Published online Feb 21, 2024. doi: 10.3748/wjg.v30.i7.685
Peer-review started: December 11, 2023
First decision: December 14, 2023
Revised: December 19, 2023
Accepted: January 17, 2024
Article in press: January 17, 2024
Published online: February 21, 2024
Processing time: 71 Days and 15.9 Hours
For compensated advanced chronic liver disease (cACLD) patients, the first decompensation represents a dramatically worsening prognostic event. Based on the first decompensation event (DE), the transition to decompensated advanced chronic liver disease (dACLD) can occur through two modalities referred to as acute decompensation (AD) and non-AD (NAD), respectively. Clinically Signifi
In this observational study, we investigated the clinical usage of RPR in predicting DEs in MASLD-related cACLD patients.
Fourty controls and 150 MASLD-cACLD patients were consecutively enrolled and followed up (FUP) semiannually for 3 years. At baseline, biochemical, clinical, and Liver Stiffness Measurement (LSM), Child-Pugh (CP), Model for End-Stage Liver Disease (MELD), aspartate aminotransferase/platelet count ratio index (APRI), Fibrosis-4 (FIB-4), Albumin-Bilirubin (ALBI), ALBI-FIB-4, and RPR were collected. During FUP, DEs (timing and modaities) were recorded. CSPH was assessed at the baseline and on DE occurrence according to the available Clinical Practice Guidelines.
Of 150 MASLD-related cACLD patients, 43 (28.6%) progressed to dACLD at a median time of 28.9 months (29 NAD and 14 AD). Baseline RPR values were significantly higher in cACLD in comparison to controls, as well as MELD, CP, APRI, FIB-4, ALBI, ALBI-FIB-4, and LSM in dACLD-progressing compared to cACLD individuals [all P < 0.0001, except for FIB-4 (P: 0.007) and ALBI (P: 0.011)]. Receiving operator curve analysis revealed RPR > 0.472 and > 0.894 as the best cut-offs in the prediction respectively of 3-year first DE, as well as its superiority compared to the other non-invasive tools examined. RPR (P: 0.02) and the presence of baseline-CSPH (P: 0.04) were significantly and independently associated with the DE. Patients presenting baseline-CSPH and RPR > 0.472 showed higher risk of decompensation (P: 0.0023).
Altogether these findings suggest the RPR as a valid and potentially applicable non-invasive tool in the prediction of timing and modalities of decompensation in MASLD-related cACLD patients.
Core Tip: The availability of non-invasive tools predicting the first decompensation event (DE) in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)-related compensated advanced chronic liver disease (cACLD) context is still demanded. Red cell distribution width to platelet ratio (RPR) has been shown to predict fibrosis in MASLD. Herein, we demonstrate that: (1) RPR predicts the first DE in MASLD-cACLD; (2) RPR predicts acute decompensation as the first DE in these patients; and (3) Patients presenting baseline Clinically Significant Portal Hypertension and RPR > 0.472 show higher risk of 3-year decompensation occurrence. Overall, RPR predicts time and modalities of DE in MASLD-related-ACLD patients, presenting the potential to be a valuable, easy-to perform, non-invasive clinical index.
- Citation: Dallio M, Romeo M, Vaia P, Auletta S, Mammone S, Cipullo M, Sapio L, Ragone A, Niosi M, Naviglio S, Federico A. Red cell distribution width/platelet ratio estimates the 3-year risk of decompensation in Metabolic Dysfunction-Associated Steatotic Liver Disease-induced cirrhosis. World J Gastroenterol 2024; 30(7): 685-704
- URL: https://www.wjgnet.com/1007-9327/full/v30/i7/685.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i7.685
In the last decade, the progressive development of tools non-invasively assessing the degree of hepatic fibrosis in patients with chronic liver diseases (CLDs) has allowed the identification of cirrhosis at the earlier and asymptomatic stage of compensated advanced CLD (cACLD), revolutionizing the clinical management and conditioning the therapeutic interven
For cACLD patients, the transition to decompensated advanced CLD (dACLD), represents a dramatic prognosis-affecting event as the liver-related mortality occurring almost exclusively after this watershed episode[3]. Based on the first decompensation event (DE), the transition to dACLD can occur through two modalities with relatively different long-term consequences: The more prognostically burdensome acute decompensation (AD); the more progressive non-AD (NAD)[4].
Metabolic dysfunction-associated Steatotic Liver Disease (MASLD), encompassing a spectrum of disease manifestations ranging from simple steatosis to steatohepatitis (MASH) and advanced fibrosis (AF), represents the most common cause of liver cirrhosis worldwide with a severe healthy and socioeconomic burden[5,6]. To make matters worse, recent evidence indicates that MAFLD/MASH-related cACLD may progress more rapidly than other etiologies and a relatively earlier decompensation has been reported in these patients[7,8]. Therefore, determining the probability of decom
Clinically significant portal hypertension (CSPH) defined by a Hepatic Venous Pressure Gradient (HVPG) value 0 mmHg has been revealed as the strongest predictor of decompensation in several CLDs etiologies, including MASH[9]. However, HVPG measurement is a nuanced, not-routinely performed procedure with a highly operator-dependent accuracy. Transient Elastography (TE)-assessed Liver Stiffness Measurement (LSM), Fibrosis-4 (FIB-4), Albumin-Bilirubin (ALBI), ALBI-FIB-4, aspartate aminotransferase (AST)/platelet (PLT) count ratio Index (APRI), Child-Pugh (CP) score, and Model for End-Stage Liver Disease (MELD), have been investigated as models non-invasively predicting decompensation[10-15]. Despite the encouraging results suggested by these findings, the development of prognostic tools including not-exclusively specialist parameters would have more appeal across healthcare systems.
Red cell distribution width (RDW) is a routinely assessed haematochemical parameter providing an analytical measure of the variability [Standard Deviation (RDW-SD) and Coefficient Variation (RDW-CV)] in the size of circulating erythrocytes whose applicability as an independent prognosis marker in cardiovascular, renal, and infectious conditions has been largely demonstrated[16]. In hepatic chronic disorders, regardless of the etiology, the perpetuation of liver injury promotes reactive oxygen species release and decreased antioxidant compounds production, determining a systemic oxidative stress imbalance and low-grade inflammation status leading to bone-marrow suppression, reduced erythropoietin functioning, and thus irregular/immature erythrocytes output[17]. In line with this, elevated RDW values have been evidenced in patients affected by viral-related and non-viral-related CLDs[17], and several findings have highlighted its usefulness as a prognostic index in CLDs of different etiologies[18,19]. However, the potential link with decompensation occurrence in cACLD individuals has never been investigated. In long-lasting CLDs, the portal hypertension-related pancytopenia determining, among the other consequences, chronic anemia, and low platelet count, has constituted the pathophysiological rationale to reveal the role of RDW-to-PLT ratio (RPR) as an RDW-derivative non-invasively predicting hepatic AF[20]. In MASLD patients, RPR has been recently shown to reflect the severity of fibrosis, correlate with main non-invasive liver-fibrosis scoring systems, and accurately predict AF[21,22]. However, the role of RPR in the prediction of decompensation in terms of timing and relative modalities (AD or NAD) in MASLD-related cACLD patients has never been explored and, the availability of tools that accurately non-invasively predict and stratify the risk of decompensation still represents an unmet need.
In this study, by focusing on MASLD-related etiology, we aimed to evaluate the accuracy of the RPR in the prediction of 3-year first DE occurrence and relative modalities (NAD or AD) in cACLD patients.
In this observational study, we consecutively enrolled patients affected by MASLD-related cACLD and a group of healthy controls. TE was adopted to non-invasively assess LSM and analytically define cACLD. The Alcohol Use Disorders Identification Test questionnaire was used to assess alcohol consumption, to exclude from the enrollment patients potentially affected by alcoholic liver disease.
As detailed below, at the enrollment, anthropometrical and clinical data were collected. Further, a 10 mL venous blood sample was collected to assess the biochemical parameters. Finally, at the baseline, MASLD-related cACLD individuals received a non-invasive evaluation of the hepatic disease severity and liver function status by computing RPR, APRI, FIB-4, ALBI, ALBI-FIB-4, MELD, and CP scores. Patients were semiannually followed up (FUP) over 3 years to record the occurrence of the first DE and the relative modalities by recognizing, according to D’Amico et al[4], two distinct modalities of decompensation: NAD and AD[4]. Liver-related events (LREs) defining decompensation, as well as NAD- and AD-specific features are detailed below.
CSPH and RPR were assessed at baseline and when the first DE occurred by using evaluation methods reported in detail in the dedicated subparagraph.
The experimental design is reported in Figure 1.
The estimation of the accuracy of the RPR in the prediction of 3-year first DE occurrence in comparison to the currently available non-invasive composite tools (APRI, FIB-4, ALBI, ALBI-FIB-4, MELD, and LSM) represented the primary study outcome.
The estimation of the accuracy of the RPR in the prediction of AD (3-year first DE) occurrence in comparison to the currently available non-invasive composite tools (APRI, FIB-4, ALBI, ALBI-FIB-4, MELD, and LSM), as well as the investigation of the relationship between RPR and baseline-CSPH with a consensual risk-stratification on DE occurrence, were the secondary study outcomes.
This study is in compliance with the Declaration of Helsinki (1975) and has been approved by the ethical committee of the University of Campania Luigi Vanvitelli in Naples (prot. n. 417/2018).
In the present study (Figure 1), after signing the informed consent, we consecutively enrolled healthy subjects as the control group and patients affected by MASLD-related cACLD. Liver Transient Elastography criteria were adopted to determine cACLD according to the Baveno VI consensus: LSM values 15 kPa defined cACLD[23]. MASLD diagnostic criteria were: (1) Overweight or obesity, defined as body mass index (BMI) > 25 kg/m2; (2) presence of type 2 diabetes mellitus (T2DM) and/or (3) presence of ≥ one metabolic risk abnormalities identified by waist circumference ≥ 102 cm in men (and ≥ 88 cm in women); blood pressure ≥ 130/85 mmHg (or specific drug treatment); plasma triglycerides (TG) ≥150 mg/dL (or specific drug treatment); plasma high-density lipoprotein (HDL) cholesterol < 40 mg/dL for men (and < 50 mg/dL for women) (or specific drug treatment); prediabetes [fasting plasma glucose (FPG) levels 100-125 mg/Dl] or 2-h post-load glucose levels 140-199 mg/dL or glycated hemoglobin 5.7%-6.4%; homeostasis model assessment for insulin resistance (HOMA-IR) score ≥ 2.5[6]. The enrollment was carried out at the Hepato-Gastroenterology Division of the University of Campania Luigi Vanvitelli between January and November 2019. Inclusion criteria were age between 18 and 80 years and MASLD-related cACLD diagnosis. Exclusion criteria were the presence of hematological disorders (particularly, autoimmune hemolytic anemia, myelodysplastic syndrome, b-thalassemia, sickle cell anemia); chronic inflammatory diseases, acute or chronic kidney diseases, rheumatoid arthritis, systemic lupus erythematosus, auto
The evaluated biochemical data were AST, alanine aminotransferase (ALT), total bilirubin (TB), PLT, plasma albumin, International Normalized Ratio (INR), total cholesterol, HDL cholesterol, Low-density lipoprotein cholesterol, TG, insulin, and FPG. Insulin levels were measured enzymatically using commercially available kits (R&D Systems, Minneapolis, MN), AST, ALT, and glucose using a colorimetric assay kit (Amplite 13801/13803 and Thermo Fisher Scientific EIAGLUC). The HOMA-IR was calculated by using the formula: fasting insulin (μU/mL) × FPG (mmol/L)/22.5[24].
RDW was determined by using a suspension of blood cells passed through a small orifice along with an electric current of the Beckman Coulter analyzer (C11137 - DxI 9000 Analyzer, Beckman Coulter, Inc©). The individual blood element generates an impedance change in the orifice, which is directly proportional to the cell size. The system counts the individual cells and provides a size distribution. The RDW is then calculated at the 20% height level above the baseline of the Red Blood Cells histogram. In particular, the RDW-CV evaluates the volumetric distribution of red blood cells considering the coefficient of variation, while the RDW-SD defines the volumetric distribution concerning the standard deviation.
MELD score, which determines prognosis and prioritizes receipt of liver transplantation, incorporates 3 widely available laboratory variables including the INR, serum creatinine, and serum bilirubin. MELD was given by the formula: [9.57 × log10 (creatinine) + 3.78 × log10 (TB) + 11.2 × log10 (INR) + 6.43][14].
CP was evaluated using five clinical and laboratory criteria: Serum bilirubin (< 2 mg/dL: 1 point; 2-3 mg/dL: 2 points; > 3 mg/dL: 3 points), serum albumin (> 3.5 mg/dL: 1 point; 2.8-3.5 mg/dL: 2 points; < 2.8 mg/dL: 3 points), ascites (none: 1 point; grade 1-2: 2 points; grade 3: 3 points), and HE (none: 1 point; grade 1-2: 2 points; grade 3-4: 3 points)[25]. CP scoring system, broke down patients into three classes: CPA - good hepatic function (CP total range: 5-6), CPB - moderately impaired hepatic function (CP total range: 8-9), and CPC- advanced hepatic dysfunction (CP total range: 10-15)[25].
APRI was calculated by using the following validated formula: [(AST/upper limit of the normal AST range) + 100]/PLT count (103/mL)[26].
The ALBI score was calculated as [-0.085 × (albumin g/L) + 0.66 × log10 (TB mmol/L)][27]. FIB-4 score, a non-invasive estimation of liver scarring, was calculated by using the originally described formula[28]: Age × AST/PLT count (103/mL) × ALT½. FIB-4 categories were: (1) Low risk for AF (< 1.45); (2) high risk for AF (> 3.25); or (3) indeterminate (1.45-3.25)[28].
The combined score ALBI-FIB-4 stratified patients as follows: I group of risk (ALBI ≤ -2.60 and FIB-4 ≤ 3.25); II group of risk (ALBI ≥ -2.60 and FIB-4 ≤ 3.25); III group of risk (ALBI ≤ -2.60 and FIB-4 ≥ 3.25); IV group of risk (ALBI ≥ -2.60 and FIB-4 ≥ 3.25)[29].
RPR was determined by using the formula: RDW-SD/PLT count (103/mL) 1000.
LSM was performed by using FibroScan® [version 502 (Echosens, Paris, France)] with M and XL probes[30]. We decided to use the XL probe when the ultrasound measured distance between the skin and the liver capsule resulted in greater than 2.5 cm and/or when the patient's BMI was > 30. FibroScan® was performed by an expert physician obtaining 10 acceptable measurements (defined as successful LSM), with the maximum number of attempts set at 20.
The criteria proposed by Boursier et al[30] were used to consider the measurement “very reliable” (IQR/M ≤ 0:1), “reliable” (0:1 < IQR/M ≤ 0:3 or IQR/M > 0:3 with LS median < 7:1 kilopascal), or “poorly reliable” (IQR/M > 0:3 with LS median ≥ 7:1 kPa[30,31].
LREs were ascites formation, hepatic encephalopathy (HE), jaundice, acute bacterial infections, and acute gastrointestinal bleeding. The onset of one (or more) LREs in cACLD patients defined the decompensation and thus the transition to dACLD. According to D’Amico et al[4], two distinct modalities of transition to decompensation were considered: (1) NAD was defined by slow/ grade 1 ascites formation, mild (grade 1 or 2) HE, or progressive jaundice in non-cholestatic cirrhosis; (2) AD was defined by grade 2/3 ascites within less than 2 wk, severe acute (i.e., in patients with previous normal consciousness) HE, acute gastrointestinal bleeding, and any type of acute bacterial infection.
According to Baveno VI Criteria, for Esophagogastroduodenoscopy-(EGDS)-naïve patients, presenting baseline LSM values ≤ 20 kPa and/or a PLT count ≤ 150.000/mm3 a screening EGDS was performed, while EGDS-not naive patients continued their regular surveillance endoscopy programs, according to the Clinical Practice Guidelines[23]. In all the cases, at the baseline, an EGDS proving esophageal varices defined CSPH. Baveno VII Criteria (CSPH-rule out if LSM ≤ 15 kPa and PLT count ≥ 150.000/mm3, CSPH-rule in if LSM values ≥ 25 kPa)[32] were not available at the time of the enrollment and were exclusively used to reassess/confirm CSPH on the occasion of first DE occurrence, independently from the endoscopic surveillance programs for each patient (Figure 1).
Finally, the Japanese Research Society for Portal Hypertension Classification estimated the entity (F1; F2; F3) of varices[33].
Continuous data were described as mean and standard deviations, while categorical variables as n (%). The Kolmogorov-Smirnov test for normality was performed to evaluate if the parametric or non-parametric analysis should be applied. Mann-Whitney and t-test for independent groups, the Kruskal-Wallis test, or ANOVA test with posthoc Tukey analysis, in the case of non-normal or normal distribution respectively, were performed to compare the continuous variables. D% RPR [(RPR on the first DE - baseline RPR)/baseline RPR 100] and D% LSM [(LSM on the first DE - baseline LSM)/baseline LSM 100]} indicated RPR and LSM% variations during the study. Linear regression analysis was adopted to evaluate the relationship (R) between continuous variables. The area under the curve (AUC), estimated by receiving operator curve (ROC) analysis with the Youden index calculation for the identification of best cut-off values, integrally with the Chi-Square test for the sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) evaluation, was performed to evaluate the accuracy of RPR in the prediction of 3-year first DE and in the prediction of AD occurrence, as well as to estimate the accuracy of the RPR in comparison to other non-invasive composite tools (APRI, FIB-4, ALBI, ALBI-FIB-4, MELD, CP, and LSM) in the prediction of both these outcomes. The adjusted odds ratio (OR) of the study variables on the just mentioned events was calculated considering the confounding variables (sex, age, BMI, diabetes, alcohol intake, the baseline/along the study administration of Non-Selective Beta Blockers) by using multinomial logistic regression models. Time-to-event analyses on DEs occurrence upper and under the RPR value ROC-analysis identified best cut-off was performed using the Kaplan-Meier method and the Log-rank test for the curve comparison considering a P value < 0.05 as statistically significant. Statistical significance was defined as P < 0.05 in a two-tailed test with a 95%CI. SPSS®vs 18.0 was used to perform the analysis. The sample size was estimated by Logistic Regression analysis (p0: 0.15; p1: 0.23; alfa: 0.05; power: 0.8) testing whether the variable (RPR) is a significant predictor of the binary (0/1) outcome (y = decompensation) performed by using wp. logistic function of STATA18 for macOS software.
A total of 150 MASLD-related-cACLD patients and 40 healthy controls were consecutively enrolled in this study. The baseline demographic data, anthropometric indexes, biochemical parameters, and non-invasive tools for liver-functional status and hepatic fibrosis assessment (CP, MELD, LSM, FIB-4, APRI, RPR, ALBI, and ALBI-FIB-4) are reported in Tables 1-4. The baseline prevalence of T2DM, primary hypertension, and dyslipidemia in the MASLD patients was respectively 54.6% (n = 82), 50.6% (n = 76), and 32 % (n = 48).
| Variables (mean ± SD) | Healthy subjects (n = 40) | cACLD patients (n = 150) | P value1 |
| BMI (kg/m2) | 24.97 ± 2.17 | 32.61 ± 23.94 | < 0.0001 |
| WhR | 0.81 ± 0.05 | 1.56 ± 3.02 | < 0.0001 |
| Systolic blood pressure (mm/Hg) | 115.3 ± 9.73 | 130.7 ± 12.57 | < 0.0001 |
| Diastolic blood pressure (mm/Hg) | 74.67 ± 10.42 | 87.33 ± 8.58 | 0.003 |
| Variables (mean ± SD) | Healthy subjects (n = 40) | cACLD patients (n = 150) | P value1 |
| AST (IU/L) | 31.30 ± 10.14 | 48.74 ± 54.09 | < 0.0001 |
| ALT (IU/L) | 39.37 ± 17.57 | 70.02 ± 15.05 | < 0.0001 |
| Bilirubin (µmol/L) | 15.98 ± 1.74 | 25.86 ± 7.53 | < 0.0001 |
| PLT count (mm3) | 242.6 ± 42.76 | 155.7 ± 61.56 | < 0.0001 |
| RDW-CV (%) | 14.40 ± 2.28 | 21.04 ± 15.20 | < 0.0001 |
| RDW-SD (fL) | 40.19 ± 4.48 | 56.27 ± 10.54 | < 0.0001 |
| Albumin (g/L) | 44.2 ± 0.29 | 26.35 ± 8.48 | < 0.0001 |
| INR | 1.02 ± 0.38 | 1.78 ± 1.11 | NS |
| HOMA-IR | 1.77 ± 0.54 | 3.15 ± 1.52 | < 0.0001 |
| Insulin (µu/mL) | 7.03 ± 1.52 | 11.67 ± 3.259 | < 0.0001 |
| FPG (mg/dL) | 100.7 ± 9.35 | 120.9 ± 17.48 | < 0.0001 |
| Total cholesterol (mg/dL) | 135.2 ± 42.07 | 185.2 ± 44.12 | < 0.0001 |
| HDL (mg/dL) | 95.93 ± 27.29 | 42.37 ± 9.92 | < 0.0001 |
| LDL (mg/dL) | 44.73 ± 9.67 | 126.1 ± 39.78 | < 0.0001 |
| Tryglicerides (mg/dL) | 109.5 ± 32.14 | 150.6 ± 63.39 | 0.002 |
| Creatinine (mg/dL) | 0.97 ± 0.23 | 1.48 ± 3.88 | 0.03 |
| Variables (mean ± SD) | Healthy subjects (n = 40) | cACLD patients (n = 150) | P value |
| LSM (kPa) | NA | 19.67 ± 3.39 | / |
| APRI | NA | 1.75 ± 0.28 | / |
| FIB-4 | NA | 3.11 ± 1.78 | / |
| ALBI | NA | -2.378 ± 0.63 | / |
| ALBI-FIB-4 | NA | 1.44 ± 0.99 | / |
| Child-Pugh | NA | 6.24 ± 1.23 | / |
| MELD | NA | 7.74 ± 2.69 | / |
| RDW (fL)/PLT ratio | 0.17 ± 0.03 | 0.458 ± 0.27 | / |
During a median follow-up of 36 (IQR: 35-36) months, 43 (28.6%) of 150 cACLD patients progressed to dACLD at a median time of 28.9 (95%CI: 27.20-32.80) months.
In 3 (21.4%) dACLD patients, community-acquired acute bacterial infections (2 Urinary Tract Infections and 1 Pneumonia) were recognized as the precipitants of decompensation configuring AD events. However, in 40 (93%) of the decompensating patients, no specific triggers could be identified. Overall survival following the first decompensation was 79.8% at 3 years. Detailed data about the first DE and relative modalities of decompensation (NAD vs AD) are described in the next subparagraph.
Tables 5-7 report the baseline demographic data, anthropometric indexes, and biochemical parameters, for remaining-cACLD and progressing-dACLD patients.
| Patients remaining compensated (n = 107) | Patients progressing to decompensation (n = 43) | P value | |
| Male [n (%)] | 66 (61.7) | 22 (51.2) | NS1 |
| Female [n (%)] | 41 (38.3) | 21 (48.8) | NS1 |
| Age (mean ± SD) | 61.81 ± 10.99 | 66.47 ± 12.01 | NS2 |
| Child-Pugh Grade A [n (%)] | 78 (72.9) | 29 (67.5) | NS1 |
| Child-Pugh Grade B [n (%)] | 29 (27.1) | 14 (32.5) | NS1 |
| Variables (mean ± SD) | Patients remaining compensated (n = 107) | Patients progressing to decompensation (n = 43) | P value1 |
| BMI (kg/m2) | 33.58 ± 2.28 | 30.18 ± 3.13 | NS |
| WhR | 1.79 ± 0.83 | 1.01 ± 0.13 | NS |
| Systolic blood pressure (mm/Hg) | 130.5 ± 13.49 | 131.2 ± 10.05 | NS |
| Diastolic blood pressure (mm/Hg) | 87.85 ± 8.85 | 86.05 ± 7.83 | NS |
| Variables (mean ± SD) | Patients remaining compensated (n = 107) | Patients progressing to decompensation | P value1 |
| AST (IU/L) | 29.28 ± 8.55 | 32.50 ± 27.15 | NS |
| ALT (IU/L) | 50.13 ± 17.5 | 54.86 ± 31.4 | NS |
| Bilirubin (µmol/L) | 23.64 ± 4.74 | 32.97 ± 7.10 | NS |
| PLT count (mm3) | 183 ± 48.77 | 87.60 ± 28.15 | < 0.0001 |
| RDW-CV (%) | 15.66 ± 3.53 | 34.41 ± 23.05 | < 0.0001 |
| RDW-SD (fL) | 53.05 ± 8.91 | 64.30 ± 10.07 | < 0.0001 |
| Albumin (g/L) | 35.02 ± 7.41 | 32.48 ± 1.54 | NS |
| INR | 1.26 ± 0.36 | 1.89 ± 0.27 | NS |
| HOMA-IR | 2.94 ± 1.57 | 3.66 ± 1.26 | NS |
| Insulin (µu/mL) | 11.50 ± 3.36 | 12.09 ± 2.98 | NS |
| FPG (mg/dL) | 121 ± 18.09 | 120.9 ± 16.07 | NS |
| Total cholesterol (mg/dL) | 185.2 ± 39.71 | 175.3 ± 54.09 | NS |
| HDL (mg/dL) | 43.23 ± 9.82 | 40.13 ± 9.97 | NS |
| LDL (mg/dL) | 125.9 ± 37.9 | 126.7 ± 44.51 | NS |
| Tryglicerides (mg/dL) | 145.8 ± 53.29 | 162.4 ± 82.99 | NS |
| Creatinine (mg/dL) | 1.13 ± 0.91 | 2.13 ± 1.07 | 0.02 |
Patients transiting to dACLD presented significantly higher baseline RPR values in comparison to controls and not-decompensating individuals (all P < 0.0001; Figure 2A), as well as MELD (P < 0.0001), CP (P < 0.0001), LSM (P < 0.0001), APRI (P < 0.0001), FIB-4 (P: 0.007), and ALBI (P: 0.011) baseline values were significantly increased in dACLD individuals compared to patients remaining compensated (Figure 2B).
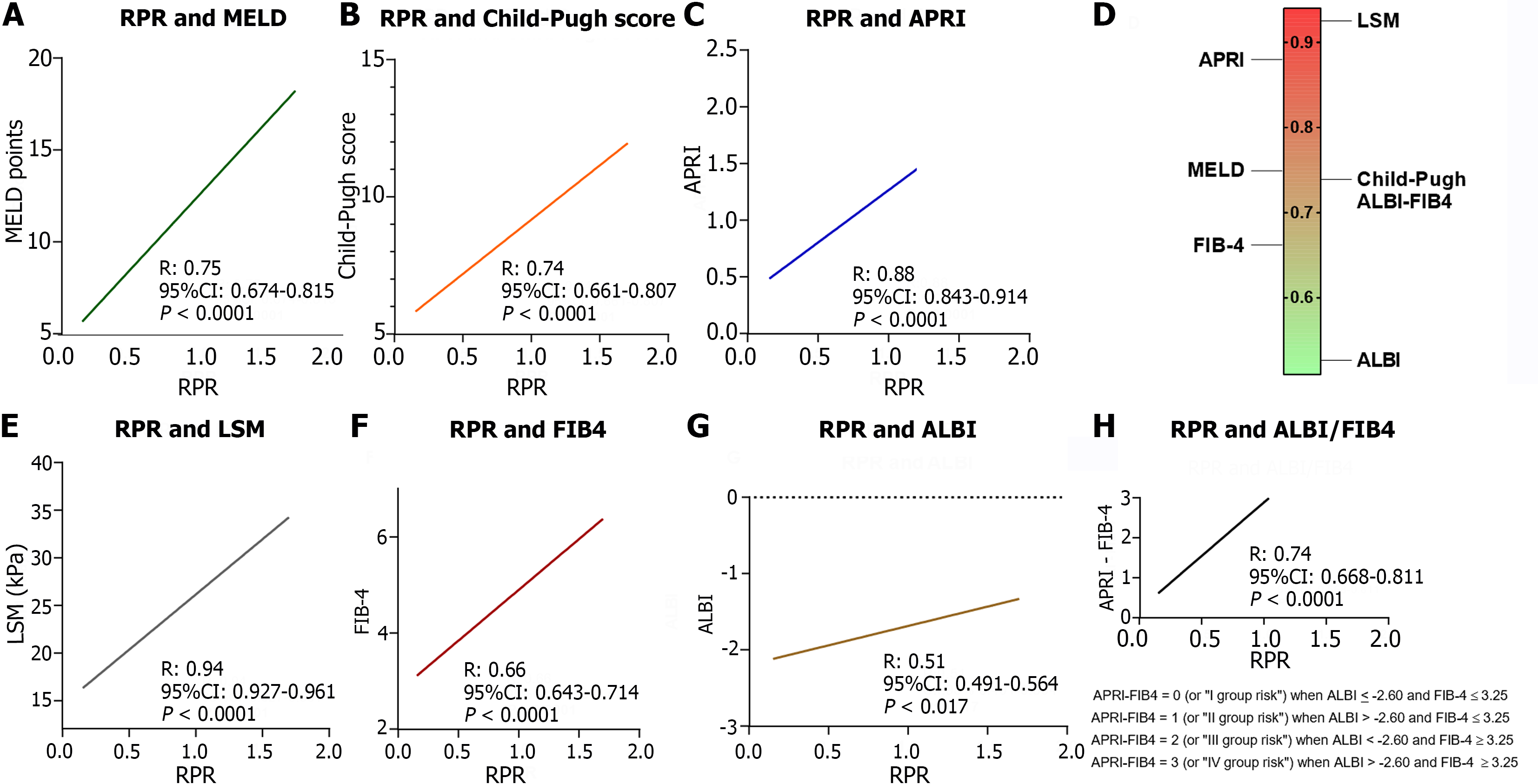
Linear regression analysis revealed the positive correlation between baseline RPR values and the others tools (CP: r = 0.74, 95%CI: 0.661- 0.807; MELD: r = 0.75, 95%CI: 0.679- 0.817; FIB-4: r = 0.66, 95%CI: 0.643-0.714; APRI: r = 0.88, 95%CI: 0.843-0.914; LSM: r = 0.94, 95%CI: 0.927-0.961; ALBI: r = 0.51, 95%CI: 0.491-0.564 ALBI-FIB-4: r = 0.74, 95%CI: 0.668-0.811; all P < 0.0001, except ALBI, P: 0.017; Figure 3).
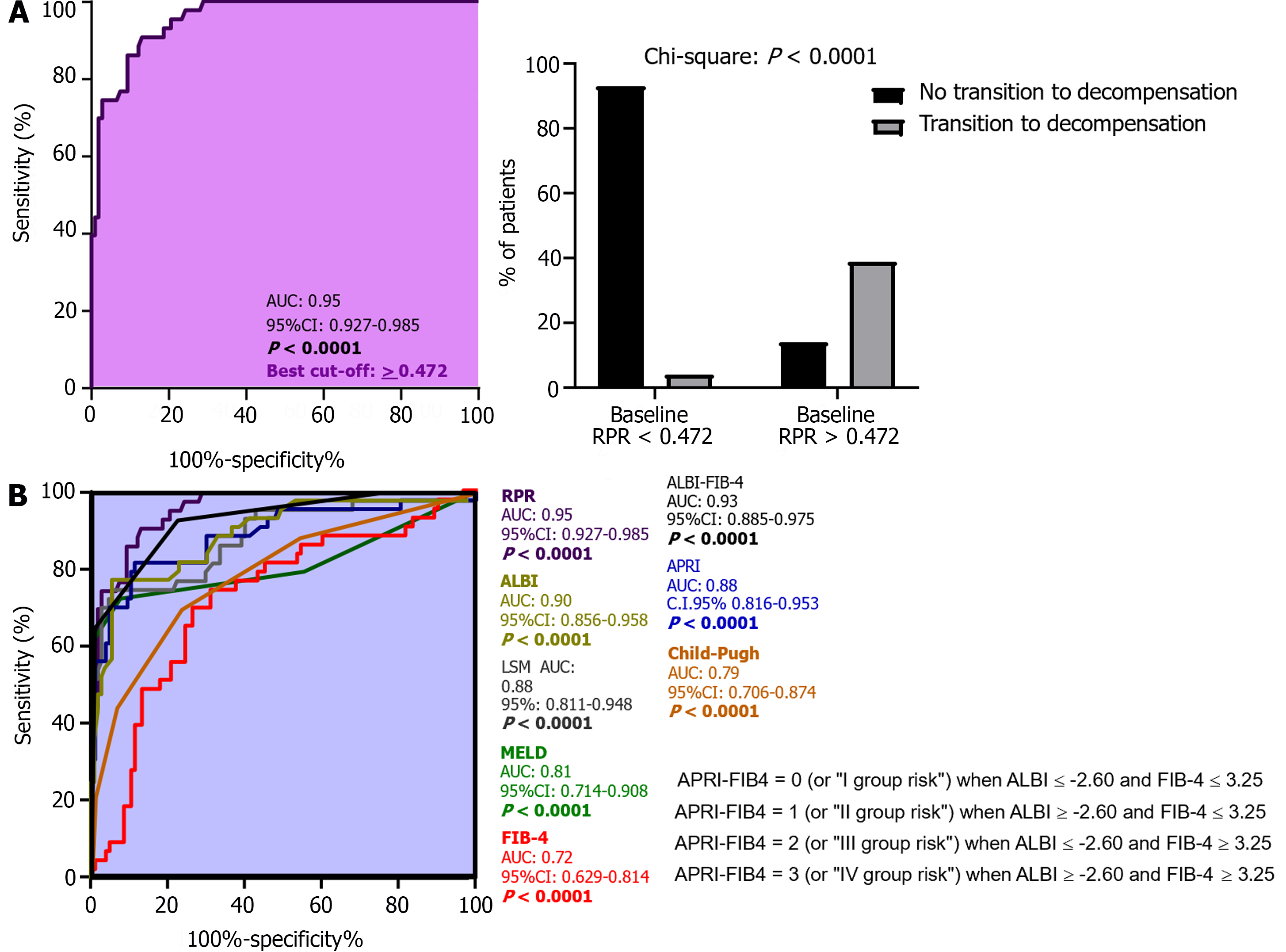
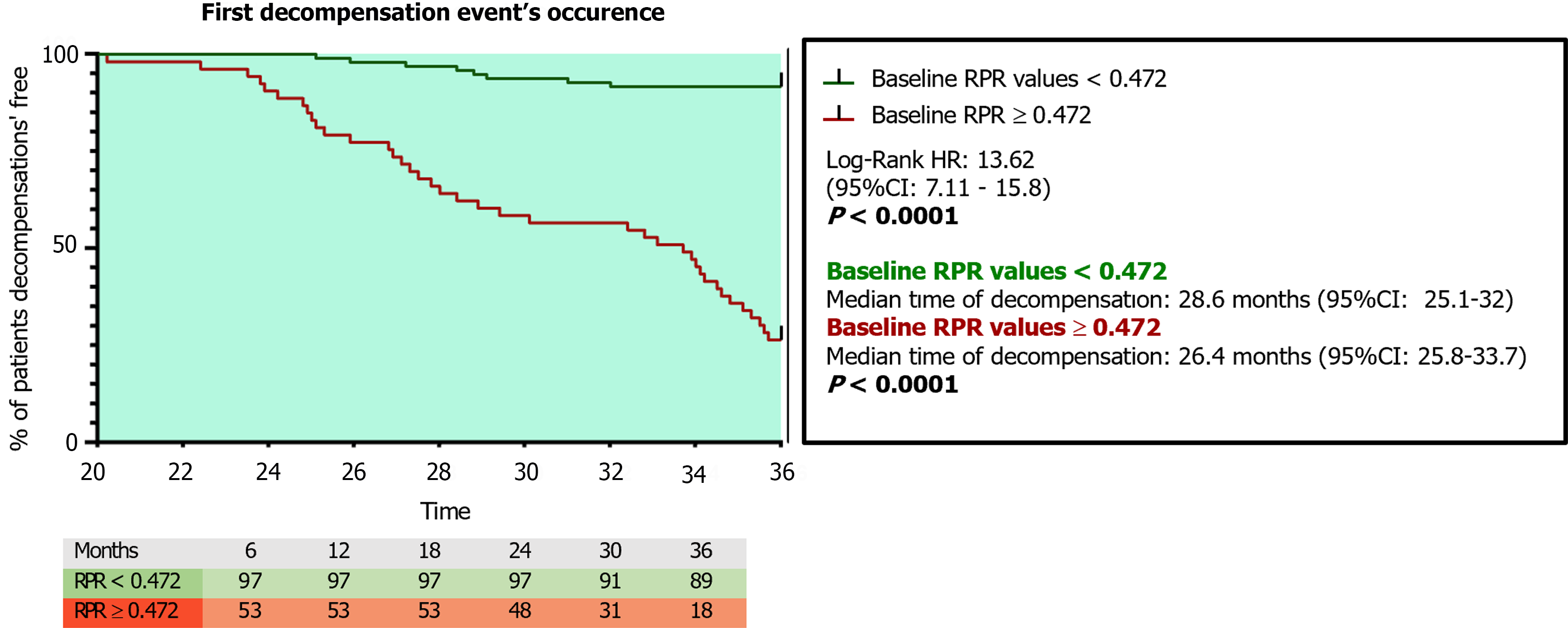
ROC analysis with the Youden index calculation for the identification of best cut-off values revealed 0.472 as the RPR threshold (AUC: 0.95; sensitivity: 86.9%; specificity: 90.7%; NPV: 73.5%; PPV: 95.8%; P < 0.0001) in the prediction of 3-year first DE, as well as a superior RPR predictive accuracy compared to APRI (AUC: 0.88), FIB-4 (AUC: 0.72), MELD (AUC: 0.81), CP (AUC: 0.79), LSM (AUC: 0.88), ALBI (AUC: 0.90), and ALBI-FIB-4 (AUC: 0.93; all P < 0.0001; Figure 4; Table 8). The RPR predictive accuracy was not statistically significantly different between male and female patients (AUC male: 0.93 vs AUC female: 0.91; P: 0.071). For patients presenting baseline RPR values 0.472, the Kaplan-Meier Log-Rank Test analysis on the first DE occurrence revealed a significantly elevated risk of this event [hazard ratio (HR): 13.62, 95%CI: 7.11-15.8; P < 0.0001], as well as a different median time of decompensation and a higher incidence ratio rate (IRR) in comparison to individuals presenting a baseline RPR < 0.472 [RPR < 0.472 vs RPR >0.472; Median time of decom
| Value | 95%CI | |
| Relative risk1 | 3.630 | 2.416 to 5.842 |
| Reciprocal of relative risk | 0.2755 | 0.1712 to 0.4140 |
| Sensitivity | 0.8692 | 0.7923 to 0.9204 |
| Specificity | 0.9070 | 0.7840 to 0.9632 |
| Positive predictive value | 0.9588 | 0.8987 to 0.9838 |
| Negative predictive value | 0.7358 | 0.6042 to 0.8356 |
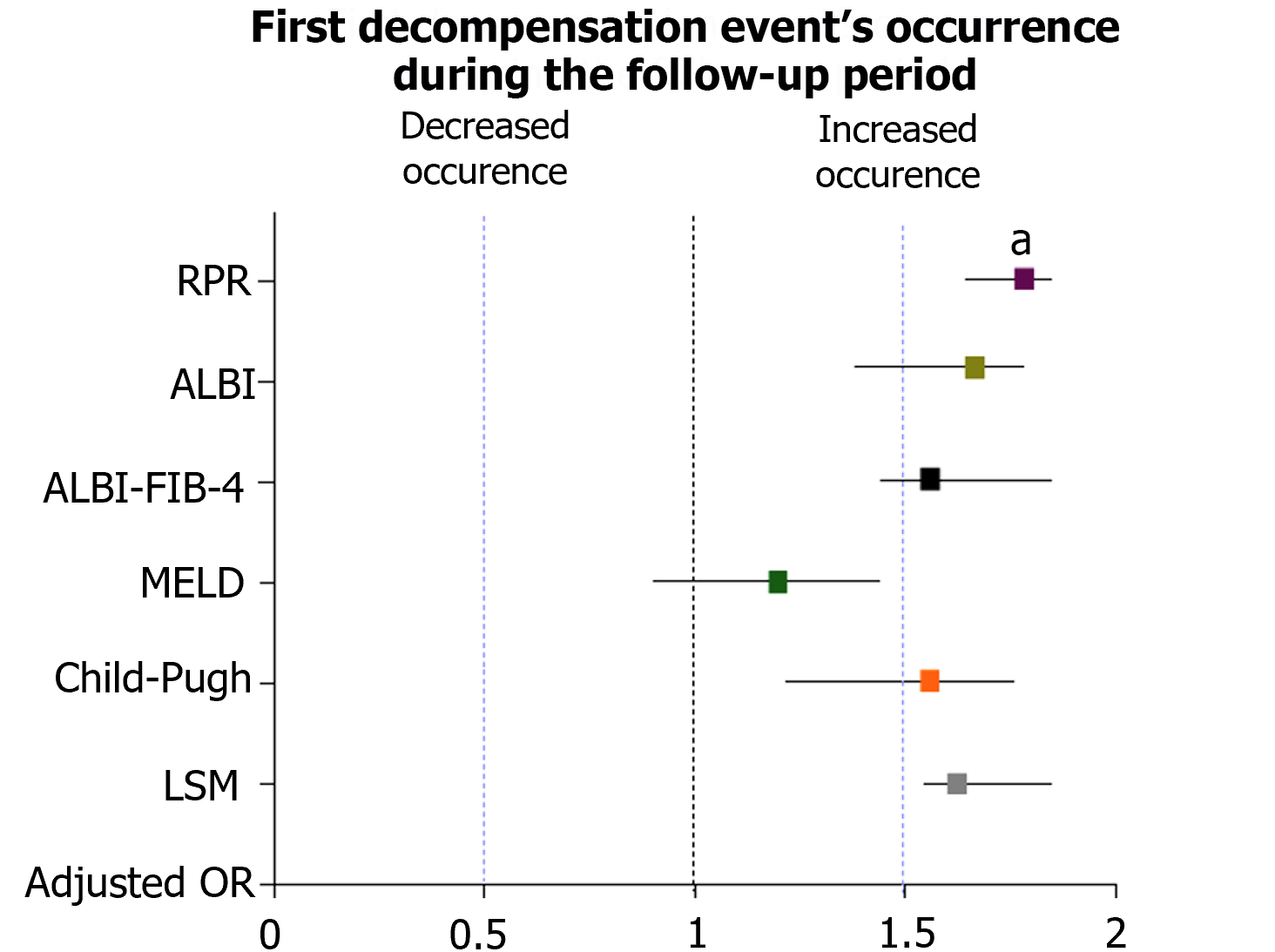
The multinomial logistic regression analysis, performed by considering the confounding variables (sex, age, BMI, diabetes, alcohol intake, the baseline/along the study administration of Non-Selective Beta Blockers), revealed the RPR (adjusted OR: 1.91; 95%CI: 1.72-1.98; P: 0.002) and the presence of baseline-assessed CSPH (adjusted OR: 1.84; 95%CI: 1.72-1.91; P: 0.04) significantly and independently associated with the outcome (Supplementary Table 2 and Figure 6).
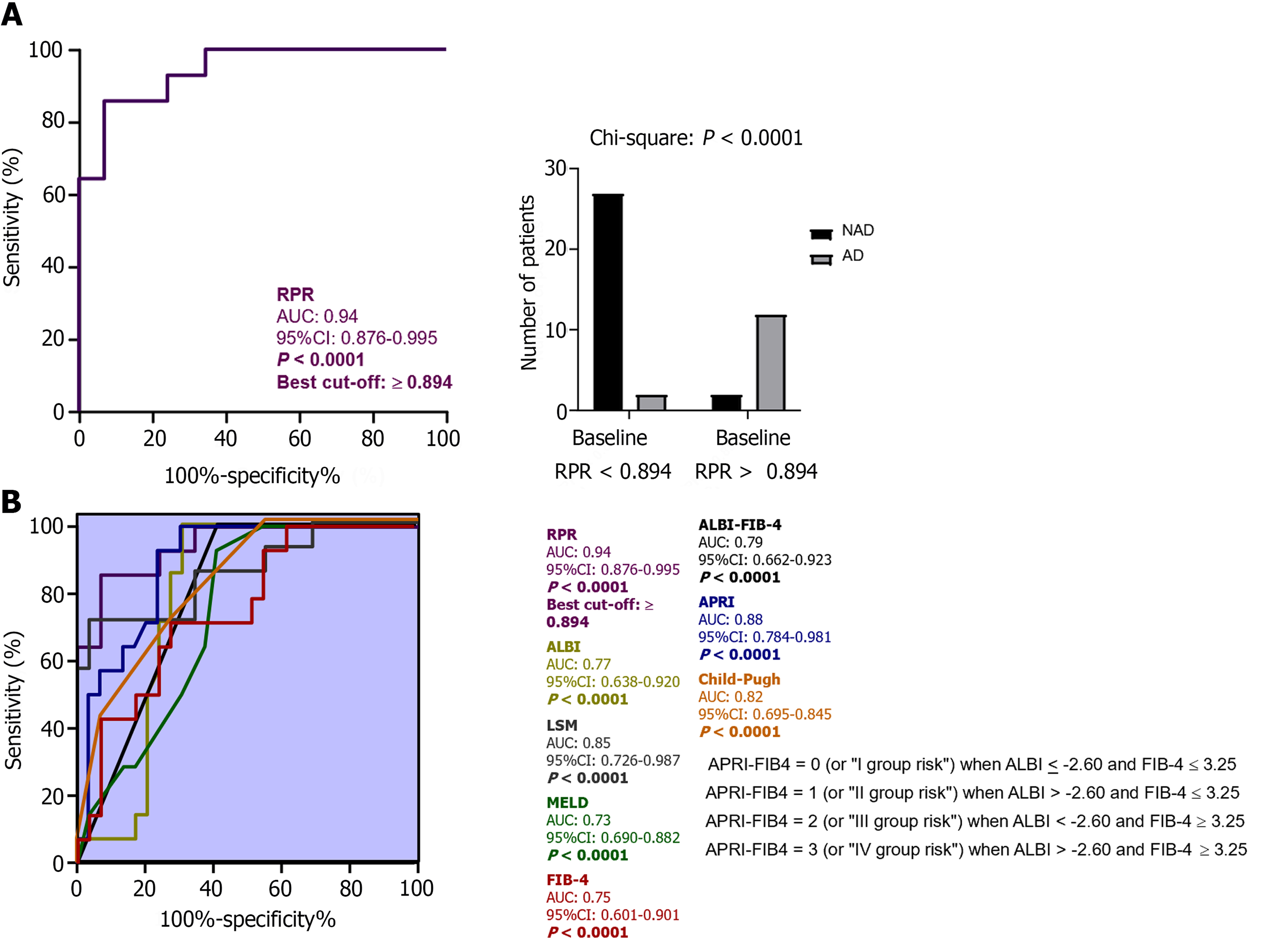
Of 43 cACLD patients progressing to dACLD, a first DE defining NAD and AD was respectively observed for 29 (NAD: 67.4 %) and 14 (AD: 32.5%) individuals. Tables 9-13 reports in detail the first DEs with the relative modalities for AD-decompensating and NAD-decompensating patients, as well as the relative baseline anthropometric indexes, biochemical parameters, and non-invasive tools for liver-functional status and hepatic fibrosis assessment (CP, MELD, LSM, FIB-4, APRI, ALBI, and RPR; Table 13). Consistently, AD-decompensating patients presented significantly higher baseline CP, MELD, APRI, LSM, ALBI, and RPR values in comparison to NAD-decompensating individuals (Table 13).
| Type of first decompensation event | NAD-decompensating patients (n = 29) | AD-decompensating patients (n = 14) |
| (A) Slow/ grade 1 ascites formation [n (%)] | 14 (48.3) | / |
| (B) Mild (grade 1/2) hepatic encephalopathy [n (%)] | 6 (20.7) | / |
| (C) Jaundice in non-cholestatic cirrhosis [n (%)] | 9 (31) | / |
| A + B/A + C | 2/5 | |
| (D) Grade 2/3 ascites within less than 2 wk [n (%)] | / | 5 (35.7) |
| (E) Severe acutea hepatic encephalopathy [n (%)] | / | 4 (28.5) |
| (F) Acute gastrointestinal bleeding [n (%)] | / | 2 (14.3) |
| (G) Acute bacterial infection | / | 3 (21.5) |
| Variables (mean ± SD) | NAD-decompensating patients (n = 29) | AD-decompensating patients (n = 14) | P value1 |
| BMI (kg/m2) | 29.57 ± 3.17 | 31.43 ± 2.72 | NS |
| WhR | 0.99 ± 0.11 | 1.05 ± 0.17 | NS |
| Systolic blood pressure (mm/Hg) | 130.3 ± 9.81 | 132.9 ± 10.59 | NS |
| Diastolic blood pressure (mm/Hg) | 86.03 ± 8.59 | 86.07 ± 6.25 | NS |
| Variables (mean ± SD) | NAD-decompensating patients (n = 29) | AD-decompensating patients (n =14) | P value1 |
| AST (IU/L) | 27.93 ± 26.99 | 44.11 ± 11.26 | 0.004 |
| ALT (IU/L) | 40.10 ± 35.52 | 54.71 ± 51.23 | NS |
| Bilirubin (µmol/L) | 21.75 ± 5.32 | 26.94 ± 1.32 | NS |
| PLT count (mm3) | 100.2 ± 24.37 | 61.57 ± 14.12 | < 0.0001 |
| RDW-CV (%) | 26.16 ± 19.26 | 51.51 ± 21.23 | < 0.0001 |
| RDW-SD (fL) | 54.39 ± 10.21 | 64.10 ± 10.14 | < 0.0001 |
| Albumin (g/L) | 28.6 ± 2.21 | 21.8 ± 2.59 | NS |
| INR | 1.76 ± 0.66 | 1.96 ± 0.62 | NS |
| HOMA-IR | 3.44 ± 1.29 | 4.12 ± 1.09 | NS |
| Total cholesterol (mg/dL) | 195.4 ± 56.20 | 164.4 ± 44.17 | NS |
| Tryglicerides (mg/dL) | 167.4 ± 92.42 | 152.1 ± 60.71 | NS |
| Creatinine (mg/dL) | 1.27 ± 1.51 | 2.61 ± 1.22 | 0.03 |
| Variables (mean ± SD) | NAD-decompensating patients (n = 29) | AD-decompensating patients (n = 14) | P value1 |
| LSM (kPa) | 20.90 ± 2.09 | 28.04 ± 3.44 | < 0.0001 |
| APRI | 1.60 ± 0.30 | 1.90 ± 0.38 | < 0.0001 |
| FIB-4 | 3.53 ± 1.75 | 3.86 ± 2.64 | NS |
| ALBI | -1.98 ± 0.62 | -1.66 ± 0.35 | 0.03 |
| Child-Pugh | 6.89 ± 0.97 | 7.42 ± 0.75 | 0.046 |
| MELD | 11.07 ± 3.35 | 13.79 ± 2.07 | 0.011 |
| RDW (fL)/PLT ratio | 0.668 ± 0.152 | 1.077 ± 0.253 | < 0.0001 |
ROC analysis with the Youden index calculation for the identification of best cut-off values revealed > 0.894 as the RPR threshold (AUC: 0.94; sensitivity: 93.1%; specificity: 85.7%; NPV: 85.71%; PPV: 93.1%; P < 0.0001) in the prediction of AD as first DE, as well as superior RPR accuracy compared to APRI (AUC: 0.88), FIB-4 (AUC: 0.75), MELD (AUC: 0.73), CP (AUC: 0.82), LSM (AUC: 0.85), ALBI (AUC: 0.77), and ALBI-FIB-4 (AUC: 0.79; all P <0.0001; Figure 7 and Table 14).
| Value | 95%CI | |
| Sensitivity | 0.9310 | 0.7804-0.9877 |
| Specificity | 0.8571 | 0.6006-0.9746 |
| Positive predictive value | 0.9310 | 0.7804-0.9877 |
| Negative predictive value | 0.8571 | 0.6006-0.9746 |
The multinomial logistic regression analysis, performed by considering the confounding variables (sex, age, BMI, diabetes, alcohol intake, the baseline/along the study administration of Non-Selective Beta Blockers), revealed the RPR baseline values (adjusted OR: 2.11; 95%CI: 1.72-2.22; P: 0.03), the presence of baseline-assessed CSPH (adjusted OR: 2.04; 95%CI: 1.92-2.11; P: 0.003), and the entity of varices (adjusted OR: 1.98; 95%CI: 1.79-2.06; P: 0.073) as the variables significantly and independently associated with the outcome (Supplementary Table 3).
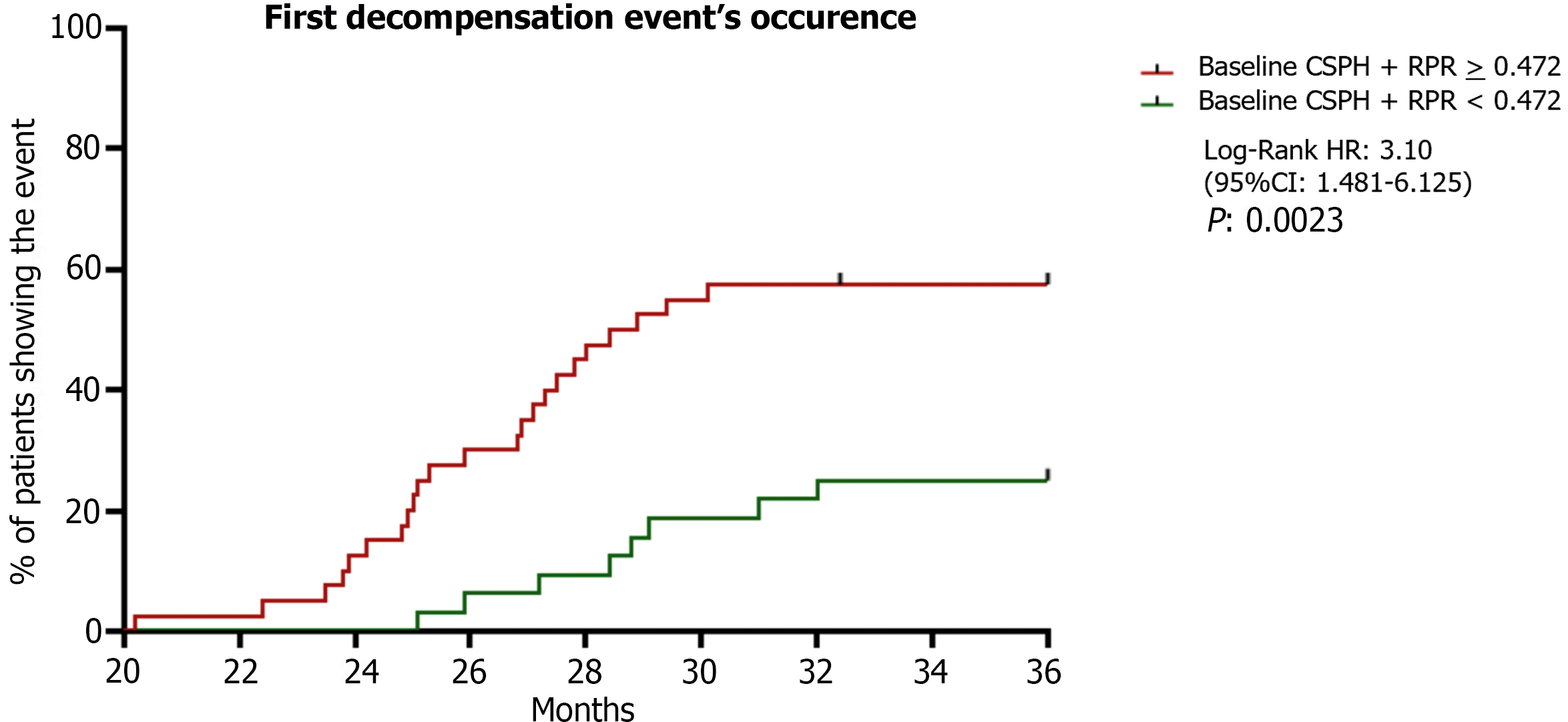
Therefore, considering these relevant findings, individuals presenting baseline CSPH were considered as “high-risk of decompensation” patients and included in a further sub-analysis investigating the relationship between RPR, liver disease progression, CSPH, and decompensation.
Regarding RPR modifications and liver disease progression, a statistically significant positive correlation between D% RPR and D% LSM was highlighted (R: 0.84; 95%CI: 0.732-0.91; P < 0.000; Supplementary Figure 1). Concerning CSPH evaluation, of 150 patients, 71 (47.3%) underwent a screening EGDS [41 (57.7%) because of PLT-count-established Baveno VI criteria, 10 (14.1%) because of LSM-established Baveno VI Criteria, and 20 (28.2%) because of both criteria][23]. According to Baveno VII Criteria (32), CSPH was non-invasively assumable in 7 of 10 patients presenting LSM values 25 kPa; however, given the non-availability of these criteria at the time of the enrollment, an EGDS was performed. EGDS revealed the presence of varices in 52 (73%) of individuals (38: F1 varices; 14: F2 varices). Twenty of 150 cACLD individuals showed esophageal varices in anamnesis (14: F1 varices; 6: F2 varices). Hence, a total of 78 individuals (52%) were baseline-CSPH free and, of these, 12 (15.3%) progressed to decompensation; a total of 72 (48%) presented baseline-CSPH, and, of these, 21 (29.1%) progressed to decompensation [with 11 (37.9%) presenting AD as the first DE]. In a mirror way, the prevalence of baseline CSPH in decompensating patients was significantly higher in patients progressing to dACLD in comparison to individuals remaining compensated (P: 0.0001), and in AD-decompensating subjects in comparison to NAD-decompensating patients (P: 0.0035; Supplementary Figure 2). On the first DE, independently from the endoscopic surveillance programs, the Baveno VII CSPH-rule in criteria[32] were adopted and CSPH was assumable in 41 (95.3 %) of decompensating patients.
Regarding RPR and CSPH, baseline RPR values were significantly higher in patients presenting baseline CSPH compared to individuals without esophageal varices (P < 0.04), and the prevalence of baseline CSPH in decompensating patients was significantly higher in patients presenting RPR baseline values > 0.472 (the best cut-off; Supplement
The irrepressible spreading of MASLD worldwide[5], in synergy with the evidence that MASLD/MASH-related cirrhosis may more rapidly progress to dACLD[7,8], remark the identification of tools predicting the decompensation in these patients as an absolute global priority. Up to now, in scientific literature, various emerging findings suggested the RPR as a predictor of severe fibrosis and cirrhosis in MASLD[21,22]. However, the link between RPR and liver decompensation in MASLD patients has never been investigated.
In the present observational study, we investigated the accuracy of RPR in the prediction of 3-year first DE occurrence in MASLD-related cACLD patients as a non-invasive tool stratifying the risk of decompensation in this setting. For this purpose, 40 controls and 150 MAFLD-cACLD patients were enrolled and followed semi-annually for 3 years. At baseline, MAFLD-cACLD individuals received a complete liver-disease status assessment including the determination of MELD, CP, APRI, ALBI, FIB-4, ALBI-FIB-4, LSM, and RPR; DE were subsequently recorded along the entire follow-up.
As expected, RPR values were shown significantly higher (P < 0.0001) in ACLD patients in comparison to healthy controls. Moreover, RPR and the baseline values of all the other non-invasive tools appeared significantly (all P < 0.0001, except for FIB-4, P: 0.007 and ALBI, P: 0.011) increased in patients progressing to decompensation in comparison to subjects who completed the follow-up remaining compensated. In line with these findings, a direct positive linear relationship between baseline RPR values and the other non-invasive tools was also highlighted and, consistently with the pre-existing evidence exploring predominantly the RPR role in the prediction of hepatic fibrosis[21], the correlation between RPR and LSM emerged as the most strict (R: 0.94). However, in comparison to all the other non-invasive tools (MELD, CP, APRI, ALBI, FIB-4, ALBI-FIB-4, and LSM), ROC analysis with the Youden index calculation evidenced a significantly higher accuracy [AUC: 0.95; P < 0.0001] of RPR in the prediction of 3-year first DE occurrence, without statistically significant differences between male and female MASLD individuals. RPR optimal cut-off (≥ 0.472) was also highlighted, as well as the relatively excellent prognostic performance suggested by very high levels of sensitivity (86.9%), specificity (90.7%), and an elevated (95.8%) PPV of decompensation.
Relevantly, patients presenting baseline RPR values ≥ 0.472 showed an elevated risk (HR: 13.62) of decompensation at 3 years (median time of decompensation of 26.4 months), with an IRR for first DE occurrence significantly higher in comparison to individuals presenting baseline RPR values under the 0.472 threshold.
Emerging evidence has revealed that, according to the pattern of the first DE, the transition to dACLD can occur through two modalities with relatively different long-term repercussions: The prognostically burdensome AD; the progressive NAD[4]. Although AD has been reported as an event occurring more frequently in already decompensated patients, when representing the first DE, it may severely impact the prognosis[4]. Therefore, the prediction of AD was based on a solid rationale and not fueled by horror vacui, representing a concrete aim of our research. To the best of our knowledge, in fact, our study is the first to assess the accuracy of a tool in the prediction of AD in cACLD patients. Concerning this, we demonstrated that modalities (AD vs NAD) of the first decompensation can be predicted by using RPR: An RPR ≥ 0.894 was shown as the threshold more accurately predicting AD (PPV: 93.1%). Moreover, ROC analysis also revealed the superiority of RPR in comparison to the other non-invasive tools (MELD, CP, APRI, ALBI, FIB-4, ALBI-FIB-4, and LSM) in the prediction of this outcome.
Altogether these findings suggest the RPR is a valid and potentially applicable non-invasive tool in the prediction of timing and modalities of decompensation in MASLD-related cACLD patients.
The importance of predicting whether and how the patient affected by MASLD-related-cACLD will move to dACLD is related to various management aspects. First, decompensation constitutes a turning point in the natural history of ACLD, and an extremely relevant feature during the clinical course of cirrhosis, which should be managed as quickly and appropriately as possible, to improve the possibility of care; early detection of this transition phase would enable targeted therapeutic interventions, potential improving life expectancy, and improving their prognosis[34]. Secondly, it’s also essential to highlight that risk of death strongly increases when a patient shifts to dACLD: 9.7 times as high as the risk in the general population, and it’s double compared to cACLD subjects[35]. In these terms, the decompensation marks a significant worsening of patient prognosis from a median survival exceeding 12 years and a preserved quality of life in compensated patients to a median survival of 2-4 years in the decompensated stage with several socioeconomic and healthy repercussions: admission rate, hospital stay, and costs considerably increased in a stepwise manner after the first episode of AD[36]; hospitalizations for the dACLD increase by a third in the total healthcare costs compared to cACLD individuals[37].
A plethora of studies have tried to explain which could be the most accurate predictor of decompensation in these patients[9,38]. The strongest predictor of transition to dACLD is, for values of ≥ 10 mmHg, the HVPG, well-studied as a marker of CSPH. However, due to the limitations related to justifying invasive HVPG measurement and its expensive costs, it is almost never evaluated in daily clinical practice in most centers[9,39]. However, while if for patients with viral- and alcohol-related cirrhosis, HVPG measurement is the gold-standard method to determine the presence of CSPH, in MASLD/MASH individuals the question is still widely debated[32,38]. Moreover, in patients with MASH- related cirrhosis, although an HVPG 10 mmHg remains strongly associated with the presence of clinical signs of portal hyper
Different research investigated the role of other non-invasive and routinely tools in the prediction of decompensation. Guha et al[12] in a recent study, also including patients with aetiologies other than MASLD, introduced a new model to predict the risk of decompensation in patients with compensated cirrhosis based on the combination of two (ALBI + FIB-4) previously identified scores: ALBI-FIB-4.
In our study, following the original ALBI-FIB-4 proposed group stratification, we compared the accuracy of RPR with ALBI-FIB-4 in the prediction of decompensation revealing a higher RPR performance in the prediction of this outcome (AUC: 0.95 vs AUC: 0.93). The NAFLD decompensation risk score (the Iowa Model) was recently developed to identify patients with MASLD at the highest risk of developing hepatic events using three variables-age, PLT count, and diabetes[15]. In a recent study including 249 MASLD patients, the AUC of the Iowa Model (0.88) was comparable to the FIB-4 (0.87) and higher than APRI (0.76)[15]. We herein decided to not perform a comparison RPR vs Iowa model, considering the new proposed MASLD diagnostic criteria[6] supporting the non-essential presence of diabetes to perform diagnosis, as many MASLD patients may present without this comorbidity. Rather, in our study, diabetes was included as a confounding variable in the multinomial logistic regression analysis.
The multinomial logistic regression analysis, performed by considering the confounding variables (sex, age, BMI, diabetes, alcohol intake, the administration of Non-Selective Beta Blockers), revealed besides the RPR, the baseline CSPH as a variable significantly associated with the outcomes (DE and AD). These findings constituted the primum movens to perform a sub-analysis investigating the relationship between RPR, liver disease progression, CSPH, and decompensation in our study. Consistently with the chronic nature of MASLD disorder, a significant positive correlation between RPR (DRPR) and LSM (DLSM) modifications was highlighted, suggesting RPR is dynamically influenced by the course of the hepatic disease.
The inclusion of CSPH assessment represented a crucial strength of our research: In fact, none of the other previously mentioned evidence reported the proportion of patients with varices, making uncertain whether patients were comparable regarding their likelihood of having CSPH and, therefore, of decompensating. In our study, baseline RPR values were significantly higher in patients with baseline CSPH (P < 0.04) and positively correlated with esophageal varices severity (P < 0.0001). The prevalence of baseline CSPH in decompensating patients was significantly higher in patients presenting RPR baseline values 0.472. Relevantly, individuals presenting baseline CSPH and RPR values 0.472 showed a significantly elevated risk (HR: 3.10, P: 0.0023) of decompensation in comparison to baseline-CSPH individuals presenting lower RPR values supporting the following risk-stratification: (1) “High risk of decompensation” (baseline CSPH and RPR < 0.472); and (2) “very high-risk of decompensation” (baseline CSPH and RPR 0.472). Considering the discrepant modalities of CSPH definition between baseline (EGDS-evidence of esophageal varices) and on first DE occurrence (CSPH assumption according to Baveno VII criteria) with a not-negligible number (61%) of patients avoiding/not undergoing surveillance endoscopy (i.e., repetition, during the 3-years follow-up, of a new EGDS for patients presenting baseline CSPH) also due to SARS CoV2 pandemic-related logistic difficulties, the not-availability of HVPG data, and, even more relevant, the limited sample size of the sub-analysis, the RPR baseline accuracy in the prediction of baseline CSPH and CSPH development along the observational period did not represent an aim of our study and was herein not investigated. The PREDESCI trial evidenced the role of non-selective beta-blockers in the prevention of decompensation in patients with CSPH[40]. Considering this, after the inclusion of the administration of propranolol and carvedilol (recorded at the baseline and on every semiannual follow-up visit) in the logistic regression model, no influence on our predictive results was highlighted.
Our study presents some limitations. First, it is based on a single-center cohort of patients, so further prospective studies at multiple centers are required to validate the clinical use of RPR in validation cohorts. Second, our population, even if a representative MASLD cohort, could represent a relatively small sample size. Finally, the accuracy of RPR was not compared with HVPG; unfortunately, in fact, the SARS CoV2 Lock-down negatively limited the availability of this tool in our center during the pandemic and we were able to collect HVPG data for a very restricted number of the enrolled patients.
As a final consideration, in the wake of our results and looking ahead to future scenarios, considering the elevated high risk of major cardiovascular events occurrence in MASLD patients[41], and the RDW well-consolidated association with cardiovascular diseases-related complications[42], it appears also reasonable to hypothesize the designation of studies investigating the potential relationship between the RPR and risk of cardiovascular acute events in MASLD individuals. The developing of tools simultaneously identifying MASLD subjects at higher risk of hepatic decompensation and acute cardiovascular events occurrence would represent a cornerstone element in the prognostic tailored management of these patients.
In the era of Precision Medicine, the development of tools non-invasively predicting decompensation in cACLD patients represents an unmet need and appears a paramount challenge for the hepatological research. Our study suggests RPR accurately predicts the time and modalities of decompensation in MASLD-related-ACLD patients, presenting the potential to be a valuable, easy-to perform, non-invasive clinical index.
In clinical practice, the availability of non-invasive tools predicting the first decompensation event (DE) in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)-related compensated advanced chronic liver disease (cACLD) context is still an unmet need.
Red cell distribution width to platelet ratio (RPR) has been recently shown to predict fibrosis in MASLD patients; however, its role in predicting DE has never been explored.
Herein, we investigated the clinical usage of RPR in predicting DEs in MASLD-related cACLD patients.
MASLD-cACLD patients were consecutively enrolled and followed up for 3 years. Biochemical, clinical, and Liver Stiffness Measurement were collected.
RPR accurately predicts [area under the curve (AUC): 0.94; best cut-off 0.472) the first DE in MASLD-cACLD. RPR accurately predicts acute decompensation (AD; AUC: 0.94; best cut-off 0.894) as the first DE in these patients. Patients presenting baseline clinically significant portal hypertension and RPR 0.472 show higher risk (hazard ratio: 3.10) of 3-year decompensation occurrence.
Altogether these findings suggest RPR as a valid and potentially applicable non-invasive tool in the prediction of decompensation in MASLD-related cACLD patients.
The potential availability of RPR as non-invasive, not expensive, and routinely assessable tool in the prediction of timing and modalities of decompensation in MASLD-cACLD patients could remodel the management of these patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng J, China S-Editor: Lin C L-Editor: A P-Editor: Cai YX
| 1. | Lurie Y, Webb M, Cytter-Kuint R, Shteingart S, Lederkremer GZ. Non-invasive diagnosis of liver fibrosis and cirrhosis. World J Gastroenterol. 2015;21:11567-11583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 189] [Cited by in RCA: 255] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 2. | Segna D, Mendoza YP, Lange NF, Rodrigues SG, Berzigotti A. Non-invasive tools for compensated advanced chronic liver disease and portal hypertension after Baveno VII - an update. Dig Liver Dis. 2023;55:326-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 3. | Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1441] [Article Influence: 180.1] [Reference Citation Analysis (3)] |
| 4. | D'Amico G, Bernardi M, Angeli P. Towards a new definition of decompensated cirrhosis. J Hepatol. 2022;76:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 113] [Article Influence: 37.7] [Reference Citation Analysis (1)] |
| 5. | Clayton-Chubb D, Kemp WW, Majeed A, Lubel JS, Woods RL, Tran C, Ryan J, Hodge A, Schneider HG, McNeil JJ, Roberts SK. Metabolic dysfunction-associated steatotic liver disease in older adults is associated with frailty and social disadvantage. Liver Int. 2024;44:39-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 6. | Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab JP, Arrese M, Bataller R, Beuers U, Boursier J, Bugianesi E, Byrne CD, Castro Narro GE, Chowdhury A, Cortez-Pinto H, Cryer DR, Cusi K, El-Kassas M, Klein S, Eskridge W, Fan J, Gawrieh S, Guy CD, Harrison SA, Kim SU, Koot BG, Korenjak M, Kowdley KV, Lacaille F, Loomba R, Mitchell-Thain R, Morgan TR, Powell EE, Roden M, Romero-Gómez M, Silva M, Singh SP, Sookoian SC, Spearman CW, Tiniakos D, Valenti L, Vos MB, Wong VW, Xanthakos S, Yilmaz Y, Younossi Z, Hobbs A, Villota-Rivas M, Newsome PN; NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79:1542-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1301] [Article Influence: 650.5] [Reference Citation Analysis (1)] |
| 7. | Harrison SA, Abdelmalek MF, Caldwell S, Shiffman ML, Diehl AM, Ghalib R, Lawitz EJ, Rockey DC, Schall RA, Jia C, McColgan BJ, McHutchison JG, Subramanian GM, Myers RP, Younossi Z, Ratziu V, Muir AJ, Afdhal NH, Goodman Z, Bosch J, Sanyal AJ; GS-US-321-0105 and GS-US-321-0106 Investigators. Simtuzumab Is Ineffective for Patients With Bridging Fibrosis or Compensated Cirrhosis Caused by Nonalcoholic Steatohepatitis. Gastroenterology. 2018;155:1140-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 281] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 8. | Loomba R, Wong R, Fraysse J, Shreay S, Li S, Harrison S, Gordon SC. Nonalcoholic fatty liver disease progression rates to cirrhosis and progression of cirrhosis to decompensation and mortality: a real world analysis of Medicare data. Aliment Pharmacol Ther. 2020;51:1149-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 134] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 9. | Ripoll C, Groszmann R, Garcia-Tsao G, Grace N, Burroughs A, Planas R, Escorsell A, Garcia-Pagan JC, Makuch R, Patch D, Matloff DS, Bosch J; Portal Hypertension Collaborative Group. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 810] [Article Influence: 45.0] [Reference Citation Analysis (1)] |
| 10. | Sharma S, Agarwal S, Saraya A. An LSM Based Strategy is Comparable to HVPG Measurement to Predict Further Events in Patients with Cirrhosis with Variceal Bleeding as Their Index Decompensation. J Clin Exp Hepatol. 2023;13:774-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Kamada Y, Munekage K, Nakahara T, Fujii H, Sawai Y, Doi Y, Hyogo H, Sumida Y, Imai Y, Miyoshi E, Ono M; Japan Study Group of NAFLD (JSG-NAFLD). The FIB-4 Index Predicts the Development of Liver-Related Events, Extrahepatic Cancers, and Coronary Vascular Disease in Patients with NAFLD. Nutrients. 2022;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Guha IN, Harris R, Berhane S, Dillon A, Coffey L, James MW, Cucchetti A, Harman DJ, Aithal GP, Elshaarawy O, Waked I, Stewart S, Johnson PJ. Validation of a Model for Identification of Patients With Compensated Cirrhosis at High Risk of Decompensation. Clin Gastroenterol Hepatol. 2019;17:2330-2338.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | Wan SZ, Nie Y, Zhang Y, Liu C, Zhu X. Assessing the Prognostic Performance of the Child-Pugh, Model for End-Stage Liver Disease, and Albumin-Bilirubin Scores in Patients with Decompensated Cirrhosis: A Large Asian Cohort from Gastroenterology Department. Dis Markers. 2020;2020:5193028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Kamath PS, Kim WR; Advanced Liver Disease Study Group. The model for end-stage liver disease (MELD). Hepatology. 2007;45:797-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1075] [Cited by in RCA: 1228] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 15. | Ahmed HS, Gangasani N, Jayanna MB, Long MT, Sanchez A, Murali AR. The NAFLD Decompensation Risk Score: External Validation and Comparison to Existing Models to Predict Hepatic Events in a Retrospective Cohort Study. J Clin Exp Hepatol. 2023;13:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. 2015;52:86-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 714] [Article Influence: 64.9] [Reference Citation Analysis (1)] |
| 17. | Aslam H, Oza F, Ahmed K, Kopel J, Aloysius MM, Ali A, Dahiya DS, Aziz M, Perisetti A, Goyal H. The Role of Red Cell Distribution Width as a Prognostic Marker in Chronic Liver Disease: A Literature Review. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 18. | Hu Z, Sun Y, Wang Q, Han Z, Huang Y, Liu X, Ding C, Hu C, Qin Q, Deng A. Red blood cell distribution width is a potential prognostic index for liver disease. Clin Chem Lab Med. 2013;51:1403-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 19. | Milić S, Mikolasević I, Radić M, Hauser G, Stimac D. Clinical utility of red cell distribution width in alcoholic and non-alcoholic liver cirrhosis. Coll Antropol. 2011;35 Suppl 2:335-338. [PubMed] |
| 20. | Taefi A, Huang CC, Kolli K, Ebrahimi S, Patel M. Red cell distribution width to platelet ratio, a useful indicator of liver fibrosis in chronic hepatitis patients. Hepatol Int. 2015;9:454-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Michalak A, Guz M, Kozicka J, Cybulski M, Jeleniewicz W, Lach T, Cichoż-Lach H. Red blood cell distribution width derivatives in alcohol-related liver cirrhosis and metabolic-associated fatty liver disease. World J Gastroenterol. 2022;28:5636-5647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Yuyun D, Zhihua T, Haijun W, Zhaoping L, Xiaoli Z, Wenfang X, Faxiang J, Hongmei L. Predictive value of the red blood cell distribution width-to-platelet ratio for hepatic fibrosis. Scand J Gastroenterol. 2019;54:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2293] [Article Influence: 229.3] [Reference Citation Analysis (3)] |
| 24. | Salgado AL, Carvalho Ld, Oliveira AC, Santos VN, Vieira JG, Parise ER. Insulin resistance index (HOMA-IR) in the differentiation of patients with non-alcoholic fatty liver disease and healthy individuals. Arq Gastroenterol. 2010;47:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 195] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 25. | Maggi U, Rossi G, Colledan M, Fassati LR, Gridelli B, Reggiani P, Basadonna G, Colombo A, Doglia M, Ferla G. Child-Pugh score and liver transplantation. Transplant Proc. 1993;25:1769-1770. [PubMed] |
| 26. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3246] [Article Influence: 147.5] [Reference Citation Analysis (0)] |
| 27. | Demirtas CO, D'Alessio A, Rimassa L, Sharma R, Pinato DJ. ALBI grade: Evidence for an improved model for liver functional estimation in patients with hepatocellular carcinoma. JHEP Rep. 2021;3:100347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 122] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 28. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 3566] [Article Influence: 187.7] [Reference Citation Analysis (0)] |
| 29. | Liao R, Li DW, Du CY, Li M. Combined Preoperative ALBI and FIB-4 Is Associated with Recurrence of Hepatocellular Carcinoma After Curative Hepatectomy. J Gastrointest Surg. 2018;22:1679-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Boursier J, Zarski JP, de Ledinghen V, Rousselet MC, Sturm N, Lebail B, Fouchard-Hubert I, Gallois Y, Oberti F, Bertrais S, Calès P; Multicentric Group from ANRS/HC/EP23 FIBROSTAR Studies. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology. 2013;57:1182-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 496] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 31. | Sasso M, Beaugrand M, de Ledinghen V, Douvin C, Marcellin P, Poupon R, Sandrin L, Miette V. Controlled attenuation parameter (CAP): a novel VCTE™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36:1825-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 638] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 32. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1494] [Article Influence: 498.0] [Reference Citation Analysis (2)] |
| 33. | Beppu K, Inokuchi K, Koyanagi N, Nakayama S, Sakata H, Kitano S, Kobayashi M. Prediction of variceal hemorrhage by esophageal endoscopy. Gastrointest Endosc. 1981;27:213-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 553] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 34. | Mandorfer M, Simbrunner B. Prevention of First Decompensation in Advanced Chronic Liver Disease. Clin Liver Dis. 2021;25:291-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 35. | Ge PS, Runyon BA. Treatment of Patients with Cirrhosis. N Engl J Med. 2016;375:767-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 233] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 36. | Lee H, Kim BK. Real-world clinical features, health-care utilization, and economic burden in decompensated cirrhosis patients: A national database. J Gastroenterol Hepatol. 2022;37:2154-2163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Desai AP, Mohan P, Nokes B, Sheth D, Knapp S, Boustani M, Chalasani N, Fallon MB, Calhoun EA. Increasing Economic Burden in Hospitalized Patients With Cirrhosis: Analysis of a National Database. Clin Transl Gastroenterol. 2019;10:e00062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 38. | Rodrigues SG. Baveno VII criteria to predict decompensation in compensated advanced chronic liver disease: Still some shades of grey. Clin Mol Hepatol. 2023;29:110-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 39. | Suk KT. Hepatic venous pressure gradient: clinical use in chronic liver disease. Clin Mol Hepatol. 2014;20:6-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 40. | Villanueva C, Albillos A, Genescà J, Garcia-Pagan JC, Calleja JL, Aracil C, Bañares R, Morillas RM, Poca M, Peñas B, Augustin S, Abraldes JG, Alvarado E, Torres F, Bosch J. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2019;393:1597-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 451] [Article Influence: 75.2] [Reference Citation Analysis (0)] |
| 41. | Targher G, Byrne CD, Tilg H. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut. 2020;69:1691-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 513] [Article Influence: 102.6] [Reference Citation Analysis (0)] |
| 42. | Abrahan LL 4th, Ramos JDA, Cunanan EL, Tiongson MDA, Punzalan FER. Red Cell Distribution Width and Mortality in Patients With Acute Coronary Syndrome: A Meta-Analysis on Prognosis. Cardiol Res. 2018;9:144-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |