Published online Jan 7, 2025. doi: 10.3748/wjg.v31.i1.98561
Revised: October 8, 2024
Accepted: October 25, 2024
Published online: January 7, 2025
Processing time: 163 Days and 3.7 Hours
Esophageal stricture ranks among the most significant complications following endoscopic submucosal dissection (ESD). Excessive fibrotic repair is a typical pa
To examine the effectiveness and underlying mechanism of Kangfuxin solution (KFX) in mitigating excessive fibrotic repair of the esophagus post-ESD.
Pigs received KFX at 0.74 mL/kg/d for 21 days after esophageal full circumferential ESD. Endoscopic examinations occurred on days 7 and 21 post-ESD. In vitro, recombinant transforming growth factor (TGF)-β1 (5 ng/mL) induced a fibrotic microenvironment in primary esophageal fibroblasts (pEsF). After 24 hours of KFX treatment (at 1.5%, 1%, and 0.5%), expression of α-smooth muscle actin-2 (ACTA2), fibronectin (FN), and type collagen I was assessed. Profibrotic signaling was analyzed, including TGF-β1, Smad2/3, and phosphor-smad2/3 (p-Smad2/3).
Compared to the Control group, the groups treated with KFX and prednisolone exhibited reduced esophageal stenosis, lower weight loss rates, and improved food tolerance 21 d after ESD. After treatment, Masson staining revealed thinner and less dense collagen fibers in the submucosal layer. Additionally, the expression of fibrotic effector molecules was notably inhibited. Mechanistically, KFX downregulated the transduction levels of fibrotic functional molecules such as TGF-β1, Smad2/3, and p-Smad2/3. In vitro, pEsF exposed to TGF-β1-induced fibrotic microenvironment displayed increased fibrotic activity, which was reversed by KFX treatment, leading to reduced activation of ACTA2, FN, and collagen I. The 1.5% KFX treatment group showed decreased expression of p-Smad 2/3 in TGF-β1-activated pEsF.
KFX showed promise as a therapeutic option for post-full circumferential esophageal ESD strictures, potentially by suppressing fibroblast fibrotic activity through modulation of the TGF-β1/Smads signaling pathway.
Core Tip: This study investigated the therapeutic effects and mechanisms of Kangfuxin solution (KFX) on esophageal stenosis following endoscopic submucosal dissection (ESD). Pigs underwent full circumferential ESD and received KFX treatment for 21 days. Endoscopic examinations assessed esophageal repair, while the expression of α-smooth muscle actin-2, fibronectin, and collagen I was evaluated. Profibrotic signaling was analyzed, including transforming growth factor (TGF)-β1, Smad2/3, and phosphor-smad2/3. In vitro, TGF-β1 induced a fibrotic microenvironment in primary esophageal fibroblasts, and KFX treatment reduced the expression of fibrotic markers in these cells.
- Citation: Zhou X, Ma D, He YX, Jin J, Wang HL, Wang YF, Yang F, Liu JQ, Chen J, Li Z. Kangfuxin solution alleviates esophageal stenosis after endoscopic submucosal dissection: A natural ingredient strategy. World J Gastroenterol 2025; 31(1): 98561
- URL: https://www.wjgnet.com/1007-9327/full/v31/i1/98561.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i1.98561
Endoscopic submucosal dissection (ESD) extensively manages early esophageal cancer and precursor lesions[1,2]. The incidence of esophageal stricture increases with the increase in the area treated with ESD[3,4]. The esophageal stricture rate after full circumferential ESD can reach 100%[5,6]. Hence, preventing and treating esophageal stricture following extensive ESD have emerged as pressing clinical concerns demanding immediate attention.
In recent years, progress has been made in treating esophageal stricture after ESD, but it still falls short of clinical needs. Studies have shown that metallic and biodegradable stents have shown promise clinically, yet they are costly and carry risks such as perforation and bleeding[7-9]. Biomedical materials such as polyethylene glycol sheets[10], high-density collagen patches[11], and carboxymethyl cellulose membranes[12] aid in re-epithelialization but are still in pre-clinical stages. Finding safer, more effective treatments remains a challenge.
According to traditional Chinese medicine (TCM) principles, esophageal stricture post-ESD stems from Qi and Yin deficiency alongside toxic stasis obstruction. The Periplaneta americana, a TCM insect remedy, is recognized for its heat-clearing, detoxifying, and blood-stasis-resolving properties. The Kangfuxin solution (KFX) is the brand name of a Chinese patent medicine whose primary component is the ethanol extract of Periplaneta americana. It has been shown that KFX enhances mucosal healing in ulcers. Li et al[13] reported that KFX promotes full-thickness healing of experimental skin wounds by facilitating the regeneration of epithelial tissues and skin appendages. Recent studies have found that KFX shows dual regulatory effects on fibrosis[14]. Su et al[15] discovered that KFX promotes wound healing and suppresses scar formation by optimizing fibroblast function and inhibiting excessive collagen synthesis. In the late stage of wound healing, KFX effectively inhibits collagen production[16,17]. Given the histological structure and biological functions similarity between esophageal mucosa and skin[11], we postulated that KFX could enhance the remodeling of artificial ulcers and inhibit excessive fibrotic repair following esophageal ESD.
This research investigated the potential impact and mechanisms of KFX in preventing esophageal stenosis after full circumferential resection during esophageal ESD, utilizing both in vivo and in vitro experiments.
KFX has been approved for market by the China Food and Drug Administration. The administration standard for KFX (produced by Good Doctor Panxi Pharmaceuticals, China) specifies a 30 mL/d dosage for adults (60 kg).
The study adhered to animal ethical guidelines and was approved by the Animal Ethics Committee of Southwest Medical University (SWMU20210381). Thirteen healthy male Chinese experimental minipigs, averaging 15.9 ± 1.122 kg, were acquired from Shanghai JiaGan Biotechnology Co., Ltd. and adaptively fed for 1 week before surgery and treatment.
The disease model chosen for this study involved using a porcine model with full circumferential esophageal ESD. Before surgery, all animals underwent a 24-h fast and were deprived of water for 6 hours. After general anesthesia (Virbac, China), an upper gastrointestinal endoscope (Olympus, GIF-Q260J) with a transparent cap (Olympus, D-201-11, 804) was inserted into the esophagus of the pig in the lateral position. Circumferential marking, submucosal injection, initial incision, establishing two submucosal tunnels, and submucosal tissue dissection between the two tunnels were per
Twelve pigs were randomly divided into three groups: The esophageal ESD-induced stenosis model group (Control, n = 4), KFX treatment group after ESD (KFX, n = 4), and the prednisolone (PDNN) treatment group after ESD, n = 4. After the ESD surgery, animals in the KFX group were treated with KFX 0.74 mL/kg/d for 21 continuous days. The doses administered in the PDNN group were[19]: 0.74 mg/kg/d for the week 1, 0.49 mg/kg/d for week 2, and 0.25 mg/kg/d for week of 3 prednisone (Xinyi Pharmaceutical Co., Ltd., China).
The food tolerance of pigs was assessed utilizing a swallowing difficulty scoring system, as reported before[20]. It is based on a score of 0 for regular diet (ability to swallow solid foods), 1 for granular diet, 2 for semi-liquid diet, 3 for liquid diet, and 4 for inability to swallow any food.
The pigs in each group underwent endoscopic re-examination on 7 and 21 days after ESD. The essential condition of the artificial ulcer, including bleeding, perforation, mucosal regeneration, and luminal patency, was visually observed.
When esophageal stenosis develops, endoscopic evaluation may be hindered, making it difficult to objectively determine the degree of stenosis. The stricture rate was evaluated by the ratio of the narrowest part of the artificial ulcer to the normal mucosa[11].
The four um-thick paraffin-embedded esophageal tissue sections were processed according to Beyotime’s manual (cat# C0105M, China), including deparaffinization, nuclear and cytoplasm staining, dehydration, and mounting. Histological features were observed by experienced researchers using an optical microscope, and a semi-quantitative analysis of inflammatory cells and fibroblast count was performed using Image J software.
Following Masson’s trichrome staining protocol (Solarbio, China), esophageal tissue paraffin sections underwent sequential staining with hematoxylin solution, Masson’s Ponceau fuchsin solution, and aniline blue solution for cell nuclei, collagen fibers, and muscle fibers, respectively. Rinsing was conducted with a 0.2% aqueous acetic acid solution, and light microscopy was employed to capture the images (Leica, Germany, ICC50W).
The paraffin-embedded esophageal tissue blocks underwent sectioning, dehydration, rehydration, antigen retrieval in citric acid buffer at 100 °C, peroxidase blocking, and bovine serum albumin blocking. Primary antibodies were applied at appropriate concentrations (Table 1), followed by overnight incubation at 4 °C[21-24]. The following day, the sections were treated with a secondary antibody conjugated to horseradish peroxidase (HRP). A brown precipitate formed within the sections upon exposure to a 3,3’-Diaminobenzidine chromogenic solution (Bioss, Cat# C-0010, China). Subsequently, sections were examined under a light microscope, and nuclear fast red counterstaining was applied. Semi-quantitative analysis was performed using Image-Pro Plus software.
| Antibody name | Host | Brand, category No. | Country | Applications and dilution | ||
| Western blot | IHC | IF | ||||
| ACTA2 | Rabbit | Proteintech | China | 1:5000 | 1:800 | 1:100 |
| FN | Rabbit | Proteintech | China | 1:1000 | 1:200 | 1:100 |
| Collagen Ⅰ | Rabbit | Proteintech | China | 1:1000 | 1:500 | 1:100 |
| TGF-β1 | Mouse | Novus | United States | 1:1000 | - | - |
| Smad2 | Rabbit | CST | United States | 1:1000 | - | - |
| Smad3 | Rabbit | CST | United States | 1:1000 | - | - |
| P-Smad2 | Rabbit | Affinity Biotech | United States | 1:1000 | 1:100 | 1:50 |
| P-Smad3 | Rabbit | Affinity Biotech | United States | 1:1000 | 1:100 | 1:50 |
| Vimentin | Rabbit | Proteintech | China | - | - | 1:200 |
| Cytokerin | Rabbit | Abcam | China | - | - | 1:200 |
| GAPDH | Rabbit | CST | United States | 1:1000 | - | - |
For RNA extraction, tissue or cellular samples were processed using the Trizol reagent kit (Simgen, cat# 5003050, China). RNA samples’ absorbance was measured with a UV spectrophotometer at 260 and 280 nm, ensuring an OD260/OD280 ratio greater than 1.8 for suitability. RNA that was extracted underwent reverse transcription to produce cDNA, utilizing a reverse transcription kit (Takara, cat# RR036Q, Japan), followed by RT-PCR analysis on the Light Cycler 480 system (Roche, Switzerland) using the Green® Premix EX Tap TM II kit (Takara, cat# RR820A, Japan) according to manufacturer instructions. The amplification process had an initial denaturation at 95 °C for 30 seconds, then 40 cycles of denaturation at 95 °C for 5 seconds, annealing at 60 °C, and extension for 30 seconds. The expression levels of mRNA were normalized against GAPDH, and the relative expression levels were evaluated by applying the 2 -ΔΔCt method. The primer sequences used in this analysis are listed in Table 2.
| Primer name | Forward 5’ to 3’ | Reverse 5’ to 3’ |
| ACTA2 | TAGAACACGGCATCATCACCAACTG | TGGGGCAACACGAAGCTCATTG |
| FN | GTGTGGATGTCCTGCCTGTCAAC | GCTTGCTTTCCCTGCCCTGATG |
| Collagen I | CTCAAGATGTGCCACTCCGACTG | GTCTCGCCTGTCTCCATGTTGC |
| TGF-β1 | GACCGCAACAACGCAATCTATGAC | GGCACTGCTTCCCGAATGTCTG |
| Smad2 | ATGTCGTCCATCTTGCCATTCACTC | TGCTTTCTCACACCACTTCTCTTCC |
| Smad3 | TGTCGTCCATCCTGCCCTTCAC | ACTTCTCCTCCTGCCCGTTCTG |
| GAPDH | ATGATTCCACCCACGGCAAGTTC | AGCACCAGCATCACCCCATTTG |
The tissue or cell samples were lysed using RIPA lysis buffer to isolate total proteins, and the protein concentrations were assessed with a BCA protein quantification kit (Epizyme, cat# ZJ102 L, China). Following denaturation at 100 °C, 35 µg of protein was subjected to separation via 12% SDS-PAGE and subsequently transferred onto a PVDF membrane. The membrane was subsequently blocked using BSA for 2 hours, followed by overnight incubation with primary antibodies at 4 °C. Post washing with TBST, a specific secondary antibody conjugated with HRP was applied. The detection of signals was facilitated through the High-Sensitive ECL Chemiluminescence Detection Kit (Vazyme, Cat# E412, China) and detected by the ChemiScope 600 Exp system (ClinX, China).
The submucosal tissue of the normal pig esophagus was isolated and preserved in pre-cooled sterile phosphate-buffered saline (PBS) (containing 100000 U/L penicillin G and 100 mg/L streptomycin). After washing the tissue clean of blood in a centrifuge tube, it was cut into 1-3 mm3 fragments. The tissue fragments were positioned on the base of a culture dish, and high-glucose DMEM (Gibco, United States) supplemented with 10% fetal bovine serum (Gibco) was gradually introduced. The cells were co-cultured in a CO2 incubator (Thermo, United States).
The primary esophageal fibroblasts (pEsF) cells in the logarithmic growth phase were seeded in a 96-well plate at a density of 3 × 10 3 cells per well. Varying concentrations of the KFX (0%-5%) were incorporated into experimental wells at equivalent volumes of the culture medium. Additionally, blank and control wells were established. Following a 24 hours incubation, the culture medium was refreshed, and serum-free medium supplemented with 10% CCK-8 reagent (Dojindo, Japan) was administered to each well at 100 µL. The absorbance at 450 nm was subsequently measured using a microplate reader. The reagent instructions determined Cell viability by applying the absorbance values to the formula.
Recombinant transforming growth factor (TGF)-β1 (5 ng/mL) (Peprotech, United States) was used to induce a fibrotic environment based on pEsF. After 1 hour of TGF-β1 stimulation, different doses of the KFX selected through CCK-8 assay were added and co-incubated for 24 hours, with solvent treatment as the negative control (NC).
Following the specified treatment, pEsF were fixed in 4% paraformaldehyde for 10 minutes and then washed with PBS. Cells were permeabilized using 0.1% Triton X-100 for 10 minutes. After blocking with a protein-free blocking solution, primary antibodies at suitable concentrations were added and incubated for 14 hours. In a darkroom, specific secondary antibodies labeled with fluorescent markers were added and incubated for 1 hour. DAPI dye (Byotime, Cat# DAPI, China) was diluted 1:1000 to stain cell nuclei. The observations are made using a Leica DM4B fluorescence microscope equipped with a Leica DMC 6200 camera.
Statistical analysis was conducted using SPSS 27.0 software. Normally distributed continuous data are presented as mean ± SD. One-way analysis of variance was performed for intergroup comparison. Post hoc testing was performed using the least significant difference method, with P < 0.05 considered significant for detecting differences.
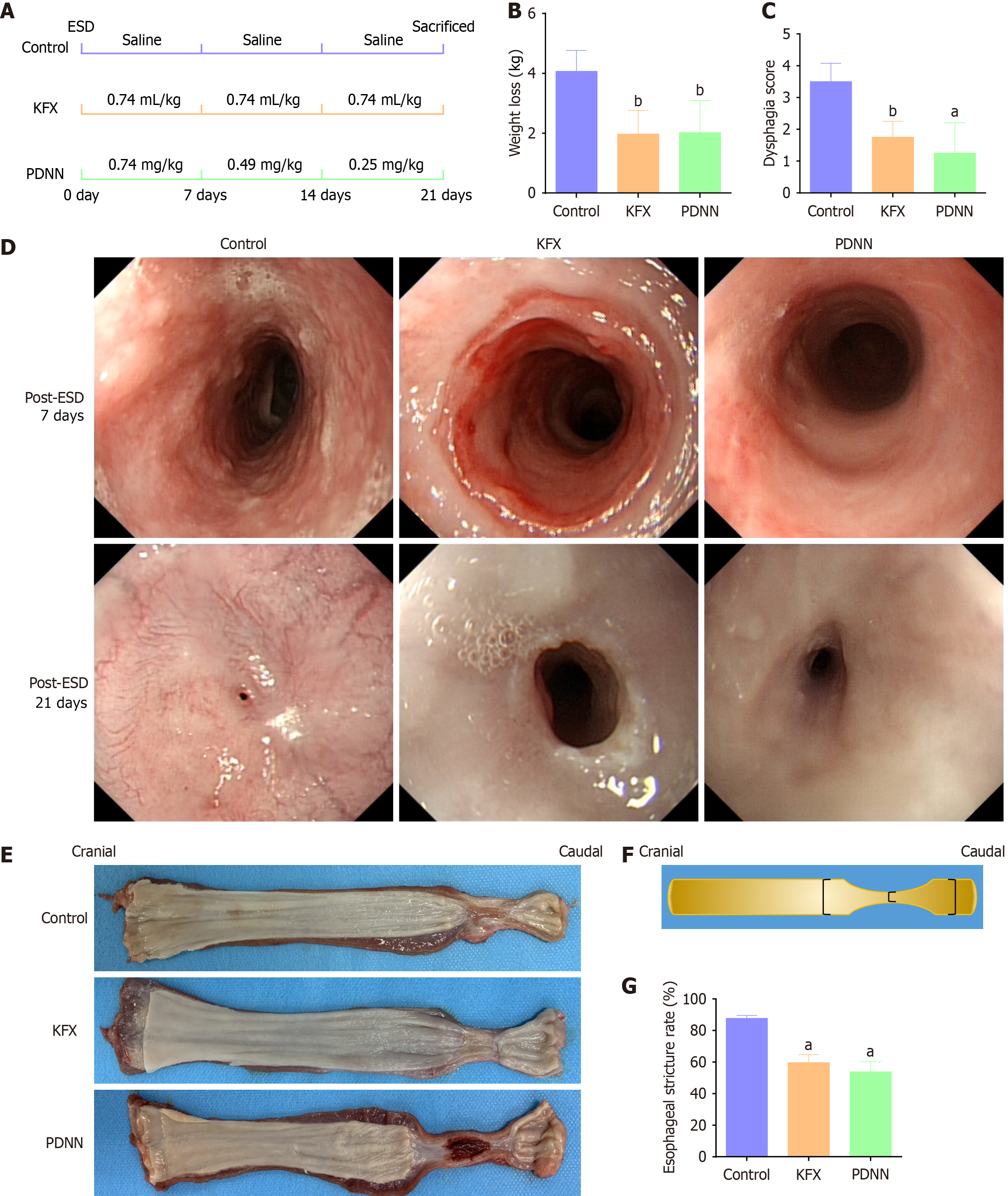
We established a porcine model of full circumferential esophageal ESD and treated animals orally with KFX and PDNN for 21 days post-ESD (Figure 1A).
Before ESD, the groups had no significant differences in body weight. However, by day 21 post-ESD, the Control group showed substantial weight loss, with the KFX and PDNN groups also experiencing weight reduction, albeit less severe (Figure 1B, Table 3).
| Control | KFX | PDNN | P value | |
| Weight loss, kg | 4.08 ± 0.69 | 1.98 ± 0.79b | 2.03 ± 1.07b | 0.011 |
| Dysphagia score | 3.50 ± 0.58 | 1.75 ± 0.50b | 1.25 ± 0.96a | 0.004 |
| Esophageal stricture rate | 87.68 ± 1.90 | 59.67 ± 5.05a | 53.90 ± 6.29a | < 0.001 |
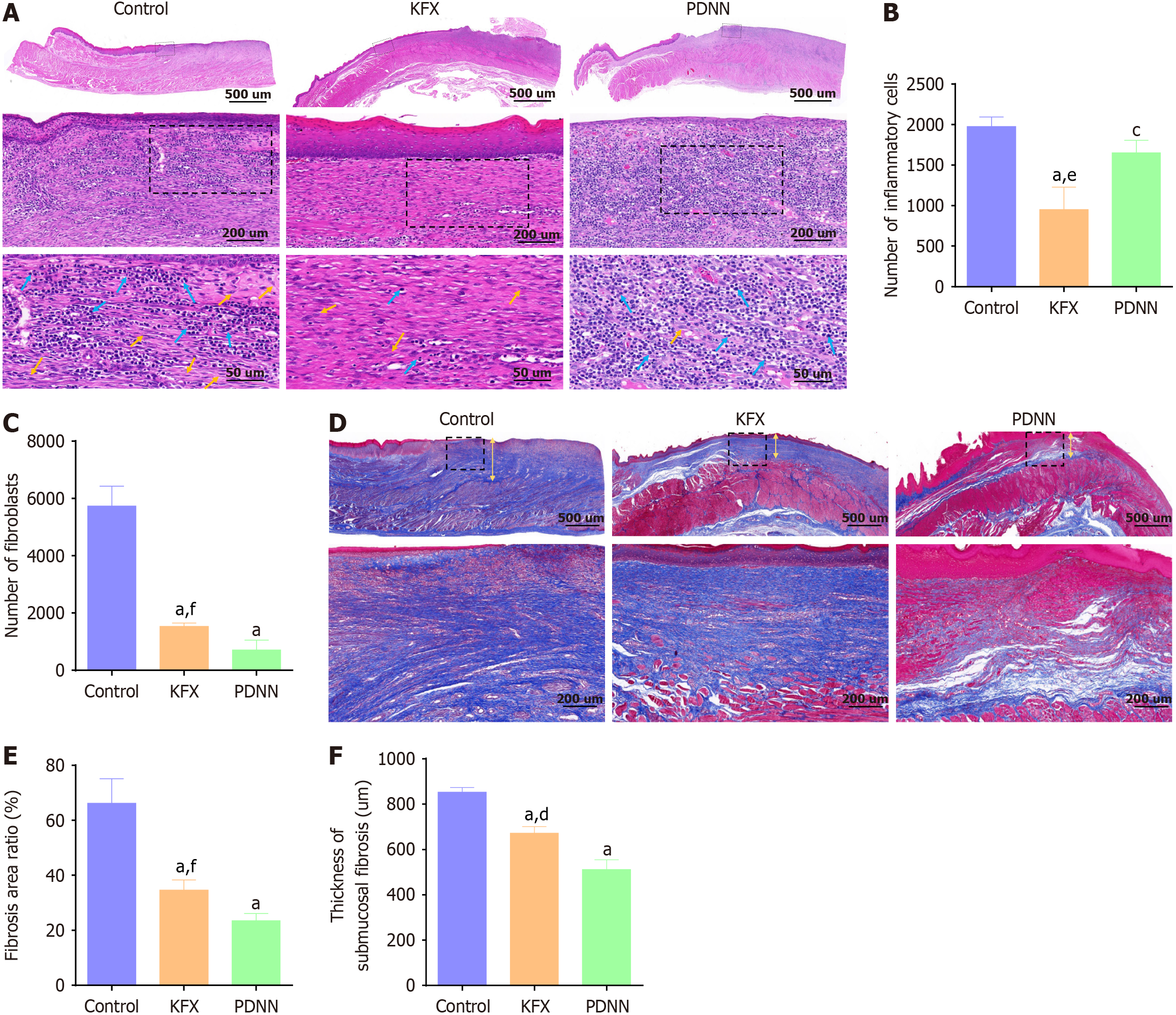
| Number of inflammatory cells | 1976.25 ± 116.39 | 950.25 ± 276.92a,e | 1651.50 ± 153.97c | < 0.001 |
| Number of fibroblasts | 5736.25 ± 691.56 | 1537.00 ± 118.89a, f | 710.00 ± 347.14a | < 0.001 |
| Fibrosis area ratio | 66.20 ± 8.95 | 34.63 ± 3.64a,f | 23.47 ± 2.65a | < 0.001 |
| Thickness of submucosal fibrosis, um | 853.93 ± 20.16 | 672.20 ± 28.69a,d | 512.83 ± 42.73a | < 0.001 |
Swallowing difficulty was assessed using a scoring system (Figure 1C, Table 3). After KFX and PDNN treatment, pigs showed improved tolerance to semi-liquid diets compared to the Control group, which struggled with liquid sustenance.
Endoscopic monitoring post-ESD showed no abnormalities on day 7. However, by day 21, the Control group developed a pinpoint-sized ulcer, causing water passage difficulty and premature termination of observation. In contrast, KFX and PDNN treatment significantly improved esophageal luminal contraction (Figure 1D).
Macroscopic examination of esophageal specimens (Figure 1E) revealed reduced strictures and contractions in the KFX and PDNN groups compared to the Control group. Additionally, the KFX group exhibited thicker newly formed esophageal mucosa than the PDNN group, with both treatment groups showing a lower incidence of esophageal mucosal stricture (Figure 1F and G, Table 3). These findings indicate that KFX and PDNN alleviate esophageal stricture after ESD.
ESD led to a fragmented distribution of regenerated epithelium, a lack of muscular mucosae, and atrophy of the muscular propria in the esophageal tissue. Compared to the Control group, KFX treatment significantly decreased the infiltration of inflammatory cells and the atrophy of the intrinsic muscle layer in the artificial ulcers. Consistent with the delayed healing observed in the esophageal specimens, compared to the PDNN group, hematoxylin-eosin (HE) staining showed a significant inflammatory cell infiltration in the KFX group (Figure 2A and B, Table 3). The morphologically polygonal or stellate fibroblasts in the submucosal layer of the esophagus were significantly reduced in the KFX and PDNN groups (Figure 2C, Table 3). These findings indicate that KFX positively influences the regeneration of submucosal in the esophagus following ESD.
The collagen fiber structure of the esophageal tissue was observed after Masson staining (Figure 2D-F, Table 3). The Control group displayed larger and denser fibrotic regions, with thicker collagen fibers filling the submucosal layer. In contrast to the Control group, the KFX and PDNN groups exhibited decreased fibrotic areas and a more loosely arranged collagen structure. However, compared to PDNN treatment, KFX demonstrated a less pronounced effect on fiber inhibition. These results suggest that KFX may benefit the reformation of collagen structure following full circumferential ESD in the esophagus.
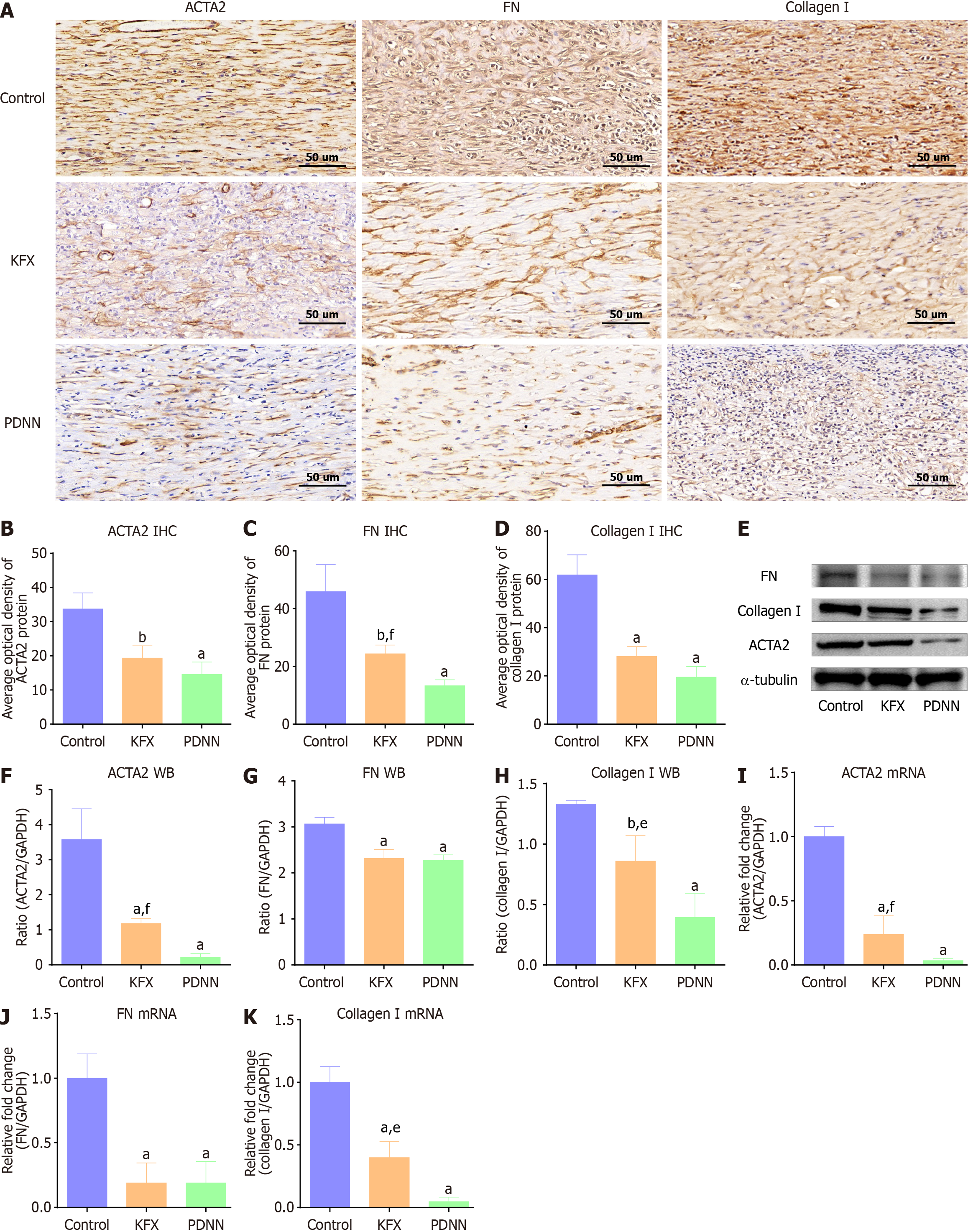
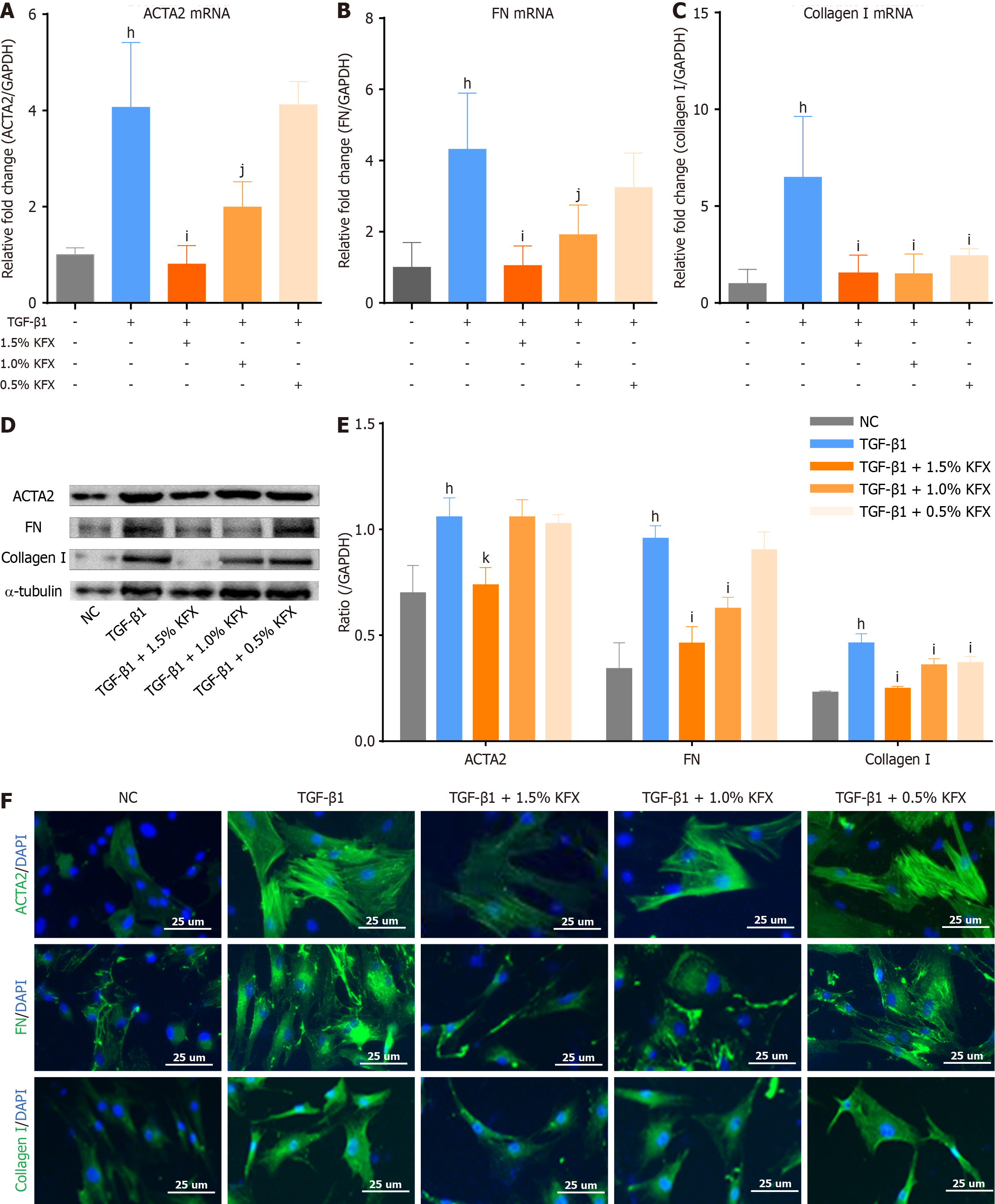
Consistent with the reduced Masson staining, the ulcer site of the esophagus in the KFX group showed decreased distribution of α-smooth muscle actin-2 (ACTA2)-positive myofibroblast, as revealed by immunohistochemical (Figure 3A and B). KFX treatment also reduced the protein levels of extracellular matrix components fibronectin (FN) and collagen I (Figure 3A, C, and D). Similarly, western blotting and RT-PCR validated the suppressed expression of ACTA2, FN, and collagen I (Figure 3E-K). However, KFX inhibited ACTA2 and collagen I expression slightly less than PDNN treatment. These data indicate that administration of KFX significantly suppressed the fibrosis-related remodeling of the esophageal ulcer. The positive drug PDNN exhibited a similar anti-fibrotic effect.
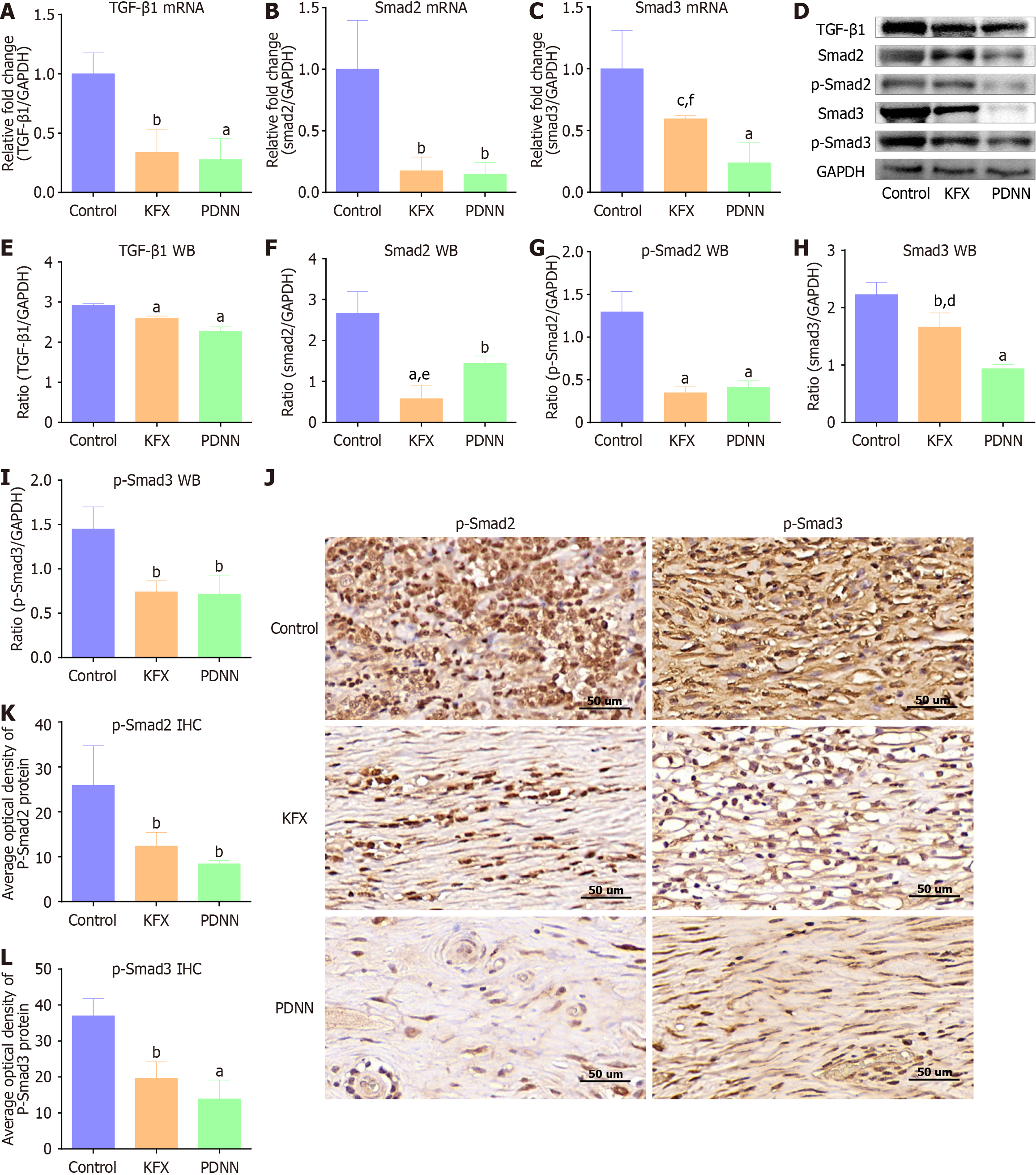
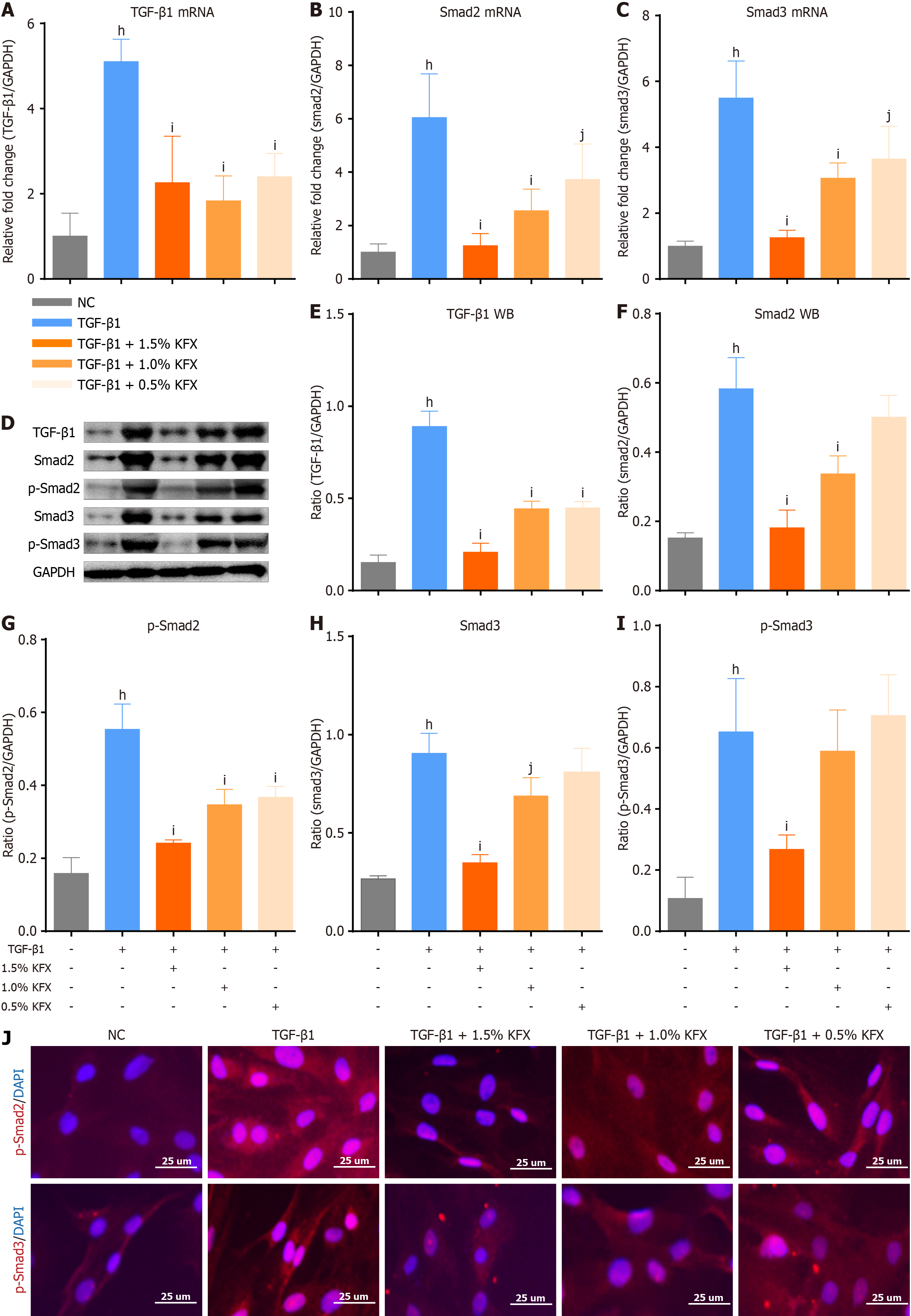
We studied the underlying mechanism by which KFX improves the esophageal stricture. The expression levels of TGF-β1, Smad2, and Smad3 were reduced in the KFX and PDNN groups when compared to the Control group (Figure 4A-C). p-Smad2 and p-Smad3 proteins were also decreased by KFX treatment (Figure 4D-I). Compared with the PDNN group, Smad3 expression in esophageal tissue was down-regulated to a lesser extent following KFX treatment. However, there was no notable difference in p-Smad 2/3 expression between the KFX and PDNN groups. Immunohistochemical staining indicated reduced nuclear expression of p-Smad2 and p-Smad3 in the KFX group (Figure 4J-L). These data suggest that KFX inhibits the activation of the TGF-β1/Smad2/3 signaling pathway in the esophagus following full circumferential ESD.
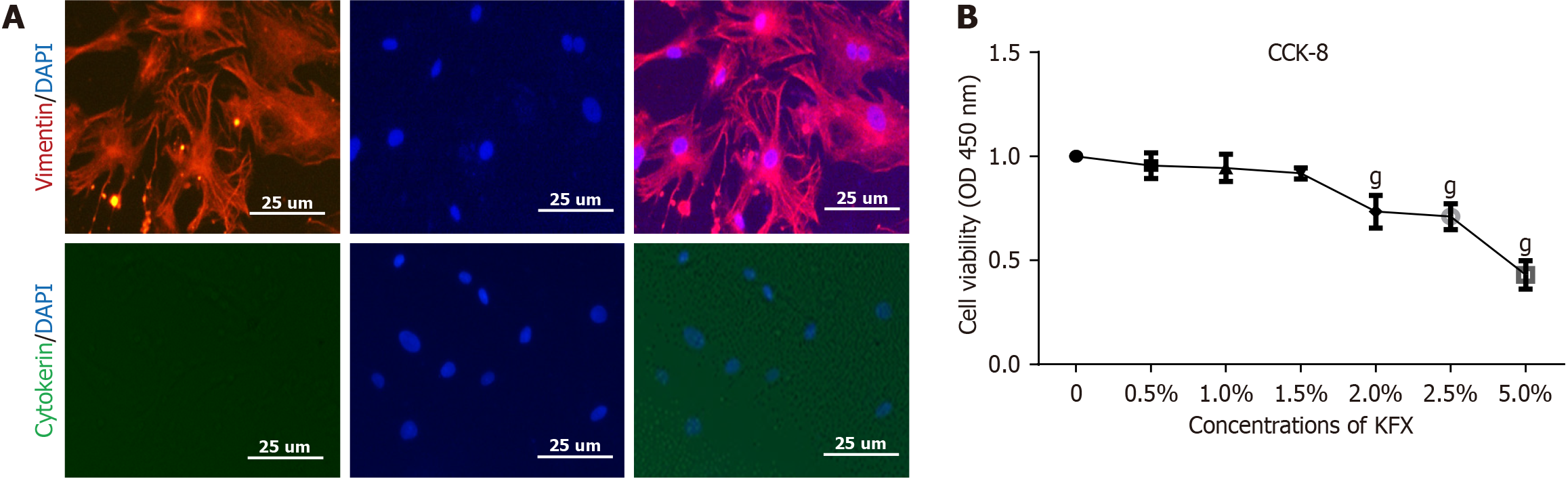
We isolated and cultured primary cells from the submucosal tissue of the esophagus (Supplementary Figure 2). Immunofluorescence revealed that most primary cells exhibited specific expression of vimentin. Cytokeratin, an epithelial marker, was used as a NC (Figure 5A).
The CCK-8 assay revealed that KFX concentrations < 1.5% exhibited minimal inhibitory effects on the viability of pEsF after 24 hours of co-culture (Figure 5B). Consequently, KFX concentrations of 0.5%, 1%, and 1.5% were chosen for subsequent experiments.
Compared to the NC group, TGF-β1 significantly increased ACTA2, FN, and collagen I expression, while KFX inter
We further discovered the expression of Smad2/3 and phosphor-smad2/3 (p-Smad2/3) in pEsF. TGF-β1 stimulation upregulated expression of Smad2 and Smad3, whereas KFX intervention resulted in a dose-dependent decrease in Smad2 and Smad3 mRNA transcription (Figure 7A-C). Compared to the TGF-β1 group, KFX significantly downregulated p-Smad2/3 protein expression (Figure 7D-I). Immunofluorescence demonstrated a dose-dependent decrease in the fluorescence signal in the KFX group (Figure 7J). These findings suggest that KFX similarly inhibits the hyperactivation of TGF-β1/Smads signaling in pEsF cells.
Esophageal strictures following ESD often entail structural abnormalities and functional impairment of the esophagus, inflicting significant distress upon patients. While the pathogenesis of ESD-induced esophageal stricture remains incompletely understood, numerous factors contributing to submucosal fibrous hyperplasia are widely acknowledged[25]. Our previous reports indicated that the typical pathological features of esophageal stenosis post-ESD involve myofibroblast proliferation and excessive extracellular matrix deposition, constituting an exaggerated fibrotic repair process[26]. Hence, addressing the inhibition of exaggerated fibrotic repair in the submucosa is crucial to prevent and treat esophageal strictures.
Corticosteroids are recognized as effective agents that can improve scarring by decreasing collagen synthesis and inhibiting fibroblast proliferation[27]. However, the oral corticosteroid route is unreliable and may cause delayed repair, immunosuppression, and abnormal glucose metabolism[28]. In addition, after esophageal full circumferential ESD, the mucosal defect area is extensive, and continuous use of steroid therapy significantly increases the risk of infection. Patients who receive systemic steroid treatment for 21 days or take > 700 mg PDNN are at increased risk of infection[29]. Other therapeutic agents, including thymosin β4, botulinum toxin type A, and mitomycin C, are still in the nascent stages of research and necessitate multicenter, large-sample, prospective randomized controlled trials to substantiate their efficacy[30-32]. Therefore, it is still the focus of researchers to study more safe and reliable drugs against esophageal strictures after ESD.
KFX is a Chinese patent medicine approved for clinical use in China (GYZ51021834). The main ingredient of KFX is the ethanol extract of the natural insect medicine Periplaneta americana, which contains abundant active compounds, including amino acids (such as leucine, alanine, proline, and lysine)[33-35], polyols (such as a polyol, glycerol, and 1, 2, 3-propanetriol)[36], nucleosides (such as uracil, guanosine, and xanthosine)[37,38], and peptides[36]. The positive role of KFX in repairing the original structure of mucosal injury is widely recognized. Studies have demonstrated that KFX not only facilitates mucosal repair by promoting the organized arrangement of new stroma but also enhances the anti-irritation function of the stratified squamous epithelium[39]. During wound healing, it stimulates the repair of dermal ultrastru
The anatomy of the swine esophagus is more akin to that of humans than that of conventional rodent models. Furthermore, the esophageal size in pigs is particularly suitable for models of ESD-associated diseases. The full circumferential ESD procedure was successfully conducted on porcine esophagi as described previously[12,18]. Subsequent endoscopic reevaluations revealed no bleeding, perforation, mucosal re-generations, or obstruction in any esophagi. Under the conditions of this study, the degree of esophageal stricture in pigs decreased after 21 days of KFX treatment. In the KFX group, the new tissue in the esophagus exhibited a closer resemblance to normal tissue, with increased softness, enhanced tension resistance, and improved mucosal integrity. Masson staining revealed decreased fibrotic areas and a more relaxed collagen arrangement in the esophageal submucosa following KFX treatment. Consistent with this reduction, KFX treatment suppressed the expression of ACTA2, FN, and collagen I at both mRNA and protein levels. Moreover, in vitro experiments demonstrated that KFX inhibited the expression of ACTA2, FN, and collagen I in pEsF in a dose-dependent manner. While PDNN treatment also exhibited a significant anti-esophageal fibrosis effect, a delay in esophageal ulcer healing was observed in the PDNN group. After PDNN therapy, a gross examination of the esophagus revealed artificial ulcers not fully covered by epithelium, along with areas of dark red ecchymosis in the middle. Honda et al[20,42] found that steroid treatment deepened and delayed the healing of esophageal ulcers induced by endoscopic mucosal resection, with no improvement seen with systemic administration. Additionally, in contrast to the KFX group, HE staining revealed significant infiltration of inflammatory cells in the esophageal submucosa of the PDNN group. This observation may be attributed to heightened inflammation in regions where external stimuli (such as saliva, food, and microorganisms) contribute to delayed healing of the esophagus. Therefore, KFX effectively inhibits excessive fibrotic repair of esophageal ESD ulcers and promotes healing.
We further investigated the mechanism of action of KFX against esophageal stricture. TGF-β1, known for promoting organ fibrosis, activates Smad upon receptor binding[26,43]. Phosphorylated Smad forms a complex with other Smads proteins, translocating into the nucleus to induce fibrosis-related gene transcription. Research indicates a notable increase in serum TGF-β1 levels in esophageal stricture, correlating with its severity[12]. Previous findings have highlighted heightened TGF-β1 expression in ESD-induced esophageal segments, enhancing Smad 2/3 phosphorylation and nuclear translocation, thereby amplifying fibrotic signaling. Consequently, targeting TGF-β1/Smads or their downstream effectors is a promising therapeutic avenue for inhibiting fibrosis in esophageal injury[26]. In this study, we observed that administering KFX in a porcine post-ESD esophageal stricture model significantly suppressed TGF-β1-driven fibrosis and downstream fibrotic molecule Smad2/3. In vitro, our findings consistently show that KFX treatment effectively curbs the overactivation of pEsF via TGF-β1/Smad2/3-dependent mechanisms within the fibrotic microenvironment induced by TGF-β1, thus regulating fibrotic function.
Our study had some limitations. We did not compare the treatment response differences between direct application and oral administration of KFX, a Chinese patent medicine approved for both routes. It is well established that the dosage form of KFX is a Chinese herbal liquid. Researchers may consider the incorporation of KFX with a reliable biomaterial to enhance its concentration within the esophageal mucosa. Other assessment methods for esophageal strictures, such as esophagography, could be utilized to obtain more comprehensive and objective evidence. Our findings suggest that KFX mitigates excessive esophageal fibrosis post-full circumferential ESD. Combining KFX with fasting, acid suppression, and physical expansion might enhance therapeutic outcomes. Further clinical and safety evaluations of KFX to prevent strictures post-esophageal ESD are planned.
In summary, KFX holds promise in ameliorating esophageal stricture following full circumferential ESD by mitigating excessive fibrous deposition. Its mechanism potentially involves the downregulation of the TGF-β1/Smads signaling pathway.
We thank Dr. Yang WX and Dr. Li L for their excellent technical assistance. We also thank Mr. Su SC for the valuable discussions on the anesthesia methods and dosage for the experimental pigs.
| 1. | Tsujii Y, Nishida T, Nishiyama O, Yamamoto K, Kawai N, Yamaguchi S, Yamada T, Yoshio T, Kitamura S, Nakamura T, Nishihara A, Ogiyama H, Nakahara M, Komori M, Kato M, Hayashi Y, Shinzaki S, Iijima H, Michida T, Tsujii M, Takehara T. Clinical outcomes of endoscopic submucosal dissection for superficial esophageal neoplasms: a multicenter retrospective cohort study. Endoscopy. 2015;47:775-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 157] [Article Influence: 15.7] [Reference Citation Analysis (1)] |
| 2. | Sanghi V, Amin H, Sanaka MR, Thota PN. Resection of early esophageal neoplasms: The pendulum swings from surgical to endoscopic management. World J Gastrointest Endosc. 2019;11:491-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 3. | Kuwano H, Nishimura Y, Oyama T, Kato H, Kitagawa Y, Kusano M, Shimada H, Takiuchi H, Toh Y, Doki Y, Naomoto Y, Matsubara H, Miyazaki T, Muto M, Yanagisawa A. Guidelines for Diagnosis and Treatment of Carcinoma of the Esophagus April 2012 edited by the Japan Esophageal Society. Esophagus. 2015;12:1-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 345] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 4. | Kitagawa Y, Uno T, Oyama T, Kato K, Kato H, Kawakubo H, Kawamura O, Kusano M, Kuwano H, Takeuchi H, Toh Y, Doki Y, Naomoto Y, Nemoto K, Booka E, Matsubara H, Miyazaki T, Muto M, Yanagisawa A, Yoshida M. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 1. Esophagus. 2019;16:1-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 390] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 5. | National Health Commission of the People’s Republic of China. [Guidelines for Diagnosis and Treatment of Esophageal Cancer (2022)]. Zhonghua Xiaohua Waike Zazhi. 2022;21:1247-1268. [DOI] [Full Text] |
| 6. | Endoscopic Tunneling Technique Collaboration Group of the Chinese Society of Digestive Endoscopy; Chinese Medical Association Endoscopy Physicians Branch. [Expert Consensus on Endoscopic Prevention and Treatment of Benign and Malignant Esophageal Strictures in China (2020, Beijing)]. Zhonghua Xiaohuaneijing Zazhi. 2021;38:173-185. [DOI] [Full Text] |
| 7. | Yano T, Yoda Y, Nomura S, Toyosaki K, Hasegawa H, Ono H, Tanaka M, Morimoto H, Horimatsu T, Nonaka S, Kaneko K, Sato A. Prospective trial of biodegradable stents for refractory benign esophageal strictures after curative treatment of esophageal cancer. Gastrointest Endosc. 2017;86:492-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Wen J, Lu Z, Yang Y, Liu Q, Yang J, Wang S, Wang X, Du H, Meng J, Wang H, Linghu E. Preventing stricture formation by covered esophageal stent placement after endoscopic submucosal dissection for early esophageal cancer. Dig Dis Sci. 2014;59:658-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Walter D, van den Berg MW, Hirdes MM, Vleggaar FP, Repici A, Deprez PH, Viedma BL, Lovat LB, Weusten BL, Bisschops R, Haidry R, Ferrara E, Sanborn KJ, O'Leary EE, van Hooft JE, Siersema PD. Dilation or biodegradable stent placement for recurrent benign esophageal strictures: a randomized controlled trial. Endoscopy. 2018;50:1146-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Nakano Y, Takao T, Morita Y, Sakaguchi H, Tanaka S, Ishida T, Toyonaga T, Umegaki E, Kodama Y. Endoscopic plombage with polyglycolic acid sheets and fibrin glue for gastrointestinal fistulas. Surg Endosc. 2019;33:1795-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Aoki S, Sakata Y, Shimoda R, Takezawa T, Oshikata-Miyazaki A, Kimura H, Yamamoto M, Iwakiri R, Fujimoto K, Toda S. High-density collagen patch prevents stricture after endoscopic circumferential submucosal dissection of the esophagus: a porcine model. Gastrointest Endosc. 2017;85:1076-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Tang J, Ye S, Ji X, Liu F, Li Z. Deployment of carboxymethyl cellulose sheets to prevent esophageal stricture after full circumferential endoscopic submucosal dissection: A porcine model. Dig Endosc. 2018;30:608-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Li LJ, Xu XH, Yuan TJ, Hou J, Yu CL, Peng LH. Periplaneta Americana L. as a novel therapeutics accelerates wound repair and regeneration. Biomed Pharmacother. 2019;114:108858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Yu X, Wang C, Wang Y, Li L, Gao X, Zhu T, An P, Meng Z, Wang W, Wu T, Hao Y. Microneedle Array Patch Made of Kangfuxin/Chitosan/Fucoidan Complex Enables Full-Thickness Wound Healing. Front Chem. 2022;10:838920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 15. | Su JR, Chen J, Huang XS, Shen YM, Tan J, Zeng CJ. [Effect of Kangfuxin Liquid on Pathological Scar Formation of Gastric Ulcer in Rats]. Zhongyaocai. 2013;36:979-982. [DOI] [Full Text] |
| 16. | Li WH, Wu WM, Liu X, Yang M, Zhou WT, Zeng JN. [Study of American Cockroach Extraction Solution in Regulating the TypeⅠ/Ⅲ Collagen of Chronic Wound]. Hunan Zhongyioyao Daxue Xuebao. 2018;38:250-253. [DOI] [Full Text] |
| 17. | Wei C, Cui SZ, Yi SY, Xiao YS, Zhang CL. [Explore on the mechanisms of Periplaneta Americana extract on skin wound repair in rats]. Anhui Yike Dauxe Xuebao. 2018;53:1161-1167. [DOI] [Full Text] |
| 18. | Su S, Pang T, Wang Y, Chen J. Prevention of Esophageal Stricture after Endoscopic Submucosal Dissection with an Autologous Esophageal Epithelial Cell Suspension: An Animal Study. Discov Med. 2023;35:1026-1034. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Kataoka M, Anzai S, Shirasaki T, Ikemiyagi H, Fujii T, Mabuchi K, Suzuki S, Yoshida M, Kawai T, Kitajima M. Efficacy of short period, low dose oral prednisolone for the prevention of stricture after circumferential endoscopic submucosal dissection (ESD) for esophageal cancer. Endosc Int Open. 2015;3:E113-E117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Honda M, Hori Y, Nakada A, Uji M, Nishizawa Y, Yamamoto K, Kobayashi T, Shimada H, Kida N, Sato T, Nakamura T. Use of adipose tissue-derived stromal cells for prevention of esophageal stricture after circumferential EMR in a canine model. Gastrointest Endosc. 2011;73:777-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Tomaszewska E, Hułas-Stasiak M, Dobrowolski P, Świątkiewicz M, Muszyński S, Tomczyk-Warunek A, Blicharski T, Donaldson J, Arciszewski MB, Świetlicki M, Puzio I, Bonior J. Does Chronic Pancreatitis in Growing Pigs Lead to Articular Cartilage Degradation and Alterations in Subchondral Bone? Int J Mol Sci. 2024;25. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Chen Y, McCauley SR, Johnson SE, Rhoads RP, El-Kadi SW. Downregulated Translation Initiation Signaling Predisposes Low-Birth-Weight Neonatal Pigs to Slower Rates of Muscle Protein Synthesis. Front Physiol. 2017;8:482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Herrmann J, Wohlert C, Saguner AM, Flores A, Nesbitt LL, Chade A, Lerman LO, Lerman A. Primary proteasome inhibition results in cardiac dysfunction. Eur J Heart Fail. 2013;15:614-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 24. | Wang S, Yan H, Fang B, Gu C, Guo J, Qiu P, Song N, Xu W, Zhang J, Lin X, Fang X. A myogenic niche with a proper mechanical stress environment improves abdominal wall muscle repair by modulating immunity and preventing fibrosis. Biomaterials. 2022;285:121519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (1)] |
| 25. | Miwata T, Oka S, Tanaka S, Kagemoto K, Sanomura Y, Urabe Y, Hiyama T, Chayama K. Risk factors for esophageal stenosis after entire circumferential endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Surg Endosc. 2016;30:4049-4056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 26. | Zhou X, Ma D, Fu J, Wang YF, Su SC, Tang ZX, Li XY, Chen J, Li Z. [Effect of TGF-β1/Smads/ACTA 2 signaling pathway on artificial ulcer fibrosis of stenosis after full circumcision of esophageal mucosal dissection: A porcine model]. Jiefangjun Yixue Zazhi. 2022;47:764-770. [DOI] [Full Text] |
| 27. | Ishihara R. Prevention of esophageal stricture after endoscopic resection. Dig Endosc. 2019;31:134-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 28. | Qiu Y, Shi R. Roles of Steroids in Preventing Esophageal Stricture after Endoscopic Resection. Can J Gastroenterol Hepatol. 2019;2019:5380815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Oliveira JF, Moura EG, Bernardo WM, Ide E, Cheng S, Sulbaran M, Santos CM, Sakai P. Prevention of esophageal stricture after endoscopic submucosal dissection: a systematic review and meta-analysis. Surg Endosc. 2016;30:2779-2791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 30. | Zhou X, Chen H, Chen M, Ding C, Zhang G, Si X. Comparison of endoscopic injection of botulinum toxin and steroids immediately after endoscopic submucosal dissection to prevent esophageal stricture: a prospective cohort study. J Cancer. 2021;12:5789-5796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 31. | Wang H, Shuai Q, Tang J, Long D, Xu C, Liu F, Li ZS. Local Thymosin β4 Gel Injection Prevents Esophageal Stricture after Circumferential Endoscopic Submucosal Dissection in a Porcine Model. Dig Dis. 2019;37:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Machida H, Tominaga K, Minamino H, Sugimori S, Okazaki H, Yamagami H, Tanigawa T, Watanabe K, Watanabe T, Fujiwara Y, Arakawa T. Locoregional mitomycin C injection for esophageal stricture after endoscopic submucosal dissection. Endoscopy. 2012;44:622-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Su H, Zhou XY, Chen L, Nai X, Li Q, Yu LS. [Detection of Amino Acids in Kangfuxin Liguid by Reverse Micellar Electrokinetic Capillary Chromatography - Direct UV Detection]. Fenxi Kexue Xuebao. 2019;35:90-94. [DOI] [Full Text] |
| 34. | Wang T, Zou Y, Sun XY, Wang KY, Xu L, Luo ZW, Lv DY. [Optimization of Extraction Process of Periplaneta americana Protein and Its Effect on Wound Healing]. Anhui Nongye Kexue. 2017;45:124-127. [DOI] [Full Text] |
| 35. | Wu J, Lu AD, Zhang LP, Zuo YX, Jia YP. [Study of clinical outcome and prognosis in pediatric core binding factor-acute myeloid leukemia]. Zhonghua Xue Ye Xue Za Zhi. 2019;40:52-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 130] [Reference Citation Analysis (0)] |
| 36. | Lv N, Shen JG, Li GZ, Wang JC, Si JY. [Studies on Chemical Components of Kangfuxin Liquid]. Zhongguo Xiandai Zhongyao. 2017;19:488-490. [DOI] [Full Text] |
| 37. | Ma Q, Luo Y, Guo P, Gao G, Yang M, Sablok G, Zhang Y, Zhou F. Clinical effects of Xinmailong therapy in patients with chronic heart failure. Int J Med Sci. 2013;10:624-633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Qi J, Yu J, Tan Y, Chen R, Xu W, Chen Y, Lu J, Liu Q, Wu J, Gu W, Zhang M. Mechanisms of Chinese Medicine Xinmailong's protection against heart failure in pressure-overloaded mice and cultured cardiomyocytes. Sci Rep. 2017;7:42843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 39. | Li ZG, Zhu H. [Research Progress on Chemical Constituents and Pharmacological Activity of Kangfuxin Solution]. Shijie Zuixin Yixue Xinxi Wenzhai. 2019;19:26-27. [DOI] [Full Text] |
| 40. | Li K, Ma X, Li Z, Liu Y, Shen G, Luo Z, Wang D, Xia L, Wang Z, Tian M, Liu H, Geng F, Li B. A Natural Peptide from A Traditional Chinese Medicine Has the Potential to Treat Chronic Atrophic Gastritis by Activating Gastric Stem Cells. Adv Sci (Weinh). 2024;11:e2304326. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 41. | Zhao B, Cai MQ, Cui YR, Liu JY. [Efficacy and safety of Kangfuxin liquid in postoperative gastrointestinal endoscopy patients: a Meta-analysis]. Yaowu Liuxuebingxue Zazhi. 2024;. [DOI] [Full Text] |
| 42. | Honda M, Nakamura T, Hori Y, Shionoya Y, Yamamoto K, Nishizawa Y, Kojima F, Shigeno K. Feasibility study of corticosteroid treatment for esophageal ulcer after EMR in a canine model. J Gastroenterol. 2011;46:866-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 43. | Urban ML, Manenti L, Vaglio A. Fibrosis--A Common Pathway to Organ Injury and Failure. N Engl J Med. 2015;373:95-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 185] [Article Influence: 18.5] [Reference Citation Analysis (0)] |