Published online Mar 14, 2025. doi: 10.3748/wjg.v31.i10.103420
Revised: January 31, 2025
Accepted: February 12, 2025
Published online: March 14, 2025
Processing time: 99 Days and 19.4 Hours
In the recent issue of the World Journal of Gastroenterology, Han et al compared the efficacy of and adverse reactions to bevacizumab versus lenvatinib as molecularly targeted agents in combination with interventional therapy and immunotherapy (IMT) to treat intermediate-to-advanced unresectable hepatocellular carcinoma. No significant differences in efficacy or adverse reactions were observed between bevacizumab and lenvatinib. This study is highly promising because in some regions, e.g., Japan, the combination of molecularly targeted therapy with IMT is fixed because of insurance restrictions, and some molecularly targeted agents cannot be combined with IMT. Further studies using these three modalities are expected to be conducted in the future. Additionally, because advanced radio
Core Tip: Many treatment approaches for intermediate-to-advanced unresectable hepatocellular carcinoma are advancing, and there is an increasing amount of evidence regarding the use of combination therapies. Although there may be limitations on combinations in some regions, there is a growing need for research on multimodal treatments, which serve as the cornerstone of personalized medicine. Based on this evidence, we believe that effective combination therapies should be sought for each patient.
- Citation: Sato K. Treatment of intermediate-to-advanced unresectable hepatocellular carcinoma is shifting toward a multidisciplinary strategy that includes multiple modalities as needed. World J Gastroenterol 2025; 31(10): 103420
- URL: https://www.wjgnet.com/1007-9327/full/v31/i10/103420.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i10.103420
In this article, I comment on the article by Han et al[1] published in the recent issue of the World Journal of Gastroenterology. They reported a retrospective study to compare bevacizumab with lenvatinib in combination with sintilimab, a programmed death-1 (PD-1) inhibitor, and interventional treatment for intermediate-to-advanced unresectable hepatocellular carcinoma (HCC). They investigated the differences in clinical efficacy and safety between the two molecularly targeted agents combined with an immune checkpoint inhibitor and interventional therapy. The results revealed no significant differences in progression-free survival (PFS), which was the primary endpoint. There were also no significant differences in overall survival (OS), the objective response rate (ORR), the disease control rate, and treatment-related adverse events (TRAEs), which were the secondary endpoints. These results indicated that bevacizumab and lenvatinib exhibited comparable efficacy and acceptable adverse reactions. In the future, we should aim to further improve the outcomes by changing the type of immunotherapy (IMT) used or considering various combinations.
In some regions, combining molecularly targeted therapy (MTT) with IMT and interventional treatment is impossible. For example, according to the algorithm for therapy for HCC in the 2021 edition of the Japanese Society of Hepatology's guidelines, if combined MTT and IMT are indicated, the primary drug therapy should be either atezolizumab as a programmed death ligand 1 (PD-L1) inhibitor plus bevacizumab (atezo-bev) or tremelimumab as a cytotoxic T lymphocyte-associated antigen-4 inhibitor plus durvalumab as a PD-L1 inhibitor. The complications of severe autoimmune disease may contraindicate IMT. If IMT is not indicated in such cases, monotherapy with MTTs such as sorafenib or lenvatinib is used. As PD-1 and PD-L1 inhibitors cannot be combined with sorafenib or lenvatinib under medical insurance, the use of combinations of MTTs and IMT such as checkpoint inhibitors is limited in Japan. However, combining interventional treatments is possible in Japan. A technique that combines lenvatinib and transarterial chemoembolization (TACE) as an interventional therapy is becoming more widespread in Japan. Tachiiri et al[2] reported that TACE combined with lenvatinib, which entails 4 days of lenvatinib administration before TACE without an intervening interval, was feasible and safe, had a high complete response (CR) rate (75% overall), improved lipiodol deposition after conventional TACE, and consequently prolonged the 12-month PFS rate to 75.0%. The following mechanisms are hypothesized: TACE following lenvatinib administration may order deformed tumor vasculature, consequently refining the intra-arterial drug distribution, and may also reduce the increase in vascular endothelial growth factor caused by hypoxia due to TACE[3,4]. Therefore, in regions where combinations of treatment modalities are limited, it is necessary to develop better combination regimens by optimizing the use of the modalities available.
However, a previous meta-analysis comparing TACE combined with MTT and IMT (TACE-MTT-IMT) versus TACE combined with MTT (TACE-MTT) revealed that add-on IMT provided additional clinical merits and survival benefits in unresectable HCC[5]. Feng et al[5] suggested the following reasons for the greater survival benefit of TACE-MTT-IMT than of TACE-MTT: TACE causes considerable local tumor necrosis and thereby increases antitumor immune responses through PD-1 inhibitors, which results in comprehensive treatment synergy[6]. The antiproliferative and antiangiogenic effects of multikinase inhibitors as MTT can act against hypoxia-induced angiogenesis due to TACE[7]. Additionally, they can regulate the tumor immune microenvironment and magnify the immune response to PD-1 inhibitors in HCC[8,9]. This synergistic antitumor activity improves the prognosis of HCC patients[5]. TACE-MTT-IMT exerts immunostimulatory effects on local and systemic immune activation by decreasing the tumor burden, increasing CD8+ T-cell infiltration, and reducing the suppressive effects of CD8+ T cells, thereby prolonging survival in HCC patients[10].
Notably, advances in therapy for HCC have been made, from conventional external beam radiotherapy (EBRT) techniques to advanced modalities such as intensity-modulated radiation therapy (IMRT), stereotactic body radiation therapy (SBRT), and innovative particle therapies such as proton therapy and carbon-ion radiation therapy[11], which can be combined with TACE-MTT-IMT to provide personalized treatment for each patient. A review by Kim and Kim[11] synthesized the results of the following clinical studies. A multicenter phase II trial conducted in Korea evaluated the combination of nivolumab that is a PD-1 inhibitor and EBRT in advanced HCC patients with macrovascular invasion[12]. The primary endpoints were PFS and safety[12]. The median PFS was 5.6 (90%CI: 3.6-9.9) months. Among the 50 patients included in this study, 40 (80.0%) and 6 (12.0%) patients experienced any grade TRAEs and grade 3/4 TRAEs, respectively[12]. Pruritus (38.0%) and rash (16.0%) were common TRAEs, and there were no deaths associated with the therapy[12]. Thus, the combination of nivolumab and EBRT was associated with favorable PFS and safety[12]. Additionally, a study conducted in Hong Kong compared the combination of SBRT and nivolumab in locally advanced HCC patients with TACE alone[13]. The primary outcome was PFS[13]. The 12- and 24-month PFS rates were significantly greater in the combination group than in the TACE alone group (93.3% vs 16.7% and 77.8% vs 2.1%, respectively, P < 0.001)[13]. The 12- and 24-month OS rates, which were secondary outcomes, were also better in the combination group than in the TACE alone group (93.8% vs 31.3% and 80.4% vs 8.3%, respectively, P < 0.001)[13]. There were fewer discontinuation of treatment (25% vs 12.5%, P = 0.295) because of adverse events (AEs) and ≥ grade 3 TRAEs (60.4% vs 18.8%, P = 0.004) in the combination group than in the TACE alone group[13]. Thus, the combination of SBRT and nivolumab was associated with significantly longer survival and fewer AEs than TACE alone[13]. As a safety-focused study, Zhang et al[14] compared the frequency of radiation-induced hepatic toxicity (RIHT) associated with RT plus PD-1 inhibitors with that associated with RT alone in patients with unresectable HCC[14]. After propensity score matching, aspartate aminotransferases ≥ grade 1 developed more often in the combination group (P = 0.020), and other hepatotoxicity metrics were not significantly different between the two groups[14]. They concluded that the incidence of RIHT in the combination therapy group was comparable to that in the RT alone group and was considered tolerable[14]. Thus, the combination of RT and IMT has considerable potential for further development in the treatment of HCC.
Regarding the combination of RT and MTT (RT-MTT), a systematic review and meta-analysis on the combined treatment of external RT and sorafenib in the treatment of HCC were conducted on the basis of 11 studies[15]. This study revealed that the median OS and PFS as the primary observation endpoints were 19.45 months and 8.20 months, respectively[15]. The incidence of AEs as the secondary observation endpoint was 0.34 (95%CI: 0.25-0.44)[15]. The study concluded that the survival of patients receiving combination therapy was significantly prolonged, and few severe AEs were observed[15]. Another systematic review and meta-analysis of 46 studies examined the use of sorafenib plus other therapeutic modalities for the treatment of advanced unresectable HCC[16]. OS, PFS, and AEs were set as the primary observation endpoints. Compared with sorafenib alone, SOF plus TACE, SOF plus RT, and SOF plus hepatic artery infusion chemotherapy were associated with improved OS and PFS (HR and 95%CI: 0.58, 0.48-0.70; 0.31, 0.21-0.47; 0.53, 0.45-0.62; HR and 95%CI: 0.69, 0.56-0.84; 0.44, 0.27-0.73; 0.45, 0.38-0.54), respectively[16]. Among these three combination therapies, a network of pairwise comparisons among seven studies revealed that SOF plus RT significantly improved OS and PFS[16]. SOF alone and SOF with combination therapies caused numerous AEs, although the frequency of AEs was lower in the SOF plus RT group[16]. Therefore, RT-MTT can be considered a promising treatment.
In terms of the combination of RT and TACE, a meta-analysis of nine studies compared SBRT plus TACE (SBRT-TACE) with TACE monotherapy for unresectable HCC with portal vein tumor thrombus[17]. Compared with TACE monotherapy, combination therapy resulted in significantly better 1-year OS rates [RR, 1.52 (95%CI: 1.33-1.74)], 2-year OS rates [RR, 2.00 (95%CI: 1.48-2.70)], ORRs [RR = 1.22 (95%CI: 1.08-1.37)], and a decreased rate of disease progression (PD) [RR = 0.45 (95%CI: 0.26-0.79)]. CR, partial response (PR), and stable disease were not significantly different between the two groups[17]. No significant differences in AEs were observed[17]. A phase 2 trial (NCT02513199) conducted SBRT-TACE for Barcelona Clinic Liver Cancer early-stage A patients with a solitary HCC ranging from 4 to 7 cm[18]. The primary endpoint was the best ORR[18]. Among the 30 patients who were enrolled, 91% had an ORR for the target lesion: 63% CR (n = 20), 28% PR (n = 9), and 3% PD (n = 1), while few toxic effects were observed[18]. Additionally, a single-center, prospective, randomized, controlled, parallel-group superiority trial (NCT02323360)[19] compared SBRT following incomplete transarterial embolization (TAE)/TACE with exclusive TAE or TACE for the treatment of unresectable HCC. The primary endpoint was 1-year local control (LC)[19]. LC was better in the group that switched treatment to SBRT than in the standard TAE/TACE rechallenge group (median not reached vs 8 months, P = 0.0002)[19]. No grade > 3 AEs were observed in either group[19]. Thus, SBRT-TACE is considered very promising.
There have been studies on the combination of RT and two other therapeutic modalities. Compared with sorafenib monotherapy, atezo-bev has been proven to achieve better 1-year OS and median PFS outcomes[20]. A retrospective study[21] compared concurrent atezo-bev and high-dose EBRT (atezo-bev-ERBT) to atezo-bev alone for highly advanced HCC with Vp4 portal vein thrombosis or tumors. The primary endpoints were ORR, OS, and safety[21]. The atezo-bev-ERBT group had a better ORR (50.0% vs 11.8%, P < 0.01) and a longer OS (not reached vs 5.5 months, P = 0.01)[21]. The rates of any-grade AEs (78.6% vs 58.8%, P = 0.19) and grade ≥ 3 AEs (14.3% vs 14.7%, P = 0.97) were not significantly different between the combination group and the atezo-bev alone group[21]. The combination of TACE, IMRT, with sorafenib (TACE-IMRT-sorafenib) was compared with the combination without sorafenib (TACE-IMRT) for advanced HCC patients with macrovascular invasion retrospectively[22]. The TACE-IMRT-sorafenib group had longer median PFS and OS than did the TACE-IMRT group (17.2 vs 9.4 months; P < 0.001, 24.1 vs 17.3 months; P < 0.001), respectively[22]. Although the TACE-IMRT-sorafenib group presented a significantly greater frequency of grade 1 to 2 AEs, such as hand-foot syndrome, no grade 4 or higher AEs were observed in either group[22]. Thus, the combination of TACE-IMRT and MTT is also worth considering in the future.
However, there are limitations with respect to the use of ERBT in combination with other therapeutic modalities. For example, contraindications for SBRT include Eastern Cooperative Oncology Group performance status score ≥ 3, hepatic decompensation, albumin-bilirubin grade 3, Child-Pugh score C, hepatic encephalopathy, bowel infiltration, significant ascites, and pregnancy[23]. Additionally, the liver and adjacent gastrointestinal organs or structures such as the stomach, duodenum, and bowel are susceptible to radiation-induced toxicity, which may cause ulcers, fistulas, bleeding, and rupture[23]. SBRT may cause radiation-induced liver disease, which is recognized as the most severe form of RIHT[14,24], and central hepatobiliary tract toxicity, which is characterized by complications such as biliary stenosis, biliary obstruction, cholangitis, and sepsis[23]. Additionally, intermediate-to-advanced unresectable HCC is likely associated with deteriorated liver function. Thus, the indications for RT must be carefully determined on a case-by-case basis.
Table 1[25-43] presents a summary of ongoing prospective trials of EBRT combined with TACE and/or MTT and/or IMT. Single-arm studies or SBRT-based treatments are common. The target diseases are not necessarily limited to unresectable HCC. Safety or tolerability as well as efficacy are selected as the primary endpoints. Notably, studies using combinations of these four modalities are also currently underway.
| Ref. | Clinical trial name | Countries | Start year | Design | Target diseases | Actual or estimated enrollment | Interventions | Primary endpoints |
| Dawson[25] | NCT01730937 | United States, Australia, Canada, China, and Korea | 2013 | Randomized, two-arm, phase 3 | HCC | 193 | Sorafenib vs SBRT followed by sorafenib | OS |
| Knox[26] | NCT03316872 | Canada | 2018 | Single-arm, phase 2 | Advanced HCC | 19 | Pembrolizumab (PD-1 inhibitor) and SBRT | ORR |
| Hong[27] | NCT03482102 | United States | 2018 | Single-arm, phase 2 | HCC or biliary tract cancer | 70 | Tremelimumab (CTLA-4 inhibitor), Durvalumab (PD-L1 inhibitor), and RT | Best ORR |
| Lock[28] | NCT03895359 | Canada | 2019 | Randomized, two-arm, phase 3 | Primary or secondary liver carcinoma | 128 | TACE vs TACE and SBRT | OS |
| Chan[29] | NCT04988945 | China | 2020 | Single-arm, phase 2 | Unresectable HCC | 33 | TACE, SBRT, Durvalumab, and Tremelimumab | Downstaging for liver resection |
| Sun[30] | NCT04387695 | China | 2020 | Randomized, two-arm, phase 3 | Unresectable HCC with PVTT | 54 | SBRT, TACE, and Sorafenib vs Sorafenib | PFS rate |
| Wo[31] | NCT04857684 | United States | 2021 | Single-arm, early phase 1 | Resectable HCC | 20 | Atezolizumab (PD-L1 inhibitor), Bevacizumab, and SBRT | Proportion of patients with grade 3-4 TRAE |
| [32] | NCT05010434 | China | 2021 | Single-arm, phase 2 | Advanced HCC | 46 | Sintilimab (PD-1 inhibitor), Bevacizumab, and RT | ORR |
| Welsh[33] | NCT04785287 | United States | 2021 | Randomized, two-arm, phase 1/2 | Advanced solid malignancies including Stage III/IV or metastatic liver cancer | 13 | Anti-CTLA4 monoclonal antibody BMS-986218 and SBRT with Nivolumab vs those without | Incidence of AE |
| Zhao[34] | NCT05185531 | China | 2022 | Single-arm, phase 1b | Resectable HCC | 20 | Tislelizumab (PD-1 inhibitor) and SBRT | Delay to surgery, ORR, pathologic response rate on evaluation of the resected specimen, and TEAE |
| Ben-Josef[35] | NCT05488522 | United States | 2022 | Single-arm, phase 1 | Advanced HCC | 18 | SBRT, Atezolizumab, and Bevacizumab | Tolerability and safety |
| Wo[36] | NCT05096715 | United States | 2022 | Single-arm, phase 1 | Unresectable HCC | 20 | Atezolizumab, Bevacizumab, and SBRT | Dose limiting toxicity rate |
| [37] | NCT05225116 | China | 2023 | Single-arm, phase 1 | HCC with PVTT | 20 | Sintilimab, Lenvatinib, and RT | Safety and number of patients who complete pre-op treatment and proceed to surgery |
| [38] | NCT05917431 | China | 2023 | Single-arm, phase 2 | Unresectable or oligometastatic HCC | 39 | SBRT, Tislelizumab, and Regorafenib | PFS |
| Xi[39] | NCT06261125 | China | 2024 | Two-arm, phase 2 | HCC with abdominal lymph node metastases | 60 | SBRT, Adebrelimab (PD-L1 inhibitor), and Lenvatinib | PFS |
| Wei[40] | NCT06561399 | China | 2024 | Single-arm, phase 2 | Unresectable HCC | 28 | TACE, Lenvatinib, Sintilimab, and RT | ORR |
| [41] | NCT06664996 | China | 2024 | Single-arm, phase 2 | Resectable HCC with PVTT | 33 | SBRT and Sintilimab | 1-year DFS rate |
| Zeng[42] | NCT06349317 | China | 2024 | Single-arm, phase 2 | Resectable HCC with PVTT | 33 | IMRT, Camrelizumab (PD-1 inhibitor), and Apatinib | 1-year EFS rate |
| [43] | NCT06349044 | China | 2024 | Randomized, two-arm, phase 2 (liver adenocarcinoma) | Advanced digestive system malignancies including liver adenocarcinoma | 120 (overall) | Hypo-RT/SBRT, Sintilimab, and Bevacizumab with Probio-M9 microbial agents vs those with placebo | ORR |
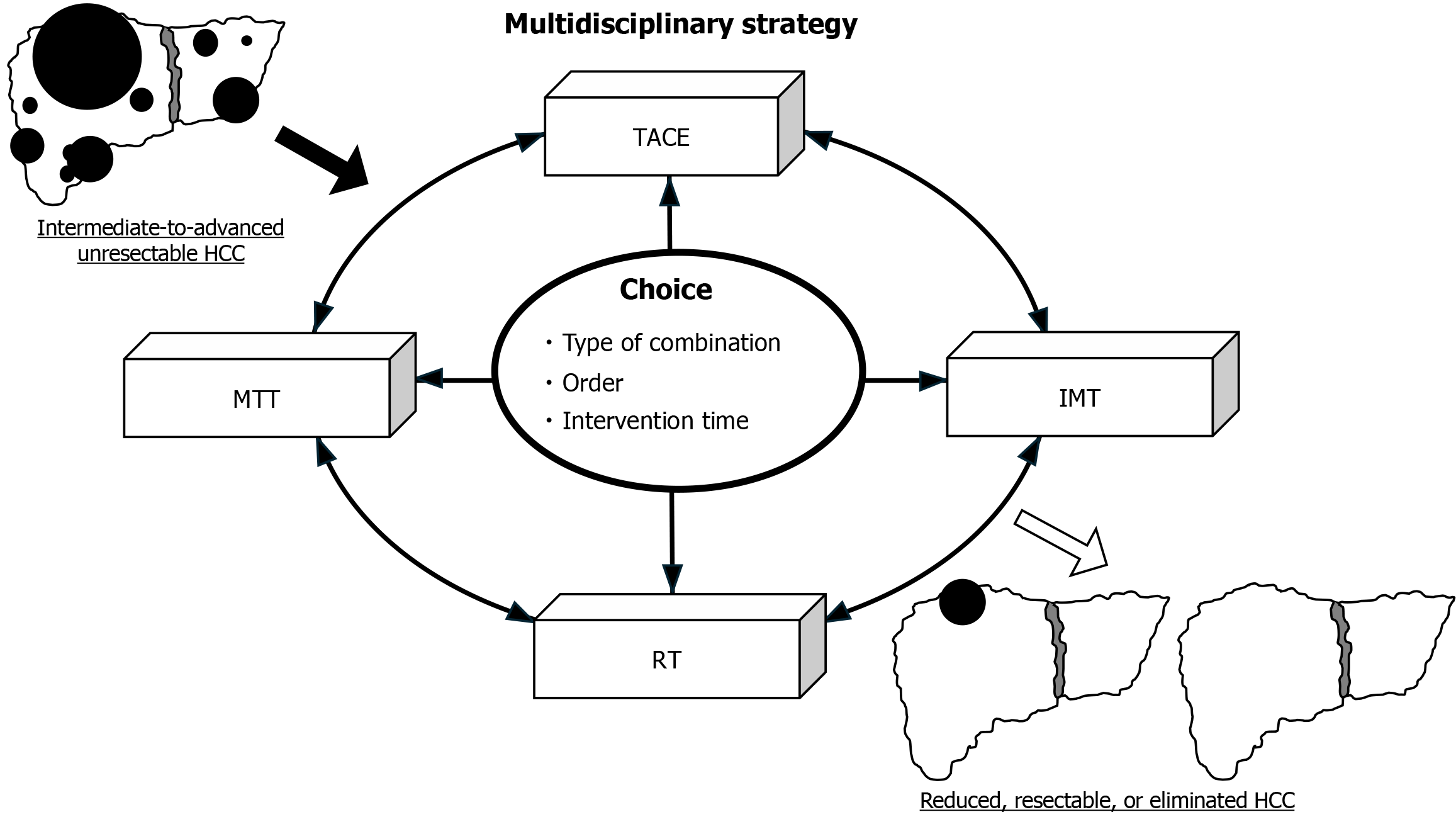
In conclusion, a multidisciplinary approach in which each patient is treated individually via a multimodal approach, such as combining TACE-MTT-IMT with RT and/or other modalities, is expected to further improve the prognosis of intermediate-to-advanced unresectable HCC patients (Figure 1). Improvements in the selection of combination modalities, the ordering of interventions, the timing of interventions, and the development of better regimen choices in areas where combination therapy is restricted should be investigated in many retrospective and prospective studies in the future.
| 1. | Han RY, Gan LJ, Lang MR, Ren SH, Liu DM, Li GT, Liu YY, Tian XD, Zhu KW, Sun LY, Chen L, Song TQ. Lenvatinib, sintilimab combined interventional treatment vs bevacizumab, sintilimab combined interventional treatment for intermediate-advanced unresectable hepatocellular carcinoma. World J Gastroenterol. 2024;30:4620-4635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | Tachiiri T, Minamiguchi K, Taiji R, Sato T, Toyoda S, Matsumoto T, Chanoki Y, Kunichika H, Yamauchi S, Shimizu S, Nishiofuku H, Marugami N, Tsuji Y, Namisaki T, Yoshiji H, Tanaka T. Effects of Short-Term Lenvatinib Administration Prior to Transarterial Chemoembolization for Hepatocellular Carcinoma. Cancers (Basel). 2024;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Kudo M. A New Treatment Option for Intermediate-Stage Hepatocellular Carcinoma with High Tumor Burden: Initial Lenvatinib Therapy with Subsequent Selective TACE. Liver Cancer. 2019;8:299-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 117] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 4. | Kudo M. Current Therapeutic Strategies for Hepatocellular Carcinoma in Japan. Liver Cancer. 2023;12:497-509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 5. | Feng J, Zhao Y, Zhai L, Zhou J. Efficacy and safety of transarterial chemoembolization combined with targeted therapy and immunotherapy versus with targeted monotherapy in unresectable hepatocellular carcinoma: A systematic review and meta-analysis. Medicine (Baltimore). 2024;103:e38037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 6. | Cheu JW, Wong CC. Mechanistic Rationales Guiding Combination Hepatocellular Carcinoma Therapies Involving Immune Checkpoint Inhibitors. Hepatology. 2021;74:2264-2276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 7. | Chang Y, Jeong SW, Young Jang J, Jae Kim Y. Recent Updates of Transarterial Chemoembolilzation in Hepatocellular Carcinoma. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 203] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 8. | Kimura T, Kato Y, Ozawa Y, Kodama K, Ito J, Ichikawa K, Yamada K, Hori Y, Tabata K, Takase K, Matsui J, Funahashi Y, Nomoto K. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 2018;109:3993-4002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 260] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 9. | Yi C, Chen L, Lin Z, Liu L, Shao W, Zhang R, Lin J, Zhang J, Zhu W, Jia H, Qin L, Lu L, Chen J. Lenvatinib Targets FGF Receptor 4 to Enhance Antitumor Immune Response of Anti-Programmed Cell Death-1 in HCC. Hepatology. 2021;74:2544-2560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 214] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 10. | Sun B, Zhang L, Sun T, Ren Y, Cao Y, Zhang W, Zhu L, Guo Y, Gui Y, Liu F, Chen L, Xiong F, Zheng C. Safety and efficacy of lenvatinib combined with camrelizumab plus transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: A two-center retrospective study. Front Oncol. 2022;12:982948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 11. | Kim D, Kim JS. Current perspectives on radiotherapy in hepatocellular carcinoma management: a comprehensive review. J Liver Cancer. 2024;24:33-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 12. | Kim BH, Park HC, Kim TH, Koh YH, Hong JY, Cho Y, Sinn DH, Park B, Park JW. Concurrent nivolumab and external beam radiation therapy for hepatocellular carcinoma with macrovascular invasion: A phase II study. JHEP Rep. 2024;6:100991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 13. | Chiang CL, Chiu KW, Lee FA, Kong FS, Chan AC. Combined Stereotactic Body Radiotherapy and Immunotherapy Versus Transarterial Chemoembolization in Locally Advanced Hepatocellular Carcinoma: A Propensity Score Matching Analysis. Front Oncol. 2021;11:798832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Zhang RJ, Zhou HM, Lu HY, Yu HP, Tang WZ, Qiu MQ, Yan LY, Long MY, Su TS, Xiang BD, He ML, Wang XT, Liang SX, Li JX. Radiotherapy plus anti-PD1 versus radiotherapy for hepatic toxicity in patients with hepatocellular carcinoma. Radiat Oncol. 2023;18:129. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Chen J, He K, Han Y, Guo L, Su K, Wu Z. Clinical efficacy and safety of external radiotherapy combined with sorafenib in the treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Ann Hepatol. 2022;27:100710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Li H, Wu Z, Chen J, Su K, Guo L, Xu K, Gu T, Jiang Y, Wang P, Zeng H, Chi H, He K, Han Y. External radiotherapy combined with sorafenib has better efficacy in unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Clin Exp Med. 2023;23:1537-1549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 17. | Zhang XF, Lai L, Zhou H, Mo YJ, Lu XQ, Liu M, Lu YX, Hou EC. Stereotactic body radiotherapy plus transcatheter arterial chemoembolization for inoperable hepatocellular carcinoma patients with portal vein tumour thrombus: A meta-analysis. PLoS One. 2022;17:e0268779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 18. | Buckstein M, Kim E, Özbek U, Tabrizian P, Gunasekaran G, Facciuto M, Rosenzweig K, Llovet JM, Schwartz M. Combination Transarterial Chemoembolization and Stereotactic Body Radiation Therapy for Unresectable Single Large Hepatocellular Carcinoma: Results From a Prospective Phase 2 Trial. Int J Radiat Oncol Biol Phys. 2022;114:221-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 19. | Comito T, Loi M, Franzese C, Clerici E, Franceschini D, Badalamenti M, Teriaca MA, Rimassa L, Pedicini V, Poretti D, Solbiati LA, Torzilli G, Ceriani R, Lleo A, Aghemo A, Santoro A, Scorsetti M. Stereotactic Radiotherapy after Incomplete Transarterial (Chemo-) Embolization (TAE\TACE) versus Exclusive TAE or TACE for Treatment of Inoperable HCC: A Phase III Trial (NCT02323360). Curr Oncol. 2022;29:8802-8813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 20. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4706] [Article Influence: 941.2] [Reference Citation Analysis (2)] |
| 21. | Su CW, Teng W, Shen EY, Huang BS, Lin PT, Hou MM, Wu TH, Tsan DL, Hsieh CH, Wang CT, Chai PM, Lin CY, Lin SM, Lin CC. Concurrent Atezolizumab Plus Bevacizumab and High-Dose External Beam Radiotherapy for Highly Advanced Hepatocellular Carcinoma. Oncologist. 2024;29:e922-e931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 22. | Yang D, Du J, Nie W, Wang C, Ma Z. Combination treatment of transcatheter arterial chemoembolization, intensity-modulated radiotherapy, and sorafenib for hepatocellular carcinoma with macrovascular invasion. Medicine (Baltimore). 2023;102:e35713. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Sharma D, Khosla D, Meena BL, Yadav HP, Kapoor R. Exploring the Evolving Landscape of Stereotactic Body Radiation Therapy in Hepatocellular Carcinoma. J Clin Exp Hepatol. 2025;15:102386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 24. | Liang SX, Zhu XD, Xu ZY, Zhu J, Zhao JD, Lu HJ, Yang YL, Chen L, Wang AY, Fu XL, Jiang GL. Radiation-induced liver disease in three-dimensional conformal radiation therapy for primary liver carcinoma: the risk factors and hepatic radiation tolerance. Int J Radiat Oncol Biol Phys. 2006;65:426-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 198] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 25. | Dawson L. Sorafenib tosylate with or without stereotactic body radiation therapy in treating patients with liver cancer. [accessed 2025 Jan 19]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT01730937 ClinicalTrials.gov Identifier: NCT01730937. |
| 26. | Knox JJ. Study of pembrolizumab and radiotherapy in liver cancer. [accessed 2025 Jan 19]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT03316872 ClinicalTrials.gov Identifier: NCT03316872. |
| 27. | Hong TS. Durvalumab (MEDI4736) and tremelimumab and radiation therapy in hepatocellular carcinoma and biliary tract cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT03482102 ClinicalTrials.gov Identifier: NCT03482102. |
| 28. | Lock M. Transarterial chemoembolization (TACE) versus TACE plus stereotactic body radiation therapy (SBRT) in liver carcinoma (TACE). In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT03895359 ClinicalTrials.gov Identifier: NCT 03895359. |
| 29. | Chan A. TACE and SBRT followed by double immunotherapy for downstaging hepatocellular carcinoma. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT04988945 ClinicalTrials.gov Identifier: NCT04988945. |
| 30. | Sun JH. SBRT+TACE+sorafenib vs sorafenib in the treatment of uHCC with PVTT. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT04387695 ClinicalTrials.gov Identifier: NCT04387695. |
| 31. | Wo JY. SBRT + atezolizumab + bevacizumab in resectable HCC. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT04857684 ClinicalTrials.gov Identifier: NCT04857684. |
| 32. | Sintilimab and bevacizumab combined with radiotherapy for advanced hepatocellular carcinoma. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT05010434 ClinicalTrials.gov Identifier: NCT05010434. |
| 33. | Welsh J. Anti-CTLA4-NF mAb (BMS986218), nivolumab, and stereotactic body radiation therapy for the treatment of metastatic solid malignancies. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT04785287 ClinicalTrials.gov Identifier: NCT04785287. |
| 34. | Zhao L. A study of neoadjuvant tislelizumab with SBRT in patients with resectable hepatocellular carcinoma (Notable-HCC). In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT05185531 ClinicalTrials.gov Identifier: NCT05185531. |
| 35. | Ben-Josef E. SBRT With Atezo/Bev for HCC. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT05488522 ClinicalTrials.gov Identifier: NCT05488522. |
| 36. | Wo JY. Atezolizumab+bevacizumab+SBRT in unresectable HCC. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT05096715 ClinicalTrials.gov Identifier: NCT05096715. |
| 37. | Safety and efficacy of lenvatinib and anti-PD1 antibody combined with radiotherapy neoadjuvant treatment for resectable hepatocellular carcinoma with PVTT. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT05225116 ClinicalTrials.gov Identifier: NCT05225116. |
| 38. | Phase 2 study of SBRT plus tislelizumab and regorafenib in unresectable or oligometastatic HCC. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT05917431 ClinicalTrials.gov Identifier: NCT05917431. |
| 39. | Xi M. Combination of SBRT, PD-L1 inhibitor, and lenvatinib in hepatocellular carcinoma (HSBRT2401) (HSBRT2401). In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT06261125 ClinicalTrials.gov Identifier: NCT06261125. |
| 40. | Wei SM. Triple therapy sequential radiotherapy in unresectable HCC (TALENP003). In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT06561399 ClinicalTrials.gov Identifier: NCT06561399. |
| 41. | Sintilimab combined SBRT as neoadjuvant therapy for resectable HCC with PVTT. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT06664996 ClinicalTrials.gov Identifier: NCT06664996. |
| 42. | Zeng Y. Neoadjuvant IMRT combined with camrelizumab and apatinib for resectable HCC with PVTT. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT06349317 ClinicalTrials.gov Identifier: NCT06349317. |
| 43. | A randomized, multicenter phase II basket study of hypofractionated radiotherapy/stereotactic body radiotherapy followed by immunotherapy-based systemic therapy +/- L. Rhamnosus M9 for the first-line treatment of advanced digestive system malignancies. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT06349044 ClinicalTrials.gov Identifier: NCT06349044. |