Published online Mar 7, 2024. doi: 10.3748/wjg.v30.i9.1132
Peer-review started: December 21, 2023
First decision: January 13, 2024
Revised: January 15, 2024
Accepted: February 8, 2024
Article in press: February 8, 2024
Published online: March 7, 2024
Processing time: 75 Days and 13.3 Hours
Melanocortin 3 and 5 receptors (i.e., MC3R and MC5R) belong to the melanocortin family. However, data regarding their role in inflammatory bowel diseases (IBD) are currently unavailable.
This study aims to ascertain their expression profiles in the colonic mucosa of Crohn’s disease (CD) and ulcerative colitis (UC), aligning them with IBD disease endoscopic and histologic activity.
Colonic mucosal biopsies from CD/UC patients were sampled, and immunohistochemical analyses were conducted to evaluate the expression of MC3R and MC5R. Colonic sampling was performed on both traits with endoscopic scores (Mayo endoscopic score and CD endoscopic index of severity) consistent with inflamed mucosa and not consistent with disease activity (i.e., normal appearing mucosa).
In both CD and UC inflamed mucosa, MC3R (CD: + 7.7 fold vs normal mucosa, P < 0.01; UC: + 12 fold vs normal mucosa, P < 0.01) and MC5R (CD: + 5.5 fold vs normal mucosa, P < 0.01; UC: + 8.1 fold vs normal mucosa, P < 0.01) were significantly more expressed compared to normal mucosa.
MC3R and MC5R are expressed in the colon of IBD patients. Furthermore, expression may differ according to disease endoscopic activity, with a higher degree of expression in the traits affected by disease activity in both CD and UC, suggesting a potential use of these receptors in IBD pharmacology.
Core Tip: This study sought to examine the expression levels of Melanocortin 3 and 5 receptors (MC3R and MC5R) in the colons of individuals diagnosed with Crohn's disease and ulcerative colitis. Analysis of tissue samples obtained from both inflamed and non-inflamed sections of the colon revealed a notable increase in the expression of both receptors within inflamed regions compared to non-inflamed areas, with the extent of expression suggesting a potential association with the severity of disease activity. These findings imply that MC3R and MC5R may serve as potential targets for pharmacological interventions in the context of inflammatory bowel diseases.
- Citation: Gravina AG, Panarese I, Trotta MC, D'Amico M, Pellegrino R, Ferraraccio F, Galdiero M, Alfano R, Grieco P, Federico A. Melanocortin 3,5 receptors immunohistochemical expression in colonic mucosa of inflammatory bowel disease patients: A matter of disease activity? World J Gastroenterol 2024; 30(9): 1132-1142
- URL: https://www.wjgnet.com/1007-9327/full/v30/i9/1132.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i9.1132
The etiology of inflammatory bowel diseases (IBD), predominantly encompassing Crohn’s disease (CD) and ulcerative colitis (UC), remains enigmatic, primarily due to the intricate interplay of various factors and molecular pathways. Inhibitors of the tumor necrosis factor (TNF)-induced inflammatory pathway continue to constitute a significant component of the treatment protocols employed in current clinical practice[1,2]. In any case, various lines of evidence have demonstrated that the modulation of the melanocortin system can influence inflammatory pathways, thereby unveiling the therapeutic potential of melanocortins[3-6].
The melanocortin system constitutes a sophisticated and evolutionarily antique network of peptides, encompassing α, β, γ-Melanocyte-Stimulating-Hormone (MSH), and adrenocorticotropic hormone (ACTH). These peptides stem from a common protein precursor called pro-opiomelanocortin[7]. It functions via five melanocortin receptors (MC1-5R) that belong to the family of G protein-coupled receptor proteins, distributed diversely in both animals and humans. They are promiscuously activated by [Nle4, DPhe7]-α-MSH, α-MSH, β-MSH, γ-MSH, ACTH, and agouti-related protein[3]. The MC1R receptor exhibits expression within a heterogeneous spectrum of cell types, encompassing fibroblasts, melanocytes, keratinocytes, neutrophils, monocytes, dendritic cells, B lymphocytes, gliocytes, endotheliocytes, and additionally, neoplastic cells. In the context of melanogenesis, it primarily contributes to the synthesis of eumelanin by activating the enzyme tyrosinase. Additionally, its expression is observed in the colon, where it plays a role in gut inflammation in rats[8,9].
In contrast, MC2R, primarily expressed in the adrenal cortex and adipocytes, is implicated in steroid synthesis[7,10] and does not have a purported role in inflammation. MC3R displays a comprehensive expression profile within the central nervous system and immune cells, specifically in B lymphocytes and macrophages. Additionally, its presence extends to diverse anatomical locations in the rat, including the gut, heart, and placenta[7,11]. Its primary roles are associated with metabolic control and inflammatory response. MC4R is located within the vagal nerve afferents to the stomach and small intestine, participating in postprandial functions in mice[7,12].
MC5R exhibits a primarily widespread distribution and is implicated in the immunomodulation of B-T lymphocytic responses, along with the regulation of secretions from exocrine glands[7,13-19], with no clear available information on its localization and role at the intestinal level. The extent of expression of MC3R and MC5R in the colon and their roles in the intestinal microenvironment of IBD are not well understood.
This study aims to assess the immunohistochemical expression of MC3R and MC5R in the colons of patients with IBD and to determine whether there is a greater immunohistochemical expression in segments affected by the disease compared to those with apparently normal mucosa.
Patients exhibiting symptoms consistent with and suspected of having IBD (e.g., increased bowel movements, rectal bleeding, abdominal pain) and receiving a specialist recommendation for colonoscopy, subsequently diagnosed with IBD (either CD or UC), were included in the study. The study was carried out at the Hepatogastroenterology Division of the University of Campania Luigi Vanvitelli from January to December 2020. The research adhered to the principles of the Declaration of Helsinki and received approval from the Ethics Committee of the University of Campania Luigi Vanvitelli (protocol code 795 on December 23, 2019).
Several inclusion and exclusion criteria were established. IBD participants aged 18 or older, who were treatment-naïve for any IBD, were included. Specifically, patients were required not to have received conventional therapy (e.g., mesalazine, budesonide, conventional immunosuppressants like azathioprine, systemic-acting steroids), advanced therapies such as biologics (e.g., infliximab, adalimumab, ustekinumab), or small molecules (e.g., tofacitinib, filgotinib, upadacitinib).
Furthermore, to prevent study exclusion, all IBD diagnoses had to be confirmed by a pathologist in accordance with current guidelines[20].
Hospitalized patients or those with clinically significant infections (e.g., tuberculosis, SARS-CoV-2, HIV, Clostridioides difficile) within the preceding six months, or with clinical or instrumental evidence of neoplasm, were excluded. Additionally, patients with subsequent endoscopic and/or histological diagnoses of ischemic colitis, radiation colitis, microscopic colitis, undetermined colitis, colonic dysplasia, and benign or malignant colonic neoplasms were also excluded from our study. Patients presenting conditions that, according to the investigators' assessment, could potentially introduce bias into the study (for instance, individuals with decompensated comorbidities or those currently experiencing severe acute conditions such as decompensated diabetes or severe cardiovascular disease) were also excluded. Moreover, patients with other digestive diseases (e.g., celiac disease, autoimmune atrophic gastritis) were excluded. Individuals with known psychiatric disorders, legal incapacity to provide informed consent, or, lastly, those with any contraindication to undergoing colonoscopy were omitted.
The following clinical-demographic variables were recorded: age (in years), gender, weight (in kg), height (in cm), body mass index (in kg/m2), smoking status (i.e., active smoker or non-smoker), IBD type (i.e., CD or UC), and comorbidity.
During a colonoscopy (conducted for diagnostic purposes, as mentioned above, unrelated specifically to this study), in addition to the standard biopsies required by current clinical practice (i.e., two segmental biopsies in the cecum, ascending, transverse, descending, sigmoid colon, and rectum)[21], additional mucosal biopsy samples were obtained for the study. Specifically, two biopsies were taken in colonic/rectal tracts displaying clear signs of mucosal inflammatory involvement (i.e., inflamed mucosa), and two in tracts with endoscopically normal-appearing mucosa. Consequently, for the study's sampling criteria, only patients with a Montreal classification for CD of L2 (colic) or L3 (ileocolonic) were included. In contrast, only patients with E1 (proctitis) or E2 (left colitis, distal colitis) were admitted for UC.
The difference between inflamed and normal-appearing mucosa was made using validated endoscopic scores to evaluate IBD[22]. In detail, in the case of suspected UC (continuous inflammatory involvement without skip lesions and with rectal involvement), the colonic mucosa was assessed using the Mayo endoscopic subscore at the endoscopic examination[23]. We performed biopsies on Mayo endoscopic subscore 0 compatible traits and Mayo endoscopic subscore ≥ 1 compatible tracts. Colonic mucosa was evaluated using the CD endoscopic index of severity (CDEIS)[24] for suspected CD (i.e., segmental colitis and ileum involvement at retrograde ileoscopy). Biopsies were conducted on tracts with CDEIS < 3 (compatible) and tracts with CDEIS ≥ 3 (consistent). The endoscopic procedures were carried out by a gastroenterologist experienced in IBD digestive endoscopy with extensive casuistry. The histological diagnosis of IBD (i.e., CD or UC) was established based on histological criteria validated by current European guidelines[20].
As previously mentioned, supplementary histological and immunohistochemical investigations were conducted on the latter. In addition to routine assessments on the same samples, these investigations aimed to identify the expression profiles of MC3R and MC5R. Following the collection of biopsy samples, they were fixed in formalin and subsequently embedded in paraffin. Sections of 5 μm were cut and then stained with haematoxylin-eosin for morphological evaluation. Two slides were chosen for each patient, one representing healthy tissue and the other depicting the site of the inflammatory process. Immunohistochemical staining for anti-MC3R and anti-MC5R antibodies was carried out on these slides. For this purpose, sections with a thickness of 4-5 μm were prepared, and paraffin was removed using a xylene substitute (Hemo-De; Thermo-Fisher Scientific, Darmstadt, Germany). The immunohistochemistry procedure was conducted using the BenchMark Automated IHC/ISH slide staining system, following the manufacturer’s instructions (BenchMark Ventana, Tucson, AZ, United States)[25].
In brief, tissue sections underwent sequential rehydration with ethanol gradient washes, pre-heating, and staining with haematoxylin and eosin. Citrate antigen retrieval was conducted by placing slides in citrate buffer (0.1 M citric acid monohydrate and 0.1 M sodium citrate; pH 6) in a water-filled steamer for 20 min. Endogenous peroxidase activity was quenched in a 3% hydrogen peroxide aqueous solution for 15 min, and non-specific antibody binding was inhibited by one-hour incubation at room temperature in a blocking solution (1% BSA, 0.2% powdered skim milk, 0.3% Triton-X 100 in PBS).
Sections were incubated with specific anti-MC3R (1:100 in blocking solution; ab140864, Abcam, United Kingdom) and anti-MC5R (1:100 in blocking solution; sc-28994, Santa Cruz, United States) antibodies, washed with PBS, incubated with biotin-conjugated secondary antibodies and DBA (avidin-biotin-peroxidase complex, Milan, Italy), and DAB (3,3 diaminobenzidine) reaction was employed to visualize the specific antigens in each section[25]. Slides were counterstained with haematoxylin. Immunostaining analysis was conducted by an expert pathologist (intraobserver variability 5%). As no existing data on the immunohistochemical expression of MC3R and MC5R in colonic mucosa are available in the literature, all staining was assessed, with particular attention to the inflammatory infiltrate. The data are expressed as a percentage ± SEM (standard error of the mean) of MC3R or MC5R positive cells relative to the total cells counted.
Descriptive statistics were employed for data presentation. Continuous variables were reported as the median (interquartile range). The receptor expression profile was presented as a percentage ± SEM. Data distribution was assessed to determine whether parametric or non-parametric tests were more appropriate. The comparison between ordinal and continuous variables across groups was conducted using the Mann-Whitney U-test.
The accepted level of statistical significance was set at a two-tailed P-value less than 0.05. IBM® SPSS® was utilized as the software for data analysis, while GraphPad Prism 9® was used for graph processing.
Forty-six patients underwent initial screening for inclusion criteria to be enrolled in the study; however, twenty did not subsequently meet the inclusion criteria, resulting in a final enrolment of twenty-six patients overall. Among the twenty excluded patients, the reasons for exclusion were as follows: Three patients had a diagnosis of significant comorbidity (gastric cancer in one case and systemic sclerosis in two cases), in five cases, exclusion was dictated by the diagnosis of colonic dysplasia, in two cases by the diagnosis of non-specific colitis, in one case of ischemic colitis, and finally, nine patients had a completely negative colonoscopy.
Of the 26 patients finally enrolled, 13 (50%) had CD, and 13 (50%) had UC. The overall median age was 49.5 (40.75-69.5) years. Table 1 displays the clinical and demographic characteristics of included patients, and categorized on IBD type.
| Parameter | CD (n = 13) | UC (n = 13) | P value1 |
| Age (yr) | 49 (45-66.5) | 50 (30.5-81) | 0.801 |
| Gender | 0.762 | ||
| Male | 8 (61.5) | 7 (53.8) | |
| Female | 5 (38.5) | 6 (46.2) | |
| Body weight (kg) | 63.2 (58-68) | 81 (79-86.5) | 0.001 |
| Body height (cm) | 174 (159.5-185.5) | 165 (158.5-178) | 0.489 |
| BMI (kg/m2) | 23 (18-25.5) | 30 (24-33) | 0.002 |
| Smoking status | 0.762 | ||
| Active smoker | 2 (15.4) | 1 (7.7) | |
| Non-smoker | 11 (84.6) | 12 (92.3) | |
| Comorbidity | |||
| Hypertension | 2 (15.4) | 1 (7.7) | 0.724 |
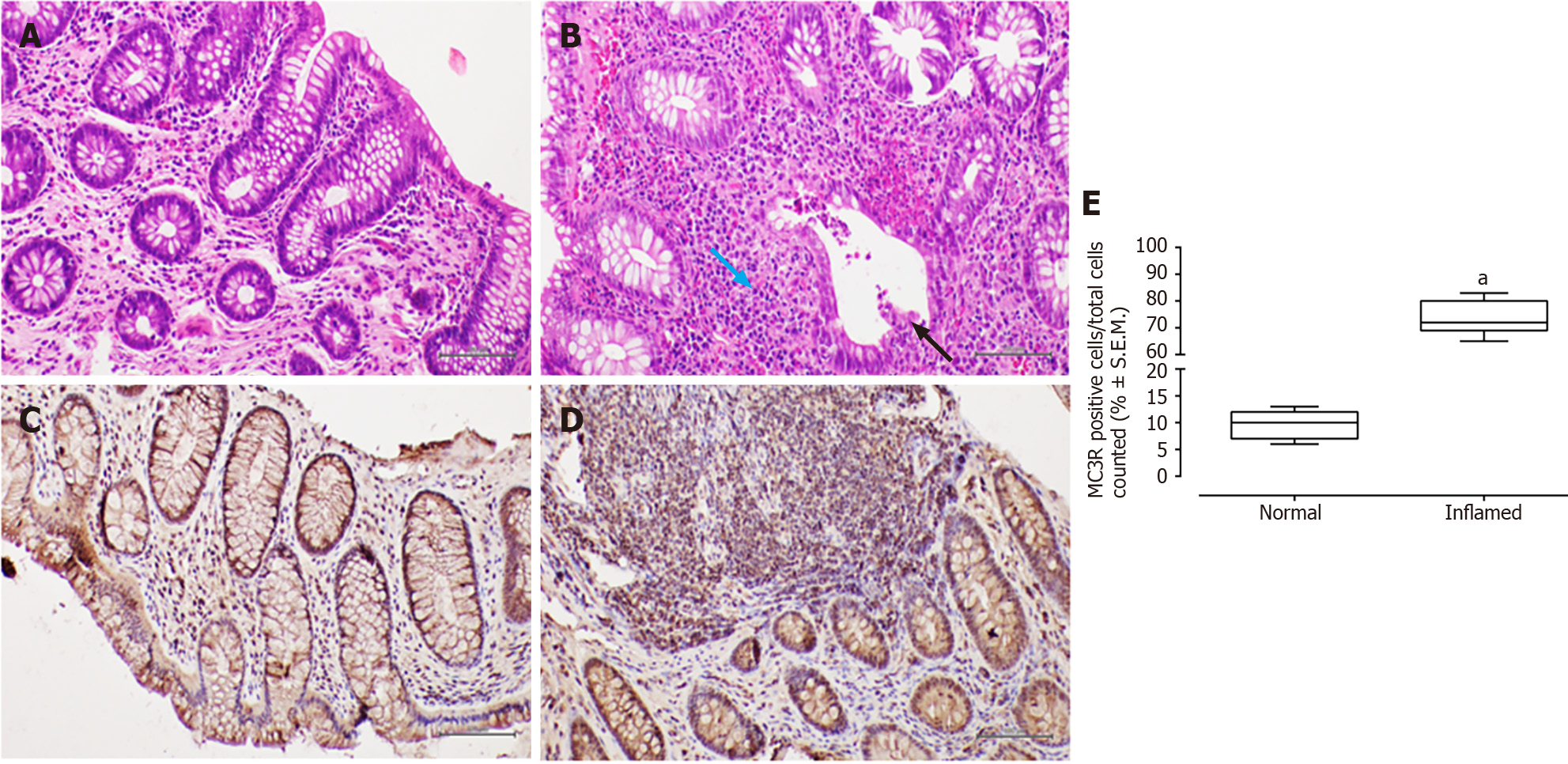
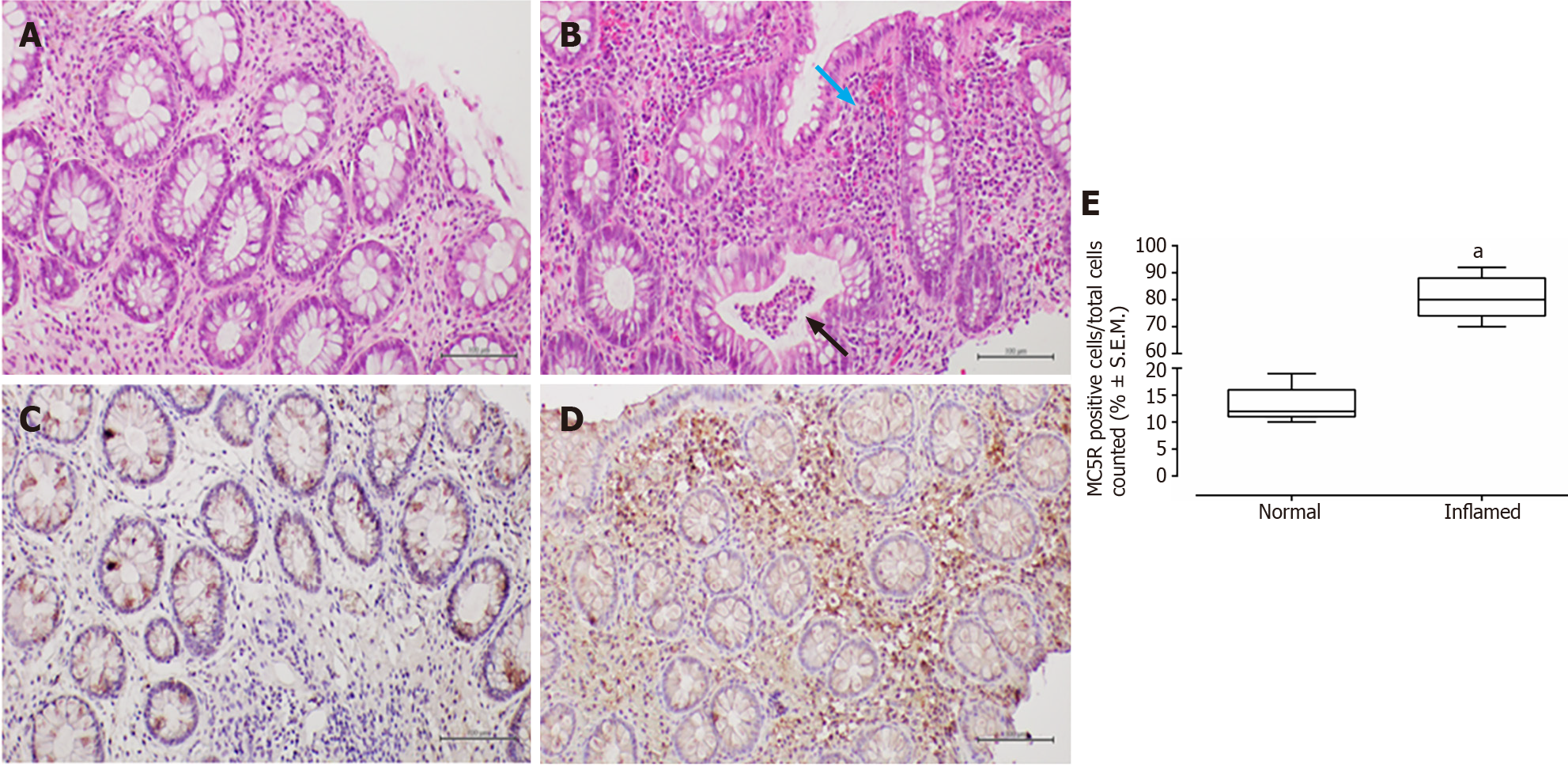
In patients with CD, haematoxylin-eosin staining revealed surface erosion and de-epithelialisation in the colonic mucosa, particularly on the right side. This was accompanied by a dense chronic inflammatory infiltrate and histological activity featuring cryptitis. Additionally, non-necrotising epithelioid granulomas were observed.
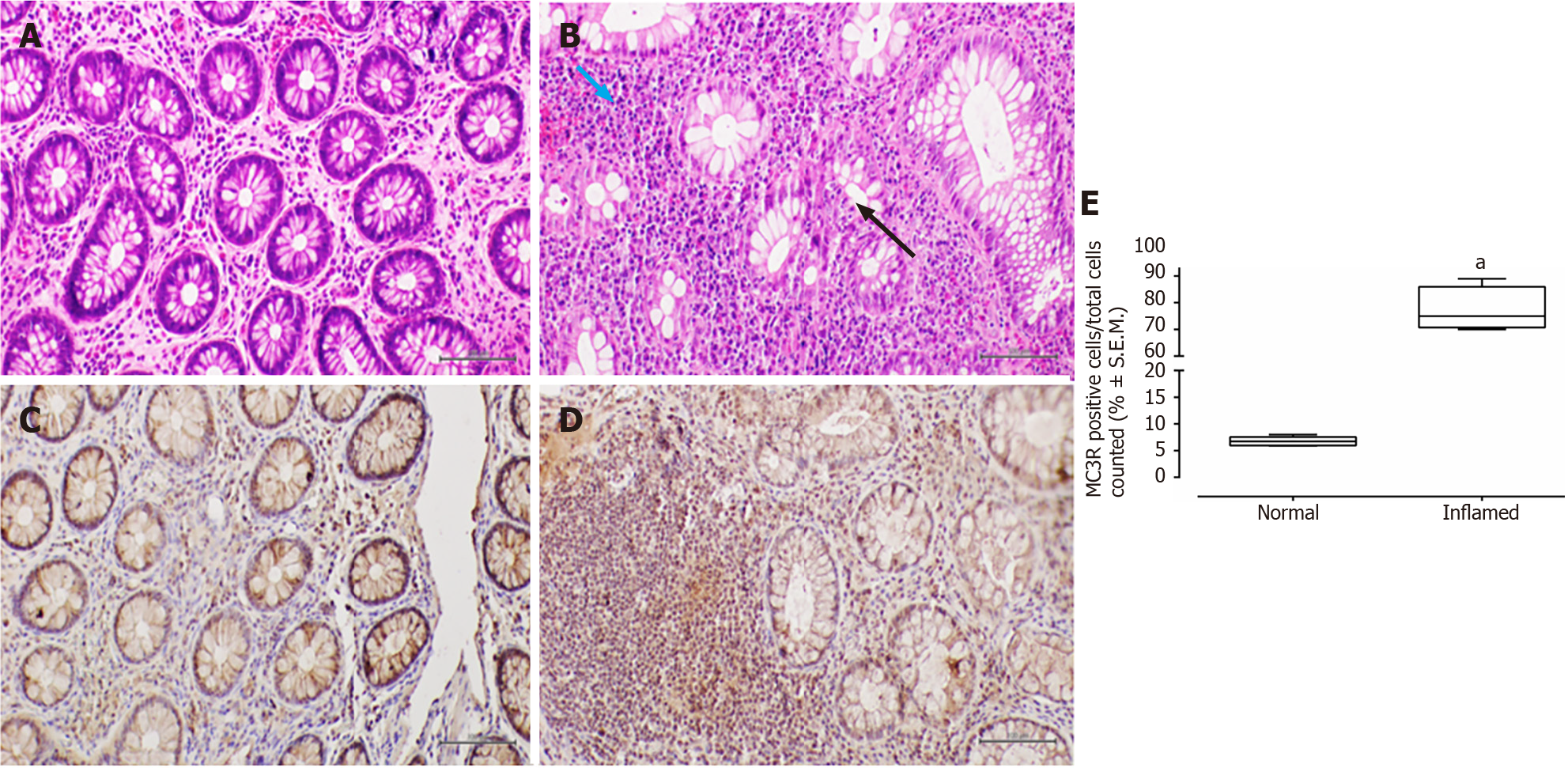
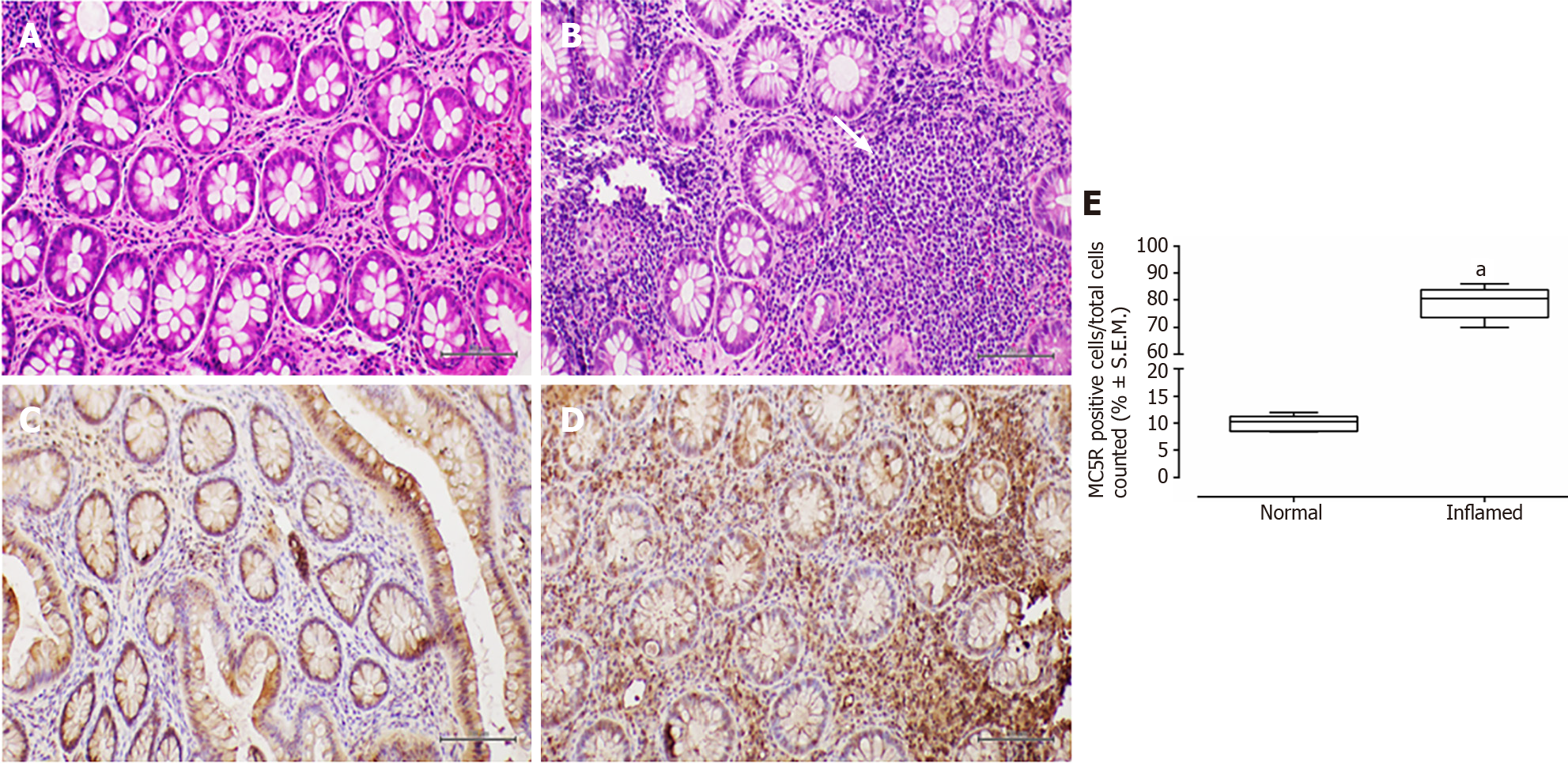
Conversely, in patients with UC, the colonic mucosa displayed erosions of the surface epithelium, characterized by a significant reduction in the glandular muciparous quota and distortion of crypt architecture. Within the lamina propria, a severe chronic inflammatory infiltrate exhibited histological activity, including cryptitis and cryptic microabscesses.
In all examined samples from individuals with IBD, no signs of epithelial dysplasia were evident (Figures 1-4).
All patients, 100% (26/26), tested positive for MC3R, and positivity was observed in all colonic samples within the segments affected by the disease (Figures 1 and 2). Interestingly, this positivity was not observed in the tracts where the disease was inactive (Figures 1 and 2).
Furthermore, immunohistochemistry for MC3R in CD samples exhibited cytoplasmic staining at the level of both mononuclear and polymorphonuclear inflammatory infiltrates, with a significant prevalence in "pathological" (i.e., inflamed mucosa) slides compared to healthy tissue obtained from normal-appearing mucosa (fold: + 7.7, P < 0.01 vs normal mucosa; Figure 1).
Similarly, MC3R labelling in UC samples was significantly higher in inflamed mucosa (fold: + 12, P < 0.01 vs normal mucosa), particularly at the level of the rectal and sigmoidal tracts (Figure 2).
MC5R tested positive in 22 out of 26 patients (84%), and like MC3R, it exhibited positivity in tracts affected by both CD (Figure 3) and UC (Figure 4).
Similar to MC3R, immunohistochemistry for MC5R displayed cytoplasmic staining at the mononuclear and poly
The present study suggests a distinctive expression pattern of MC3R and MC5R in the colorectum of patients with IBD. It proposes that their expression could be hypothetically linked to disease activity, indicating a heightened presence in segments of the large bowel affected by histological damage. To the best of our knowledge, such a profile of immunohistochemical expression has not been previously reported[7].
The current evidence on the expression profile of melanocortin receptors at the level of body tissues is, in fact, not yet wholly conclusive[7]. Still, it needs to be studied, and in particular, the evidence on the relationship between MC3R and MC5R receptors and IBD is scarce[9]. However, our results do not fully clarify the role of these two types of receptors in specific inflammatory pathogenesis. A mechanistic breakthrough is necessary to assess the mechanisms through which these receptors may exert immunomodulatory effects.
Numerous studies have previously highlighted the participation of MC3R in inflammation. Furthermore, various molecules with the capability to engage with MC3R have shown anti-inflammatory characteristics linked to this receptor. Specifically, fragments of ACTH that activate MC3R have demonstrated the ability to inhibit cytokine synthesis in peritoneal macrophages, thereby indirectly impeding neutrophilic diapedesis[26]. Furthermore, at the cardiac level, where macrophages express MC3R, the application of agonists during instances of acute myocardial infarction in mice has exhibited a protective function, even amidst reperfusion. This demonstration revealed that the protection was associated with a reduction in systemic and local inflammatory markers, including interleukin-1 and myeloperoxidase[27]. In other mice models, the same protective effects were identified in gouty arthritis[28]. The anti-inflammatory function of MC3R has been substantiated in the context of metabolic syndrome in mice lacking MC3R, regardless of weight gain[29].
The MC5R receptor is predominantly linked to the modulation of immune-mediated inflammation and the initiation of the JAK2-mediated pathway[30]. This latter pathway has been effectively utilized pharmacologically for the treatment of UC[31].
MC5R has been associated with ocular immunity, although its involvement in inflammatory responses remains incompletely understood. Selective agonists of MC5R have demonstrated promising positive effects in conditions characterized by immune dysregulation[32].
Earlier studies have attempted to assess the pharmacological role of certain melanocortin receptors in IBD. This includes PL-8177 (a selective MC1R ligand), which, in a study involving induced murine colitis, demonstrated the ability to reduce bowel inflammation with effects comparable to sulfasalazine[9].
Upon observing a noteworthy expression of the receptors in the impaired tissue, it is tempting to speculate that, from a translational standpoint, the expression of MC3R and MC5R may not correspond to an activity adequate for resolving colonic inflammation. Therefore, substantial stimulation with specific agonists may be necessary to overcome the burst of inflammation underlying IBD. This considers their reviewed role in fighting inflammation in several conditions[9,32-35].
The capacity of these receptors to respond to various endogenous agonists such as [Nle4, DPhe7]-α-MSH, α-MSH, and γ-MSH may underscore the importance of regarding these receptors as crucial responders to inflammation. However, Montero-Melendez[36], in a systematic review, has emphasized the concept that α-MSH can serve as an excellent illustration of "endogenous-based pro-resolving therapy". Unlike biological drugs targeted against a single entity, this molecule can simultaneously modulate interleukin-1β, prostaglandins, TNF-α, cell adhesion molecules, and inflammatory cells such as monocytes, macrophages, and neutrophils, as previously elucidated.
Given a well-determined receptor expression higher in patients with CD and UC samples, one would argue that new MC3R-MC5R drugs could be molecules in rectal and gastro-resistant oral formulations. However, this is a remote hypothesis that should be considered only after further preclinical studies demonstrate mechanistically a genuine anti-inflammatory potential for IBD. It is known that a proportion of IBD patients experience primary failure or secondary loss of response to several lines of biological therapy. Therefore, as a consequence, the exploration of new therapeutic agents and, particularly, new therapeutic mechanisms for IBD is desirable. Presently, there are specific MC3R and MC5R agonists already developed and documented in the literature, which could be regarded as potential therapeutic agents. Specifically, DTrp-α-MSH and the macrocyclic peptide PG911 are well-established agonists at hMC3R and hMC5R, respectively[37,38].
In CD, the data in this study are, by its design, restricted to patients with disease extension L2 or L3 according to the Montreal classification and, in any case, exclusively within the colonic microenvironment. Consequently, it is important to consider this observation and the non-applicability of the data to ileal or ileojejunal locations or other non-colonic disease localizations. Therefore, a greater understanding of the expression of these receptors in the small intestine is absolutely necessary as an additional piece in this already limited research context. A preliminary study by Gantz et al[39] suggested MC3R intestinal expression (specifically in the duodenum) through northern blot hybridization and polymerase chain reaction. However, subsequently, there haven't been robust studies that thoroughly detail the expression profiles of these two receptors in different gastrointestinal segments.
This study possesses several strengths. The majority of investigations concerning the melanocortin system and IBD have been conducted using pre-clinical cell models or mouse models[7]. The assessment of MC3R-MC5R expression profiles was conducted in treatment-naïve patients diagnosed with IBD for the first time and highly selected for comorbidities. This implies that the evaluation was carried out in patients with a diminished risk of bias attributable to prior treatments or comorbidities. However, this study is subject to several limitations. Firstly, the patient sample needs to be expanded to encompass individuals expressing varying levels of these receptors. Additionally, the correlation between these expression profiles and the IBD therapies undertaken by patients should be explored in future investigations. Moreover, it is noteworthy that this study, despite its novelty, did not incorporate assessments through polymerase chain reaction or Western blot analysis. Subsequent studies should consider incorporating these techniques to thoroughly evaluate the expression of MC3R and MC5R. Nevertheless, the high specificity demonstrated by the MC3R-MC5R kits employed in our study leads us to presume that analyses of this nature would likely corroborate the observed expression profile at the colonic level.
Moreover, it could be intriguing from a translational standpoint to investigate whether there exists a correlation between MC3R-MC5R expression and endoscopic disease activity, assessed using validated tools and scores[22], aiming to identify a potential direct association. However, it is crucial to highlight that the sample size within our dataset impedes the undertaking of correlation analyses essential for revealing statistically significant findings.
Therefore, we await the execution of future, more extensive studies and randomized double-blind trials to test new melanocortin agonists and verify their immunomodulatory potentialities.
In conclusion, this study delves into the distinctive immunohistochemical expression patterns of MC3R and MC5R in the colorectum of patients with IBD. The observed expression of these receptors potentially appears to be linked to disease activity, with heightened presence in segments of the large bowel affected by histological damage. This unique expression profile has not been previously reported, indicating a novel avenue for exploration in the context of IBD and the melanocortin system.
However, the study has limitations, such as a small sample size and a lack of correlation analyses with endoscopic disease activity. Future investigations with larger cohorts, incorporating mechanicistics and molecular analyses like PCR and Western blot, could provide a more comprehensive understanding. Additionally, the observed expression patterns prompt consideration for the development of specific MC3R and MC5R ligands as potential therapeutic agents for IBD, necessitating further exploration in preclinical and clinical settings.
Inflammatory bowel diseases (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), present complex etiologies. The modulation of the melanocortin system, involved in inflammatory pathways, offers therapeutic potential.
Understanding the immunohistochemical expression of melanocortin 3 and 5 receptors (MC3R and MC5R) in colonic mucosa of IBD patients is crucial for unraveling potential immunomodulatory roles.
To assess immunohistochemical expression patterns of MC3R and MC5R in colons of IBD patients, exploring associations with disease activity.
A study involving treatment naïve IBD patients was conducted. Biopsies were taken from inflamed and normal mucosa, and immunohistochemical staining for MC3R and MC5R was performed.
Both MC3R and MC5R exhibited significant positivity in inflamed mucosa compared to normal mucosa, suggesting a potential correlation with disease activity. The expression pattern was distinct in CD and UC samples.
The study proposes a unique expression profile of MC3R and MC5R in IBD, indicating potential links to disease activity. However, limitations include a small sample size and a lack of correlation analyses with endoscopic disease activity.
Future investigations with larger cohorts and mechanistic analyses, such as polymerase chain reaction and Western blot, are necessary for a comprehensive understanding. The observed expression patterns suggest potential avenues for developing specific MC3R and MC5R ligands as therapeutic agents for IBD, warranting exploration in preclinical and clinical settings.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: United European Gastroenterology; Società Italiana Di Gastroenterologia Ed Endoscopia Digestiva.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rathnaswami A, India; Valek V, Czech Republic S-Editor: Lin C L-Editor: A P-Editor: Yu HG
| 1. | Harbord M, Eliakim R, Bettenworth D, Karmiris K, Katsanos K, Kopylov U, Kucharzik T, Molnár T, Raine T, Sebastian S, de Sousa HT, Dignass A, Carbonnel F; European Crohn’s and Colitis Organisation [ECCO]. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J Crohns Colitis. 2017;11:769-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 938] [Cited by in RCA: 875] [Article Influence: 109.4] [Reference Citation Analysis (0)] |
| 2. | Torres J, Bonovas S, Doherty G, Kucharzik T, Gisbert JP, Raine T, Adamina M, Armuzzi A, Bachmann O, Bager P, Biancone L, Bokemeyer B, Bossuyt P, Burisch J, Collins P, El-Hussuna A, Ellul P, Frei-Lanter C, Furfaro F, Gingert C, Gionchetti P, Gomollon F, González-Lorenzo M, Gordon H, Hlavaty T, Juillerat P, Katsanos K, Kopylov U, Krustins E, Lytras T, Maaser C, Magro F, Marshall JK, Myrelid P, Pellino G, Rosa I, Sabino J, Savarino E, Spinelli A, Stassen L, Uzzan M, Vavricka S, Verstockt B, Warusavitarne J, Zmora O, Fiorino G. ECCO Guidelines on Therapeutics in Crohn's Disease: Medical Treatment. J Crohns Colitis. 2020;14:4-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 991] [Cited by in RCA: 903] [Article Influence: 180.6] [Reference Citation Analysis (2)] |
| 3. | Holder JR, Haskell-Luevano C. Melanocortin ligands: 30 years of structure-activity relationship (SAR) studies. Med Res Rev. 2004;24:325-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Panaro BL, Tough IR, Engelstoft MS, Matthews RT, Digby GJ, Møller CL, Svendsen B, Gribble F, Reimann F, Holst JJ, Holst B, Schwartz TW, Cox HM, Cone RD. The melanocortin-4 receptor is expressed in enteroendocrine L cells and regulates the release of peptide YY and glucagon-like peptide 1 in vivo. Cell Metab. 2014;20:1018-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 136] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 5. | Rajora N, Boccoli G, Catania A, Lipton JM. alpha-MSH modulates experimental inflammatory bowel disease. Peptides. 1997;18:381-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Oktar BK, Ercan F, Yeğen BC, Alican I. The effect of alpha-melanocyte stimulating hormone on colonic inflammation in the rat. Peptides. 2000;21:1271-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Gravina AG, Pellegrino R, Durante T, Palladino G, Imperio G, D'Amico G, Trotta MC, Dallio M, Romeo M, D'Amico M, Federico A. The Melanocortin System in Inflammatory Bowel Diseases: Insights into Its Mechanisms and Therapeutic Potentials. Cells. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 8. | Maaser C, Kannengiesser K, Specht C, Lügering A, Brzoska T, Luger TA, Domschke W, Kucharzik T. Crucial role of the melanocortin receptor MC1R in experimental colitis. Gut. 2006;55:1415-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Spana C, Taylor AW, Yee DG, Makhlina M, Yang W, Dodd J. Probing the Role of Melanocortin Type 1 Receptor Agonists in Diverse Immunological Diseases. Front Pharmacol. 2018;9:1535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Wang W, Guo DY, Lin YJ, Tao YX. Melanocortin Regulation of Inflammation. Front Endocrinol (Lausanne). 2019;10:683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 11. | Hiramoto K, Kobayashi H, Ishii M, Sato E, Inoue M. Increased alpha-melanocyte-stimulating hormone (alpha-MSH) levels and melanocortin receptors expression associated with pigmentation in an NC/Nga mouse model of atopic dermatitis. Exp Dermatol. 2010;19:132-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Gautron L, Lee C, Funahashi H, Friedman J, Lee S, Elmquist J. Melanocortin-4 receptor expression in a vago-vagal circuitry involved in postprandial functions. J Comp Neurol. 2010;518:6-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Catania A. Neuroprotective actions of melanocortins: a therapeutic opportunity. Trends Neurosci. 2008;31:353-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Videira IF, Moura DF, Magina S. Mechanisms regulating melanogenesis. An Bras Dermatol. 2013;88:76-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 354] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 15. | Lam CW, Getting SJ. Melanocortin receptor type 3 as a potential target for anti-inflammatory therapy. Curr Drug Targets Inflamm Allergy. 2004;3:311-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Loos RJ, Lindgren CM, Li S, Wheeler E, Zhao JH, Prokopenko I, Inouye M, Freathy RM, Attwood AP, Beckmann JS, Berndt SI; Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, Jacobs KB, Chanock SJ, Hayes RB, Bergmann S, Bennett AJ, Bingham SA, Bochud M, Brown M, Cauchi S, Connell JM, Cooper C, Smith GD, Day I, Dina C, De S, Dermitzakis ET, Doney AS, Elliott KS, Elliott P, Evans DM, Sadaf Farooqi I, Froguel P, Ghori J, Groves CJ, Gwilliam R, Hadley D, Hall AS, Hattersley AT, Hebebrand J, Heid IM; KORA, Lamina C, Gieger C, Illig T, Meitinger T, Wichmann HE, Herrera B, Hinney A, Hunt SE, Jarvelin MR, Johnson T, Jolley JD, Karpe F, Keniry A, Khaw KT, Luben RN, Mangino M, Marchini J, McArdle WL, McGinnis R, Meyre D, Munroe PB, Morris AD, Ness AR, Neville MJ, Nica AC, Ong KK, O'Rahilly S, Owen KR, Palmer CN, Papadakis K, Potter S, Pouta A, Qi L; Nurses' Health Study, Randall JC, Rayner NW, Ring SM, Sandhu MS, Scherag A, Sims MA, Song K, Soranzo N, Speliotes EK; Diabetes Genetics Initiative, Syddall HE, Teichmann SA, Timpson NJ, Tobias JH, Uda M; SardiNIA Study, Vogel CI, Wallace C, Waterworth DM, Weedon MN; Wellcome Trust Case Control Consortium, Willer CJ; FUSION, Wraight, Yuan X, Zeggini E, Hirschhorn JN, Strachan DP, Ouwehand WH, Caulfield MJ, Samani NJ, Frayling TM, Vollenweider P, Waeber G, Mooser V, Deloukas P, McCarthy MI, Wareham NJ, Barroso I, Jacobs KB, Chanock SJ, Hayes RB, Lamina C, Gieger C, Illig T, Meitinger T, Wichmann HE, Kraft P, Hankinson SE, Hunter DJ, Hu FB, Lyon HN, Voight BF, Ridderstrale M, Groop L, Scheet P, Sanna S, Abecasis GR, Albai G, Nagaraja R, Schlessinger D, Jackson AU, Tuomilehto J, Collins FS, Boehnke M, Mohlke KL. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet. 2008;40:768-775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1032] [Cited by in RCA: 947] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 17. | Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2235] [Cited by in RCA: 2209] [Article Influence: 78.9] [Reference Citation Analysis (0)] |
| 18. | Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O'Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348:1085-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1184] [Cited by in RCA: 1167] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 19. | Branson R, Potoczna N, Kral JG, Lentes KU, Hoehe MR, Horber FF. Binge eating as a major phenotype of melanocortin 4 receptor gene mutations. N Engl J Med. 2003;348:1096-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 207] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 20. | Magro F, Langner C, Driessen A, Ensari A, Geboes K, Mantzaris GJ, Villanacci V, Becheanu G, Borralho Nunes P, Cathomas G, Fries W, Jouret-Mourin A, Mescoli C, de Petris G, Rubio CA, Shepherd NA, Vieth M, Eliakim R; European Society of Pathology (ESP); European Crohn's and Colitis Organisation (ECCO). European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis. 2013;7:827-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 466] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 21. | Pouw RE, Bisschops R, Gecse KB, de Hertogh G, Iacucci M, Rutter M, Barret M, Biermann K, Czakó L, Hucl T, Jansen M, Savarino E, Spaander MCW, Schmidt PT, Dinis-Ribeiro M, Vieth M, van Hooft JE. Endoscopic tissue sampling - Part 2: Lower gastrointestinal tract. European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2021;53:1261-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 22. | Kishi M, Hirai F, Takatsu N, Hisabe T, Takada Y, Beppu T, Takeuchi K, Naganuma M, Ohtsuka K, Watanabe K, Matsumoto T, Esaki M, Koganei K, Sugita A, Hata K, Futami K, Ajioka Y, Tanabe H, Iwashita A, Shimizu H, Arai K, Suzuki Y, Hisamatsu T. A review on the current status and definitions of activity indices in inflammatory bowel disease: how to use indices for precise evaluation. J Gastroenterol. 2022;57:246-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 23. | Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR, de Villiers WJ, Present D, Sands BE, Colombel JF. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2744] [Cited by in RCA: 2884] [Article Influence: 144.2] [Reference Citation Analysis (2)] |
| 24. | Mary JY, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn's disease: a prospective multicentre study. Groupe d'Etudes Thérapeutiques des Affections Inflammatoires du Tube Digestif (GETAID). Gut. 1989;30:983-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 665] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 25. | Di Filippo C, Zippo MV, Maisto R, Trotta MC, Siniscalco D, Ferraro B, Ferraraccio F, La Motta C, Sartini S, Cosconati S, Novellino E, Gesualdo C, Simonelli F, Rossi S, D'Amico M. Inhibition of ocular aldose reductase by a new benzofuroxane derivative ameliorates rat endotoxic uveitis. Mediators Inflamm. 2014;2014:857958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Getting SJ, Gibbs L, Clark AJ, Flower RJ, Perretti M. POMC gene-derived peptides activate melanocortin type 3 receptor on murine macrophages, suppress cytokine release, and inhibit neutrophil migration in acute experimental inflammation. J Immunol. 1999;162:7446-7453. [PubMed] |
| 27. | Getting SJ, Di Filippo C, Christian HC, Lam CW, Rossi F, D'Amico M, Perretti M. MC-3 receptor and the inflammatory mechanisms activated in acute myocardial infarct. J Leukoc Biol. 2004;76:845-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Getting SJ, Lam CW, Chen AS, Grieco P, Perretti M. Melanocortin 3 receptors control crystal-induced inflammation. FASEB J. 2006;20:2234-2241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Ellacott KL, Murphy JG, Marks DL, Cone RD. Obesity-induced inflammation in white adipose tissue is attenuated by loss of melanocortin-3 receptor signaling. Endocrinology. 2007;148:6186-6194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Buggy JJ. Binding of alpha-melanocyte-stimulating hormone to its G-protein-coupled receptor on B-lymphocytes activates the Jak/STAT pathway. Biochem J. 1998;331 ( Pt 1):211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 96] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Sandborn WJ, Su C, Sands BE, D'Haens GR, Vermeire S, Schreiber S, Danese S, Feagan BG, Reinisch W, Niezychowski W, Friedman G, Lawendy N, Yu D, Woodworth D, Mukherjee A, Zhang H, Healey P, Panés J; OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med. 2017;376:1723-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 1186] [Article Influence: 148.3] [Reference Citation Analysis (0)] |
| 32. | Xu Y, Guan X, Zhou R, Gong R. Melanocortin 5 receptor signaling pathway in health and disease. Cell Mol Life Sci. 2020;77:3831-3840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 33. | Maisto R, Gesualdo C, Trotta MC, Grieco P, Testa F, Simonelli F, Barcia JM, D'Amico M, Di Filippo C, Rossi S. Melanocortin receptor agonists MCR(1-5) protect photoreceptors from high-glucose damage and restore antioxidant enzymes in primary retinal cell culture. J Cell Mol Med. 2017;21:968-974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Trotta MC, Maisto R, Alessio N, Hermenean A, D'Amico M, Di Filippo C. The Melanocortin MC5R as a New Target for Treatment of High Glucose-Induced Hypertrophy of the Cardiac H9c2 Cells. Front Physiol. 2018;9:1475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Rossi S, Maisto R, Gesualdo C, Trotta MC, Ferraraccio F, Kaneva MK, Getting SJ, Surace E, Testa F, Simonelli F, Grieco P, Merlino F, Perretti M, D'Amico M, Di Filippo C. Activation of Melanocortin Receptors MC 1 and MC 5 Attenuates Retinal Damage in Experimental Diabetic Retinopathy. Mediators Inflamm. 2016;2016:7368389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Montero-Melendez T. ACTH: The forgotten therapy. Semin Immunol. 2015;27:216-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 37. | Grieco P, Han G, Weinberg D, Van der Ploeg LH, Hruby VJ. Design and synthesis of highly potent and selective melanotropin analogues of SHU9119 modified at position 6. Biochem Biophys Res Commun. 2002;292:1075-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Can VC, Locke IC, Kaneva MK, Kerrigan MJP, Merlino F, De Pascale C, Grieco P, Getting SJ. Novel anti-inflammatory and chondroprotective effects of the human melanocortin MC1 receptor agonist BMS-470539 dihydrochloride and human melanocortin MC3 receptor agonist PG-990 on lipopolysaccharide activated chondrocytes. Eur J Pharmacol. 2020;872:172971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Gantz I, Konda Y, Tashiro T, Shimoto Y, Miwa H, Munzert G, Watson SJ, DelValle J, Yamada T. Molecular cloning of a novel melanocortin receptor. J Biol Chem. 1993;268:8246-8250. [PubMed] |