Published online Mar 21, 2025. doi: 10.3748/wjg.v31.i11.102795
Revised: January 25, 2025
Accepted: February 20, 2025
Published online: March 21, 2025
Processing time: 128 Days and 20.4 Hours
Hepatic steatosis, characterized by fat accumulation in hepatocytes, can result from metabolic dysfunction-associated steatotic liver disease (MASLD), infections, alcoholism, chemotherapy, and toxins. MASLD is diagnosed via imaging or biopsy with metabolic criteria and may progress to metabolic dysfunction–asso
To investigate the disparity of ATI for assessing biopsy-based hepatic steatosis in CHB patients and MASLD patients.
The study enrolled 249 patients who underwent both ATI and liver biopsy, including 78 with CHB and 171 with MASLD. Hepatic steatosis was classified into grades S0 to S3 according to the proportion of fat cells present. Liver fibrosis was staged from 0 to 4 according to the meta-analysis of histological data in viral hepatitis scoring system. The diagnostic performance of attenuation coefficient (AC) values across different groups was compared for each grade of steatosis. Factors associated with the AC values were determined through linear regression analysis. A multivariate logistic regression model was established to predict ≥ S2 within the MASLD group.
In both the CHB and the MASLD groups, AC values increased significantly with higher steatosis grade (P < 0.001). In the CHB group, the areas under the curve (AUCs) of AC for predicting steatosis grades ≥ S1, ≥ S2 and S3 were 0.918, 0.960 and 0.987, respectively. In contrast, the MASLD group showed AUCs of 0.836, 0.774, and 0.688 for the same steatosis grades. The diagnostic performance of AC for detecting ≥ S2 and S3 indicated significant differences between the two groups (both P < 0.001). Multivariate linear regression analysis identified body mass index, triglycerides, and steatosis grade as significant factors for AC. When the steatosis grade is ≥ S2, it can progress to more serious liver conditions. A clinical model integrating blood biochemical parameters and AC was developed in the MASLD group to enhance the prediction of ≥ S2, achieving an AUC of 0.848.
The AC could effectively discriminate the degree of steatosis in both the CHB and MASLD groups. In the MASLD group, when combined with blood biochemical parameters, AC exhibited better predictive ability for moderate to severe steatosis.
Core Tip: Our study demonstrated that for the same steatosis grade, the attenuation coefficient (AC) value was significantly higher in the metabolic dysfunction-associated steatotic liver disease (MASLD) group than that in the chronic hepatitis B group. AC effectively discriminated between the degree of steatosis in steatotic liver disease of various etiologies. In the MASLD group, to improve the ability to predict ≥ S2, a clinical model incorporating blood biochemical parameters and AC was established, with an area under the curve of 0.848. The predictive model demonstrated a sensitivity of 91.2% and a specificity of 71.8%.
- Citation: Li XQ, Cheng GW, Akiyama I, Huang XJ, Liang J, Xue LY, Cheng Y, Kudo M, Ding H. Attenuation imaging for hepatic steatosis in chronic hepatitis B vs metabolic dysfunction-associated steatotic liver disease. World J Gastroenterol 2025; 31(11): 102795
- URL: https://www.wjgnet.com/1007-9327/full/v31/i11/102795.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i11.102795
Hepatic steatosis is characterized by the accumulation of triglyceride-rich fat vesicles within hepatocytes. Hepatic steatosis can occur because of fatty liver disease, infections, alcoholism, chemotherapy, and toxins.
A consensus group has redefined fatty liver disease as metabolic dysfunction-associated steatotic liver disease (MASLD)[1,2]. MASLD diagnosis requires hepatic steatosis confirmed through imaging or biopsy, accompanied by at least one cardiometabolic criterion. A subset of patients with MASLD develops metabolic dysfunction-associated steatohepatitis, which has the potential to progress to advanced hepatic fibrosis and potentially result in cirrhosis or hepatocellular carcinoma[3,4]. Fatty liver disease also affects extrahepatic organs, increasing the risk of cardiovascular diseases, chronic kidney disease, and hypertension[5,6].
MASLD is now a leading cause of chronic liver diseases worldwide, driven by the global rise in obesity and metabolic syndrome, and often coexists with other conditions. Hepatitis B virus (HBV) infection continues to be widespread in China. Consequently, the coexistence of chronic HBV infection and hepatic steatosis is expected to rise[7]. In patients with chronic hepatitis B (CHB), the occurrence of hepatic steatosis is comparable to that in the general population and is mainly associated with metabolic factors instead of viral factors. Patients with CHB and liver steatosis face an increased risk of all-cause mortality and cancer progression compared to those without steatosis, irrespective of their initial HBV viral load. Patients with CHB and liver steatosis require close monitoring regardless of viral load.
Liver biopsy histological evaluation is the definitive method for diagnosing and staging liver steatosis[8]. Liver biopsy limitations encompass morbidity, mortality, sampling variability, and interobserver variation[9]. Noninvasive alternatives to liver biopsy include serum markers and imaging-based technologies. Attenuation imaging (ATI) has recently emerged as an innovative ultrasound-based technique for the quantitative assessment of hepatic fat deposition in real time[10]. ATI quantifies fat deposition by measuring the attenuation coefficient (AC) in dB × cm-1 × MHz-1, indicating changes in ultrasound beam intensity. Recent adult studies show that ATI effectively diagnoses and correlates with steatosis severity, using liver biopsy or magnetic resonance imaging-derived proton density fat fraction (MRI-PDFF) as benchmarks[11-13]. To our knowledge, no studies have compared the performance of ATI in detecting hepatic steatosis between CHB patients and MASLD patients.
The aim of this study was to highlight the disparity in the accuracy of ATI in assessing hepatic steatosis between patients with CHB and patients with MASLD.
From June 2021 to December 2022, patients with liver disorders who underwent liver biopsy and ultrasound examinations were prospectively enrolled. All patients in our study underwent ATI examination before liver biopsy. The first group consisted of CHB patients aged over 18 years who tested positive for hepatitis B surface antigen. The second group consisted of MASLD patients meeting diagnostic criteria who had bariatric surgery according to standard National Institutes of Health guidelines. According to the recent Delphi consensus, MASLD is diagnosed in patients exhibiting hepatic steatosis on ultrasound alongside at least one cardiometabolic risk factor, excluding other causes of hepatic steatosis. The study excluded patients with concurrent liver conditions, including hepatitis C, alcohol-related liver disease, drug-induced liver injury, liver transplantation history, or any cancer type, such as hepatocellular carcinoma or cholangiocarcinoma. Patients lacking ATI data or without a liver biopsy conducted within two weeks of the ATI examination were excluded. CHB patients who were already receiving antiviral therapy were also excluded. Figure 1 shows the flowchart of the study patients.
Data on age, sex, body mass index (BMI), fasting glucose, alanine aminotransferase (ALT), aspartate transaminase (AST), alkaline phosphatase, γ-glutamyl transpeptidase (GGT), triglyceride (TG), and high-density lipoprotein cholesterol (HDL-C), collected within 7 days of the ATI examination, were retrospectively reviewed from medical records.
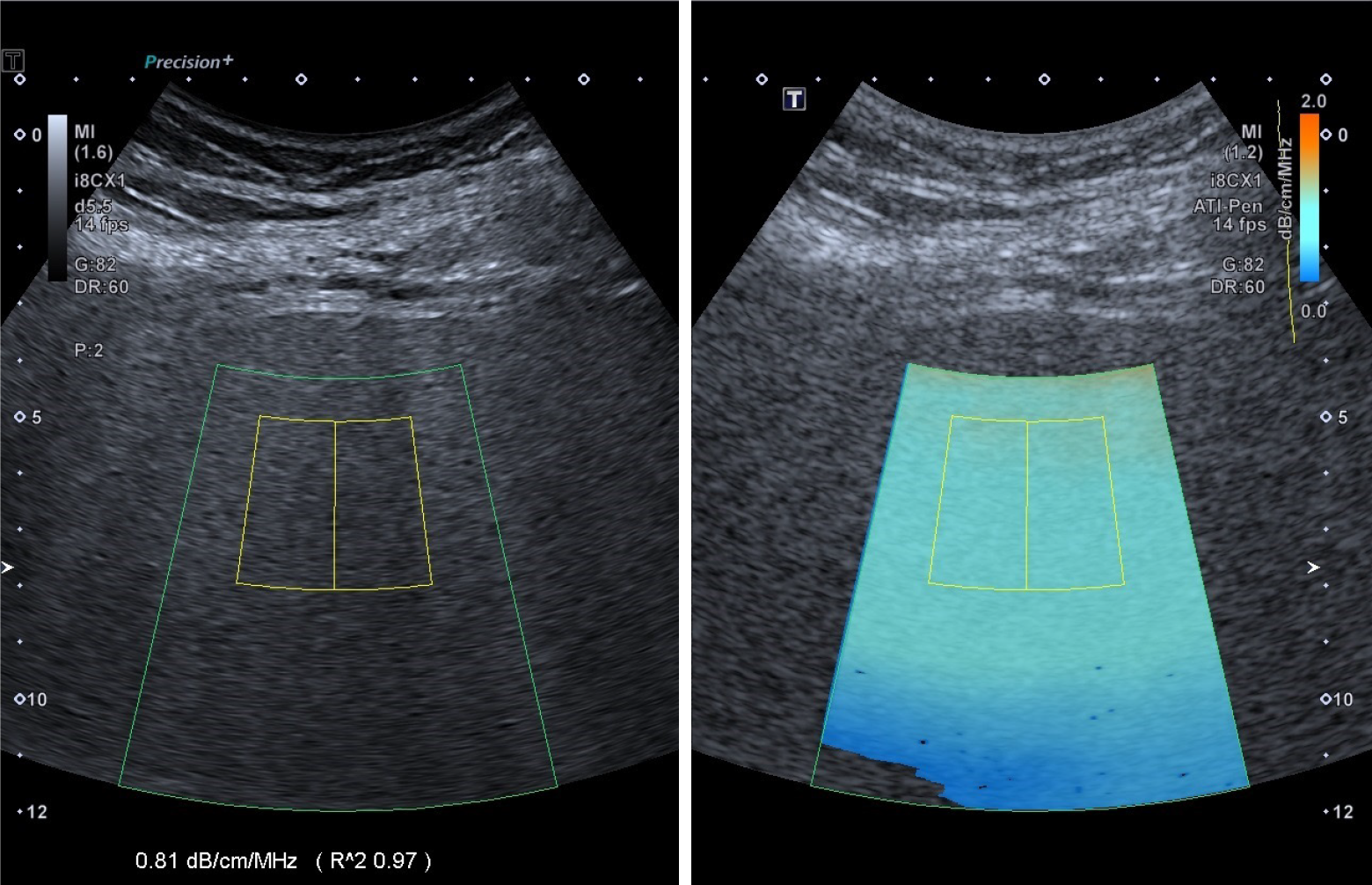
A radiologist with 5 years of experience performed ATI examinations using an i8CX1 convex probe connected to the Aplio i900 ultrasound system (Canon Medical Systems, Tochigi, Japan). Patients were required to fast for 6 hours prior to the ATI examination. The operator was unaware of the clinical details. The patients were positioned supine with their right upper limbs lifted. The probe was placed perpendicular to the skin in the right intercostal space to reduce shadowing and artifacts. Upon activation of the ATI mode, patients were instructed to hold their breath for 5 seconds, prompting the automatic appearance of a large, fan-shaped, color-coded sampling box within the liver parenchyma. A fan-shaped region of interest measuring 2 cm by 4 cm was positioned to encompass sufficient liver parenchyma (Figure 2). The display’s bottom left corner presented the AC value in dB × cm-1 × MHz-1. Each AC measurement included a quality coefficient, with the reliability of the result indicated by an R² value. An AC value with R2 ≥ 0.80 was considered valid. Five valid measurements were obtained, and the median values were selected as representative after five measurements.
Liver biopsy samples were collected from the left lobe using an 18-gauge Tru-Cut needle during laparoscopic bariatric surgery in MASLD patients within 2 weeks post-ATI examination. Samples from CHB patients were collected through percutaneous liver biopsy of the right lobe. The specimens were fixed in paraffin wax for histopathological evaluation. Steatosis (S) was classified by the percentage of liver fat cells observed on the glass slide as follows: None (S0, < 5%), mild (S1, 5%-33%), moderate (S2, 34%-66%), and severe (S3, > 66%)[14]. Liver fibrosis was assessed using the meta-analysis of histological data in viral hepatitis scoring system, ranging from stage 0 to 4: F0 indicates no fibrosis; F1 represents portal fibrosis without septa; F2 involves portal fibrosis with few septa; F3 is characterized by numerous septa without cirrhosis; and F4 denotes cirrhosis[15].
Statistical analyses were performed using the statistical product and service solutions (version 26.0) and R-Language (version 4.3.1) software. Patient characteristics were summarized using median and interquartile range for continuous variables, and absolute counts with percentages for categorical variables. Continuous variables were evaluated using analysis of variance and the Kruskal-Wallis test, whereas categorical variables were analyzed with the χ² test. A receiver operating characteristic curve was created to assess the diagnostic efficacy of AC values in determining liver steatosis grade. The areas under the curve (AUCs) were evaluated to establish cutoff values and assess ATI’s diagnostic efficacy in predicting fatty liver. An optimal cutoff value for each parameter was established to enhance combined performance metrics, such as sensitivity and specificity, in the AUC analysis. The AUCs were compared using DeLong’s test. Linear regression analyses were performed to identify the significant factors affecting AC values. Univariate logistic regression was conducted on each potential factor for steatosis grade ≥ 2, followed by a multivariate logistic regression model to compute prediction scores for steatosis ≥ 2. P values below 0.05 were deemed statistically significant.
A total of 249 patients were enrolled in the study, including 78 with CHB and 171 with MASLD. The characteristics of the participants were summarized in Table 1. Among them, 90 (36.1%) were male. The median age was 33 years (range: 28-39), and the median BMI was 33.71 kg/m² (range: 26.75-38.03). According to the pathological analysis of hepatic steatosis grades, the distribution of patients was as follows: 62 (24.90%) with S0, 84 (33.73%) with S1, 79 (31.73%) with S2, and 24 (9.64%) with S3. Additionally, the fibrosis stages were categorized as follows: 31 (12.45%) with F0, 71 (28.51%) with F1, 96 (38.55%) with F2, 46 (18.47%) with F3, and 5 (2.01%) with F4. As shown in Table 1, the sex, age, BMI, HDL-C, steatosis grade, and fibrosis stage were significantly different between the CHB and MASLD groups (all P < 0.05).
| Characteristic | Total (n = 249) | CHB group (n = 78) | MASLD group (n = 171) | P value |
| Male | 90 (36.14) | 51 (65.38) | 39 (22.81) | < 0.001a |
| Age (years) | 33 (28-39) | 40 (33-47) | 31 (26-35) | < 0.001a |
| BMI (kg/m2) | 33.71 (26.75-38.03) | 23.48 (21.65-25.69) | 36.33 (33.27-40.81) | < 0.001a |
| Fasting glucose (mmol/L) | 5.50 (5.10-6.40) | 5.65 (5.20-6.27) | 5.50 (5.10-6.40) | 0.457 |
| ALT (U/L) | 41 (25-73) | 37 (28-79) | 43 (22-70) | 0.325 |
| AST (U/L) | 26 (19-41) | 27 (17-43) | 29 (22-48) | 0.101 |
| ALP (U/L) | 79 (67-95) | 82 (69-106) | 76 (65-93) | 0.055 |
| GGT (U/L) | 35 (23-61) | 31 (19-63) | 35 (26-59) | 0.199 |
| TG (mmol/L) | 1.53 (1.07-2.10) | 1.47 (0.98-2.25) | 1.55 (1.08-2.04) | 0.788 |
| HDL-C (mmol/L) | 1.08 (0.93-1.30) | 1.05 (0.89-1.26) | 1.14 (0.97-1.52) | 0.016a |
| Steatosis grade | < 0.001a | |||
| S0 | 62 (24.90) | 45 (57.69) | 17 (9.94) | |
| S1 | 84 (33.73) | 16 (20.51) | 68 (39.77) | |
| S2 | 79 (31.73) | 15 (19.23) | 64 (37.43) | |
| S3 | 24 (9.64) | 2 (2.56) | 22 (12.87) | |
| Fibrosis stage | < 0.001a | |||
| F0 | 31 (12.45) | 19 (24.36) | 12 (7.02) | |
| F1 | 71 (28.51) | 29 (37.18) | 42 (24.56) | |
| F2 | 96 (38.55) | 14 (17.95) | 82 (47.95) | |
| F3 | 46 (18.47) | 11 (14.10) | 35 (20.47) | |
| F4 | 5 (2.01) | 5 (6.41) | 0 (0.00) |
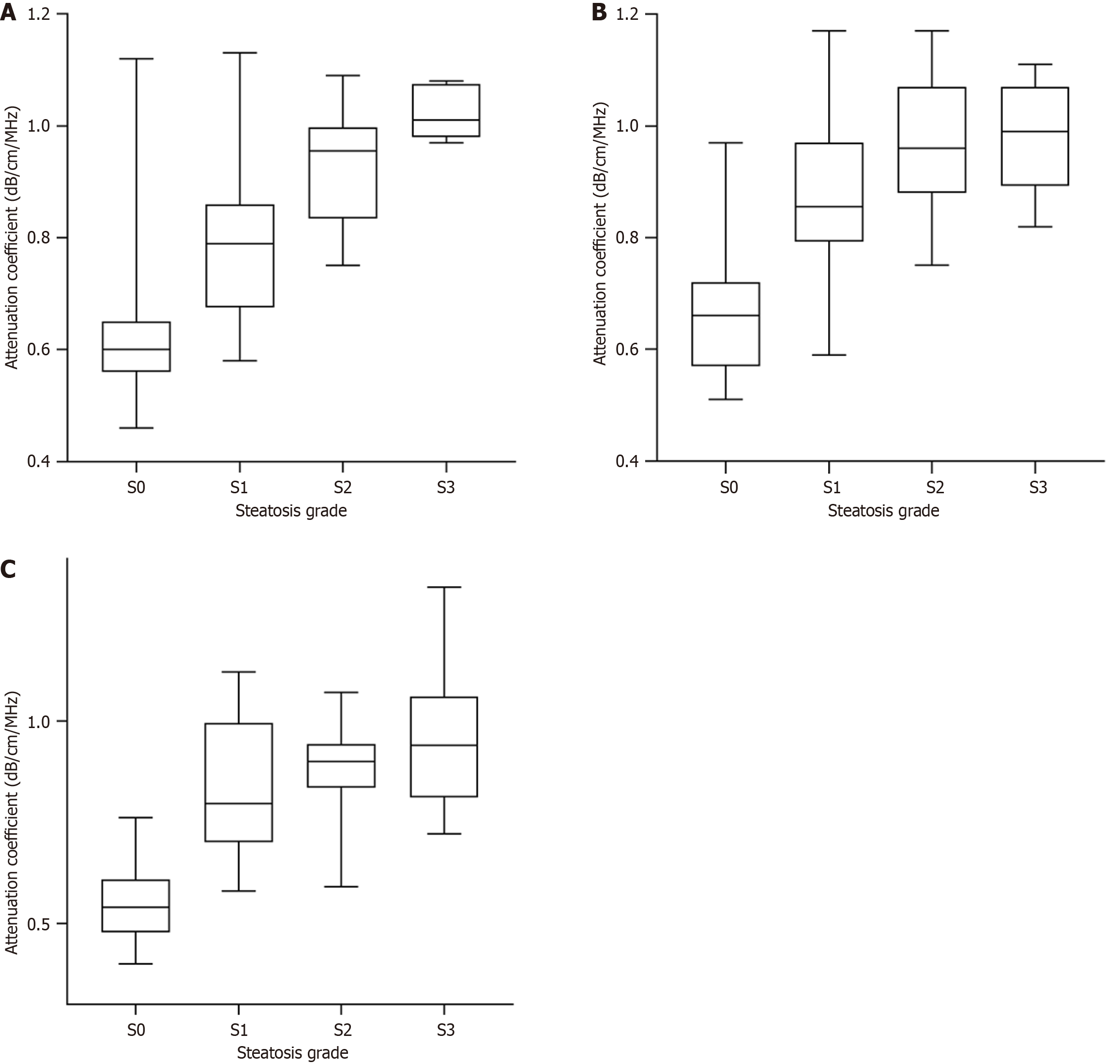
In the CHB group, median AC values significantly increased with the increased severity of steatosis (0.58, 0.69, 0.83, and 0.98 dB × cm-1 × MHz-1 for S1, S2, S3, and S4, respectively, P < 0.001). In the MASLD group, similar results were obtained, with median AC values of (0.71, 0.85, 0.96, and 0.99 dB × cm-1 × MHz-1 for S1, S2, S3, and S4, respectively (P < 0.001). The results revealed that the distribution of AC values in S0, S1, and S2 was statistically significant between the two groups (P < 0.05). For the same steatosis grade, the AC value was significantly higher in the MASLD group than in the CHB group.
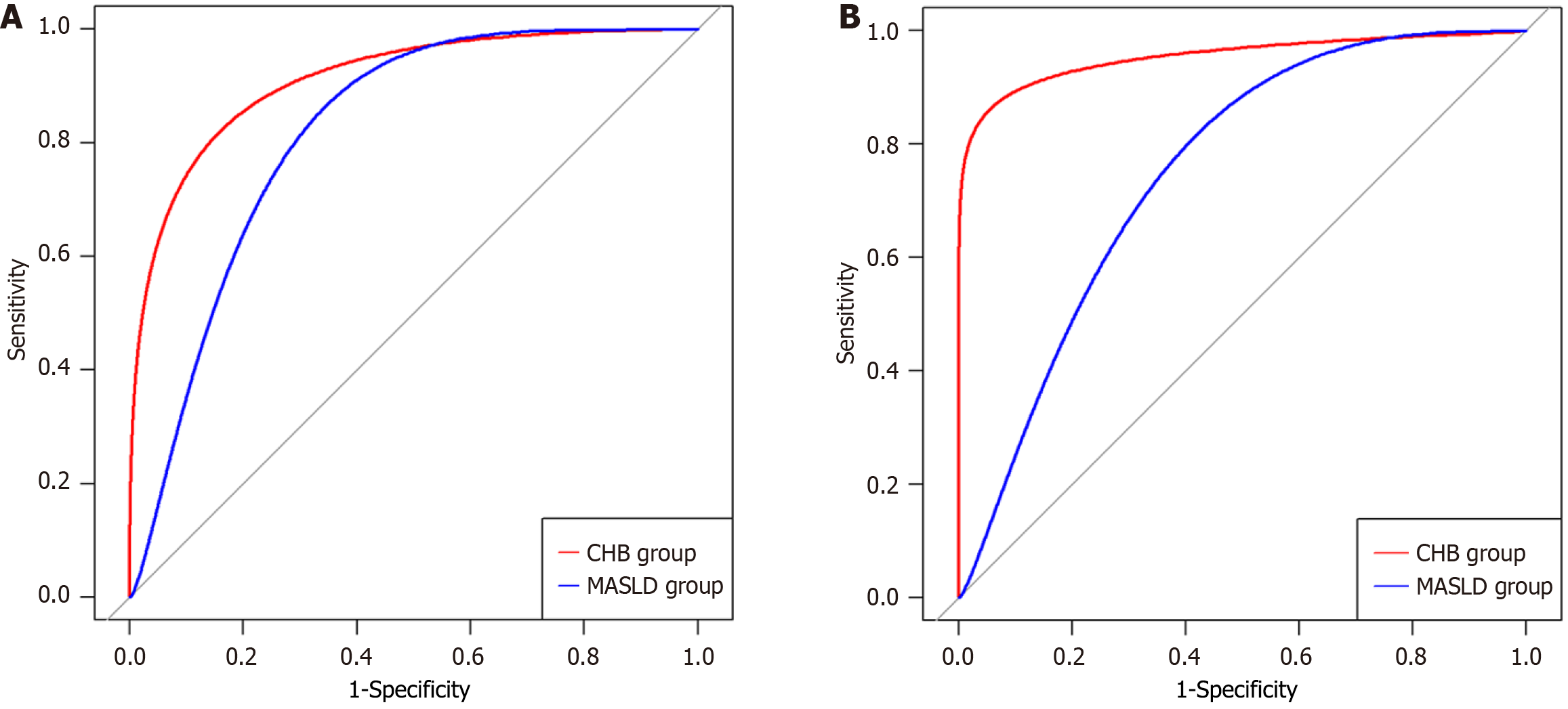
As presented in Figure 3, the AUCs of AC for predicting steatosis grade ≥ S1 were 0.918 [95% confidence interval (CI): 0.859-0.977] in the CHB group and 0.836 (95%CI: 0.709-0.963) in the MASLD group. No significant difference was observed between the AUC values of the two groups (P = 0.251). In the CHB and MASLD groups, the AUCs of AC for predicting steatosis grade ≥ S2 were 0.960 (95%CI: 0.893-1.000) and 0.774 (95%CI: 0.702-0.846), respectively, and the AUCs for predicting steatosis grade S3 were 0.987 (95%CI: 0.961-1.000) and 0.688 (95%CI: 0.579-0.798), respectively. The AUCs of AC for detecting hepatic steatosis ≥ S2 and S3 were significantly different between the two groups (both P < 0.001); the AUCs of AC were higher in the CHB group. The corresponding sensitivity, specificity, positive predictive value, negative predictive value, and accuracy values are presented in Table 2.
| Characteristic | AUC | Cutoff value | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | Accuracy (%) |
| CHB | |||||||
| ≥ S1 | 0.918 | 0.64 | 90.9 | 77.8 | 75 | 92.1 | 83.3 |
| ≥ S2 | 0.960 | 0.77 | 88.2 | 98.4 | 93.7 | 96.7 | 96.1 |
| S3 | 0.987 | 0.94 | 100 | 98.7 | 66.7 | 100 | 98.7 |
| MASLD | |||||||
| ≥ S1 | 0.836 | 0.79 | 84.4 | 82.3 | 97.7 | 36.8 | 84.2 |
| ≥ S2 | 0.774 | 0.88 | 80.2 | 68.2 | 71.8 | 77.3 | 74.2 |
| S3 | 0.688 | 0.93 | 72.7 | 63.8 | 22.8 | 94.1 | 64.9 |
For the total patient population, univariate linear regression analysis identified age, BMI, TG, HDL-C, fasting glucose, steatosis grade, and fibrosis stage as factors associated with AC (Table 3). In the multivariate linear regression analysis, these variables were included because all the variance inflation factors were < 5. As a result, BMI, TG level, and steatosis grade were found to be significant factors for increased AC values (P < 0.001, P = 0.008, and P < 0.001, respectively).
| Characteristic | Univariate analysis | Multivariate analysis | ||||
| Coefficient | 95%CI | P value | Coefficient | 95%CI | P value | |
| Male gender | 0.040 | -0.006 to 0.087 | 0.090 | |||
| Age | -0.005 | -0.007 to -0.003 | < 0.001a | 0 | -0.002 to 0.002 | 0.770 |
| BMI | 0.012 | -0.009 to 0.014 | < 0.001a | 0.006 | 0.004 to 0.008 | < 0.001a |
| Fasting glucose | 0.017 | 0.006 to 0.027 | 0.002a | 0.004 | -0.003 to 0.012 | 0.250 |
| AST | 0 | 0 to 0 | 0.060 | |||
| ALT | 0 | 0 to 0 | 0.341 | |||
| ALP | 0 | -0.001 to 0 | 0.169 | |||
| GGT | 0 | 0 to 0 | 0.946 | |||
| TG | 0.016 | 0.009 to 0.024 | < 0.001a | 0.008 | 0.002 to 0.014 | 0.008a |
| HDL-C | -0.201 | -0.277 to -0.123 | < 0.001a | -0.024 | -0.088 to 0.040 | 0.462 |
| Steatosis grade | 0.134 | 0.116 to 0.151 | < 0.001a | 0.097 | 0.077 to 0.116 | < 0.001a |
| Fibrosis stage | 0.040 | 0.018 to 0.063 | < 0.001a | -0.003 | -0.020 to 0.015 | 0.769 |
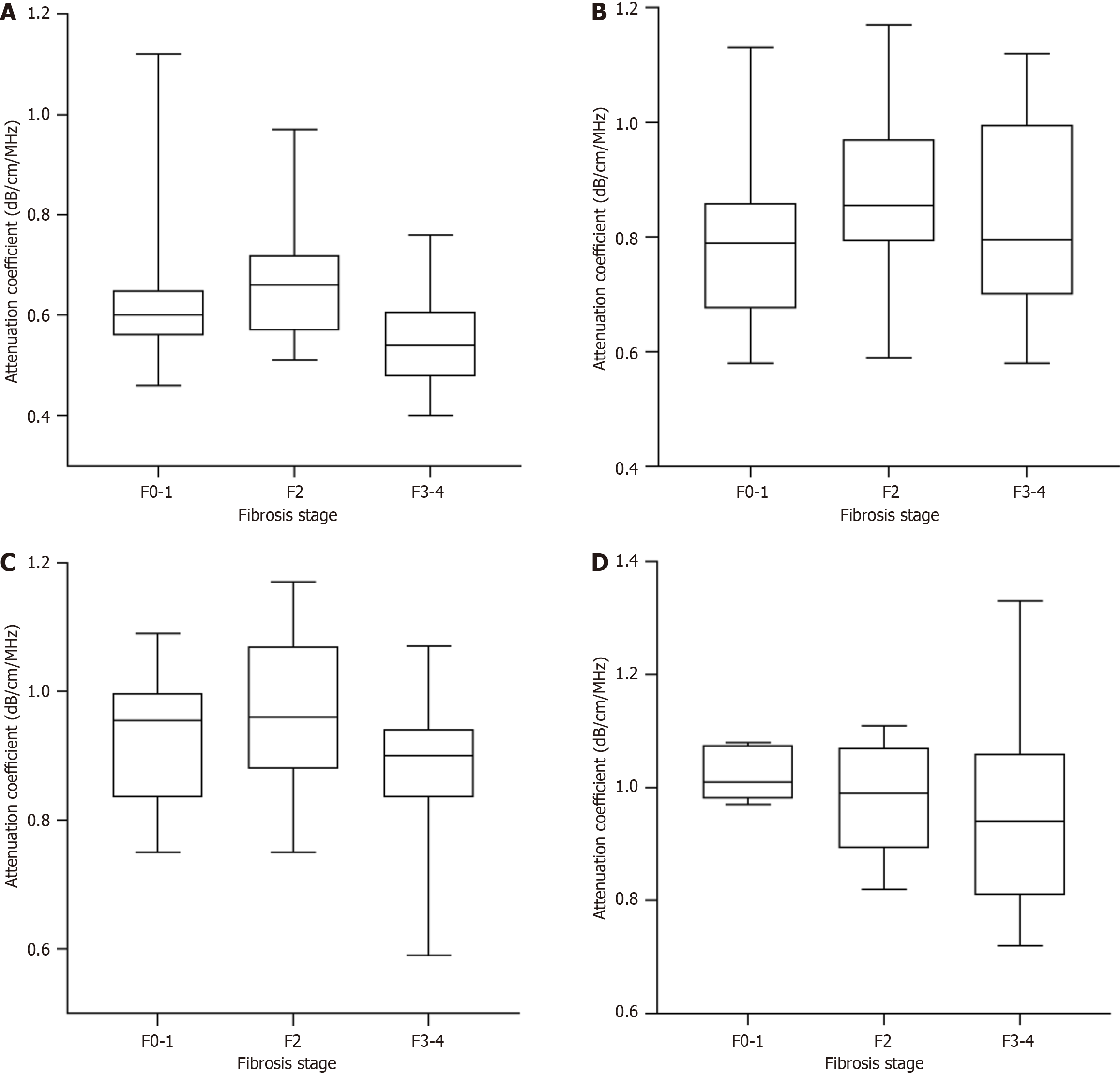
We analyzed the AC values in the patients with different fibrosis stages. As presented in Table 4 and Figure 4, AC values did not significantly differ between the patients with different fibrosis stages within each steatosis grade. However, within each fibrosis stage, the AC values significantly increased with the progression of steatosis grade (Table 4 and Figure 5). These results revealed that the AC values may not be affected by the fibrosis stage.
We performed univariate logistic regression analysis to assess steatosis ≥ 2 against each continuous variable in the MASLD group. The data revealed that fasting glucose, AST, ALT, GGT, TG, and AC were predictors of steatosis ≥ S2 (Supplementary Table 1). Multivariate logistic regression using current data provided a scoring system for predicting steatosis ≥ S2. The optimized model included fasting glucose, AST, ALT, and AC. The model was represented by the following equation: Logit (P) = 0.7 × (fasting glucose) + 0.7 × (AST) - 0.1 × (ALT) + 80 × (AC).
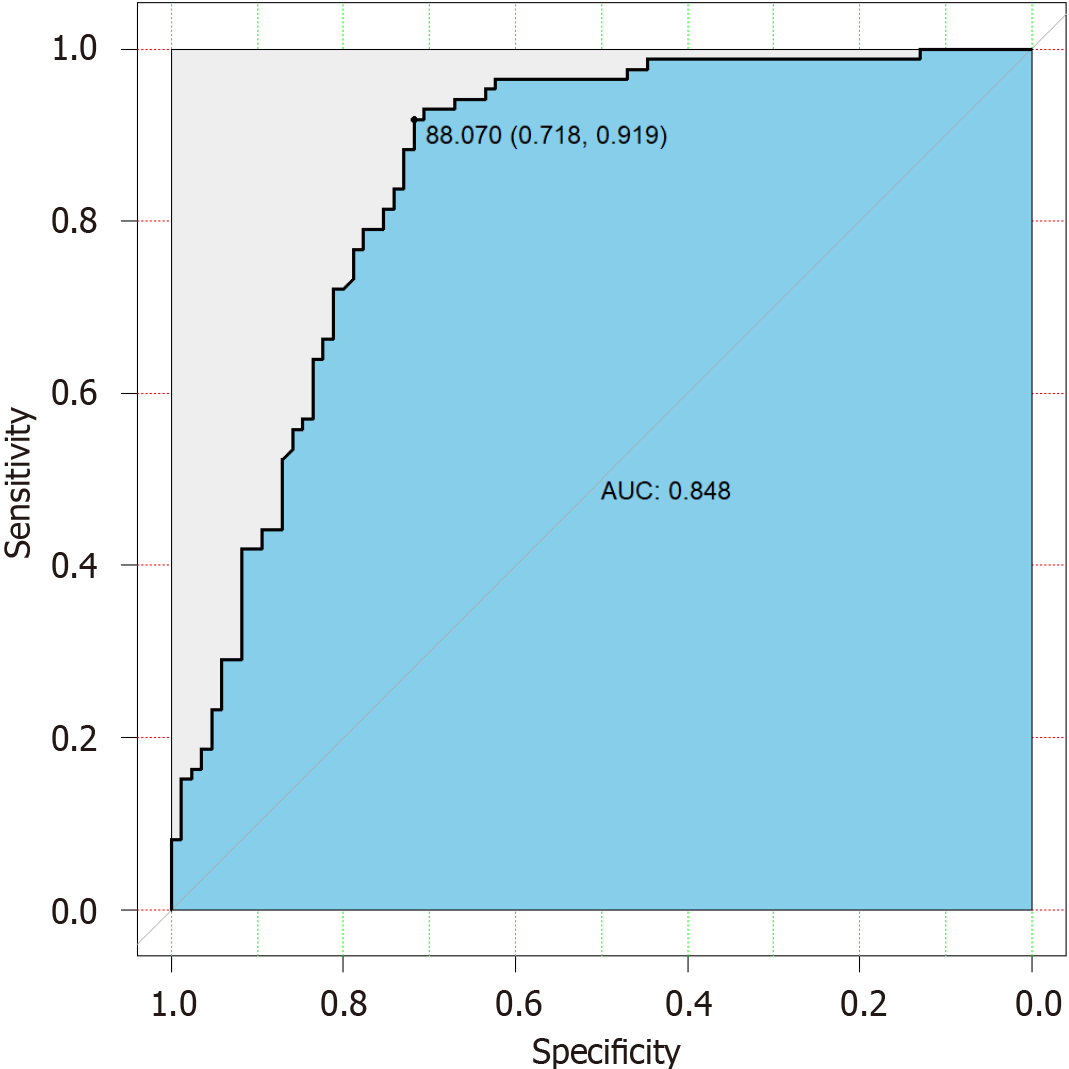
The combination of these individual predictors yielded a total score that significantly predicted ≥ S2 (P < 0.001, AUC = 0.848, Figure 6). Analysis of all cutoff scores determined the optimized sensitivity and specificity using a score of 88.07. The sensitivity and specificity of the predictive score were 91.2% and 71.8%, respectively.
Steatotic liver disease is the leading cause of chronic liver disease globally. MASLD impacts nearly a quarter of the global adult population and is the primary cause of cirrhosis[3]. The concurrent presence of HBV infection and hepatic steatosis is increasingly recognized as a characteristic of chronic liver diseases[16]. Liver steatosis is strongly associated with higher risks of all-cause mortality and cancer in CHB patients[16]. Therefore, patients diagnosed with both HBV infection and hepatic steatosis should be closely monitored for liver function and disease progression. Early detection and proactive management are critical for preventing severe liver complications.
Liver biopsy is the definitive method for quantifying hepatic steatosis. Due to the limitations of liver biopsy, alternatives such as MRI-PDFF are being explored. Nonetheless, MRI-PDFF evaluations are costly and time-consuming. Recently, ultrasound-based methods for quantifying hepatic steatosis, such as ATI, have been developed[17,18]. ATI provides objective information on hepatic steatosis, along with conventional grayscale echogenicity and structural data[19]. Compared with other methods, ATI offers several advantages for evaluating hepatic steatosis, including its noninvasiveness, objectivity, availability, and ease of measurement. Previous research on ATI has mainly concentrated on its effectiveness in diagnosing hepatic steatosis[13,17,19]. Notably, our study is the first to compare the assessment of hepatic steatosis using ATI between CHB patients and MASLD patients.
Our study suggests that ATI may be a novel method for detecting hepatic steatosis according to different fatty liver etiologies. In our study, AC values significantly increased with the progression of steatosis grade in both the CHB and MASLD groups. However, for the same steatosis grade, median AC values were significantly higher in the MASLD group than in the CHB group. To explore this result, we assessed the factors associated with AC values. In the multiple regression analysis, AC values were significantly associated with BMI, TG, and steatosis grade in the total patient population. The question of whether hepatic fibrosis affects the evaluation of steatosis via the AC value was important. Our study indicated that AC values, when used to evaluate steatosis, were not significantly affected by the presence of hepatic fibrosis. This finding was consistent with previous results from Yuri et al[20], suggesting that AC can reliably measure steatosis even in patients with concurrent fibrosis.
Regarding AC performance, our results revealed excellent diagnostic accuracy in the CHB group. However, our AUCs and cutoff values were not entirely consistent with those reported in other studies[20]. This inconsistency may be explained by differences in ultrasound instrument versions and study populations. However, both studies revealed that the diagnostic capability of ATI for predicting steatosis grade was better in the viral hepatitis group than in the MASLD group. Tada et al[11] reported that the AUCs for ATI diagnosis of ≥ S1, ≥ S2, and S3 in obese patients (BMI > 25 kg/m2) were 0.72, 0.72, and 0.78, respectively, whereas in the general population, the AUCs were 0.85, 0.91, and 0.91, respectively. This indicated that the diagnostic capability of ATI was lower in the obese group[10]. Similar results were obtained in our study with MASLD patients with high BMI. A meta-analysis on the accuracy of AC for evaluating hepatic steatosis by Jang et al[21] reported that the proportions of patients with high BMI were significantly associated with study heterogeneity. Therefore, differences in the diagnostic performance of ATI across different populations may be related to BMI.
While both CHB and MASLD involve hepatic fat accumulation, the pathophysiology of fat buildup is quite different. In CHB, viral infection and immune-mediated liver inflammation are the driving factors, leading to disrupted lipid metabolism and fat storage. In contrast, MASLD is primarily caused by metabolic dysfunction, with insulin resistance and obesity playing a central role in the accumulation of fat in the liver[22,23]. These factors can affect the attenuation properties of the liver tissue, leading to higher ACs even when the degree of steatosis is similar. The nature and distribution of fat in the liver can also differ between MASLD and CHB patients. In MASLD, the fat is typically more concentrated in the hepatocytes and might be more heterogeneous in terms of its composition, with a higher proportion of toxic lipids (such as free fatty acids or TGs)[24]. This can influence the liver's ability to attenuate signals differently than in CHB, where the fat distribution may be less severe or different in composition.
Steatosis, especially in its moderate to severe stages, can progress to more serious liver conditions such as steatohepatitis, fibrosis, cirrhosis, and potentially liver failure. It is crucial to identify and manage steatosis at an earlier stage to prevent or slow down disease progression. In our result, the performance of AC in predicting steatosis grade ≥ S2 was moderate in the MASLD group. To improve prediction, we developed a model based on fasting glucose, AST, ALT, and AC to reliably predict liver steatosis ≥ 2. The predictive model exhibited a sensitivity of 91.2% and a specificity of 71.8%. These results suggested that this easy-to-use scoring model, which incorporated blood biochemical parameters and quantitative ultrasound parameter, had meaningful clinical implications for the management of patients with steatosis ≥ S2.
Our study is significant because ATI had not previously been assessed to compare diagnostic performance between CHB and MASLD patients. However, there are several limitations. First, our study included a relatively small number of CHB patients, with only two cases of severe liver steatosis. This may affect the generalizability of our findings and introduce potential bias in the interpretation of the results. Second, liver biopsy samples were obtained from different lobes in the two groups-samples from the right lobe in CHB patients and from the left lobe in MASLD patients-which may have introduced potential bias into our findings. Most studies suggested that the right lobe should be chosen for ATI examination. Therefore, in future experiments, we will unify the location of liver biopsy in the right lobe of the liver. Finally, our study population consisted exclusively of MASLD patients with high BMI and obesity, lacking MASLD patients with normal BMI. Moreover, a limitation of our study was the significant differences in gender and age between the two groups. These demographic disparities could potentially introduce bias into the results. In future studies, it would be beneficial to include MASLD patients with a normal BMI and aim for a more balanced representation across genders and age groups. This will help to reduce potential biases and enhance the applicability of the results to a broader population.
A multicenter trial with diverse fatty liver disease patients is necessary to confirm our findings.
Our study demonstrated that at the same degree of hepatic steatosis, the distribution of AC values differed between the CHB and MASLD groups. ATI effectively discriminated between the degrees of steatosis in steatotic liver disease of various etiologies.
| 1. | Suzuki A, Diehl AM. Nonalcoholic Steatohepatitis. Annu Rev Med. 2017;68:85-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 2. | Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab JP, Arrese M, Bataller R, Beuers U, Boursier J, Bugianesi E, Byrne CD, Castro Narro GE, Chowdhury A, Cortez-Pinto H, Cryer DR, Cusi K, El-Kassas M, Klein S, Eskridge W, Fan J, Gawrieh S, Guy CD, Harrison SA, Kim SU, Koot BG, Korenjak M, Kowdley KV, Lacaille F, Loomba R, Mitchell-Thain R, Morgan TR, Powell EE, Roden M, Romero-Gómez M, Silva M, Singh SP, Sookoian SC, Spearman CW, Tiniakos D, Valenti L, Vos MB, Wong VW, Xanthakos S, Yilmaz Y, Younossi Z, Hobbs A, Villota-Rivas M, Newsome PN; NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79:1542-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1167] [Article Influence: 583.5] [Reference Citation Analysis (1)] |
| 3. | Noureddin M, Truong E, Mayo R, Martínez-Arranz I, Mincholé I, Banales JM, Arrese M, Cusi K, Arias-Loste MT, Bruha R, Romero-Gómez M, Iruzubieta P, Aller R, Ampuero J, Calleja JL, Ibañez-Samaniego L, Aspichueta P, Martín-Duce A, Kushner T, Ortiz P, Harrison SA, Anstee QM, Crespo J, Mato JM, Sanyal AJ. Serum identification of at-risk MASH: The metabolomics-advanced steatohepatitis fibrosis score (MASEF). Hepatology. 2024;79:135-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 4. | Hagiwara S, Nishida N, Ueshima K, Minami Y, Komeda Y, Aoki T, Takita M, Morita M, Chishina H, Yoshida A, Ida H, Kudo M. Accumulation of Genetic and Epigenetic Alterations in the Background Liver and Emergence of Hepatocellular Carcinoma in Patients with Non-Alcoholic Fatty Liver Disease. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Tsutsumi T, Eslam M, Kawaguchi T, Yamamura S, Kawaguchi A, Nakano D, Koseki M, Yoshinaga S, Takahashi H, Anzai K, George J, Torimura T. MAFLD better predicts the progression of atherosclerotic cardiovascular risk than NAFLD: Generalized estimating equation approach. Hepatol Res. 2021;51:1115-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 6. | Kang JH, Park SJ, Jeong S, Park YJ, Kim HJ, Song J, Choi J, Park S, Kim J, Lee H, Chang J, Son JS, Park SM. Association between antibiotic use and cardiovascular diseases in metabolic dysfunction-associated steatotic liver disease: A nationally representative retrospective cohort study. Hepatol Res. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Branchi F, Conti CB, Baccarin A, Lampertico P, Conte D, Fraquelli M. Non-invasive assessment of liver fibrosis in chronic hepatitis B. World J Gastroenterol. 2014;20:14568-14580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Merriman RB, Ferrell LD, Patti MG, Weston SR, Pabst MS, Aouizerat BE, Bass NM. Correlation of paired liver biopsies in morbidly obese patients with suspected nonalcoholic fatty liver disease. Hepatology. 2006;44:874-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 240] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 9. | Lurie Y, Webb M, Cytter-Kuint R, Shteingart S, Lederkremer GZ. Non-invasive diagnosis of liver fibrosis and cirrhosis. World J Gastroenterol. 2015;21:11567-11583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 189] [Cited by in RCA: 251] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 10. | Ferraioli G, Soares Monteiro LB. Ultrasound-based techniques for the diagnosis of liver steatosis. World J Gastroenterol. 2019;25:6053-6062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 324] [Cited by in RCA: 287] [Article Influence: 47.8] [Reference Citation Analysis (7)] |
| 11. | Tada T, Kumada T, Toyoda H, Nakamura S, Shibata Y, Yasuda S, Watanuki Y, Tsujii K, Fukuda N, Fujioka M, Takeshima K, Niwa F, Ogawa S, Hashinokuchi S, Kataoka S, Ichikawa H, Iijima H. Attenuation imaging based on ultrasound technology for assessment of hepatic steatosis: A comparison with magnetic resonance imaging-determined proton density fat fraction. Hepatol Res. 2020;50:1319-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 12. | Li X, Huang X, Cheng G, Liang J, Qiu L, Zhang J, Yao Q, Ding H. Optimizing the number of valid measurements for the attenuation coefficient to assess hepatic steatosis in MAFLD patients: A study of 139 patients who underwent liver biopsy. Ultraschall Med. 2024;45:395-404. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Bae JS, Lee DH, Lee JY, Kim H, Yu SJ, Lee JH, Cho EJ, Lee YB, Han JK, Choi BI. Assessment of hepatic steatosis by using attenuation imaging: a quantitative, easy-to-perform ultrasound technique. Eur Radiol. 2019;29:6499-6507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 14. | Bedossa P; FLIP Pathology Consortium. Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology. 2014;60:565-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 453] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 15. | Ravaioli F, Dajti E, Mantovani A, Newsome PN, Targher G, Colecchia A. Diagnostic accuracy of FibroScan-AST (FAST) score for the non-invasive identification of patients with fibrotic non-alcoholic steatohepatitis: a systematic review and meta-analysis. Gut. 2023;72:1399-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 16. | Peleg N, Issachar A, Sneh Arbib O, Cohen-Naftaly M, Braun M, Leshno M, Barsheshet A, Shlomai A. Liver steatosis is a strong predictor of mortality and cancer in chronic hepatitis B regardless of viral load. JHEP Rep. 2019;1:9-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 17. | Dioguardi Burgio M, Ronot M, Reizine E, Rautou PE, Castera L, Paradis V, Garteiser P, Van Beers B, Vilgrain V. Quantification of hepatic steatosis with ultrasound: promising role of attenuation imaging coefficient in a biopsy-proven cohort. Eur Radiol. 2020;30:2293-2301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 18. | Bo JM, Yiman DM, Xiang FM, Jianing ZM, Lianhua ZM, Qiuyang LM, Yukun LMP. Ultrasound-Guided Attenuation Parameter May Replace B-mode Ultrasound in Diagnosing Nonalcoholic Fatty Liver Disease. AUDT. 2023;7:260. [DOI] [Full Text] |
| 19. | Hsu PK, Wu LS, Yen HH, Huang HP, Chen YY, Su PY, Su WW. Attenuation Imaging with Ultrasound as a Novel Evaluation Method for Liver Steatosis. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Yuri M, Nishimura T, Tada T, Yoshida M, Fujiwara A, Kawata S, Yoshihara K, Yoshioka R, Ota S, Nakano R, Yuri Y, Takashima T, Aizawa N, Ikeda N, Shiomi H, Ide YH, Enomoto H, Yasuhiro F, Yano H, Iijima H. Diagnosis of hepatic steatosis based on ultrasound attenuation imaging is not influenced by liver fibrosis. Hepatol Res. 2022;52:1009-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Jang JK, Choi SH, Lee JS, Kim SY, Lee SS, Kim KW. Accuracy of the ultrasound attenuation coefficient for the evaluation of hepatic steatosis: a systematic review and meta-analysis of prospective studies. Ultrasonography. 2022;41:83-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 22. | Wang Y, Li J, Wang S, Pang Y, Liu P, Xie B, Dou S, Yang T, Liu X, Shi Y, Chen D. The hepatitis B virus promotes the progression of non-alcoholic fatty liver disease through incomplete autophagy. Free Radic Biol Med. 2023;204:326-336. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Diao Y, Tang J, Wang X, Deng W, Tang J, You C. Metabolic Syndrome, Nonalcoholic Fatty Liver Disease, and Chronic Hepatitis B: A Narrative Review. Infect Dis Ther. 2023;12:53-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 24. | Alves-Bezerra M, Cohen DE. Triglyceride Metabolism in the Liver. Compr Physiol. 2017;8:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 410] [Article Influence: 51.3] [Reference Citation Analysis (0)] |