Published online Feb 21, 2024. doi: 10.3748/wjg.v30.i7.705
Peer-review started: August 25, 2023
First decision: November 20, 2023
Revised: December 18, 2023
Accepted: January 23, 2024
Article in press: January 23, 2024
Published online: February 21, 2024
Processing time: 179 Days and 16.7 Hours
The detection rate of peptic ulcer in children is improving, with development of diagnostic procedures. Gastroscopy is the gold standard for the diagnosis of peptic ulcer, but it is an invasive procedure. Gastrointestinal contrast-enhanced ultrasonography (CEUS) has the advantages of being painless, noninvasive, nonradioactive, easy to use, and safe.
To investigate the clinical value of CEUS for diagnosis and treatment of peptic ulcer in children.
We investigated 43 children with digestive tract symptoms in our hospital from January 2021 to June 2022. All children were examined by routine ultrasound, gastrointestinal CEUS, and gastroscopy. The pathological results of gastroscopy were taken as the gold standard. Routine ultrasonography was performed before gastrointestinal CEUS. Conventional ultrasound showed the thickness of the gastroduodenal wall, gastric peristalsis, and the adjacent organs and tissues around the abdominal cavity. Gastrointestinal CEUS recorded the thickness of the gastroduodenal wall; the size, location and shape of the ulcer; gastric peristalsis; and adjacent organs and tissues around the abdominal cavity. The results of routine ultrasound and gastrointestinal ultrasound were compared with those of gastroscopy to evaluate the diagnostic results and coincidence rate of routine ultrasound and gastrointestinal CEUS. All children received informed consent from their guardians for CEUS. This study was reviewed and approved by the hospital medical ethics committee.
Among the 43 children, 17 (15 male, 2 female) were diagnosed with peptic ulcer by gastroscopy. There were 26 children with nonpeptic ulcer. There were eight cases of peptic ulcer and 35 of nonpeptic ulcer diagnosed by conventional ultrasound. The diagnostic coincidence rate of peptic ulcer in children diagnosed by conventional ultrasound was 79.1% (34/43), which was significantly different from that of gastroscopy (P = 0.033). It indicates that the coincidence rate of gastrointestinal contrast-enhanced ultrasound and gastroscope is low. Fifteen cases of peptic ulcer and 28 of nonpeptic ulcer were diagnosed by CEUS. The diagnostic coincidence rate of peptic ulcer in children was 95.3% (41/43). There was no significant difference between CEUS and gastroscopy (P = 0.655). It indicates that the coincidence rate of gastrointestinal contrast-enhanced ultrasound and gastroscope is high.
Gastrointestinal CEUS has a high coincidence rate in the diagnosis of peptic ulcer in children, and can be used as a preliminary examination method.
Core Tip: In this study, routine gastrointestinal ultrasound and contrast-enhanced ultrasonography (CEUS) in children were compared with gastroscopy. The clinical coincidence rate between gastrointestinal CEUS and gastroscopy was higher, which provided a new examination method for pediatricians to screen upper gastrointestinal diseases. This method is painless, noninvasive, nonradioactive, simple to operate, accepted by children and parents, and can be used as a preliminary screening method for children with epigastric pain. It is expected to be an effective supplement to gastroscopy and provide a reference for clinical selection of appropriate treatment.
- Citation: Zhang YH, Xu ZH, Ni SS, Luo HX. Gastrointestinal contrast-enhanced ultrasonography for diagnosis and treatment of peptic ulcer in children. World J Gastroenterol 2024; 30(7): 705-713
- URL: https://www.wjgnet.com/1007-9327/full/v30/i7/705.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i7.705
Diseases of the digestive system are common in childhood, and peptic ulcer is also common in clinical practice. However, the clinical symptoms of peptic ulcer in children are not typical and there is a lack of specific symptoms and signs in the early stage. Children cannot accurately express conscious symptoms or it is difficult to accurately describe the location and nature of the symptoms, resulting in missed diagnosis or misdiagnosis of peptic ulcer. Gastroscopy can directly observe the gastric and duodenal mucosa and the degree of pathological changes, which is the gold standard for diagnosis of gastrointestinal diseases[1]. However, as gastroscopy is an invasive method, clinicians and parents have some concerns about whether children can tolerate the examination process, and its safety[2]. At present, with the improvement of ultrasonic image resolution and the continuous improvement and development of ultrasonic diagnostic technology, contrast-enhanced ultrasonography (CEUS) is safe, simple and noninvasive, which makes the acceptance of patients higher[3].
This was a analysis of 43 children with digestive tract symptoms treated in our hospital from January 2021 to June 2022, to explore the clinical diagnostic value of routine ultrasound and gastrointestinal CEUS in children with peptic ulcer.
We investigated 43 children with gastrointestinal symptoms in our hospital from January 2021 to June 2022. All patients were examined by routine ultrasound, gastrointestinal CEUS, and gastroscopy. Eight patients (all male) with peptic ulcer were diagnosed by routine ultrasound, The age was 8-15 years, with an average of 10.8 ± 2.5 years. Fifteen patients (13 male, 2 female) with peptic ulcer were diagnosed by gastrointestinal CEUS. The age was 8-15 years, with an average of 11.4 ± 2.3 years. The above cases all had different degrees of upper gastrointestinal symptoms, such as epigastric fullness, nausea, vomiting, and epigastric pain. Some children showed periodic epigastric pain, empty abdominal pain or nocturnal pain, intermittent black stools, and anemia. The above children were compared with those who were examined by gastroscopy.
The gastroscopy method was based on the consensus of experts on gastroscopy and colonoscopy for children in Europe[4].
Color Doppler ultrasound was performed using a Philips EPIQ7 color Doppler ultrasound diagnostic instrument (Philips, Netherlands), L12-5 Linear array probe, at a frequency of 5-12 MHz. The patients ate a light diet the day before the inspection, and avoided food that can produce gas and is not easy to digest. Patients fasted for 8 h and refrained from drinking for > 4 h before examination. Contrast agent produced by Huqingtang (Hangzhou, China) was chosen. Before taking the contrast agent, the contraindications such as gastrointestinal perforation, acute gastric dilatation and intestinal obstruction were eliminated by whole abdominal scan. The contrast agent was added to 150-200 mL water at 35-45 °C, stirred well, and hot water at > 90 °C was added to make 400-500 mL. This was stirred again and set aside. After cooling to a suitable temperature, the patient took the fluid orally. The dose depended on the age of the child: 3-10 years, 200-400 mL; 10-15 years, 400-500 mL.
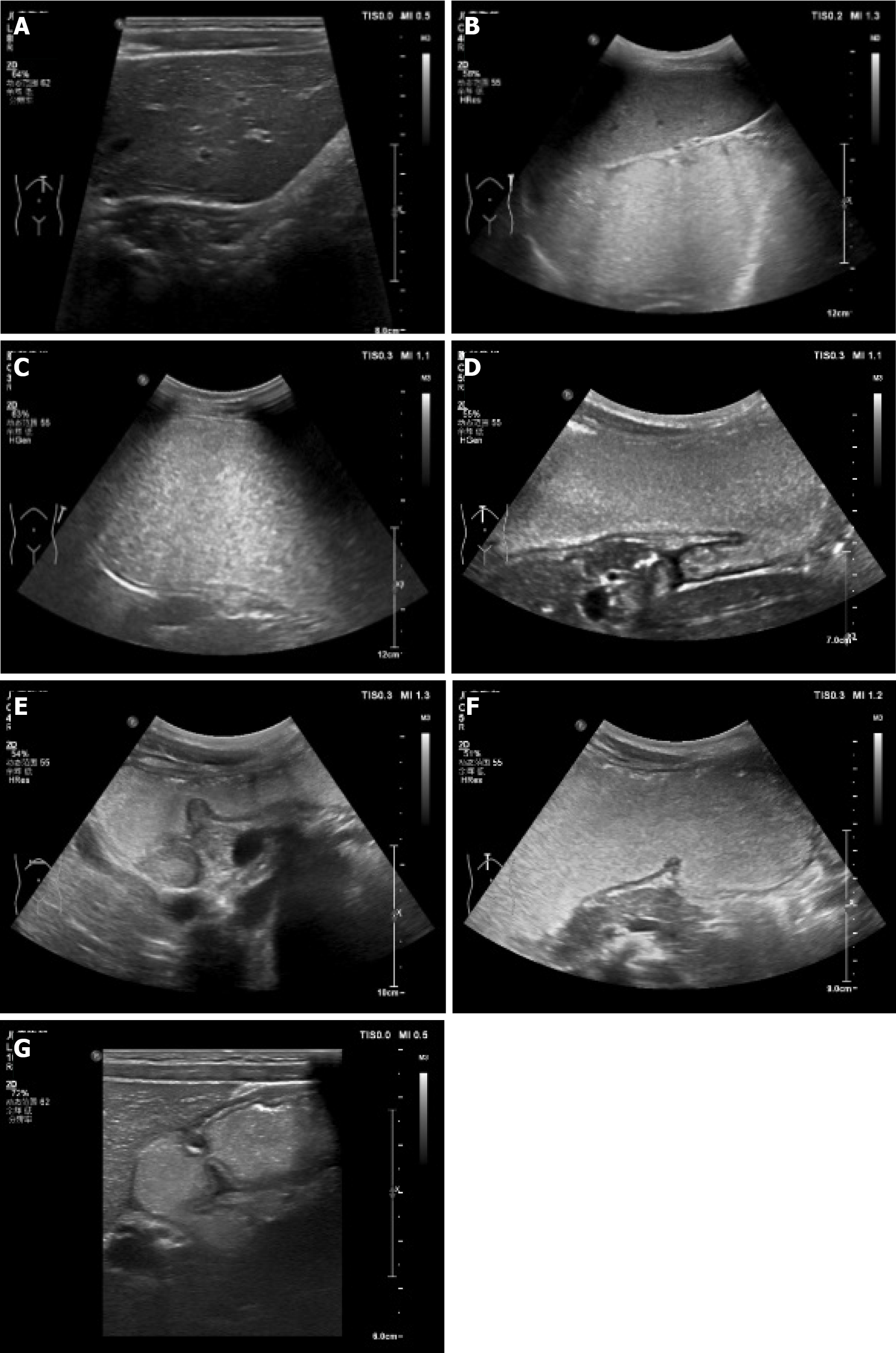
CEUS was performed by the same ultrasound physician on the same machine, and they did not know the pathological results of the child before the examination. Mainly in sitting, supine and right supine positions, a series of vertical and transverse and oblique scans were performed in the left middle and upper abdomen. There were the following scanning sections: (1) Cardia and lower esophagus (Figure 1A). The probe was placed obliquely under the left costal region near the xiphoid process and rotated to the left and rear to obtain the long-axis sonogram of the lower esophagus and cardia, and then the cross-exchange scan was performed to obtain the short-axis section and sonogram of the cardia and lower esophagus; (2) gastric fundus (Figure 1B). The probe was tilted to the left quarter rib and rotated to the left, posterior and upper, with an angle range of 0–80°. This section showed the fundus sonogram more completely; (3) gastric body (Figure 1C). The long axis of the gastric body can be displayed when the probe is positioned longitudinally on the left upper abdomen, and the short axis of the gastric body can be displayed when the probe is moved horizontally on the left upper abdomen; (4) gastric angle (Figure 1D). The probe was placed horizontally on the abdomen and scanned contin
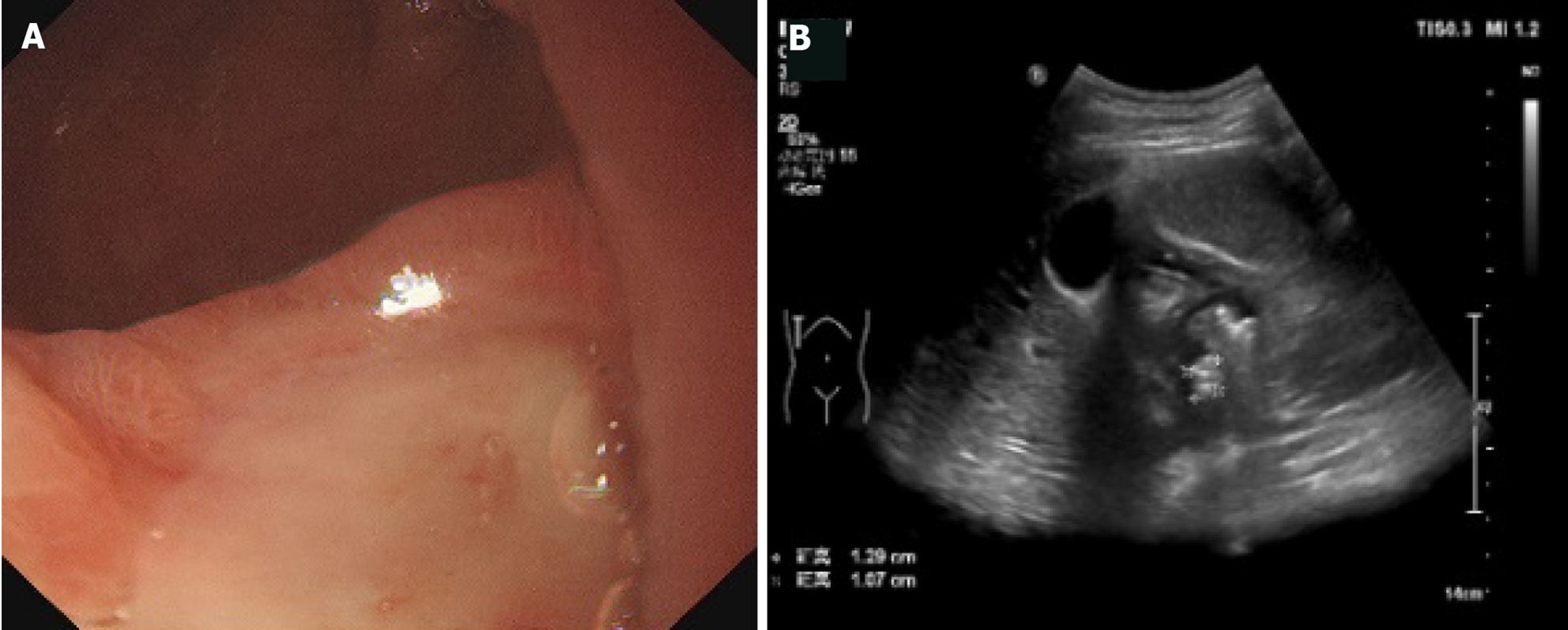
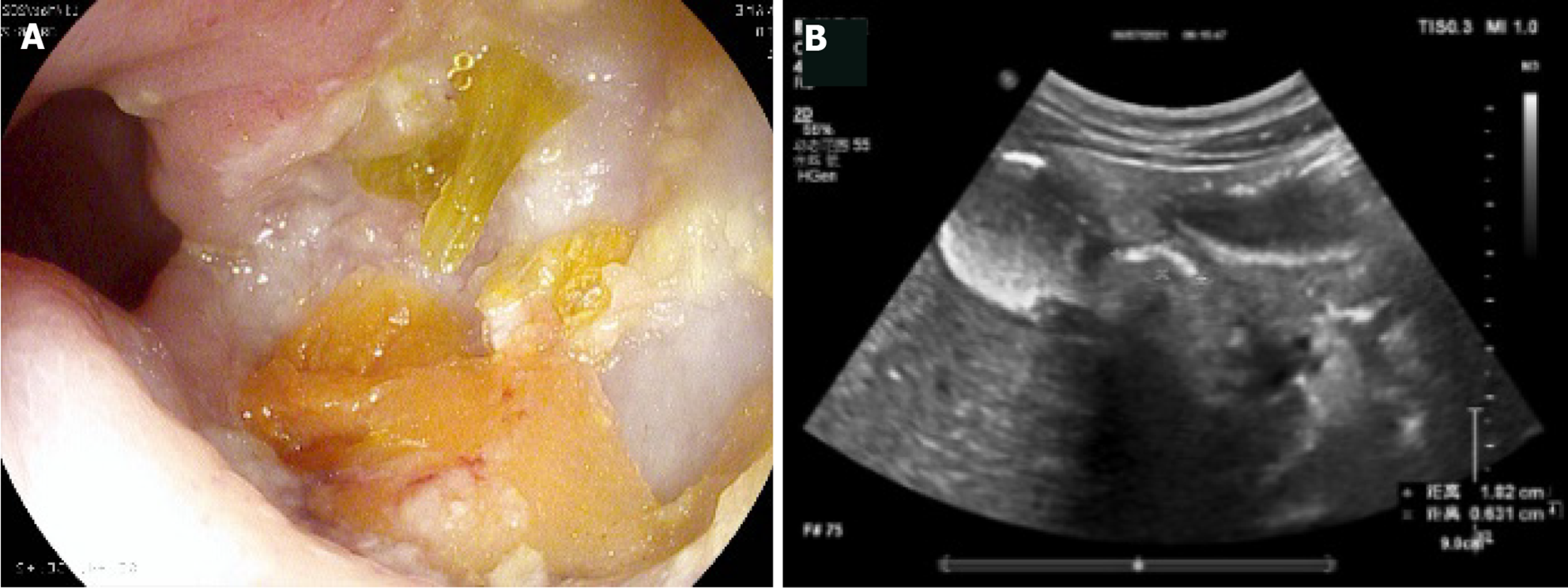
We observed local thickening of the gastric wall, and interruption and depression of the gastric mucosa at the bottom of the ulcer. The diameter of the ulcer was 5-10 mm, the shape was fairly regular, the edge was symmetrical and slightly raised, and it had a crater-like appearance. The thickened gastric wall at the base of the depression and around it showed low echo. The concave surface of the mucous membrane was flat. Peptic ulcer showed speckled hyperecho under CEUS. The local peristalsis of the gastric wall was weakened or disappeared. Duodenal bulbar ulcer showed localized thickening of the intestinal wall, deformed bulb, poor fluid filling, localized depression on the surface of duodenal bulbar ulcer with a diameter of 10 mm, and strong echo spots on the surface. These are typical CEUS and gastroscopy findings of peptic ulcer (Figures 2 and 3).
SPSS version 16.0 was used for statistical analysis. The numerical data (diagnostic results, coincidence rate, and diagnostic accuracy) were tested by χ2 test and expressed as percentages. P < 0. 05 indicated a significant difference.
Seventeen cases (15 male, 2 female) of peptic ulcer were diagnosed by gastroscopy. Fifteen children were positive for Helicobacter pylori (H. pylori) antibody and had different degrees of abdominal pain. Hemoglobin decreased by varying degrees in laboratory examination. Hemoglobin level was 51-105 g/L, with an average of 82.1 ± 13.8 g/L. There were also 26 cases of nonpeptic ulcer.
Routine ultrasound diagnosed eight cases of peptic ulcer, 35 cases of nonpeptic ulcer and there was nine missed diagnoses. The results are compared with gastroscopy in Table 1.
| Method (%) | Total | χ2 | P value | |||
| Routine ultrasound | Gastroscopy | |||||
| Group | Positive | 8 (18.60) | 17 (39.53) | 25 (29.07) | 4.568 | 0.033a |
| Negative | 35 (81.40) | 26 (60.47) | 61 (70.93) | |||
| Total | 43 | 43 | 86 | |||
Gastrointestinal CEUS diagnosed 15 cases of peptic ulcer, 28 cases of nonpeptic ulcer, and there were two missed diagnoses. The results are compared with gastroscopy in Table 2.
| Method (%) | Total | χ2 | P value | |||
| Gastrointestinal contrast-enhanced ultrasound | Gastroscopy | |||||
| Group | Positive | 15 (34.88) | 17 (39.53) | 32 (37.21) | 0.199 | 0.655 |
| Negative | 28 (65.12) | 26 (60.47) | 54 (62.79) | |||
| Total | 43 | 43 | 86 | |||
Peptic ulcer refers to chronic ulcer in the stomach and duodenum. It is a common disease in adults that is mainly caused by the erosion of gastrointestinal mucosa by gastric acid and pepsin. In the past, it was considered that peptic ulcer was rare in children, but the development of diagnostic procedures has increased detection rates continuously. The lifetime prevalence rate of peptic ulcer in the general population is 5%-10%, with an annual incidence rate of 0.1%-0.3%[5]. Peptic ulcer in children is caused by many factors. The common cause is H. pylori infection. Oral and fecal transmission is the main route. H. pylori infection is mainly acquired in childhood, and adults can transmit H. pylori to children. H. pylori infection is still highly prevalent in children and adolescents globally[6]. In this study, the number of children with H. pylori infection was 88%. Overseas studies have also shown that H. pylori is the main cause of peptic ulcer, and > 75% of duodenal ulcers and > 17% of gastric ulcers are associated with this infection[7]. In children with H. pylori-infected duodenal ulcers, the mucosal microbiota of the duodenal bulb is altered, characterized by an increased abundance of H. pylori and decreased abundance of Clostridium and Streptococcus, which possibly alters the biological function of the commensal microbiota through specific metabolic pathways[8]. A systematic review and meta-analysis that assessed the global prevalence of H. pylori infection found that more than half the population is infected[9]. Therefore, it is essential to develop a plan for early detection of H. pylori to reduce the risk of peptic ulcer[10].
In our study, there were more male than female children with peptic ulcer, which is consistent with a previous study[11]. This may be because boys exercise more, eat too much and eat fast, which leads to excessive and rapid gastric acid secretion, which in turn destroys the duodenal mucosal barrier. Girls have higher estrogen levels, which can stimulate the duodenal mucosa to secrete bicarbonate, enhance mucosal barrier function, inhibit gastric and duodenal juice secretion, and reduce pepsin activity, thus protecting gastrointestinal mucosa to reduce the occurrence of ulcers[12,13].
The clinical symptoms of peptic ulcer in children vary with age. Studies have shown that younger children may be irritable, eat a poor diet, and have gastroesophageal reflux but no significant weight gain, and older children may be characterized by abdominal pain, flatulence, hematemesis and black stools[14]. In this study, all children had varying degrees of abdominal pain, because most children cannot accurately describe the degree, location and duration of abdominal pain, it is easy to miss diagnosis or misdiagnose, resulting in delayed treatment, seriously affecting the health of children. Moreover, the proportion of perforation, massive hemorrhage, severe anemia and other serious complications of peptic ulcer in children is higher than in adults[15,16]. It has been reported that most cases of duodenal ulcer perforation are in teenagers; less than half the cases have a history of abdominal pain, and most of them have ulcer perforation at the beginning of acute disease, which may be related to the inability of children to accurately describe abdominal discomfort[17].
The results of gastroscopy are used as the gold standard for the diagnosis of peptic ulcer in children. However, gastroscopy is an invasive examination method. Because the anatomical structure of the upper digestive tract in children is different from that in adults, it is difficult to operate with the narrow gastrointestinal tract and thin gastrointestinal wall, and cooperation from children is difficult. It is reported that only 55% of children with gastrointestinal symptoms have abnormal gastroscopy results[18]. Age is also a risk factor for children in capsule endoscopy[19]. Children do not cooperate well with ordinary gastroscopy, and even painless electronic gastroscopy may have adverse reactions such as hypotension, myocardial ischemia, drug allergy, and arrhythmia. Therefore, ultrasound as a safe, simple, noninvasive and rapid examination method for screening peptic ulcer in children is particularly important. Due to the presence of intestinal gases and feces, the diagnostic quality of conventional gastrointestinal ultrasound may be affected, and the diagnostic sensitivity is lower in older or obese children. Gastrointestinal CEUS can eliminate the interference of gas and contents in the gastrointestinal cavity and improve the diagnostic value of gastroduodenal diseases by filling the gastrointestinal cavity with oral contrast agent.
A total of 43 children were included in this study; 17 cases of duodenal ulcer were diagnosed by gastroscopy, and eight cases were diagnosed by routine ultrasound. The diagnostic accuracy was 47.1% (8/17), and the coincidence rate was 79.1% (34/43). Ultrasonography showed thickening of the local intestinal wall of the duodenum, decreased echo, stiffness and decreased peristalsis. Routine gastrointestinal ultrasound has some limitations, and the coincidence rate of diagnosis is low. Transabdominal ultrasonography is an effective method for detecting peptic ulcer in low-weight children[20]. Therefore, sonographers should carefully evaluate indirect findings around the stomach or duodenum[21]. Fifteen cases were diagnosed by gastrointestinal CEUS, the diagnostic accuracy was 88.2% (15/17), and the coincidence rate was 95.3% (41/43). Ultrasound showed that the shape of the duodenal bulb was irregular, the area was small, the local intestinal wall showed hypoechoic thickening, the mucosal folds of the duodenal wall were thickened, the ulcer surface had local depression, and strong echo spots could be seen on the surface. Gastrointestinal CEUS missed diagnosis in two cases, which may be because the ulcer area was smaller, and the gastric and duodenal ulcer in children is more difficult to see than in adults[22]. The lesion is small, with a diameter of 3-4 mm, and the ulcer is superficial, the bottom is smooth, and the thickening of the gastric wall around the ulcer is not obvious, so it is easy to miss diagnosis. The occurrence of these missed cases shows the limitations of CEUS. It is less sensitive to small and superficial upper digestive tract ulcers and requires a high level of operator skill. However, compared with gastroscopy, CEUS has advantages in compliance, repeatability, incidence of complications and tolerance in children.
Our study had some limitations. Only a few cases were selected. Only the examination results and coincidence rate were analyzed, and the correlation between the size, location, age, weight and diagnostic accuracy of ulcer was not studied in depth. The children with peptic ulcer were not re-examined to evaluate the clinical treatment effect and supervise the recurrence.
Gastrointestinal CEUS in children has high accuracy, which provides pediatricians with a new and simple method for screening upper gastrointestinal diseases in children, which is easily accepted by children and parents, and is an effective supplement to gastroscopy. For children with recurrent abdominal pain and other upper gastrointestinal symptoms with unknown etiology, gastrointestinal CEUS should be performed to provide a reference for clinical selection of appropriate treatment.
In a larger study, we will investigate the correlation between the size, location, age, weight and diagnostic accuracy of ulcers, and re-examine the children with peptic ulcer after regular treatment by gastrointestinal contrast-enhanced ultrasonography (CEUS).
CEUS has advantages in compliance, repeatability, incidence of complications, and tolerance of children, and has a high coincidence rate for clinical diagnosis. it can be used as a preliminary screening method for children with epigastric pain and an effective supplement to gastroscopy.
This study found that the diagnostic coincidence rate of conventional ultrasound was lower than that of gastrointestinal CEUS, and the results of gastrointestinal CEUS and gastroscopy were highly consistent, which confirmed that gastrointestinal CEUS had some advantages. The research on gastrointestinal CEUS in children was supplemented and improved. At present, gastrointestinal CEUS is not widely used in children, and it is necessary to establish the norms and standards of CEUS examination in children, which is helpful to better guide ultrasound physicians to carry out examination and improve the accuracy of examination.
The contrast agents used in CEUS examination are food-grade contrast agents, which are safe, with no adverse effects or smell, and easy to drink. The contrast medium is a little sweet, easy for children to accept, and there is no need for intravenous sedative or general anesthesia. The examination process is painless, greatly reducing the anxiety of children and their families. The sound velocity and impedance of the contrast medium are similar to those of the liver. After oral administration of the contrast medium, the stomach and duodenum show uniform, medium and high dotted echoes, and at the same time, the gastric emptying time is prolonged. Under the gastrointestinal transmission window, the gastrointestinal wall structure and its pathological changes can be displayed more clearly. CEUS can also observe gastric peristalsis and extragastric tissue, and improve the diagnosis of gastroduodenal diseases.
The main goal of this study was to find the most suitable preliminary screening method for the diagnosis of peptic ulcer in children. For children who have contraindications for gastroscopy, CEUS can be a new option. For children with recurrent abdominal pain and other upper gastrointestinal symptoms with unknown etiology, gastrointestinal CEUS can provide a reference for clinical selection of appropriate treatment. For children with peptic ulcer who have been diagnosed and received regular drug treatment, the curative effect can be observed and evaluated repeatedly.
The common examination methods for upper gastrointestinal ulcer in children include upper gastrointestinal X-ray barium meal examination, gastroscopy, gastric computed tomography, and gastric CEUS. In children, it is particularly important to find a simple, noninvasive examination method. CEUS is simple and noninvasive, the examination process is not painful, and there is no need for sedation or anesthesia, especially for children. It is expected to become a routine examination method for the diagnosis of digestive diseases in children.
The detection rate of peptic ulcer in children is increasing, with developments in diagnostic procedures. Most children show abdominal pain, but cannot accurately describe it, so it is easy to miss diagnosis, misdiagnose, and delay treatment. Gastroscopy is the gold standard for the diagnosis of peptic ulcer, but it is an invasive examination. To maximize the diagnostic efficiency and reduce the risk, gastrointestinal CEUS screening is feasible before gastroscopy as an effective supplement to gastroscopy.
The authors thank Huang KY and Huang JQ from the Department of Pediatrics who contributed to data collection and conduct of the study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Skrypnyk I, Ukraine S-Editor: Qu XL L-Editor: A P-Editor: Qu XL
| 1. | Kalach N, Bontems P, Koletzko S, Mourad-Baars P, Shcherbakov P, Celinska-Cedro D, Iwanczak B, Gottrand F, Martinez-Gomez MJ, Pehlivanoglu E, Oderda G, Urruzuno P, Casswall T, Lamireau T, Sykora J, Roma-Giannikou E, Veres G, Wewer V, Chong S, Charkaluk ML, Mégraud F, Cadranel S. Frequency and risk factors of gastric and duodenal ulcers or erosions in children: a prospective 1-month European multicenter study. Eur J Gastroenterol Hepatol. 2010;22:1174-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Hagiwara S, Nakayama Y, Tagawa M, Arai K, Ishige T, Murakoshi T, Sekine H, Abukawa D, Yamada H, Inoue M, Saito T, Kudo T, Seki Y. Pediatric Patient and Parental Anxiety and Impressions Related to Initial Gastrointestinal Endoscopy: A Japanese Multicenter Questionnaire Study. Scientifica (Cairo). 2015;2015:797564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Nielsen MB, Søgaard SB, Bech Andersen S, Skjoldbye B, Hansen KL, Rafaelsen S, Nørgaard N, Carlsen JF. Highlights of the development in ultrasound during the last 70 years: A historical review. Acta Radiol. 2021;62:1499-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Thomson M, Tringali A, Dumonceau JM, Tavares M, Tabbers MM, Furlano R, Spaander M, Hassan C, Tzvinikos C, Ijsselstijn H, Viala J, Dall'Oglio L, Benninga M, Orel R, Vandenplas Y, Keil R, Romano C, Brownstone E, Hlava Š, Gerner P, Dolak W, Landi R, Huber WD, Everett S, Vecsei A, Aabakken L, Amil-Dias J, Zambelli A. Paediatric Gastrointestinal Endoscopy: European Society for Paediatric Gastroenterology Hepatology and Nutrition and European Society of Gastrointestinal Endoscopy Guidelines. J Pediatr Gastroenterol Nutr. 2017;64:133-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 163] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 5. | Lanas A, Chan FKL. Peptic ulcer disease. Lancet. 2017;390:613-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 555] [Article Influence: 69.4] [Reference Citation Analysis (37)] |
| 6. | Yuan C, Adeloye D, Luk TT, Huang L, He Y, Xu Y, Ye X, Yi Q, Song P, Rudan I; Global Health Epidemiology Research Group. The global prevalence of and factors associated with Helicobacter pylori infection in children: a systematic review and meta-analysis. Lancet Child Adolesc Health. 2022;6:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 96] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 7. | Robinson K, Atherton JC. The Spectrum of Helicobacter-Mediated Diseases. Annu Rev Pathol. 2021;16:123-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 8. | Zheng W, Peng KR, Li FB, Zhao H, Jiang MZ. [The effect of Helicobacter pylori infection on duodenal bulbar microbiota in children with duodenal ulcer]. Zhonghua Er Ke Za Zhi. 2023;61:49-55. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, Chan FKL, Sung JJY, Kaplan GG, Ng SC. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 2032] [Article Influence: 254.0] [Reference Citation Analysis (0)] |
| 10. | Nguyen TC, Tang NLC, Le GKN, Nguyen VT, Nguyen KHG, Che TH, Phan VTT, Nguyen NM, Truong DQ, Ngo XM, Nguyen HT, Robert A, Bontems P, Nguyen PNV. Helicobacter pylori Infection and Peptic Ulcer Disease in Symptomatic Children in Southern Vietnam: A Prospective Multicenter Study. Healthcare (Basel). 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Wang EH, Sun M. [Upper gastrointestinal ulcer in children: a clinical analysis of 173 cases]. Zhongguo Dang Dai Er Ke Za Zhi. 2022;24:372-376. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Sorokman TV, Sokolnyk SV, Moldovan PM, Chernei NY, Ostapchuk VG. IMPROVEMENT OF ERADICATION THERAPY IN CHILDREN WITH DUODENAL ULCER ASSOCIATED WITH HELICOBACTER PYLORI. Wiad Lek. 2022;75:215-222. [PubMed] [DOI] [Full Text] |
| 13. | Tuo B, Wen G, Wei J, Liu X, Wang X, Zhang Y, Wu H, Dong X, Chow JY, Vallon V, Dong H. Estrogen regulation of duodenal bicarbonate secretion and sex-specific protection of human duodenum. Gastroenterology. 2011;141:854-863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Sierra D, Wood M, Kolli S, Felipez LM. Pediatric Gastritis, Gastropathy, and Peptic Ulcer Disease. Pediatr Rev. 2018;39:542-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Peetsalu A, Kirsimägi U, Peetsalu M. Methods of emergency surgery in high-risk stigmata peptic ulcer hemorrhage. Minerva Chir. 2014;69:177-184. [PubMed] |
| 16. | Yang HR. Updates on the Diagnosis of Helicobacter pylori Infection in Children: What Are the Differences between Adults and Children? Pediatr Gastroenterol Hepatol Nutr. 2016;19:96-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Shen Q, Liu T, Wang S, Wang L, Wang D. Experience in diagnosis and treatment of duodenal ulcer perforation in children. BMC Pediatr. 2023;23:144. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Lyons H, Zhang Y, Szpunar S, Dharmaraj R. Predictors of positive esophagogastroduodenoscopy outcomes in children and adolescents: a single center experience. BMC Res Notes. 2017;10:356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Wang H, Xie J, Ren L, Liang D, Xiong L, Liu L, Xu W, Gong S, Geng L, Chen P. Age Is a Risk Factor for Gastroscopy-Assisted Capsule Endoscopy in Children. Turk J Gastroenterol. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 20. | Lee EJ, Lee YJ, Park JH. Usefulness of Ultrasonography in the Diagnosis of Peptic Ulcer Disease in Children. Pediatr Gastroenterol Hepatol Nutr. 2019;22:57-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Hosokawa T, Tanami Y, Sato Y, Hara T, Iwama I, Ishimaru T, Kawashima H, Oguma E. Diagnostic Accuracy of Ultrasound for Detecting Gastric or Duodenal Ulcers in Pediatric Patients. J Ultrasound Med. 2022;41:457-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Huang SC, Sheu BS, Lee SC, Yang HB, Yang YJ. Etiology and treatment of childhood peptic ulcer disease in Taiwan: a single center 9-year experience. J Formos Med Assoc. 2010;109:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |