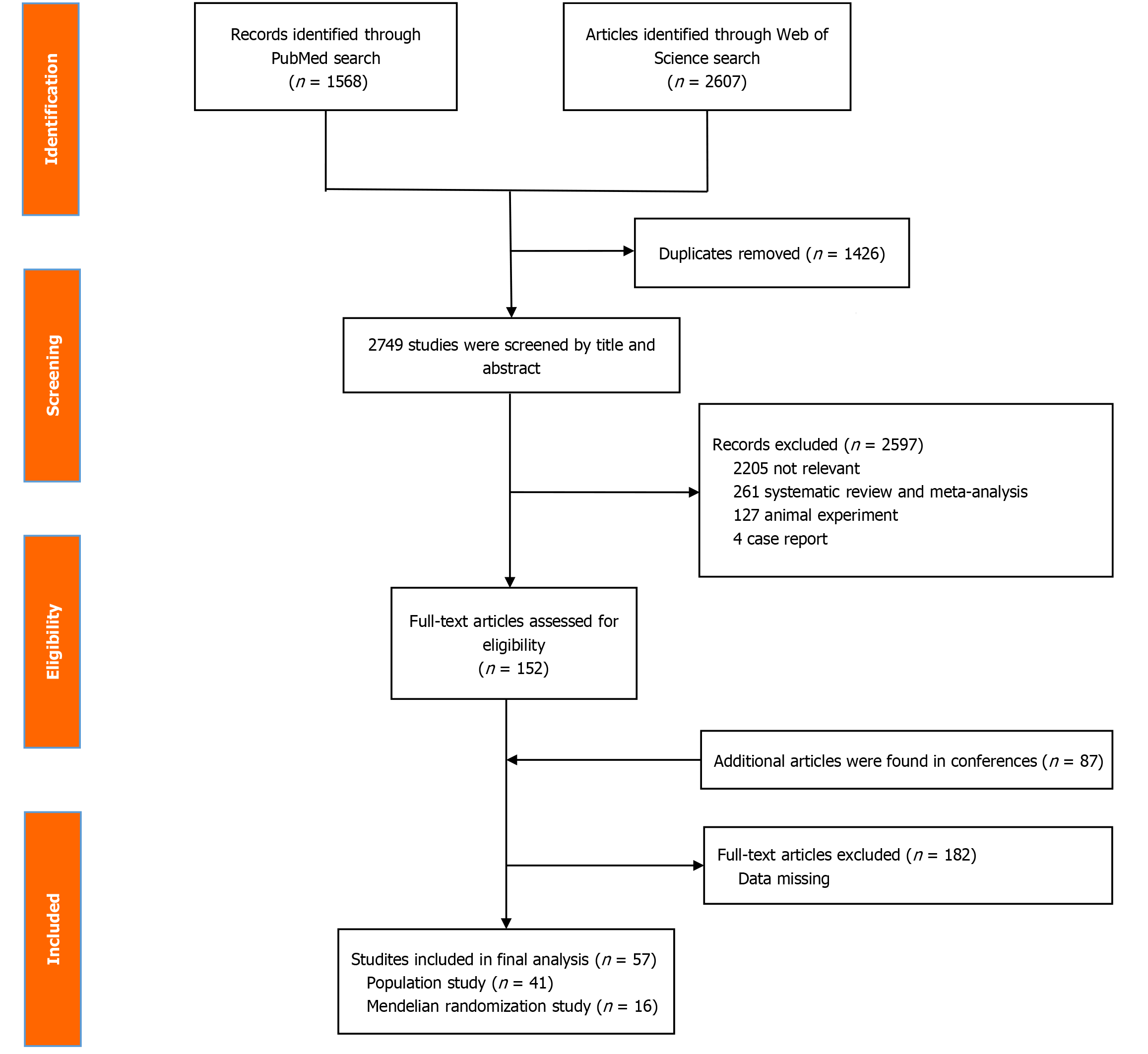
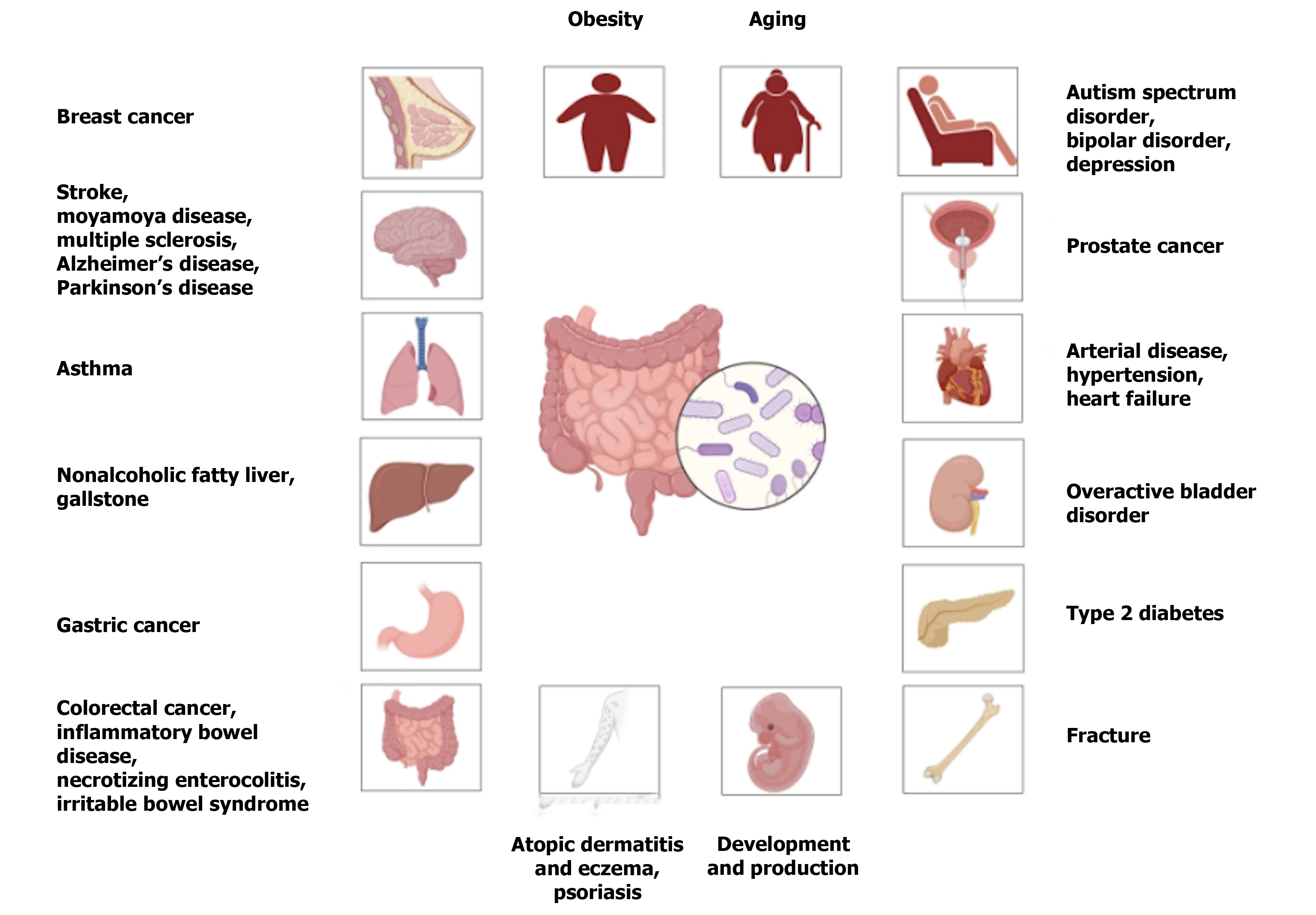
Among the 82 articles that were initially screened (Supplementary material), 14, 37, 18, and 13 were conducted in China, the United States, Japan, and Ghana and other countries, respectively. Of these, 57 articles met the selection criteria, as shown in Figure 1, and were included for analysis. The final analysis included 8, 12, 21, and 16 cross-sectional, cohort, case-control, and Mendelian randomization studies, respectively, collectively involving more than one million participants to assess the correlation of the IF with the incidences of various cancers[16,20-28], cardiovascular diseases[17,29-34], cerebrovascular diseases[35-37], neurological diseases[18,38-40], mental and behavioral disorders[41-43], digestive diseases[18,44-54], respiratory diseases[55], urinary diseases[56], endocrine, nutritional, and metabolic diseases[29,57-64], skin and subcutaneous tissue diseases[55,65-70], skeletal and muscular system diseases[71], and reproductive and fertility disorders[72-74] (Figures 2, 3, and 4).
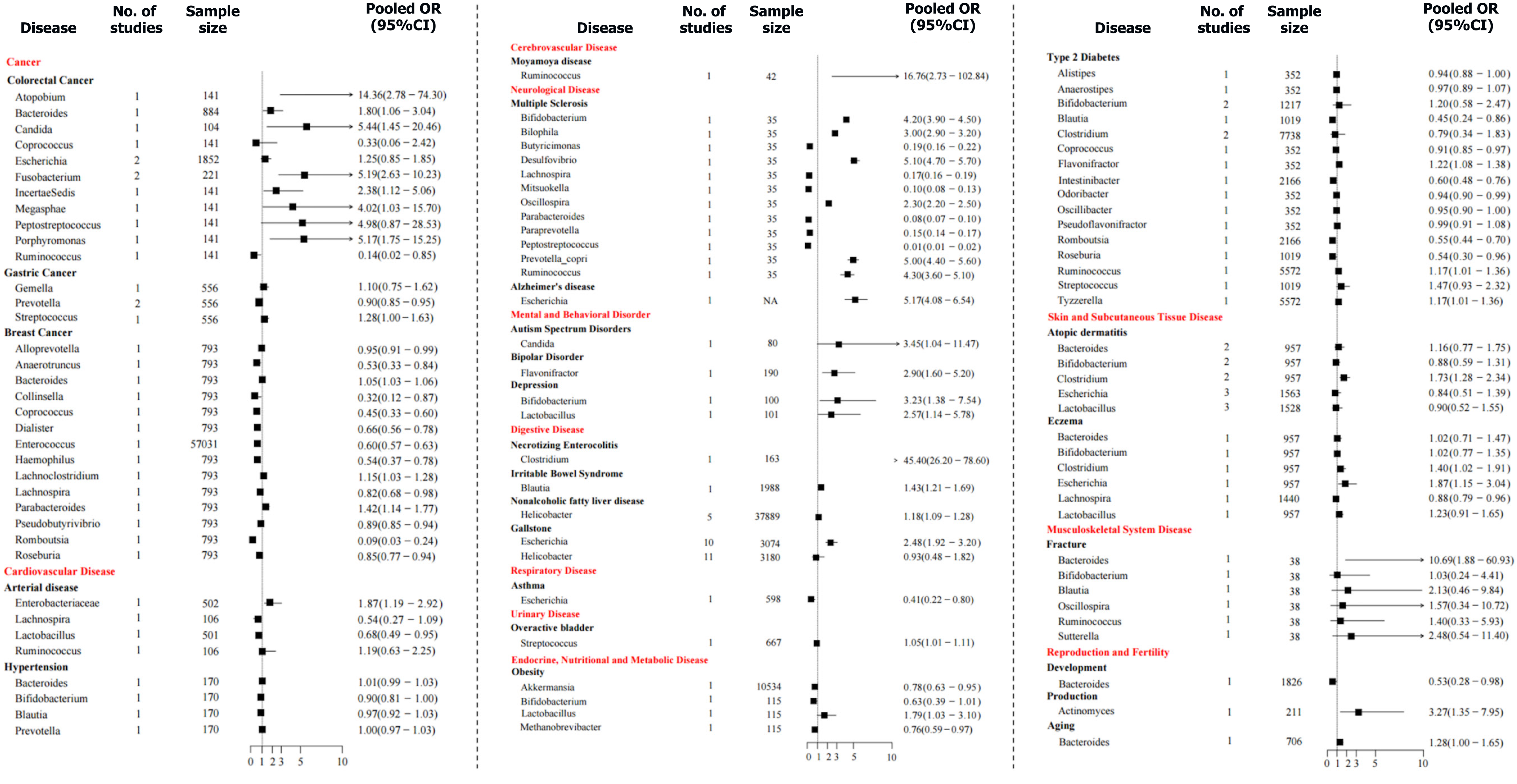
Colorectal cancer: Five cross-sectional studies[16,20-23] reported positive correlations of colorectal cancer (CRC) with Fusobacterium (pooled OR = 5.19, 95%CI: 2.63-10.23), Atopobium (pooled OR = 14.36, 95%CI: 2.78-74.30), Incertae sedis (pooled OR = 2.38, 95%CI: 1.12-5.06), Porphyromonas (pooled OR = 5.17, 95%CI: 1.75-15.25), Megasphaera (pooled OR = 4.02, 95%CI: 1.03-15.70), Candida (pooled OR = 5.44, 95%CI: 1.45-20.46), and Bacteroides (pooled OR = 1.80, 95%CI: 1.06-3.04); a negative association with Ruminococcus (pooled OR = 0.14, 95%CI: 0.02-0.85) and uncertain associations with Escherichia (pooled OR = 1.25, 95%CI: 0.85-1.85), Peptostreptococcus (pooled OR = 4.98, 95%CI: 0.87-28.53), and Coprococcus (pooled OR = 0.33, 95%CI: 0.06-2.36).
Gastric cancer: One case-control study[24] reported that that Prevotella (pooled OR = 0.90, 95%CI: 0.85-0.95) was negatively associated with gastric cancer, while Streptococcus (pooled OR = 1.28, 95%CI: 1.00-1.63) and Gemella (pooled OR = 1.10, 95%CI: 0.75-1.62) were not significantly associated.
Breast cancer: Two observational studies[25,26] found that Bacteroides (pooled OR = 1.05, 95%CI: 1.03-1.06), Parabacteroides (pooled OR = 1.42, 95%CI: 1.14-1.77), and Lachnoclostridium (pooled OR = 1.15, 95%CI: 1.03-1.28) were positively associated with breast cancer, while Enterococcus (pooled OR = 0.60, 95%CI: 0.57-0.63), Coprococcus (pooled OR = 0.45, 95%CI: 0.33-0.60), Romboutsia (pooled OR = 0.09, 95%CI: 0.03-0.24), Collinsella (pooled OR = 0.32, 95%CI: 0.12-0.87), Alloprevotella (pooled OR = 0.95, 95%CI: 0.91-0.99), Lachnospira (pooled OR = 0.82, 95%CI: 0.68-0.98), Roseburia (pooled OR = 0.85, 95%CI: 0.77-0.94), Pseudobutyrivibrio (pooled OR = 0.89, 95%CI: 0.85-0.94), Dialister (pooled OR = 0.66, 95%CI: 0.56-0.78), Anaerotruncus (pooled OR = 0.53, 95%CI: 0.33-0.84), and Haemophilus (pooled OR = 0.54, 95%CI: 0.37-0.78) were negatively associated. A two-sample Mendelian randomization study indicated that increased abundances of Erysipelatoclostridium (pooled OR = 1.25, 95%CI: 1.10-1.41) and Sellimonas (pooled OR = 1.09, 95%CI: 1.04-1.14) were causally associated with breast cancer risk, while Adlercreutzia (pooled OR = 0.88, 95%CI: 0.81-0.95) and Ruminococcus_2 (pooled OR = 0.77, 95%CI: 0.67-0.89) were negatively associated[27].
Prostate cancer: A two-sample Mendelian randomization study suggested that the relative abundance of Eubacterium (pooled OR = 1.16, 95%CI: 1.01-1.34) was positively associated with the risk of prostate cancer, while Akkermansia (pooled OR = 0.79, 95%CI: 0.67-0.99) and Bacteroides (pooled OR = 0.90, 95%CI: 0.83-0.99) were negatively associated, whereas Alistipes (pooled OR = 1.17, 95%CI: 0.91-1.52), Prevotella (pooled OR = 0.82, 95%CI: 0.63-1.06), Roseburia (pooled OR = 0.90, 95%CI: 0.74-1.09), Veillonella (pooled OR = 1.07, 95%CI: 0.96-1.19), and Ruminococcus (pooled OR = 0.96, 95%CI: 0.87-1.05) were unrelated[28].
Arterial disease: Two case-control studies[30,31] found that the Enterobacteriaceae (pooled OR = 1.87, 95%CI: 1.19-2.92) may increase the risk of arterial disease, while Lactobacillus (pooled OR = 0.68, 95%CI: 0.49-0.95) may reduce this risk, and both Lachnospira (pooled OR = 0.54, 95%CI: 0.27-1.10) and Ruminococcus (pooled OR = 1.19, 95%CI: 0.63-2.25) were not related to disease risk. Two Mendelian randomization studies[29,32] reported that Oxalobacter (pooled OR = 1.06, 95%CI: 1.03-1.10) was positively correlated with arterial disease, whereas Bifidobacterium (pooled OR = 0.96 95%CI: 0.94-0.98) was negatively correlated and Lachnospira (pooled OR = 1.10, 95%CI: 1.00-1.20) was not correlated.
Hypertension: One case-control study[33] found that the relative abundances of Bifidobacterium (pooled OR = 0.90, 95%CI: 0.81-1.00), Bacteroides (pooled OR = 1.01, 95%CI: 0.99-1.03), Blautia (pooled OR = 0.97, 95%CI: 0.92-1.03), and Prevotella (pooled OR = 1.00, 95%CI: 0.97-1.03) were not associated with the risk of hypertensive disorders of pregnancy.
Heart failure: Two Mendelian randomization studies[17,34] reported that heart failure was associated with the abundances of Bacteroides (pooled OR = 1.06, 95%CI: 1.02-1.10) and Lachnospiraceae_noname (pooled OR = 1.09, 95%CI: 1.03-1.14), while Bilophila (pooled OR = 0.94, 95%CI: 0.89-1.00) may be a protective factor. However, Dorea (pooled OR = 1.11, 95%CI: 1.00-1.23), Alistipes (pooled OR = 1.06, 95%CI: 1.00-1.13), Eubacterium (pooled OR = 1.04, 95%CI: 1.00-1.09), Candida (pooled OR = 0.99, 95%CI: 0.97-1.02), Shigella (pooled OR = 0.99, 95%CI: 0.98-1.01), and Campylobacter (pooled OR = 1.01, 95%CI: 0.91-1.13) were not associated with heart failure.
Stroke: Two Mendelian randomization studies[35,36] showed that Alistipes (pooled OR = 1.52, 95%CI: 1.04-2.24), Dorea (pooled OR = 1.53, 95%CI: 1.25-1.86), Eisenbergiella (pooled OR = 1.24, 95%CI: 1.05-1.48), Eubacterium_rectale_group (pooled OR = 1.46, 95%CI: 1.01-2.09), Fusicatenibacter (pooled OR = 1.42, 95%CI: 1.02-1.97), Gordonibacter (pooled OR = 1.36, 95%CI: 1.05-1.76), Lachnospiraceae_UCG_008 (pooled OR = 1.28, 95%CI: 1.01-1.62), Parabacteroides (pooled OR = 1.78, 95%CI: 1.15-2.75), Parasutterella (pooled OR = 1.27, 95%CI: 1.06-1.52), and Prevotella_7 (pooled OR = 1.23, 95%CI: 1.08-1.41) were positively correlated with stroke risk. In contrast, Butyrivibrio (pooled OR = 0.80, 95%CI: 0.65-0.99), Candidatus_Soleaferrea (pooled OR = 0.74, 95%CI: 0.59-0.92), Coprobacter (pooled OR = 0.71, 95%CI: 0.57-0.89), Eubacterium_hallii_group (pooled OR = 0.79, 95%CI: 0.65-0.96), FamilyXIIIAD3011 (pooled OR = 0.73, 95%CI: 0.55-0.97), Intestinimonas (pooled OR = 0.83, 95%CI: 0.73-0.94), Lachnospiraceae_NK4A136_group (pooled OR = 0.78, 95%CI: 0.67-0.91), Methanobrevibacter (pooled OR = 0.70, 95%CI: 0.54-0.91), Rikenellaceae_RC9_gut_group (pooled OR = 0.81, 95%CI: 0.67-0.99), and Tyzzerella_3 (pooled OR = 0.81, 95%CI: 0.68-0.97) were negatively associated, whereas Eubacterium_xylanophilum_group (pooled OR = 1.26, 95%CI: 0.91-1.74). Finally, Lachnospiraceae_ND_3007 (pooled OR = 0.96, 95%CI: 0.61-1.51), and Ruminococcaceae_UCG_009 (pooled OR = 0.94, 95%CI: 0.80-1.10) were not associated with an increased risk of stroke.
Moyamoya disease: One case-control study[37] reported that Ruminococcus was highly associated with an increased incidence of moyamoya disease (OR = 16.76, 95%CI: 2.73-102.84).
Multiple sclerosis: A matched case-control study[75] found that Bilophila (pooled OR = 3.00, 95%CI: 2.90-3.20), Bifidobacterium (pooled OR = 4.20, 95%CI: 3.90-4.50), Desulfovibrio (pooled OR = 5.10, 95%CI: 4.70-5.70), Prevotella_copri (pooled OR = 5.00, 95%CI: 4.40-5.60), Oscillospira (pooled OR = 2.30, 95%CI: 2.20-2.50), and Ruminococcus (pooled OR = 4.30, 95%CI: 3.60-5.10) were all positively associated with the risk of multiple sclerosis. By contrast, Lachnospira (pooled OR = 0.17, 95%CI: 0.16-0.19), Paraprevotella (pooled OR = 0.15, 95%CI: 0.14-0.17), Peptostreptococcus (pooled OR = 0.01, 95%CI: 0.01-0.02), Butyricimonas (pooled OR = 0.19, 95%CI: 0.16-0.22), Parabacteroides (pooled OR = 0.08, 95%CI: 0.07-0.10), and Mitsuokella (pooled OR = 0.10, 95%CI: 0.08-0.13) were all negatively associated. One Mendelian randomization study revealed that higher relative abundances of Bifidobacterium and Ruminococcus were associated with an increased risk of multiple sclerosis by 1.38 fold (pooled OR = 1.38, 95%CI: 1.13-1.70) and 2.89 fold (pooled OR = 2.89, 95%CI: 1.67-5.00), respectively[18].
Alzheimer’s disease: One cross-sectional study[39] reported that Escherichia was positively associated with the risk of Alzheimer’s disease (pooled OR = 5.17, 95%CI: 4.08-6.54).
Parkinson’s disease: One two-sample bidirectional Mendelian randomization study[40] found that Candidatus_Soleaferrea (pooled OR = 1.32, 95%CI: 1.04-1.68), Clostridium_sensustricto_1 (pooled OR = 1.38, 95%CI: 1.07-1.78), Eubacteri
Autism spectrum disorder: One case-control study[41] found that Candida albicans was associate with an increased incidence of autism spectrum disorder by about 3.45 fold (pooled OR = 3.45, 95%CI: 1.04-11.47).
Bipolar disorder: One case-control study[42] reported that the relative abundance of Flavonifractor was greater in patients with bipolar disorder as compared to a healthy population (pooled OR = 2.90, 95%CI: 1.60-5.20).
Depression: One case-control study[43] demonstrated a possible association of Bifidobacterium (pooled OR = 3.23, 95%CI: 1.38-7.54) and Lactobacillus (pooled OR = 2.57, 95%CI: 1.14-5.78) with the risk of major depression.
Inflammatory bowel disease: One two-sample Mendelian randomization study[18] showed that Bifidobacterium (pooled OR = 1.18, 95%CI: 1.04-1.35) and Ruminococcus (pooled OR = 2.14, 95%CI: 1.43-3.22) were positively correlated with inflammatory bowel disease.
Necrotizing enterocolitis: One case-control study[44] demonstrated that Clostridium could significantly increase the risk of necrotizing enterocolitis (pooled OR = 45.40, 95%CI: 26.20-78.60).
Irritable bowel syndrome: A large cohort study[45] reported that Blautia was positively correlated with irritable bowel syndrome (IBS) (pooled OR = 1.43, 95%CI: 1.21-1.69). One Mendelian randomization study[46] showed that Eubac
Nonalcoholic fatty liver disease: Five studies[47-51] found a positive correlation between Helicobacter pylori (H. pylori) and nonalcoholic fatty liver disease (pooled OR = 1.18, 95%CI: 1.09-1.28).
Gallstone: Twenty-four articles reported a positive association of Escherichia with gallstone formation (pooled OR = 2.48, 95%CI: 1.92-3.20)[52]. Helicobacter was not correlated with gallstones (pooled OR = 0.93, 95%CI: 0.48-1.82)[52]. One Mendelian randomization study[53] indicated that Butyrivibrio (pooled OR = 1.00, 95%CI: 1.00-1.00), Lachno
Asthma: One cohort study[55] found a negative correlation of Escherichia with the risk of asthma (pooled OR = 0.41, 95%CI: 0.22-0.80).
Overactive bladder disorder: One three-year longitudinal study[56] demonstrated that Streptococcus was an independent risk factor for overactive bladder disorder (pooled OR = 1.05, 95%CI: 1.01-1.11).
Obesity: Two observational studies determine whether changes in the gut microbiota are associated with obesity[57,58]. Lactobacillus was positively correlated with obesity (pooled OR = 1.79, 95%CI: 1.03-3.10), Akkermansia (pooled OR = 0.78, 95%CI: 0.63-0.95) and Methanobrevibacter were negatively correlated with obesity (pooled OR = 0.76, 95%CI: 0.59-0.97), while Bifidobacterium was not statistically correlated with obesity (pooled OR = 0.63, 95%CI: 0.39-1.01). A Mendelian randomization analysis found that Bifidobacterium (pooled OR = 0.99, 95%CI: 0.98-1.00) and Faecalibacterium (pooled OR = 0.99, 95%CI: 0.98-1.00) were not correlated with obesity[29].
Type 2 diabetes mellitus: Five observational studies[59-61,63,64] indicated that Flavonifractor (pooled OR = 1.22, 95%CI: 1.08-1.38), Tyzzerella (pooled HR = 1.17, 95%CI: 1.01-1.36), and Ruminococcus (pooled HR = 1.17, 95%CI: 1.01-1.36) were positively correlated with the risk of type 2 diabetes mellitus (T2DM), while Intestinibacter (pooled OR = 0.60, 95%CI: 0.48-0.76), Romboutsia (pooled OR = 0.55, 95%CI: 0.44-0.70), Coprococcus (pooled OR = 0.91, 95%CI: 0.85-0.97), Odoribacter (pooled OR = 0.94, 95%CI: 0.90-0.99), Blautia (pooled OR = 0.45, 95%CI: 0.24-0.86), and Roseburia (pooled OR = 0.54, 95%CI: 0.30-0.96) were negatively associated. However, Clostridium (pooled OR = 0.79, 95%CI: 0.34-1.83), Alistipes (pooled OR = 0.94, 95%CI: 0.88-1.00), Anaerostipes (pooled OR = 0.97, 95%CI: 0.89-1.07), Bifidobacterium (pooled OR = 1.20, 95%CI: 0.58-2.47), Oscillibacter (pooled OR = 0.95, 95%CI: 0.90-1.00), Pseudoflavonifractor (pooled OR = 0.99, 95%CI: 0.91-1.08), and Streptococcus (pooled OR = 1.47, 95%CI: 0.93-2.32) were not statistically associated with the risk of T2DM. Two Mendelian randomization studies[29,64] found that Streptococcaceae (pooled OR = 1.17, 95%CI: 1.04-1.31) and Acidaminococcaceae (pooled OR = 1.17, 95%CI: 1.04-1.31) were both positively associated with T2DM, but not Anaerostipes (pooled OR = 0.96, 95%CI: 0.93-1.00)[29,64].
Atopic dermatitis and eczema: Five studies[55,65-68] concurred that Clostridium was positively correlated with atopic dermatitis (pooled OR = 1.73, 95%CI: 1.28-2.34), while Bifidobacterium (pooled OR = 0.88, 95%CI: 0.59-1.31), Escherichia (pooled OR = 0.84, 95%CI: 0.51-1.39), Bacteroides (pooled OR = 1.16, 95%CI: 0.77-1.75), and Lactobacillus (pooled OR = 0.90, 95%CI: 0.52-1.55) were not. Meanwhile, Escherichia (pooled OR = 1.87, 95%CI: 1.15-3.04) and Clostridium (pooled OR = 1.40, 95%CI: 1.02-1.91) were positively correlated with eczema, while Lachnospira was negatively correlated (pooled OR = 0.88, 95%CI: 0.79-0.96), whereas Bifidobacterium (pooled OR = 1.02, 95%CI: 0.77-1.35), Bacteroides (pooled OR = 1.02, 95%CI: 0.71-1.47), and Lactobacillus (pooled OR = 1.23, 95%CI: 0.91-1.65) were not statistically correlated.
Psoriasis and psoriatic arthritis: Two Mendelian randomization studies[69,70] demonstrated that Eusicatena_fi
One cross-sectional study[71] reported that Bacteroides was associated with an increased risk of fracture by about 10.69 fold (pooled OR = 10.69, 95%CI: 1.88-60.93), while Blautia (pooled OR = 2.13, 95%CI: 0.46-9.84), Oscillospira (pooled OR = 1.57, 95%CI: 0.34-10.72), Ruminococcus (pooled OR = 1.40, 95%CI: 0.33-5.93), Bifidobacterium (pooled OR = 1.03, 95%CI: 0.24-4.41), and Sutterella (pooled OR = 2.48, 95%CI: 0.54-11.40) were not significantly associated with fracture.
Development: One cohort study[72] showed that the Bacteroide-dominated enterotype was correlated with later menarche and voice break (pooled OR = 0.53, 95%CI: 0.28-0.98).
Reproduction: One cohort study[73] found that a greater abundance of Actinomyces was positively associated with spontaneous preterm birth (pooled OR = 3.27, 95%CI: 1.35-7.95).
Aging: One cohort study[74] demonstrated that Bacteroides was not associated with the risk of death of community-dwelling participants at any age (pooled HR = 1.28, 95%CI: 1.00-1.65).
The current meta-analysis included more than 57 articles to assess the impact of the IF on the risks of 30 non-communicable disease during different stages of growth, development, and aging. Population and Mendelian randomization studies simultaneously confirmed positive associations of Bifidobacterium and Ruminococcus with multiple sclerosis. These findings provide more effective strategies for disease control and promote healthy growth and development.
Fusobacterium nucleatum (F. nucleatum) has been implicated in the pathogenesis of CRC by stimulating growth of CRC cells via increased expression of the transcription factor nuclear factor-kappaB and various oncogenes, such as c-Myc and cyclin D1, by binding fadA and E-cadherin[76]. The proliferation of CRC cells can also be increased by up-regulating expression of microRNA-21[76]. F. nucleatum can also down-regulate T cells and natural killer cells to inhibit cellular anti-tumor immunity[76]. In addition, F. nucleatum can induce the production of pro-inflammatory cytokines and form a pro-inflammatory microenvironment, which can influence the efficacy of anti-tumor chemotherapy drugs and treatment outcomes[76]. Escherichia coli (E. coli) can promote oncogenesis by inducing the mucosal antibody response of immunoglobulin A and/or the systemic antibody response of immunoglobulin G[23]. The carcinogenic potential of H. pylori is realized primarily through the virulence factors cytotoxin-associated gene A and vacuolating cytotoxin A, which inhibit expression of immune and pro-inflammatory cytokines[24]. In addition, lymphocytic gastritis caused by Propionibacterium acnes may enhance the development of gastric cancer by increased production of pro-inflammatory cytokines, such as interleukin (IL)-15[24]. On the contrary, Lactobacillus lactis can cause cell cycle arrest at the G0/G1 phase, thereby playing an anti-tumor role by inducing apoptosis[24]. For breast cancer, the IF plays an important role in regulating systemic estrogen[77]. Bacteria with estrogen-decoupling enzyme activity, characterized by the presence of the β-glucuronidase or β-galactosidase genes, have enzyme activity that promotes the reabsorption of unbound estrogen into the circulation, thereby increasing the estrogen load, which promotes the occurrence and development of breast cancer[77]. Members of the genus Ruminococcus produce butyrate, which can promote fat deposition and subsequently accelerate the growth of prostate tumors by inducing inflammation[78]. In addition, alterations to lipid metabolism, especially excessive accumulation of cholesterol and fatty acids, promote the malignant transformation of prostate cancer through the formation of cholesterol esters[78,79]. Akkermansia_muciniphila and Amuc_1100 may reduce the risk of prostate cancer by improving inflammation and regulating glucose and lipid metabolism[28].
Arterial and cerebrovascular diseases are mostly due to the breakdown of Proteobacteria in dietary metabolism to produce trimethylamine-N-oxide, which promotes oxidative stress, inflammatory responses, and the formation of atherosclerotic plaques[80]. An imbalance to the IF will cause intestinal barrier dysfunction, increase the proportions of harmful bacteria, as well as concentrations of hydrogen sulfide and lipopolysaccharides, while decreasing the abundances of beneficial bacteria, contents of short-chain fatty acids (SCFAs), and formation of intestinal tight junction proteins, thereby increasing intestinal permeability, which will lead to inflammatory responses, resulting in endothelial dysfunction and subsequent hypertension[81]. The intestinal hypothesis suggests that damage to the intestinal barrier may increase intestinal permeability and promote microbial translocation, resulting in the entry of microorganisms and related metabolites into the blood circulation, triggering low-grade chronic inflammation, ultimately inducing heart failure[81].
The gut-brain axis is thought to be a bidirectional biochemical signaling pathway between the gut and brain[15]. Disturbances to the IF can alter brain function by changing the levels of neuroactive compounds, increasing the levels of pro-inflammatory cytokines, and altering microbial metabolism[82]. Changes to the IF of multiple sclerosis patients may be associated with changes to the gene expression profiles of circulating T cells and monocytes involved in dendritic cell maturation, interferon signaling, and the nuclear factor-kappaB signaling pathway[83]. Alzheimer’s disease is characterized by decreased abundances of protective microbiota (e.g., Bacteroides, Spirillum, and Ruminococcus) and an increased proportion of Prevotella, which promotes inflammation[84]. Patients with depressive episodes generally have decreased proportions of bacteria that produce SCFAs and increased proportions of pro-inflammatory bacteria that are involved in lipid metabolism[85].
Many studies have shown that disruption to the IF is the main environmental driver of inflammatory bowel disease. A decrease in the number of butyrate-producing bacteria results in decreased activity of peroxisome-enhanced activator receptor γ and the agonist level/activity of the nuclear transcription factor aromatic receptor (AhR), increased glycolysis, decreased oxygen consumption, and increased production of carbon monoxide, leading to increased abundances of pathogenic facultative anaerobic bacteria (e.g., Enterobacteriaceae and Proteobacteria)[1]. Inflammatory bowel disease is characterized by reduced function of regulatory T cells, increased inflammation, and dysregulation of the intestinal barrier and detoxification function[1]. Several animal models have shown that Clostridium_butyricum can reproduce NEC-like lesions, which have significant cytotoxicity and cell adhesion, resulting in increased oxidative stress and immunosuppression[44]. An elevated Firmicutes/Bacteroidetes ratio in subgroups of patients with IBS are associated with changes in epithelial permeability and low-grade inflammation, which are considered possible mechanisms of IBS[44]. In addition, changes to the bidirectional interaction of the brain-gut axis play an important role in the pathophysiology of IBS[86]. H. pylori infection is associated with chronic inflammation, insulin resistance, diabetes, dyslipidemia, and metabolic syndrome, which are also risk factors for nonalcoholic fatty liver disease[47]. In addition, H. pylori infection can alter lipid metabolism, resulting in increased triacylglycerol accumulation in the liver[48], as well as local infiltration of lymphocytes and granulocytes by releasing inflammatory factors, such as IL-6, IL-1, and tumor necrosis factor-α, thereby inducing inflammation and exacerbating liver damage[47].
For asthma, lipopolysaccharides of Gram-negative bacteria, such as E. coli, activate signaling of toll-like receptor (TLR)-4 and cytokine production mediated by the myeloid differentiation primary response 88 protein[55]. Persistent exposure to lipopolysaccharides leads to tolerance and can cause downregulation of the TLR-4 signaling pathway[55]. Thus, colonization of the neonatal intestine by E. coli may lead to downregulation of TLR-4 signaling and remission of inflammatory responses, thereby reducing the risk of respiration-related inflammation[55]. An overactive bladder is closely associated with IBS and explained by crosstalk between the bladder and gastrointestinal tract via parasympathetic and sympathetic nerves[56]. Since IBS is thought to be closely related to nerve substances produced by the IF, dysregulation of the IF may also affect bladder function through convergent sensory pathways among the peripheral intestine, bladder, and spine[56].
The mechanisms of the IF leading to metabolic and inflammatory diseases, such as obesity and T2DM, may include decreased abundances of SCFA-producing bacteria, decreased activity of peroxisome proliferator-activated receptor gamma, increased glycolysis, increased proportions of pathogenic facultative anaerobic bacteria and anaerobic bacteria, decreased T cell function, and increased inflammatory responses[80]. Decreased levels/activities of AhR decreases intestinal barrier function and increases the abundance of harmful bacteria[80]. Endotoxins produced by Gram-negative bacteria can activate inflammation via specific pattern recognition receptors, such as TLR2 and TLR4[80]. Decreased activity of the myeloid differentiation primary response 88 protein results in decreased production of antimicrobial peptides and T cell activities, which can result in metabolic disorders[80]. Changes to the endocannabinoid system signaling pathway increases production of arachidonic acid glycolamide, fluctuations in intestinal permeability, and increased lipogenesis, which can destroy the intestinal barrier[80]. In addition, the disturbance to the IF acts on the enteric nervous system, altering the brain-gut axis and promoting glucose absorption, leading to hyperglycemia[80].
In recent years, the concept of the “gut-skin axis” has been gradually expanded. Disturbance to the IF can affect the gut-skin axis, resulting in decreased AhR, impaired differentiation and repair of the epidermis, and vulnerability to skin infections and barrier damage[80]. In psoriatic arthritis, harmful bacteria can reach the joints through the circulation or intestinal lymphatic system[70]. Dysregulation of the IF and gut inflammation may lead to reduced production of SCFAs, upregulation of the IL-23/Th17 axis and tumor necrosis factor-α, leading to inflammation, thereby increasing the risk of psoriasis[87,88]. Low blood vitamin K concentrations have been linked to higher incidences of femoral, cervical, and vertebral compression fractures in older women[71]. Notably, the abundance of Bacteroides was significantly correlated to vitamin K levels, which may explain the influence of Bacteroides on the occurrence of fractures[71].
The composition of the IF changes with age. Prevotella and Staphylococcus can cause inflammation, while Bacteroides produce SCFAs with anti-inflammatory properties[72]. Inflammation can alter the function of neurons via gonadotropin-releasing hormone, ultimately altering the course of pubertal development[72]. For preterm mothers, low IF diversity may be one of the root causes of inflammation, as an increased abundance of Actinomycetes has been linked to intense inflammation and energy loss[73]. Spontaneous preterm birth has been attributed to the higher tissue inflammatory status of obese women[89]. The microbiome of healthy older adults is characterized by the depletion of core genera found in most humans and an increase in rare taxa capable of synthesizing bioactive microbial metabolites[74]. There is an increasing burden of intestinal heterogenic metabolites in aging hosts, such as mildly toxic phenylalanine/tyrosine microbial fermentation products[90].
This study has illuminated the connections between gut microbiota and disease risks, but it is not without limitations. Firstly, common confounding factors such as diet, lifestyle, and medication use, which can influence the relationship between gut microbiota and disease risk, were not fully accounted for in previous studies due to inadequate data acquisition. Secondly, inherent limitations such as small sample sizes, flawed study designs, and suboptimal microbiological analysis techniques have led to significant heterogeneity among studies. Consequently, the findings should be interpreted with caution. Thirdly, the inherent limitations of observational studies affect the establishment of causal temporal relationships. Future research should include a multitude of prospective cohort studies to further elucidate the link between gut microbiota and chronic disease risk. Additionally, while most current studies have examined the correlation between IF and disease risk, there is a notable absence of discussion regarding the underlying biological mechanisms. Future investigations should incorporate more animal and in vitro experiments to explore the biological mechanisms by which IF may treat diseases.
The present study revealed significant associations of the IF and numerous chronic non-communicable diseases, including various cancers, cardiovascular disorders, and neurological conditions during different stages of growth, development, and aging. These findings provide valuable insights into the pathogenic mechanisms of specific bacterial genera on the host, facilitating enhanced disease prevention and control strategies for related disorders. Nevertheless, in the future, more standardized analytical techniques need to be explored to discover the specific mechanisms by which gut microbiota induce diseases. Additionally, longitudinal studies and interventional studies should be increased. Moreover, by combining multi-omics data (such as genomics and metabolomics) with machine learning methods, we can gain a deeper understanding of the temporal relationship between changes in gut microbiota and disease onset, understand the dynamic changes of the microbiota over time or under therapeutic interventions, and thus clarify the causal relationship more definitively.
We are deeply indebted to the funding agencies that provided financial support for this study. Their generous support enabled us to conduct extensive experiments, access necessary resources, and complete the data collection and analysis.
| 1. | Adak A, Khan MR. An insight into gut microbiota and its functionalities. Cell Mol Life Sci. 2019;76:473-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 829] [Article Influence: 138.2] [Reference Citation Analysis (0)] |
| 2. | Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3394] [Cited by in RCA: 3545] [Article Influence: 177.3] [Reference Citation Analysis (5)] |
| 3. | Yan X, Jin J, Su X, Yin X, Gao J, Wang X, Zhang S, Bu P, Wang M, Zhang Y, Wang Z, Zhang Q. Intestinal Flora Modulates Blood Pressure by Regulating the Synthesis of Intestinal-Derived Corticosterone in High Salt-Induced Hypertension. Circ Res. 2020;126:839-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 152] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 4. | Ye X, Wang A, Lin W, Xu Y, Dong X, Zhou Y, Tian K, Xu X. The Role of Intestinal Flora in Anti-Tumor Antibiotic Therapy. Front Biosci (Landmark Ed). 2022;27:281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 5. | Zhou B, Yuan Y, Zhang S, Guo C, Li X, Li G, Xiong W, Zeng Z. Intestinal Flora and Disease Mutually Shape the Regional Immune System in the Intestinal Tract. Front Immunol. 2020;11:575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 238] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 6. | Lynch SV, Pedersen O. The Human Intestinal Microbiome in Health and Disease. N Engl J Med. 2016;375:2369-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1826] [Cited by in RCA: 2325] [Article Influence: 258.3] [Reference Citation Analysis (0)] |
| 7. | Kåhrström CT, Pariente N, Weiss U. Intestinal microbiota in health and disease. Nature. 2016;535:47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 150] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 8. | Si H, Yang Q, Hu H, Ding C, Wang H, Lin X. Colorectal cancer occurrence and treatment based on changes in intestinal flora. Semin Cancer Biol. 2021;70:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 9. | Wong CC, Yu J. Gut microbiota in colorectal cancer development and therapy. Nat Rev Clin Oncol. 2023;20:429-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 277] [Reference Citation Analysis (0)] |
| 10. | Rahman MM, Islam F, -Or-Rashid MH, Mamun AA, Rahaman MS, Islam MM, Meem AFK, Sutradhar PR, Mitra S, Mimi AA, Emran TB, Fatimawali, Idroes R, Tallei TE, Ahmed M, Cavalu S. The Gut Microbiota (Microbiome) in Cardiovascular Disease and Its Therapeutic Regulation. Front Cell Infect Microbiol. 2022;12:903570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 133] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 11. | Chen X, Zhang H, Ren S, Ding Y, Remex NS, Bhuiyan MS, Qu J, Tang X. Gut microbiota and microbiota-derived metabolites in cardiovascular diseases. Chin Med J (Engl). 2023;136:2269-2284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 12. | Peh A, O'Donnell JA, Broughton BRS, Marques FZ. Gut Microbiota and Their Metabolites in Stroke: A Double-Edged Sword. Stroke. 2022;53:1788-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 137] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 13. | Zhu S, Jiang Y, Xu K, Cui M, Ye W, Zhao G, Jin L, Chen X. The progress of gut microbiome research related to brain disorders. J Neuroinflammation. 2020;17:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 275] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 14. | Liu L, Wang H, Chen X, Zhang Y, Zhang H, Xie P. Gut microbiota and its metabolites in depression: from pathogenesis to treatment. EBioMedicine. 2023;90:104527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 276] [Reference Citation Analysis (0)] |
| 15. | Socała K, Doboszewska U, Szopa A, Serefko A, Włodarczyk M, Zielińska A, Poleszak E, Fichna J, Wlaź P. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol Res. 2021;172:105840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 402] [Article Influence: 100.5] [Reference Citation Analysis (0)] |
| 16. | Iwasaki M, Kanehara R, Yamaji T, Katagiri R, Mutoh M, Tsunematsu Y, Sato M, Watanabe K, Hosomi K, Kakugawa Y, Ikematsu H, Hotta K, Kunisawa J, Wakabayashi K, Matsuda T. Association of Escherichia coli containing polyketide synthase in the gut microbiota with colorectal neoplasia in Japan. Cancer Sci. 2022;113:277-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Luo Q, Hu Y, Chen X, Luo Y, Chen J, Wang H. Effects of Gut Microbiota and Metabolites on Heart Failure and Its Risk Factors: A Two-Sample Mendelian Randomization Study. Front Nutr. 2022;9:899746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 18. | Xu Q, Ni JJ, Han BX, Yan SS, Wei XT, Feng GJ, Zhang H, Zhang L, Li B, Pei YF. Causal Relationship Between Gut Microbiota and Autoimmune Diseases: A Two-Sample Mendelian Randomization Study. Front Immunol. 2021;12:746998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 178] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 19. | Painold A, Mörkl S, Kashofer K, Halwachs B, Dalkner N, Bengesser S, Birner A, Fellendorf F, Platzer M, Queissner R, Schütze G, Schwarz MJ, Moll N, Holzer P, Holl AK, Kapfhammer HP, Gorkiewicz G, Reininghaus EZ. A step ahead: Exploring the gut microbiota in inpatients with bipolar disorder during a depressive episode. Bipolar Disord. 2019;21:40-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 148] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 20. | Ahn J, Sinha R, Pei Z, Dominianni C, Wu J, Shi J, Goedert JJ, Hayes RB, Yang L. Human gut microbiome and risk for colorectal cancer. J Natl Cancer Inst. 2013;105:1907-1911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 713] [Article Influence: 59.4] [Reference Citation Analysis (1)] |
| 21. | Kashani N, Bezmin Abadi AT, Rahimi F, Forootan M. FadA-positive Fusobacterium nucleatum is prevalent in biopsy specimens of Iranian patients with colorectal cancer. New Microbes New Infect. 2020;34:100651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Starý L, Mezerová K, Vysloužil K, Zbořil P, Skalický P, Stašek M, Raclavský V. Candida albicans culture from a rectal swab can be associated with newly diagnosed colorectal cancer. Folia Microbiol (Praha). 2020;65:989-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Butt J, Jenab M, Werner J, Fedirko V, Weiderpass E, Dahm CC, Tjønneland A, Olsen A, Boutron-Ruault MC, Rothwell JA, Severi G, Kaaks R, Turzanski-Fortner R, Aleksandrova K, Schulze M, Palli D, Pala V, Panico S, Tumino R, Sacerdote C, Bueno-de-Mesquita B, Van Gils CH, Gram IT, Lukic M, Sala N, Sánchez Pérez MJ, Ardanaz E, Chirlaque MD, Palmquist R, Löwenmark T, Travis RC, Heath A, Cross AJ, Freisling H, Zouiouich S, Aglago E, Waterboer T, Hughes DJ. Association of Pre-diagnostic Antibody Responses to Escherichia coli and Bacteroides fragilis Toxin Proteins with Colorectal Cancer in a European Cohort. Gut Microbes. 2021;13:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 24. | Gunathilake M, Lee J, Choi IJ, Kim YI, Yoon J, Sul WJ, Kim JF, Kim J. Alterations in Gastric Microbial Communities Are Associated with Risk of Gastric Cancer in a Korean Population: A Case-Control Study. Cancers (Basel). 2020;12:2619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Byrd DA, Vogtmann E, Wu Z, Han Y, Wan Y, Clegg-Lamptey JN, Yarney J, Wiafe-Addai B, Wiafe S, Awuah B, Ansong D, Nyarko K, Hullings AG, Hua X, Ahearn T, Goedert JJ, Shi J, Knight R, Figueroa JD, Brinton LA, Garcia-Closas M, Sinha R. Associations of fecal microbial profiles with breast cancer and nonmalignant breast disease in the Ghana Breast Health Study. Int J Cancer. 2021;148:2712-2723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 26. | Cardeiro M, Ardeljan AD, Frankel L, Kim E, Takabe K, Rashid OM. Incidence of Breast Cancer and Enterococcus Infection: A Retrospective Analysis. World J Oncol. 2023;14:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 27. | Zhang S, Zhang W, Ren H, Xue R, Wang Z, Wang Z, Lv Q. Mendelian randomization analysis revealed a gut microbiota-mammary axis in breast cancer. Front Microbiol. 2023;14:1193725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 28. | Xie Q, Hu B. Effects of gut microbiota on prostatic cancer: a two-sample Mendelian randomization study. Front Microbiol. 2023;14:1250369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 29. | Yang Q, Lin SL, Kwok MK, Leung GM, Schooling CM. The Roles of 27 Genera of Human Gut Microbiota in Ischemic Heart Disease, Type 2 Diabetes Mellitus, and Their Risk Factors: A Mendelian Randomization Study. Am J Epidemiol. 2018;187:1916-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 30. | Toya T, Corban MT, Marrietta E, Horwath IE, Lerman LO, Murray JA, Lerman A. Coronary artery disease is associated with an altered gut microbiome composition. PLoS One. 2020;15:e0227147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 31. | Gao J, Wang J, Zhao LL, Yao TT, Chen Y, Ma J, Zhang X, Wang JX, Wang Y, Cui Z, Liu Y. Gut Lactobacillus Level Is a Predictive Marker for Coronary Atherosclerotic Lesions Progress and Prognosis in Patients With Acute Coronary Syndrome. Front Cell Infect Microbiol. 2021;11:687827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Zhang Y, Zhang X, Chen D, Lu J, Gong Q, Fang J, Jiang J. Causal associations between gut microbiome and cardiovascular disease: A Mendelian randomization study. Front Cardiovasc Med. 2022;9:971376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 33. | Yu J, Zhang B, Miao T, Hu H, Sun Y. Dietary Nutrition and Gut Microbiota Composition in Patients With Hypertensive Disorders of Pregnancy. Front Nutr. 2022;9:862892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 34. | Dai H, Hou T, Wang Q, Hou Y, Wang T, Zheng J, Lin H, Zhao Z, Li M, Wang S, Zhang D, Dai M, Zheng R, Lu J, Xu Y, Chen Y, Ning G, Wang W, Bi Y, Xu M. Causal relationships between the gut microbiome, blood lipids, and heart failure: a Mendelian randomization analysis. Eur J Prev Cardiol. 2023;30:1274-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 35. | Meng C, Deng P, Miao R, Tang H, Li Y, Wang J, Wu J, Wang W, Liu S, Xia J, Lu Y. Gut microbiome and risk of ischaemic stroke: a comprehensive Mendelian randomization study. Eur J Prev Cardiol. 2023;30:613-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 36. | Shen Y, Liu H, Meng X, Gao A, Liu Y, Ma W, Liang H, Hu F. The causal effects between gut microbiota and hemorrhagic stroke: a bidirectional two-sample Mendelian randomization study. Front Microbiol. 2023;14:1290909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 37. | Mineharu Y, Nakamura Y, Sato N, Kamata T, Oichi Y, Fujitani T, Funaki T, Okuno Y, Miyamoto S, Koizumi A, Harada KH. Increased abundance of Ruminococcus gnavus in gut microbiota is associated with moyamoya disease and non-moyamoya intracranial large artery disease. Sci Rep. 2022;12:20244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 38. | Tremlett H, Fadrosh DW, Faruqi AA, Hart J, Roalstad S, Graves J, Lynch S, Waubant E; US Network of Pediatric MS Centers. Gut microbiota composition and relapse risk in pediatric MS: A pilot study. J Neurol Sci. 2016;363:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 39. | Katz J, Gao H. The Alzheimer-E. coli Axis: What Can We Learn from an Electronic Health Record Platform. J Alzheimers Dis. 2021;84:717-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Jiang L, Li JC, Tang BS, Guo JF. Associations between gut microbiota and Parkinson disease: A bidirectional Mendelian randomization analysis. Eur J Neurol. 2023;30:3471-3477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 41. | Hughes HK, Ashwood P. Anti-Candida albicans IgG Antibodies in Children With Autism Spectrum Disorders. Front Psychiatry. 2018;9:627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Coello K, Hansen TH, Sørensen N, Munkholm K, Kessing LV, Pedersen O, Vinberg M. Gut microbiota composition in patients with newly diagnosed bipolar disorder and their unaffected first-degree relatives. Brain Behav Immun. 2019;75:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 43. | Aizawa E, Tsuji H, Asahara T, Takahashi T, Teraishi T, Yoshida S, Ota M, Koga N, Hattori K, Kunugi H. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J Affect Disord. 2016;202:254-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 412] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 44. | Cassir N, Benamar S, Khalil JB, Croce O, Saint-Faust M, Jacquot A, Million M, Azza S, Armstrong N, Henry M, Jardot P, Robert C, Gire C, Lagier JC, Chabrière E, Ghigo E, Marchandin H, Sartor C, Boutte P, Cambonie G, Simeoni U, Raoult D, La Scola B. Clostridium butyricum Strains and Dysbiosis Linked to Necrotizing Enterocolitis in Preterm Neonates. Clin Infect Dis. 2015;61:1107-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 45. | Brunkwall L, Ericson U, Nilsson PM, Orho-Melander M, Ohlsson B. Self-reported bowel symptoms are associated with differences in overall gut microbiota composition and enrichment of Blautia in a population-based cohort. J Gastroenterol Hepatol. 2021;36:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 46. | Liu B, Ye D, Yang H, Song J, Sun X, He Z, Mao Y, Hao G. Assessing the relationship between gut microbiota and irritable bowel syndrome: a two-sample Mendelian randomization analysis. BMC Gastroenterol. 2023;23:150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 47. | Chen CX, Mao YS, Foster P, Zhu ZW, Du J, Guo CY. Possible association between Helicobacter pylori infection and nonalcoholic fatty liver disease. Appl Physiol Nutr Metab. 2017;42:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 48. | Kim TJ, Sinn DH, Min YW, Son HJ, Kim JJ, Chang Y, Baek SY, Ahn SH, Lee H, Ryu S. A cohort study on Helicobacter pylori infection associated with nonalcoholic fatty liver disease. J Gastroenterol. 2017;52:1201-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 49. | Cai O, Huang Z, Li M, Zhang C, Xi F, Tan S. Association between Helicobacter pylori Infection and Nonalcoholic Fatty Liver Disease: A Single-Center Clinical Study. Gastroenterol Res Pract. 2018;2018:8040262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 50. | Fan N, Peng L, Xia Z, Zhang L, Wang Y, Peng Y. Helicobacter pylori Infection Is Not Associated with Non-alcoholic Fatty Liver Disease: A Cross-Sectional Study in China. Front Microbiol. 2018;9:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 51. | Kang SJ, Kim HJ, Kim D, Ahmed A. Association between cagA negative Helicobacter pylori status and nonalcoholic fatty liver disease among adults in the United States. PLoS One. 2018;13:e0202325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 52. | Wu S, Zhu W, Wang X, Zhao K, He B, Peng Z, Yang J. Relationship between different intestinal microflora and cholelithiasis: A systematic review and meta-analysis. Asian J Surg. 2023;46:4780-4782. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 53. | Li W, Ren A, Qin Q, Zhao L, Peng Q, Ma R, Luo S. Causal associations between human gut microbiota and cholelithiasis: a mendelian randomization study. Front Cell Infect Microbiol. 2023;13:1169119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 54. | Cen L, Wu J, Zhu S, Pan J, Zhou T, Yan T, Shen Z, Yu C. The potential bidirectional association between Helicobacter pylori infection and gallstone disease in adults: A two-cohort study. Eur J Clin Invest. 2023;53:e13879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 55. | Orivuori L, Mustonen K, de Goffau MC, Hakala S, Paasela M, Roduit C, Dalphin JC, Genuneit J, Lauener R, Riedler J, Weber J, von Mutius E, Pekkanen J, Harmsen HJM, Vaarala O; PASTURE Study Group. High level of fecal calprotectin at age 2 months as a marker of intestinal inflammation predicts atopic dermatitis and asthma by age 6. Clin Exp Allergy. 2015;45:928-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 56. | Okuyama Y, Okamoto T, Sasaki D, Ozaki K, Songee J, Hatakeyama S, Mikami T, Ohyama C. The influence of gut microbiome on progression of overactive bladder symptoms: a community-based 3-year longitudinal study in Aomori, Japan. Int Urol Nephrol. 2022;54:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 57. | Million M, Maraninchi M, Henry M, Armougom F, Richet H, Carrieri P, Valero R, Raccah D, Vialettes B, Raoult D. Obesity-associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii. Int J Obes (Lond). 2012;36:817-825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 516] [Cited by in RCA: 493] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 58. | Zhou Q, Zhang Y, Wang X, Yang R, Zhu X, Zhang Y, Chen C, Yuan H, Yang Z, Sun L. Gut bacteria Akkermansia is associated with reduced risk of obesity: evidence from the American Gut Project. Nutr Metab (Lond). 2020;17:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 59. | Chen Z, Radjabzadeh D, Chen L, Kurilshikov A, Kavousi M, Ahmadizar F, Ikram MA, Uitterlinden AG, Zhernakova A, Fu J, Kraaij R, Voortman T. Association of Insulin Resistance and Type 2 Diabetes With Gut Microbial Diversity: A Microbiome-Wide Analysis From Population Studies. JAMA Netw Open. 2021;4:e2118811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 191] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 60. | Tabasi M, Eybpoosh S, Sadeghpour Heravi F, Siadat SD, Mousavian G, Elyasinia F, Soroush A, Bouzari S. Gut Microbiota and Serum Biomarker Analyses in Obese Patients Diagnosed with Diabetes and Hypothyroid Disorder. Metab Syndr Relat Disord. 2021;19:144-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 61. | Cui J, Ramesh G, Wu M, Jensen ET, Crago O, Bertoni AG, Gao C, Hoffman KL, Sheridan PA, Wong KE, Wood AC, Chen YI, Rotter JI, Petrosino JF, Rich SS, Goodarzi MO. Butyrate-Producing Bacteria and Insulin Homeostasis: The Microbiome and Insulin Longitudinal Evaluation Study (MILES). Diabetes. 2022;71:2438-2446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 62. | Ruuskanen MO, Erawijantari PP, Havulinna AS, Liu Y, Méric G, Tuomilehto J, Inouye M, Jousilahti P, Salomaa V, Jain M, Knight R, Lahti L, Niiranen TJ. Gut Microbiome Composition Is Predictive of Incident Type 2 Diabetes in a Population Cohort of 5,572 Finnish Adults. Diabetes Care. 2022;45:811-818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 63. | Tamura A, Murabayashi M, Nishiya Y, Mizushiri S, Hamaura K, Ito R, Ono S, Terada A, Murakami H, Tanabe J, Yanagimachi M, Tokuda I, Sawada K, Ihara K, Daimon M. Correction: Tamura et al. Interrelations between Gut Microbiota Composition, Nutrient Intake and Diabetes Status in an Adult Japanese Population. J. Clin. Med. 2022, 11, 3216. J Clin Med. 2022;12:3. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 64. | Xiang K, Zhang JJ, Xu YY, Zhong X, Ni J, Pan HF. Genetically Predicted Causality of 28 Gut Microbiome Families and Type 2 Diabetes Mellitus Risk. Front Endocrinol (Lausanne). 2022;13:780133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 65. | Penders J, Thijs C, van den Brandt PA, Kummeling I, Snijders B, Stelma F, Adams H, van Ree R, Stobberingh EE. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut. 2007;56:661-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 572] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 66. | Penders J, Thijs C, Mommers M, Stobberingh EE, Dompeling E, Reijmerink NE, van den Brandt PA, Kerkhof M, Koppelman GH, Postma DS. Intestinal lactobacilli and the DC-SIGN gene for their recognition by dendritic cells play a role in the aetiology of allergic manifestations. Microbiology (Reading). 2010;156:3298-3305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 67. | Penders J, Gerhold K, Stobberingh EE, Thijs C, Zimmermann K, Lau S, Hamelmann E. Establishment of the intestinal microbiota and its role for atopic dermatitis in early childhood. J Allergy Clin Immunol. 2013;132:601-607.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 217] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 68. | Hu C, van Meel ER, Medina-Gomez C, Kraaij R, Barroso M, Kiefte-de Jong J, Radjabzadeh D, Pasmans SGMA, de Jong NW, de Jongste JC, Moll HA, Nijsten T, Rivadeneira F, Pardo LM, Duijts L. A population-based study on associations of stool microbiota with atopic diseases in school-age children. J Allergy Clin Immunol. 2021;148:612-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 69. | Zang C, Liu J, Mao M, Zhu W, Chen W, Wei B. Causal Associations Between Gut Microbiota and Psoriasis: A Mendelian Randomization Study. Dermatol Ther (Heidelb). 2023;13:2331-2343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 70. | Qian X, Fu Z, Diao C, Zhang W, Tao W, Hu J, Zhang S, Zhao D. Genetic causal relationship between gut microbiome and psoriatic arthritis: a bidirectional two-sample Mendelian randomization study. Front Microbiol. 2023;14:1265786. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 71. | Ozaki D, Kubota R, Maeno T, Abdelhakim M, Hitosugi N. Association between gut microbiota, bone metabolism, and fracture risk in postmenopausal Japanese women. Osteoporos Int. 2021;32:145-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 72. | Xu Y, Xiong J, Shan S, Wang X, He F, Cheng G. Age-Dependent and Body Composition-Dependent Association of Child Gut Microbial Enterotype With Puberty Timing: A Chinese Cohort. J Clin Endocrinol Metab. 2023;108:2363-2370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 73. | Yu HR, Tsai CC, Chan JYH, Lee WC, Wu KLH, Tain YL, Hsu TY, Cheng HH, Huang HC, Huang CH, Pan WH, Yeh YT. A Higher Abundance of Actinomyces spp. in the Gut Is Associated with Spontaneous Preterm Birth. Microorganisms. 2023;11:1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 74. | Wilmanski T, Diener C, Rappaport N, Patwardhan S, Wiedrick J, Lapidus J, Earls JC, Zimmer A, Glusman G, Robinson M, Yurkovich JT, Kado DM, Cauley JA, Zmuda J, Lane NE, Magis AT, Lovejoy JC, Hood L, Gibbons SM, Orwoll ES, Price ND. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat Metab. 2021;3:274-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 391] [Article Influence: 97.8] [Reference Citation Analysis (0)] |
| 75. | Tremlett H, Fadrosh DW, Faruqi AA, Zhu F, Hart J, Roalstad S, Graves J, Lynch S, Waubant E; US Network of Pediatric MS Centers. Gut microbiota in early pediatric multiple sclerosis: a case-control study. Eur J Neurol. 2016;23:1308-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 265] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 76. | Lee SA, Liu F, Riordan SM, Lee CS, Zhang L. Global Investigations of Fusobacterium nucleatum in Human Colorectal Cancer. Front Oncol. 2019;9:566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 77. | Kwa M, Plottel CS, Blaser MJ, Adams S. The Intestinal Microbiome and Estrogen Receptor-Positive Female Breast Cancer. J Natl Cancer Inst. 2016;108:djw029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 206] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 78. | Hayashi T, Fujita K, Nojima S, Hayashi Y, Nakano K, Ishizuya Y, Wang C, Yamamoto Y, Kinouchi T, Matsuzaki K, Jingushi K, Kato T, Kawashima A, Nagahara A, Ujike T, Uemura M, Pena MDCR, Gordetsky JB, Morii E, Tsujikawa K, Netto GJ, Nonomura N. High-Fat Diet-Induced Inflammation Accelerates Prostate Cancer Growth via IL6 Signaling. Clin Cancer Res. 2018;24:4309-4318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 124] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 79. | Wang X, Sun B, Wei L, Jian X, Shan K, He Q, Huang F, Ge X, Gao X, Feng N, Chen YQ. Cholesterol and saturated fatty acids synergistically promote the malignant progression of prostate cancer. Neoplasia. 2022;24:86-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 80. | de Vos WM, Tilg H, Van Hul M, Cani PD. Gut microbiome and health: mechanistic insights. Gut. 2022;71:1020-1032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 1255] [Article Influence: 418.3] [Reference Citation Analysis (0)] |
| 81. | Yang Z, Wang Q, Liu Y, Wang L, Ge Z, Li Z, Feng S, Wu C. Gut microbiota and hypertension: association, mechanisms and treatment. Clin Exp Hypertens. 2023;45:2195135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 64] [Reference Citation Analysis (0)] |
| 82. | Mehra A, Arora G, Sahni G, Kaur M, Singh H, Singh B, Kaur S. Gut microbiota and Autism Spectrum Disorder: From pathogenesis to potential therapeutic perspectives. J Tradit Complement Med. 2023;13:135-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 83. | Jangi S, Gandhi R, Cox LM, Li N, von Glehn F, Yan R, Patel B, Mazzola MA, Liu S, Glanz BL, Cook S, Tankou S, Stuart F, Melo K, Nejad P, Smith K, Topçuolu BD, Holden J, Kivisäkk P, Chitnis T, De Jager PL, Quintana FJ, Gerber GK, Bry L, Weiner HL. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. 2016;7:12015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 703] [Cited by in RCA: 936] [Article Influence: 104.0] [Reference Citation Analysis (0)] |
| 84. | Guo M, Peng J, Huang X, Xiao L, Huang F, Zuo Z. Gut Microbiome Features of Chinese Patients Newly Diagnosed with Alzheimer's Disease or Mild Cognitive Impairment. J Alzheimers Dis. 2021;80:299-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 115] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 85. | Huang TT, Lai JB, Du YL, Xu Y, Ruan LM, Hu SH. Current Understanding of Gut Microbiota in Mood Disorders: An Update of Human Studies. Front Genet. 2019;10:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 173] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 86. | Labus JS, Hollister EB, Jacobs J, Kirbach K, Oezguen N, Gupta A, Acosta J, Luna RA, Aagaard K, Versalovic J, Savidge T, Hsiao E, Tillisch K, Mayer EA. Differences in gut microbial composition correlate with regional brain volumes in irritable bowel syndrome. Microbiome. 2017;5:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 199] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 87. | de Alcantara CC, Reiche EMV, Simão ANC. Cytokines in psoriasis. Adv Clin Chem. 2021;100:171-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 88. | Ney LM, Wipplinger M, Grossmann M, Engert N, Wegner VD, Mosig AS. Short chain fatty acids: key regulators of the local and systemic immune response in inflammatory diseases and infections. Open Biol. 2023;13:230014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 95] [Reference Citation Analysis (0)] |
| 89. | Tersigni C, Neri C, D'Ippolito S, Garofalo S, Martino C, Lanzone A, Scambia G, Di Simone N. Impact of maternal obesity on the risk of preterm delivery: insights into pathogenic mechanisms. J Matern Fetal Neonatal Med. 2022;35:3216-3221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 90. | Nemet I, Saha PP, Gupta N, Zhu W, Romano KA, Skye SM, Cajka T, Mohan ML, Li L, Wu Y, Funabashi M, Ramer-Tait AE, Naga Prasad SV, Fiehn O, Rey FE, Tang WHW, Fischbach MA, DiDonato JA, Hazen SL. A Cardiovascular Disease-Linked Gut Microbial Metabolite Acts via Adrenergic Receptors. Cell. 2020;180:862-877.e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 488] [Article Influence: 97.6] [Reference Citation Analysis (0)] |