INTRODUCTION
The National Bureau of Statistics has indicated that the number of people with chronic liver disease in China has reached nearly 400 million, and various end-stage diseases, such as cirrhosis (including portal hypertension [PHT]) and liver cancer, have greatly increased social costs[1,2]. PHT is a deleterious syndrome characterized by chronically elevated portal pressure (PP), which is the main consequence of liver cirrhosis[3]. Liver cirrhosis leads to PHT due to increased intrahepatic vascular resistance and heightened blood flow through the portal vein[1]. During liver cirrhosis, hepatic inflammatory factors increase, which promotes the secretion of hepatic stellate cells and the deposition of the extracellular matrix. This process culminates in compression of the hepatic sinuses, resulting in heightened resistance to hepatic blood flow and the subsequent onset of PHT. The exacerbation of PHT can lead to a range of complications, including gastrointestinal bleeding, ascites, hepatorenal syndrome and hepatic encephalopathy, which can potentially be life-threatening.
In liver fibrosis, the lymphatic system undergoes various structural and functional alterations that exhibit a compensatory effect during the initial phases of fibrosis, thereby partially mitigating local inflammation levels and hepatic circulatory pressure[4]. The lymphatic system has anchoring filaments that sustain the aperture of the lumen under high pressure, thereby potentially diminishing inflammatory factors[5]. Previous studies have demonstrated that increasing the number of lymphatic vessels during liver fibrosis and cirrhosis alleviates the deposition of fibers. However, further research is needed to investigate the specific mechanisms underlying lymphatic vascular dysfunction and remodeling in PHT. In addition, there is a lack of efficacious methods for increasing the number of lymphatic vessels in patients with liver cirrhosis and PHT. To elucidate the mechanism and discover disease progression and intervention methods, the present study utilized whole-transcriptome sequencing to measure alterations in the lymphatic system in cirrhotic rats[6]. Based on differentially expressed genes (DEGs) and hub genes in cirrhotic lymphatic samples, Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) enrichment analyses revealed that platelets and their related pathways were enriched, indicating that they have the potential to address lymphatic insufficiency associated with liver cirrhosis and cirrhotic PHT.
Platelets possess a diverse array of bioactive molecules within their granules and exhibit distinct receptors on their surfaces; therefore, these receptors play a significant role in the processes of inflammation, cancer progression, and metastasis[7]. As an important component of the anticoagulant system, platelet production decreases during cirrhosis because of reduced production of thrombopoietin, bone marrow suppression, hypersplenism, and autoantibody-mediated platelet destruction[8,9]. Platelets contain a diverse array of cytokines and have the capacity to modulate the process of lymphatic vessel formation, thereby exerting potential therapeutic effects in the context of liver cirrhosis[10]. Previous studies have suggested that platelets have the potential to stimulate lymphangiogenesis through the release of vascular endothelial growth factor C (VEGF-C) and subsequent activation of the VEGF-C/VEGF receptor 3 (VEGFR-3) pathway[11]. In the present study, the quantity and quality of platelets within the livers of cirrhotic rats were increased via treatment with platelet-rich plasma (PRP) to induce lymphangiogenesis to investigate the therapeutic impact of platelet-induced lymphangiogenesis on liver cirrhosis and PHT.
MATERIALS AND METHODS
Induction of PHT and drug treatment of rats
Six- to eight-week old male Sprague Dawley (SD) rats, weighing 200-250 g, were purchased from Shanghai Laboratory Animal Centre (Shanghai, Center). The rats were kept under controlled conditions (20-22 °C, 60% humidity, and a 12:12 hour light/dark cycle) with free access to food and water. All SD rats were divided into the following groups: Sham-operated (control), common bile duct ligation (BDL-vehicle [VEH]/BDL), adeno-associated virus-VEGF-C (AAV-VEGF-C) combined with BDL (BDL-AAV-VEGF-C), PRP combined with BDL (BDL-PRP), and MAZ-51 + PRP combined with BDL (BDL-MAZ-51) groups. The specific protocol of BDL was previously described[12]. At the beginning of the surgery and drug treatments, the number of rats in each group was 10, and shortly before sacrificing, the number of live rats in each group was 10 (control), 7 (BDL-VEH/BDL), 8 (BDL-AAV-VEGF-C), 8 (BDL-PRP), and 6 (BDL-MAZ-51).
A rat-VEGFC gene-overexpressing AAV vector serotype 8 (AAV8) was constructed by Jiman Company (Shanghai, China). The characteristics of the AAV8-VEGF-C vector were as follows: PGMAAV-4892: GPAAV-CMV-MCS-3 × Flag-T2A-eGFP-WPRE. At 1 day after BDL, the rats in the BDL-AAV-VEGF-C group were given 100 μL of an AAV-VEGF-C solution (1 × 1013 vg/mL) via tail vein injection. At 1 day after BDL, the rats in the BDL-PRP group received an intraperitoneal injection of PRP in phosphate-buffered saline (PBS) twice a week for 4 weeks at a rate of 0.5 mL/kg after activation with calcium chloride. At 1 day after BDL, the rats in the BDL-MAZ-51 group were given MAZ-51 (10 mg/mL, 1:10 dissolved in PBS; GMBIO) once a week for 4 weeks on the basis of exposure to PRP simultaneously. Ethical approval was granted by the Ethical Committee of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (Shanghai, China).
Preparation of PRP
PRP was obtained from the blood of donor rats. Approximately 10 mL blood was collected from each rat. The ratio of blood to sodium citrate used in the sodium citrate anticoagulant solution was 10:1. PRP was obtained through the following two-step centrifugation method: (1) PRP was centrifuged at 1600 rpm for 10 minutes, and the top layer of plasma was extracted after stratification; and (2) PRP was centrifuged at 2000 rpm for 10 minutes, and the lower part of the plasma, known as PRP, was obtained. PRP was mixed with PBS at a 1:1 ratio, resulting in a PRP concentration of 3.8 × 106/μL. PRP was activated by mixing PRP with 10% calcium chloride (10:1) before use.
Histology and immunohistochemistry
The right hepatic lobe and mesenteric samples from each rat were fixed in 10% formalin neutral buffered solution (potential of hydrogen = 7.4) and embedded in paraffin blocks. The liver and mesenteric samples were stained with hematoxylin and eosin (H&E). Masson’s trichrome staining and Sirius red staining were employed to evaluate liver fibrosis and collagen deposition regions, respectively. Deparaffinized samples were rehydrated and then microwaved for 20 minutes to extract antigens for immunohistochemistry. After the washing and blocking procedures, the slides were incubated with the following primary antibodies: lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1) (1:100; Affinity, Inc., Milwaukee, WI, United States), VEGF-C (1:50; Novus Biologicals, Centennial, CO, United States), VEGFR-3 (1:800; Boster Bio, Pleasanton, CA, United States), transforming growth factor beta (TGF-β) (1:2000; Proteintech Group, Wuhan, China), fibronectin (FN) (1:4000; Abcam, Cambridge, United Kingdom), cluster of differentiation 41 (CD41, 1:200; Abcam), CD31 (1:100; Abcam), matrix metalloproteinase 2 (MMP2, 1:250; Abcam), von Willebrand factor (vWF) (1:600; Abcam), VEGF-A (1:200; Abcam), and CD68 (1:200; Novus Biologicals). The slides were subsequently incubated with a secondary antibody (D-3004; Long Island Biotech, Shanghai, China).
Hemodynamic measurements
A polyethylene 50 tube was inserted into the portal vein of each rat following anesthesia with 2% isoflurane, as previously described[13]. After the polyethylene 50 was connected to a pressure transducer and analyzed automatically via the ALC-MPA multichannel biological information analysis system (Shanghai Alcott Biotech Co., Ltd., Shanghai, China). A P value was obtained.
Lymphatic isolation and RNA whole-transcriptome sequencing
The present study focused on lymphatic vessels associated with the superior mesenteric artery. Prior to sampling after 4 weeks of BDL, the rats were fasted for 8-12 hours to ensure an empty stomach. The rats subsequently received an intragastric administration of olive oil in accordance with the standard dosage of 5 mL per 100 g of body weight. Following a 1-hour waiting period, the abdomen was surgically opened, revealing the milky white appearance of the lymphatic vessels that accompany the superior mesenteric artery. The lymphatic vessels were isolated from control rats (n = 6) and BDL rats (n = 6). RNA sequencing analysis was conducted by TIANGEN Biotech (Beijing, China). Total RNA was extracted via the TRNzol reagent (TIANGEN). A NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, United States) and the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, United States) were used to ensure the concentration, purity, and integrity of the total RNA. One microgram of RNA per sample was used for RNA transcriptome sequencing library construction. After trimming the reads, adapters and low-quality regions were removed to ensure that the data were clean for subsequent analyses.
Identification of DEGs
According to the comprehensive mRNA expression profiles of each sample, principal component analysis was conducted to assess the similarity or dissimilarity between the control and BDL groups. EdgeR software (http://www.r-project.org) was used for the identification of DEGs. The read counts for each sequenced library were adjusted via the EdgeR program package, which applies a scaling-normalized factor. The screening standards for DEGs were |log2 fold change|> 1 and P < 0.05. Heatmaps and volcano plots were generated via the pheatmap and ggplot2 R packages, respectively.
Functional enrichment analyses
The protein-protein interaction network was constructed via the STRING database (http://string-db.org/), which is renowned for its ability to predict protein-protein interactions. KEGG and GO enrichment analyses of the DEGs were conducted via the cluster Profiler R package. P < 0.05 was set as the cutoff value for the GO and KEGG analyses. Gene set enrichment analysis (GSEA) was performed via GSEA software (http://www.broadinstitute.org/gsea).
Western blot analysis
Proteins were extracted using radio immunoprecipitation assay buffer (Beyotime, Shanghai, China) and phenylmethylsulfonyl fluoride (Beyotime). The protein concentrations were measured with a bicinchoninic acid assay™ protein assay kit (Pierce, Appleton, WI, United States). The proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to polyvinylidene difluoride membranes. Primary antibodies against alpha smooth muscle actin (SMA) (1:2000; Abcam) and glyceraldehyde-3-phosphate dehydrogenase (1:8000; Sungene Biotech, China) were used as protein loading controls.
Statistical analyses
All data are presented as the mean ± SD. The Student’s t test was used to evaluate differences between two groups, and P < 0.05 was considered statistically significant. The statistical methods of this study were reviewed by Fan Q, Zheng L, and Luo M from Department of General Surgery, Shanghai Ninth People’s Hospital Affiliated with Shanghai Jiao Tong University School of Medicine (Shanghai, China).
RESULTS
Enhanced proliferation of lymphatic vessels was observed in the early stage of BDL in rats
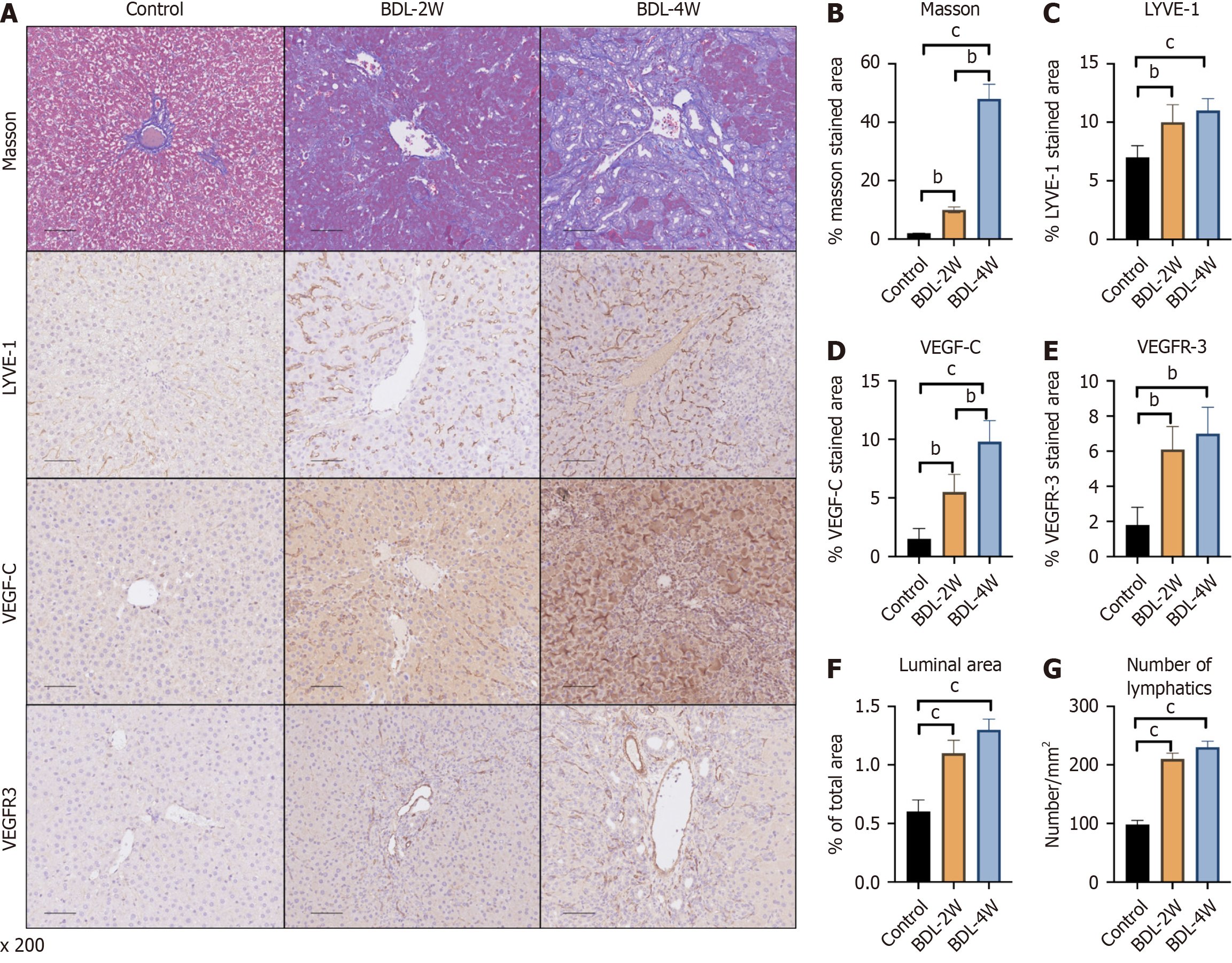
The number and area of lymphatic vessels in the liver were evaluated after BDL, a method for inducing PHT in rats. Masson’s trichrome staining identified liver fibrosis 2 weeks after BDL, and liver cirrhosis was successfully induced after 4 weeks (Figure 1A and B). Compared with that of the sham-operated-VEH group, the area of LYVE-1 was increased at 2 weeks after surgery, and no changes were observed at 4 weeks compared with 2 weeks (Figure 1A and C). However, VEGF-C increased at 2 weeks and was further upregulated at 4 weeks compared with 2 weeks (Figure 1A and D). A similar trend was observed for VEGFR-3 (Figure 1A and E). In addition, the expression of genes related to the lymphatic system, was increased at 2 weeks but did not change at 4 weeks according to the luminal area and number (Figure 1F and G).
Figure 1 Trends in the number of rat lymphatic vessels at 2 weeks and 4 weeks after bile duct ligation.
A: Representative immunohistochemistry images of Masson’s trichrome, lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1), vascular endothelial growth factor C (VEGF-C) and VEGF receptor 3 (VEGFR-3) staining in the livers of the different groups; B: Quantitative analysis of Masson’s trichrome; C: Quantitative analysis of LYVE-1; D: Quantitative analysis of VEGF-C; E: Quantitative analysis of VEGFR-3 staining; F: Luminal area of lymphatic vessels were also measured; G: The number of lymphatic vessels was also measured. The data represent the mean ± SD. aP < 0.05. bP < 0.01. cP < 0.001. BDL: Bile duct ligation; W: Week.
Application of VEGF-C ameliorates liver fibrosis and PHT via an increased lymphatic system
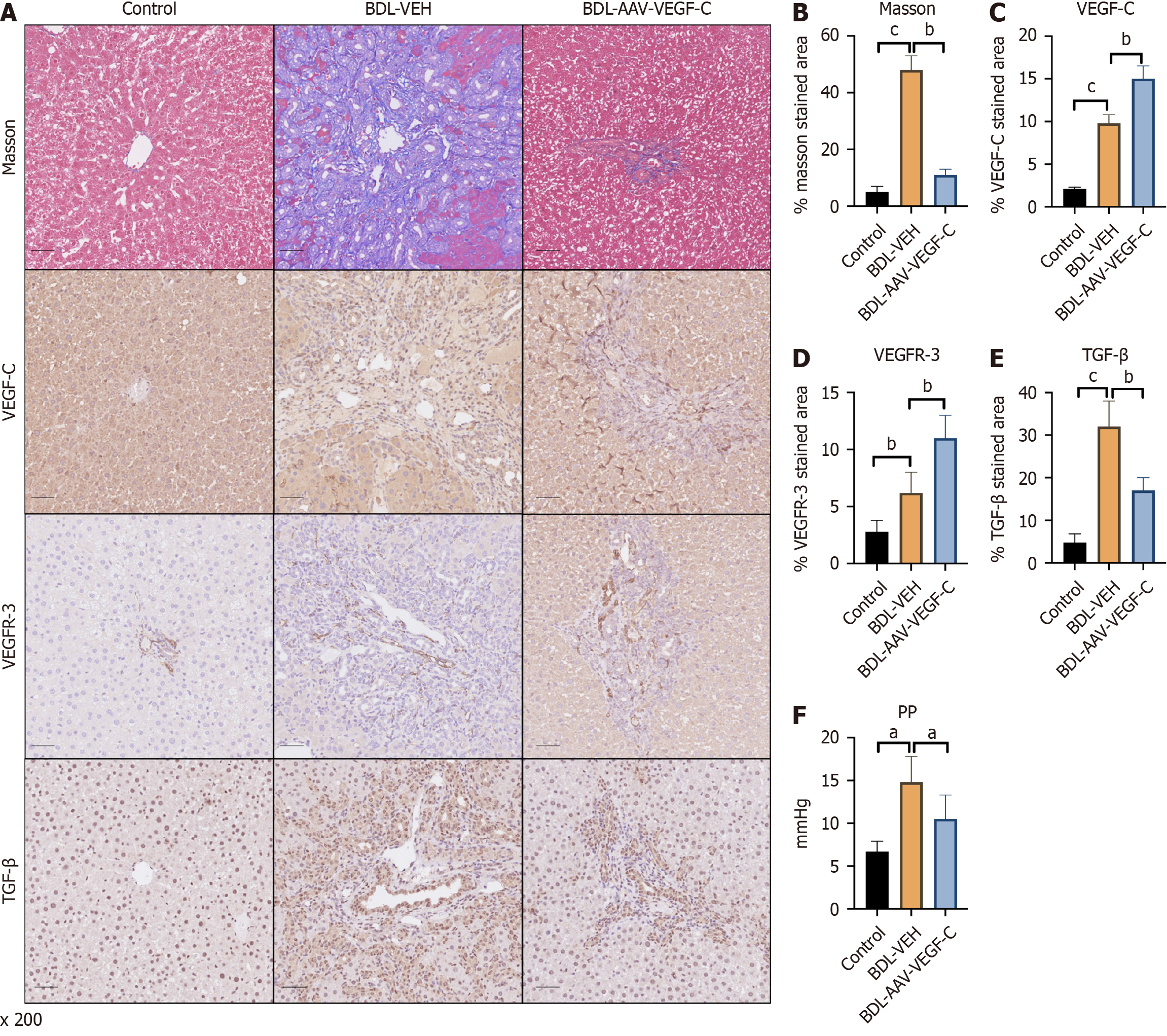
To investigate the function of the lymphatic system in rats with liver fibrosis and PHT, VEGF-C was combined with VEGFR-3 to increase the lymphatic system. Collagen deposition was significantly reduced after VEGF-C intervention (Figure 2A and B). Along with the increase in VEGF-C, the expression of VEGFR-3 also increased compared with that in the BDL-VEH group at the same time (Figure 2A, C and D). These results indicated that the proliferation of intrahepatic lymphatic vessels was strongly enhanced after treatment with AAV-VEGF-C. In addition, the expression of TGF-β, a marker that promotes liver fibrosis, was lower in the AAV-VEGF-C group than in the BDL-VEH group (Figure 2A and E), which indirectly confirmed the high drainage of the lymphatic system due to AAV infection. Moreover, PP was reversed after treatment with AAV-VEGF-C compared with that in the BDL-VEH group (Figure 2F).
Figure 2 Intervention with adeno-associated virus-vascular endothelial growth factor C relieves liver fibrosis and portal hypertension by increasing the number of lymphatic vessels involved in bile duct ligation.
A: Representative immunohistochemistry images of Masson’s trichrome, virus-vascular endothelial growth factor C (VEGF-C), vascular endothelial growth factor receptor 3 (VEGFR-3) and transforming growth factor beta (TGF-β) staining in the livers of the different groups; Quantitative analysis of B: Masson’s trichrome; C: VEGF-C; D: VEGFR-3; E: TGF-β staining; F: The levels of portal pressure were also measured. The data represent the mean ± SD. aP < 0.05. bP < 0.01. cP < 0.001. AAV: Adeno-associated virus; BDL: Bile duct ligation; PP: Portal pressure; TGF-β: Transforming growth factor beta; VEGF-C: Vascular endothelial growth factor C; VEGFR-3: Vascular endothelial growth factor receptor 3; VEH: Vehicle.
Landscape of DEGs in the lymphatic system of BDL rats
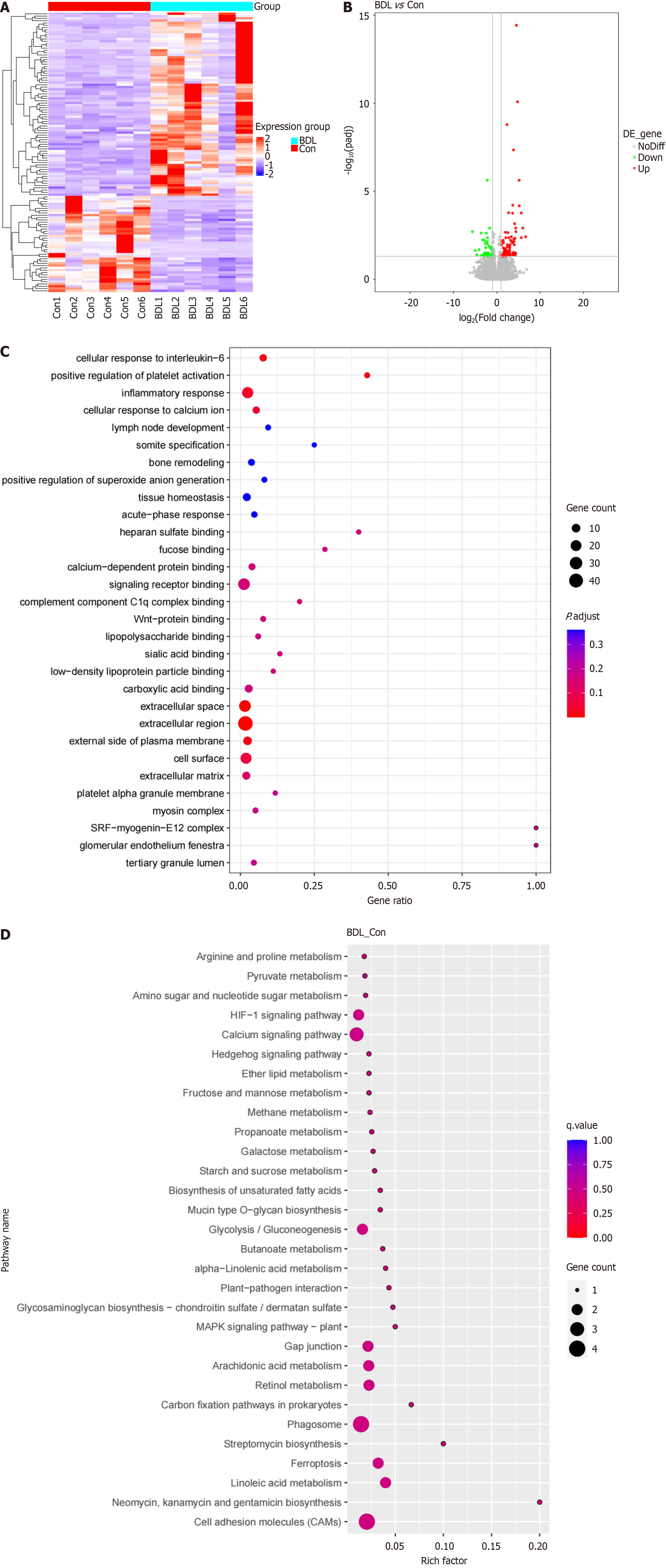
Considering the difficulties in obtaining the lymphatic system in the liver, the lymphatic vessels accompanied by the superior mesenteric artery were collected for whole-transcriptome sequencing. A total of 122 DEGs were screened out after whole-transcriptome sequencing. Between the BDL group and the control group, approximately 42 DEGs were downregulated and 80 DEGs were upregulated (Figure 3A and B). Furthermore, GO and KEGG analyses were performed, and the biological processes were annotated via meta scape. GO analysis revealed the top 30 enriched biological processes related to positive regulation of platelet activation, the inflammatory response, and the cellular response to interleukin-6 (Figure 3C). KEGG analysis revealed that cell adhesion molecules and various metabolic pathways were enriched (Figure 3D).
Figure 3 Identification of the differentially expressed genes in the bile duct ligation lymphatic system and related biological processes.
A: Heatmap comparison of differentially expressed genes (DEGs) in the lymphatic system in the bile duct ligation (BDL) and control groups; B: Volcano map of DEG expression in the lymphatic system between the BDL and control groups; C: Bubble plot for gene ontology enrichment of DEGs; D: Bubble plot for Kyoto encyclopaedia of genes and genomes enrichment of DEGs. BDL: Bile duct ligation; Con: Control.
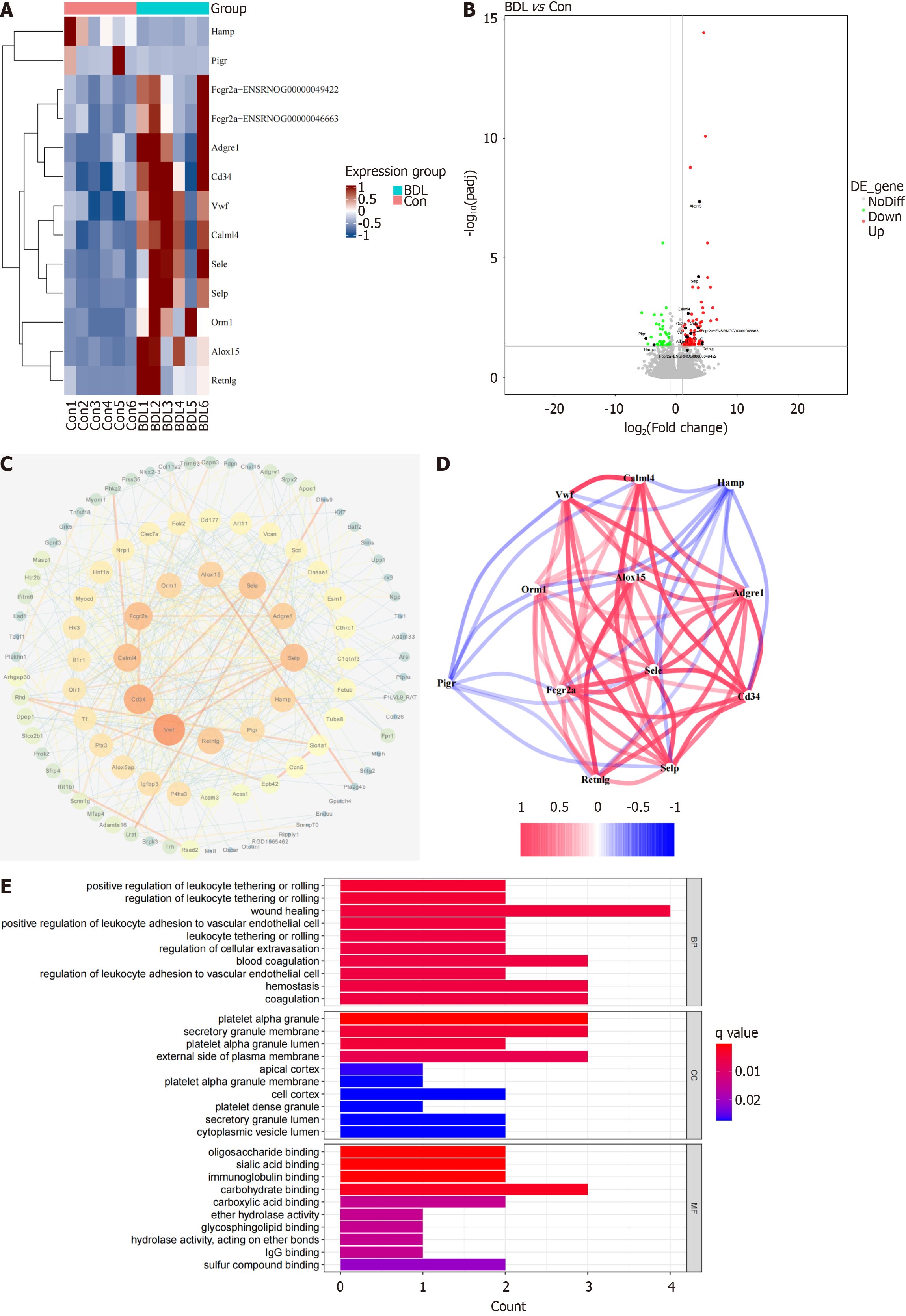
Twelve core DEGs were filtered out according to the degree of gene expression via Cytoscape (Figure 4A). Compared with the control group, the volcano map revealed that HAMP and polymeric immunoglobulin receptor (PIGR) were downregulated in the BDL group, while Fc gamma receptor IIa, adhesion G protein-coupled receptor E1, CD34, vWF, calmodulin like 4, E-selectin, selectin P, orosomucoid 1, arachidonate 15-lipoxygenase, and resistin like gamma were upregulated in the BDL group (Figure 4B). Twelve core genes closely interacted with each other (Figure 4C and D). To further investigate the biological functions of the 12 core genes, GO and KEGG analyses were conducted. GO analysis identified the enrichment of wound healing, platelet alpha granules, platelet alpha granule lumen, platelet alpha granule membrane, and platelet dense granules (Figure 4E). GO analysis also identified the enrichment of cell adhesion molecules, phagosomes (Supplementary Figure 1A). and platelets. GSEA revealed that the calcium signaling pathway, cell adhesion molecules, the cyclic guanosine monophosphate-protein kinase G signaling pathway, focal adhesion and the phosphatidylinositol 3-kinase-AKT serine/threonine kinase 1 signaling pathway were mostly correlated with platelet adherence, aggregation, and activation (Supplementary Figure 1B-F).
Figure 4 Twelve core differentially expressed genes in the bile duct ligation lymphatic system and their related biological processes.
A: Heatmap of 12 core differentially expressed genes (DEGs) in the lymphatic system between the bile duct ligation (BDL) and control groups; B: Volcano map of core DEGs in the lymphatic system between the BDL and control groups; C: Protein-protein interaction network between the DEGs and the 12 core DEGs; D: Co-expression of these core DEGs; E: Bar plot of the gene ontology enrichment of the core DEGs. BDL: Bile duct ligation; BP: Biological process; CC: Cellular component; Con: Control; MF: Molecular function.
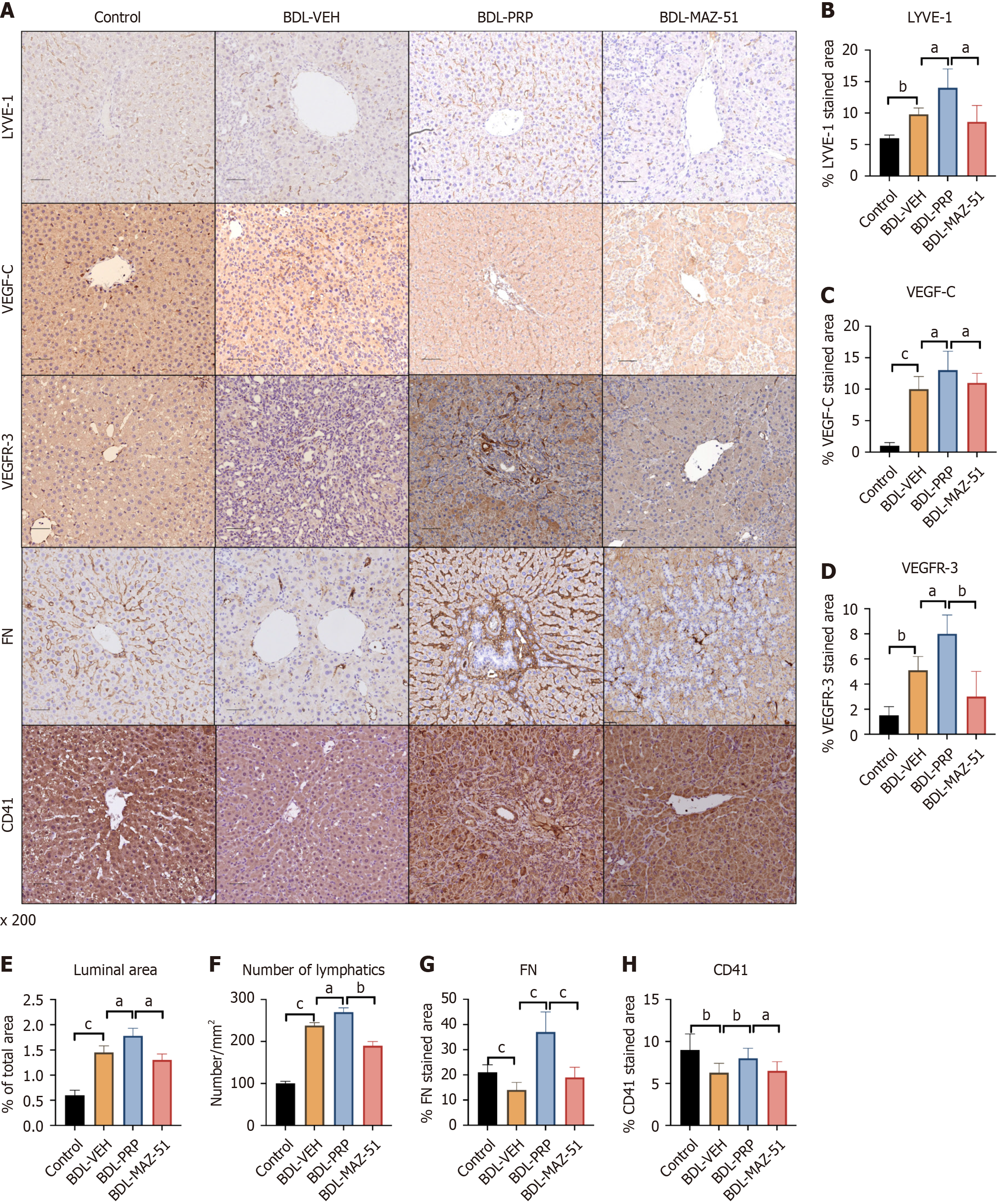
PRP-induced platelets promote intrahepatic lymphangiogenesis through the VEGF-C/VEGFR-3 pathway
Significantly greater expression of LYVE-1, VEGF-C, and VEGFR-3 was detected in the BDL-PRP group than in the BDL-VEH group, which was reversed after treatment with MAZ-51 (Figure 5A-D). Quantitative analysis of intrahepatic lymphatic vessels revealed a significant increase in both the area and number of lymphatic vessels following treatment with PRP compared with the BDL-VEH group. Conversely, compared with the BDL-PRP group, the MAZ-51 group presented a significant decrease in the number of lymphatic vessels (Figure 5E and F). In addition, the expression of CD41 and FN was increased in the BDL-PRP group compared to the BDL-VEH group. After MAZ-51 intervention, the expression of both platelet markers decreased (Figure 5A, G and H). Due to the enhanced proliferation of lymphatic vessels, the potential phenotypic changes in PHT need to be further assessed.
Figure 5 Platelet-rich plasma-induced platelets increase intrahepatic lymphatic vessels via the vascular endothelial growth factor C/vascular endothelial growth factor receptor 3 pathway.
A: Representative immunohistochemistry images of lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1), vascular endothelial growth factor C (VEGF-C) and vascular endothelial growth factor receptor 3 (VEGFR-3), fibronectin (FN) and cluster of differentiation 41 (CD41) in the livers of the different groups; Quantitative analysis of B: LYVE-1; C: VEGF-C; D: VEGFR-3; E: The luminal area; F: The number of lymphatic vessels were also measured; G: FN; H: CD41 expression. The data represent the mean ± SD. aP < 0.05. bP < 0.01. cP < 0.001. BDL: Bile duct ligation; PRP: Platelet-rich plasma; VEH: Vehicle.
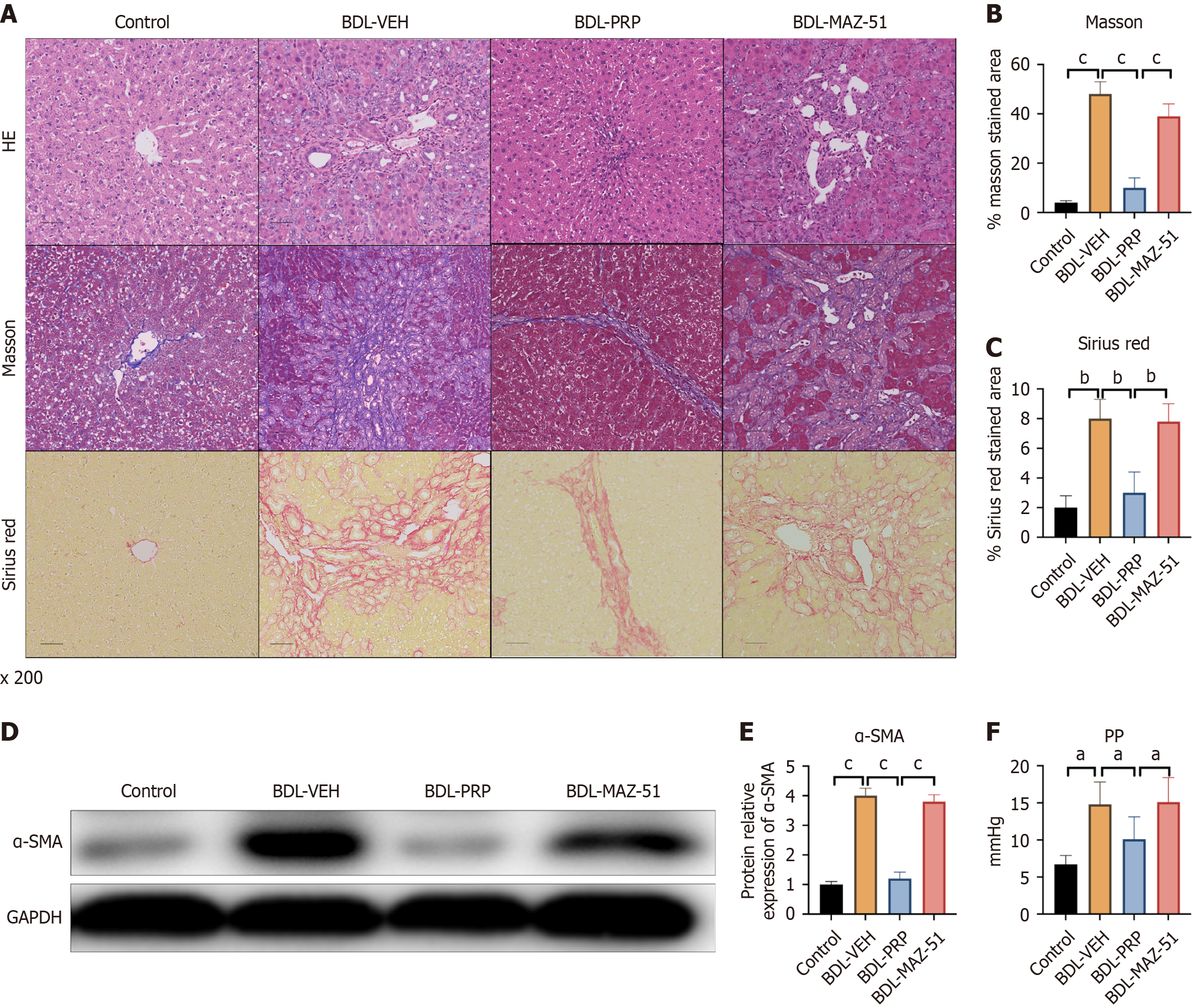
PRP-induced platelets improve liver cirrhosis and PHT
The BDL-VEH group exhibited notable liver fibrosis after BDL surgery. Following PRP intervention, a significant decrease in collagen deposition was evidenced by a decrease in Masson’s trichrome, H&E, and Sirius red staining (Figure 6A-C). However, MAZ-51 treatment induced collagen deposition in the liver, as indicated by increased H&E, Masson’s trichrome, and Sirius red staining compared with that in the BDL-PRP group (Figure 6A-C). The protein expression of α-SMA, a marker of liver fibrosis, showed a similar trend in the BDL-PRP and BDL-MAZ-51 groups (Figure 6D and E). PRP significantly decreased the BDL-induced increase in PP, and this phenomenon was reversed by MAZ-51 (Figure 6F).
Figure 6 Inhibition of vascular endothelial growth factor C/vascular endothelial growth factor receptor 3 reversed the amelioration of liver fibrosis and portal hypertension in platelet-rich plasma-induced platelets.
A: Representative immunohistochemistry images of hematoxylin and eosin (H&E), Masson’s trichrome, and Sirius red staining in liver samples from the different groups; Quantitative analysis of B: Masson’s trichrome; C: Sirius Red; D: Protein expression of alpha smooth muscle actin (α-SMA) in the different groups; Quantitative analysis of E: α-SMA staining; F: The levels of portal pressure were also measured. The data represent the mean ± SD. aP < 0.05. bP < 0.01. cP < 0.001. BDL: Bile duct ligation; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; PP: Portal pressure; PRP: Platelet-rich plasma; VEH: Vehicle.
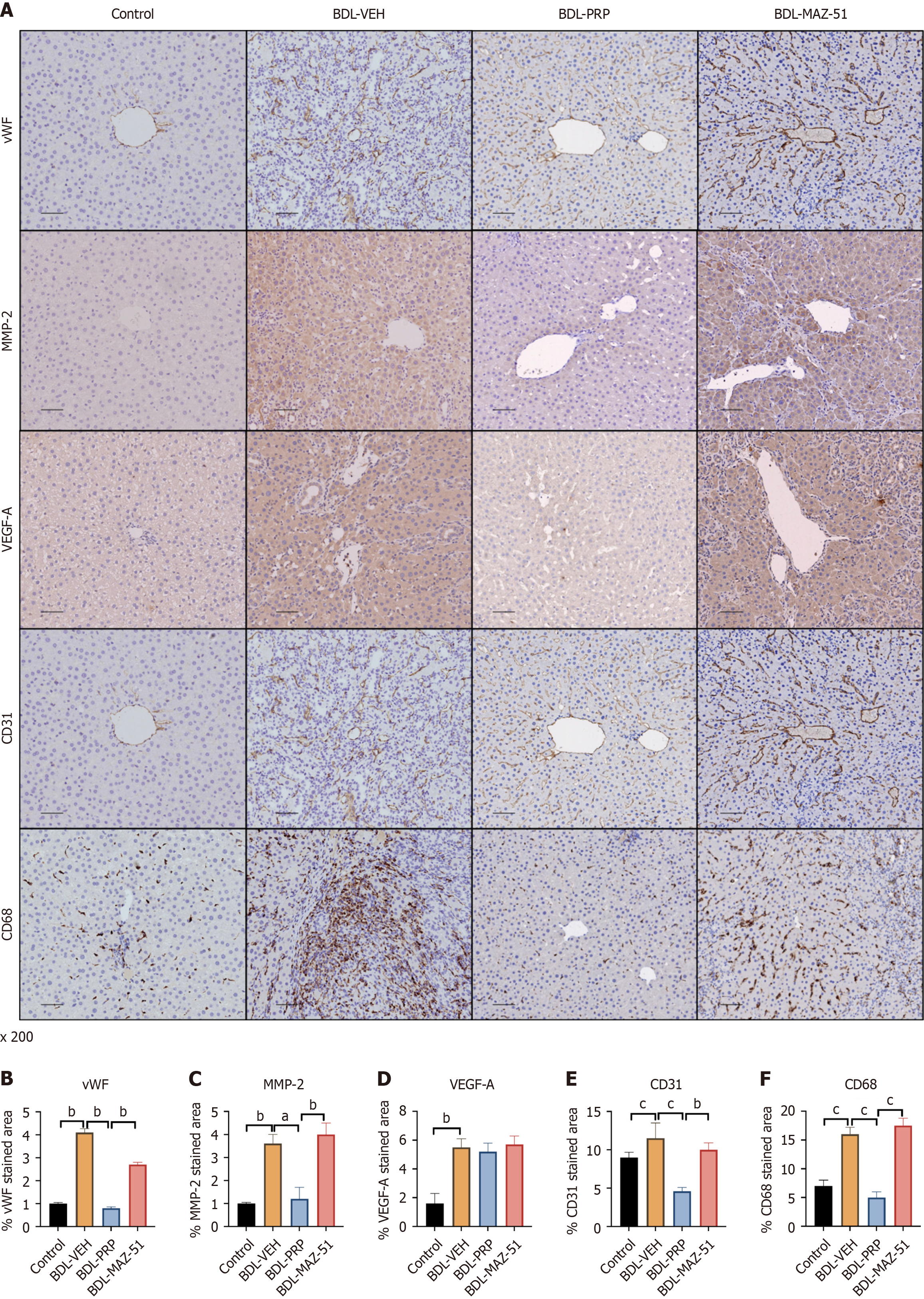
PRP-induced platelets inhibit intrahepatic angiogenesis and alleviate the inflammatory response in cirrhotic rats
Immunohistochemistry analysis revealed a significant reduction in the elevated levels of angiogenesis- and remodeling-related proteins, namely, vWF, MMP2, VEGF-A, and CD31, in the BDL-PRP group compared with those in the BDL-VEH group. Furthermore, MAZ-51 treatment reversed this phenomenon (Figure 7A-E). In addition, hepatic expression of CD68 was elevated in the BDL-VEH group. However, upon intervention with PRP, the hepatic expression of CD68 was subsequently downregulated. Conversely, the PRP + MAZ-51 intervention, which inhibited lymphangiogenesis, increased CD68 expression in the liver (Figure 7A and F).
Figure 7 Inhibition of vascular endothelial growth factor C/vascular endothelial growth factor receptor 3 reverses the amelioration of intrahepatic angiogenesis and inflammation by platelet-rich plasma-induced platelets.
A: Representative immunohistochemistry images of von Willebrand factor (vWF), matrix metalloproteinase 2 (MMP2), vascular endothelial growth factor A (VEGF-A), cluster of differentiation 31 (CD31) and CD68 in the livers of different groups; Quantitative analysis of B: vWF; C: MMP2; D: VEGF-A; E: CD31; F: CD68. The data represent the mean ± SD. aP < 0.05. bP < 0.01. cP < 0.001. BDL: Bile duct ligation; PRP: Platelet-rich plasma; VEH: Vehicle.
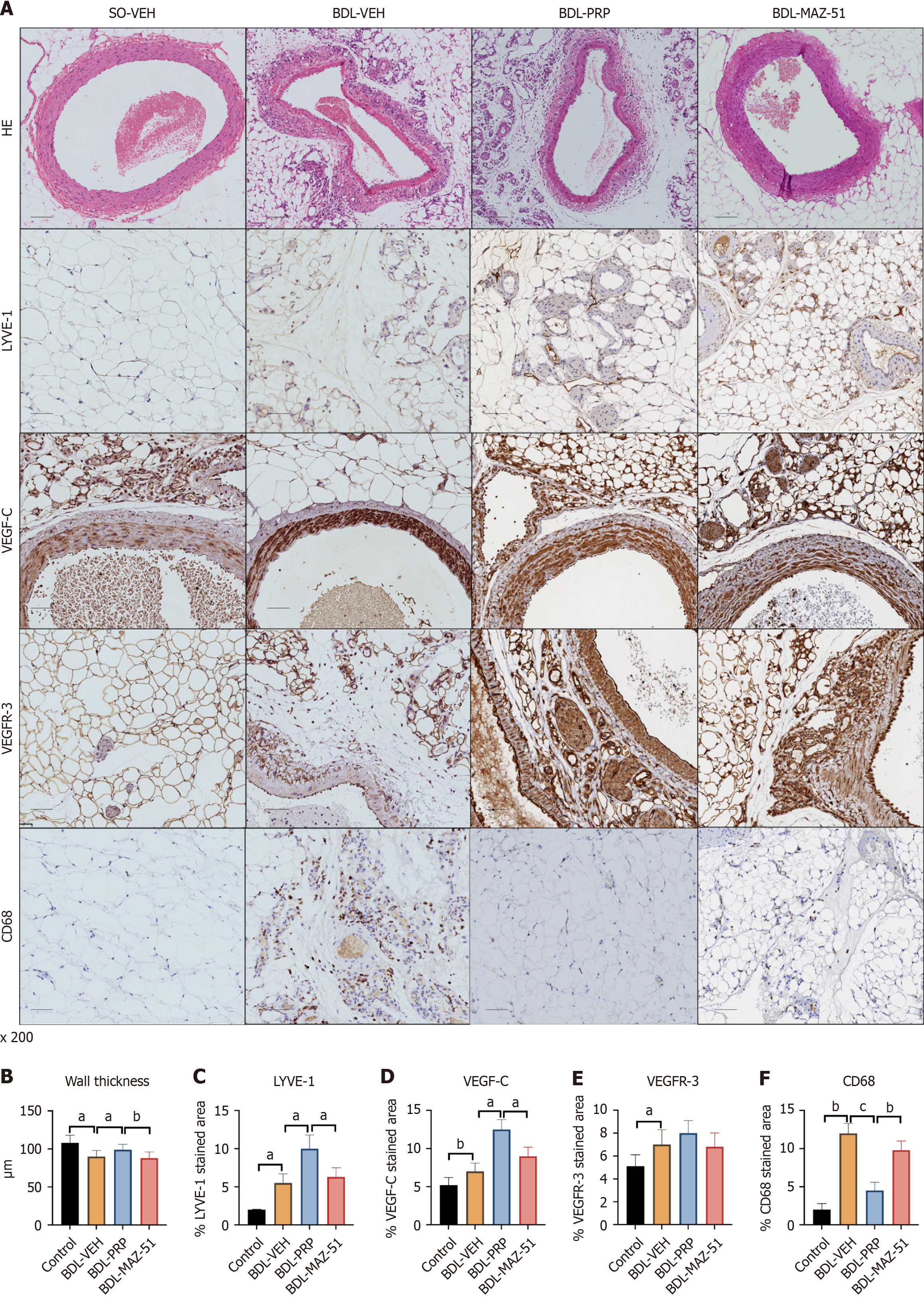
PRP-induced platelets alleviate mesenteric vascular remodeling and immune inflammatory pathways in cirrhotic rats
Compared with the control group, the BDL-VEH group presented a reduction in the SMA lumen thickness and disruption of the vascular structure. After PRP intervention, the SMA of the BDL-PRP group was restored towards a relatively normal state, as characterized by a thicker wall. Conversely, the utilization of PRP + MAZ-51 to inhibit lymphatic vessel formation led to a subsequent decrease in wall thickness (Figure 8A and B). Similarly, the levels of the LYVE-1, VEGF-C, and VEGFR-3 lymphatic system markers were increased in the BDL-VEH group and further increased in the BDL-PRP group; these effects were inhibited by MAZ-51 treatment (Figure 8A, C-E). In addition, the levels of the CD68 macrophage marker decreased after PRP intervention, while MAZ-51 treatment increased the CD68 levels in the mesentery (Figure 8A and F).
Figure 8 Inhibition of vascular endothelial growth factor C/vascular endothelial growth factor receptor 3 reverses the amelioration of mesenteric angiogenesis and inflammation by platelet-rich plasma-induced platelets.
A: Representative immunohistochemistry images of hematoxylin and eosin, lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1), vascular endothelial growth factor C (VEGF-C) and vascular endothelial growth factor receptor 3 (VEGFR-3) and cluster of differentiation 68 (CD68) in the mesentery of the different groups; Quantitative analysis of B: Wall thickness; C: LYVE-1; D: VEGF-C; E: VEGFR-3; F: CD68. The data represent the mean ± SD. aP < 0.05. bP < 0.01. cP < 0.001. BDL: Bile duct ligation; PRP: Platelet-rich plasma; SO-VEH: Sham-operated-vehicle; VEH: Vehicle.
DISCUSSION
Cirrhosis and its associated complications contribute significantly to global mortality rates on an annual basis. Approximately 90% of individuals diagnosed with cirrhosis ultimately experience the onset of PHT, a condition that serves as a precursor to the majority of mortalities observed in cirrhotic patients. The onset of PHT is triggered by an elevation in intrahepatic vascular resistance and the presence of a hyperdynamic circulatory state. During the initial phase of liver cirrhosis, there is a notable increase in both the quantity and density of lymphatic vessels within the hepatic tissue of patients and rats[14-16]. Nevertheless, advanced liver cirrhosis could result in compromised lymphatic drainage functionality. Henriksen[17] reported a three-fold increase in the lymphatic drainage flow of the thoracic duct in early-stage patients without ascites; conversely, in patients with late-stage tension ascites, the total drainage flow of the thoracic duct returns to the original normal level. These results suggest an essential role of the lymphatic system in the progression of cirrhosis and PHT. Nevertheless, the potential consequences of actively regulating the quantity of lymphatic vessels on the progression of liver cirrhosis and PHT remain unknown. In the present study, the number of lymphatic vessels in the liver were increased via the injection of AAV-VEGF-C, which resulted in the recovery of liver fibrosis and PHT compared to the BDL-VEH group. These findings were consistent with those of Juneja et al[18]. However, the specific genetic alterations that transpire in the lymphatic vessels of individuals afflicted with liver cirrhosis remain unknown. The present study used whole-transcriptome sequencing to explore changes in gene expression between the normal and BDL groups. In addition, on the basis of the DEGs, KEGG analysis, GO analysis, and GSEA were performed to explore functional pathways related to DEGs and hub genes[19]. Comparison of gene expression identified 122 DEGs, and 12 hub genes were obtained according to the degree nodes. GO and KEGG enrichment analyses identified enrichment in the inflammation response (including interleukin 6 and Wnt-protein binding) and immune system processes (including leukocyte tethering or rolling, positive regulation of leukocyte adhesion to vascular endothelial cells, Ig binding, and IgG binding). Biological process analysis of both the DEGs and hub genes revealed an enrichment of platelet-related pathways, including positive regulation of platelet activation, the platelet alpha granule membrane, platelet alpha granules, the platelet alpha granule lumen and platelet dense granules, indicating a potentially important role of platelets in the lymphatic system after individuals suffer from BDL. In addition, the regulation of platelet function plays a pivotal role in achieving effective and proficient hemostasis and coagulation, which were both enriched in the analysis of biological processes. According to the literature, there is a complex relationship between lymphatic vessels and platelets. Activated platelets release various cytokines, including VEGF-C, to induce lymphangiogenesis and hemostasis[11]. Recent studies have demonstrated that platelets play a protective role in the development of blood vessels, lymphatic vessels, and microvasculature in areas where leukocytes infiltrate, such as inflamed organs and tumors[20]. By contrast, the exacerbation of intestinal inflammation is attributed to the suppression of lymphangiogenesis through platelet interactions with lymphatic vessels[21]. Moreover, the activation of the lymphatic system may in turn affect platelets. Stimulation of lymphatic vessels and neutrophils induces the secretion of PDPN, which subsequently binds to the C-type lectin-like receptor 2 located on platelet surfaces. This binding event triggers platelet activation, thereby facilitating the progression of lymphatic vessel function and thrombus formation[22]. However, it remains uncertain whether elevated platelets alleviate the symptoms of liver cirrhosis and PHT.
The present study employed a method to augment the platelet count in rats, namely, PRP infusion. PRP effectively improves various liver diseases, such as ischemia-reperfusion injury, cirrhosis, cholestatic liver injury, viral hepatitis, and the generation of liver tissue[23-27]. Because platelets directly interact with liver cells through the hepatic sinuses, the growth factors carried by platelets in PRP effectively promote liver cell proliferation and prevent liver cell apoptosis[28,29]. PRP has positive effects on ameliorating carbon tetrachloride (CCl4)-induced liver injury, thioacetamide (TAA)-related liver injury, and liver fibrosis. However, the potential impact of PRP on PHT in advanced cirrhosis remains unexplored[30]. In addition to improving liver tissue, PRP may also promote lymphangiogenesis. Platelets in PRP have been reported to facilitate local lymphangiogenesis during wound healing through the release of VEGF-C[11]. VEGF-C is released upon activation by peripheral blood platelets, thereby contributing to the processes of wound healing and lymphangiogenesis[31]. In the present study, functional enrichment analysis revealed that the wound healing signaling pathway was enriched. The present study demonstrated that PRP-induced platelets led to intrahepatic lymphangiogenesis, mesenteric lymphangiogenesis, inflammation suppression, vascular remodeling, platelet aggregation and fiber deposition inhibition. In addition, the present study revealed that PRP-induced platelets relieve liver cirrhosis and PHT through the VEGF-C/VEGFR-3 pathway. The present findings are consistent with reports in clinical practice that platelet transfusion improves liver cirrhosis[32]. In addition, the present study demonstrated that the application of PRP suppresses the formation of intrahepatic blood vessels. The increase in hepatic angiogenesis is likely a repair mechanism, but it aggravates liver pathology and does not alleviate PHT[33,34]. These newly formed vessels are different from the sinusoidal vasculature, and they do not increase the blood perfusion of the cirrhotic liver; however, they further impair the homeostasis of hepatocytes and aggravate liver damage, further fulling fibrosis and inflammation[35]. Various investigations have shown that antiangiogenic drugs alleviate preclinical PHT models by reducing fibrosis in the liver[36], which is consistent with the present findings. However, it is imperative to acknowledge that in individuals afflicted with liver cirrhosis, the administration of a standard adult platelet dose merely yields a marginal elevation in platelet count. Consequently, additional platelet transfusions or alternative pharmacological interventions might be needed to enhance clinical indicators[32,37].
The present study had several limitations. BDL, a classic PHT model, represents acute cholestatic cirrhosis, but the causes of PHT vary greatly. Poison-induced (CCl4, TAA, and alcohol), chronic cholestasis and genetic defect-induced PHT models need to be investigated. Due to time constraints, the present study lacked a comprehensive investigation into the impact of PRP on lymphangiogenesis and liver fibrosis at various stages.