Published online Feb 21, 2024. doi: 10.3748/wjg.v30.i7.742
Peer-review started: November 13, 2023
First decision: December 7, 2023
Revised: December 19, 2023
Accepted: January 16, 2024
Article in press: January 16, 2024
Published online: February 21, 2024
Processing time: 100 Days and 24 Hours
In hepatology, the clinical use of endoscopic ultrasound (EUS) has experienced a notable increase in recent times. These applications range from the diagnosis to the treatment of various liver diseases. Therefore, this systematic review sum
To examine and summarize the current available evidence of the possible roles of the EUS in making a suitable diagnosis in liver diseases as well as the therapeutic accuracy and efficacy.
PubMed, Medline, Cochrane Library, Web of Science, and Google Scholar databases were extensively searched until October 2023. The methodological quality of the eligible articles was assessed using the Newcastle-Ottawa scale or Cochrane Risk of Bias tool. In addition, statistical analyses were performed using the Comprehensive Meta-Analysis software.
Overall, 45 articles on EUS were included (28 on diagnostic role and 17 on therapeutic role). Pooled analysis demonstrated that EUS diagnostic tests had an accuracy of 92.4% for focal liver lesions (FLL) and 96.6% for parenchymal liver diseases. EUS-guided liver biopsies with either fine needle aspiration or fine needle biopsy had low complication rates when sampling FLL and parenchymal liver diseases (3.1% and 8.7%, respectively). Analysis of data from four studies showed that EUS-guided liver abscess had high clinical (90.7%) and technical success (90.7%) without significant complications. Similarly, EUS-guided interventions for the treatment of gastric varices (GV) have high technical success (98%) and GV obliteration rate (84%) with few complications (15%) and rebleeding events (17%).
EUS in liver diseases is a promising technique with the potential to be considered a first-line therapeutic and diagnostic option in selected cases.
Core Tip: This is an extensive systematic review to assess the efficacy and accuracy of the endoscopic ultrasound (EUS) in dealing with different liver pathologies. The EUS guided liver abscess drainage (EUS-AD) was highly accurate (90.7%) and very safe, with more than 90% of patients experienced no complications post EUS-AD. The safety profiles of the EUS guided aspiration and EUS guided biopsy was very promising with very low complication rate. EUS guided interventions is a safe and accurate procedure and this was demonstrated in different interventions such as EUS guided gastric varies obliteration which was successful in 84% with only 15% rebreeding risk.
- Citation: Gadour E, Awad A, Hassan Z, Shrwani KJ, Miutescu B, Okasha HH. Diagnostic and therapeutic role of endoscopic ultrasound in liver diseases: A systematic review and meta-analysis. World J Gastroenterol 2024; 30(7): 742-758
- URL: https://www.wjgnet.com/1007-9327/full/v30/i7/742.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i7.742
Since its introduction in the 1980s, endoscopic ultrasonography (EUS) has emerged as a pivotal diagnostic and therapeu
EUS offers advantages that distinguish it from other diagnostic tools. EUS is performed by inserting the probe into the GI tract; therefore, it can provide close proximity to the target tissues[5]. This close proximity is particularly valuable for evaluating lesions within the GI wall, adjacent lymph nodes, and surrounding vasculature. It is also valuable in guiding FNA and fine-needle biopsy (FNB) for the collection of tissue samples from lesions and suspicious areas identified during the course of examination[6]. Furthermore, EUS can provide real-time imaging, which allows for dynamic assessment and precise localization of lesions[7].
Despite its advantages, evidence of the role of EUS in liver disease is limited. Therefore, this systematic review aimed to evaluate the diagnostic and therapeutic roles of EUS in liver disease.
PubMed, Medline, Cochrane Library, Web of Science, and Google Scholar databases were comprehensively searched for all randomized and nonrandomized studies published from inception to October 2023. The bibliographies of potential articles were also scrutinized for additional studies. Studies with the following MeSH terms and keywords were retrieved from the electronic databases: (Endoscopic ultrasound OR endosonography OR EUS OR endoscopic ultrasound-guided fine needle aspiration OR EUS-FNA OR endoscopic ultrasound-guided fine needle biopsy OR EUS-FNB) AND (diagnosis OR diagnostic OR detection OR treatment OR interventional OR therapeutic) AND (hepatic OR liver). The gray literature and duplicates were not retrieved, as they would have interfered with the scientific purpose of the current study.
Two independent reviewers scrutinized potential studies using predefined inclusion and exclusion criteria. Studies were eligible for review and analysis if they were full articles published in English, included human participants, or reported on the role of EUS in the diagnosis or treatment of liver diseases, including portal hypertension. On the other hand, articles that went against these criteria or were designed as case reports, systematic reviews, conference abstracts, and letters to the editors or reported the therapeutic and diagnostic role of EUS in extrahepatic structures such as bile duct and gall bladder were excluded. In the event of differences between the reviewers, a third reviewer was consulted to harmonize discrepancies.
Two impartial reviewers examined all included records and abstracted the data required for review and analysis into separate Excel files. Discrepancies in the extracted data were resolved through constructive discussions or by consulting a third reviewer. The extracted data included the Author ID (surname of the primary author and publication date), study design, study location (country), characteristics of the enrolled patients (sample size, sex distribution, mean/median age, and indication for conducting EUS/EUS-guided diagnostic tests), diagnostic tests used, intervention, treated liver disorder, and outcomes.
The outcomes of our study were divided into the therapeutic and diagnostic groups. The diagnostic endpoints included diagnostic accuracy and yield. Diagnostic accuracy was defined as the ratio of true positives to true negatives for an accurate cytological or histological diagnosis in the total number of patients. Therapeutic outcomes included procedure-related complications, technical and clinical success, gastric varices (GV) obliteration, and rebleeding.
Randomized and nonrandomized studies were included in the current review; therefore, quality assessment was performed using two different tools. The Newcastle-Ottawa scale was used to assess the methodological quality of non-randomized studies. This tool evaluates studies according to the selection, comparability, and outcome domains. For every domain, a maximum of one star was assigned for a fully answered criterion; otherwise, no stars were assigned. In the selection domain, a maximum of 4 stars could be attained, whereas a maximum of two and three stars could be achieved for the comparability and outcome domains, respectively.
On the other hand, bias assessment of randomized trials was performed using Cochrane’s risk of bias (RoB) tool embedded within the Review Manager software. RoB was assessed based on selection, attrition, performance, reporting, and other biases. A low RoB was assigned to a domain that was sufficiently addressed within the study, whereas a high and unclear risk was assigned to domains that were not entirely addressed or had insufficient information to make a judgment.
The comprehensive meta-analysis software (CMA V3) was used to conduct all statistical analyses in the present study. The random-effects model was used to pool the estimated weighted effect size and counter-anticipated heterogeneity. The inter-study heterogeneity was calculated using the I2 statistics, of which values > 50% were regarded as significant[8]. Moreover, the effect sizes were calculated together with their 95% confidence intervals, and when possible, subgroup analyses were performed according to diagnostic tests or EUS-guided interventions.
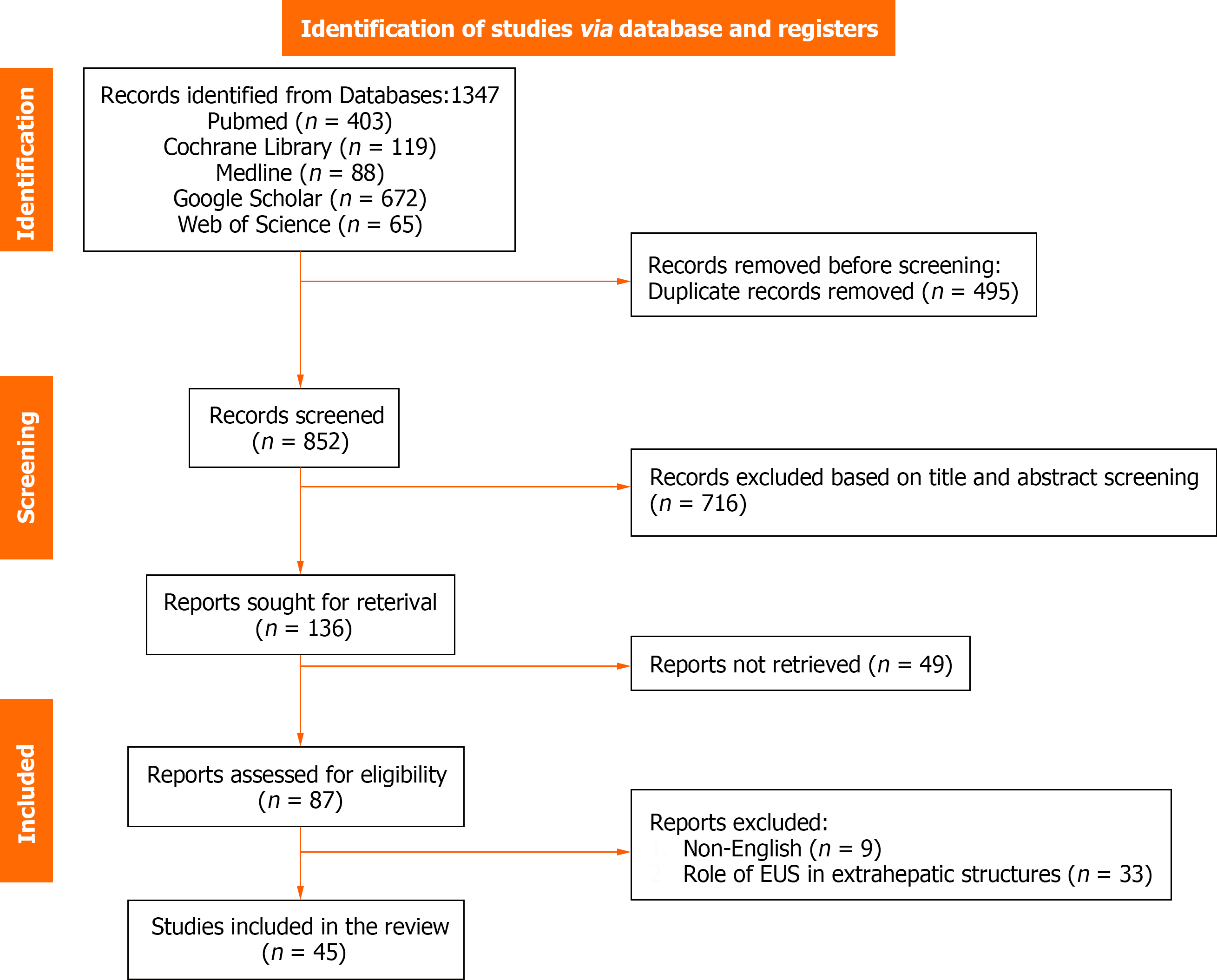
An extensive database search identified 1347 potential articles. Duplicate screening resulted in the exclusion of 495 duplicate studies. Subsequently, 716 records were eliminated based on title, abstract, and title screening, and 49 were not retrieved as they were either case reports, reviews, conference abstracts, or letters to the editor. Finally, 45 records were included and the remaining 42 were excluded for the following reasons: nine were published in different languages and 33 evaluated the diagnostic or therapeutic role of EUS in extrahepatic structures and other parts of the body (Figure 1).
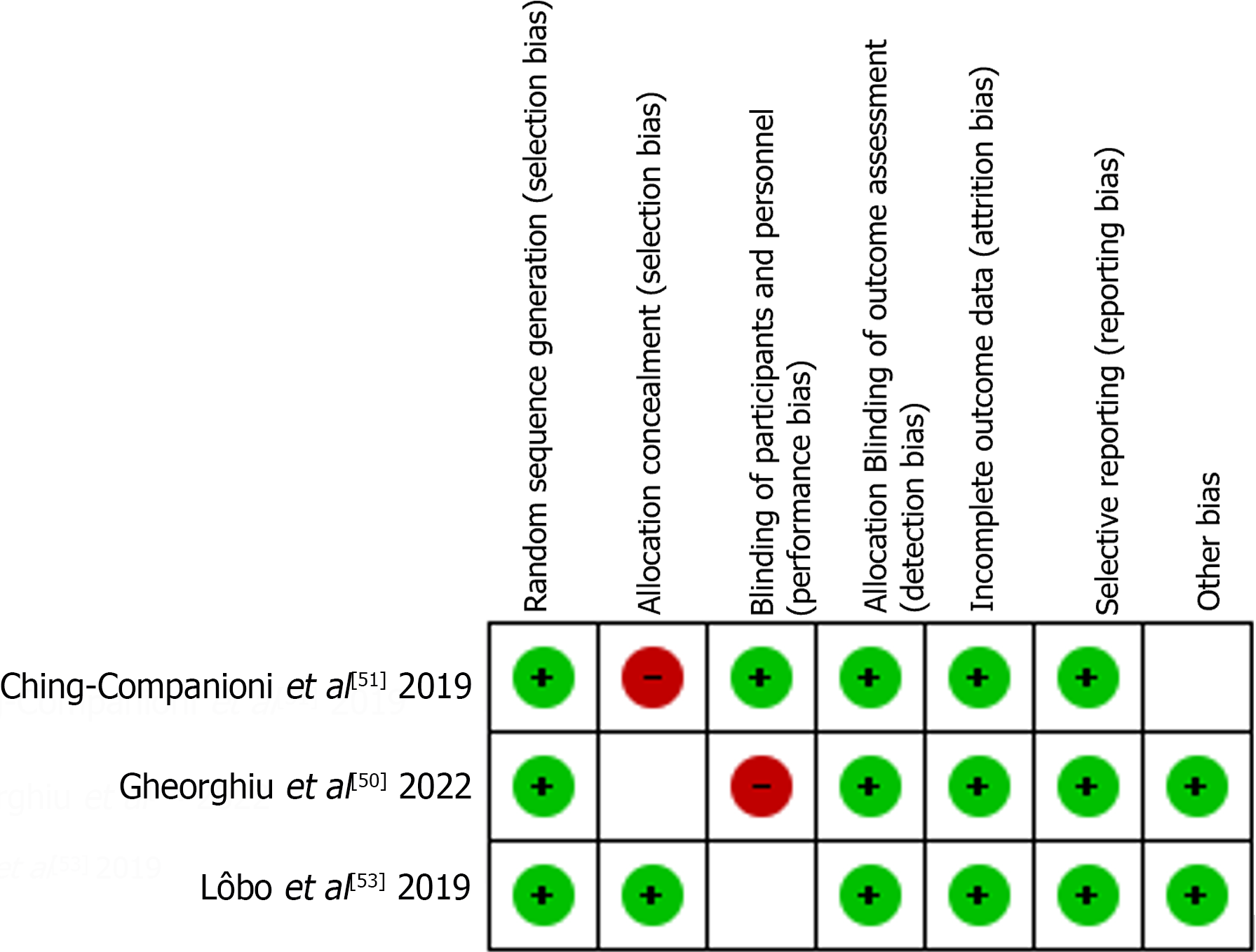
Using the Newcastle–Ottawa scale and Cochrane RoB, we found that most studies were of good or fair quality. Table 1 presents the Newcastle-Ottawa scale results and Figure 2 summarizes the RoB.
| Ref. | Selection (/4) | Comparability (/2) | Outcome (/3) | Overall methodological quality |
| Ichim et al[9], 2022 | 3 | 1 | 2 | Good |
| Minaga et al[10], 2021 | 2 | 1 | 1 | Poor |
| Takano et al[11], 2021 | 3 | 1 | 2 | Good |
| Ichim et al[12], 2020 | 3 | 1 | 2 | Good |
| Facciorusso et al[13], 2021 | 3 | 1 | 3 | Good |
| Chon et al[14], 2019 | 3 | 1 | 2 | Good |
| Akay et al[15], 2021 | 3 | 1 | 3 | Good |
| Chen et al[16], 2020 | 3 | 1 | 3 | Good |
| Hollerbach et al[17], 2003 | 3 | 1 | 2 | Good |
| Singh et al[18], 2007 | 2 | 1 | 2 | Fair |
| tenBerge et al[19], 2002 | 2 | 1 | 2 | Fair |
| Lee et al[20], 2015 | 3 | 2 | 1 | Poor |
| Oh et al[21], 2018 | 3 | 2 | 2 | Good |
| Singh et al[22], 2009 | 3 | 1 | 2 | Good |
| Okasha et al[23], 2023 | 3 | 1 | 2 | Good |
| Hasan et al[24], 2019 | 2 | 1 | 3 | Good |
| Bhogal et al[25], 2020 | 3 | 1 | 3 | Good |
| Diehl et al[26], 2015 | 2 | 1 | 2 | Fair |
| Sundaram et al[27], 2023 | 4 | 1 | 2 | Good |
| Saab et al[28], 2017 | 2 | 1 | 1 | Poor |
| Sey et al[29], 2016 | 3 | 2 | 1 | Poor |
| Shah et al[30], 2017 | 2 | 1 | 1 | Poor |
| Sisman et al[31], 2020 | 2 | 1 | 2 | Fair |
| Stavropoulos et al[32], 2012 | 3 | 2 | 1 | Poor |
| Zhang et al[33], 2021 | 3 | 1 | 2 | Good |
| Huang et al[34], 2017 | 3 | 1 | 2 | Good |
| Ogura et al[35], 2016 | 3 | 1 | 2 | Good |
| Tanikawa et al[36], 2023 | 3 | 1 | 2 | Good |
| Tonozuka et al[37], 2015 | 2 | 1 | 2 | Fair |
| Carbajo et al[38], 2019 | 3 | 1 | 2 | Good |
| Nakaji et al[39], 2016 | 3 | 1 | 2 | Good |
| Frost et al[40], 2018 | 2 | 1 | 2 | Fair |
| Bhat et al[41], 2016 | 3 | 1 | 2 | Good |
| Bick et al[42], 2019 | 3 | 1 | 2 | Good |
| Binmoeller et al[43], 2011 | 3 | 1 | 2 | Good |
| Bazarbashi et al[44], 2020 | 3 | 2 | 1 | Poor |
| Mukkada et al[45], 2018 | 3 | 1 | 2 | Good |
| Lee et al[46], 2000 | 2 | 2 | 2 | Fair |
| Gubler et al[47], 2014 | 2 | 1 | 2 | Fair |
| Kozieł et al[48], 2019 | 3 | 1 | 2 | Good |
| Romero-Castro et al[49], 2013 | 4 | 2 | 2 | Good |
Twenty-eight studies reported the diagnostic role of EUS, of which 16 evaluated its value in detecting FLL, 10 in detecting parenchymal liver diseases (PLD), and two in detecting portal hypertension. Furthermore, all the studies were conducted in individual countries (11 in the United States, 2 in Japan, 3 in Romania, 2 in Turkey, 3 in Korea, 1 in Italy, 1 in Germany, 1 in India, 2 in China, and 1 in Egypt; Table 2).
| Ref. | Study design | Study location | Participants characteristics | Diagnostic test | Outcomes | |||
| Sample | M/F | Age (yr) | Indication | |||||
| Ichim et al[9], 2022 | Single-arm observational study | Romania | 30 | 17/13 | 64.3 | FLL | EUS-FNA | Diagnostic accuracy: 97% |
| Complications: 1 patient | ||||||||
| Minaga et al[10], 2021 | Retrospective study | Japan | 426 | 248/178 | 69 (63–75) | FLL | CEH-EUS | Diagnostic accuracy: 98.4% |
| Takano et al[11], 2021 | Retrospective study | Japan | 106 | 60/46 | 68 (32–87) | FLL | EUS-FNA | Diagnostic accuracy: 96% |
| Complications: 1 patient | ||||||||
| Ichim et al[12], 2020 | Prospective study | Romania | 48 | 27/21 | 66.3 (40–83) | FLL | EUS-FNA | Diagnostic accuracy: 98% |
| Complications: None | ||||||||
| Facciorusso et al[13], 2021 | Retrospective study | Italy | 116 | 70/46 | NR | FLL | EUS-FNB | Diagnostic accuracy: 88.8% |
| Complications: None | ||||||||
| Chon et al[14], 2019 | Retrospective study | Korea | 58 | 35/23 | 68.1 (42–86) | FLL | EUS-FNB | Diagnostic accuracy: 89.7% |
| Complications: 1 patient | ||||||||
| Akay et al[15], 2021 | Retrospective study | Turkey | 25 | 15/10 | 62.73 ± 15.24 | FLL | EUS-FNA | Diagnostic accuracy: 86.3% |
| Complications: None | ||||||||
| Gheorghiu et al[50], 2022 | Prospective RCT | Romania | 30 | 21/9 | 60 (37–84) | FLL | EUS-FNA and EUS-FNB | Diagnostic accuracy: 100% and 86.7% for EUS-FNB and EUS-FNA, respectively |
| Complications: None | ||||||||
| Chen et al[16], 2020 | Retrospective study | China | 38 | 35/3 | 55.7 ± 11.8 | FLL | EUS-FNB | Diagnostic accuracy: 90% |
| Complications: 3 patients | ||||||||
| Hollerbach et al[17], 2003 | Prospective study | Germany | 41 | NR | 66 ± 7 | FLL | EUS-FNA | Diagnostic accuracy: 94% |
| Complications: 2 patients | ||||||||
| Singh et al[18], 2007 | Prospective study | United States | 17 | NR | 56 (43–85) | FLL | EUS and EUS-FNA | Diagnostic accuracy: 65% and 94% for EUS and EUS-FNA, respectively |
| Complications: None | ||||||||
| tenBerge et al[19], 2002 | Retrospective study | 26 | NR | NR | FLL | EUS-FNA | Diagnostic accuracy: 89% | |
| Complications: 6 patients | ||||||||
| Lee et al[20], 2015 | Retrospective study | Korea | 21 | 9/12 | 63 (37–81) | FLL | EUS-FNB | Diagnostic accuracy: 90.5% |
| Complications: None | ||||||||
| Oh et al[21], 2018 | Prospective study | Korea | 30 | 19/11 | 66.5 (55.5–74) | FLL | CEH-EUS and CEH-EUS-FNA | Diagnostic accuracy: 80% and 86.7% for CEH-EUS and CEH-EUS-FNA, respectively |
| Complications: None | ||||||||
| Singh et al[22], 2009 | Prospective study | United States | 131 | 128/3 | 67 (45–86) | FLL | EUS and EUS-FNA | Diagnostic accuracy: 97% and 98% for EUS and EUS-FNA, respectively |
| Complications: None | ||||||||
| Okasha et al[23], 2023 | Cross-sectional study | Egypt | 43 | 32/11 | 56 | FLL | EUS and EUS-FNA/FNB | Diagnostic accuracy: 94%, and 100% for EUS and EUS-FNA/FNB |
| Complications: None | ||||||||
| Ching-Companioni et al[51], 2019 | Prospective RCT | United States | 40 | NR | NR | PLD | EUS-FNA and EUS-FNB | Diagnostic accuracy: 100% |
| Complications: 13 patients | ||||||||
| Hasan et al[24], 2019 | Prospective study | United States | 40 | 14/26 | 61 (46.7–68.2) | PLD | EUS-FNB | Diagnostic accuracy: 100% |
| Complications: 9 patients | ||||||||
| Bhogal et al[25], 2020 | Retrospective study | United States | 513 | 244/269 | NR | PLD | EUS-FNA and EUS-FNB | Diagnostic accuracy: 99% |
| Diehl et al[26], 2015 | Prospective study | United States | 110 | 48/62 | 53 (9–87) | PLD | EUS-FNA | Diagnostic accuracy: 98% |
| Complications: 1 patient | ||||||||
| Sundaram et al[27], 2023 | Retrospective study | India | 74 | 37/37 | 44.5 (18–79) | PLD | EUS-FNA | Diagnostic accuracy: 97.3% |
| Complications: 5 patients | ||||||||
| Saab et al[28], 2017 | Retrospective study | United States | 47 | 16/31 | 54 | PLD | EUS-FNB | Diagnostic accuracy: 100% |
| Complications: 2 patients | ||||||||
| Sey et al[29], 2016 | Cross-sectional study | United States | 75 | 24/51 | 51 | PLD | EUS-FNB | Diagnostic accuracy: 82.7% |
| Complications: 2 patients | ||||||||
| Shah et al[30], 2017 | Retrospective study | United States | 24 | NR | NR | PLD | EUS-FNB | Diagnostic accuracy: 96% |
| Complications: 2 patients | ||||||||
| Sisman et al[31], 2020 | Retrospective study | Turkey | 40 | 24/16 | 44 (22–72) | PLD | EUS-FNB | Diagnostic accuracy: 100% |
| Complications: 2 patients | ||||||||
| Stavropoulos et al[32], 2012 | Prospective case series | United States | 22 | 6/16 | 61 (32–79) | PLD | EUS-FNA | Diagnostic accuracy: 91% |
| Complications: None | ||||||||
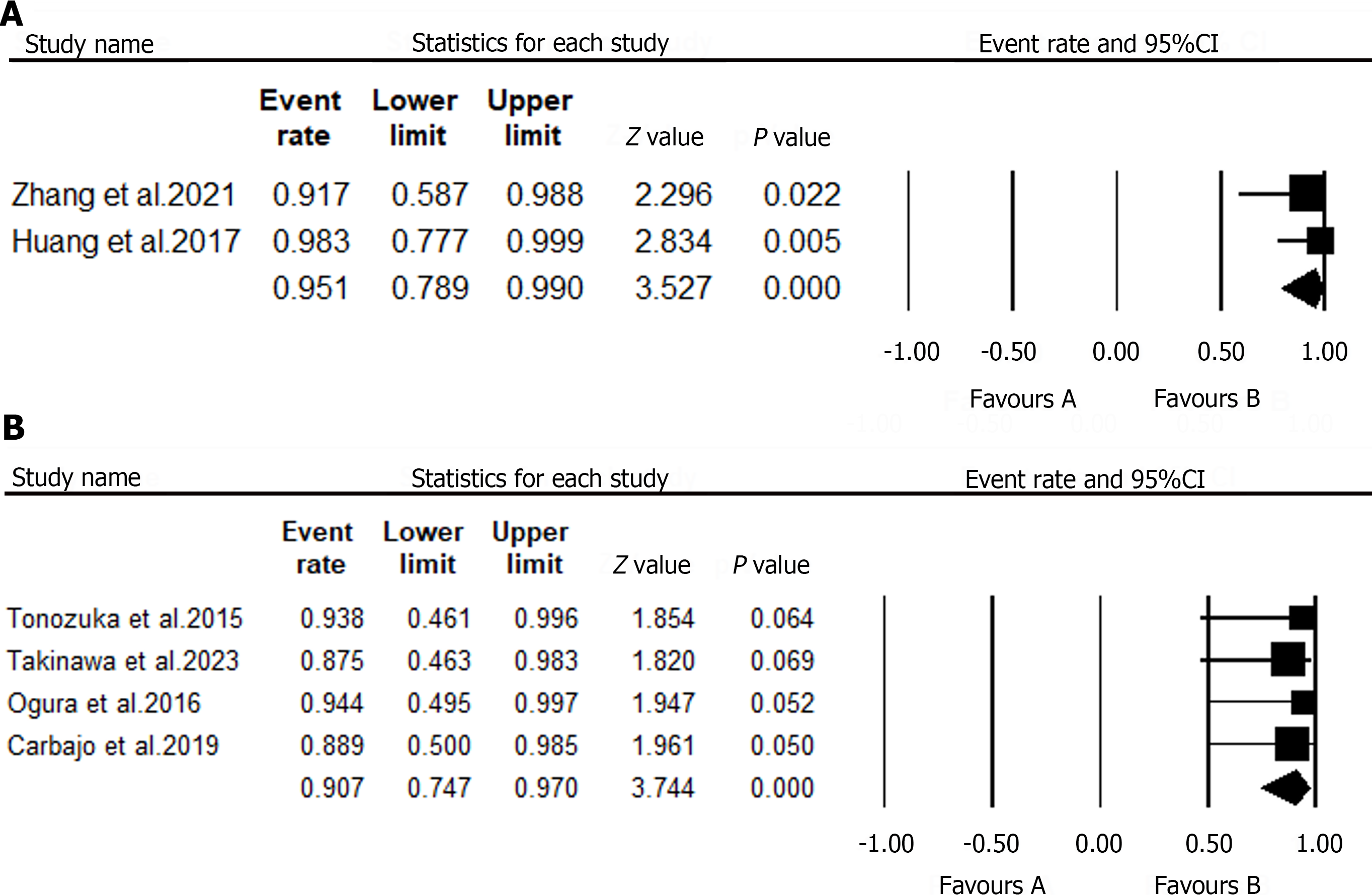
| Zhang et al[33], 2021 | Prospective study | China | 12 | 9/3 | NR | PH | EUS-PPG | Technical success rate: 91.7% |
| EUS-PPG correlates well with HVPG (r = 0.923) | ||||||||
| Complications: None | ||||||||
| Huang et al[34], 2017 | Prospective study | United States | 28 | 18/10 | 63 (30–80) | PH | EUS-PPG | Technical success rate: 100% |
| EUS-PPG correlates well with clinical parameters of PH | ||||||||
| Complications: None | ||||||||
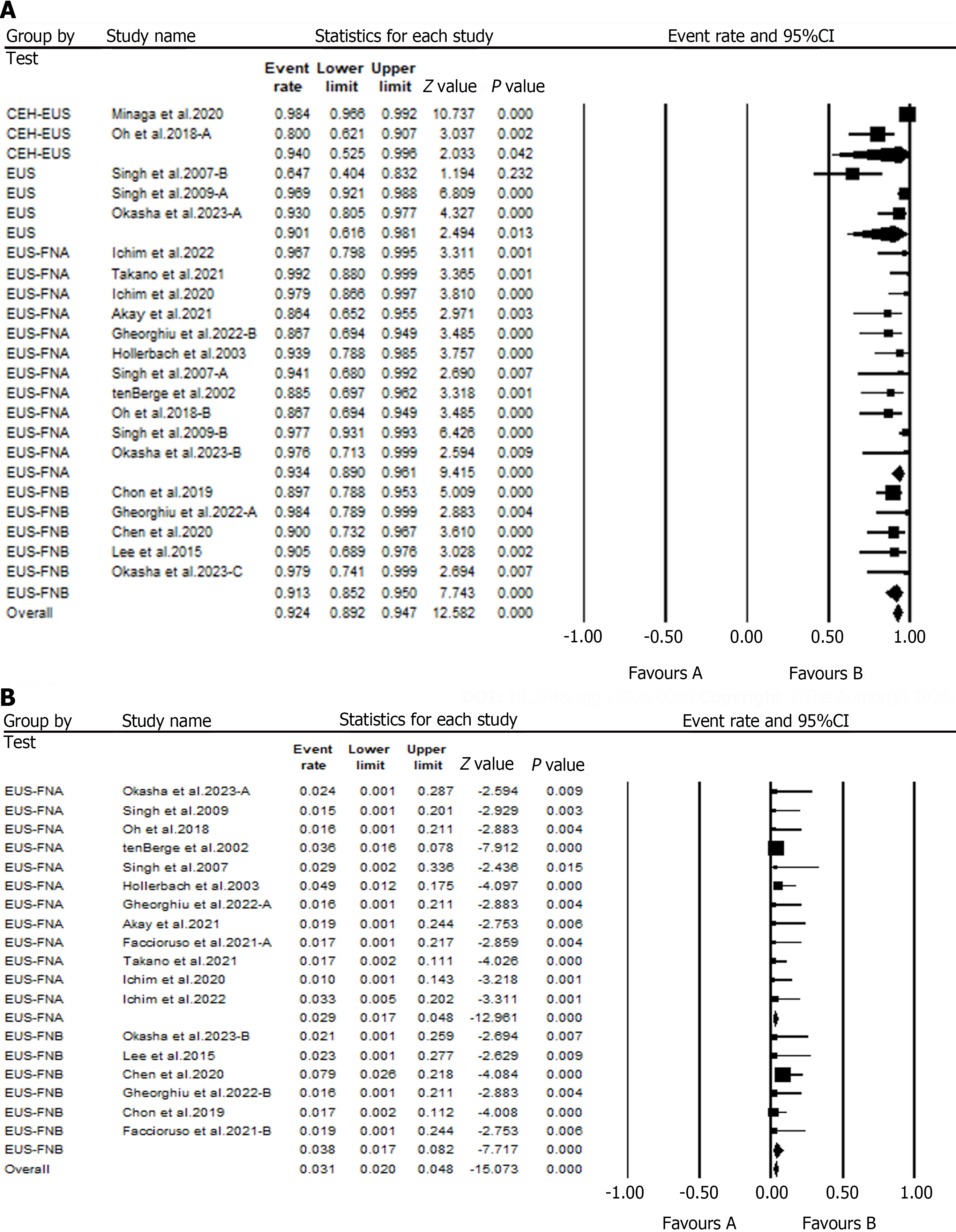
The cumulative analyses on the role of EUS in detecting FLL have shown an overall diagnostic accuracy rate of 92.4% (95%CI: 89.2 – 0.95). A subgroup analysis of the EUS diagnostic tests has shown that EUS alone had a diagnostic accuracy of 90.1%, whereas EUS-FNA and EUS-FNB had diagnostic accuracies of 93.4% and 98%, respectively. Furthermore, analysis of data from two studies has shown that Contrast-enhanced EUS (CEH-EUS) had a diagnostic accuracy of 94% for detecting FLL (Figure 3A).
Additionally, a safety analysis was performed to determine the safety of EUS-FNA and EUS-FNB in diagnosing FLL. Our subgroup analysis suggested that EUS-FNA had a complication rate of 2.9%, whereas the rate of complications when using EUS-FNA was 3.8% (Figure 3B).
Seventeen studies assessing the value of EUS in detecting parenchymal liver disease reported an overall diagnostic accuracy of 96.6%. A subgroup analysis of data from these studies showed that EUS-FNA had a diagnostic accuracy of 96.6%, whereas EUS-FNB had a diagnostic accuracy of 97.6% for the detection of PLD (Figure 4A). Furthermore, a safety evaluation of these diagnostic tests has shown complication rates of 6.2% and 9.6% for EUS-FNA and EUS-FNB, respectively (Figure 4B).
Although studies on the role of EUS in portal hypertension are limited, we to identify two human studies evaluating the efficacy of EUS-guided portal pressure gradient (PPG) measurements. A meta-analysis of data from these studies revealed that 40 patients underwent EUS-PPG, with a technical success rate of 95.1% (Figure 5A). No complications related to this procedure have been previously reported.
In the current review, the role of EUS in the treatment of liver diseases was reported in 17 studies. Four of these studies reported the efficacy of EUS-guided liver abscess drainage (EUS-AD), whereas two reported the value of EUS-guided interventions for the treatment of liver lesions. Additionally, 11 studies reported the therapeutic efficacy of various EUS-guided treatments of GV (Table 3).
| Ref. | Study design | Study location | Participant characteristics | Condition | Intervention | Outcomes | |
| Sample | M/F | ||||||
| Ogura et al[35], 2016 | Retrospective study | Japan | 27 | 20/7 | Liver abscess | EUS-AD | Clinical success: 100% |
| Technical success: 100% | |||||||
| Complications: None | |||||||
| Tanikawa et al[36], 2023 | Retrospective study | Japan | 8 | 4/4 | Liver abscess | EUS-AD | Clinical success: 87.5% |
| Technical success: 87.5% | |||||||
| Tonozuka et al[37], 2015 | Retrospective case series | Japan | NR | Liver abscess | EUS-AD | Clinical success: 100% | |
| Technical success: 100% | |||||||
| Complications: None | |||||||
| Carbajo et al[38], 2019 | Retrospective study | Spain | 9 | NR | Liver abscess | EUS-AD | Clinical success: 88.9% |
| Technical success: 88.9% | |||||||
| Nakaji et al[39], 2016 | Retrospective study | Japan | 12 | 10/2 | Solid liver lesions | EUS-guided ethanol injection | Complications: 2 |
| Overall survival: 91.7%, 75%, and 53.3% at 1, 2, and 3 years | |||||||
| Jiang et al[52], 2016 | Case series | China | 26 | 17/9 | Solid liver lesions | EUS-guided ethanol injection and iodine-125 brachytherapy | Complications: None |
| Frost et al[40], 2018 | Case series | Ireland | 8 | 7/1 | GV | EUS-guided thrombin injection | Complications: None |
| Obliteration: 75% | |||||||
| Rebleeding: 1 patient | |||||||
| Bhat et al[41], 2016 | Retrospective study | United States | 152 | 97/55 | GV | EUS-guided CYA and coil embolization | Technical success: 99% |
| Obliteration: 93% | |||||||
| Rebleeding: 20 patients | |||||||
| Complications: 9 patients | |||||||
| Bick et al[42], 2019 | Retrospective study | United States | 104 | 62/42 | GV | EUS-guided CYA | Obliteration: 79% |
| Rebleeding: 12 patients | |||||||
| Complications: 13 patients | |||||||
| Binmoeller et al[43], 2011 | Retrospective study | United States | 30 | 19/11 | GV | EUS-guided CYA and coil embolization | Technical success: 100% |
| Obliteration: 95.8% | |||||||
| Rebleeding: 4 patients | |||||||
| Complications: None | |||||||
| Bazarbashi et al[44], 2020 | Prospective study | United States | 40 | 27/13 | GV | EUS-Guided coil embolization | Technical success: 100% |
| Obliteration: 100% | |||||||
| Complications: 1 patient | |||||||
| Lôbo et al[53], 2019 | RCT | Brazil | 32 | 13/19 | GV | EUS-guided CYA and coil embolization | Complications: 13 patients |
| Obliteration: 93.3% | |||||||
| Mukkada et al[45], 2018 | Retrospective study | India | 30 | NR | GV | EUS-Guided coil embolization | Rebleeding: 6 patients |
| Lee et al[46], 2000 | Prospective study | China | 101 | 69/32 | GV | EUS-guided CYA | Obliteration: 79.6% |
| Complications: 22 patients | |||||||
| Rebleeding: 19 patients | |||||||
| Gubler et al[47], 2014 | Retrospective study | Switzerland | 40 | 25/15 | GV | EUS-guided CYA | Complications: 2 patients |
| Kozieł et al[48], 2019 | Retrospective study | Poland | 16 | 9/7 | GV | EUS-guided CYA and coil embolization | Technical success: 94% |
| Complications: 6 patients | |||||||
| Romero-Castro et al[49], 2013 | Retrospective study | Germany | 30 | 22/8 | GV | EUS-guided coil embolization | Obliteration: 90.9% |
| Complications: 1 patient | |||||||
| Rebleeding: None | |||||||
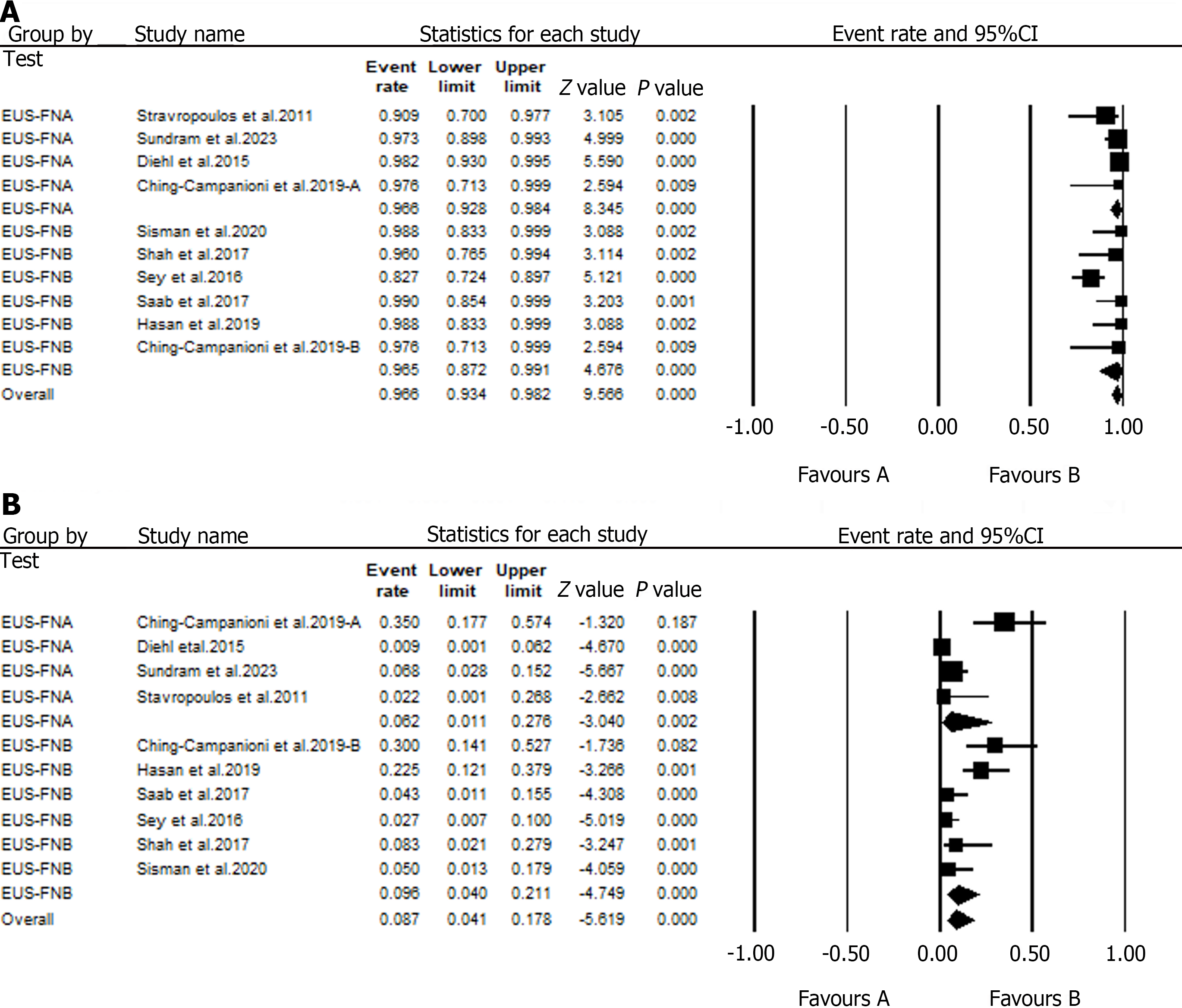
The efficacy of EUS-AD was reported in four studies[35-38]. A pooled analysis of data from these studies has shown that EUS-AD had a high technical (90.7%) and clinical success (90.7%; Figure 5B and C). Furthermore, two studies that included patients with hepatic abscesses reported that EUS-AD did not have any immediate or delayed complications.
The use of EUS to guide the treatment of FLL is a new and evolving field that has mostly been reported in case reports and animal studies. However, we identified two human studies[39,52] reporting the efficacy of EUS-guided interventions for solid liver lesions. Jiang et al[52] reported that EUS-guided therapy (ethanol injection, n = 10; iodine-125 seed brachytherapy, n = 13) was successful in most cases of left-sided liver tumors (23/25) without any procedure-related complications. Furthermore, complete tumor response was achieved in 65.2% of the patients, whereas partial response was achieved in 34.8%[52].
Nakaji et al[39] studied the efficacy of EUS-guided ethanol injections in the treatment of hepatocellular carcinoma (HCC). They found that the overall survival at 1, 2, and 3 years after the EUS-guided intervention was 91.7%, 75%, and 53.3%, respectively. Moreover, they reported two episodes of fever related to the procedure. However, no serious complications, such as intra-abdominal hemorrhage, abscesses, or bilomas were recorded[39].
The role of EUS in GV treatment has not yet been fully established and remains an area of investigation. Therefore, we evaluated the efficacy and safety of EUS-guided interventions [cyanoacrylate (CYA), coil embolization, thrombin, and a combination of CYA and coil embolization] for GV. The pooled analyses revealed that EUS-guided interventions had a technical success rate of 98%. In addition, the rate of complication, GV obliteration, and rebleeding events were 15%, 84%, and 17%, respectively. Subgroup analyses of individual EUS-guided interventions are presented in Table 4.
| Outcome | Cumulative analyses (95%CI) | Subgroup analyses (95%CI) | |||
| EUS-CYA | EUS-Coil | EUS-CYA + Coil | EUS-thrombin | ||
| Technical success | 0.98 (0.92–0.99) | NR | 0.96 (0.55–0.99) | 0.98 (0.92–0.99) | NR |
| Obliteration | 0.84 (0.79–0.88) | 0.78 (0.70–0.85) | 0.93 (0.71–0.99) | 0.93 (0.88–0.97) | 0.75 (0.38–0.94) |
| Complications | 0.15 (0.07–0.28) | 0.20 (0.07–0.44) | 0.10 (0.02–0.31) | 0.22 (0.04–0.69) | 0.06 (0.003–0.51) |
| Rebleeding | 0.17 (0.13–0.23) | 0.26 (0.13–0.49) | 0.08 (0.02–0.34) | 0.16 (0.11–0.23) | 0.13 (0.02–0.54) |
This systematic review and meta-analysis summarizes the evidence for the therapeutic and diagnostic roles of EUS in hepatic diseases. The pooled analysis showed that EUS is an effective and safe tool for the diagnosis of FLL, PLD, and portal hypertension. We found that EUS-guided interventions were effective and safe for the treatment of liver diseases.
Despite the establishment of transabdominal US, CT, and magnetic resonance imaging as diagnostic tools for liver diseases, the use of EUS as a diagnostic and therapeutic modality has increased considerably in recent years. In our analysis, we found that EUS-guided liver biopsy (FNA and FNB) for parenchymal liver disease had high diagnostic accuracy (96.6%) and low complication rates (8.7%). This finding is consistent with that reported in the first meta-analysis of nine studies published between 2009 and 2016[54]. According to that meta-analysis, EUS-liver biopsy (EUS-LB) had an overall diagnostic yield of 93.9% and a complication rate of 2.3%. Similarly, a more recent meta-analysis evaluating the efficacy and safety of EUS-LB in patients with parenchymal liver disease and FLL revealed that EUS-LB had a high diagnostic yield (95%) and low adverse event rate (3%)[55]. The evidence from these studies and our analysis suggests that EUS-LB may be a safer diagnostic alternative for PLD. However, our subgroup analysis has shown that adverse events were more prevalent when using FNB needles than FNA needles (9.6% vs 6.2%). Therefore, high-quality randomized trials are needed to evaluate the safety of EUS-FNA compared with EUS-FNB in the diagnosis of PLD.
EUS is also a valuable diagnostic tool for FLL. EUS can provide high-resolution images of the liver anatomy, enabling the identification and characterization of focal lesions. In our analyses, we found that EUS-guided biopsy had an overall diagnostic accuracy of 92.4% and a low complication rate (3.1%). This finding is consistent with a previous review article reporting that the diagnostic yield of EUS-guided biopsy of FLL ranges from 89.7% to 100%[7]. Furthermore, our subgroup analysis has shown that both EUS-FNA and EUS-FNB used in sampling FLL had excellent diagnostic accuracy (93.4% and 98%, respectively). However, a recent prospective trial found that a 22G EUS-FNB had significantly better diagnostic accuracy than a 22G EUS-FNA for FLL (100% vs 83.3%)[50]. However, these findings cannot be used independently to guide the clinical diagnosis of FLL owing to various limitations. First, the trial was carried out in a single center and had a limited number of patients, indicating that it is not representative of all FLL cases worldwide. Second, cytology was not performed on the EUS-FNA samples; thus, the diagnostic accuracy of EUS-FNA may have decreased. Finally, rapid on-site or macroscopic on-site evaluation was not conducted; hence, it is possible that the diagnostic accuracy decreased.
In addition, the use of CEH-EUS for FLL examination has gained interest. Owing to the dual blood supply to the liver, US contrast agents help examine the FLL in the arterial, portal, and venous phases. A pooled analysis of data from two studies in our review has shown that CEH-EUS achieved a diagnostic accuracy of 94% without any reported complications. Therefore, CEH-EUS has the potential to be integrated into daily clinical practice for the detection of suspected FLLs and for maximizing the management of these patients. However, further studies are required to confirm these findings.
EUS has several clinical applications in portal hypertension, including assessment of GV, assessment of collateral veins, and measurement of hemodynamic changes. It is also valuable for direct measurement of the PPG, which reflects the severity of portal hypertension and is an excellent prognostic factor in hepatic disease[56]. The two human studies[33,34] in the current review have shown that EUS can be used to guide the measurement of PPG, with a technical success rate of 95.1% and minimal complications. Zhang et al[33] observed a strong correlation between EUS-PPG using a 22G FNA needle and the hepatic venous pressure gradient (Pearson correlation, r = 0.93). Therefore, EUS is safe and has a potential significance in the management and understanding of portal hypertension. However, larger clinical trials are needed to confirm these findings.
In addition to its use as a diagnostic tool, EUS plays an important role in the treatment of liver diseases. Percutaneous drainage (PCD) is considered the first-line therapy for liver abscess drainage because it is minimally invasive and has a considerably high technical success[57,58]. However, this is disadvantageous because external drainage and self-tube removal may cause patient discomfort. Therefore, EUS-AD was developed to address these challenges. Although the efficacy of EUS-AD has largely been examined in case reports[59-65], we identified four small case series. The pooled analysis of data from these studies has shown that it has a high clinical (90.7%) and technical success rate (90.7%), and no major complications. This finding has been supported by a previous review that found that EUS-AD has a technical success rate of 97.5% for draining liver abscesses that are difficult to access[64]. Therefore, EUS-AD is a safe and viable intervention, especially for abscesses inaccessible by PCD.
EUS has also been used to treat FLL using various techniques. However, this is a relatively new and expanding field, with the majority of information obtained from case reports and animal research. In the present study, only two studies reported EUS-guided interventions for solid liver lesions. A case series by Jiang et al[52] reported that EUS-guided iodine-125 brachytherapy was a safer and more effective treatment modality than EUS-guided ethanol injection for refractory left-sided liver lesions[52]. However, this finding warrants further large-scale clinical trials and comparative studies. In contrast, Nakaji et al[39] revealed that EUS-guided ethanol injection may be an effective and safe treatment option for early-stage HCC located in the caudate lobe[39].
GV in portal hypertension and cirrhosis can be catastrophic if not managed appropriately. Currently, therapeutic methods for managing GV include medical techniques, endoscopic interventions, and interventional radiology-guided procedures, such as transjugular intrahepatic portosystemic shunt and balloon retrograde transvenous obliteration. However, in recent years, EUS-guided interventions, such as EUS-guided coil embolization, thrombin, and CYA injections, have gained interest. Our pooled analysis has shown that EUS-guided interventions for GV had high technical success (98%), high obliteration rates (84%), low complications (15%), and low rebleeding events (17%). Furthermore, the subgroup analysis revealed that EUS-guided coil embolization alone was associated with fewer complications than EUS-guided CYA alone (10% vs 20%, respectively). Additionally, we noticed that combining CYA with coil embolization was associated with improved technical success, obliteration rates, and complication rates compared to EUS-guided CYA alone.
Similar to other scientific research articles, our review has several limitations that should be considered when interpreting our findings. First, we observed high inter-study heterogeneity in our statistical analysis, which may be due to the varied and limited sample sizes. However, we used a random-effects model to account for this heterogeneity and obtained conservative results. Second, most studies included in the present research were conducted in single centers; hence, they are not entirely representative of the general population and community. Third, most studies were retrospective or prospective in nature, indicating that they were subject to selection and confounding biases. Finally, conference abstracts and articles published in different languages were eliminated, indicating that the data from these studies improved the scientific and statistical power of the meta-analysis.
EUS plays a significant role in the diagnosis and treatment of hepatic disorders. Notably, EUS-LB with FNA or FNB provides excellent diagnostic precision for FLL and PLD. Accumulated evidence indicates that EUS-FNB may be more effective than EUS-FNA for FLL diagnosis, and the addition of contrast enhancement can improve the diagnostic accuracy of EUS. However, these findings need extensive validation through larger clinical trials and comparative studies. EUS-guided interventions tend to be effective in the treatment of liver abscesses, GV, and FLL, with reduced complication risks. Nevertheless, the potential efficacy of EUS-guided interventions requires further large-scale randomized trials.
Endoscopic ultrasound (EUS) is a diagnostic and therapeutic procedure. The use of the EUS in the field of liver disease is recognizably increasing. However, the safety and efficacy are not well addressed.
We aimed to explore the safety and accuracy profile of the EUS in hepatology by comparing 28 articles evaluating the diagnostic role and 17 evaluating the therapeutic role of EUS.
To examine and explore the accuracy and efficacy of the role of the EUS in liver disease including the international aspects.
We independently conducted an extensive systematic review using an electronic search on PubMed, Medline, Cochrane Library, Web of Science, and Google Scholar databases were extensively scoured for studies until October 2023. The methodological quality of the eligible articles was performed using the Newcastle-Ottawa scale or Cochrane’s Risk of Bias tool. In addition, statistical analyses were performed with the comprehensive meta-analysis software.
The pooled analysis demonstrated that EUS diagnostic tests have an accuracy of 92.4% for focal liver lesions (FLL) and 96.6% for parenchymal liver diseases. In addition, the cumulative analyses showed that EUS-guided liver biopsies with either fine needle aspiration or fine needle biopsy have low complication rates when sampling FLL and parenchymal liver diseases (3.1% and 8.7%, respectively). Furthermore, analysis of data from four studies has shown that EUS-guided liver abscess has a high clinical (90.7%) and technical success (90.7%) without significant complications. Similarly, EUS-guided interventions for the treatment of gastric varices (GV) have a high technical success (98%) and GV obliteration rates (84%), with low complications (15%) and rebleeding events (17%).
The role of EUS in the liver disease is well established with promising accuracy and efficacy profile. We found that EUS-guided interventions are effective and safe in treating liver diseases.
EUS in liver diseases is a promising technique with the potential to be considered as a first-line therapeutic and diagnostic option in selected cases.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: British Society of Gastroenterology; United European Gastroenterology.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Saudi Arabia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang GX, China S-Editor: Li L L-Editor: A P-Editor: Cai YX
| 1. | Ryozawa S, Fujita N, Irisawa A, Hirooka Y, Mine T. Current status of interventional endoscopic ultrasound. Dig Endosc. 2017;29:559-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Saraireh HA, Bilal M, Singh S. Role of endoscopic ultrasound in liver disease: Where do we stand in 2017? World J Hepatol. 2017;9:1013-1021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Nguyen P, Feng JC, Chang KJ. Endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration (FNA) of liver lesions. Gastrointest Endosc. 1999;50:357-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 123] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Awad SS, Fagan S, Abudayyeh S, Karim N, Berger DH, Ayub K. Preoperative evaluation of hepatic lesions for the staging of hepatocellular and metastatic liver carcinoma using endoscopic ultrasonography. Am J Surg. 2002;184:601-4; discussion 604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Andanappa HK, Dai Q, Korimilli A, Panganamamula K, Friedenberg F, Miller L. Acoustic liver biopsy using endoscopic ultrasound. Dig Dis Sci. 2008;53:1078-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Choudhary N, Bansal RK, Puri R, Singh RR, Nasa M, Shah V, Sarin H, Guleria M, Saigal S, Saraf N, Sud R, Soin AS. Impact and safety of endoscopic ultrasound guided fine needle aspiration on patients with cirrhosis and pyrexia of unknown origin in India. Endosc Int Open. 2016;4:E953-E956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Sbeit W, Kadah A, Mahamid M, Pellicano R, Mari A, Khoury T. A State-of-the-Art Review on the Evolving Utility of Endoscopic Ultrasound in Liver Diseases Diagnosis. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46284] [Article Influence: 2103.8] [Reference Citation Analysis (3)] |
| 9. | Ichim VA, Chira RI, Nagy GA, Chira A, Mircea PA. Endoscopic Ultrasound-guided Biopsy of Liver Tumors. In Vivo. 2022;36:890-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Minaga K, Kitano M, Nakai A, Omoto S, Kamata K, Yamao K, Takenaka M, Tsurusaki M, Chikugo T, Matsumoto I, Chiba Y, Watanabe T, Kudo M. Improved detection of liver metastasis using Kupffer-phase imaging in contrast-enhanced harmonic EUS in patients with pancreatic cancer (with video). Gastrointest Endosc. 2021;93:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Takano Y, Noda J, Yamawaki M, Azami T, Kobayashi T, Niiya F, Maruoka N, Norose T, Ohike N, Wakabayashi T, Matsuo K, Tanaka K, Nagahama M. Comparative Study of an Ultrasound-guided Percutaneous Biopsy and Endoscopic Ultrasound-guided Fine-needle Aspiration for Liver Tumors. Intern Med. 2021;60:1657-1664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Ichim VA, Chira RI, Mircea PA, Nagy GA, Crisan D, Socaciu MA. Accuracy of endoscopic ultrasound-guided biopsy of focal liver lesions. Med Ultrason. 2020;22:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Facciorusso A, Ramai D, Conti Bellocchi MC, Bernardoni L, Manfrin E, Muscatiello N, Crinò SF. Diagnostic Yield of Endoscopic Ultrasound-Guided Liver Biopsy in Comparison to Percutaneous Liver Biopsy: A Two-Center Experience. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Chon HK, Yang HC, Choi KH, Kim TH. Endoscopic Ultrasound-Guided Liver Biopsy Using a Core Needle for Hepatic Solid Mass. Clin Endosc. 2019;52:340-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Akay E, Atasoy D, Altınkaya E, Koç A, Ertan T, Karaman H, Caglar E. Endoscopic Ultrasound-Guided Fine Needle Aspiration Using a 22-G Needle for Hepatic Lesions: Single-Center Experience. Clin Endosc. 2021;54:404-412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Chen F, Bao H, Deng Z, Zhao Q, Tian G, Jiang TA. Endoscopic ultrasound-guided sampling using core biopsy needle for diagnosis of left-lobe hepatocellular carcinoma in patients with underlying cirrhosis. J Cancer Res Ther. 2020;16:1100-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Hollerbach S, Willert J, Topalidis T, Reiser M, Schmiegel W. Endoscopic ultrasound-guided fine-needle aspiration biopsy of liver lesions: histological and cytological assessment. Endoscopy. 2003;35:743-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Singh P, Erickson RA, Mukhopadhyay P, Gopal S, Kiss A, Khan A, Ulf Westblom T. EUS for detection of the hepatocellular carcinoma: results of a prospective study. Gastrointest Endosc. 2007;66:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | tenBerge J, Hoffman BJ, Hawes RH, Van Enckevort C, Giovannini M, Erickson RA, Catalano MF, Fogel R, Mallery S, Faigel DO, Ferrari AP, Waxman I, Palazzo L, Ben-Menachem T, Jowell PS, McGrath KM, Kowalski TE, Nguyen CC, Wassef WY, Yamao K, Chak A, Greenwald BD, Woodward TA, Vilmann P, Sabbagh L, Wallace MB. EUS-guided fine needle aspiration of the liver: indications, yield, and safety based on an international survey of 167 cases. Gastrointest Endosc. 2002;55:859-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Lee YN, Moon JH, Kim HK, Choi HJ, Choi MH, Kim DC, Lee TH, Cha SW, Kim SG, Kim YS. Usefulness of endoscopic ultrasound-guided sampling using core biopsy needle as a percutaneous biopsy rescue for diagnosis of solid liver mass: Combined histological-cytological analysis. J Gastroenterol Hepatol. 2015;30:1161-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Oh D, Seo DW, Hong SM, Jun JH, Song TJ, Park DH, Son BK, Lee SS, Lee SK, Kim MH. The usefulness of contrast-enhanced harmonic EUS-guided fine-needle aspiration for evaluation of hepatic lesions (with video). Gastrointest Endosc. 2018;88:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Singh P, Mukhopadhyay P, Bhatt B, Patel T, Kiss A, Gupta R, Bhat S, Erickson RA. Endoscopic ultrasound vs CT scan for detection of the metastases to the liver: results of a prospective comparative study. J Clin Gastroenterol. 2009;43:367-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Okasha HH, Delsa H, Alsawaf A, Hashim AM, Khattab HM, Abdelfatah D, Abdellatef A, Albitar A. Role of endoscopic ultrasound and endoscopic ultrasound-guided tissue acquisition in diagnosing hepatic focal lesions. World J Methodol. 2023;13:287-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Reference Citation Analysis (2)] |
| 24. | Hasan MK, Kadkhodayan K, Idrisov E, Ali S, Rafiq E, Ben-Ami Shor D, Abdel-Jalil A, Navaneethan U, Bang J, Varadarajulu S, Hawes R, Pernicone P. Endoscopic ultrasound-guided liver biopsy using a 22-G fine needle biopsy needle: a prospective study. Endoscopy. 2019;51:818-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 25. | Bhogal N, Lamb B, Arbeiter B, Malik S, Sayles H, Lazenby AJ, Chandan S, Dhaliwal A, Singh S, Bhat I. Safety and adequacy of endoscopic ultrasound-guided random liver biopsy in comparison with transjugular and percutaneous approaches. Endosc Int Open. 2020;8:E1850-E1854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Diehl DL, Johal AS, Khara HS, Stavropoulos SN, Al-Haddad M, Ramesh J, Varadarajulu S, Aslanian H, Gordon SR, Shieh FK, Pineda-Bonilla JJ, Dunkelberger T, Gondim DD, Chen EZ. Endoscopic ultrasound-guided liver biopsy: a multicenter experience. Endosc Int Open. 2015;3:E210-E215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 27. | Sundaram S, Shah B, Jagtap N, Angadi S, Jain AK, Afzalpurkar S, Giri S. Diagnostic efficacy of endoscopic ultrasound-guided liver biopsy for diffuse liver diseases and its predictors - a multicentric retrospective analysis. Clin Exp Hepatol. 2023;9:243-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 28. | Saab S, Phan J, Jimenez MA, Grotts JF, Walters L, Hathaway KA, Patel KR, Lankarani A, Herman M, Holloman DA, Nieto JM. Endoscopic Ultrasound Liver Biopsies Accurately Predict the Presence of Fibrosis in Patients With Fatty liver. Clin Gastroenterol Hepatol. 2017;15:1477-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Sey MS, Al-Haddad M, Imperiale TF, McGreevy K, Lin J, DeWitt JM. EUS-guided liver biopsy for parenchymal disease: a comparison of diagnostic yield between two core biopsy needles. Gastrointest Endosc. 2016;83:347-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Shah ND, Sasatomi E, Baron TH. Endoscopic Ultrasound-guided Parenchymal Liver Biopsy: Single Center Experience of a New Dedicated Core Needle. Clin Gastroenterol Hepatol. 2017;15:784-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Sisman G, Barbur E, Saka D, Piyade B, Besli S, Boynukara C, Kirimlioglu H. Endoscopic ultrasound-guided liver biopsy using a 20-gauge fine needle biopsy needle with the wet-heparinized suction technique. Eur J Gastroenterol Hepatol. 2020;32:1470-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Stavropoulos SN, Im GY, Jlayer Z, Harris MD, Pitea TC, Turi GK, Malet PF, Friedel DM, Grendell JH. High yield of same-session EUS-guided liver biopsy by 19-gauge FNA needle in patients undergoing EUS to exclude biliary obstruction. Gastrointest Endosc. 2012;75:310-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 33. | Zhang W, Peng C, Zhang S, Huang S, Shen S, Xu G, Zhang F, Xiao J, Zhang M, Zhuge Y, Wang L, Zou X, Lv Y. EUS-guided portal pressure gradient measurement in patients with acute or subacute portal hypertension. Gastrointest Endosc. 2021;93:565-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 34. | Huang JY, Samarasena JB, Tsujino T, Lee J, Hu KQ, McLaren CE, Chen WP, Chang KJ. EUS-guided portal pressure gradient measurement with a simple novel device: a human pilot study. Gastrointest Endosc. 2017;85:996-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 35. | Ogura T, Masuda D, Saori O, Wataru T, Sano T, Okuda A, Miyano A, Kitano M, Abdel-Aal UM, Takeuchi T, Fukunishi S, Higuchi K. Clinical Outcome of Endoscopic Ultrasound-Guided Liver Abscess Drainage Using Self-Expandable Covered Metallic Stent (with Video). Dig Dis Sci. 2016;61:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | Tanikawa T, Kawada M, Ishii K, Urata N, Nishino K, Suehiro M, Kawanaka M, Haruma K, Kawamoto H. Efficacy of endoscopic ultrasound-guided abscess drainage for non-pancreatic abscesses: A retrospective study. JGH Open. 2023;7:470-475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 37. | Tonozuka R, Itoi T, Tsuchiya T, Sofuni A, Ishii K, Ikeuchi N, Umeda J, Tanaka R, Mukai S, Gotoda T, Moriyasu F. EUS-guided drainage of hepatic abscess and infected biloma using short and long metal stents (with videos). Gastrointest Endosc. 2015;81:1463-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 38. | Carbajo AY, Brunie Vegas FJ, García-Alonso FJ, Cimavilla M, Torres Yuste R, Gil-Simón P, de la Serna-Higuera C, Fernández Pérez GC, Pérez-Miranda M. Retrospective cohort study comparing endoscopic ultrasound-guided and percutaneous drainage of upper abdominal abscesses. Dig Endosc. 2019;31:431-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Nakaji S, Hirata N, Mikata R, Kobayashi M, Shiratori T, Ogasawara S, Ooka Y, Tsuyuguchi T, Yamaguchi T, Yokosuka O. Clinical outcomes of endoscopic ultrasound-guided ethanol injection for hepatocellular carcinoma in the caudate lobe. Endosc Int Open. 2016;4:E1111-E1115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Frost JW, Hebbar S. EUS-guided thrombin injection for management of gastric fundal varices. Endosc Int Open. 2018;6:E664-E668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Bhat YM, Weilert F, Fredrick RT, Kane SD, Shah JN, Hamerski CM, Binmoeller KF. EUS-guided treatment of gastric fundal varices with combined injection of coils and cyanoacrylate glue: a large U.S. experience over 6 years (with video). Gastrointest Endosc. 2016;83:1164-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 161] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 42. | Bick BL, Al-Haddad M, Liangpunsakul S, Ghabril MS, DeWitt JM. EUS-guided fine needle injection is superior to direct endoscopic injection of 2-octyl cyanoacrylate for the treatment of gastric variceal bleeding. Surg Endosc. 2019;33:1837-1845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 43. | Binmoeller KF, Weilert F, Shah JN, Kim J. EUS-guided transesophageal treatment of gastric fundal varices with combined coiling and cyanoacrylate glue injection (with videos). Gastrointest Endosc. 2011;74:1019-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 44. | Bazarbashi AN, Wang TJ, Jirapinyo P, Thompson CC, Ryou M. Endoscopic Ultrasound-Guided Coil Embolization With Absorbable Gelatin Sponge Appears Superior to Traditional Cyanoacrylate Injection for the Treatment of Gastric Varices. Clin Transl Gastroenterol. 2020;11:e00175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 45. | Mukkada RJ, Antony R, Chooracken MJ, Francis JV, Chettupuzha AP, Mathew PG, Augustine P, Koshy A. Endoscopic ultrasound-guided coil or glue injection in post-cyanoacrylate gastric variceal re-bleed. Indian J Gastroenterol. 2018;37:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Lee YT, Chan FK, Ng EK, Leung VK, Law KB, Yung MY, Chung SC, Sung JJ. EUS-guided injection of cyanoacrylate for bleeding gastric varices. Gastrointest Endosc. 2000;52:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 128] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 47. | Gubler C, Bauerfeind P. Safe and successful endoscopic initial treatment and long-term eradication of gastric varices by endoscopic ultrasound-guided Histoacryl (N-butyl-2-cyanoacrylate) injection. Scand J Gastroenterol. 2014;49:1136-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 48. | Kozieł S, Pawlak K, Błaszczyk Ł, Jagielski M, Wiechowska-Kozłowska A. Endoscopic Ultrasound-Guided Treatment of Gastric Varices Using Coils and Cyanoacrylate Glue Injections: Results after 1 Year of Experience. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 49. | Romero-Castro R, Ellrichmann M, Ortiz-Moyano C, Subtil-Inigo JC, Junquera-Florez F, Gornals JB, Repiso-Ortega A, Vila-Costas J, Marcos-Sanchez F, Muñoz-Navas M, Romero-Gomez M, Brullet-Benedi E, Romero-Vazquez J, Caunedo-Alvarez A, Pellicer-Bautista F, Herrerias-Gutierrez JM, Fritscher-Ravens A. EUS-guided coil vs cyanoacrylate therapy for the treatment of gastric varices: a multicenter study (with videos). Gastrointest Endosc. 2013;78:711-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 50. | Gheorghiu M, Seicean A, Bolboacă SD, Rusu I, Seicean R, Pojoga C, Moșteanu O, Sparchez Z. Endoscopic Ultrasound-Guided Fine-Needle Biopsy vs Fine-Needle Aspiration in the Diagnosis of Focal Liver Lesions: Prospective Head-to-Head Comparison. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Reference Citation Analysis (0)] |
| 51. | Ching-Companioni RA, Diehl DL, Johal AS, Confer BD, Khara HS. 19 G aspiration needle vs 19 G core biopsy needle for endoscopic ultrasound-guided liver biopsy: a prospective randomized trial. Endoscopy. 2019;51:1059-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 52. | Jiang TA, Deng Z, Tian G, Zhao QY, Wang WL. Efficacy and safety of endoscopic ultrasonography-guided interventional treatment for refractory malignant left-sided liver tumors: a case series of 26 patients. Sci Rep. 2016;6:36098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 53. | Lôbo MRA, Chaves DM, DE Moura DTH, Ribeiro IB, Ikari E, DE Moura EGH. Safety and efficacy of EUS-guided coil plus cyanoacrylate versus conventional cyanoacrylate technique in the treatment of gastric varices: a randomized controlled trial. Arq Gastroenterol. 2019;56:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 54. | Mohan BP, Shakhatreh M, Garg R, Ponnada S, Adler DG. Efficacy and safety of EUS-guided liver biopsy: a systematic review and meta-analysis. Gastrointest Endosc. 2019;89:238-246.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 55. | Zeng K, Jiang Z, Yang J, Chen K, Lu Q. Role of endoscopic ultrasound-guided liver biopsy: a meta-analysis. Scand J Gastroenterol. 2022;57:545-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 56. | Campos S, Poley JW, van Driel L, Bruno MJ. The role of EUS in diagnosis and treatment of liver disorders. Endosc Int Open. 2019;7:E1262-E1275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 57. | Bertel CK, van Heerden JA, Sheedy PF 2nd. Treatment of pyogenic hepatic abscesses. Surgical vs percutaneous drainage. Arch Surg. 1986;121:554-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 83] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 58. | Cai YL, Xiong XZ, Lu J, Cheng Y, Yang C, Lin YX, Zhang J, Cheng NS. Percutaneous needle aspiration vs catheter drainage in the management of liver abscess: a systematic review and meta-analysis. HPB (Oxford). 2015;17:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 59. | Noh SH, Park DH, Kim YR, Chun Y, Lee HC, Lee SO, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided drainage of hepatic abscesses not accessible to percutaneous drainage (with videos). Gastrointest Endosc. 2010;71:1314-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 60. | Seewald S, Imazu H, Omar S, Groth S, Seitz U, Brand B, Zhong Y, Sikka S, Thonke F, Soehendra N. EUS-guided drainage of hepatic abscess. Gastrointest Endosc. 2005;61:495-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 61. | Itoi T, Ang TL, Seewald S, Tsuji S, Kurihara T, Tanaka R, Itokawa F. Endoscopic ultrasonography-guided drainage for tuberculous liver abscess drainage. Dig Endosc. 2011;23 Suppl 1:158-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 62. | Kumta NA, Torres-Ruiz F, Reinoso PJ, Kahaleh M. Endoscopic management of hepatic abscess after EUS-guided hepaticogastrostomy. Gastrointest Endosc. 2016;84:1054-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 63. | Gadour E, Hassan Z. Post-orthotopic liver transplant cholangiopathy assessment and surveillance with endoscopic ultrasonography: the way forward. Int J Innov Res Med Sci. 2023;8:269-278. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 64. | Chin YK, Asokkumar R. Endoscopic ultrasound-guided drainage of difficult-to-access liver abscesses. SAGE Open Med. 2020;8:2050312120921273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 65. | Okasha HH, Wifi MN, Awad A, Abdelfatah Y, Abdelfatah D, El-Sawy SS, Alzamzamy A, Abou-Elenin S, Abou-Elmagd A, ElHusseiny R, Wahba M, El-Feki MA, Pawlak KM. Role of EUS in detection of liver metastasis not seen by computed tomography or magnetic resonance imaging during staging of pancreatic, gastrointestinal, and thoracic malignancies. Endosc Ultrasound. 2021;10:344-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |