Published online Mar 14, 2025. doi: 10.3748/wjg.v31.i10.102725
Revised: January 16, 2025
Accepted: January 23, 2025
Published online: March 14, 2025
Processing time: 121 Days and 15.1 Hours
This article discusses the manuscript recently published in the World Journal of Gastroenterology, which explores the application of deep learning models in decision-making processes via wireless capsule endoscopy. Integrating artificial intelligence (AI) into gastrointestinal disease diagnosis represents a trans
Core Tip: Integrating artificial intelligence (AI) in gastrointestinal diagnostics enhances early detection accuracy, offering a promising tool in precision medicine. These models, such as those employed in wireless capsule endoscopy, enable real-time identification of subtle lesions that might be missed by clinicians alone. However, the ethical implications are significant. AI systems must address data security and biases, particularly regarding equitable care across diverse patient groups. Additionally, AI should support rather than overshadow clinical judgment, maintaining the critical balance in physician-patient relationships. By focusing on these ethical pillars, AI’s role can evolve responsibly, delivering both innovative and fair diagnostics while safeguarding patient trust.
- Citation: Ramoni D, Scuricini A, Carbone F, Liberale L, Montecucco F. Artificial intelligence in gastroenterology: Ethical and diagnostic challenges in clinical practice. World J Gastroenterol 2025; 31(10): 102725
- URL: https://www.wjgnet.com/1007-9327/full/v31/i10/102725.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i10.102725
The landscape of diagnostic medicine, particularly in gastroenterology, is rapidly evolving with the integration of artificial intelligence (AI). The capacity of AI and deep learning models to elevate early diagnosis and detection of gastrointestinal (GI) diseases, most notably GI cancers, marks a revolutionary stride toward precision medicine. In the field of diagnostic imaging, AI technologies have proven to be invaluable, enhancing the accuracy of procedures like endoscopy throughout the explorable GI tract[1,2]. These AI-driven advancements facilitate real-time analysis and allow for the identification of early-stage precancerous polyps and subtle lesions that might otherwise escape detection by the human eye.
Recent studies underscore the significant potential of these AI-technologies in gastroenterology. In a retrospective study recently published in the World Journal of Gastroeneterology, Xiao et al[3] proposed a deep learning-based multi-lesion classification model that successfully identified and labeled 23 distinct types of digestive tract lesions via wireless capsule endoscopy (WCE). This model not only improves lesion detection rates but also provides a nuanced analysis capable of simultaneously categorizing multiple lesion types. Evaluating the clinical value and developmental potential of this WCE detection model reveals its impact on diagnostic precision and efficiency, leveraging advanced deep learning techniques, such as the Swin Transformer[4], to accurately and rapidly detect a wide variety of lesions. These advan
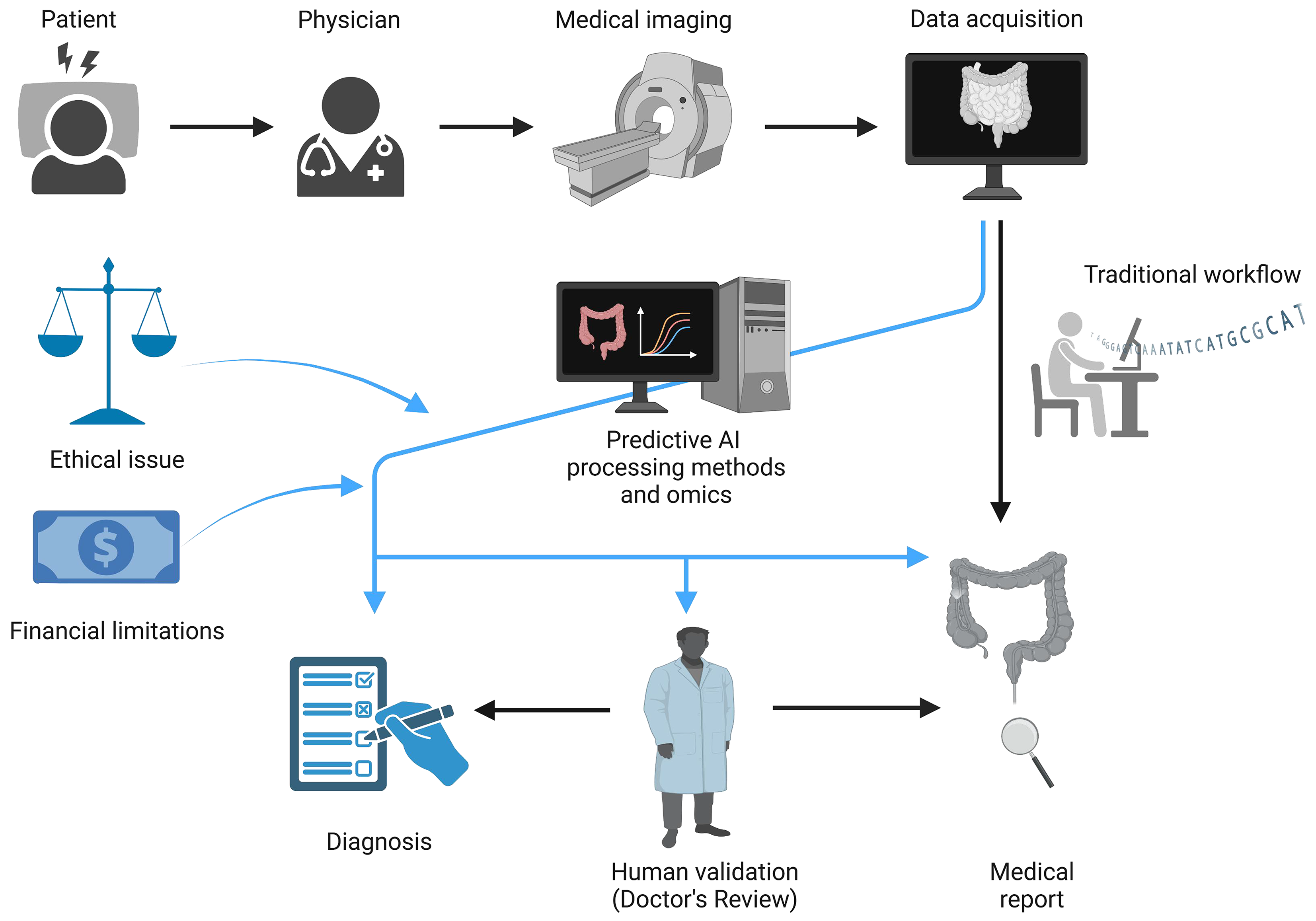
The implications of integrating AI into GI diagnostics extend beyond improved detection; they reshape the entire diagnostic workflow (Figure 1). AI-driven diagnostic tools are already transforming clinical workflows by streamlining the diagnostic process and enabling faster, more accurate analyses. Traditionally, clinicians have spent significant time and resources manually analyzing high-resolution imaging data from procedures like WCE. However, AI-powered models now automate much of this work, reducing the time required to reach a diagnosis and freeing up valuable time for clinicians to focus on treatment planning and patient care[6]. This efficiency is crucial in managing the growing number of GI cases, where a timely diagnosis can significantly influence treatment options and outcomes. However, the integration of these technologies into routine clinical practice necessitates robust validation studies and the development of user-friendly interfaces for healthcare providers.
At present, it is reasonable to anticipate that AI could find applications across various domains of gastroenterology. One of the most compelling applications of AI in GI diagnostics is its capacity for predictive analysis, an innovation that aligns with the core principles of precision medicine. By leveraging large datasets that include imaging data, genetic information, and patient medical histories, AI can assist in predicting disease progression and recurrence risks for con
Other potential application includes the diagnosis of structural and functional disorders, such as hiatal hernia and achalasia. In the case of hiatal hernia, AI algorithms could be applied to imaging data to enable precise assessments of anatomical abnormalities, facilitating early detection and personalized treatment strategies[11]. Similarly, for achalasia, AI-assisted analysis of high-resolution esophageal manometry significantly enhances the classification of motility disorders[12]. Building on this, AI-driven diagnostic systems have also emerged as transformative tools to address the substantial challenges posed by upper GI cancers, which often progress asymptomatically in their early stages. As previously mentioned, AI demonstrates exceptional proficiency in analyzing high-resolution endoscopic images, enabling the detection of early neoplastic changes with remarkable sensitivity[13,14]. Therefore, machine learning models trained on extensive datasets of upper GI images have achieved impressive accuracy, contributing to the prediction of postoperative complications and disease progression following upper GI surgeries for malignancies[15].
In addition to these diagnostic advancements, upper GI cancers often lead to nutritional complications, such as progressive dysphagia and cachexia. AI-based predictive models can assess malnutrition risk and provide personalized nutritional intervention recommendations[16,17]. By addressing both diagnostic and supportive care needs, AI offers a comprehensive approach to managing upper GI cancers, with the potential to enhance clinical outcomes and improve patient quality of life.
The integration of omics sciences and AI is reshaping the landscape of biomedical research and clinical practice, particularly in the pursuit of precision medicine[18]. This convergence leverages the capabilities of genomics, proteomics, and metabolomics to uncover genetic predispositions, monitor disease progression, and provide detailed insights into disease mechanisms. AI, in turn, plays a crucial role in managing and analyzing the vast datasets generated by these omics technologies, extracting actionable insights for improved diagnostics and therapeutics.
Genomics has facilitated the identification of specific genetic predispositions to diseases, while proteomics and metabolomics provide insights into the functional dynamics of disease progression. Together, these disciplines produce complex, data-rich outputs that require advanced computational tools for interpretation. AI-driven machine learning models, including support vector machines and random forests, are increasingly being used to identify biomarkers and patterns in these datasets[19]. This is particularly evident in conditions like GI cancers and inflammatory bowel disease, where AI has been instrumental in detecting mutations and expression patterns associated with disease onset and progression[20,21].
A significant strength of AI lies in its ability to integrate multi-omics datasets. By linking genetic, proteomic, and clinical variables, AI offers a comprehensive perspective on disease mechanisms, enabling more precise diagnoses and personalized treatment plans. This integrative approach significantly enhances physicians’ ability to detect diseases at an early stage and predict their trajectories with unprecedented accuracy.
Additionally, AI-assisted imaging technologies further extend the benefits of this integration. Advanced techniques like radiomics and deep learning-based imaging analysis allow for the identification of disease-specific patterns in non-invasive modalities such as radiological scans and histopathological slides[22]. These tools are transforming diagnostics by moving beyond symptom-based assessments to molecularly informed, evidence-based decisions.
While the advancements discussed are undoubtedly transformative, they also present challenges. Issues like data standardization, ethical concerns surrounding AI-driven decision-making, and ensuring equitable access to these technologies remain critical. Addressing these challenges is essential to maximize the potential of omics and AI integration in global healthcare. This paradigm shift underscores the need for continued investment in research, technology, and policy development to ensure its benefits are universally realized.
Ethical concerns also require attention to ensure equitable access and unbiased care. The ethical implications of using AI in gastroenterology are broad, raising concerns that go beyond technological feasibility and diagnostic accuracy to encompass privacy, equity, data security, patient autonomy, and the dynamics of the physician-patient relationship[23]. As AI-based diagnostic tools like multi-lesion classification models become more embedded in clinical practice, they necessitate robust frameworks to protect patient privacy, especially given their reliance on extensive data for training and operational efficacy[24]. Most AI models draw from vast collections of patient records, including imaging and clinical history data, to build algorithms capable of highly accurate lesion identification. Even with standard practices in anonymization, the risk of re-identifying patient information remains, particularly as datasets grow in size and detail. The challenge lies in implementing stringent data security measures and ensuring compliance with data protection regulations like the General Data Protection Regulation law to mitigate the risks of data breaches and unauthorized data use[25]. Such regulatory frameworks are crucial for establishing trust and accountability in how patient data is handled within AI-driven healthcare[26].
Moreover, the potential for bias within AI models poses another significant ethical concern. If AI algorithms are trained on datasets that overrepresent certain populations—such as a particular age group, ethnicity, or geographic region—their diagnostic accuracy may significantly vary across demographic groups[27]. This variability risks exacerbating healthcare disparities, as some patients might receive less accurate or less effective diagnostics than others[28]. AI models require extensive datasets for training, many with the potential for bias within AI algorithms. AI models are only as unbiased as the data on which they are trained; if a model is trained on data that overrepresents a particular demographic, it may perform sub-optimally or inaccurately when applied to a broader population. This is particularly concerning in a field as diverse as gastroenterology, where diagnostic and disease progression patterns can vary across demographics and ethnicities[29]. Addressing these biases requires that training datasets be representative of diverse patient groups and that models undergo continuous validation to ensure equitable outcomes.
Additionally, as AI tools take on more central roles in diagnostic processes, there is growing concern about the potential for diminished clinical judgment and the impact on physician-patient dynamics. With the increasing accuracy of AI-driven models, there is a risk of over-reliance on machine-generated outputs, which could inadvertently reduce the role of clinician expertise in decision-making[30]. This shift could weaken the nuanced, individualized approach that is often crucial in complex diagnostic cases, where factors beyond raw data, such as the patient’s full clinical picture, comorbidities, and preferences, need careful consideration. To mitigate this risk, AI should be implemented as a complementary tool that augments, rather than replaces, clinical judgment[31]. Ethical guidelines that promote a collaborative approach, where AI provides support rather than prescriptive recommendations, can help clinicians maintain a balanced and comprehensive approach to patient care[32].
Furthermore, patient autonomy and informed consent also play essential roles in the ethical application of AI in gastroenterology. Patients have the right to understand how AI is influencing their diagnosis and treatment options. The use of AI in diagnostics, particularly in sensitive areas like cancer detection, underscores the need for transparency around AI’s role and capabilities. Informed consent protocols should reflect the complexity of AI integration, ensuring that patients are not only aware of its benefits but also of potential limitations or areas where human oversight is critical[33].
Lastly, the integration of AI into gastroenterology raises regulatory and accountability concerns that extend beyond the clinical setting. As AI models continually learn and adapt, their decision-making processes may become opaque, creating an effect where even the developers or clinicians cannot fully explain why certain recommendations or classifications were made[34,35]. This lack of transparency can impact both clinician trust and patient confidence, particularly in high-stakes diagnoses. Establishing regulatory clinical guidelines and ethical standards that require explainability and accountability in AI algorithms is essential for preserving the integrity of AI-based diagnostics.
In addition to ethical concerns, the practical application of AI in clinical practice is influenced by significant financial limitations. Implementing AI systems often requires substantial investment in technology infrastructure, ongoing maintenance, and specialized training, which may limit accessibility, especially in resource-constrained settings. Furthermore, the attitudes of various stakeholders, including healthcare providers, patients, and regulatory organizations, play a crucial role in the adoption and success of AI in everyday practice. Clinicians’ acceptance of AI tools depends on their trust in technology’s reliability and the perceived impact on workflow and patient care. Patient attitudes, shaped by concerns over data privacy and the impersonality of AI-driven diagnoses, may also affect adoption. Thus, addressing both the financial implications and stakeholder perspectives is essential for an understanding of AI's potential and limitations in clinical practice.
In summary, the ethical landscape surrounding AI in gastroenterology is complex, underscoring the need for a balanced approach that respects patient rights, fosters equitable care, and preserves the integrity of clinical decision-making. Addressing these ethical challenges is essential to ensuring that AI fulfills its potential to transform diagnostics without compromising fairness, transparency, or patient trust. Through responsible data practices, efforts to mitigate bias, safeguards for patient autonomy, and strong regulatory frameworks, the integration of AI in gastroenterology can align with the core principles of ethical, patient-centered care.
Looking to the future, the integration of AI and omics in GI disease management is poised to deepen as technologies advance. Future developments could enable even more comprehensive diagnostic systems that incorporate multimodal data, combining genetic, imaging, and clinical information, for a holistic view of patient health. With these capabilities, AI can become an indispensable tool in personalized medicine, offering tailored treatment plans based on a patient’s unique risk factors and disease profile. Yet, as this technology becomes more embedded in clinical practice, it is essential to address the ethical and operational challenges it presents. By fostering a collaborative approach that respects both technological innovation and human expertise, the field of gastroenterology stands to benefit from AI’s contributions, ultimately enhancing patient care and outcomes in ways previously thought unattainable.
Regulatory guidelines will also play a crucial role in supporting the safe and effective use of AI, ensuring transparency in AI’s decision-making processes and establishing quality standards for AI applications in healthcare.
| 1. | Moen S, Vuik FER, Kuipers EJ, Spaander MCW. Artificial Intelligence in Colon Capsule Endoscopy-A Systematic Review. Diagnostics (Basel). 2022;12:1994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Yamaguchi D, Shimoda R, Miyahara K, Yukimoto T, Sakata Y, Takamori A, Mizuta Y, Fujimura Y, Inoue S, Tomonaga M, Ogino Y, Eguchi K, Ikeda K, Tanaka Y, Takedomi H, Hidaka H, Akutagawa T, Tsuruoka N, Noda T, Tsunada S, Esaki M. Impact of an artificial intelligence-aided endoscopic diagnosis system on improving endoscopy quality for trainees in colonoscopy: Prospective, randomized, multicenter study. Dig Endosc. 2024;36:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 3. | Xiao ZG, Chen XQ, Zhang D, Li XY, Dai WX, Liang WH. Image detection method for multi-category lesions in wireless capsule endoscopy based on deep learning models. World J Gastroenterol. 2024;30:5111-5129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (1)] |
| 4. | Liu Z, Lin Y, Cao Y, Hu H, Wei Y, Zhang Z, Lin S, Guo B. Swin Transformer: Hierarchical Vision Transformer using Shifted Windows. 2021 IEEE/CVF International Conference on Computer Vision (ICCV), 2021. [DOI] [Full Text] |
| 5. | Repici A, Spadaccini M, Antonelli G, Correale L, Maselli R, Galtieri PA, Pellegatta G, Capogreco A, Milluzzo SM, Lollo G, Di Paolo D, Badalamenti M, Ferrara E, Fugazza A, Carrara S, Anderloni A, Rondonotti E, Amato A, De Gottardi A, Spada C, Radaelli F, Savevski V, Wallace MB, Sharma P, Rösch T, Hassan C. Artificial intelligence and colonoscopy experience: lessons from two randomised trials. Gut. 2022;71:757-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 134] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 6. | Mohan BP, Khan SR, Kassab LL, Ponnada S, Chandan S, Ali T, Dulai PS, Adler DG, Kochhar GS. High pooled performance of convolutional neural networks in computer-aided diagnosis of GI ulcers and/or hemorrhage on wireless capsule endoscopy images: a systematic review and meta-analysis. Gastrointest Endosc. 2021;93:356-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Zeng Q, Klein C, Caruso S, Maille P, Allende DS, Mínguez B, Iavarone M, Ningarhari M, Casadei-Gardini A, Pedica F, Rimini M, Perbellini R, Boulagnon-Rombi C, Heurgué A, Maggioni M, Rela M, Vij M, Baulande S, Legoix P, Lameiras S; HCC-AI study group, Bruges L, Gnemmi V, Nault JC, Campani C, Rhee H, Park YN, Iñarrairaegui M, Garcia-Porrero G, Argemi J, Sangro B, D'Alessio A, Scheiner B, Pinato DJ, Pinter M, Paradis V, Beaufrère A, Peter S, Rimassa L, Di Tommaso L, Vogel A, Michalak S, Boursier J, Loménie N, Ziol M, Calderaro J. Artificial intelligence-based pathology as a biomarker of sensitivity to atezolizumab-bevacizumab in patients with hepatocellular carcinoma: a multicentre retrospective study. Lancet Oncol. 2023;24:1411-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 8. | Waljee AK, Wallace BI, Cohen-Mekelburg S, Liu Y, Liu B, Sauder K, Stidham RW, Zhu J, Higgins PDR. Development and Validation of Machine Learning Models in Prediction of Remission in Patients With Moderate to Severe Crohn Disease. JAMA Netw Open. 2019;2:e193721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 9. | Rimondi A, Gottlieb K, Despott EJ, Iacucci M, Murino A, Tontini GE. Can artificial intelligence replace endoscopists when assessing mucosal healing in ulcerative colitis? A systematic review and diagnostic test accuracy meta-analysis. Dig Liver Dis. 2024;56:1164-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 10. | Deding U, Herp J, Havshoei AL, Kobaek-Larsen M, Buijs MM, Nadimi ES, Baatrup G. Colon capsule endoscopy versus CT colonography after incomplete colonoscopy. Application of artificial intelligence algorithms to identify complete colonic investigations. United European Gastroenterol J. 2020;8:782-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Kafetzis I, Fuchs KH, Sodmann P, Troya J, Zoller W, Meining A, Hann A. Efficient artificial intelligence-based assessment of the gastroesophageal valve with Hill classification through active learning. Sci Rep. 2024;14:18825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Popa SL, Surdea-Blaga T, Dumitrascu DL, Chiarioni G, Savarino E, David L, Ismaiel A, Leucuta DC, Zsigmond I, Sebestyen G, Hangan A, Czako Z. Automatic Diagnosis of High-Resolution Esophageal Manometry Using Artificial Intelligence. J Gastrointestin Liver Dis. 2022;31:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Xin Y, Zhang Q, Liu X, Li B, Mao T, Li X. Application of artificial intelligence in endoscopic gastrointestinal tumors. Front Oncol. 2023;13:1239788. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Luo H, Xu G, Li C, He L, Luo L, Wang Z, Jing B, Deng Y, Jin Y, Li Y, Li B, Tan W, He C, Seeruttun SR, Wu Q, Huang J, Huang DW, Chen B, Lin SB, Chen QM, Yuan CM, Chen HX, Pu HY, Zhou F, He Y, Xu RH. Real-time artificial intelligence for detection of upper gastrointestinal cancer by endoscopy: a multicentre, case-control, diagnostic study. Lancet Oncol. 2019;20:1645-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 253] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 15. | Bektaş M, Burchell GL, Bonjer HJ, van der Peet DL. Machine learning applications in upper gastrointestinal cancer surgery: a systematic review. Surg Endosc. 2023;37:75-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Yin L, Song C, Cui J, Lin X, Li N, Fan Y, Zhang L, Liu J, Chong F, Wang C, Liang T, Liu X, Deng L, Li W, Yang M, Yu J, Wang X, Liu X, Yang S, Zuo Z, Yuan K, Yu M, Cong M, Li Z, Jia P, Li S, Guo Z, Shi H, Xu H; Investigation on Nutrition Status and Clinical Outcome of Common Cancers (INSCOC) Group. A fusion decision system to identify and grade malnutrition in cancer patients: Machine learning reveals feasible workflow from representative real-world data. Clin Nutr. 2021;40:4958-4970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 17. | Zheng P, Wang B, Luo Y, Duan R, Feng T. Research progress on predictive models for malnutrition in cancer patients. Front Nutr. 2024;11:1438941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | Ozaki Y, Broughton P, Abdollahi H, Valafar H, Blenda AV. Integrating Omics Data and AI for Cancer Diagnosis and Prognosis. Cancers (Basel). 2024;16:2448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 19. | Ouyang F, Zhang L. AI-driven learning analytics applications and tools in computer-supported collaborative learning: A systematic review. Educ Res Rev. 2024;44:100616. [DOI] [Full Text] |
| 20. | Iacucci M, Santacroce G, Zammarchi I, Maeda Y, Del Amor R, Meseguer P, Kolawole BB, Chaudhari U, Di Sabatino A, Danese S, Mori Y, Grisan E, Naranjo V, Ghosh S. Artificial intelligence and endo-histo-omics: new dimensions of precision endoscopy and histology in inflammatory bowel disease. Lancet Gastroenterol Hepatol. 2024;9:758-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 21. | Wang S, Wang S, Wang Z. A survey on multi-omics-based cancer diagnosis using machine learning with the potential application in gastrointestinal cancer. Front Med (Lausanne). 2022;9:1109365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Wong PK, Chan IN, Yan HM, Gao S, Wong CH, Yan T, Yao L, Hu Y, Wang ZR, Yu HH. Deep learning based radiomics for gastrointestinal cancer diagnosis and treatment: A minireview. World J Gastroenterol. 2022;28:6363-6379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 23. | Jeyaraman M, Balaji S, Jeyaraman N, Yadav S. Unraveling the Ethical Enigma: Artificial Intelligence in Healthcare. Cureus. 2023;15:e43262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 51] [Reference Citation Analysis (0)] |
| 24. | Ueda D, Kakinuma T, Fujita S, Kamagata K, Fushimi Y, Ito R, Matsui Y, Nozaki T, Nakaura T, Fujima N, Tatsugami F, Yanagawa M, Hirata K, Yamada A, Tsuboyama T, Kawamura M, Fujioka T, Naganawa S. Fairness of artificial intelligence in healthcare: review and recommendations. Jpn J Radiol. 2024;42:3-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 127] [Article Influence: 127.0] [Reference Citation Analysis (0)] |
| 25. | Vlahou A, Hallinan D, Apweiler R, Argiles A, Beige J, Benigni A, Bischoff R, Black PC, Boehm F, Céraline J, Chrousos GP, Delles C, Evenepoel P, Fridolin I, Glorieux G, van Gool AJ, Heidegger I, Ioannidis JPA, Jankowski J, Jankowski V, Jeronimo C, Kamat AM, Masereeuw R, Mayer G, Mischak H, Ortiz A, Remuzzi G, Rossing P, Schanstra JP, Schmitz-Dräger BJ, Spasovski G, Staessen JA, Stamatialis D, Stenvinkel P, Wanner C, Williams SB, Zannad F, Zoccali C, Vanholder R. Data Sharing Under the General Data Protection Regulation: Time to Harmonize Law and Research Ethics? Hypertension. 2021;77:1029-1035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 26. | Youssef A, Nichol AA, Martinez-Martin N, Larson DB, Abramoff M, Wolf RM, Char D. Ethical Considerations in the Design and Conduct of Clinical Trials of Artificial Intelligence. JAMA Netw Open. 2024;7:e2432482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 27. | Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291:2720-2726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1452] [Cited by in RCA: 1692] [Article Influence: 80.6] [Reference Citation Analysis (1)] |
| 28. | Khoury MJ, Bowen S, Dotson WD, Drzymalla E, Green RF, Goldstein R, Kolor K, Liburd LC, Sperling LS, Bunnell R. Health equity in the implementation of genomics and precision medicine: A public health imperative. Genet Med. 2022;24:1630-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 99] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 29. | Istasy P, Lee WS, Iansavichene A, Upshur R, Gyawali B, Burkell J, Sadikovic B, Lazo-Langner A, Chin-Yee B. The Impact of Artificial Intelligence on Health Equity in Oncology: Scoping Review. J Med Internet Res. 2022;24:e39748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 30. | Aggarwal N, Drew DA, Parikh RB, Guha S. Ethical Implications of Artificial Intelligence in Gastroenterology: The Co-pilot or the Captain? Dig Dis Sci. 2024;69:2727-2733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 31. | Fehr J, Jaramillo-Gutierrez G, Oala L, Gröschel MI, Bierwirth M, Balachandran P, Werneck-Leite A, Lippert C. Piloting a Survey-Based Assessment of Transparency and Trustworthiness with Three Medical AI Tools. Healthcare (Basel). 2022;10:1923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | van Delden JJ, van der Graaf R. Revised CIOMS International Ethical Guidelines for Health-Related Research Involving Humans. JAMA. 2017;317:135-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 33. | Ahmad OF, Stoyanov D, Lovat LB. Barriers and pitfalls for artificial intelligence in gastroenterology: Ethical and regulatory issues. Tech Innovat Gastroi. 2020;22:80-84. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 34. | Bond RR, Novotny T, Andrsova I, Koc L, Sisakova M, Finlay D, Guldenring D, McLaughlin J, Peace A, McGilligan V, Leslie SJ, Wang H, Malik M. Automation bias in medicine: The influence of automated diagnoses on interpreter accuracy and uncertainty when reading electrocardiograms. J Electrocardiol. 2018;51:S6-S11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 35. | Dratsch T, Chen X, Rezazade Mehrizi M, Kloeckner R, Mähringer-Kunz A, Püsken M, Baeßler B, Sauer S, Maintz D, Pinto Dos Santos D. Automation Bias in Mammography: The Impact of Artificial Intelligence BI-RADS Suggestions on Reader Performance. Radiology. 2023;307:e222176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 114] [Reference Citation Analysis (0)] |