Published online Mar 21, 2025. doi: 10.3748/wjg.v31.i11.102848
Revised: January 17, 2025
Accepted: February 11, 2025
Published online: March 21, 2025
Processing time: 130 Days and 3.1 Hours
Hepatocellular carcinoma (HCC) is a prevalent and aggressive malignancy in the Chinese population; the severe vascularization by the tumor makes it difficult to cure. The high incidence and poor survival rates of this disease indicate the search for new therapeutic alternatives. Apatinib became a drug of choice because it inhibits tyrosine kinase activity, mainly through an effect on vascular endothelial growth factor receptor-2, thereby preventing tumor angiogenesis. This mecha
To investigate the effect of apatinib on the glycolysis of vascular endothelial cells (VECs).
This present study has investigated the effects of HCC cells on VECs, paying particular attention to changes in the glycolytic activity of VECs. The co-culture system established in the present study examined key cellular functions such as extracellular acidification rate and oxygen consumption rate. It also discusses participation of apatinib in the above processes. Core to the findings is the phosphatidylinositol 3-kinase (PI3K)/AKT/6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3) signaling pathway, emphasizing the function of phosphorylated AKT and its interaction with PFKFB3, an essential regulator of glycolysis. In the investigation, molecular mechanisms by which such a pathway could influence the above VECs functions of proliferation, migration, and tube formation were underlined through coimmunoprecipitation analysis. Besides, supplementary in vivo experiments on nude mice provided additional biological relevance to the obtained results.
The glycolytic metabolism in VECs co-cultured with HCC cells is highly active, and the increased glycolysis in these endothelial cells accelerates the malignant transformation of HCC cells. Apatinib has been shown to inhibit this glycolytic activity in the VECs. It also hinders the development, multiplication, and movement of these cells while encouraging their programmed cell death. Moreover, biological analysis revealed that apatinib mainly influences VECs by regulating the PI3K/AKT signaling pathway. Subsequent research indicated that apatinib blocks the PI3K/AKT/PFKEB3 pathway, which in turn reduces glycolysis in these cells.
Apatinib influences the glycolytic pathway in the VECs of HCC a through the PI3K/AKT/PFKFB3 signaling pathway.
Core Tip: Hepatocellular carcinoma (HCC) is characterized by its rich vascularization and highly active metabolic processes. As a vascular endothelial growth factor receptor-2-targeting agent, apatinib serves as a primary therapeutic option for HCC patients. Nevertheless, the impact of apatinib on the metabolic activities of vascular endothelial cells remains poorly understood. The study revealed that apatinib modulates glycolysis in HCC-associated vascular endothelial cells through the phosphatidylinositol 3-kinase/AKT/6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 signaling cascade. These findings provide insights into the molecular mechanism of apatinib’s action on endothelial cells and uncover a potential therapeutic target for HCC patients with high 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 expression.
- Citation: Wu Y, Xie BB, Zhang BL, Zhuang QX, Liu SW, Pan HM. Apatinib regulates the glycolysis of vascular endothelial cells through PI3K/AKT/PFKFB3 pathway in hepatocellular carcinoma. World J Gastroenterol 2025; 31(11): 102848
- URL: https://www.wjgnet.com/1007-9327/full/v31/i11/102848.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i11.102848
Hepatocellular carcinoma (HCC) represents the predominant contributor to global cancer mortality rates[1]. This malignancy constitutes roughly 90% of total liver cancer cases[2]. It is a complex environment that includes both malignant and non-malignant cells. Angiogenesis refers to the process where endothelial cells multiply and move to create new blood vessel sprouts from existing vessels. Tumor progression is frequently associated with angiogenesis, where the tumor microenvironment (TME) exhibits a complex network of interactions between pro-angiogenic elements, including fibroblast growth factor and vascular endothelial growth factor (VEGF), and anti-angiogenic components. Such dynamic interactions play a crucial role in regulating angiogenesis, a process essential for tumor growth and metastasis[3]. Numerous regulatory factors present in the TME have been shown to facilitate critical processes like angiogenesis and epithelial-mesenchymal transition[4]. Consequently, these factors collectively promote tumour progression. The process of tumour angiogenesis, in particular, facilitates the provision of oxygen and nutrients that are essential for cell proliferation. Moreover, it establishes pathways that promote tumour metastasis and generates hypoxic zones, which in turn can lead to drug resistance. The vascular system is far more than a passive channel for uniformly distributing oxygen and nutrients[5]. In typical physiological environments, the process of angiogenesis plays a crucial role in facilitating wound repair and ensuring the delivery of essential nutrients throughout the organism. However, in pathological states, angiogenesis has been shown to be a critical driver of tumor growth and metastasis[6,7]. It is well established that tumour blood vessels frequently exhibit abnormal characteristics, including high clutter, a lack of clear demarcation between arterioles and venules, and high permeabilit. The progression from a small cluster of cancerous cells to a potentially fatal tumor predominantly relies on the activation of angiogenesis, a process that entails an imbalance in the regulation of angiogenesis[7].
The development of new blood vessels, known as angiogenesis, is a hallmark of cancer. As a result, disrupting pathways such as VEGF in endothelial cells has emerged as a viable approach for anti-angiogenic treatments[8]. In 1971, Folkman[9] proposed that therapies targeting angiogenesis could dismantle existing blood vessels, inhibit the creation of new ones, and cut off the essential oxygen and nutrients to cancer cells, thereby impeding tumor growth and aiding in cancer management. This therapeutic promise has spurred the creation of two main types of anti-angiogenic drugs: Monoclonal antibodies and small molecule tyrosine kinase inhibitors. Apatinib is the first tyrosine kinase inhibitor developed and approved for market in China, which has strong and highly selective inhibitory activity against VEGF receptor-2 (VEGFR-2) tyrosine kinase (IC50 is 1 nmol/L). The tyrosine kinases c-kit (IC50 429 nmol/L), c-src (IC50 530 nmol/L), and RET (IC50 13 nmol/L) were also suppressed[10]. Apatinib significantly hinders the growth, movement, and tube formation of human umbilical vein endothelial cells stimulated by fetal bovine serum. In addition, it has been shown to possess anti-tumour growth activity in several established human tumour xenotransplantation models[11,12]. Research has shown that apatinib exhibits anti-tumor properties and triggers autophagy in lung cancer cells characterized by elevated VEGFR-2 levels[13]. Additionally, in the context of thyroid cancer, apatinib has been found to inhibit cell proliferation, migration, invasion, and angiogenesis by reducing the expression of pyruvate kinase M[14].However, the study of apatinib on liver cancer, whether from the perspective of radiosensitization or tumor inhibition, is limited to the tumor cells themselves[15,16]. HCC is a vascular rich tumor, and apatinib is a highly selective target drug for VEGFR2. However, the impact of apatinib on the metabolic processes of vascular endothelial cells (VECs) is still not fully understood.
These cells, which form the interior surface of blood vessels, play a critical role in sustaining the overall functionality of the circulatory system. In adults, these cells typically remain in a predominantly quiescent state. However, under conditions of injury or pathology, these cells possess the capacity to promptly initiate the formation of new blood vessels. This dynamic process, known as “germinal angiogenesis”, is meticulously orchestrated and is initiated by a migrating tip cell that extrudes a filamentous foot protrusion. This is then followed by the further extension of the bud and the multiplication of stem cells. The significant influence of endothelial cell metabolism on angiogenesis has only been recognized in recent times. Different metabolic processes, including glycolysis, fatty acid breakdown, and glutamine utilization, have been pinpointed as having distinct and crucial functions in the development of new blood vessels[17]. A groundbreaking study by De Bock et al[18] revealed that endothelial cells predominantly depend on glycolysis rather than oxidative phosphorylation for adenosine triphosphate (ATP) production. The absence of the glycolysis activator 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3) was shown to hinder blood vessel formation, especially in tumor environments. Nonetheless, the impact of apatinib on the glycolytic activity of VECs and the underlying molecular mechanisms remain unexplored.
This study used a variety of human cell lines, including human umbilical vein endothelial cells (HUVEC), HCC cell lines (PLC/PRF/5, HCCLM3, HLE, and Huh7), and a normal liver cell line LO2. All of these cell lines were provided by the Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences. Cells were cultured in Dulbecco’s modified eagle medium (DMEM) containing 4500 mg/L D-glucose, 100 mL/L fetal bovine serum (Gibco), and 1% antibiotic solution containing 100 U penicillin and 100 μg streptomycin per mL (Sigma-Aldrich). The environmental conditions for cell culture were 37 °C, 50 mL/L CO2, and appropriate humidity. Cells were passaged at 80%-90% confluence using phosphate buffered saline (PBS) without calcium and magnesium and trypsin (1 mL of a solution containing 2.5 μL trypsin and 0.2 μL EDTA). Trypsin treatment was performed for 1-3 minutes at 37 °C, and the enzyme reaction was terminated by adding fresh medium. During the subculturing process, the cell morphology was regularly observed under a microscope to ensure that the cells could detach normally and maintain a healthy growth state.
In order to observe the effect of normal liver cells/HCC cells on HUVEC, a co-culture experiment was conducted. When L-02/HCC-LM3 cells were cultured in a petri dish to reach 90% confluence, a new DMEM was used for a further 24 hours. The cell culture media from both cell types were collected in equal volumes and added to HUVEC, which were then cultured for a further 48 hours to detect changes in HUVEC. In a similar manner, HUVEC of NC/PFKFB3-OE was co-cultured with HUVEC of HCC cells, HCCLM3 and PLC/PRF/5, to observe the effect of HUVEC glycolysis on HCC cells. HUVEC of NC/PFKFB3-OE was cultured to 90% fusion in petri dishes and then cultured with a new DMEM for 24 hours. The cell supernatant from equal volumes of the two types of cells were then collected and added to the prepared liver cancer cells, which were subsequently cultured for a further 24 hours to detect changes in the liver cancer cells.
PFKFB3 cDNA (GenBank Accession No. L77662.1) was polymerase chain reaction (PCR)-amplified with the 2× Phanta® Flash Master Mix (cat. No. P520, Vazyme, Nanjing, China) and the primers (sense: 5’-GGAGACCCAAGCTGGCTAGCATGCCGTTGGAACTGACGC-3’; antisense: 5’-GCTGGATATCTGCAGAATTCTCAGTGTTTCCTGGAGGAGTCAG-3’) followed by being subcloned into the NheI and EcoRI sites of pcDNA3.1(+) (Invitrogen) with the ClonExpress MultiS One Step Cloning Kit (cat. No. C113, Vazyme, Nanjing, China). A pcDNA3.1(+) vector served as the control. HUVEC transfection was carried out with Lipofectamine 3000, following the procedure outlined by the manufacturer (Invitrogen, United States). The transfection efficiency of the plasmid was observed under fluorescence microscope (Supplementary Figure 1).
A 48-well culture plate was prepared by dispensing 100 μL of thawed basement membrane matrix (BD Biosciences, San Jose, CA, United States) into each well, followed by incubation at 37 °C with 50 mL/L CO2 for 30 minutes to allow solidification. Subsequently, HUVEC cells at a density of 5 × 104 were plated into each well and exposed to varying concentrations of apatinib for a 24-hour period. Tube formation by endothelial cells was subsequently assessed through phase-contrast microscopic observation.
The experiment involves a series of key steps to assess cell behavior and migration. Initially, cell proliferation is evaluated using a Cell Counting Kit-8 assay in a 96-well plate, where absorbance measurements are taken to track cell growth. Migration is studied through Transwell analysis, where cells are placed in a medium containing or lacking fetal bovine serum to assess their movement. Serum-free medium is used for specific conditions. In another part of the experiment, crystal violet staining is performed to visualize the cells, while wound closure is monitored using a 6-well plate. Optical microscopy allows for detailed observation of cell movement and monolayer formation, and wound creation is carefully performed to simulate cellular migration in response to injury. These techniques together provide comprehensive insights into cell behavior and responses under different conditions.
The main purpose of the present study was to investigate the effects of apatinib on HUVEC cells, with a special focus on inducing cell apoptosis. TdT-mediated dUTP nick-end labeling (TUNEL) staining and Annexin V-fluorescein iso
RNA was extracted from HUVEC using TRIzol reagent (Thermo Fisher Scientific, MA, United States) and mRNA was reverse transcribed into cDNA using HiScript III RT SuperMix for quantitative PCR (qPCR) Kit (Vazyme, Beijing, China). CDNA was subjected to real-time qPCR (RT-qPCR) analysis using Ultra SYBR One-Step qPCR Kit (CWBIO). Primer sequences are listed in Supplementary Table 1.
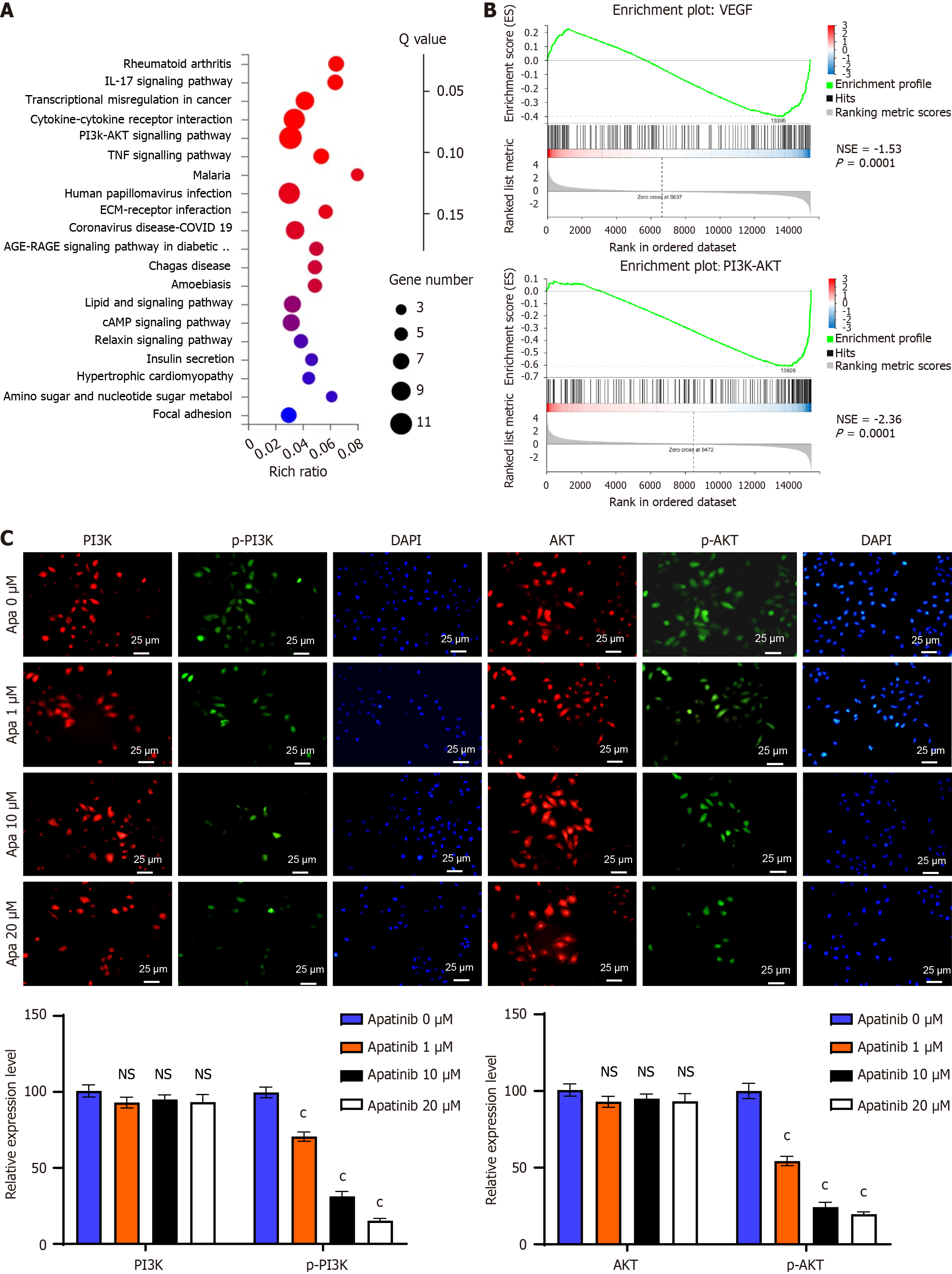
Fluorescent antibody staining was utilized to assess the presence and distribution of proteins within the phosphatidylinositol 3-kinase (PI3K)/AKT signaling cascade. HUVEC cells were cultured on conventional glass slides and exposed to varying doses of apatinib (0 μM, 1 μM, 10 μM, 20 μM) for a period of 24 hours. Following cell fixation with absolute ethyl alcohol for 10 minutes and a single PBS wash, specific antibodies were used for incubation. The primary antibodies employed included anti-PI3 kinase p85 (Invitrogen, catalog No. MA5-32917), anti-phospho-PI3K p85 (Invitrogen, catalog No. PA5-104853), anti-AKT1 (Invitrogen, catalog No. 44-609G), and anti-phospho-AKT S473 (Abcam, catalog No. ab81283), with a dilution of 1:200. These were incubated at room temperature for 1 hour or overnight at 4 °C. Subsequently, goat anti-rabbit Immunoglobulin G H&L (horseradish peroxidase) secondary antibody (Abcam, catalogue No. ab205718) at a dilution of 1:500 was applied for 30 minutes at room temperature for staining. Finally, protein expression levels and localization were analysed by observing and capturing images with an Olympus microscope. The experiment was independently repeated on three separate occasions.
According to the experimental protocol, cellular proteins were extracted using Nonidet P-40 (NP40) buffer (Meilun Bio, Dalian, China). Ricinus communis agglutinin (RCA)-I/II lectin, from Vector Laboratories, United States, is a lectin that specifically binds to galactose and N-acetylglucosamine in glycoproteins. Next, RCA-I/II lectin was mixed with protein A/G agarose beads and cell lysate to form a complex. After incubating the complex overnight at 4 °C, the beads were washed five times with NP40 buffer and centrifuged at 15000 rpm. Finally, 2 × concentration of sodium-dodecyl sulfate solution was added and the complex was analyzed by Western blot.
According to the experimental protocol, cellular proteins were extracted by using NP40 buffer (Meilun Bio, Dalian, China). RCA-I/II lectin was from Vector Laboratories, United States, and is a lectin that specifically binds to galactose and N-acetylglucosamine present in glycoproteins. Next, RCA-I/II lectin was mixed with protein A/G agarose beads and cell lysate to form a complex. The complex was incubated overnight at 4 °C, after which the beads were washed five times with NP40 buffer and separated by centrifugation at 15000 rpm. Finally, for Western blot analysis, two times the concentration of sodium-dodecyl sulfate solution was added to the samples.
The levels of lactate, pyruvate, ATP, and glucose were assessed using assay kits from Jiancheng Bioengineering Institute for lactate and pyruvate, Beyotime Biotechnology for ATP, and Elabscience for glucose uptake, all in accordance with the specific protocols provided by the manufacturers. Each experiment was conducted three times to ensure accuracy.
A suitable quantity of tissue samples was collected, rinsed with pre-chilled PBS (0.02 mol/L, pH 7.0-7.2) to eliminate blood, and then weighed for subsequent use. Larger tissue pieces were sectioned before homogenization. The tissue sections were placed into a glass homogenizer, and 5-10 mL of pre-chilled PBS was added (a tissue-to-PBS ratio of 1:5 is advised, meaning 1 g of tissue should be mixed with 5 mL of PBS). The grinding process was conducted on ice. The resulting homogenate could undergo further processing through ultrasonic disruption. The concentration of alpha-fetoprotein (AFP) was measured using a mouse AFP enzyme linked immunosorbent assay (ELISA) kit (BY-M01542, Baiyibio), while the CD44 concentration was assessed with a mouse CD44 ELISA kit (BY-M02667, Baiyibio). All ELISA procedures followed the manufacturer's guidelines, and each experiment was replicated three times.
The nude mice utilized in this research were procured from the Shanghai Laboratory Animal Center (Shanghai, China) and housed under sterile conditions at Zhejiang University’s animal research facility. This investigation strictly followed all established institutional and national protocols governing the ethical use of laboratory animals, with approval granted by the Medical Ethics Committee of Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University (No. SRRSH202302100). Huh7 cells, prepared at a concentration of 1 × 105, were resuspended in 100 μL of PBS and subsequently injected into the nude mice. Upon reaching a tumor volume between 150-200 mm3, the experimental animals were randomly allocated into four treatment groups (n = 5 per group), receiving daily oral doses of either 100 mg/kg/day apatinib or 1,3-dicaffeoylquinic acid for a 21-day period. Tumor progression and animal body mass were assessed at 72-hour intervals. The calculation of tumor volume employed the standard formula: Volume = (length × width2)/2, where length and width correspond to the maximum and minimum tumor diameters, respectively. Following the completion of treatment protocols, animals were humanely euthanized after 24 hours, with subsequent tumor excision and photographic documentation.
In the present study, SPSS and GraphPad Prism 10 were used for statistical analysis. All data are presented as mean ± SD. Comparisons between two groups were analyzed by unpaired Student’s t-test, and multiple comparisons among groups were made using analysis of variance. The P value was used to determine the significance of the difference, and when it was less than 0.05, the difference was considered to be statistically significant.
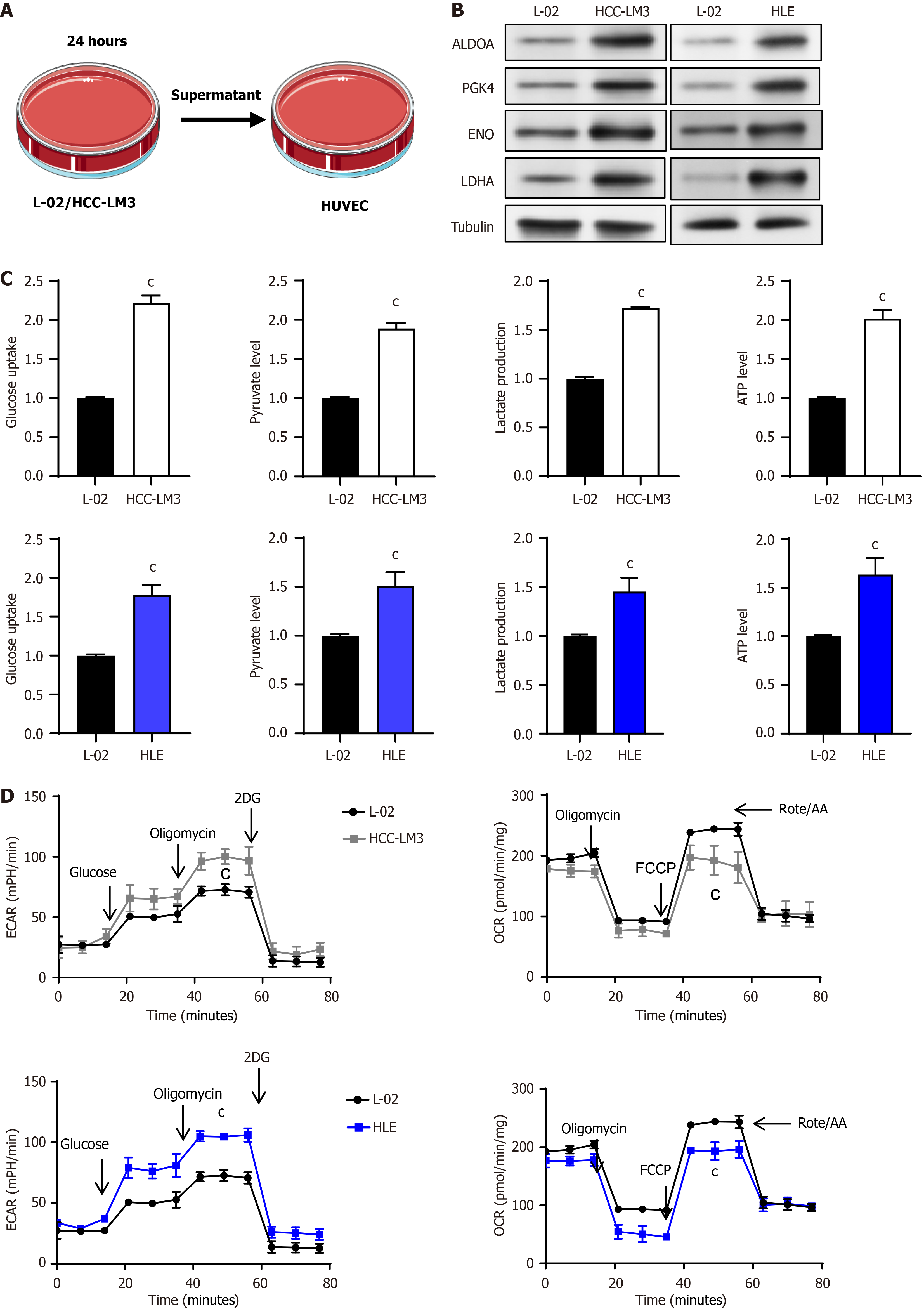
Normal liver cells and liver cancer cells were cultured for 24 hours, and the VECs were cultured with the supernatant of these two cells respectively (Figure 1A), and their effects on VECs were observed. Western blot results showed that VECs HUVEC, cultured with the conditioned medium from liver cancer cells, exhibited enhanced expression of glycolytic-related molecules aldolase A, phosphoglycerate kinase, enolase, and lactate dehydrogenase A (LDHA) (Figure 1B). Glycolysis involves the degradation of glucose in the cytoplasm into pyruvate, where a single glucose molecule yields two pyruvate molecules and generates two ATP molecules. We quantified the utilization of glucose and the formation of pyruvate, lactate, and ATP throughout this metabolic pathway. The results demonstrated that following treatment with the conditioned medium from liver cancer cells, HUVEC exhibited a heightened glucose consumption and elevated levels of pyruvate, lactate, and ATP in comparison to treatment with the conditioned medium from normal liver cells (Figure 1C). Utilising a Seahorse instrument, extracellular acidification rate (ECAR) and the oxygen consumption rate (OCR) of these two distinct cell groups were subsequently measured. The results demonstrated that VECs treated with the conditioned medium from HCCLM3 or HLE cells exhibited significantly higher ECAR curves in comparison to those treated with the conditioned medium from L-02 normal liver cells (Figure 1D). With regard to OCR, following the addition of the oxidative phosphorylation uncoupler carbonylcyanide-p-trifluoromethoxyphenylhydrazone, VECs treated with conditioned medium from HCCLM3 or HLE cells exhibited significantly lower OCR curves in comparison to those treated with conditioned medium from L-02 normal liver cells. In conclusion, our findings demonstrate that co-culturing VECs with liver tumor cells enhances their glycolytic activity.
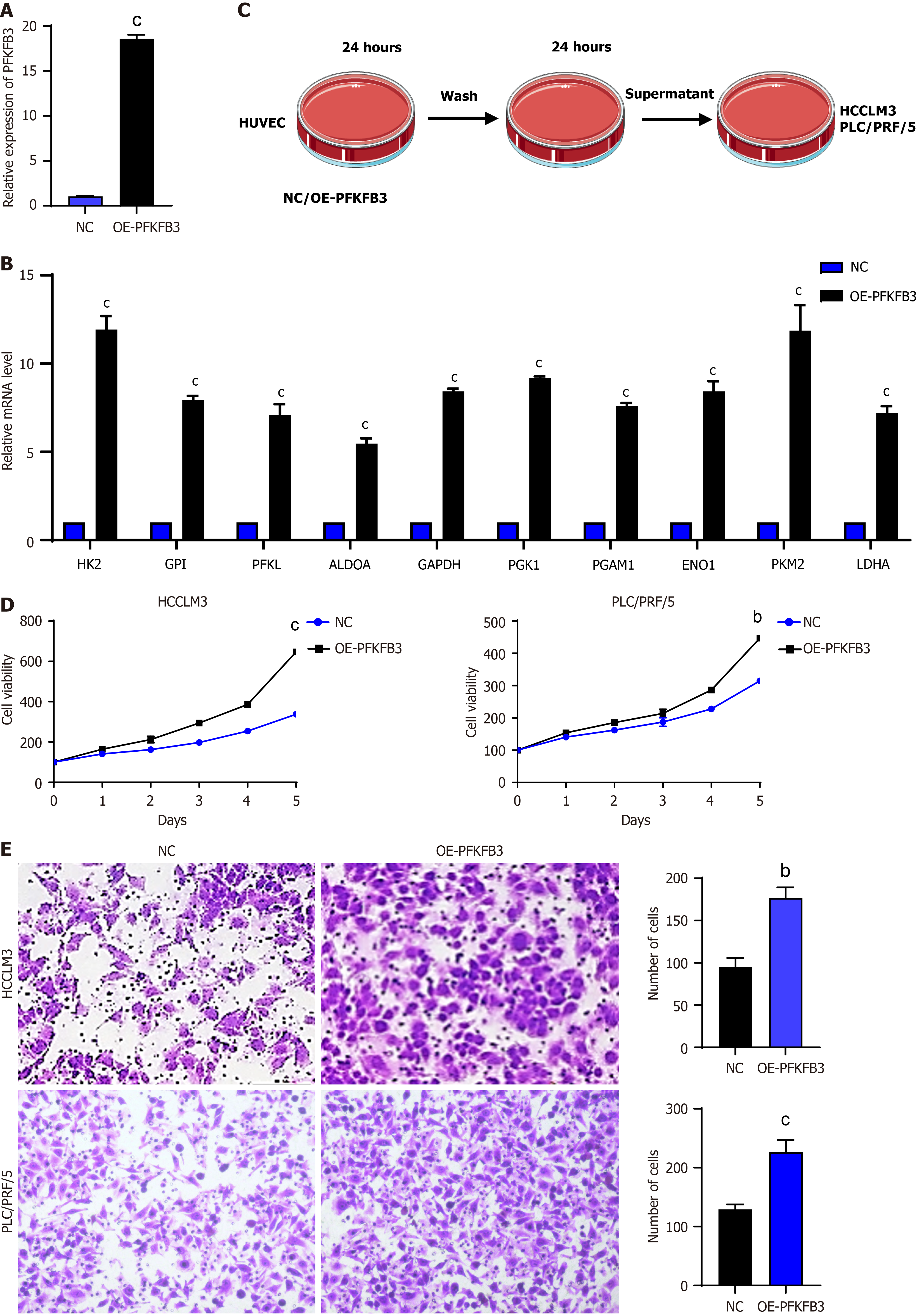
As previously stated, glycolysis is the predominant metabolic type of VECs. The present study investigates the impact of high glycolysis in VECs on tumour cells. In order to investigate this, we first introduced PFKFB3 overexpressing plasmids into HUVEC to construct high glycolysis VECs (Figure 2A). RT-qPCR analysis revealed a substantial upregulation in the expression levels of key glycolytic enzymes in VECs following the introduction of PFKFB3, suggesting elevated glycolytic activity (Figure 2B). Subsequently, normal VECs or high glycolysis VECs were cultured overnight, after which the conditioned media were separately used to culture liver cancer cells to establish co-culture systems with two different cell types (Figure 2C). The Cell Counting Kit-8 assay results demonstrated that co-culturing with high glycolysis VECs enhanced the proliferation capacity of two liver cancer cell lines, HCCLM3 and PLC/PRF/5 (Figure 2D). Furthermore, the Transwell assay results demonstrated that co-culturing with high glycolysis VECs augmented the migratory capacity of HCCLM3 and PLC/PRF/5 liver cancer cells (Figure 2E). In conclusion, our findings indicate that co-culturing with high glycolysis VECs enhances the proliferation and migratory abilities of liver cancer cells.
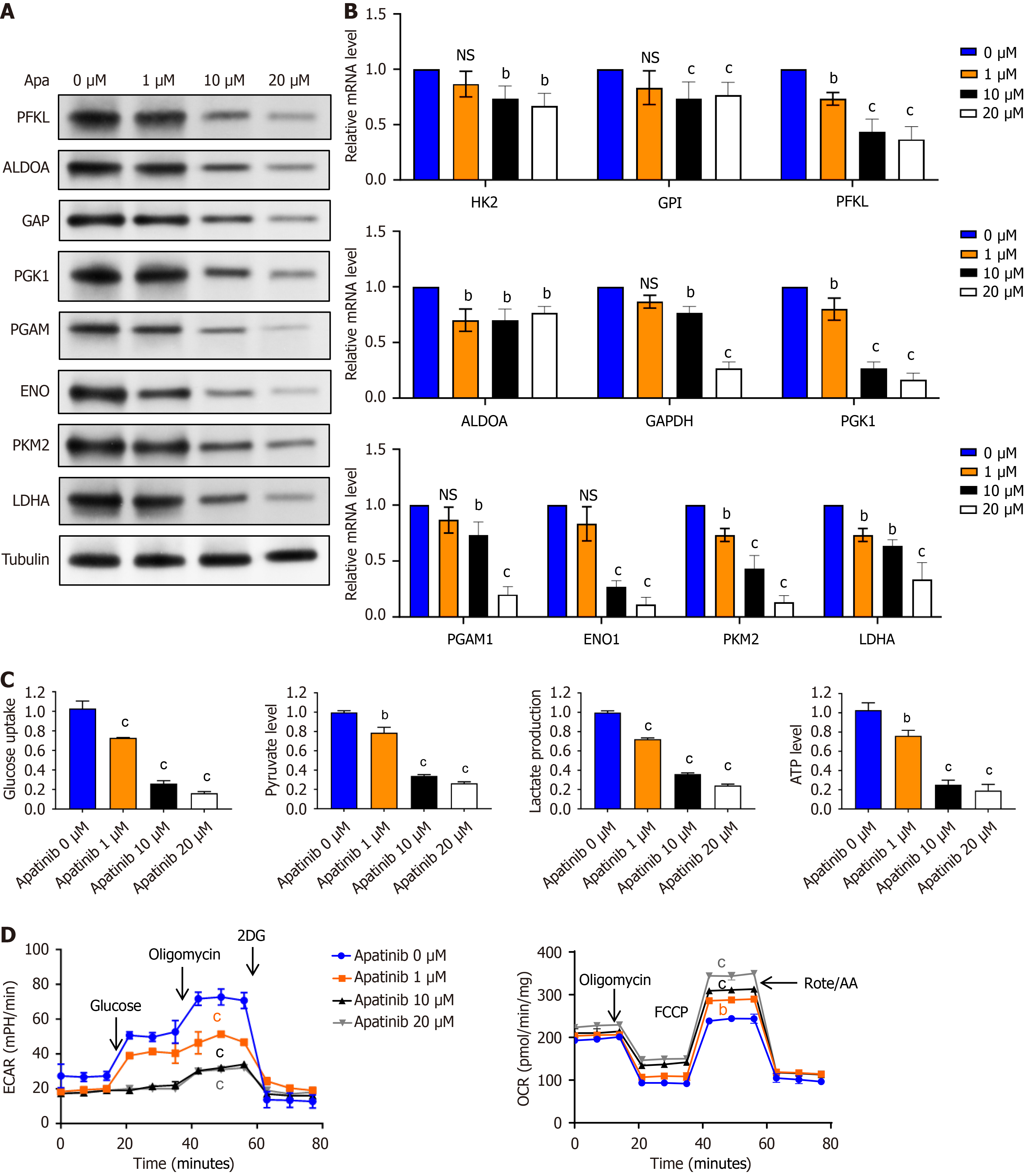
Apatinib, as a small molecular tyrosine kinase inhibitor, shows potency in liver cancer therapy targeting the receptor of VEGFR2 that is important for tumor angiogenesis. This kind of therapy influences VECs and alters their metabolic characteristics. In other words, apatinib changes energy metabolism by shifting the expression of the main catalytic enzymes participating in glycolysis and oxidative phosphorylation. The following enzymes involved in the process of glycolysis-phosphofructokinase liver type, aldolase A, glyceraldehyde-3-phosphate dehydrogenase, phosphoglycerate kinase 1, phosphoglycerate mutase, enolase, pyruvate kinase M2, and LDHA-are significantly changed under treatment (Figure 3A). It was shown that the effects of apatinib on these metabolic pathways occur in a dose-dependent manner. These changes are related to modifications within the basic molecular mechanisms shown by RT-qPCR (Figure 3B), and reflecting the changes in mRNA of key enzymes of glycolysis. These findings underline the fact that apatinib could be of potential interest not only in targeted therapy but also in metabolic reprogramming in liver cancer cells.
The glycolytic pathway initiates with the uptake of a single glucose molecule, subsequently generating two molecules each of pyruvate, lactate, and ATP. This investigation quantitatively assessed the glucose utilization rates and the formation of lactate, pyruvate, and ATP through glycolysis. Experimental data demonstrated a concentration-dependent effect of apatinib, showing reduced glucose consumption and diminished production of lactate, pyruvate, and ATP with increasing apatinib concentrations, as illustrated in Figure 3C. Furthermore, ECAR analysis revealed a decline in ECAR curves with increasing apatinib concentrations following oligomycin addition, suggesting the inhibition of mitochondrial oxidative phosphorylation and a compensatory increase in glycolytic rate to maintain energy production (Figure 3D). Conversely, OCR analysis demonstrated an enhancement in OCR curves with the incorporation of the oxidative phosphorylation uncoupler carbonylcyanide-p-trifluoromethoxyphenylhydrazone, resulting in a swift escalation in oxygen consumption. Treatment with 10 μM and 20 μM apatinib resulted in a significant elevation of the OCR curves of VECs (Figure 3D). Collectively, these results demonstrate that apatinib can inhibit glycolysis in VECs.
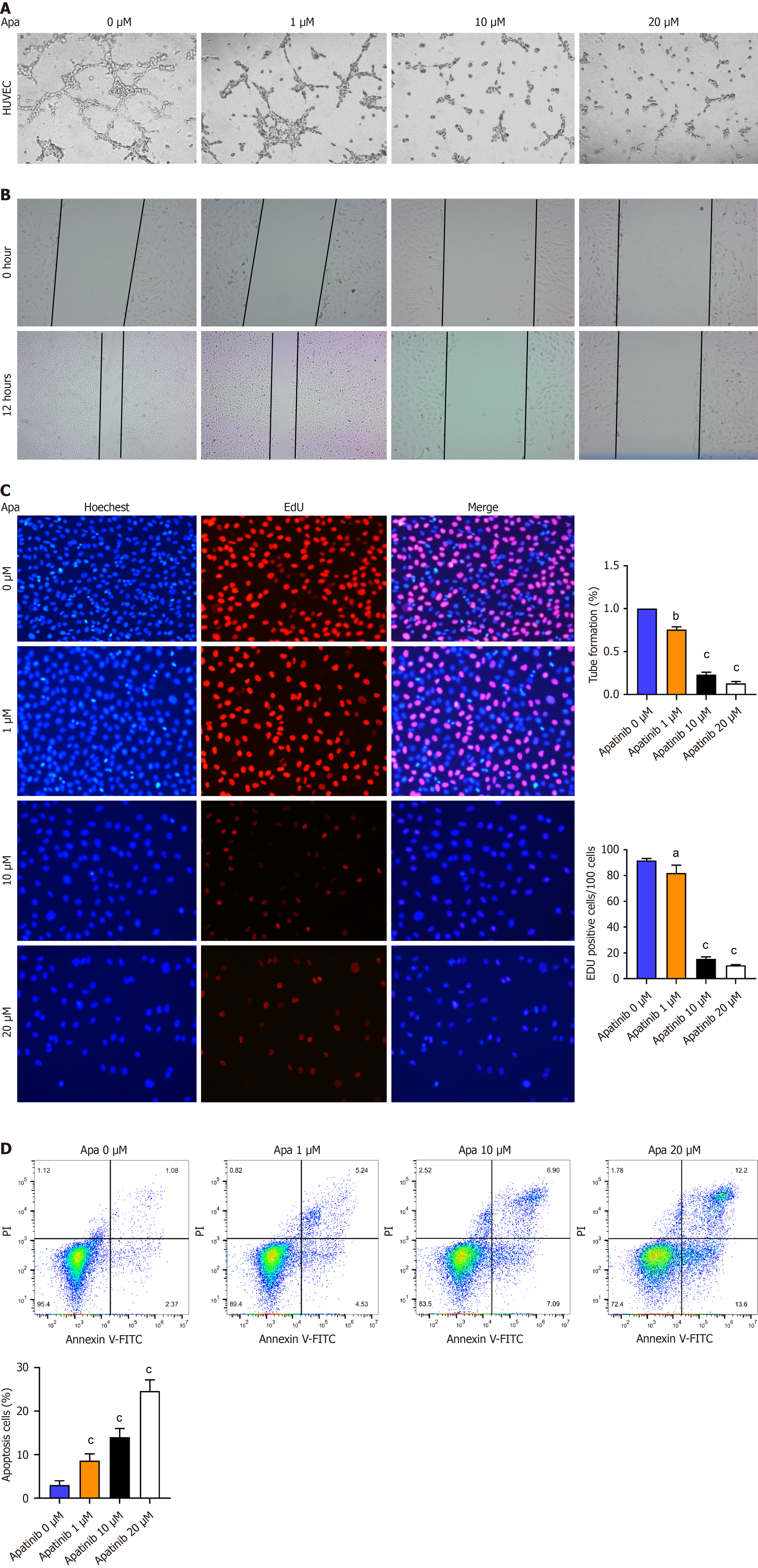
During the research related to the effect of apatinib on VECs, some of the important processes which were closely watched included glycolysis, proliferation, migration, and tube formation. Apatinib was noted to affect the energy supply of VECs, especially concerning their glycolytic activity, an important part of cellular metabolism. EdU incorporation assays detected the proliferating cells that, during the assay, had incorporated the EdU-DNA complex in their nucleus. The analysis of the cell cycle was made with the goal of highlighting how apatinib could modulate the cell growth and division. Further VEC behavior research has shown that apatinib induces changes in the migratory capacity assayed by scratch test but mostly impairs tube formation (Figure 4A), a crucial step in the angiogenesis process. It also detected the apoptosis rate with the Annexin V-FITC/PI double-staining kit and by flow cytometry in order to understand how apatinib mediates an apoptosis event via a dose-dependent process. Three dosages for treatment were prepared: Low dose (1 μM), medium dose (10 μM), and high dose (20 μM), reflecting different trends of apoptosis and cell survival (Figure 4D). Of note, Annexin V and PI staining differentiated apoptotic and necrotic cells, respectively, thus clearly showing the action of the compound at different concentrations. These assays, including EdU incorporation (Figure 4C), scratch assays (Figure 4B), and flow cytometry, have been combined in order to comprehensively analyze the role of apatinib in cell migration, proliferation, and apoptosis of HUVEC cells, contributing to the understanding of its therapeutic potential in targeting angiogenesis. In conclusion, it can be concluded that apatinib inhibits the formation, proliferation and migration of VECs, and promotes their apoptosis.
As previously stated, apatinib exerts a negative regulatory effect on the glycolysis of VECs and demonstrates the capacity to impede circularization, proliferation, and migration. The molecules that drive these phenotypes remain to be elucidated. To address this question, VECs were collected, with some samples being untreated and others being treated with 10 μM apatinib. MRNA transcriptome sequencing was then performed. Utilising a filtering criterion of log fold change absolute value > 5, 16 differentially expressed genes were identified (Supplementary Figure 2). Detailed se
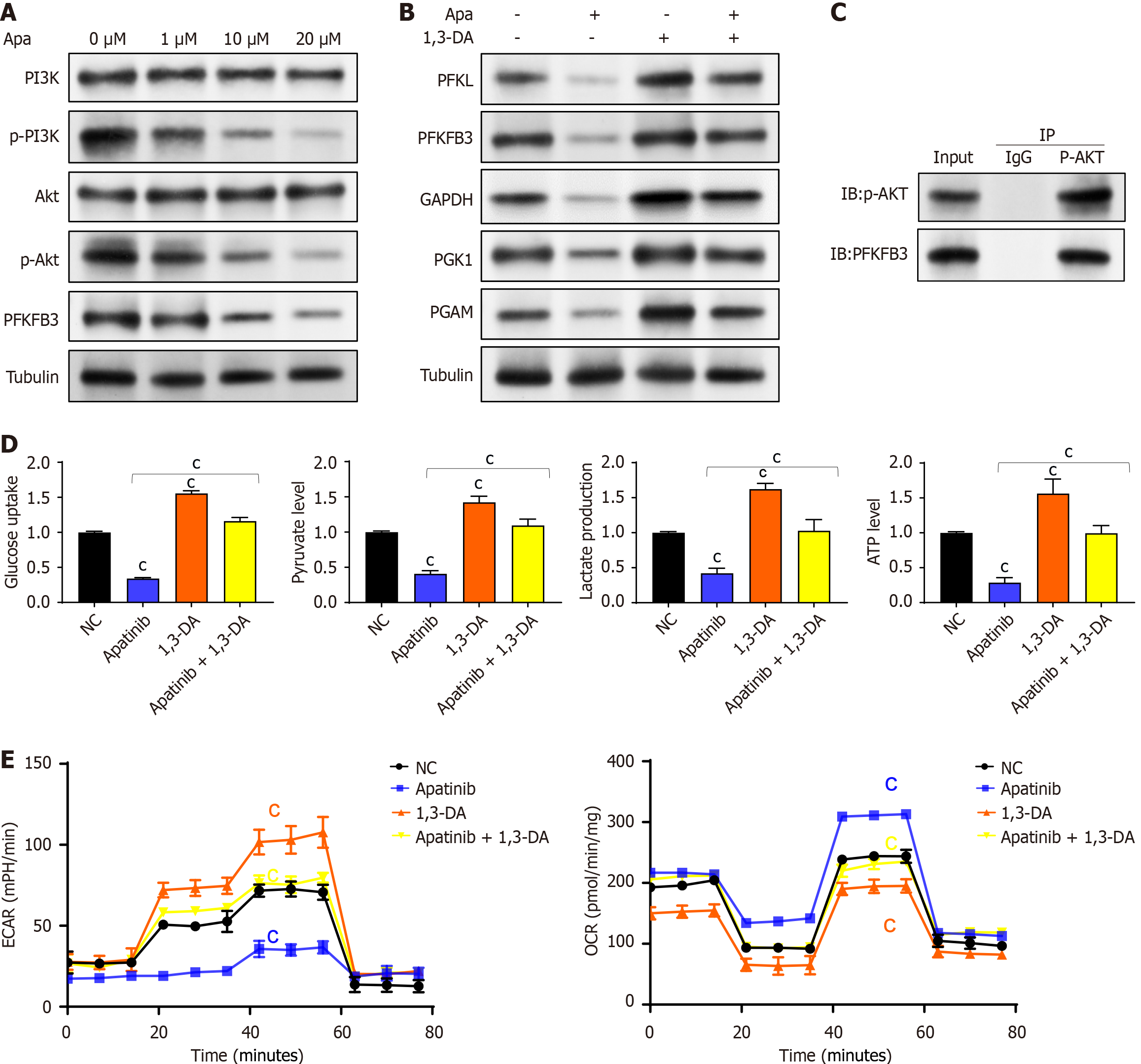
To validate the hypothesis derived from bioinformatics analysis, VECs were treated with varying concentrations of apatinib, and the expression of critical components in the PI3K/AKT pathway was assessed. The results indicated that high concentrations of apatinib did not markedly change the expression levels of PI3K and AKT. Nonetheless, apatinib was found to inhibit the phosphorylation of the PI3K/AKT pathway and PFKFB3 (Figure 6A). To explore the role of the PI3K/AKT pathway in mediating the effects of apatinib on endothelial cell glycolysis, further experiments were performed using 1,3-dicaffeoylquinic acid as a PI3K/AKT pathway activator. It was observed that treating endothelial cells with 1,3-dicaffeoylquinic acid increased the expression of key glycolysis enzymes. Moreover, the combined treatment of apatinib and 1,3-dicaffeoylquinic acid led to a notable increase in the expression of glycolysis-related enzymes (Figure 6B). Coimmunoprecipitation experiments revealed that phosphorylated AKT interacts with PFKFB3 (Figure 6C). Therefore, apatinib is concluded to modulate vascular endothelial cell glycolysis via the PI3K/AKT/PFKFB3 pathway. To further confirm this conclusion, experiments were designed to analyze glycolysis products and processes. The findings showed that the impacts of apatinib on glucose uptake, pyruvate production, lactate generation, and ATP synthesis in endothelial cell glycolysis could all be counteracted by 1,3-dicaffeoylquinic acid (Figure 6D). A comparable outcome was noted in the reversal of apatinib’s effects on the extracellular acidification rate and cellular oxygen consumption rate in endothelial cells, with these alterations also being reversed by 1,3-dicaffeoylquinic acid (Figure 6E). Thus, it can be inferred that apatinib inhibits endothelial cell glycolysis by suppressing the PI3K/AKT pathway and PFKFB3.
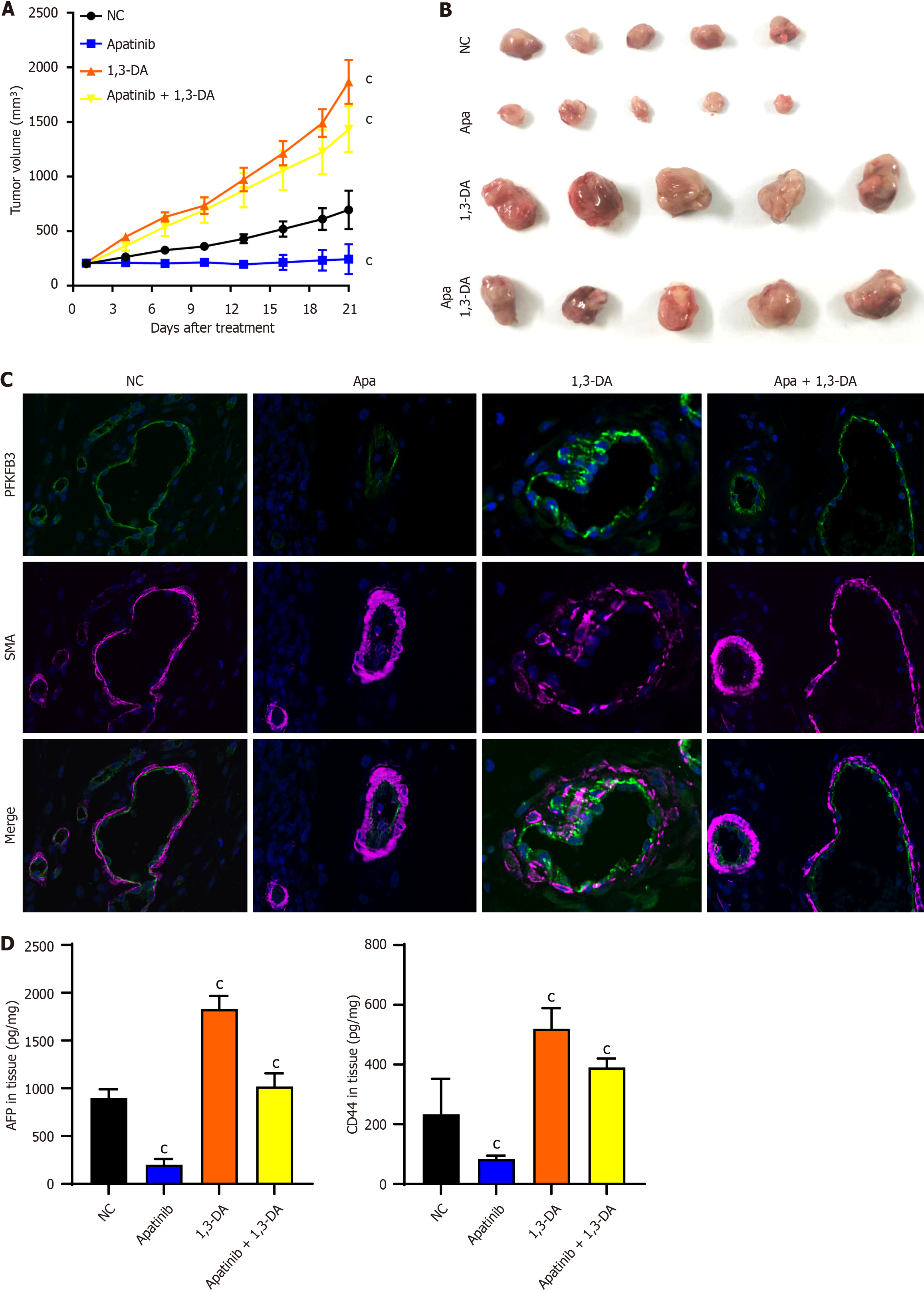
The impact of apatinib on VECs has been validated through cellular studies, and it is anticipated that similar outcomes will be observed in animal trials. Considering the presence of PFKFB3 molecules in HCC cell lines, the aim is to emphasize the influence of this medication on VECs. For this purpose, HCC cell lines with minimal PFKFB3 expression were chosen[19]. A week prior to the initiation of the experiment, the HCC cell line Huh7 was injected subcutaneously into nude mice. The mice were subsequently divided into four treatment groups at random. The treatment protocol included the oral delivery of 100 mg/kg of apatinib and 1,3-diacetylquinic acid, given every alternate day for a total of 21 days (Figure 7A). The results obtained demonstrated that apatinib exerts a therapeutic effect on liver cancer in terms of tumour size, while PI3K/AKT activators promote tumour proliferation (Figure 7B). The tumor samples from each group were preserved, sliced, and then stained with fluorescent dyes. The statistical evaluation revealed notable variations in PFKFB3 expression among the endothelial cells from different tumor groups (Figure 7C). Apatinib can effectively inhibit AFP and CD44 in tumor tissue, and 1,3-diacetylquinic acid can unblock this inhibition (Figure 7D). Apatinib has been shown to inhibit the expression of PFKFB3, a key glycolytic molecule, in tumour vessels, while PI3K/AKT activator 1,3-dicaffeoylquinic acid has been demonstrated to promote the expression of PFKFB3 in tumour vessels. Furthermore, the combination of apatinib and 1,3-dicaffeoylquinic acid resulted in a consistent expression of PFKFB3 in tumor blood vessels. As previously mentioned, apatinib functions by inhibiting the phosphorylation activation of PI3K/AKT. Collectively, these observations indicate that apatinib modulates endothelial cell glycolysis in mice by regulating PFKFB3, a pivotal glycolysis molecule, through the PI3K/AKT/PFKFB3 pathway.
The present research demonstrated that apatinib markedly reduced the glycolytic function of VECs by blocking the PI3K/AKT/PFKFB3 signaling pathway. Consequently, this inhibition resulted in decreased VEC proliferation, tube formation, and migration, while also enhancing apoptosis. The endothelial cells in tumor blood vessels exhibit high glycolytic activity and demonstrate an enhanced glycolytic phenotype compared to healthy endothelial cells. This is evidenced by the heightened presence of the glucose transporter glucose transporter 1 and the glycolytic activator PFKFB3[20,21]. Additionally, tumor endothelial cells show a significant upregulation of the pentose phosphate pathway and the serine biosynthesis pathway, both of which play crucial roles in nucleotide production and biomass generation. It has been demonstrated that certain signals within the TME, including hypoxia, pro-inflammatory cytokines, and hormonal signals, can upregulate PFKFB3[22]. These findings have significant implications for cancer treatment. Indeed, the inhibition of PFKFB3 function in tumor endothelial cells has been shown to reduce their proliferation[23], reduce endothelial cell permeability, tighten the vascular barrier, and restore perfusion, all of which are signs of tumor vessel normalization. It is imperative to note that the objective of this therapeutic strategy is to reduce glycolysis to levels observed in normal endothelial cells, as opposed to reducing it excessively or completely abolishing glycolytic flux. The latter approach would lead to endothelial cell death and the disruption of tumor vasculature, thereby facilitating cancer cell evasion and metastasis[24].
Our research also uncovered that endothelial cells with high glycolytic activity contribute to tumor malignancy. Despite the fact that a comprehensive understanding of tumor endothelial cell metabolism is still in its infancy, it is believed to mirror the metabolic processes of highly activated endothelial cells. This similarity is due to the significant metabolic demands during the transition from a quiescent state to proliferation and migration, which is induced by tumors. Typically, stationary endothelial cells rely on high glycolysis rates to generate sufficient energy for essential functions, such as maintaining the tight barrier of specific vascular beds. Tumor endothelial cells, however, upregulate PFKFB3 and further enhance glycolysis[18]. Lactate, generated through glycolysis and used in energy metabolism, plays a crucial role in the metabolic processes of cancer cells and the TME. These cells rely on monocarboxylate transporter protein 1 to absorb lactate, which is subsequently transformed into pyruvate by intracellular LDHB, thereby fueling their proliferation and metabolic activities[25]. Glycolytic endothelial cells express metabolic-promoting molecules like PFKFB3[26]. Exosomes, acting as intercellular communication vessels, can facilitate interactions between tumor cells and endothelial cells[27,28]. These vesicles play a crucial role in tumor angiogenesis. HCC cells exhibit the Warburg effect[29,30], relying on anaerobic glycolysis for energy and the synthesis of necessary biomolecules[31-35].
Our findings offer new therapeutic possibilities for HCC. HCC cells demonstrate the Warburg effect[29,30], using anaerobic glycolysis to produce energy and synthesize essential biomolecules[31-35]. Some HCC patients show elevated PFKFB3 expression. This research highlights the promising application of PFKFB3 inhibitors as a substitute for apatinib in targeted treatments. Apatinib, a robust and specific small molecule tyrosine kinase inhibitor, targets and hinders VEGFR-2. This inhibition disrupts the VEGF signaling pathway, thereby significantly reducing the growth, movement, and tubule development of human umbilical VECs[11]. In line with these observations, our study confirms the anti-proliferative, anti-migratory, and anti-motility effects of apatinib on endothelial cells. Although VEGFR is expressed at low levels on tumor cells, evidence indicates that apatinib affects both endothelial and tumor cells. Research findings indicate that apatinib exerts inhibitory effects on glycolysis in ovarian cancer cells through downregulation of the VEGFR2/AKT1/SRY-box transcription factor 5/glucose transporter 4 signaling cascade[36]. Our study shows that apatinib has minimal impact on the proliferation of liver cancer cell lines, likely due to the generally low VEGFR2 expression in these cells (Supplementary Figure 3). However, additional research indicates that apatinib can trigger G2/M phase cell cycle arrest and enhance apoptosis in HCC cells[37]. Furthermore, it has been shown that apatinib can hinder the growth and migration of liver cancer cells by modulating the PI3K/AKT pathway[16]. Our study highlights the PI3K/AKT pathway as the primary target of apatinib, which reduces glycolytic activity in HUVECs by blocking the PI3K pathway. Consequently, targeting the PI3K/AKT pathway could present a novel approach for treating liver cancer.
In an initial study with 31 patients suffering from advanced HCC, a daily dose of 500 mg of apatinib led to a response rate of 32.26% and a disease control rate of 80.65%. The median time until tumor progression was 4.8 months. Furthermore, the survival rates at 6 and 12 months were 73.8% and 55.4%, respectively. The most frequent adverse reactions included grade 3 thrombocytopenia, which affected 6.45% of patients, and hypertension, observed in 48.39% of cases, representing the main hematologic and non-hematologic side effects[38]. In a separate retrospective analysis, 25 individuals with inoperable or relapsed HCC were given apatinib until their condition worsened or they experienced intolerable toxicity. The median overall survival and progression-free survival durations were 13 months and 5 months, respectively. Frequently reported adverse events comprised hypertension, liver enzyme elevation, proteinuria, and hand-foot skin reaction[39]. A phase 3 clinical study involving 400 subjects, conducted under randomized, double-blind, and placebo-controlled conditions, revealed that apatinib significantly enhanced overall survival rates in patients with advanced HCC when used as a second-line or subsequent therapy. The median survival duration was 8.7 months [95% confidence interval (CI): 7.5-9.8] for the apatinib group vs 6.8 months (95%CI: 5.7-9.1) for placebo recipients, with a hazard ratio of 0.785 (95%CI: 0.617-0.998, P = 0.048). Among treatment-emergent adverse events of grade 3 or 4 severity, hypertension was the most prevalent, occurring in 71 patients[40]. Overall, apatinib is a highly effective treatment for liver cancer, with the most common adverse reactions being secondary hypertension, proteinuria, and hand-foot syndrome, which may be associated with intracellular and extracellular calcium ion imbalance. Our study focuses on the impact of apatinib on the glycolytic activity of endothelial cells, while liver cancer itself is a tumor characterized by the Warburg effect. In the future, metabolic inhibitors may replace VEGFR2 tyrosine kinase inhibitors as a therapeutic approach.
This study has several limitations that warrant attention. Apatinib, commonly utilized in HCC treatment, will be further investigated through expanded clinical data collection to assess its effects on endothelial cell glycolysis. The results demonstrate that apatinib inhibits glycolysis in VECs through the PI3K/AKT/PFKFB3 signaling pathway. Considering the overexpression of PFKFB3 in some liver cancer patients, our subsequent research will focus on comparing the therapeutic outcomes and adverse reactions between apatinib and PFKFB3 inhibitors in liver cancer management.
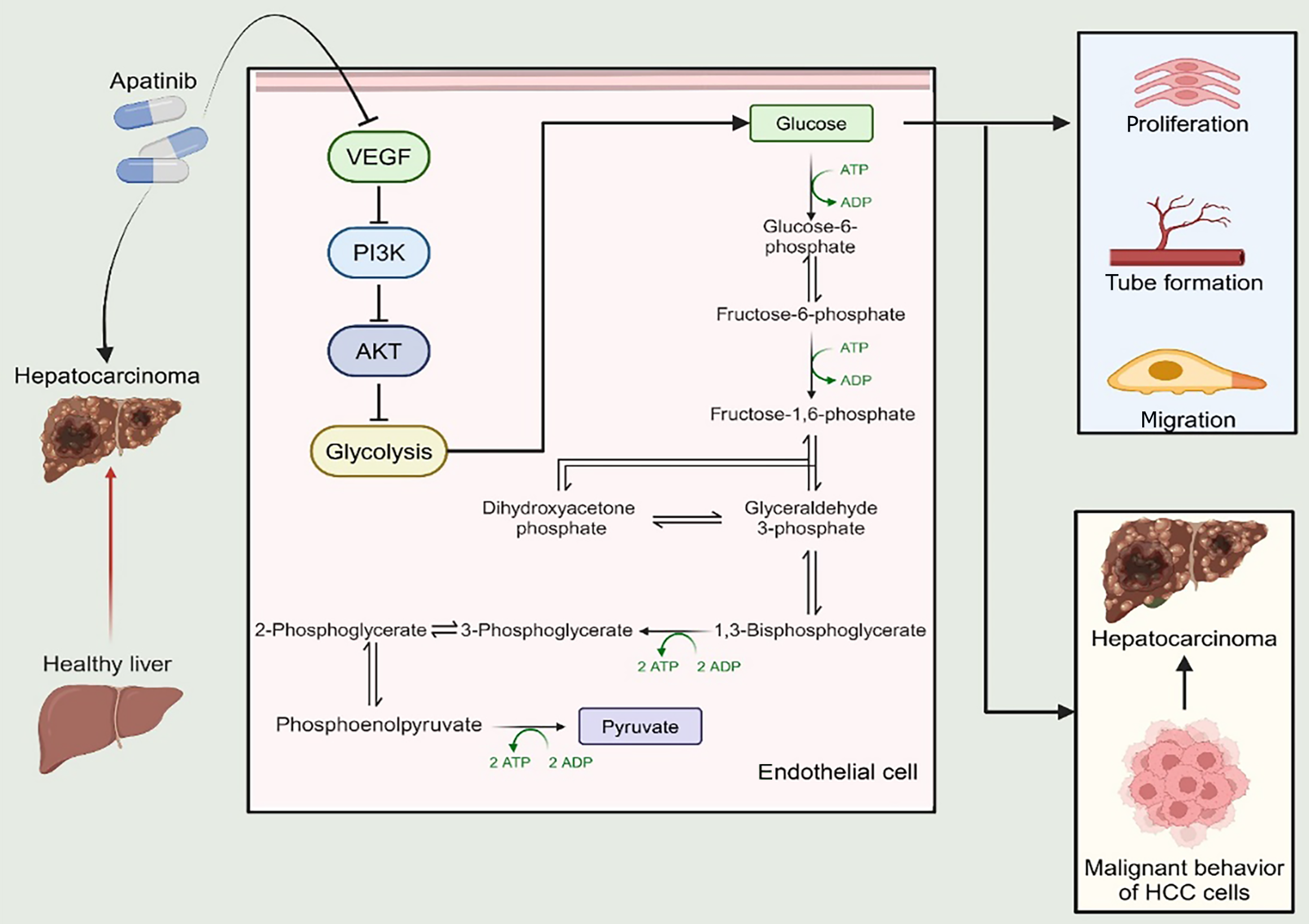
In summary, apatinib demonstrates its anti-cancer properties by suppressing the glycolytic activity in VECs, primarily by acting on the PI3K/AKT/PFKFB3 signaling axis. This interference hinders the formation of tubular structures, curtails cell growth and movement, and induces programmed cell death (Figure 8). The insights from this research could provide a novel and potentially valuable therapeutic target for addressing HCC.
The authors extend their appreciation to Dr. Liu Hao for her assistance in creating the visual elements for this manuscript.
| 1. | Rich NE. Changing Epidemiology of Hepatocellular Carcinoma Within the United States and Worldwide. Surg Oncol Clin N Am. 2024;33:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 26] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 2. | Yang C, Zhang H, Zhang L, Zhu AX, Bernards R, Qin W, Wang C. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2023;20:203-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 374] [Reference Citation Analysis (0)] |
| 3. | Kaur G, Roy B. Decoding Tumor Angiogenesis for Therapeutic Advancements: Mechanistic Insights. Biomedicines. 2024;12:827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 4. | Yan T, Shi J. Angiogenesis and EMT regulators in the tumor microenvironment in lung cancer and immunotherapy. Front Immunol. 2024;15:1509195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 5. | Eelen G, Treps L, Li X, Carmeliet P. Basic and Therapeutic Aspects of Angiogenesis Updated. Circ Res. 2020;127:310-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 332] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 6. | Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4014] [Cited by in RCA: 4088] [Article Influence: 120.2] [Reference Citation Analysis (0)] |
| 7. | Lawler J. Counter regulation of tumor angiogenesis by vascular endothelial growth factor and thrombospondin-1. Semin Cancer Biol. 2022;86:126-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 8. | Lopes-Coelho F, Martins F, Pereira SA, Serpa J. Anti-Angiogenic Therapy: Current Challenges and Future Perspectives. Int J Mol Sci. 2021;22:3765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 235] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 9. | Folkman J. Successful treatment of an angiogenic disease. N Engl J Med. 1989;320:1211-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 127] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Scott LJ. Apatinib: A Review in Advanced Gastric Cancer and Other Advanced Cancers. Drugs. 2018;78:747-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 153] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 11. | Tian S, Quan H, Xie C, Guo H, Lü F, Xu Y, Li J, Lou L. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011;102:1374-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 428] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 12. | Xie C, Zhou X, Liang C, Li X, Ge M, Chen Y, Yin J, Zhu J, Zhong C. Apatinib triggers autophagic and apoptotic cell death via VEGFR2/STAT3/PD-L1 and ROS/Nrf2/p62 signaling in lung cancer. J Exp Clin Cancer Res. 2021;40:266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 128] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 13. | Liu M, Li H. Apatinib has anti-tumor effects and induces autophagy in lung cancer cells with high expression of VEGFR-2. Iran J Basic Med Sci. 2024;27:1370-1379. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Yang X, Li W, Han X, Wang J, Dai J, Ye X, Meng M. Apatinib weakens proliferation, migration, invasion, and angiogenesis of thyroid cancer cells through downregulating pyruvate kinase M2. Sci Rep. 2024;14:879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 15. | Liao J, Jin H, Li S, Xu L, Peng Z, Wei G, Long J, Guo Y, Kuang M, Zhou Q, Peng S. Apatinib potentiates irradiation effect via suppressing PI3K/AKT signaling pathway in hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38:454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | Song J, Guan Z, Song C, Li M, Gao Z, Zhao Y. Apatinib suppresses the migration, invasion and angiogenesis of hepatocellular carcinoma cells by blocking VEGF and PI3K/AKT signaling pathways. Mol Med Rep. 2021;23:429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Rohlenova K, Veys K, Miranda-Santos I, De Bock K, Carmeliet P. Endothelial Cell Metabolism in Health and Disease. Trends Cell Biol. 2018;28:224-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 222] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 18. | De Bock K, Georgiadou M, Schoors S, Kuchnio A, Wong BW, Cantelmo AR, Quaegebeur A, Ghesquière B, Cauwenberghs S, Eelen G, Phng LK, Betz I, Tembuyser B, Brepoels K, Welti J, Geudens I, Segura I, Cruys B, Bifari F, Decimo I, Blanco R, Wyns S, Vangindertael J, Rocha S, Collins RT, Munck S, Daelemans D, Imamura H, Devlieger R, Rider M, Van Veldhoven PP, Schuit F, Bartrons R, Hofkens J, Fraisl P, Telang S, Deberardinis RJ, Schoonjans L, Vinckier S, Chesney J, Gerhardt H, Dewerchin M, Carmeliet P. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell. 2013;154:651-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 838] [Cited by in RCA: 1140] [Article Influence: 95.0] [Reference Citation Analysis (0)] |
| 19. | Shi WK, Zhu XD, Wang CH, Zhang YY, Cai H, Li XL, Cao MQ, Zhang SZ, Li KS, Sun HC. PFKFB3 blockade inhibits hepatocellular carcinoma growth by impairing DNA repair through AKT. Cell Death Dis. 2018;9:428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 20. | Cantelmo AR, Conradi LC, Brajic A, Goveia J, Kalucka J, Pircher A, Chaturvedi P, Hol J, Thienpont B, Teuwen LA, Schoors S, Boeckx B, Vriens J, Kuchnio A, Veys K, Cruys B, Finotto L, Treps L, Stav-Noraas TE, Bifari F, Stapor P, Decimo I, Kampen K, De Bock K, Haraldsen G, Schoonjans L, Rabelink T, Eelen G, Ghesquière B, Rehman J, Lambrechts D, Malik AB, Dewerchin M, Carmeliet P. Inhibition of the Glycolytic Activator PFKFB3 in Endothelium Induces Tumor Vessel Normalization, Impairs Metastasis, and Improves Chemotherapy. Cancer Cell. 2016;30:968-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 483] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 21. | Yeh WL, Lin CJ, Fu WM. Enhancement of glucose transporter expression of brain endothelial cells by vascular endothelial growth factor derived from glioma exposed to hypoxia. Mol Pharmacol. 2008;73:170-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 22. | Trenti A, Tedesco S, Boscaro C, Ferri N, Cignarella A, Trevisi L, Bolego C. The Glycolytic Enzyme PFKFB3 Is Involved in Estrogen-Mediated Angiogenesis via GPER1. J Pharmacol Exp Ther. 2017;361:398-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 23. | Schoors S, De Bock K, Cantelmo AR, Georgiadou M, Ghesquière B, Cauwenberghs S, Kuchnio A, Wong BW, Quaegebeur A, Goveia J, Bifari F, Wang X, Blanco R, Tembuyser B, Cornelissen I, Bouché A, Vinckier S, Diaz-Moralli S, Gerhardt H, Telang S, Cascante M, Chesney J, Dewerchin M, Carmeliet P. Partial and transient reduction of glycolysis by PFKFB3 blockade reduces pathological angiogenesis. Cell Metab. 2014;19:37-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 419] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 24. | Conradi LC, Brajic A, Cantelmo AR, Bouché A, Kalucka J, Pircher A, Brüning U, Teuwen LA, Vinckier S, Ghesquière B, Dewerchin M, Carmeliet P. Tumor vessel disintegration by maximum tolerable PFKFB3 blockade. Angiogenesis. 2017;20:599-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 25. | Wang X, Liu H, Ni Y, Shen P, Han X. Lactate shuttle: from substance exchange to regulatory mechanism. Hum Cell. 2022;35:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 26. | Feng Y, Zou R, Zhang X, Shen M, Chen X, Wang J, Niu W, Yuan Y, Yuan F. YAP promotes ocular neovascularization by modifying PFKFB3-driven endothelial glycolysis. Angiogenesis. 2021;24:489-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 27. | Lu Y, Han G, Zhang Y, Zhang L, Li Z, Wang Q, Chen Z, Wang X, Wu J. M2 macrophage-secreted exosomes promote metastasis and increase vascular permeability in hepatocellular carcinoma. Cell Commun Signal. 2023;21:299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 71] [Reference Citation Analysis (0)] |
| 28. | Huang XY, Huang ZL, Huang J, Xu B, Huang XY, Xu YH, Zhou J, Tang ZY. Exosomal circRNA-100338 promotes hepatocellular carcinoma metastasis via enhancing invasiveness and angiogenesis. J Exp Clin Cancer Res. 2020;39:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 299] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 29. | Zheng J, Yan X, Lu T, Song W, Li Y, Liang J, Zhang J, Cai J, Sui X, Xiao J, Chen H, Chen G, Zhang Q, Liu Y, Yang Y, Zheng K, Pan Z. CircFOXK2 promotes hepatocellular carcinoma progression and leads to a poor clinical prognosis via regulating the Warburg effect. J Exp Clin Cancer Res. 2023;42:63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 30. | Xu Y, Hao X, Ren Y, Xu Q, Liu X, Song S, Wang Y. Research progress of abnormal lactate metabolism and lactate modification in immunotherapy of hepatocellular carcinoma. Front Oncol. 2022;12:1063423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 31. | Xia P, Zhang H, Lu H, Xu K, Jiang X, Jiang Y, Gongye X, Chen Z, Liu J, Chen X, Ma W, Zhang Z, Yuan Y. METTL5 stabilizes c-Myc by facilitating USP5 translation to reprogram glucose metabolism and promote hepatocellular carcinoma progression. Cancer Commun (Lond). 2023;43:338-364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 81] [Reference Citation Analysis (0)] |
| 32. | Dong F, Li H, Liu L, Yao LL, Wang J, Xiang D, Ma J, Zhang G, Zhang S, Li J, Jiang SH, Hu X, Chen J, Bao Z. ACE2 negatively regulates the Warburg effect and suppresses hepatocellular carcinoma progression via reducing ROS-HIF1α activity. Int J Biol Sci. 2023;19:2613-2629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 33. | Ma WK, Voss DM, Scharner J, Costa ASH, Lin KT, Jeon HY, Wilkinson JE, Jackson M, Rigo F, Bennett CF, Krainer AR. ASO-Based PKM Splice-Switching Therapy Inhibits Hepatocellular Carcinoma Growth. Cancer Res. 2022;82:900-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 34. | Zuo Q, He J, Zhang S, Wang H, Jin G, Jin H, Cheng Z, Tao X, Yu C, Li B, Yang C, Wang S, Lv Y, Zhao F, Yao M, Cong W, Wang C, Qin W. PPARγ Coactivator-1α Suppresses Metastasis of Hepatocellular Carcinoma by Inhibiting Warburg Effect by PPARγ-Dependent WNT/β-Catenin/Pyruvate Dehydrogenase Kinase Isozyme 1 Axis. Hepatology. 2021;73:644-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 35. | Zhou Y, Lin F, Wan T, Chen A, Wang H, Jiang B, Zhao W, Liao S, Wang S, Li G, Xu Z, Wang J, Zhang J, Ma H, Lin D, Li Q. ZEB1 enhances Warburg effect to facilitate tumorigenesis and metastasis of HCC by transcriptionally activating PFKM. Theranostics. 2021;11:5926-5938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 95] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 36. | Chen L, Cheng X, Tu W, Qi Z, Li H, Liu F, Yang Y, Zhang Z, Wang Z. Apatinib inhibits glycolysis by suppressing the VEGFR2/AKT1/SOX5/GLUT4 signaling pathway in ovarian cancer cells. Cell Oncol (Dordr). 2019;42:679-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 37. | Yang C, Qin S. Apatinib targets both tumor and endothelial cells in hepatocellular carcinoma. Cancer Med. 2018;7:4570-4583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 38. | Yu WC, Zhang KZ, Chen SG, Liu WF. Efficacy and Safety of apatinib in patients with intermediate/advanced hepatocellular carcinoma: A prospective observation study. Medicine (Baltimore). 2018;97:e9704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 39. | Zhen L, Jiali C, Yong F, Han X, Hongming P, Weidong H. The Efficacy and Safety of Apatinib Treatment for Patients with Unresectable or Relapsed Liver Cancer: a retrospective study. J Cancer. 2018;9:2773-2777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 40. | Qin S, Li Q, Gu S, Chen X, Lin L, Wang Z, Xu A, Chen X, Zhou C, Ren Z, Yang L, Xu L, Bai Y, Chen L, Li J, Pan H, Cao B, Fang W, Wu W, Wang G, Cheng Y, Yu Z, Zhu X, Jiang D, Lu Y, Wang H, Xu J, Bai L, Liu Y, Lin H, Wu C, Zhang Y, Yan P, Jin C, Zou J. Apatinib as second-line or later therapy in patients with advanced hepatocellular carcinoma (AHELP): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Gastroenterol Hepatol. 2021;6:559-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 154] [Article Influence: 38.5] [Reference Citation Analysis (0)] |