Published online Mar 7, 2024. doi: 10.3748/wjg.v30.i9.1121
Peer-review started: November 22, 2023
First decision: December 7, 2023
Revised: January 14, 2024
Accepted: February 1, 2024
Article in press: February 1, 2024
Published online: March 7, 2024
Processing time: 104 Days and 13.2 Hours
Traditional esophagogastroduodenoscopy (EGD), an invasive examination method, can cause discomfort and pain in patients. In contrast, magnetically controlled capsule endoscopy (MCE), a noninvasive method, is being applied for the detection of stomach and small intestinal diseases, but its application in treating esophageal diseases is not widespread.
To evaluate the safety and efficacy of detachable string MCE (ds-MCE) for the diagnosis of esophageal diseases.
Fifty patients who had been diagnosed with esophageal diseases were pros
Using EGD as the gold standard, the sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy of ds-MCE for esophageal disease detection were 85.71%, 86.21%, 81.82%, 89.29%, and 86%, respectively. ds-MCE was more comfortable and convenient than EGD was, with 80% of patients feeling that ds-MCE examination was very comfortable or comfortable and 50% of patients believing that detachable string v examination was very convenient.
This study revealed that ds-MCE has the same diagnostic effects as traditional EGD for esophageal diseases and is more comfortable and convenient than EGD, providing a novel noninvasive method for treating esophageal diseases.
Core Tip: Our study highlights detachable string magnetically controlled capsule endoscopy (ds-MCE) as an innovative, noninvasive technique for esophageal diagnosis that matches the 86% accuracy of traditional esophagogastroduodenoscopy while significantly enhancing patient comfort. With 80% of patients reporting a favorable experience, ds-MCE stands to increase compliance and revolutionize the approach to esophageal disease management, offering a promising leap forward in gastroenterological diagnostics with minimal patient discomfort.
- Citation: Yang YL, Qin HW, Chen ZY, Fan HN, Yu Y, Da W, Zhu JS, Zhang J. Detachable string magnetically controlled capsule endoscopy for the noninvasive diagnosis of esophageal diseases: A prospective, blind clinical study. World J Gastroenterol 2024; 30(9): 1121-1131
- URL: https://www.wjgnet.com/1007-9327/full/v30/i9/1121.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i9.1121
Esophageal diseases, including gastroesophageal reflux disease, Barrett’s esophagus, and esophageal tumors, pose serious threats to human health[1]. Long-term chronic inflammation is strongly associated with an increased risk of Barrett's esophagus and esophageal cancer[2]. Most malignant cases can metastasize to distant sites, leading to poor prognosis[3,4]. Consequently, early diagnosis of esophageal disease is crucial for preventing the occurrence of esophageal cancer.
Esophagogastroduodenoscopy (EGD) is the "gold standard" for detecting upper gastrointestinal diseases; this method allows visualization of the mucosa and sampling, but EGD requires intubation and even anesthesia, resulting in poor compliance. Esophageal capsule endoscopy (ECE) is a well-tolerated and safe procedure[5] that was initially used to diagnose small intestinal diseases[6]. In 2004, the first-generation ECE was authorized, and video images were acquired from both ends of the device at a rate of 2 frames/second/end[7]. Second-generation ECE was approved in 2007 to provide improved image quality at 15 frames/second and high spatial resolution[8]. A third-generation ECE features a 174° angle of view and a 35 frames/second rate[9] but fails to improve detection rates[10]. ECE, as a screening tool for esophageal diseases, has shown a moderate sensitivity and specificity of 77% and 86%, respectively, of cases, whereas EGD has a sensitivity and specificity of 76% and 90%[11], respectively; however, ECE has a high false-positive rate[8], making it ineffective as an alternative to EGD. In 2013, a magnetically controlled capsule endoscopy (MCE) system was developed and approved in China[12]; this system involves a capsule manipulated by an external magnetic field to visualize the mucosa. MCE is nearly equivalent to conventional EGD due to its diagnostic accuracy[13] and high sensitivity for detecting minor erosions[12]. Another study demonstrated that the diagnostic accuracy of MCE for esophageal lesions can reach 86%[14]. Moreover, detachable string MCE (ds-MCE) was developed to control the movement of the capsule, allowing direct and repeated observation of the esophagus[15].
Our previous study showed that MCE is a safe and noninvasive endoscopic imaging method with a highly accurate detection rate for gastric and small intestinal diseases[16]. In the present study, ds-MCE was applied for the detection of esophageal diseases, and we found that ds-MCE had the same diagnostic effects as traditional EGD for esophageal diseases and was more comfortable and convenient than EGD was, providing a novel noninvasive method for detecting esophageal diseases.
This was a prospective, blinded, self-controlled clinical study conducted at Shanghai Sixth People's Hospital from November 29th, 2019, to September 20th, 2021. This study was approved by the Ethics Committee of Shanghai Sixth People's Hospital, No. 2019-082-(1).
Participants aged 18-75 years with previously diagnosed esophageal diseases were eligible for this clinical study. Patients with one of the following conditions were excluded: (1) No surgical condition or refusal to undergo any abdominal surgery; (2) an intracorporeal pacemaker; (3) intracorporeal implantation of electronic devices such as cochlear implants, magnetic metal drug infusion pumps, neurostimulators, or magnetic metal foreign bodies; (4) pregnancy; (5) dysphagia, known or suspected gastrointestinal obstruction, stricture, or fistula; significant gastrointestinal bleeding; a history of gastrointestinal surgery or history of abdominal surgery with altered gastrointestinal anatomy; or history of abdominal radiation; (6) contraindication to electrogastroscopy; and (7) other conditions that the investigator believed were not appropriate for this study. All the subjects understood and agreed to the study protocol and voluntarily signed the informed consent form.
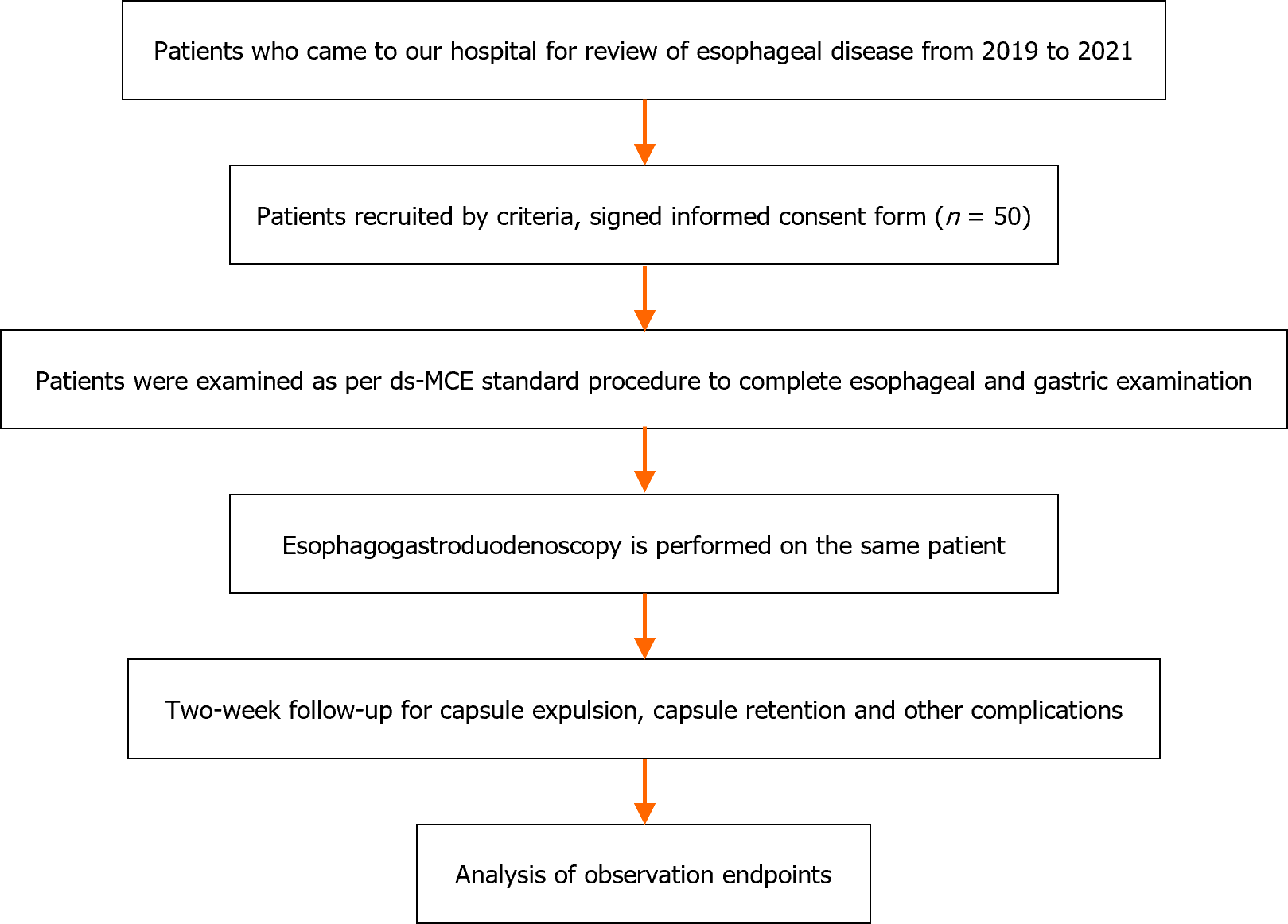
After providing informed consent, each subject underwent ds-MCE followed by EGD, which is the standard diagnostic method for comparison with ds-MCE. The EGD operating and diagnosing physicians were unblinded to the ds-MCE operating and reviewing physicians. The procedure for this study is shown in Figure 1.
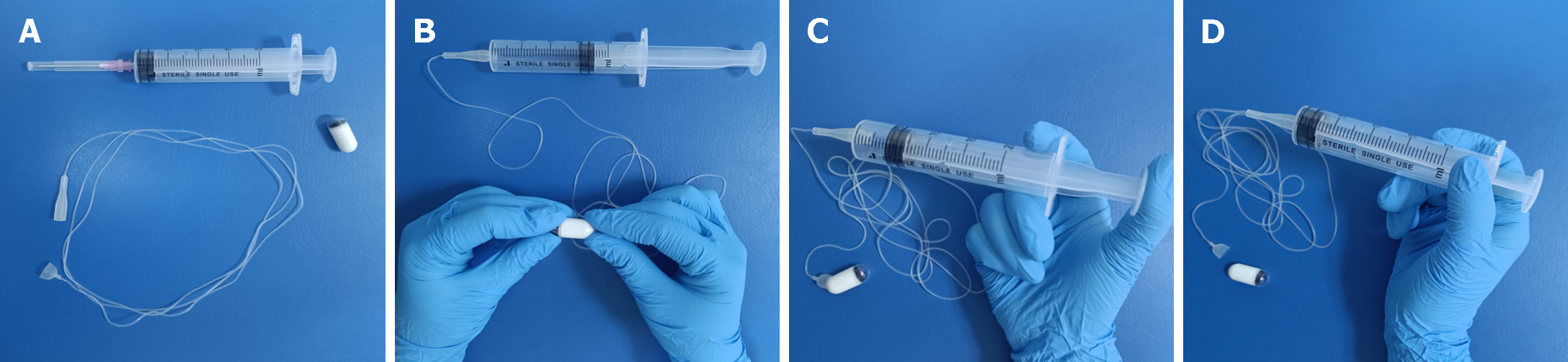
The ds-MCE (specification: NU-1/CEE-1), which consisted of an MCE system and a single-use detachable string (Figure 2), was obtained from Shanghai Ankon Medical Technology Co., Ltd. (Shanghai, China). The examiner used only one capsule to complete the upper gastrointestinal examination. The detachable string of the single-use capsule endoscope was connected to one end of the capsule, and the other end was attached to a syringe, which moved the capsule up and down to examine the esophagus. Following the examination of the esophagus, air was injected through the syringe to separate the capsule from the string, and the capsule was then inserted into the stomach to examine the stomach and small intestine.
Esophageal lesions were detected by ds-MCE and EGD, and EGD was used as the gold standard for the diagnosis of esophageal disease. The primary endpoints of this study included the sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy of ds-MCE for detecting esophageal disease (Table 1): (1) Sensitivity: The probability of a positive EGD and a positive MCE result, which was an indicator of the ability to detect a positive result in this test; (2) Specificity: The specificity is the probability of a negative EGD and a negative MCE, which is an indicator of the ability to detect a negative result; (3) Predictive value: Includes positive and negative predictive values. The higher the value was, the more likely the probability of the subject having the disease was to be estimated; and (4) Diagnostic accuracy: The probability that the ds-MCE was consistent with the EGD findings.
| EGD | ||||
| Positive | Negative | Total | ||
| ds-MCE | Positive | a | b | a + b (R1) |
| Negative | c | d | c + d (R2) | |
| Total | a + c (C1) | b + d (C2) | a + b + c + d (N) | |
Secondary endpoints: (1) Esophageal visualization: The ability to clearly capture and preserve images of the upper, middle, and lower esophagus; (2) Dentate line visualization: Clear capture of the dentate line images; and (3) Subject tolerance: Participants completed a questionnaire to assess their level of comfort with EGD and ds-MCE, with ratings ranging from 0 to 4 (0 = very comfortable; 1 = comfortable; 2 = tolerable; 3 = uncomfortable; 4 = very uncomfortable); the ease of inspection ranged from 0 to 3 (0 = very convenient; 1 = convenient; 2 = inconvenient; 3 = very inconvenient). This study evaluated the safety of the interventions by evaluating the incidence of adverse events and complications during and after capsule endoscopy and EGD.
The statistical analysis of the data was conducted with SPSS 26.0 and GraphPad Prism 9. All hypothesis tests were performed at a two-sided 5% level of significance. Continuous variables were summarized using descriptive statistics. For normally distributed variables, the mean and standard deviation were calculated; for variables with a skewed distribution, the median and interquartile spacing were calculated. The Kruskal-Wallis test was used for comparisons between groups.
A total of 50 patients (average age of 53.26 years; 46% female) were included; 48 patients had a history of esophagitis, 1 patient had a history of Barrett's esophagus, 2 patients had a history of esophageal hiatal hernia, 8 patients had superficial gastritis, and 2 patients had atrophic gastritis. The demographic data of the 50 subjects are presented in Table 2.
| Subjects (n = 50) | |
| Sex, % (n) | |
| Male | 54% (27/50) |
| Female | 46% (23/50) |
| Age (yr) | |
| n | 50 |
| Mean | 53.26 |
| Median | 56 |
| Min, Max | 22, 73 |
| Height (cm) | |
| n | 50 |
| Mean | 164.3 |
| Median | 162 |
| Min, Max | 150.0, 182.0 |
| Weight (kg) | |
| n | 50 |
| Mean | 63.76 |
| Median | 63.5 |
| Min, Max | 42, 93 |
| BMI (kg/m2) | |
| n | 50 |
| Mean | 23.5 |
| Median | 23.34 |
| Min, Max | 16.41, 31.25 |
| Medical history | |
| Esophagitis | 48 |
| Barrett's esophagus | 1 |
| Esophageal hiatal hernia | 2 |
| Superficial gastritis | 8 |
| Atrophic gastritis | 2 |
Fifty participants successfully underwent ds-MCE and did not experience any adverse reactions or device defects associated with ds-MCE or EGD. A total of 21 patients (42%) with esophageal diseases were detected by EGD, and 22 patients (44%) were detected by ds-MCE. Among them, 3 patients were diagnosed with esophageal disease by EGD but were negative by ds-MCE, while 4 patients were diagnosed with esophageal disease by ds-MCE but were negative by EGD. The detection of specific esophageal diseases is shown in Supplementary Table 1.
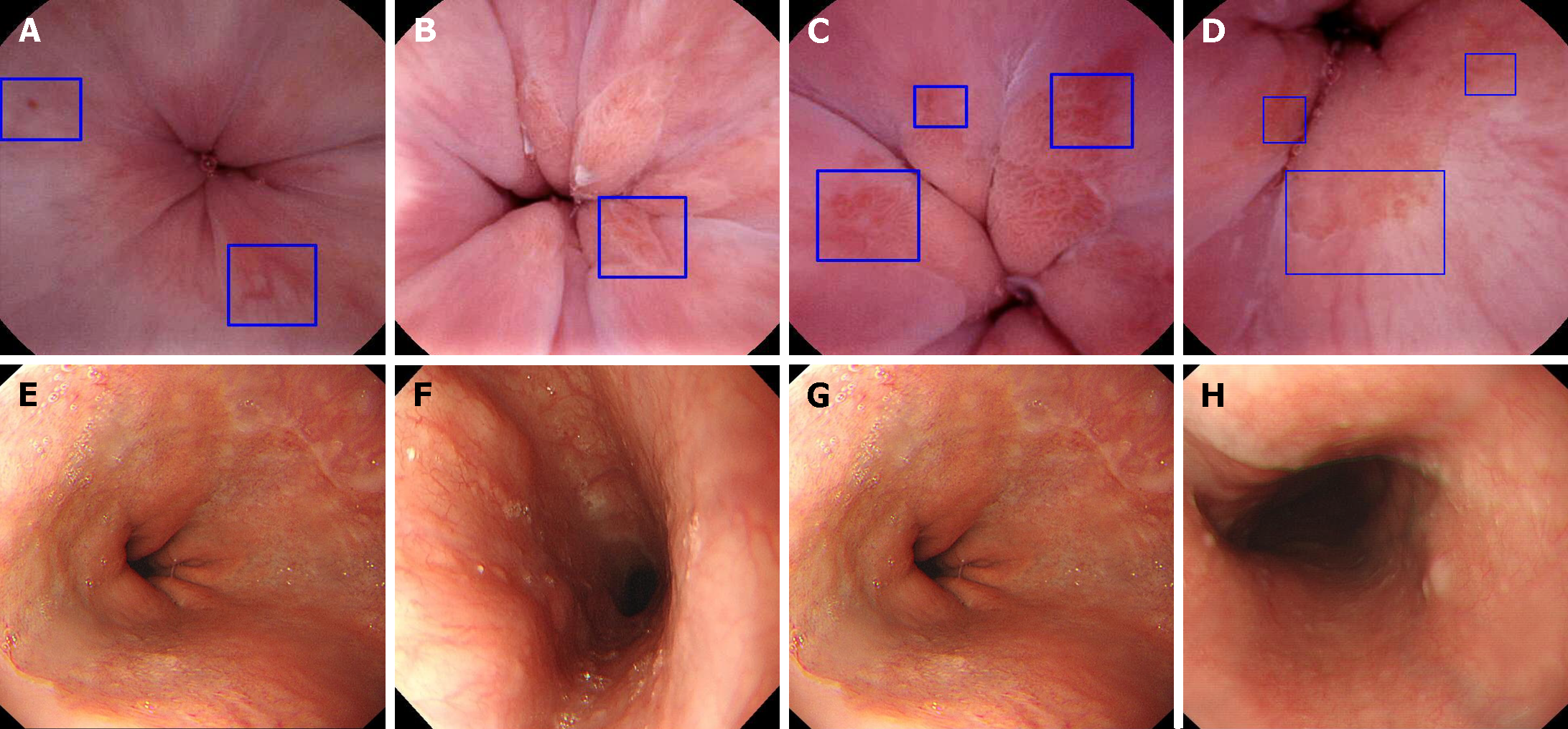
Using EGD as the gold standard, the sensitivity of ds-MCE for detecting esophageal disease was 85.71% (95%CI: 62.64%, 96.24%), the specificity was 86.21% (95%CI: 67.43%, 95.49%), the positive predictive value was 81.82% (95%CI: 58.99%, 94.01%), the negative predictive value was 89.29% (95%CI: 70.63%, 97.19%), and the diagnostic concordance was 86% (95%CI: 72.64%, 93.72%; Tables 3 and 4). Moreover, ds-MCE was also capable of clearly capturing and preserving images of the upper, middle, and lower esophagus and the dentate line with 100% integrity (Figure 3).
| EGD | ||||
| Positive | Negative | Total | ||
| ds-MCE | Positive | 18 | 4 | 22 |
| Negative | 3 | 25 | 28 | |
| Total | 21 | 29 | 50 | |
| Primary endpoint | 95%CI |
| Sensitivity | 85.71% (62.64%, 96.24%) |
| Specificity | 86.21% (67.43%, 95.49%) |
| Positive predictive value | 81.82% (58.99%,94.01%) |
| Negative predictive value | 89.29% (70.63%, 97.19%) |
| Diagnostic accuracy | 86% (72.64%,93.72%) |
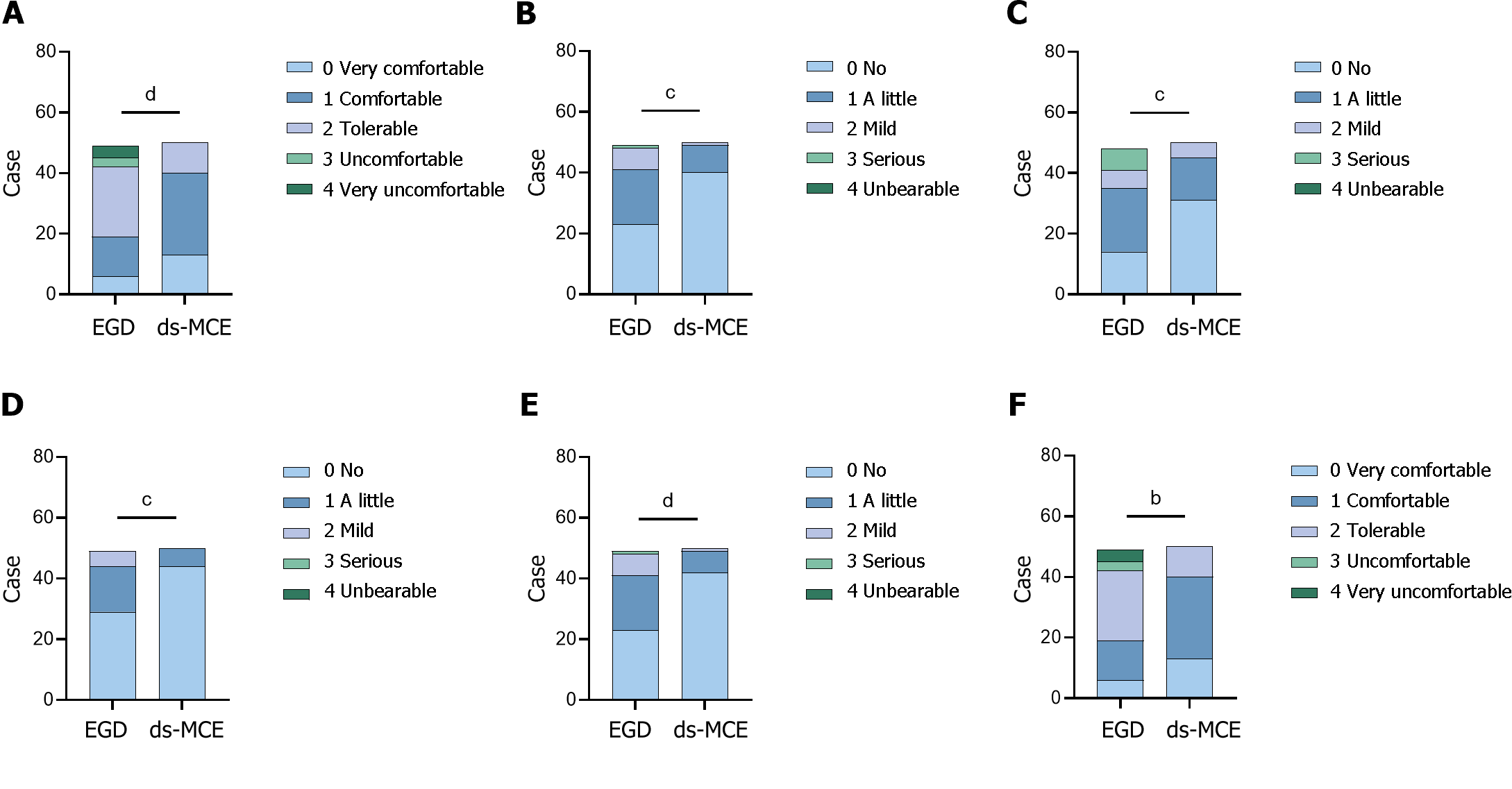
The perceived comfort of the subjects who underwent EGD and ds-MCE examinations was assessed via a questionnaire. The overall comfort level of EGD was lower than that of the ds-MCE examination. Among them, 40 patients (80%) perceived that it was very comfortable or comfortable to undergo a ds-MCE examination, but only 19 patients (38.7%) perceived that it was very comfortable or comfortable to undergo an EGD. In addition, 7 patients (14.3%) considered undergoing EGD to be uncomfortable or very uncomfortable, and the data were significantly different between the two sets (P < 0.0001). Regarding the convenience of the ds-MCE examination, 25 patients (50%) found it very convenient, and no patients found it inconvenient (Figure 4 and Table 5).
| Projects | EGD (n = 49) | ds-MCE (n = 50) |
| Comfort level | ||
| 0 Very comfort | 6 | 13 |
| 1 Comfort | 13 | 27 |
| 2 Tolerable | 23 | 10 |
| 3 Uncomfortable | 3 | 0 |
| 4 Very uncomfortable | 4 | 0 |
| P value (Kruskal-Wallis test) | < 0.0001 | |
| Pain during the examination | ||
| 0 No | 23 | 40 |
| 1 A little | 18 | 9 |
| 2 Mild | 7 | 1 |
| 3 Serious | 1 | 0 |
| 4 Unbearable | 0 | 0 |
| P value (Kruskal-Wallis test) | 0.0004 | |
| Discomfort during the examination | ||
| 0 No | 14 | 31 |
| 1 A little | 21 | 14 |
| 2 Mild | 6 | 5 |
| 3 Serious | 7 | 0 |
| 4 Unbearable | 0 | 0 |
| P value (Kruskal-Wallis test) | 0.0003 | |
| Pain after the examination | ||
| 0 No | 29 | 44 |
| 1 A little | 15 | 6 |
| 2 Mild | 5 | 0 |
| 3 Serious | 0 | 0 |
| 4 Unbearable | 0 | 0 |
| P value (Kruskal-Wallis test) | 0.0008 | |
| Discomfort after the examination | ||
| 0 No | 23 | 42 |
| 1 A little | 18 | 7 |
| 2 Mild | 7 | 1 |
| 3 Serious | 1 | 0 |
| 4 Unbearable | 0 | 0 |
| P value (Kruskal-Wallis test) | < 0.0001 | |
| Convenience of inspection | ||
| 0 Very convenient | 14 | 25 |
| 1 Convenient | 29 | 25 |
| 2 Inconvenient | 5 | 0 |
| 3 Very inconvenient | 1 | 0 |
| P value (Kruskal-Wallis test) | 0.0074 | |
The evolution of diagnostic modalities in gastroenterology is driven by the dual requirements of improving patient comfort and expanding clinicians' diagnostic capabilities. In this context, the development of ds-MCE represents a detection innovation offering patients a less invasive option and improving compliance with recommended diagnostic procedures. Our clinical study evaluated the safety and effectiveness of ds-MCE, a novel capsule endoscopy technique, for diagnosing esophageal diseases. Our findings confirmed the clinical utility of ds-MCE as a feasible, accurate, and safe noninvasive diagnostic modality that may provide tremendous benefits in terms of patient comfort and diagnostic value.
Our study identified specific patient populations that may benefit from ds-MCE detection. These included individuals at lower risk for esophageal pathology who did not require tissue sampling, such as those undergoing routine surveillance for conditions such as Barrett's esophagus. Furthermore, ds-MCE presented a safer diagnostic option for patients at increased risk of complications from traditional endoscopy or sedation, including elderly individuals, pregnant women, and those with hemodynamic instability. This approach might meet the growing demand for patient-friendly diagnostics that minimize discomfort and risk.
The unique design of ds-MCE features a steerable capsule endoscope tethered by a detachable cord, which facilitates detailed examination of targeted esophageal areas. ds-MCE has demonstrated enhanced visualization rates across various esophageal segments, including the Z-line, with one study reporting significantly greater visualization than standard MCE[17]. This ability to obtain high-quality mucosal images without anesthesia represents a significant advance in patient-centered care, especially for those who are averse to traditional endoscopy, confirming the results of previous studies[14]. Comparative analysis with existing technologies such as the N-scope demonstrated that ds-MCE can enhance the patient experience by minimizing discomfort. However, direct comparative studies are needed to confirm these claims.
The design of ds-MCE allows for extended examination times within the esophagus, which is a substantial improvement over traditional capsule endoscopy and often provides limited esophageal imaging due to rapid transit. The limitations inherent in current methods underscore the need for innovation in diagnostic endoscopy. The ds-MCE system in our study addressed these limitations by allowing extended visualization of the esophageal mucosa and was particularly advantageous for assessing areas that are traditionally challenging to evaluate using standard endoscopic techniques.
Despite these promising results, we acknowledge that our study has several limitations. First, patients with esophageal or gastric varices, which are commonly found in the lower esophagus, were not included, leaving it unclear whether their presence would alter the interpretation of the dentate line by ds-MCE. Second, most of the participants in this study were older adults with high levels of anxiety, and whether the comfort ratings would be similar for younger individuals needs to be investigated. Third, this pilot study recruited adults but did not address the potential challenges of capsule ingestion in groups known for high rates of swallowing difficulties, such as children. Fourth, ds-MCE takes longer than does EGD, with median examination times of 14.3 and 6.2 min, respectively[18]. Fifth, as a noninvasive procedure, ds-MCE does not provide histological samples or therapeutic options[19], and such considerations should be weighed within the broader clinical workflow and diagnostic strategy.
The clinical significance of false negatives in diagnosing esophageal pathology, especially for high-risk lesions such as early-stage malignancies, is profound. Although the sensitivity shown in our study is encouraging, the observed false-negative rate of approximately 15% necessitates a cautious approach when considering ds-MCE for the detection of such critical conditions. Rigorous evaluation of the diagnostic accuracy of ds-MCE is required in subsequent research efforts. Prospective, multicenter studies with larger sample sizes and the inclusion of high-risk patient groups are essential for validating our findings. Such studies should also perform head-to-head comparisons with established gold-standard diagnostic procedures to firmly establish the reliability and clinical applicability of ds-MCE.
In summary, our study highlights ds-MCE as a viable alternative to conventional EGD, as it has comparable efficacy in detecting esophageal lesions and has the potential to improve patient compliance in clinical settings. The continuous improvement and development of capsule endoscopy technology is expected to revolutionize the detection and management of upper gastrointestinal pathology[20]. To fully realize the potential of ds-MCE, future studies should include diverse patient groups, such as children and elderly individuals, to ensure robust and generalizable results. Investigating the cost-effectiveness of ds-MCE and its integration into standard diagnostic algorithms will be pivotal for its widespread adoption in clinical practice. Preliminary cost analysis models, accounting for potential reductions in anesthesia use and increased patient throughput, can offer valuable insights into the economic viability of ds-MCE. Through these coordinated research efforts, ds-MCE is likely to redefine the paradigm of esophageal disease diagnosis in the coming years.
Esophagogastroduodenoscopy (EGD), the gold standard for diagnosing esophageal diseases, is invasive and can cause patient discomfort. Magnetically controlled capsule endoscopy (MCE) is a noninvasive alternative, yet its application for esophageal conditions is limited.
This study was motivated by the need to enhance patient compliance and comfort during esophageal examinations while maintaining high diagnostic accuracy.
The primary aim was to assess the safety and diagnostic efficacy of detachable string MCE (ds-MCE) for identifying esophageal diseases.
A prospective, blinded, self-controlled clinical study was conducted to compare ds-MCE with EGD across various diagnostic parameters and patient tolerance levels in 50 subjects with known esophageal diseases.
Ds-MCE demonstrated diagnostic accuracy comparable to that of EGD (86%) and was preferred by patients, with 80% reporting a comfortable or very comfortable experience.
Ds-MCE is an effective and patient-friendly diagnostic tool for esophageal diseases, offering a noninvasive alternative to traditional EGD with similar diagnostic outcomes.
Further studies should expand patient demographics to validate the utility of ds-MCE across various populations and evaluate its cost-effectiveness for broader clinical adoption.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Oh SH, South Korea S-Editor: Li L L-Editor: A P-Editor: Chen YX
| 1. | Zagari RM, Fuccio L, Wallander MA, Johansson S, Fiocca R, Casanova S, Farahmand BY, Winchester CC, Roda E, Bazzoli F. Gastro-oesophageal reflux symptoms, oesophagitis and Barrett's oesophagus in the general population: the Loiano-Monghidoro study. Gut. 2008;57:1354-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 326] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 2. | Eusebi LH, Cirota GG, Zagari RM, Ford AC. Global prevalence of Barrett's oesophagus and oesophageal cancer in individuals with gastro-oesophageal reflux: a systematic review and meta-analysis. Gut. 2021;70:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 3. | Shaheen O, Ghibour A, Alsaid B. Esophageal Cancer Metastases to Unexpected Sites: A Systematic Review. Gastroenterol Res Pract. 2017;2017:1657310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 4. | Thrift AP. Global burden and epidemiology of Barrett oesophagus and oesophageal cancer. Nat Rev Gastroenterol Hepatol. 2021;18:432-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 201] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 5. | Cardey J, Le Gall C, Michaud L, Dabadie A, Talbotec C, Bellaiche M, Lamireau T, Mas E, Bridoux-Henno L, Viala J, Restier-Miron L, Lachaux A. Screening of esophageal varices in children using esophageal capsule endoscopy: a multicenter prospective study. Endoscopy. 2019;51:10-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Bouchard S, Ibrahim M, Van Gossum A. Video capsule endoscopy: perspectives of a revolutionary technique. World J Gastroenterol. 2014;20:17330-17344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Eliakim R, Sharma VK, Yassin K, Adler SN, Jacob H, Cave DR, Sachdev R, Mitty RD, Hartmann D, Schilling D, Riemann JF, Bar-Meir S, Bardan E, Fennerty B, Eisen G, Faigel D, Lewis BS, Fleischer DE. A prospective study of the diagnostic accuracy of PillCam ESO esophageal capsule endoscopy vs conventional upper endoscopy in patients with chronic gastroesophageal reflux diseases. J Clin Gastroenterol. 2005;39:572-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Gralnek IM, Adler SN, Yassin K, Koslowsky B, Metzger Y, Eliakim R. Detecting esophageal disease with second-generation capsule endoscopy: initial evaluation of the PillCam ESO 2. Endoscopy. 2008;40:275-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Hosoe N, Naganuma M, Ogata H. Current status of capsule endoscopy through a whole digestive tract. Dig Endosc. 2015;27:205-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Duvvuri A, Desai M, Vennelaganti S, Higbee A, Gorrepati VS, Dasari C, Chandrasekar VT, Vennalaganti P, Kohli D, Sathyamurthy A, Rai T, Sharma P. Diagnostic accuracy of a novel third generation esophageal capsule as a non-invasive detection method for Barrett's esophagus: A pilot study. J Gastroenterol Hepatol. 2021;36:1222-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Park J, Cho YK, Kim JH. Current and Future Use of Esophageal Capsule Endoscopy. Clin Endosc. 2018;51:317-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Zou WB, Hou XH, Xin L, Liu J, Bo LM, Yu GY, Liao Z, Li ZS. Magnetic-controlled capsule endoscopy vs. gastroscopy for gastric diseases: a two-center self-controlled comparative trial. Endoscopy. 2015;47:525-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 13. | Liao Z, Hou X, Lin-Hu EQ, Sheng JQ, Ge ZZ, Jiang B, Hou XH, Liu JY, Li Z, Huang QY, Zhao XJ, Li N, Gao YJ, Zhang Y, Zhou JQ, Wang XY, Liu J, Xie XP, Yang CM, Liu HL, Sun XT, Zou WB, Li ZS. Accuracy of Magnetically Controlled Capsule Endoscopy, Compared With Conventional Gastroscopy, in Detection of Gastric Diseases. Clin Gastroenterol Hepatol. 2016;14:1266-1273.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 152] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 14. | Beg S, Card T, Warburton S, Rahman I, Wilkes E, White J, Ragunath K. Diagnosis of Barrett's esophagus and esophageal varices using a magnetically assisted capsule endoscopy system. Gastrointest Endosc. 2020;91:773-781.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 15. | Chen YZ, Pan J, Luo YY, Jiang X, Zou WB, Qian YY, Zhou W, Liu X, Li ZS, Liao Z. Detachable string magnetically controlled capsule endoscopy for complete viewing of the esophagus and stomach. Endoscopy. 2019;51:360-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Chen XY, Da W, Liang R, Fan HN, Yi YC, Chen M, Qin HW, Zhang J, Zhu JS. The Detective Value of Magnetically Controlled Robotic Capsule Endoscopy in Patients With Suspected Small Intestinal Disease. Front Med (Lausanne). 2021;8:610563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 17. | Song J, Bai T, Zhang L, Xiang XL, Xie XP, Hou XH. Better view by detachable string magnetically controlled capsule endoscopy for esophageal observation: a retrospective comparative study. Dis Esophagus. 2020;33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Jiang X, Qian YY, Liu X, Pan J, Zou WB, Zhou W, Luo YY, Chen YZ, Li ZS, Liao Z. Impact of magnetic steering on gastric transit time of a capsule endoscopy (with video). Gastrointest Endosc. 2018;88:746-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Wang S, Huang Y, Hu W, Mao H, McAlindon ME, Liu Y, Yang L, Zhang C, Xu M, He C, Dang T, Wu B, Ji D, Zhang L, Mao X, Liu C, Xu D, Li Y, Li G, Han J, Lv F, Liang X, Jin S, Zhang S, Tai FWD, Xu Q, Yang C, Wang G, Wang L, Li B, Yang H, Xie P, Deng L, Ren L, Chang Z, Wang X, Wang S, Gao X, Li J, Zhu L, Wang F, Zhang G, Jiang X, Pan J, Meng W, Li X, Hou J, Dray X, Liao Z, Qi X. Detachable string magnetically controlled capsule endoscopy for detecting high-risk varices in compensated advanced chronic liver disease (CHESS1801): A prospective multicenter study. Lancet Reg Health West Pac. 2021;6:100072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Alsayid M, Melson J. Will magnet-assisted capsule endoscopy become a viable screening tool for Barrett's esophagus and esophageal varices? Gastrointest Endosc. 2020;91:782-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |