Published online Feb 28, 2024. doi: 10.3748/wjg.v30.i8.984
Peer-review started: September 28, 2023
First decision: December 28, 2023
Revised: January 5, 2024
Accepted: January 25, 2024
Article in press: January 25, 2024
Published online: February 28, 2024
Processing time: 150 Days and 21.8 Hours
Cronkhite-Canada syndrome (CCS) is a rare, noninherited disease characterized by gastrointestinal polyposis with diarrhea and ectodermal abnormalities. CCS polyps are distributed through the whole digestive tract, and they are common in the stomach and colon but very uncommon in the esophagus.
Here, we present a case of a 63-year-old man with skin hyperpigmentation accompanied by diarrhea, alopecia, and loss of his fingernails. Laboratory data indicated anemia, hypoalbuminemia, hypocalcemia, hypokalemia, and positive fecal occult blood. Endoscopy showed numerous polyps scattered throughout the digestive tract, including the esophagus. He was treated with nutritional support and glucocorticoids with remission of his symptoms.
Comprehensive treatment led by hormonal therapy can result in partial or full remission of clinical symptoms. Treatment should be individualized for each patient according to their therapy response. Surveillance endoscopy is necessary for assessing mucosal disease activity and detecting malignant transformation.
Core Tip: Cronkhite-Canada syndrome is a rare, noninherited disease characterized by gastrointestinal polyposis with diarrhea and ectodermal abnormalities. We report a case of a 63-year-old man with numerous polyps scattered throughout the digestive tract, including the esophagus. Comprehensive corticosteroid treatment can result in partial or full remission of clinical symptoms.
- Citation: Tang YC. Cronkhite-Canada syndrome with esophagus involvement and six-year follow-up: A case report. World J Gastroenterol 2024; 30(8): 984-990
- URL: https://www.wjgnet.com/1007-9327/full/v30/i8/984.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i8.984
First reported in 1955, Cronkhite-Canada syndrome (CCS), also known as polyposis pigmentation-alopecia-onycholrophia syndrome, is characterized by gastrointestinal polyposis with diarrhea and ectodermal abnormalities[1]. It is a rare, noninherited disease with an incidence of 1 in a million[2]. More than 500 cases have been reported worldwide, with most of the cases being reported in Asian countries[3]. Although the incidence of CCS is low, it is associated with high mortality, mainly because of the difficulty in diagnosis and delay in treatment[4]. The diagnosis of CCS is based on history, physical examination, endoscopic findings of gastrointestinal polyposis, and histopathology. Studies have suggested that corticosteroids and immunomodulators are the most effective treatments thus far[5,6]. Long-term surveillance is needed because of the relatively poor prognosis and the increased risk of intestinal malignancy[7].
A 63-year-old male was admitted to our hospital with a two-month history of skin hyperpigmentation and one month of diarrhea.
The patient complained of skin hyperpigmentation for two months and loose stool two to four times per day for one month. There was no associated blood in the stool, abdominal distension, nausea, vomiting, appetite loss, or weight loss. The abnormal skin pigmentation was unevenly distributed throughout the body, with the face and both sides of the hands mainly affected.
Segmental resection of the small intestine was performed twenty-three years prior because of severe abdominal trauma. He was diagnosed with syphilis two years previously and treated with benzathine penicillin. Other medical records revealed bradycardia and varicose veins in the lower limbs.
The patient has an 80-pack-year history and a 40-year history of drinking approximately 100 g of wine per day. There was no family history of gastrointestinal polyposis.
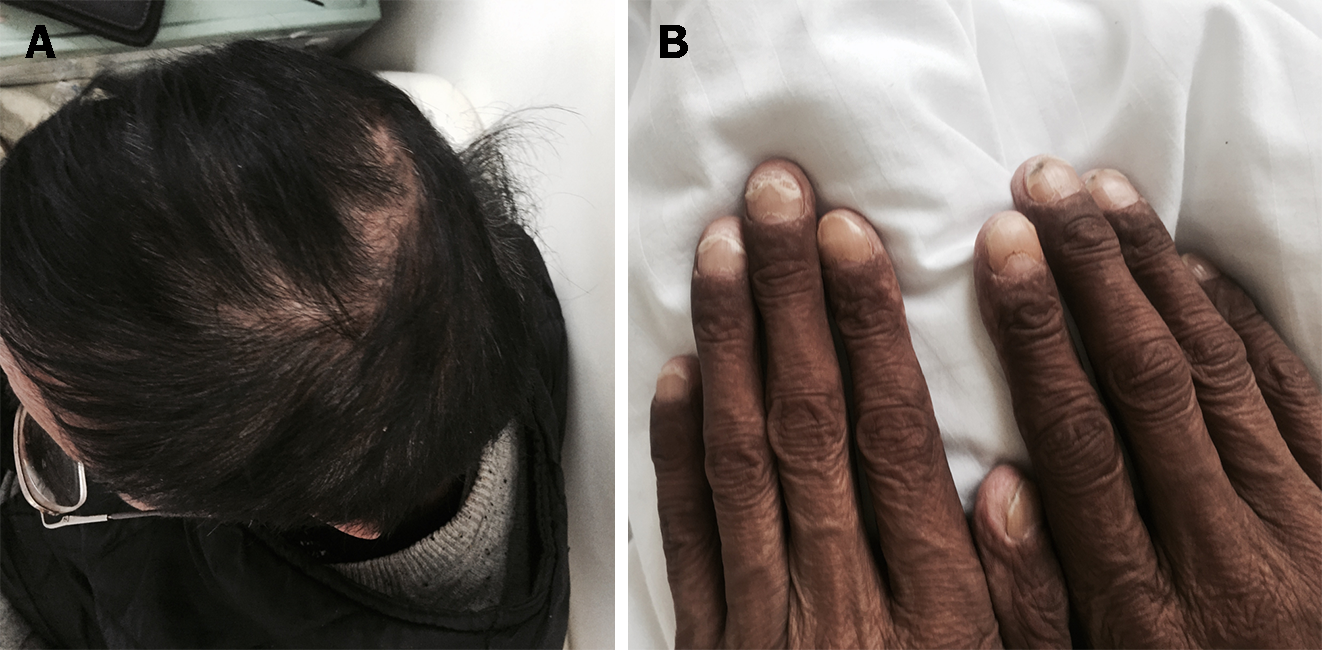
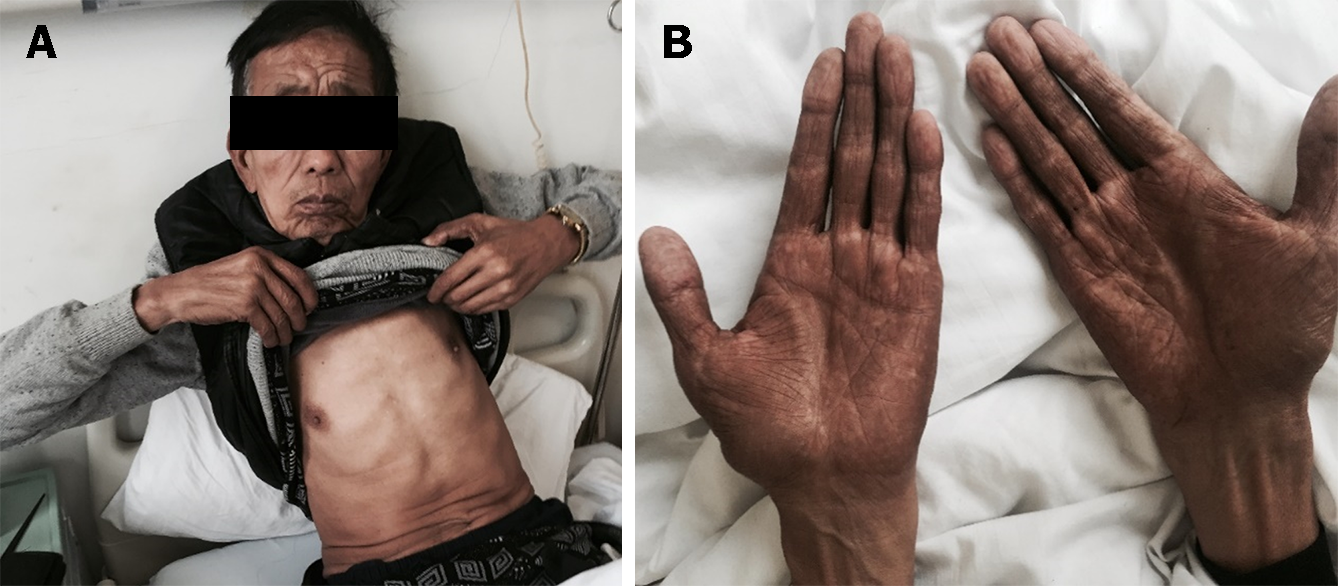
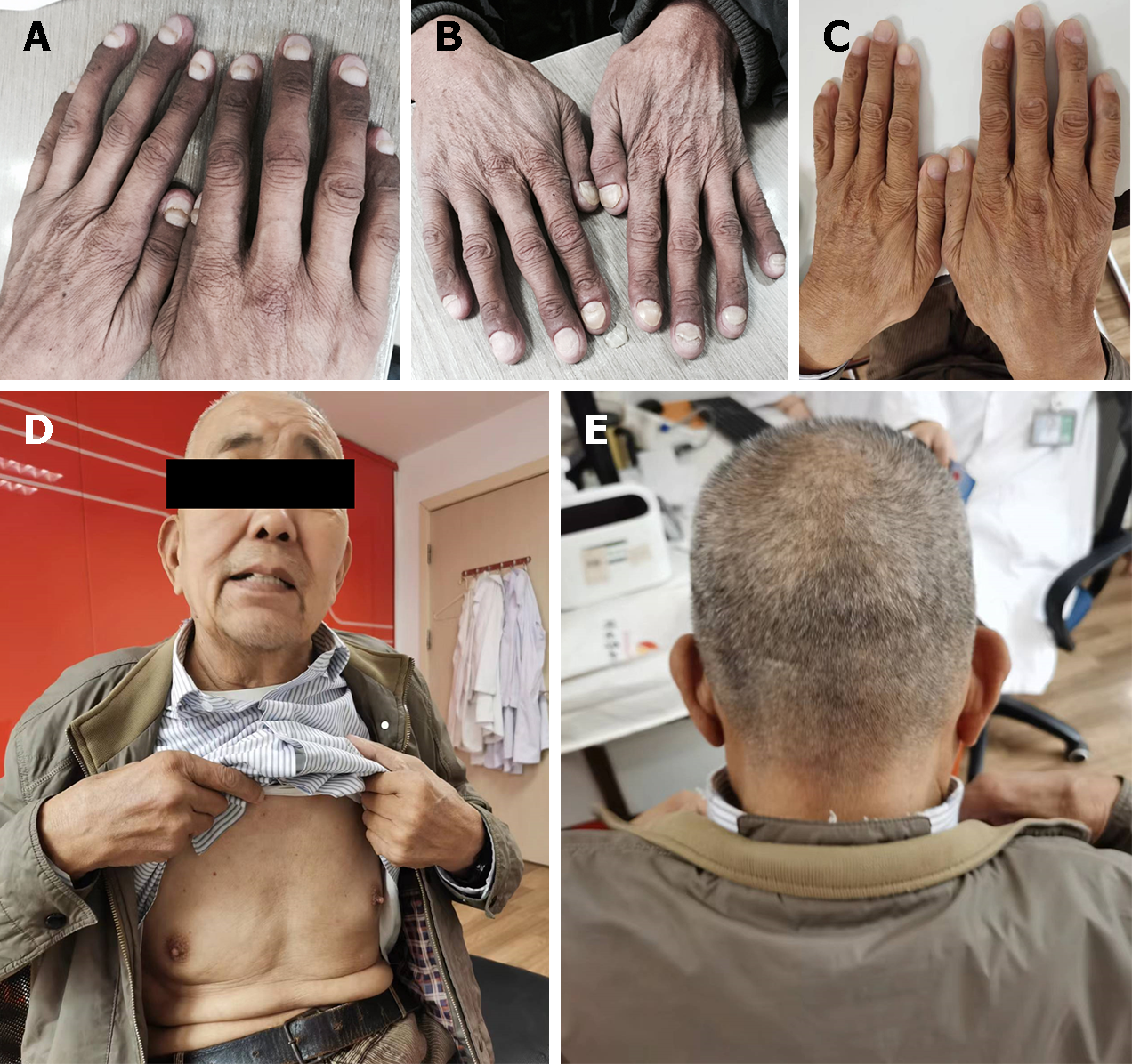
On physical examination, he had alopecia, nail dystrophy (Figure 1), and hyperpigmentation of the skin, especially on his face and bilateral upper extremities (Figure 2).
Laboratory tests revealed normal routine blood results (Table 1). The fecal occult blood test was positive (+), and C-reactive protein was elevated. Serum albumin and serum total protein were decreased. The levels of serum potassium and calcium were also reduced. Bacterial culture of the stool found no salmonella, shigella, or fungus. Cortisol secretion rhythm, erythrocyte sedimentation rate, immunoglobulin, liver function tests, and other blood chemistry panels were normal. Electromyography suggested peripheral nerve damage (sensory fibers) in the upper and lower extremities.
| Parameters | Findings | Normal range | Remarks |
| White blood cell count (/L) | 8.47 × 109 | (3.5-9.5) × 109 | Normal |
| Hemoglobin (g/L) | 132 | 130-175 | Normal |
| Platelet count (/L) | 271 × 109 | (125-350) × 109 | Normal |
| C-reactive protein (mg/L) | 51.5 | 0-8 | High |
| Fecal occult blood test | Positive | Negative | Positive |
| Serum albumin (g/L) | 36.2 | 40-55 | Low |
| Serum total protein (g/L) | 63.3 | 65-85 | Low |
| Serum potassium (mmol/L) | 3.1 | 3.5-5.3 | Low |
| Serum calcium (mmol/L) | 1.86 | 2.11-2.52 | Low |
A computer tomography scan of the abdomen showed a partially thickened gastric wall, enterostasis, and slightly enlarged inguinal lymph nodes.
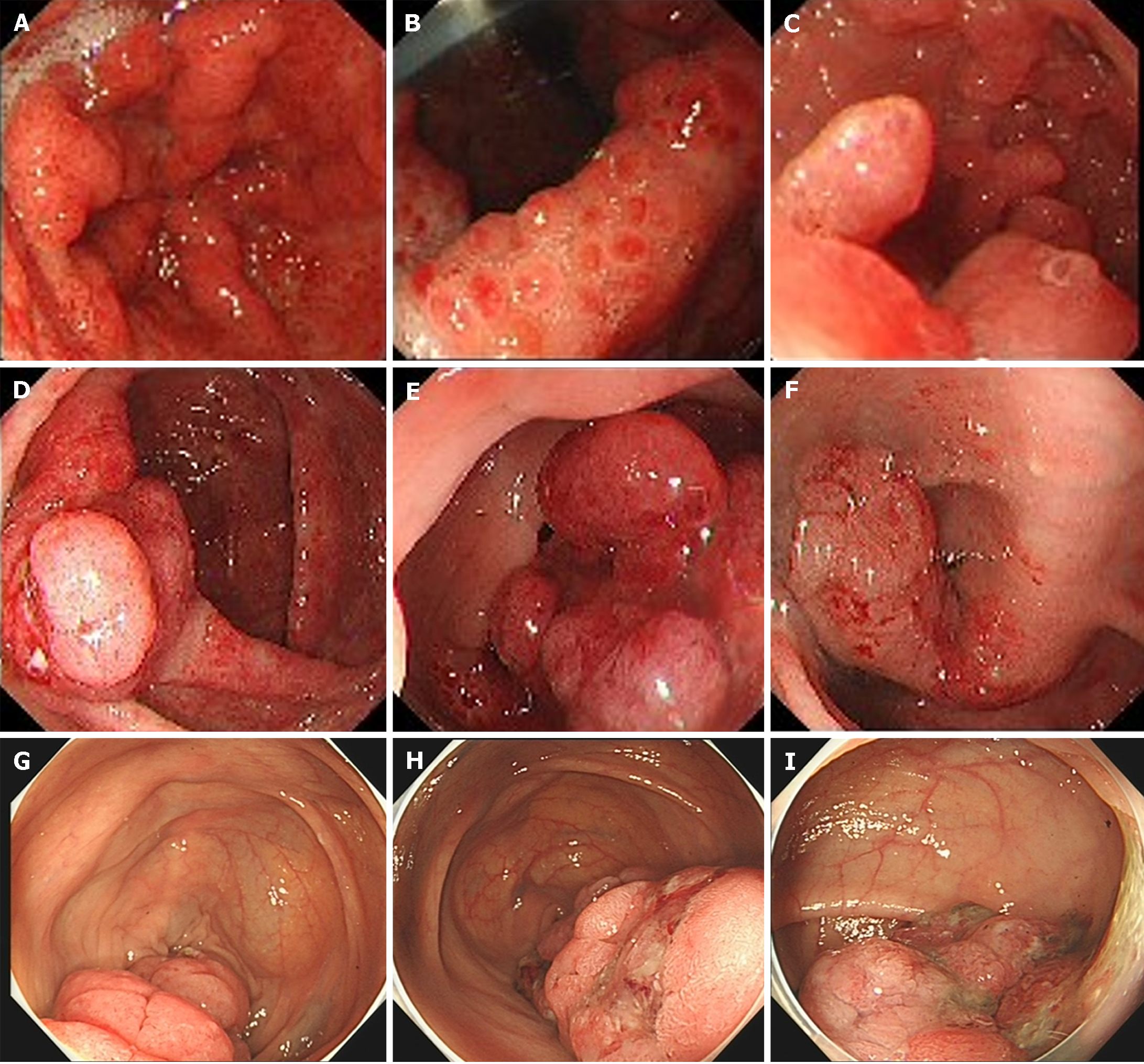
The results of gastroscopy showed multiple polyp hyperplasia of the gastric body, gastric angle, gastric antrum, and descending part of the duodenum (Figure 3B and C). Granular apophyses below the dentate line were observed by the endoscopist, although they are not as evident in Figure 3A. The results of the colonoscopy showed that mucosa erosion and multiple apophyses with the appearance of polypuses were observed in the ileocecal valve, and numerous polyps were found in the whole colon, among which the largest polyp (4.0 cm) was found 20 cm from the anus (Figure 3D-F).
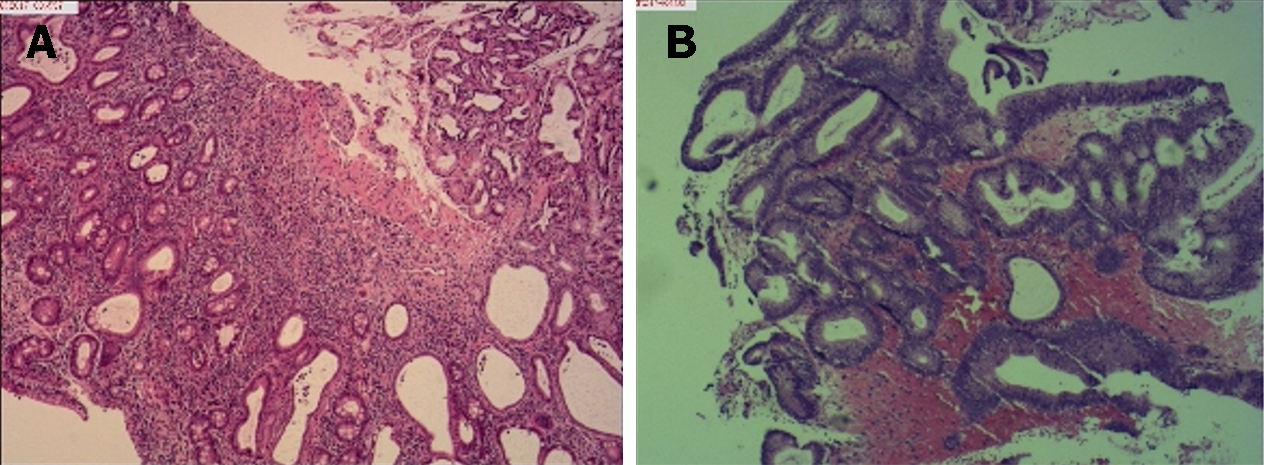
Histological examination of biopsy specimens obtained from the duodenal bulb was consistent with hyperplastic polyps, and biopsy specimens from the gastric antrum mucosa displayed moderate chronic mucosal inflammation, expansion of the glands, and mild intestinal metaplasia of the glandular epithelium (Figure 4A). Biopsy specimens obtained from the colon suggested tubular adenomas and low-grade intraepithelial neoplasia (Figure 4B).
Given the diffuse polyps in the gastrointestinal tract and ectodermal abnormalities, along with the age of onset and family history, the final diagnosis of CCS was made.
The patient was treated with corticosteroids (oral administration of prednisone initiated at 40 mg/d), nutritional support including vitamins and compound amino acid injection 18AA-II, proton pump inhibitors, gastric mucosal protective agents, antidiarrheal drugs, and Bacillus licheniformis capsule to modulate intestinal flora.
The patient was followed up irregularly for approximately six years and had adhered to the advice provided by the chief physician. The symptoms of diarrhea, alopecia, nail dystrophy, and skin hyperpigmentation were relieved within one month (Figure 5). However, the endoscopic findings showed no improvement (Figure 3G-I). The fecal occult blood test remained positive, and his hemoglobin gradually dropped to 91 g/L (normal range, 130-175 g/L) in the following years. Due to the side effects of corticosteroids, he unfortunately developed osteonecrosis of the femoral head, and it was suggested that he undergo total hip arthroplasty. However, the patient refused the surgery, considering the operation risk and his health condition. In total, the patient developed an ileus twice: One was a colonic obstruction with a right inguinal hernia, and the other was a small bowel obstruction with a right inguinal hernia. Conservative therapy was applied, and further examinations, including colonoscopy, were advised to investigate any malignant transformation of the polyps. Nevertheless, the patient refused because he could not tolerate the endoscopy. The most severe complication was an infection of the cervical, oral, and maxillofacial spaces, and he was admitted to the intensive care unit. The patient recovered after catheter drainage of the abscess and other anti-inflammatory treatment. Since diagnosis, the dose of prednisone has been decreased gradually. It has remained at the minimum effective level (10 mg per day) to control the symptoms of diarrhea, alopecia, nail dystrophy, and hyperpigmentation. Although his anemia and malnutrition had not improved, he gained weight and survived a COVID-19 infection.
CCS is a rare nonfamilial polyposis syndrome characterized by epithelial disorders in both the gastrointestinal tract and epidermis[8]. The etiology and pathogenesis are still unclear at present[9]. There is no consensus on the treatment of CCS[10]. In this study, we report a case with esophageal involvement and long-term outcomes to better understand CCS in Chinese patients.
There are no definitive criteria for diagnosing CCS, which usually depends on medical history, physical examination, endoscopic examination, and pathological results[11]. For our patient, who first went to the department of endocrinology, the differential diagnosis for the hyperpigmentation included primary adrenal insufficiency, POEMS syndrome, Peutz-Jegher syndrome (PJS), hemochromatosis and some connective tissue diseases. The hyperpigmentation of primary adrenal insufficiency is usually distributed throughout the body but can also be localized. In general, it is more obvious in exposed parts and portions of the skin that are easy to rub (such as the face, hand, palmprint, areola, and so on). The pigmentation of the tongue surface, buccal mucosa, lips, oral mucosa, gums, and cicatrix was also deepened but not distinct from normal skin. However, only a few studies described the hyperpigmentation in CCS, mainly as brownish changes with a clear boundary. Colored spots sometimes occur on the face, body, limbs, palms, soles of the foot, and oral mucosa[12].
When there are multiple polyps in the gastrointestinal tract, CCS usually needs to be differentiated from PJS, juvenile polyposis syndrome, familial adenomatous polyposis, Turcot syndrome, Cowden syndrome, and other diseases. PJS is characterized by the association of gastrointestinal (GI) polyposis, mucocutaneous pigmentation, and cancer predisposition[13]. PJS-type hamartomatous polyps are most common in the small intestine but can also occur in the stomach, large bowel, and extraintestinal sites[14]. Mucocutaneous hyperpigmentation presents in childhood as dark blue to dark brown macules around the mouth, eyes, and nostrils, in the perianal area, and on the buccal mucosa. Hyperpigmented macules on the fingers are usually common[15].
CCS polyps are distributed throughout the whole digestive tract, and they are common in the stomach and colon, less common in the small intestine and rectum, and uncommon in the esophagus[16]. In a Japanese nationwide survey, the esophagus was involved in 12.3% of cases, in contrast to prior reports[17]. However, in a retrospective study of 103 Chinese cases, the ratio was 4/103, much lower than the Japanese data[18]. Multiple changes, including but not limited to polyps, may occur in the esophagus, which might not be focused on except when there are polyps. As in our case, the endoscopist observed granular apophyses below the dentate line.
There is no consensus on the treatment of CCS, and it is still in the exploratory stage. Current medical therapies include corticosteroids, nonsteroidal anti-inflammatory drugs, immunomodulators, proton pump inhibitors, H2-receptor antagonists, antibiotics, surgery, 5-aminosalicylate acid, antitumor necrosis factor α agents, eradication of Helicobacter pylori, nutritional support or a combination of these therapies[19]. Steroid therapy is the mainstay of medical treatment. A retrospective analysis confirmed that oral corticosteroid therapy (30-49 mg/d) appeared to be effective for active CCS, and in most circumstances, a slow reduction in dosage was suggested[17]. However, the long-term efficacy and side effects of low-dose corticosteroids still require observation. In both the Japanese nationwide survey and the largest single-center cohort of Chinese patients, nearly 40% of patients failed to achieve long-term clinical remission after corticosteroid administration, and relapse occurred during or after the cessation of corticosteroid use[17,18]. A proportion of patients were prescribed low-dose (5-10 mg/d) corticosteroids or immunosuppressants to counteract the tendency to relapse[19]. In addition, the symptoms and endoscopic findings of CCS may have different hormonal responses[11]. For our patient, whose symptoms were relieved before the improvement in polyps after treatment, long-term endoscopy surveillance is needed to check the sensitivity to corticosteroids and to detect mucosal disease activity and any malignant transformation of polyps. Adverse events secondary to corticosteroid treatment were frequently reported in the medical records. Even though we tried to reduce the dosage from 10 mg/d to 5 mg/d and withdraw corticosteroids, the GI symptoms, hyperpigmentation, alopecia, and nail dystrophy relapsed. Despite the adverse effects of corticosteroids, they are still the most important therapy with the most clinical evidence and efficacy. The widespread use of corticosteroids may have contributed to the decrease in 5-year mortality from 55% to 16% and the general improvement of outcomes[4,18].
CCS is a rare disease with major clinical features of gastrointestinal polyps, diarrhea, skin hyperpigmentation, alopecia, and nail atrophy. Comprehensive treatment led by corticosteroid therapy can result in partial or full remission of clinical symptoms. Treatment should be individualized for each patient according to their responses to the therapy. Surveillance endoscopy is necessary for assessing mucosal disease activity and detecting malignant transformation.
The author would like to express the gratitude for the case. I also want to acknowledge the valuable support of Jie Dong, MD, from the Department of Gastroenterology, Zhejiang Province People’s Hospital, and Jian-Ping Chu, Chief Doctor of our department, and everyone engaged in accomplishing this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Piltcher-da-Silva R, Brazil S-Editor: Gong ZM L-Editor: A P-Editor: Chen YX
| 1. | Sweetser S, Alexander GL; Boardman LA. A case of Cronkhite-Canada syndrome presenting with adenomatous and inflammatory colon polyps. Nat Rev Gastroenterol Hepatol. 2010;7:460-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Goto A. Cronkhite-Canada syndrome: epidemiological study of 110 cases reported in Japan. Nihon Geka Hokan. 1995;64:3-14. [PubMed] |
| 3. | Slavik T, Montgomery EA. Cronkhite-Canada syndrome six decades on: the many faces of an enigmatic disease. J Clin Pathol. 2014;67:891-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Daniel ES, Ludwig SL, Lewin KJ, Ruprecht RM, Rajacich GM, Schwabe AD. The Cronkhite-Canada Syndrome. An analysis of clinical and pathologic features and therapy in 55 patients. Medicine (Baltimore). 1982;61:293-309. [PubMed] |
| 5. | Cho W, Nam K, Bang KB, Shin HD, Shin JE. Cronkhite-Canada Syndrome Showing Good Early Response to Steroid Treatment. Korean J Gastroenterol. 2018;71:239-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Dore MP, Satta R, Murino A, Pes GM. Long-lasting remission in a case of Cronkhite-Canada syndrome. BMJ Case Rep. 2018;2018:bcr2017223527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Yashiro M, Kobayashi H, Kubo N, Nishiguchi Y, Wakasa K, Hirakawa K. Cronkhite-Canada syndrome containing colon cancer and serrated adenoma lesions. Digestion. 2004;69:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Zhao R, Huang M, Banafea O, Zhao J, Cheng L, Zou K, Zhu L. Cronkhite-Canada syndrome: a rare case report and literature review. BMC Gastroenterol. 2016;16:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Ma M, Huang Y, Suo Z, Ma X. Cronkhite-Canada syndrome: A case report and review of the literature. Ann Med Surg (Lond). 2022;81:104090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 10. | Wu ZY, Sang LX, Chang B. Cronkhite-Canada syndrome: from clinical features to treatment. Gastroenterol Rep (Oxf). 2020;8:333-342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Wen XH, Wang L, Wang YX, Qian JM. Cronkhite-Canada syndrome: report of six cases and review of literature. World J Gastroenterol. 2014;20:7518-7522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | She Q, Jiang JX, Si XM, Tian XY, Shi RH, Zhang GX. A severe course of Cronkhite-Canada syndrome and the review of clinical features and therapy in 49 Chinese patients. Turk J Gastroenterol. 2013;24:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Tacheci I, Kopacova M, Bures J. Peutz-Jeghers syndrome. Curr Opin Gastroenterol. 2021;37:245-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 14. | Sandru F, Petca A, Dumitrascu MC, Petca RC, Carsote M. Peutz-Jeghers syndrome: Skin manifestations and endocrine anomalies (Review). Exp Ther Med. 2021;22:1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | McGarrity TJ, Amos CI, Baker MJ. Peutz-Jeghers Syndrome. 2001 Feb 23. In: GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2024. [PubMed] |
| 16. | Sweetser S, Ahlquist DA, Osborn NK, Sanderson SO, Smyrk TC, Chari ST; Boardman LA. Clinicopathologic features and treatment outcomes in Cronkhite-Canada syndrome: support for autoimmunity. Dig Dis Sci. 2012;57:496-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | Watanabe C, Komoto S, Tomita K, Hokari R, Tanaka M, Hirata I, Hibi T, Kaunitz JD, Miura S. Endoscopic and clinical evaluation of treatment and prognosis of Cronkhite-Canada syndrome: a Japanese nationwide survey. J Gastroenterol. 2016;51:327-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 18. | Lu Y, Huang F, Wang Y, Zhou J, Zhao Q, Liu L. Clinical and Endoscopic Characteristics of Chinese Cronkhite-Canada Syndrome Patients: A Retrospective Study of 103 Cases. Dig Dis. 2021;39:488-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Liu S, You Y, Ruan G, Zhou L, Chen D, Wu D, Yan X, Zhang S, Zhou W, Li J, Qian J. The Long-Term Clinical and Endoscopic Outcomes of Cronkhite-Canada Syndrome. Clin Transl Gastroenterol. 2020;11:e00167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |