Published online Mar 7, 2024. doi: 10.3748/wjg.v30.i9.1213
Peer-review started: November 26, 2023
First decision: December 25, 2023
Revised: December 28, 2023
Accepted: February 5, 2024
Article in press: February 5, 2024
Published online: March 7, 2024
Processing time: 101 Days and 4.9 Hours
Helicobacter pylori (H. pylori) infects over half the global population, causing gastrointestinal diseases like dyspepsia, gastritis, duodenitis, peptic ulcers, G-MALT lymphoma, and gastric adenocarcinoma. Eradicating H. pylori is crucial for treating and preventing these conditions. While conventional proton pump inhibitor (PPI)-based triple therapy is effective, there’s growing interest in longer acid suppression therapies. Potassium competitive acid blocker (P-CAB) triple and dual therapy are new regimens for H. pylori eradication. Initially used in Asian populations, vonoprazan (VPZ) has been recently Food and Drug Administration-approved for H. pylori eradication.
To assess the efficacy of regimens containing P-CABs in eradicating H. pylori infection.
This study, following PRISMA 2020 guidelines, conducted a systematic review and meta-analysis by searching MEDLINE and Scopus libraries for randomized clinical trials (RCTs) or observational studies with the following command: [(“Helicobacter pylori” OR “H pylori”) AND (“Treatment” OR “Therapy” OR “Eradication”) AND (“Vonaprazan” OR “Potassium-Competitive Acid Blocker” OR “P-CAB” OR “PCAB” OR “Revaprazan” OR “Linaprazan” OR “Soraprazan” OR “Tegoprazan”)]. Studies comparing the efficacy of P-CABs-based treatment to classical PPIs in eradicating H. pylori were included. Exclusion criteria included case reports, case series, unpublished trials, or conference abstracts. Data variables encompassed age, diagnosis method, sample sizes, study duration, intervention and control, and H. pylori eradication method were gathered by two independent reviewers. Meta-analysis was performed in R software, and forest plots were generated.
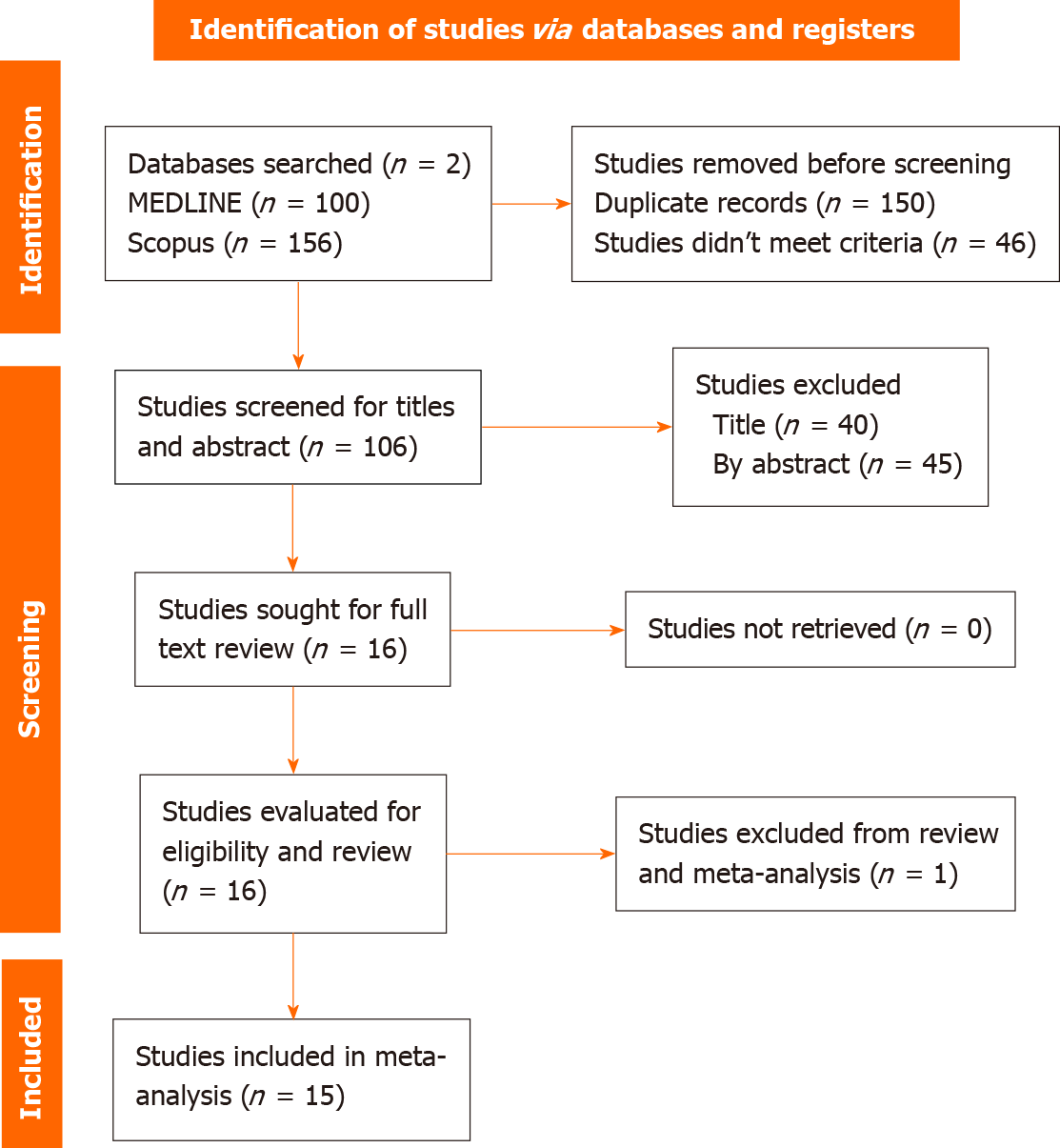
A total of 256 references were initially retrieved through the search command. Ultimately, fifteen studies (7 RCTs, 7 retrospective observational studies, and 1 comparative unique study) were included, comparing P-CAB triple therapy to PPI triple therapy. The intention-to-treat analysis involved 8049 patients, with 4471 in the P-CAB intervention group and 3578 in the PPI control group across these studies. The analysis revealed a significant difference in H. pylori eradication between VPZ triple therapy and PPI triple therapy in both RCTs and observational studies [risk ratio (RR) = 1.17, 95% confidence interval (CI): 1.11-1.22, P < 0.0001] and (RR = 1.13, 95%CI: 1.09-1.17, P < 0.0001], respectively. However, no significant difference was found between tegoprazan (TPZ) triple therapy and PPI triple therapy in both RCTs and observational studies (RR = 1.04, 95%CI: 0.93-1.16, P = 0.5) and (RR = 1.03, 95%CI: 0.97-1.10, P = 0.3), respectively.
VPZ-based triple therapy outperformed conventional PPI-based triple therapy in eradicating H. pylori, positioning it as a highly effective first-line regimen. Additionally, TPZ-based triple therapy was non-inferior to classical PPI triple therapy.
Core Tip: In the systematic review and meta-analysis on the treatment of Helicobacter pylori (H. pylori) with potassium competitive acid blockers, vonoprazan-based triple therapy demonstrated superior efficacy over conventional proton pump inhibitor (PPI)-based triple therapy, establishing itself as a highly effective first-line regimen. Conversely, tegoprazan-based triple therapy was found to be non-inferior to classical PPI triple therapy. These findings signify a potential paradigm shift in H. pylori eradication strategies.
- Citation: Kanu JE, Soldera J. Treatment of Helicobacter pylori with potassium competitive acid blockers: A systematic review and meta-analysis. World J Gastroenterol 2024; 30(9): 1213-1223
- URL: https://www.wjgnet.com/1007-9327/full/v30/i9/1213.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i9.1213
Helicobacter pylori (H. pylori), a gram-negative bacterium, which infects over 50% of the global population, with a prevalence in approximately half of the world’s inhabitants. The primary mode of transmission is person-to-person through fecal/oral exposure[1]. The presence of H. pylori in the stomach is associated with the onset of various gastroduodenal conditions, including chronic gastritis, peptic ulcers (10%-15% incidence), and gastric adenocarcinomas (less than 1% occurrence)[2].
Early childhood infection with H. pylori, ranging from 30% to 50%, significantly elevates the infection rate to over 90% during adulthood. This phenomenon is closely linked to adverse socioeconomic conditions and overcrowded living environments commonly found in developing countries[3]. As a result, while the incidence and prevalence of this infection have notably decreased in the developed world, the decline is not as pronounced in developing countries[3].
Successful eradication of H. pylori not only enhances the mucosal healing of ulcers and gastritis but also diminishes the incidence of gastric cancer[4,5]. Nevertheless, achieving successful H. pylori eradication poses challenges, leading to a rise in resistance rates to multiple antibiotics. This is compounded by factors such as host CYP2C19 gene polymorphisms and inadequate therapeutic regimens, contributing to a gradual decline in H. pylori eradication rates[6].
Vonoprazan (VPZ) stands out as an orally active, innovative potassium competitive acid blocker (P-CAB), distinguishing itself from conventional proton pump inhibitors (PPIs). Its mechanism involves binding to and inhibiting H+, K+-ATPase in the gastric parietal cells, marking the final step in the acid secretory cascade[7]. VPZ’s independence from pre or post-meal conditions allows for rapid absorption post-oral administration, achieving peak plasma levels in under 2 h and exhibiting an extended plasma half-life of 9 h. This unique pharmacokinetic profile grants VPZ a prolonged acid suppression effect compared to PPIs, and it remains unaffected by CYP2C19 polymorphisms[7].
VPZ exhibits versatile clinical applications, encompassing the treatment of gastroesophageal reflux disease, non-erosive reflux disease, erosive esophagitis, and peptic ulcer disease. It also serves as prophylaxis for upper gastroin
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA 2020) guidelines[9], a systematic review and meta-analysis were conducted, searching the literature in MEDLINE and Scopus libraries.
Inclusion criteria encompassed randomized clinical trials (RCTs) or observational studies that compared the efficacy of P-CABs-based treatment with classical PPIs-containing regimens for H. pylori eradication. Exclusion criteria involved case reports, case series, unpublished trials, or those solely presented as conference abstracts (oral presentations or posters).
A comprehensive search of various databases including MEDLINE and Scopus was conducted using the search terms: (“Helicobacter pylori” OR “H pylori”) AND (“Treatment” OR “Therapy” OR “Eradication”) AND (“Vonaprazan” OR “Potassium-Competitive Acid Blocker” OR “P-CAB” OR “PCAB” OR “Revaprazan” OR “Linaprazan” OR “Soraprazan” OR “Tegoprazan”).
The initial screening involved eliminating duplicates and non-RCTs or observational studies. Two independent reviewers then assessed titles and abstracts to exclude irrelevant papers. Subsequently, full papers were obtained, and the reviewers independently gathered data on the efficacy rates of regimens containing P-CABs for H. pylori eradication. Additional collected variables comprised age, diagnostic method, sample sizes, study duration, intervention, control group, and the method used to confirm the eradication of H. pylori.
Data were subjected to analysis and summarization employing descriptive techniques, encompassing frequency, means, and median calculations. For the meta-analysis of H. pylori treatment efficacy rates, R software version 4.3.2 and the meta package were employed.
The search command yielded 256 references, ultimately resulting in the inclusion of 15 studies: 7 RCTs, 7 retrospective observational studies, and 1 unique study that compared an RCT with an observational study, as illustrated in the PRISMA flowchart (Figure 1). The majority of these studies were conducted in Asia, with nine in Japan, four in South Korea, and one in Thailand. Notably, only one study was conducted in a Western region.
For the intention-to-treat analysis (ITT), a total of 8049 patients were involved across the 15 included studies, with 4471 in the intervention group and 3578 in the control group. The mean study duration in the intervention arm was 8.4 d, while in the control arm, it was 9.3 d.
Regarding H. pylori diagnosis, thirteen studies employed upper gastrointestinal endoscopy, utilizing biopsy with either rapid urease test, histology, or culture. Additionally, nine studies utilized the urease breath test (UBT), and another nine studies employed the H. pylori stool antigen, while four studies reported the use of H. pylori serum immunoglobulin G as one of the diagnostic methods.
The duration for H. pylori eradication control varied, ranging from 4 wk to 8 wk after the completion of treatment, using either the UBT or H. pylori stool antigen. Notably, fourteen studies utilized the UBT to assess H. pylori eradication status, while four studies employed the H. pylori stool antigen.
In eleven studies, VPZ was utilized in the intervention group, while tegoprazan (TPZ) was employed in only four studies. Notably, all four studies implementing TPZ were conducted in South Korea. Regarding the eleven VPZ studies, nine were carried out in Japan, one in Thailand, and the remaining one included both the United States and Europe. Lansoprazole (LPZ) emerged as the most frequently used PPI, featured in eleven studies, followed by rabeprazole (RPZ), which was employed in seven studies.
In ten studies, it was indicated that first-line or second-line triple therapies containing P-CABs were non-inferior to conventional PPI regimens (six studies including VPZ and four containing TPZ). Additionally, five studies demonstrated the superiority of P-CABs, with all of them utilizing VPZ compared to classic PPI therapy. Furthermore, all included studies reported that P-CABs were well-tolerated and deemed safe, with a safety profile similar to PPI regimens.
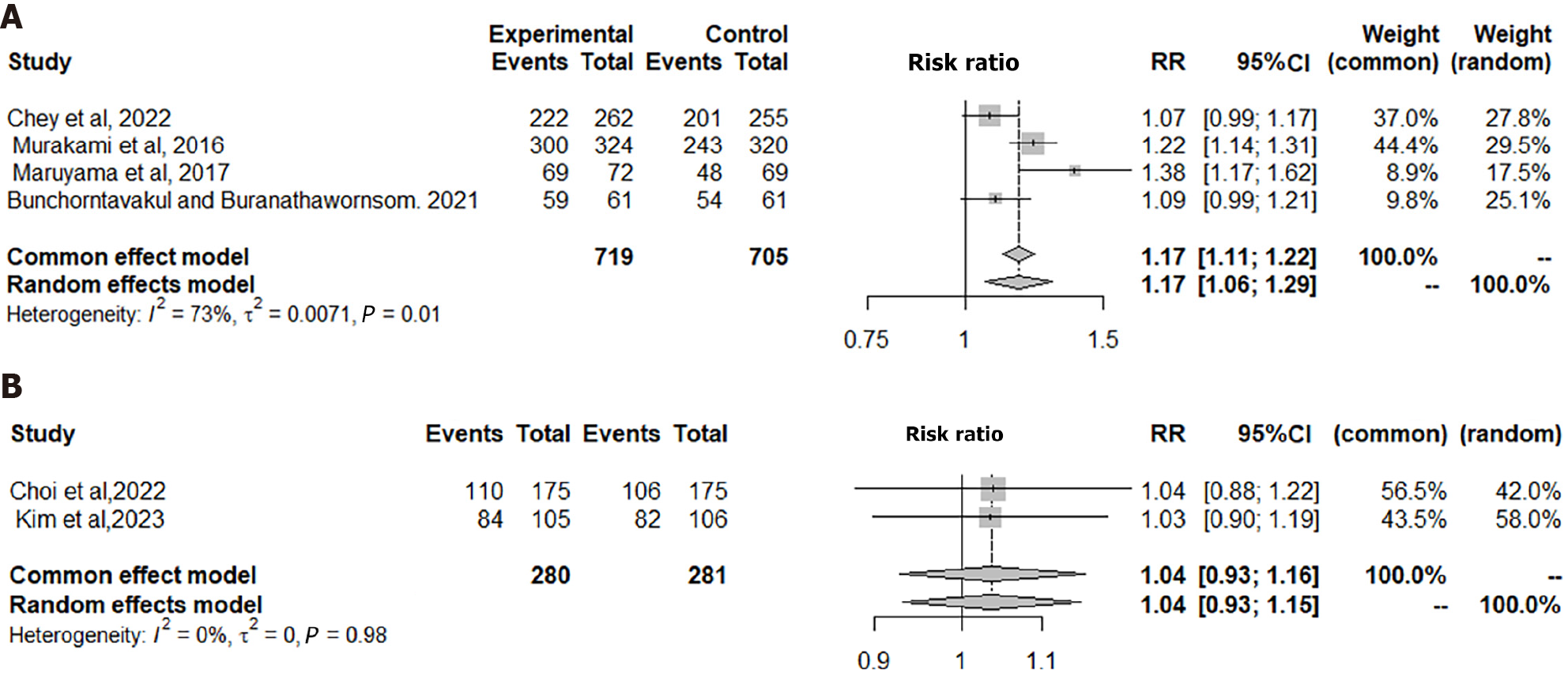
In the four included RCTs in Figure 2A, the ITT analysis comprised a total of 719 patients in the VPZ group and 705 in the PPI control group. The analysis revealed a significant difference between VPZ triple therapy and PPI triple therapy for H. pylori eradication [risk ratio (RR) = 1.17, 95% confidence interval (CI): 1.11-1.22, P = 0.0001], indicating that VPZ-based triple therapy is statistically superior to conventional PPI triple therapy for H. pylori eradication.
In the two RCTs included in Figure 2B, the ITT analysis involved a total of 280 patients in the TPZ group and 281 in the PPI control group. The analysis revealed no significant difference in H. pylori eradication therapy between the TPZ triple regimen and the PPI triple regimen (RR = 1.04, 95%CI: 0.93-1.16, P = 0.5324). Consequently, TPZ triple treatment is considered non-inferior to the classical PPI-based treatment.
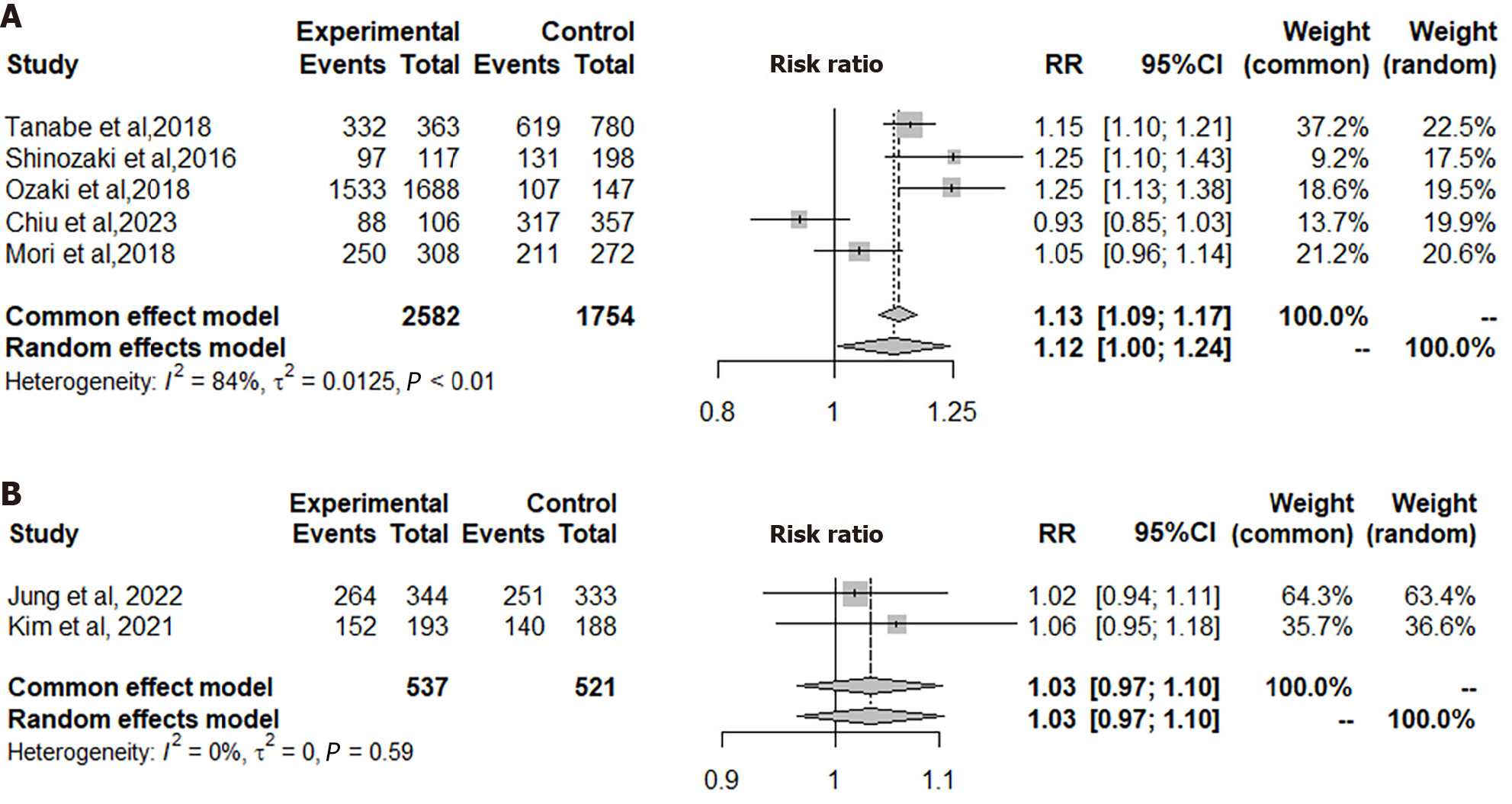
In the five observational studies included in Figure 3A, the ITT analysis encompassed a total of 2582 patients in the VPZ group and 1754 in the PPI control group. The analysis revealed a significant difference between VPZ triple therapy and PPI triple therapy for H. pylori eradication (RR = 1.13, 95%CI: 1.09-1.17, P = 0.0001). Consequently, VPZ-based triple therapy demonstrated superiority over conventional PPI triple therapy for H. pylori eradication in this context.
In the two observational studies represented in Figure 3B, the ITT analysis incorporated a total of 537 patients in the TPZ group and 521 in the PPI control group. The analysis revealed no significant difference in H. pylori eradication therapy between the TPZ triple regimen and the PPI triple regimen (RR = 1.03, 95%CI: 0.97-1.10, P = 0.3555). Consequently, TPZ triple treatment demonstrated non-inferiority to classical PPI-based treatment in this context.
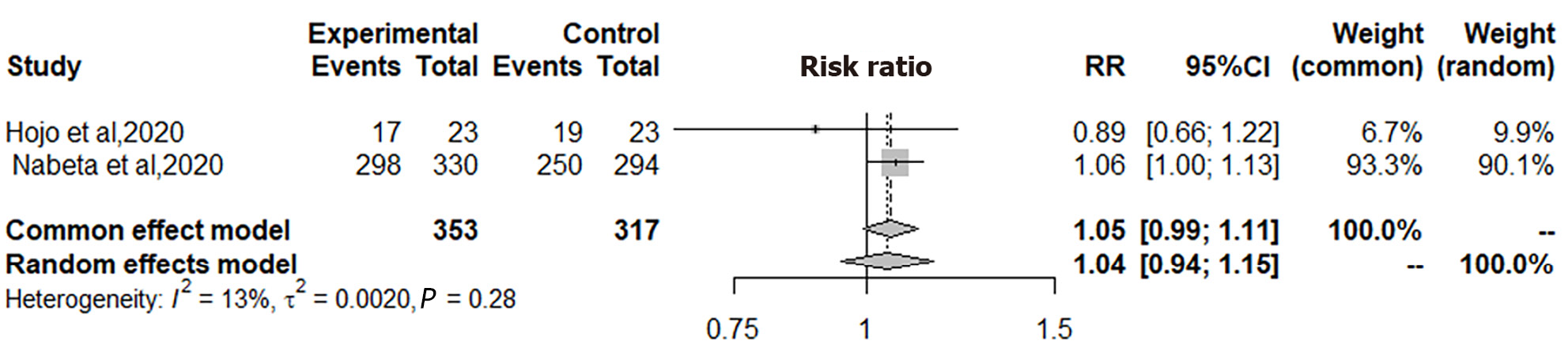
In the two studies focusing on second-line H. pylori treatment, as illustrated in Figure 4, the ITT analysis encompassed a total of 353 patients in the VPZ group and 317 in the PPI control group. The analysis revealed no significant difference in H. pylori eradication therapy between the VPZ second-line regimen and the PPI second-line regimen (RR = 1.05, 95%CI: 0.99-1.11, P = 0.1001). Consequently, VPZ second-line treatment demonstrated non-inferiority to PPI second-line treatment for H. pylori eradication.
Eradicating H. pylori is of utmost significance, serving as the primary treatment for low-grade G-MALT, mitigating the recurrence of peptic ulcers, and lowering the risk of gastric adenocarcinoma in high-risk populations[10-12]. This critical therapeutic intervention not only addresses immediate health concerns but also plays a pivotal role in preventing more severe and potentially life-threatening conditions associated with H. pylori infection.
For years, PPI-based regimens have been the primary approach for both initial and secondary treatments in H. pylori eradication. However, the effectiveness of these regimens has declined recently, notably due to increasing resistance to clarithromycin and levofloxacin. The need for alternative treatments has become evident. Although there is an ongoing debate about the practicality of conducting susceptibility testing before starting PPI-based first-line triple therapy, especially in areas where clarithromycin resistance exceeds 15%, logistical and financial challenges present obstacles, particularly in developing nations. As a result, the prevailing practice in many settings involves empirical PPI-based triple therapy without susceptibility testing[11-14].
Given the increasing difficulties associated with PPI first-line triple therapy, P-CAB-based first-line therapy emerges as a viable alternative. In 2015, the VPZ-based triple regimen received approval in Japan, and in 2022, both triple and dual VPZ-containing therapies obtained approval in the United States for the treatment of H. pylori infection and associated diseases[6-8].
In addition to the clinical complexities associated with H. pylori eradication therapy, economic considerations play a significant role, encompassing the substantial costs incurred for H. pylori testing, treatments, and follow-up requirements. A study aimed at assessing the cost-effectiveness of VPZ-based therapy compared to other first-line H. pylori eradication treatments in the United States utilized a Markov model to examine costs across various treatment options[15]. The cost analysis involved comparing VPZ-based triple therapy with alternatives such as VPZ-Amoxicillin dual treatment, rifabutin-based triple regimen, bismuth quadruple therapy, and PPI triple regimen. The study revealed that VPZ triple therapy had a higher expected cost of $1172 compared to $1048 with rifabutin triple therapy. However, VPZ triple therapy demonstrated a slightly higher expected quality-adjusted life years of 14.262 compared to 14.256 with rifabutin. The conclusion drawn from this investigation was that VPZ-based treatment is more cost-effective than the other options[15].
It’s worth noting that the cost of VPZ triple therapy (United States brand Voquezna Triple Pak) at $1172 could pose significant challenges in most developing countries, potentially limiting its prescription by clinicians even if the drugs are available. Therefore, the availability of highly government-subsidized generic forms of these drugs could prove beneficial in enhancing their utilization in the developing world.
This systematic review achieves a distinctive equilibrium by incorporating 7 RCTs, 7 retrospective observational studies, and 1 hybrid study (an RCT compared with observation). The cumulative sample size encompasses 8049 patients, with 55.5% (4471) allocated to the P-CAB group and 44.5% (3578) to the PPI control group. Notably, this review stands out from others by examining two different P-CABs, VPZ and TPZ, along with nearly all conventional PPIs, including omeprazole, esomeprazole, RPZ, LPZ, and pantoprazole.
The average duration of the studies in the experimental arm is 8.4 d, slightly shorter than the 9.3 d in the control arm. Despite its Asian dominance, this systematic review encompasses a diverse range of countries, including Japan, South Korea, and Thailand, offering a broader geographical perspective.
In this systematic review, 5 studies concluded that VPZ exhibited superiority over classical PPIs, while an additional 6 studies observed that VPZ was non-inferior to PPIs. Furthermore, TPZ was reported as non-inferior to typical PPIs in 4 other studies. The findings from this systematic review align with a previous one conducted in 2017[16]. In our meta-analysis, which included 4 RCTs and 5 observational studies comparing VPZ to PPI triple treatment, we found that VPZ-containing regimens are superior to conventional PPI-based triple therapy. Notably, in this current review, 3 studies involving VPZ were conducted outside Japan. In contrast, the previous meta-analysis from 2017 included 10 studies, all of which were conducted in Japan.
Results from this systematic review align with those published in 2022[17], suggesting that the VPZ-based triple regimen is superior to triple therapy containing PPIs. Furthermore, this meta-analysis conducted in 2022[17], which includes 7 studies from 3 countries, supports our findings. However, our systematic review provides updated data, incorporating 9 studies that compare VPZ to PPI in first-line triple therapy. Our meta-analysis reveals that TPZ-based triple therapy is non-inferior to the classical PPI-based triple regimen for H. pylori eradication. Similar results are observed for VPZ in second-line therapy, indicating its non-inferiority to PPI in the second-line H. pylori eradication treatment (Table 1).
| Ref. | Country | Methods | Results |
| Chey et al[8], 2022 | United States and Europe | Intervention: VPZ 20 mg + AMX 1 g + CLR 500 mg BD 14 d. Control: LPZ 30 mg + AMX 1 g + CLR 500 mg BD 14 d | H. pylori eradication in VPZ triple therapy in 84.7% of patients’ vs 78.8% for LPZ triple therapy (difference 5.9%; 95%CI: 0.8-12.6; noninferiority P < 0.001). VPZ triple therapy is non-inferior to LPZ triple therapy |
| Murakami et al[18], 2016 | Japan | Intervention: VPZ 20 mg + AMX 750 mg + CLR 200 mg/400 mg BD 7 d. Control: LPZ 30 mg + AMX 750 mg + CLR 200 mg/400 mg BD 7 d | H. pylori eradication rate with VPZ was 92.6% (95%CI: 89.2%-95.2%) compare to 75.9% (95%CI: 70.9%-80.5%) with LPZ with the difference being 16.7% (95%CI: 11.2%-22.1%) indicating the non-inferiority of VPZ (P < 0.0001). VPZ tripple therapy is non-inferior to LPZ. VPZ is also well tolerated and safe |
| Maruyama et al[19], 2017 | Japan | Intervention: VPZ 20 mg + AMX 750 mg, and CLR 200 mg/400 mg BD 7 d. Control: LPZ 30 mg + AMX 750 mg, and CLR 200 mg/400 mg BD 7 d | H. pylori eradication rate was significantly higher 95.8% in VPZ group compare to 69.6% in PPI group 95%CI: 88.3%-99.1%, P = 0.00003. VPZ-based therapy is superior to PPI as first line Pylori eradication and is safe |
| Bunchorntavakul et al[20], 2021 | Thailand | Intervention: VPZ 20 mg + AMX 1 g + CLR 500 mg BD 7 d. Control: OPZ 20 mg + AMX 1 g + CLR 500 mg BD 14 d | The H. pylori eradication rates was 96.7% in VPZ group compare to 88.5% in OPZ group (P = 0.083). VPZ triple therapy is non-inferior to OPZ triple therapy and it is well tolerated and safe |
| Kim et al[21], 2023 | South Korea | Intervention: TBMT group-TPZ 50 mg bid + TET 500 g qid, MTZ 500 mg tid + BBS 300 mg qid for 14 d. Control: LBMT group-LPZ 30 mg bid + TET 500 g qid, MTZ 500 mg tid + BBS 300 mg qid for 14 d | H. pylori eradication rates of TBMT group was 80.0% compare to LBMT group 77.4% with 95%CI: -8.4 to 13.7, P = 0.0124. Tegoprazan TBMT was non-inferior compare to lansoprazole LBMT and TBMT is also safe |
| Choi et al[22], 2022 | South Korea | Intervention: TPZ 50 mg + AMX 1 g + CLR 500 mg bid 7 d. Control: LPZ 30 mg + AMX 1 g + CLR 500 mg bid 7 d | The H. Pylori eradication rates in the TPZ group was 62.86% and LPZ groups was 60.57% with 95%CI: -8.53 with non-inferiority test, P = 0.009. TPZ based triple therapy is non-inferior to LPZ triple regimen and TPZ is safe |
| Tanabe et al[23], 2018 | Japan | Intervention: VPZ 20 mg + AMX 750 mg + CLR 200 mg/400 mg bid for 7 d. Control: PPI (LPZ 30 mg or RPZ 10 mg or EPZ 20 mg) + AMX 750 mg + CLR 200 mg/400 mg bid for 7 d | H. pylori eradication rates in VPZ based therapy group was 97.4% compare to the empirical PPI based therapy group 86.3% with 95%CI: 83.8-88.8 compare to with 95%CI: 95.7-99.1. The VPZ-based therapy was significantly more effective (P < 0.001) than PPI based empirical therapy |
| Shinozaki et al[24], 2016 | Japan | Intervention: VAC group VPZ 20 mg + AMX 750 mg + CLR 200 mg for 7 d. Control: PPI group [(LAC) LPZ 30 mg + AMX 750 mg + CLR 200 mg for bid 7 d or (RAC) RPZ 10 mg + AMX 750 mg + CLR 200 mg bid for 7 d or (EAC) EPZ 40 mg + AMX 750 mg + CLR 200 mg bid for 7 d] | H. pylori eradication therapy was 83% in VAC with 95%CI: 75-89 compare to LAC 66% with 95%CI: 59-72; RAC 67% with 95%CI: 58-74; EAC 85% with 95%CI: 75-89. The VAC group showed a significantly higher eradication rate compared with the LAC and RAC groups VAC 83%, LAC 66% and RAC 67%, P < 0.01. Similar eradication rate were observed in VAC group 83% compare to EAC group 83% |
| Ozaki et al[25], 2018 | Japan | Intervention: VPZ 40 mg + CLR 400 mg/800 mg + AMX 1500 mg daily for 7 d. Control: EPZ 40 mg + CLR 400 mg/800 mg + AMX 1500 daily for 7 d or RPZ 20 mg + CLR 400 mg/800 mg + AMX 1500 mg daily 7 d | H. pylori eradication rate in EPZ group 77.5% and the RPZ group 68.4%, no significant difference. There was a significantly superior eradication rate in VPZ group 90.8% compare to EPZ 77.5% and RPZ 68.4%. VPZ-based triple therapy eradication rates was remarkably higher compared with PPIs-based triple therapy in real world |
| Chiu et al[26], 2023 | Japan | Intervention: VAC group-VPZ 20 mg + AMX 1 g + CLR 500 mg bid for 7 d. Control: PPI (LPZ 30 mg or RPZ 20 mg or PPZ 40 mg) + AMX 1 g bid daily for 7 d, followed by the same PPI + CLR 500 mg + MTZ 500 mg bid for 7 d | H. pylori eradication rate was 83.0% in the VAC compare to 88.8% in the PPI group. There was no significant difference in eradication rate between VAC 7 d therapy and LAC 14 d sequential therapy P = 0.12 |
| Mori et al[27], 2018 | Japan | Intervention: VPZ 20 mg + AMX 750 mg + CLR 200 mg/400 mg bid for 7 d. Control: LPZ 30 mg + AMX 750 mg + CLR 200 mg/400 mg bid for 7 d | H. pylori eradication rate with VPZ was significantly higher than the LPZ group 91.0% compare to 84.7% P = 0.030. VPZ was significantly more effective than LPZ for first-line treatment |
| Jung et al[10], 2023 | South Korea | Intervention: TPZ 50 mg + AMX 1 g + CLR 500 mg bid for 14 d. Control: RPZ 20 mg + AMX 1 g + CLR 500 mg bid for 14 d | Eradication rate 76.7% in TPZ group with 95%CI: 72.1%-81.0% compare to 75.4% in RPZ group with 95%CI: 70.5%-79.8%, P > 0.999. The eradication rate of TPZ-based triple therapy was similar to that of RPZ-based triple therapy |
| Kim et al[28], 2021 | South Korea | Intervention: TACB-TPZ 50 mg + AMX 1 g, CLR 500 mg, and BBS 300 mg bid for 7 d. Control: LACB LPZ 30 mg + AMX 1 g + CLR 500 mg, and BBS 300 mg bid for 7 d | Eradication rates were 78.8% in the tegoprazan TACB and 74.5% in the lansoprazole LACB group (P = 0.323). Both the tegoprazan and lansoprazole group showed simlar eradication rates |
| Hojo et al[29], 2020 | Japan | Intervention: VPZ 20 mg + AMX 750 mg + MTZ 250 mg bid for 7 d - as 2nd line. Control: RPZ 10 mg + AMX 750 mg + MTZ 250 mg bid for 7 d - as 2nd line | Eradication rates in the was 73.9% in VPZ with 95%CI: 51.6%-89.8% compare to 82.6% in RPZ with 95%CI: 61.2%-95.0%, P = 0.72. VPZ based 2nd line therapy is non-inferior to RPZ 2nd line therapy and is safe |
| Nabeta et al[30], 2020 | Japan | Intervention: VPZ 20 mg + MTZ 250 mg + AMX 750 mg bid for 7 d. Control: LPZ 30 mg or RPZ 10 mg + MTZ 250 mg + AMX 750 mg bid for 7 d | H. pylori eradication rate in VPZ 2nd line therapy 90% (298/330) compare to 85% in lansoprazole 2nd line therapy (250/294), P = 0.045. P-CAB-based (VPZ) second-line H. pylori eradication is significantly better than PPI-based (lansoprazole) therapy |
VPZ-based dual therapy has emerged as a promising first-line treatment for H. pylori infection, involving the combination of VPZ and amoxicillin. Furthermore, one RCT compared a 10-d VPZ-amoxicillin dual therapy with a standard 14-d bismuth-based quadruple therapy, demonstrating noninferiority in eradication rates with fewer adverse events for the dual therapy[31]. A systematic review and meta-analysis conducted assessed the efficacy of this regimen compared to standard therapies for eradicating H. pylori. The pooled results from 15 studies involving 4568 patients demonstrated that VPZ-amoxicillin dual therapy achieved a high eradication rate of 85.0% and 90.0% by ITT and per-protocol analysis, respectively. Notably, this regimen outperformed PPIs-based triple therapy, showcasing its superiority[32]. A meta-analysis comparing VPZ-amoxicillin dual therapy with bismuth-containing quadruple therapy revealed similar eradication rates and improved safety profiles for the VPZ-based regimen. These findings collectively highlight the efficacy and safety of VPZ-based dual therapy as a compelling option for first-line H. pylori eradication[33].
Limitations of this study include the predominant focus on Eastern populations, hindering the generalization of the benefits of P-CABs to other demographic groups due to regional variations in genetics, diets, and lifestyle, which can influence gastric acidity levels. Moreover, the pooled sample size and the number of studies included in the analysis are limited. Additionally, the impact of antibiotic resistance, particularly clarithromycin resistance, wasn’t thoroughly addressed in this systematic review.
This systematic review and meta-analysis indicate that VPZ-based triple therapy is statistically superior to classical PPI-based therapy, while TPZ-based triple therapy is non-inferior to PPI-based triple regimens. The superiority of VPZ-based therapy in H. pylori eradication is demonstrated with statistical significance, while TPZ triple therapy shows non-inferiority compared to PPI-based regimens. Nevertheless, further studies are needed to comprehensively evaluate the extent of P-CAB-based triple therapy’s superiority over PPIs in eradicating H. pylori. The findings underscore the continued emphasis on the efficacy and safety of P-CABs, positioning P-CABs based triple therapy as a viable alternative to traditional PPI-based regimens for H. pylori eradication - being non-inferior and, in some instances, even superior to PPIs. Clinicians, especially in situations where VPZ-based therapy is available and affordable, should consider it as a viable option over traditional PPI-based therapy.
Helicobacter pylori (H. pylori) infection affects over 50% of the global population, with varying prevalence worldwide. The bacterium is linked to gastroduodenal conditions, including chronic gastritis, peptic ulcers, and gastric adenocarcinomas. Childhood infection, particularly in socioeconomically challenged environments, significantly elevates adult infection rates. Successful H. pylori eradication is crucial for mucosal healing and reducing gastric cancer incidence. However, rising antibiotic resistance and suboptimal therapeutic regimens pose challenges to eradication. Vonoprazan (VPZ), a potassium-competitive acid blocker (P-CAB), offers a promising alternative to conventional proton pump inhibitors (PPIs). With unique pharmacokinetics and diverse clinical applications, VPZ has gained Food and Drug Administration approval for H. pylori eradication therapy.
The increasing prevalence of H. pylori antibiotic resistance and suboptimal therapeutic outcomes necessitate innovative eradication strategies. VPZ emerges as a novel, effective alternative with distinct pharmacokinetics and versatile clinical applications. Addressing the efficacy of regimens incorporating P-CABs in H. pylori eradication is crucial. This study seeks to unravel the potential of VPZ-based therapies in overcoming challenges associated with conventional treatments, impacting future research directions in the field of H. pylori eradication.
The primary objective of this study is to evaluate the efficacy of regimens containing P-CABs, particularly focusing on VPZ, in eradicating H. pylori infection. The study aims to analyze key parameters, including eradication rates, adverse events, and compliance, to provide a comprehensive understanding of VPZ-based therapies. By achieving these objectives, the study contributes valuable insights into optimizing H. pylori eradication strategies, guiding future research towards more effective and tailored therapeutic approaches in gastroenterology and infectious diseases.
This systematic review and meta-analysis followed the PRISMA 2020 guidelines and conducted a comprehensive literature search in MEDLINE and Scopus libraries. Inclusion criteria encompassed randomized clinical trials (RCTs) or observational studies comparing the efficacy of P-CABs with classical PPIs for H. pylori eradication. Exclusion criteria ruled out case reports, case series, unpublished trials, and conference abstracts. Two independent reviewers screened titles, abstracts, and full papers, extracting data on efficacy rates and relevant variables. Descriptive techniques, including frequency, means, and medians, were employed for data summarization. The meta-analysis utilized R software version 4.3.2 and the meta package.
The systematic review and meta-analysis identified 15 studies, including 7 RCTs and 7 retrospective observational studies, contributing valuable insights into the efficacy and safety of regimens containing P-CABs for H. pylori eradication. The majority of studies were conducted in Asia, particularly in Japan and South Korea, with limited representation from Western regions. The analysis, comprising 8049 patients, demonstrated that VPZ triple therapy significantly outperformed conventional PPI triple therapy, showcasing its statistical superiority in H. pylori eradication. Conversely, tegoprazan (TPZ) triple therapy was found to be non-inferior to classical PPI-based treatment. Observational studies reinforced these findings, emphasizing the superiority of VPZ triple therapy over PPI-based regimens. Additionally, second-line therapy with VPZ demonstrated non-inferiority to PPI-based second-line treatment for H. pylori eradication. Notably, all P-CAB regimens exhibited good tolerability and safety profiles, aligning with PPI treatments. These results contribute comprehensive evidence supporting the efficacy and safety of P-CABs, particularly VPZ, in H. pylori eradication therapy. Further research may explore the application of these findings in diverse populations and regions.
This study advances the understanding of H. pylori eradication therapy by demonstrating the statistical superiority of VPZ-based triple therapy over conventional PPI-based regimens. The research concludes that TPZ-based triple therapy is non-inferior to PPI-based triple regimens. Notably, the study introduces the novel concept of P-CABs, specifically VPZ and TPZ, as effective alternatives in H. pylori eradication. By presenting robust evidence from 15 studies, including RCTs and observational studies, the research contributes to evolving treatment paradigms. The findings highlight VPZ’s superior efficacy and TPZ’s non-inferiority, offering clinicians valuable insights into alternative therapies amid rising antibiotic resistance. This study stands as a significant departure from conventional PPI-centric approaches and introduces P-CABs as viable options for H. pylori eradication, addressing the pressing need for alternative treatments in the face of declining PPI effectiveness.
Future research should delve into the broader application of P-CAB-based triple and dual therapy beyond the predominantly studied Eastern populations. Assessing the generalizability of VPZ and TPZ efficacy to diverse demographic groups is crucial for informing global H. pylori eradication strategies. Additionally, further investigations should explore the impact of P-CABs in regions with varying genetic, dietary, and lifestyle factors, influencing gastric acidity levels. Addressing the limitations of this study, such as the limited pooled sample size and focused demographic representation, will enhance the external validity of findings. Future studies could integrate comprehensive analyses of antibiotic resistance, particularly clarithromycin resistance, to refine treatment recommendations. Economical considerations, emphasized in the study, warrant exploration through cost-effectiveness analyses in diverse healthcare settings. As the study hints at the potential affordability challenges of VPZ triple therapy in developing countries, future research could explore strategies for enhancing accessibility, such as government-subsidized generic forms. These perspectives collectively guide future research to broaden the scope, applicability, and accessibility of P-CAB-based therapies, fostering a more nuanced understanding of their role in global H. pylori eradication efforts.
We extend our appreciation to the Faculty of Life Sciences and Education at the University of South Wales for the Gastroenterology MSc program and their invaluable support in our work. We sincerely acknowledge the efforts of the University of South Wales and commend them for their commitment to providing life-long learning opportunities and advanced life skills to Healthcare professionals.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: Federação Brasileira De Gastroenterologia.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng H, China; Shen YF, China; Wang D, China S-Editor: Wang JJ L-Editor: A P-Editor: Yu HG
| 1. | Shatila M, Thomas AS. Current and Future Perspectives in the Diagnosis and Management of Helicobacter pylori Infection. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (1)] |
| 2. | Bravo D, Hoare A, Soto C, Valenzuela MA, Quest AF. Helicobacter pylori in human health and disease: Mechanisms for local gastric and systemic effects. World J Gastroenterol. 2018;24:3071-3089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 109] [Cited by in RCA: 143] [Article Influence: 20.4] [Reference Citation Analysis (38)] |
| 3. | Salih BA. Helicobacter pylori infection in developing countries: the burden for how long? Saudi J Gastroenterol. 2009;15:201-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 4. | Ford AC, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer: systematic review and meta-analysis. Gut. 2020;69:2113-2121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 283] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 5. | Lee YC, Chiang TH, Chou CK, Tu YK, Liao WC, Wu MS, Graham DY. Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology. 2016;150:1113-1124.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 674] [Article Influence: 74.9] [Reference Citation Analysis (0)] |
| 6. | Zhong Z, Zhan B, Xu B, Gao H. Emphasizing the importance of successful eradication of Helicobacter pylori on initial treatment. Am J Cancer Res. 2022;12:1215-1221. [PubMed] |
| 7. | Oshima T, Miwa H. Potent Potassium-competitive Acid Blockers: A New Era for the Treatment of Acid-related Diseases. J Neurogastroenterol Motil. 2018;24:334-344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 125] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 8. | Chey WD, Mégraud F, Laine L, López LJ, Hunt BJ, Howden CW. Vonoprazan Triple and Dual Therapy for Helicobacter pylori Infection in the United States and Europe: Randomized Clinical Trial. Gastroenterology. 2022;163:608-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 169] [Article Influence: 56.3] [Reference Citation Analysis (2)] |
| 9. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40406] [Article Influence: 10101.5] [Reference Citation Analysis (2)] |
| 10. | Jung YS, Kim S, Kim HY, Noh SJ, Park JH, Sohn CI, Park CH. Efficacy and Tolerability of 14-Day Tegoprazan- versus Rabeprazole-Based Triple Therapy for Eradication of Helicobacter pylori: A Real-World Evidence Study. Gut Liver. 2023;17:711-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Coelho LGV, Marinho JR, Genta R, Ribeiro LT, Passos MDCF, Zaterka S, Assumpção PP, Barbosa AJA, Barbuti R, Braga LL, Breyer H, Carvalhaes A, Chinzon D, Cury M, Domingues G, Jorge JL, Maguilnik I, Marinho FP, Moraes-Filho JP, Parente JML, Paula-E-Silva CM, Pedrazzoli-Júnior J, Ramos AFP, Seidler H, Spinelli JN, Zir JV. IVTH BRAZILIAN CONSENSUS CONFERENCE ON HELICOBACTER PYLORI INFECTION. Arq Gastroenterol. 2018;55:97-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Malfertheiner P, Megraud F, Rokkas T, Gisbert JP, Liou JM, Schulz C, Gasbarrini A, Hunt RH, Leja M, O'Morain C, Rugge M, Suerbaum S, Tilg H, Sugano K, El-Omar EM; European Helicobacter and Microbiota Study group. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 662] [Article Influence: 220.7] [Reference Citation Analysis (0)] |
| 13. | de Moraes Andrade PV, Monteiro YM, Chehter EZ. Third-line and rescue therapy for refractory Helicobacter pylori infection: A systematic review. World J Gastroenterol. 2023;29:390-409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 14. | Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol. 2017;112:212-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 1018] [Article Influence: 127.3] [Reference Citation Analysis (1)] |
| 15. | Yunusa I, Love BL. Cost-Effectiveness of Vonoprazan-Based and Rifabutin-Based vs Other Regimens as First-Line Treatment of Helicobacter pylori Infection in the United States. Am J Gastroenterol. 2023;118:635-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Jung YS, Kim EH, Park CH. Systematic review with meta-analysis: the efficacy of vonoprazan-based triple therapy on Helicobacter pylori eradication. Aliment Pharmacol Ther. 2017;46:106-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 135] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 17. | Zhang M, Pang M, Zhang M. Efficacy and safety of potassium-competitive acid blockers versus proton pump inhibitors as Helicobacter pylori eradication therapy: a meta-analysis of randomized clinical trials. Clinics (Sao Paulo). 2022;77:100058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Murakami K, Sakurai Y, Shiino M, Funao N, Nishimura A, Asaka M. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut. 2016;65:1439-1446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 317] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 19. | Maruyama M, Tanaka N, Kubota D, Miyajima M, Kimura T, Tokutake K, Imai R, Fujisawa T, Mori H, Matsuda Y, Wada S, Horiuchi A, Kiyosawa K. Vonoprazan-Based Regimen Is More Useful than PPI-Based One as a First-Line Helicobacter pylori Eradication: A Randomized Controlled Trial. Can J Gastroenterol Hepatol. 2017;2017:4385161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 20. | Bunchorntavakul C, Buranathawornsom A. Randomized clinical trial: 7-day vonoprazan-based versus 14-day omeprazole-based triple therapy for Helicobacter pylori. J Gastroenterol Hepatol. 2021;36:3308-3313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 21. | Kim JS, Ko W, Chung JW, Kim TH. Efficacy of tegoprazan-based bismuth quadruple therapy compared with bismuth quadruple therapy for Helicobacter pylori infection: A randomized, double-blind, active-controlled study. Helicobacter. 2023;28:e12977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (2)] |
| 22. | Choi YJ, Lee YC, Kim JM, Kim JI, Moon JS, Lim YJ, Baik GH, Son BK, Lee HL, Kim KO, Kim N, Ko KH, Jung HK, Shim KN, Chun HJ, Kim BW, Lee H, Kim JH, Chung H, Kim SG, Jang JY. Triple Therapy-Based on Tegoprazan, a New Potassium-Competitive Acid Blocker, for First-Line Treatment of Helicobacter pylori Infection: A Randomized, Double-Blind, Phase III, Clinical Trial. Gut Liver. 2022;16:535-546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 23. | Tanabe H, Yoshino K, Ando K, Nomura Y, Ohta K, Satoh K, Ichiishi E, Ishizuka A, Otake T, Kohgo Y, Fujiya M, Okumura T. Vonoprazan-based triple therapy is non-inferior to susceptibility-guided proton pump inhibitor-based triple therapy for Helicobacter pylori eradication. Ann Clin Microbiol Antimicrob. 2018;17:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Shinozaki S, Nomoto H, Kondo Y, Sakamoto H, Hayashi Y, Yamamoto H, Lefor AK, Osawa H. Comparison of vonoprazan and proton pump inhibitors for eradication of Helicobacter pylori. Kaohsiung J Med Sci. 2016;32:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 25. | Ozaki H, Harada S, Takeuchi T, Kawaguchi S, Takahashi Y, Kojima Y, Ota K, Hongo Y, Ashida K, Sakaguchi M, Tokioka S, Sakamoto H, Furuta T, Tominaga K, Higuchi K. Vonoprazan, a Novel Potassium-Competitive Acid Blocker, Should Be Used for the Helicobacter pylori Eradication Therapy as First Choice: A Large Sample Study of Vonoprazan in Real World Compared with Our Randomized Control Trial Using Second-Generation Proton Pump Inhibitors for Helicobacter pylori Eradication Therapy. Digestion. 2018;97:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Chiu YT, Lee FJ, Kuo CY, Lin YC, Liang KS, Tseng LW, Chen YT, Chang CY. Seven-day vonoprazan-based triple therapy as first-lineHelicobacter pylori treatment in comparison with extended sequential therapy. JGH Open. 2023;7:105-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 27. | Mori N, Nishiura Y, Suga D, Moritani I, Yamanaka Y, Ooya Y, Inoue H, Takase K, Hioki M, Shiraki K. Second-line triple therapy in failures with vonoprazan-based triple therapy for eradication of Helicobacter pylori. Biomed Rep. 2018;9:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Kim JY, Lee SY, Kim H, Kim JH, Sung IK, Park HS. Efficacy of Seven-Day Potassium-Competitive Acid Blocker-Based First-Line Helicobacter Pylori Eradication Therapy Administered with Bismuth. Yonsei Med J. 2021;62:708-716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Hojo M, Asaoka D, Takeda T, Shimada Y, Matsumoto K, Yatagai N, Akazawa Y, Ueda K, Ueyama H, Nagahara A. Randomized controlled study on the effects of triple therapy including vonoprazan or rabeprazole for the second-line treatment of Helicobacter pylori infection. Therap Adv Gastroenterol. 2020;13:1756284820966247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Nabeta H, Shinozaki S, Abe Y, Koyanagi R, Nakamichi T, Kobayashi Y, Lefor AK, Hirashima H. A Potassium-Competitive Acid Blocker-Based Regimen as Second-Line Therapy Improves Helicobacter pylori Eradication. Digestion. 2020;101:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Yan TL, Wang JH, He XJ, Zhu YB, Lu LJ, Wang YJ, Wang ZW, Gao JG, Xu CF, Ma H, Luan SM, Li L, Chen Y. Ten-Day Vonoprazan-Amoxicillin Dual Therapy vs Standard 14-Day Bismuth-Based Quadruple Therapy for First-Line Helicobacter pylori Eradication: A Multicenter Randomized Clinical Trial. Am J Gastroenterol. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 32. | Du RC, Hu YX, Ouyang Y, Ling LX, Xu JY, Sa R, Liu XS, Hong JB, Zhu Y, Lu NH, Hu Y. Vonoprazan and amoxicillin dual therapy as the first-line treatment of Helicobacter pylori infection: A systematic review and meta-analysis. Helicobacter. 2024;29:e13039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (1)] |
| 33. | Zhou BG, Jiang X, Ding YB, She Q, Li YY. Vonoprazan-amoxicillin dual therapy versus bismuth-containing quadruple therapy for Helicobacter pylori eradication: A systematic review and meta-analysis. Helicobacter. 2024;29:e13040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (1)] |