INTRODUCTION
Liver cancer is one of the major cancers worldwide and is the most common tumor in the digestive system, characterized by high invasiveness and high mortality rates. According to the “2022 Global Cancer Statistics”, liver cancer ranks sixth in incidence and third in mortality among all cancers globally, with hepatocellular carcinoma (HCC) accounting for 85% of primary liver cancer cases[1]. The prognosis for most HCC patients is poor, primarily because the majority of patients are diagnosed at an advanced stage and effective treatment options are limited, resulting in only a small number of patients being eligible for curative treatment. This makes the treatment of HCC particularly challenging. The complexity of the HCC tumor microenvironment (TME) limits treatment efficacy for many patients, and long-term outcomes are significantly influenced by the TME. Therefore, assessing the HCC-TME is particularly important. The TME plays a critical role in cancer biology, influencing not only tumor growth, invasion, and metastasis but also the tumor’s response to systemic therapies and local treatments such as thermal ablation and resistance. The TME is a highly dynamic and heterogeneous combination of tumor cells, tumor-infiltrating immune cells, tumor-associated fibroblasts, blood and lymphatic vessels, nerve fibers, signaling molecules, and extracellular matrix, which interact and influence each other, collectively shaping the TME and affecting tumor development, progression, and cellular adaptation[2]. The TME can promote tumor growth, angiogenesis, invasion, and immune evasion by cancer cells, while also performing immune surveillance and immune editing on tumor cells[3]. In recent years, significant contributions have been made to the research and assessment of the TME, particularly in the application of imaging technologies to the TME. Imaging techniques not only assess the tumor itself but also evaluate the TME. This article will review the application of imaging technologies in HCC-TME, providing new ideas and methods for the personalized treatment, efficacy assessment, and prognosis of HCC patients.
MOLECULAR IMAGING TECHNIQUES IN THE APPLICATION OF HYPOXIC ENVIRONMENTS IN HCC
Hypoxia and low oxygen levels are significant markers and important features of solid tumors, playing a crucial role in regulating the TME. Assessing hypoxia is essential for effective cancer treatment. Due to the rapid growth of HCC cells, insufficient blood supply occurs, and the proliferation of cancer cells consumes large amounts of oxygen, leading to the formation of a hypoxic TME[4]. The tumor hypoxic microenvironment is closely related to hypoxia-inducible factors, particularly its subtype hypoxia-inducible factors-1α, whose signaling pathways have been explored as potential therapeutic targets for future HCC management[5]. Recent studies have found that low mechanical index (= 0.3) ultrasound combined with microbubble contrast agents can effectively alleviate tumor hypoxia, reduce levels of hypoxia and hypoxia-inducible factors, and inhibit radioresistance. This combined treatment represents a novel and promising approach to reversing hypoxia in HCC[6]. Fluorescent probes and nanoprobe technologies have been designed to emit detectable light signals in specific biological or chemical environments, allowing for real-time monitoring of hypoxia in the TME. An iridium-based optical probe has been found to simultaneously sense acidity and hypoxia, showing great application potential in hypoxic imaging[7]. Furthermore, researchers have synthesized a hypoxia-responsive probe based on a flavin dye containing azo groups, which detects hypoxia and normoxia conditions in cancer cells through a reaction catalyzed by reductases. Results indicate that, compared to normal conditions, the fluorescence intensity under hypoxic conditions increased approximately tenfold after 15 minutes and 26-fold after 60 minutes[8]. Hypoxia-targeted radiopharmaceuticals using positron emission tomography (PET) have been employed for detecting and treating tumor hypoxia. Nitroimidazole, a bioreducible moiety, becomes reactive under low intracellular oxygen concentrations. The reduction of O2 leads to a failure of the reoxidation of nitro free radical anions, resulting in the formation of reactive substances that covalently bind to cellular proteins or DNA, thereby distinguishing hypoxic tissues from normoxic tissues. This technique has been widely applied for hypoxia detection, and PET imaging can detect tumors as small as 1 millimeter, providing better diagnosis, staging, and monitoring of drug responses[9,10]. Related studies have shown that nitroimidazole not only has unique advantages in hypoxia but also exhibits strong anti-tumor activity. Additionally, nitroimidazole grafts can enhance targeted drug delivery[11]. Moreover, fluorine-18 fluoromisonidazole, which binds to intracellular macromolecules in hypoxic environments after reduction, cannot be reoxidized and washed out. In HCC orthotopic mouse models, significantly higher standardized uptake values of fluorine-18 fluoromisonidazole were found compared to non-cancerous liver groups, demonstrating tumor hypoxia, while 18F-fluorodeoxyglucose did not indicate tumor hypoxia[12]. Furthermore, studies have shown that tumor cells metabolize 11C-acetate under hypoxic conditions, with increased uptake of 11C-acetate in HCC orthotopic mouse models. Gene expression analysis indicated that the PET phenotype is associated with upregulation of characteristic hypoxic features, suggesting that 11C-acetate uptake correlates with hypoxic gene expression and poorer prognosis[13]. Radiomic features from contrast-enhanced computed tomography (CT) can predict hypoxia-inducible factors expression, indirectly predicting hypoxia, with an accuracy of 0.795 and an area under the curve (AUC) of 0.719[14]. This technology holds potential application value in assessing hypoxia in HCC. A study involving 30 HCC patients with cirrhosis found that blood oxygen level-dependent 3T magnetic resonance imaging (MRI) could assess changes in blood oxygen levels following transcatheter arterial embolization treatment[15]. Despite significant achievements in animal studies with PET imaging and many tracers showing great application value, high radiation exposure, high examination costs, and limitations in clinical applications for HCC remain major drawbacks. In contrast, MRI, as a non-invasive diagnostic technique, shows unique advantages in availability, repeatability, high sensitivity to varying hypoxia levels, and excellent spatial resolution, providing new insights for assessing local tumor hypoxia (Figure 1).
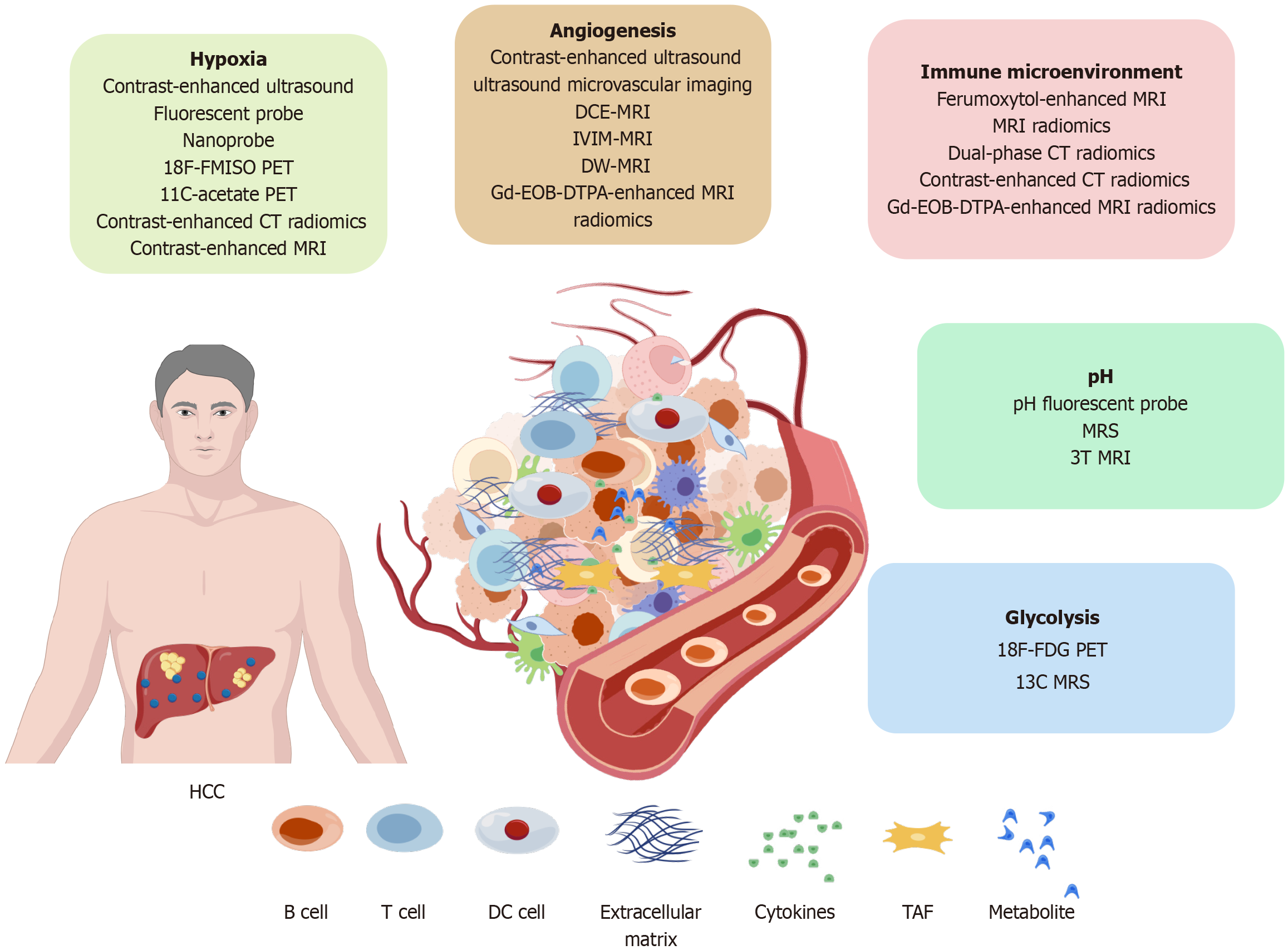
Figure 1 Hepatocellular carcinoma tumor microenvironment and imaging techniques.
HCC: Hepatocellular carcinoma; PET: Positron emission tomography; CT: Computed tomography; MRI: Magnetic resonance imaging; DCE-MRI: Dynamic contrast-enhanced magnetic resonance imaging; IVIM-MRI: Intravoxel incoherent motion magnetic resonance imaging; DW-MRI: Diffusion-weighted magnetic resonance imaging; MRS: Magnetic resonance spectroscopy; DC: Dendritic cell; TAF: Tumor-associated fibroblast; 18F-FDG PET: 18F-fluorodeoxyglucose.
ASSESSMENT OF THE IMPACT OF HCC GLYCOLYSIS
Changes in tumor metabolism are significant markers of cancer, as cancer cells strongly upregulate glucose uptake and glycolysis to produce biomolecules crucial for their survival, proliferation, and metastasis[16]. 18F-fluorodeoxyglucose is a glucose analogue that follows the same metabolic pathway as glucose. When the glycolytic rate in cancer cells increases, the uptake of 18F-fluorodeoxyglucose in tumor tissues also rises, making 18F-fluorodeoxyglucose PET useful for detecting the upregulation of glycolysis[17]. In clinical and research settings, glycolytic indicators for assessing tumor cell glucose metabolism include the maximum and average standardized uptake values of 18F-fluorodeoxyglucose, peak standardized uptake value, metabolic tumor volume, and total lesion glycolysis. These indicators can help evaluate tumor glycolysis, providing key information for diagnosis and treatment. In a study involving 47 HCC patients treated with 90Y transarterial radioembolization, researchers conducted 18F-fluorodeoxyglucose PET/CT scans before treatment and measured each patient’s maximum tumor standardized uptake value to average normal liver standardized uptake value ratio, metabolic tumor volume, and total lesion glycolysis. The results indicated that 18F-fluorodeoxyglucose PET/CT more accurately assessed tumor glycolysis, with high total lesion glycolysis being an independent prognostic factor for overall survival. The one-year overall survival rate for patients with low total lesion glycolysis was 72.9%, while it was 33.3% for patients with high total lesion glycolysis[18]. Additionally, 18F-fluorodeoxyglucose PET/CT can predict complete response in HCC patients undergoing transarterial radioembolization by measuring total lesion glycolysis[19]. Proton magnetic resonance spectroscopy (MRS) can also monitor elevated lactate levels in cirrhotic HCC patients, closely related to HCC glycolysis, with lactate levels serving as indicators for assessing glycolysis[20]. Hyperpolarized 13C MRS imaging is an emerging technology that can significantly enhance the MR signals of 13C-enriched molecules, particularly using 13C-rich pyruvate probes, providing metabolic information unattainable by non-invasive techniques and showing potential application value in monitoring downstream glycolysis[21]. In rat HCC models, Bliemsrieder et al[22] proposed 13C MRS imaging techniques for observing glycolytic changes in HCC, offering new possibilities for non-invasive assessment of HCC glycolysis. Additionally, pyruvate can serve as a promising probe molecule to distinguish particularly aggressive tumor phenotypes[22]. In clinical settings, inhibiting HCC glycolysis plays an important role in suppressing HCC development.
IMAGING TECHNIQUES FOR THE ASSESSMENT OF ANGIOGENESIS IN HCC
HCC is a typical hypervascular tumor, characterized by abnormal angiogenesis. This process is crucial for cancer cells to obtain nutrients and oxygen and serves as the basis for tumor growth, progression, invasion, and metastasis. Recent studies have shown that contrast-enhanced ultrasound can clearly visualize small lesions and surrounding vascular structures. In a prospective study involving 66 HCC patients receiving anti-angiogenic combination therapy, quantitative parameters obtained from contrast-enhanced ultrasound were found to predict the efficacy of the therapy, such as rise time ratio, time to peak ratio, decline time ratio, and peak enhancement ratio, with the rise time ratio being an independent predictive factor[23]. Furthermore, Xia et al[24] conducted a prospective assessment using contrast-enhanced ultrasound on 31 surgically confirmed HCC cases, calculating microvascular density and microvascular area percentage, discovering a strong correlation between microvascular density and HCC, enabling dynamic visualization of tumor microcirculation. Copper is an essential nutrient in mammals and acts as a cofactor in various physiological processes, playing a significant role in angiogenesis by stimulating endothelial cell proliferation in both benign and malignant conditions. The potential of 64Cu PET in diagnosing HCC angiogenesis and targeted therapy is substantial[25]. Additionally, dynamic contrast-enhanced MRI demonstrates unique advantages in assessing HCC microcirculation. In an evaluation of 52 HCC patients, its perfusion parameters (Ktrans, Kep, D*, f) showed significant positive correlations with microvascular density in HCC (r = 0.892, 0.808, 0.589, 0.543; P = 0.000, 0.000, 0.001, 0.003), with Ktrans showing the highest sensitivity, underscoring its importance in assessing HCC angiogenesis[26]. In evaluating anti-angiogenic therapies, dynamic contrast-enhanced MRI clearly demonstrated significant reductions in perfusion values. Ki67 and CD34 are histological markers reflecting tumor cell proliferation and microvascular density. In a retrospective study of HCC patients, the T1 relaxation time and signal intensity of Gadoxetate disodium (Gd-EOB-DTPA) enhanced MRI were significantly associated with Ki67 expression and microvascular density, with a 20-minute relaxation time showing high diagnostic value for high Ki67 expression and high microvascular density in HCC tissue[27,28]. MRI fat fraction quantification can also assess HCC proliferation and vascular invasion in a clinical study of 66 patients[29]. Moreover, diffusion-weighted imaging and intravoxel incoherent motion modeling also have potential application value in assessing angiogenesis in HCC, particularly in quantifying vascular endothelial growth factor. Studies indicate a significant positive correlation between the fast apparent diffusion coefficient (D*) and vascular endothelial growth factor (P < 0.001), which can be used for non-invasive assessment of angiogenesis in HCC[30]. The trabecular subtype of HCC is an aggressive variant associated with angiogenesis and an immunosuppressive TME. CT radiomics models can accurately predict the prognosis and efficacy of HCC patients with the trabecular subtype. Non-invasive radiomics models based on Gd-EOB-DTPA enhanced MRI can predict preoperative vascular endothelial growth factor status by calculating the AUC, with training set [AUC = 0.936, 95% confidence interval (CI): 0.898-0.974] and testing set (AUC = 0.836, 95%CI: 0.728-0.944), serving as reliable prognostic markers for vascular endothelial growth factor levels in HCC[31,32]. Dynamic contrast-enhanced MRI radiomics can also evaluate microvascular density in HCC patients, greatly assisting in the assessment of HCC[33]. In addition, the poor prognosis of HCC is related to angiopoietin 2, and the radiomics model based on dynamic contrast-enhanced-MRI can be used to evaluate the expression of angiopoietin 2 and indirectly evaluate the angiogenesis of HCC[34].
IMAGING OF EXTRACELLULAR PH IN HCC AND ITS APPLICATION IN MONITORING TUMOR TREATMENT
A decrease in pH is a significant characteristic of the HCC TME. HCC is often associated with severe hypoxia and high glycolytic activity, leading to the accumulation of large amounts of lactate, carbon dioxide, and pyruvate, which in turn causes a drop in pH[35]. MRS is commonly used to analyze metabolites and chemical compositions in living tissues and plays a vital role in pH quantification. Savic et al[36] detected pH values in HCC models following transarterial embolization and radiotherapy using MRS imaging. The results indicated a significant reduction in pH in untreated tissues. Multimodal imaging showed that after transarterial embolization and radiotherapy, tumors immediately exhibited sustained devascularization, with necrosis gradually increasing, suggesting that MRS-based pH measurement can serve as a longitudinal monitoring tool for viable tumors. Moreover, normalizing tumor pH through treatment may act as a biomarker for positive treatment outcomes[36]. Additionally, a 3T MRI scanner is a high-field MRI device that provides higher signal-to-noise ratios, faster scanning speeds, and improved image resolution, making it widely used in clinical diagnostics and research[37]. Coman et al[38] mapped the pH values of liver parenchyma and tumor margins in a rabbit HCC model using 3T MRI and validated the accuracy using pH electrodes in vitro, demonstrating its potential for clinical translational applications in monitoring tumor invasiveness and treatment outcomes.
APPLICATION OF IMAGING TECHNIQUES IN THE IMMUNE MICROENVIRONMENT OF HCC
In recent years, novel therapeutic strategies such as cancer immunosuppressive therapy have significantly extended the lifespan of patients. Although systemic treatment for HCC has undergone a major paradigm shift, treatment for advanced HCC remains insufficient due to the lack of evidence related to treatment resistance and response prediction. Therefore, elucidating the immune environment of HCC is critical to improving core therapeutic strategies and prognosis for HCC patients[39]. Aghighi et al[40] conducted ferumoxytol-enhanced MRI imaging on patients with osteosarcoma and lymphoma. Ferumoxytol, a superparamagnetic iron oxide nanoparticle, is efficiently taken up by tumor-associated macrophages. By using ferumoxytol as a contrast agent, MRI can capture signal changes related to the number of tumor-associated macrophages in tumors, and the T2-weighted imaging signal enhancement in MRI images was significantly correlated with the density of CD68+ and CD163+ tumor-associated macrophages. Ferumoxytol-enhanced MRI can be used as a surrogate biomarker for tumor-associated macrophages in malignant tumors. Movahedi et al[41] used macrophage mannose receptors and single-photon emission CT, and Jiang et al[42] tracked tumor-associated macrophages in mouse liver cancer using Cy7-labeled deoxyglucose and near-infrared fluorescence imaging, confirming the possibility of tumor-associated macrophage imaging in HCC. However, its application in HCC patients remains limited. Although studies quantifying tumor-associated macrophages in HCC are currently limited, this field holds great research potential. Studies have shown that forkhead box M1 is a proliferation-specific transcriptional regulator that promotes the migration of various immune cells, including macrophages, T cells, B cells, monocytes, and dendritic cells[43]. Dual-region CT radiomics can predict forkhead box M1 expression and prognosis in HCC. Researchers constructed a radiomics score significantly associated with forkhead box M1 by extracting radiomic features from both tumor and peritumoral regions. This score can serve as an independent predictor of immune cell infiltration in HCC, providing a new perspective on the characterization of the HCC immune microenvironment[44]. Programmed death receptor 1 is a key immune checkpoint molecule, mainly expressed on the surface of activated T cells, B cells, and other immune cells. Programmed death receptor 1 is an important immunosuppressive molecule that enables tumors to acquire immune escape capability. Monitoring programmed death receptor 1 expression can help assess the efficacy of immunotherapy for HCC. Related studies have established prediction models and radiomics-based immune scores using MRI radiomics to effectively quantify programmed death receptor 1 and observe the therapeutic response to anti programmed death receptor 1 immunotherapy. Research indicates that MRI radiomics models may have potential guidance in predicting programmed death receptor 1 efficacy for HCC immunotherapy[45]. Moreover, contrast-enhanced CT radiomics and Gd-EOB-DTPA-enhanced MRI radiomics can quantitatively analyze CD3+ and CD8+ T cells, further providing a basis for the non-invasive quantification and assessment of the HCC immune microenvironment, as well as for monitoring the efficacy of immunotherapy[46,47].
CONCLUSION
Although the imaging techniques discussed hold significant promise in research settings, their clinical adoption remains constrained by technical complexity and infrastructure demands. These techniques often rely on advanced equipment, intricate image processing algorithms, and specialized expertise. For example, implementing high-resolution MRI requires costly hardware and optimized scanning protocols, which pose significant challenges in resource-limited healthcare settings. To address these barriers, future research should focus on simplifying the deployment of these technologies or developing practical alternatives that reduce costs and complexity, thereby promoting broader clinical use. High costs play a pivotal role in determining the economic feasibility of integrating emerging imaging technologies into clinical practice. Comprehensive cost-effectiveness analyses are critical in this context. For early-stage liver cancer patients, imaging-based assessments of the TME may have limited impact on treatment decisions. Conversely, for patients with advanced or recurrent disease, such technologies could significantly influence clinical outcomes. Furthermore, not all HCC patients are suitable candidates for these advanced imaging methods. Studies highlight substantial variations in the demand for and benefits of imaging technologies across patient subgroups. For instance, advanced HCC patients often require imaging focused on tumor metabolic activity and immune microenvironment evaluation, while early-stage patients prioritize angiogenesis and local tissue characteristics. Future research should systematically compare the diagnostic accuracy, predictive power, and clinical feasibility of these technologies to provide more tailored recommendations for specific patient populations. Emerging imaging techniques offer not only enhanced diagnostic precision for HCC but also the potential to transform treatment strategies. By dynamically monitoring the TME, these technologies enable more accurate predictions of patient responses to targeted therapies and immunotherapies. This imaging-guided treatment model could significantly advance personalized care, facilitating the development of tailored treatment plans that adapt to individual tumor dynamics.
TME is intricately and closely linked to tumor hypoxia, energy metabolism, pH levels, and the immune environment. In-depth research into TME-related mechanisms can help identify effective therapeutic targets, formulate personalized treatment strategies, and develop new drugs. Therefore, imaging technologies, as non-invasive tools, offer new ideas and methods for studying the TME. By developing TME-related imaging biomarkers through molecular imaging technologies, clinicians can be assisted in diagnosing and monitoring tumors, adjusting personalized treatment plans, and improving patient outcomes, making this a field with broad clinical development prospects and significant importance.
ACKNOWLEDGEMENTS
We would like to express our sincere gratitude to all those who have supported and contributed to this work.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade A, Grade B, Grade B
Novelty: Grade A, Grade A, Grade A
Creativity or Innovation: Grade A, Grade B, Grade B
Scientific Significance: Grade A, Grade B, Grade B
P-Reviewer: Dong WK; Karagiannakis DS S-Editor: Wang JJ L-Editor: A P-Editor: Zheng XM