Published online Feb 21, 2024. doi: 10.3748/wjg.v30.i7.663
Peer-review started: December 7, 2023
First decision: December 18, 2023
Revised: December 22, 2023
Accepted: January 22, 2024
Article in press: January 22, 2024
Published online: February 21, 2024
Processing time: 76 Days and 0.3 Hours
Colorectal cancer liver metastasis (CRLM) presents a clinical challenge, and optimizing treatment strategies is crucial for improving patient outcomes. Surgical resection, a key element in achieving prolonged survival, is often linked to a heightened risk of recurrence. Acknowledging the potential benefits of preoperative neoadjuvant chemotherapy in managing resectable liver metastases, this approach has gained attention for its role in tumor downsizing, assessing biological behavior, and reducing the risk of postoperative recurrence. However, the use of neoadjuvant chemotherapy in initially resectable CRLM sparks ongoing debates. The balance between tumor reduction and the risk of hepatic injury, coupled with concerns about delaying surgery, necessitates a nuanced approach. This article explores recent research insights and draws upon the practical experiences at our center to address critical issues regarding considerations for initially resectable cases. Examining the criteria for patient selection and the judicious choice of neoadjuvant regimens are pivotal areas of discussion. Striking the right balance between maximizing treatment efficacy and minimizing adverse effects is imperative. The dynamic landscape of precision medicine is also reflected in the evolving role of gene testing, such as RAS/BRAF and PIK3CA, in tailoring neoadjuvant regimens. Furthermore, the review emphasizes the need for a multidisciplinary approach to navigate the comp
Core Tip: Optimizing treatment for colorectal cancer liver metastasis (CRLM) is essential. This review explores the dynamic landscape of preoperative neoadjuvant chemotherapy for initially resectable CRLM, addressing debates, criteria for patient selection, and the role of gene testing. Emphasizing a multidisciplinary approach, it navigates complexities in managing progression post-chemotherapy, contributing to ongoing discussions on refining treatment paradigms for improved outcomes.
- Citation: Cheng XF, Zhao F, Chen D, Liu FL. Current landscape of preoperative neoadjuvant therapies for initial resectable colorectal cancer liver metastasis. World J Gastroenterol 2024; 30(7): 663-672
- URL: https://www.wjgnet.com/1007-9327/full/v30/i7/663.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i7.663
Colorectal cancer liver metastasis (CRLM), identified in approximately 25% of cases at the primary diagnosis, significantly contributes to both the incidence and mortality rates of colorectal cancer (CRC)[1]. The prognosis for CRC patients with liver metastasis remains challenging, with a 5-year survival rate of less than 10%[2]. Although the outcome of metastatic CRC varies, the surgical removal of isolated CRC liver metastases holds the potential for curative effects. Recent studies have shown an upward trend in survival rates following hepatic resection for metastatic CRC, indicating improved prognoses[3]. Notably, for initially resectable CRLM patients, perioperative chemotherapy combined with surgery has been explored, emphasizing outcomes such as progression-free survival (PFS) and overall survival (OS)[4].
Despite surgery being the cornerstone for treating initially resectable CRLM, a considerable number of patients experience recurrence within a year post-surgery, highlighting the limitations of surgery alone[5]. This prompts the consideration of alternative treatment modalities, including direct surgery followed by adjuvant chemotherapy and neoadjuvant therapy followed by surgery and postoperative adjuvant chemotherapy.
The implementation of neoadjuvant chemotherapy as part of the treatment strategy offers distinct advantages and poses challenges. On the positive side, it enables the early control of microscopic metastatic lesions, facilitates the assessment of tumor response to chemotherapy, and provides patients with a "biological waiting window" to potentially avoid surgery in cases of early progression[6-9]. However, neoadjuvant chemotherapy introduces disadvantages such as the risk of chemotherapy-induced liver damage, including sinusoidal injury from oxaliplatin and irinotecan-induced steatohepatitis, thereby elevating the risk of surgical complications and mortality[10,11]. Furthermore, the disappearance of imaging lesions post-chemotherapy presents a challenge in determining the extent of surgical resection[12,13].
Thus, neoadjuvant chemotherapy acts as a double-edged sword, emphasizing the paramount importance of clinically identifying patients suitable for neoadjuvant chemotherapy vs direct surgery. The primary challenge faced by neoadjuvant treatment lies in the difficulty of patient selection, stemming from the vague definition of resectability and limited understanding of risk factors for recurrence in metastatic CRC, including biological behavior and inadequate assessment.
In clinical practice, the central question of "resectability" has traditionally focused on technical aspects. The criteria for deeming a case resectable have centered on the ability to achieve R0 resection for all liver lesions while ensuring an adequate residual liver volume[14]. However, this technical definition introduces inherent subjectivity, influenced by factors such as varying clinical volumes across different centers and the diverse technical prowess of hepatobiliary surgeons. Moreover, not all technically resectable liver metastases translate into meaningful postoperative benefits, with up to 80% of patients experiencing recurrence within three years post-resection[15-18]. This underscores the critical need for a more nuanced understanding and strategic planning of perioperative treatments to effectively mitigate the often-devastating consequences of postoperative recurrence.
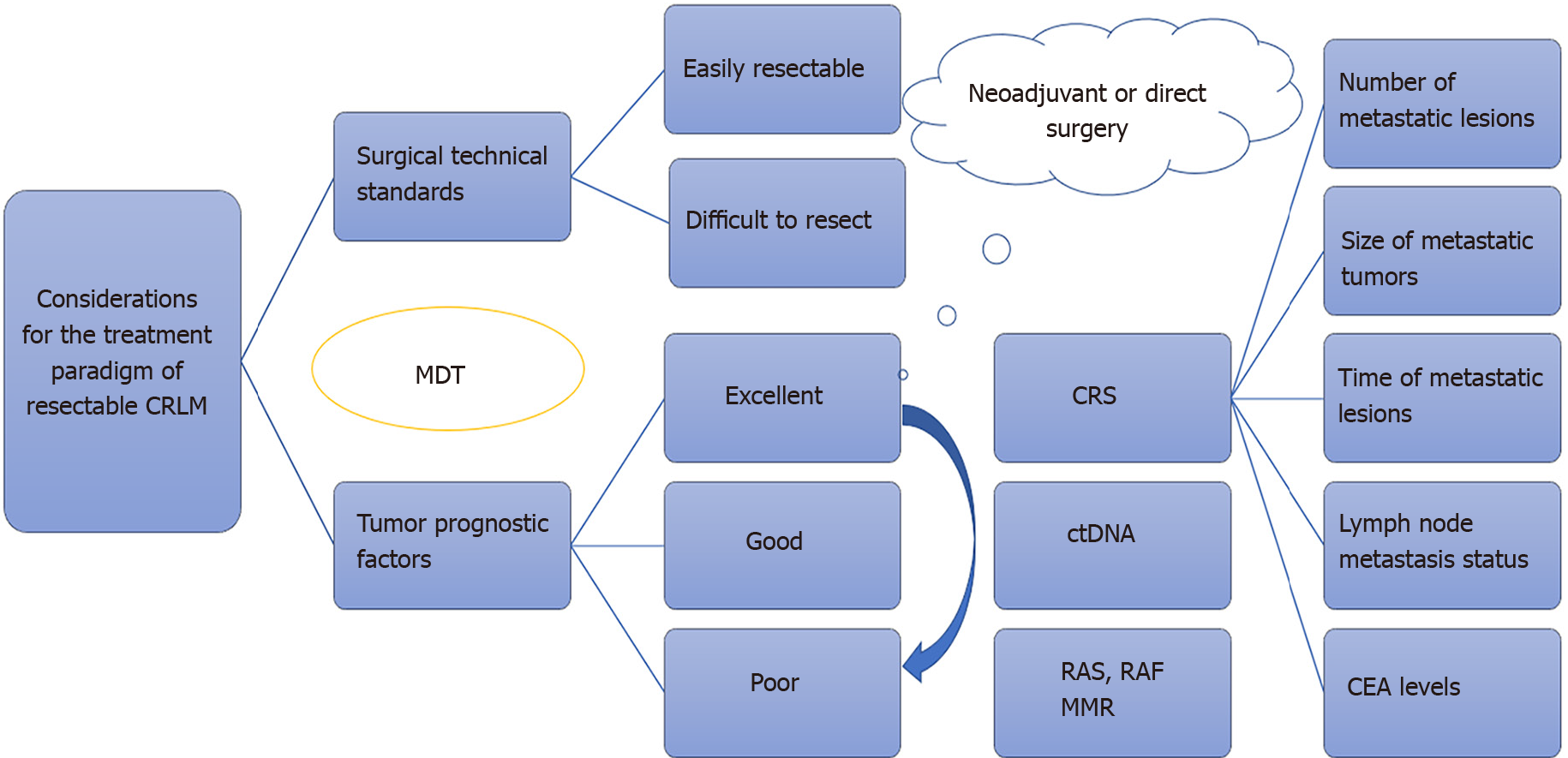
Recognizing the limitations of a purely technical definition, the European Society for Medical Oncology (ESMO) guidelines advocate for a comprehensive evaluation of initially resectable CRLM that integrates both "surgical technical standards" and "oncological prognostic factors"[19]. The surgical technical standards are further categorized into "easily resectable" and "difficult to resect," while oncological prognostic information is classified into "excellent," "good," and "poor" prognostic factors[19,20]. This dual-dimensional approach acknowledges the inherent complexity of assessing resectability, recognizing that successful surgery extends beyond technical feasibility to crucial oncological factors that significantly influence prognosis.
In 1999, Fong et al[21] introduced the Clinical risk score (CRS) to assess the risk of postoperative recurrence in CRLM patients[21]. This scoring system incorporates five clinical indicators, providing a valuable tool for identifying patients at high risk of recurrence[21]. However, despite its widespread use, there is a current gap in the literature regarding whether neoadjuvant treatment can improve outcomes for high-risk patients identified by CRS[22,23].
Addressing the biological dimension, Guinney et al[24] made a significant stride in understanding the molecular landscape of CRC with the introduction of Consensus molecular subtypes (CMS) in 2015[24]. This classification, encompassing four molecular subtypes, offers a more nuanced view of the disease[24]. However, the application of CMS in identifying beneficiaries of neoadjuvant treatment remains unexplored to date[25-28]. Bridging this gap in research is imperative for a comprehensive understanding of the molecular intricacies influencing treatment responses in CRC.
In recent years, advancements in prognostic assessment systems have been prompted by our evolving understanding of the molecular biology of tumors and treatment paradigms for CRLM. Notably, Brudvik et al[29] introduced the modified clinical score (m-CS) in 2017, an enhanced system incorporating RAS gene status, size of liver metastases, and lymph node status of the primary tumor[29]. While representing progress in simplification compared to previous scores, the m-CS lacks granularity in weighing diverse high-risk factors and fails to account for chemotherapy sensitivity. Wang et al's study[30] underscores the significance of neoadjuvant chemotherapy insensitivity, CRS > 2 points, and KRAS mutations as independent prognostic indicators, providing valuable insights for refining prognostic tools[30]. Utilizing a score-based prediction model, their findings present a concise yet effective means of predicting long-term survival postoperatively.
Moreover, a new frontier in prognostic assessment emerged in 2018 with the introduction of the tumor burden score (TBS) by Sasaki et al[31] Utilizing tumor size and number, TBS categorizes patients into low, intermediate, and high-risk groups[31]. External validation by Tsilimigras et al[32] showcased TBS's superior discriminatory ability compared to the widely used CRS[32]. The subsequent integration of genetic and morphological factors in the Genetic and morphological evaluation (GAME) score by Margonis et al[33] represents a notable step forward[33]. Though GAME scores outperformed CRS in external validation by Wang et al[34], a potential limitation lies in the exclusion of chemotherapy as an evaluation parameter. Nevertheless, this innovative integration of genetic and morphological factors in the GAME score enhances its prognostic utility in CRLM, urging further exploration of its practical implications.
The comprehensive evaluation of relapse risk (CERR) score emerges as a robust prognostic system, integrating the refined TBS (mTBS) model. Building upon TBS, the mTBS introduces parameters accounting for unilateral or bilateral metastases, addressing inherent limitations in TBS calculations. Chen et al[35] crafted the CERR score, considering factors such as KRAS/NRAS/BRAF mutation status, primary tumor lymph node involvement, presence of extrahepatic disease, and elevated carcinoembryonic antigen or carbohydrate antigen-199 Levels[35]. These elements, combined with mTBS, provide a CERR. Stratifying patients into high-risk (CERR score ≥ 4), intermediate-risk (2 ≤ CERR score ≤ 3), and low-risk groups (CERR score < 2), the CERR score exhibits superior discriminatory ability over CRS and GAME scoring systems[35]. This nuanced approach enhances our understanding of tumor biological behavior, aligning with both clinical and molecular perspectives. However, while the CERR score represents a comprehensive tool, its mathematical complexity and abstract metrics pose challenges for widespread clinical application.
Recent years have witnessed significant strides in the integration of artificial intelligence (AI) in medical practice, presenting a promising avenue for predicting patient prognosis following CRLM resection. Chakedis et al[36] pioneered a machine learning-based model that, through Bootstrap resampling and multifactorial logistic regression analysis, demonstrated remarkable accuracy in predicting recurrence risk[36]. Compared to the conventional CRS, the model exhibited a substantial increase in discriminative ability, showcasing its potential to predict postoperative recurrence. However, it is crucial to acknowledge the inherent limitations of machine learning, including the risk of model overfitting and the black-box nature of the model. These factors hinder its seamless integration into clinical practice, demanding careful consideration of its application.
In conclusion, the guidance of Multidisciplinary Teams (MDT) remains pivotal for stratified management based on patient-specific and tumor-specific factors. Adhering to diagnostic and therapeutic guidelines, respecting individual patient features, and aligning with established principles are crucial for optimizing patient care strategies and maximizing survival benefits. This comprehensive and personalized approach, incorporating the latest advancements in preoperative neoadjuvant therapies for initially resectable CRLM, underscores our commitment to achieving the highest standards in patient care. Future research should delve into refining prognostic models, addressing their limitations, and exploring innovative applications of AI in enhancing precision medicine for CRC patients. Refer to Table 1 for a detailed overview of biological behavior assessment systems, their advantages, and limitations.
| Evaluation System | Components included | Advantages | Limitations | Ref. |
| CRS | Five clinical indicators | Widely used; identifies high-risk patients | Limited evidence on improving outcomes for high-risk CRS patients | [21-23] |
| CMS | Molecular classification into 4 subtypes | Offers nuanced view of disease | Application in identifying neoadjuvant treatment beneficiaries remains unexplored | [24] |
| m-CS | RAS gene status, size of liver metastases, lymph node status of the primary tumor | Enhanced system compared to previous scores | Lacks granularity in weighing diverse high-risk factors and does not account for chemotherapy sensitivity | [29,30] |
| TBS | Categorizes patients into low, intermediate, and high-risk groups based on tumor size and number | Superior discriminatory ability | Limited by excluding chemotherapy as an evaluation parameter | [31] |
| GAME | Genetic and morphological factors | Outperformed CRS in external validation | Potential limitation in excluding chemotherapy as an evaluation parameter | [33,34] |
| CERR | Integrates mTBS with additional parameters | CERR | Mathematical complexity and abstract metrics pose challenges for widespread clinical application | [35] |
| AI model | Machine learning-based model predicting recurrence risks | Remarkable accuracy in predicting recurrence risk | Inherent limitations include model overfitting and the black-box nature, hindering seamless integration into clinical practice | [36] |
The engagement of MDT in tailoring individualized approaches for patients with initially resectable CRLM stands as a cornerstone in contemporary oncology[37]. Placing patient-centered care at the forefront, this approach involves the collaboration of a diverse team of qualified medical professionals. Ideally, the MDT should encompass experts in colorectal surgery, gastrointestinal surgery, hepatic surgery, medical oncology, radiation oncology, interventional radiology, radiology, ultrasound imaging, pathology, and other pertinent specialties. This collaborative and comprehensive team ensures a holistic evaluation of each patient, allowing for a well-rounded and specialized treatment plan that considers the intricacies of CRLM from various medical perspectives[38].
The management of CRLM has progressively incorporated MDTs for comprehensive patient care[39]. Extensive evidence supports the impact of MDT processes on patient survival, demonstrating improved OS under MDT care[40,41]. Notably, a Chinese study reported that MDT discussions contributed to prolonged OS in patients with advanced gastrointestinal cancers, including CRC[42]. Additionally, preoperative MDT assessment has shown associations with enhanced survival in patients with locally advanced colon cancer[43].
The significance of MDTs in managing CRLM has been underscored by its substantial impact on patient outcomes and treatment strategies. A retrospective study by Milana et al[44] demonstrated the effects of MDT on CRC patients with liver metastases, highlighting the potential benefits of a multidisciplinary approach in tailoring individualized treatment strategies[44]. The study identified patients undergoing liver resection or simultaneous resection for primary CRC and liver metastases with curative intent, emphasizing the role of MDT in optimizing patient care and outcomes[44]. Additionally, a review illustrated the advantages of a multidisciplinary team approach, particularly in treating patients with CRLM. The review emphasized the significance of MDTs in developing tailored treatment plans and optimizing patient management, stressing the importance of a collaborative and comprehensive approach to address the com
In conclusion, the engagement of MDTs in customizing individualized approaches for patients with initially resectable CRLM is pivotal for optimizing patient care, treatment strategies, and outcomes. The collaborative nature of MDTs allows for comprehensive assessments, personalized treatment plans, and the integration of diverse expertise, ultimately contributing to improved patient management and prognosis.
In recent years, the management of CRLM has witnessed significant advancements, prompting a nuanced consideration of neoadjuvant chemotherapy. The National Comprehensive Cancer Network guidelines, starting from the 2009 edition, suggest that patients with resectable CRLM and fewer adverse prognostic factors may benefit more from direct surgery. Conversely, those with borderline resectability might find neoadjuvant chemotherapy more suitable[28,47,48]. Notably, for patients who have not undergone chemotherapy or have been chemotherapy-free for the past 12 months, the clinical benefits of neoadjuvant chemotherapy may be more significant[49,50].
The 2009 European expert consensus proposes specific recommendations, advocating neoadjuvant chemotherapy followed by surgical resection for CRLM patients with a CRS of ≥ 2[51]. The 2012 edition of the ESMO guidelines refines these recommendations[52]. For initially resectable CRLM with a single lesion and a diameter < 2 cm, direct surgery is recommended, while other scenarios favor preoperative neoadjuvant mFOLFOX6 chemotherapy[52].
The 2016 ESMO guidelines introduce a comprehensive approach, considering both surgical complexity and tumor biology. Patients with a poor prognosis or challenging resections are recommended for preoperative neoadjuvant chemotherapy, while those with technically feasible and favorable prognosis CRLM are advised to undergo direct surgery[19]. The 2023 ESMO guidelines highlight careful consideration for patients with small metastatic lesions (10-15 mm), which may disappear after systemic treatment[53]. In such cases, neoadjuvant chemotherapy, if indicated, should not exceed two months. Alternatively, under MDT discussion, early surgery or other local treatment methods for small lesions, such as percutaneous ablative therapy, may be considered[53].
In summary, for initially R0 resectable or locally treatable CRLM, adopting the ESMO guidelines in clinical practice is recommended. While the ESMO guidelines do not provide specific criteria for classifying prognosis as good, moderate, or poor, the CRS is generally regarded as the gold standard. Patients with scores 0-2 are considered to have a 'good prognosis,' whereas those with a score of 3 or above are classified as having a 'poor prognosis.' The more adverse prognostic factors in CRLM, the more neoadjuvant chemotherapy is recommended.
In the landscape of neoadjuvant chemotherapy for initially resectable CRLM, the gold standard, as established by randomized controlled trials, remains the FOLFOX regimen, a cornerstone reaffirmed by the EPOC study[54,55]. FOLFOX's prominence persists as the standard approach, and its equivalency with oxaliplatin in combination with capecitabine (CAPOX) in palliative treatment has seamlessly integrated CAPOX into clinical neoadjuvant chemotherapy practices. However, regimens containing irinotecan are generally cautioned against for neoadjuvant use, unless necessitated by a patient's history of oxaliplatin-based adjuvant chemotherapy and subsequent development of liver metastasis within one year of completing treatment. The complexity of patient selection criteria poses a challenge, impacting the outcomes of clinical studies focused on neoadjuvant treatment for CRLM. Notable among these studies are EORTC-40983, COMET, and NEW EPOC.
The EORTC-40983 trial, initiated in 2008, delved into the perioperative use of the FOLFOX4 regimen (6 cycles preoperatively + 6 cycles postoperatively) for CRLM. Results indicated enhanced PFS with perioperative chemotherapy (median PFS 18.7 vs 11.7 months), yet failed to demonstrate conclusive OS benefits [median OS (mOS) 61.3 vs 54.3 months, P > 0.05]. The study's broad categorization without distinguishing between synchronous and metachronous liver metastases, combined with generally low overall tumor burden, introduces complexities in assessing neoadjuvant benefits[4,56,57]. The COMET trial, a phase II study exploring neoadjuvant chemotherapy and targeted therapy's impact on PFS for initially resectable CRLM, administered 6 cycles of FOLFOX or 4 cycles of XELOX preoperatively[58]. The addition of cetuximab or bevacizumab was contingent on KRAS status. While the study demonstrated a median PFS of 22.5 months for wild-type KRAS and 10.5 months for mutant KRAS, showcasing modest improvement compared to EORTC-40983, its efficacy was influenced by a significant proportion of low-risk patients, with around half having a single liver metastasis, potentially diluting the overall neoadjuvant treatment impact[59]. The NEW EPOC trial aimed to evaluate the effectiveness of adding cetuximab to chemotherapy in the neoadjuvant treatment of KRAS exon 2 wild-type CRLM. The combination group exhibited a higher proportion of synchronous liver metastases (53% vs 47%), larger metastatic volumes, and worse prognosis-related indicators (14% poorly differentiated vs 10%)[55,60]. However, statistical P-values were not provided. While the neoadjuvant treatment effective rate (Complete Response + Partial Response) was higher in both groups than COMET, it lacked statistical significance (70% for the combination group, 62% for chemotherapy alone, P > 0.05). Intriguingly, the combination group showed significantly shorter PFS than the chemotherapy-alone group (14.1 vs 20.5 months, P = 0.030). Long-term follow-up even suggested that neoadjuvant treatment with combined targeted therapy could shorten OS (mOS for the combination group 55.4 months vs chemotherapy alone 81 months, Hazard Ratio 1.45, P = 0.036). Further stratified analysis indicated that for patients with favorable prognostic factors, neoadjuvant combination targeted therapy did not confer a survival benefit. The unexpected reduction in survival with combined targeted therapy remains unclear, possibly influenced by significant differences in postoperative adjuvant treatment between the two groups[55,60].
In a retrospective analysis, Hao et al[61] evaluated 3038 cases of resectable colon cancer with single-organ metastases (either in the liver or lung). They compared patients who underwent neoadjuvant treatment (either preoperative or perioperative) with those who received postoperative adjuvant treatment alone. The neoadjuvant treatment group showed a substantial extension in mOS, reaching 47.24 months, compared to 38.08 months in the postoperative adjuvant treatment alone group[61,62]. Chakrabarti et al[63] retrospectively analyzed seven cases of Microsatellite Instability-High (MSI-H) non-metastatic digestive tract tumors treated with neoadjuvant immunotherapy at their center. Among three cases of CRC, two achieved a pathological Complete Response (pCR), showcasing promising results[63]. However, given the small sample size and the absence of M1 samples, the generalizability of these findings awaits confirmation in larger studies. This study raises parallels with a 2022 The New England Journal of Medicine publication reporting a 100% complete clinical response in 12 cases of stage II-III dMMR rectal cancer treated with single-agent programmed cell death 1 (PD-1) (dostarlimab) in the neoadjuvant setting[64]. This suggests that immunotherapy, especially for dMMR patients, might be considered earlier in the treatment timeline. Xiao et al[65] conducted an analysis on 10 cases of pMMR/Microsatellite Stable (MSS) CRLM subjected to neoadjuvant treatment involving PD-1, bevacizumab, and chemotherapy. The findings revealed a noteworthy pCR in one case, accompanied by a Disease Control Rate of 100% and an Overall Response Rate (ORR) of 62.5%[65]. Comparing these outcomes with the ORR observed in the COMET study, the addition of PD-1 appears to augment the ORR, closely approaching the results of NEW EPOC[55,58,60]. However, considering the NEW EPOC study demonstrated a 70% ORR with a regimen involving eight agents plus chemotherapy, the distinct contribution of PD-1 remains uncertain. This observation is reminiscent of a study in 2021, which explored neoadjuvant dual immunotherapy for CRLM[66]. In this study, 23 CRLM patients underwent preoperative treatment with a combination of CTLA-4 and durvalumab, followed by postoperative monotherapy involving four administrations of durvalumab. Among the four patients achieving pCR, two had pMMR tumors. This suggests that, analogous to liver cancer, differentiating between MSI-H or MSS may not be imperative for CRLM, and both subtypes could potentially derive benefits from immunotherapy[66]. Alternatively, there might be unidentified factors influencing the efficacy of immunotherapy in these specific patients.
Several ongoing studies (https://clinicaltrials.gov/) are currently investigating various neoadjuvant treatments for CRLM, encompassing different regimens such as FOLFIRINOX (NCT03487939, NCT05362825), combined immunotherapy (NCT03844750, NCT05359393), post-neoadjuvant local treatment for recurrent liver metastases (NCT05861505), and neoadjuvant treatment combined with VEGFR (NCT00659022, NCT01508000, NCT01646554).
In summary, the ORR for neoadjuvant treatment currently falls within the 50%-70% range, with traditional chemo
The management of CRLM following neoadjuvant therapy and subsequent progression poses a complex therapeutic challenge. Studies indicate that hepatectomy significantly improves OS in patients with progressive disease (PD) compared to continuing chemotherapy, especially when considering a balanced patient profile[67]. However, in patients undergoing extensive liver resection, survival analysis reveals that PFS and OS are influenced by independent prognostic factors. The acceptance of second-line chemotherapy is not an independent predictor of patient survival. This observation suggests that patients undergoing extensive liver resection, often burdened with a larger tumor load and high CRS, may not derive substantial benefits from second-line chemotherapy. Therefore, timely surgical intervention is advisable for eligible patients to achieve a disease-free state[67].
Comparing patients who achieved complete or partial response to second-line chemotherapy with those undergoing direct surgery, no significant advantage in terms of PFS and OS is evident for effective responders[68]. This implies that the efficacy of second-line chemotherapy has limited impact on the long-term outcomes of initially resectable patients, particularly those who do not achieve objective relief from neoadjuvant therapy. For patients experiencing PD after neoadjuvant treatment, the consideration of liver resection becomes pivotal. Observational studies indicate a significant improvement in OS for PD patients undergoing liver resection, particularly when patient characteristics are meticulously balanced[67]. In the context of patients progressing after first-line chemotherapy and experiencing PD following neoadjuvant treatment, the continuation of chemotherapy may diminish survival rates. Therefore, prompt consideration of surgical options is crucial to avoid unnecessary adverse effects of prolonged chemotherapy.
A detailed analysis of the type of progression during neoadjuvant therapy, including numeric, dimensional, and biological changes, aids in determining the suitability for surgery[69]. Tumor markers hold potential value in assessing treatment response and disease progression. Monitoring protein levels in blood samples, especially during progression, enhances the understanding of the patient's disease biology[70]. When formulating individualized treatment strategies, factors such as the patient's overall condition, treatment sensitivity, and biological characteristics should be considered. Personalized treatment plans may involve surgery, targeted therapy, or more potent chemotherapy regimens. This underscores the importance of a comprehensive approach, considering multiple factors, to optimize treatment strategies when dealing with progression after neoadjuvant therapy for CRLM.
With an increasingly comprehensive and precise definition of 'initially resectable', the significance of neoadjuvant therapy has gained recognition. Combining patient-specific factors and the tumor's intrinsic characteristics, a stratified management model has emerged under the guidance of MDT (Figure 1). This model emphasizes surgical intervention as the primary approach, with perioperative systemic therapy serving as auxiliary support. It facilitates more precise treatment decisions for initially resectable CRLM. Neoadjuvant therapy, tailored to individual differences, offers varying intensities of regimens.
For patients with adverse prognostic factors like a high CRS, GAME risk scores, initial treatment insensitivity, or elevated ctDNA levels, exploring two-drug combination targeted therapies or more potent three-drug chemotherapy regimens may optimize survival benefits. The adjustment of specific regimens and efficacy predictions is increasingly guided by gene testing (such as RAS/BRAF, PIK3CA), now recommended in major guidelines. Simultaneously, the promising biomarker ctDNA awaits further validation through advanced-level evidence-based research as a prospective indicator for treatment response. Future research directions should focus on validating and refining these approaches to enhance the precision and effectiveness of neoadjuvant therapy in the management of initially resectable CRLM.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mikulic D, Croatia S-Editor: Li L L-Editor: A P-Editor: Li L
| 1. | Kim TM, Jung SH, An CH, Lee SH, Baek IP, Kim MS, Park SW, Rhee JK, Chung YJ. Subclonal Genomic Architectures of Primary and Metastatic Colorectal Cancer Based on Intratumoral Genetic Heterogeneity. Clin Cancer Res. 2015;21:4461-4472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 139] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 2. | Abdel-Rahman O. Prognostic Value of Baseline ALBI Score Among Patients With Colorectal Liver Metastases: A Pooled Analysis of Two Randomized Trials. Clin Colorectal Cancer. 2019;18:e61-e68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 3. | House MG, Ito H, Gönen M, Fong Y, Allen PJ, DeMatteo RP, Brennan MF, Blumgart LH, Jarnagin WR, D'Angelica MI. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg. 2010;210:744-752, 752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 358] [Article Influence: 23.9] [Reference Citation Analysis (1)] |
| 4. | Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Collette L, Praet M, Bethe U, Van Cutsem E, Scheithauer W, Gruenberger T; EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und-tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD). Perioperative chemotherapy with FOLFOX4 and surgery vs surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1478] [Cited by in RCA: 1444] [Article Influence: 84.9] [Reference Citation Analysis (0)] |
| 5. | Imai K, Allard MA, Benitez CC, Vibert E, Sa Cunha A, Cherqui D, Castaing D, Bismuth H, Baba H, Adam R. Early Recurrence After Hepatectomy for Colorectal Liver Metastases: What Optimal Definition and What Predictive Factors? Oncologist. 2016;21:887-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 6. | Olawoyin OM, Mehta S, Chouairi F, Gabrick KS, Avraham T, Pusztai L, Alperovich M. Comparison of Autologous Breast Reconstruction Complications by Type of Neoadjuvant Chemotherapy Regimen. Plast Reconstr Surg. 2021;148:1186-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Zhu D, Zhong Y, Wei Y, Ye L, Lin Q, Ren L, Ye Q, Liu T, Xu J, Qin X. Effect of neoadjuvant chemotherapy in patients with resectable colorectal liver metastases. PLoS One. 2014;9:e86543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Takatsuki M, Tokunaga S, Uchida S, Sakoda M, Shirabe K, Beppu T, Emi Y, Oki E, Ueno S, Eguchi S, Akagi Y, Ogata Y, Baba H, Natsugoe S, Maehara Y; Kyushu Study Group of Clinical Cancer (KSCC). Evaluation of resectability after neoadjuvant chemotherapy for primary non-resectable colorectal liver metastases: A multicenter study. Eur J Surg Oncol. 2016;42:184-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Esteva FJ, Hortobagyi GN. Integration of Systemic Chemotherapy in the Management of Primary Breast Cancer. Oncologist. 1998;3:300-313. [PubMed] |
| 10. | Calistri L, Rastrelli V, Nardi C, Maraghelli D, Vidali S, Pietragalla M, Colagrande S. Imaging of the chemotherapy-induced hepatic damage: Yellow liver, blue liver, and pseudocirrhosis. World J Gastroenterol. 2021;27:7866-7893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Donati F, Cioni D, Guarino S, Mazzeo ML, Neri E, Boraschi P. Chemotherapy-Induced Liver Injury in Patients with Colorectal Liver Metastases: Findings from MR Imaging. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 12. | Ono T, Ishida H, Kumamoto K, Okada N, Ishibashi K. Outcome in disappearing colorectal cancer liver metastases during oxaliplatin-based chemotherapy. Oncol Lett. 2012;4:905-909. [PubMed] |
| 13. | Sugihara K, Uetake H. Therapeutic strategies for hepatic metastasis of colorectal cancer: overview. J Hepatobiliary Pancreat Sci. 2012;19:523-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Jones RP, Kokudo N, Folprecht G, Mise Y, Unno M, Malik HZ, Fenwick SW, Poston GJ. Colorectal Liver Metastases: A Critical Review of State of the Art. Liver Cancer. 2016;6:66-71. [PubMed] |
| 15. | Sugihara K, Hojo K, Moriya Y, Yamasaki S, Kosuge T, Takayama T. Pattern of recurrence after hepatic resection for colorectal metastases. Br J Surg. 1993;80:1032-1035. [PubMed] |
| 16. | Jones A, Findlay A, Knight SR, Rees J, O'Reilly D, Jones RP, Pathak S. Follow up after surgery for colorectal liver metastases: A systematic review. Eur J Surg Oncol. 2023;49:107103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 17. | Hansdotter P, Scherman P, Petersen SH, Mikalonis M, Holmberg E, Rizell M, Naredi P, Syk I. Patterns and resectability of colorectal cancer recurrences: outcome study within the COLOFOL trial. BJS Open. 2021;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 18. | Simmonds PC, Primrose JN, Colquitt JL, Garden OJ, Poston GJ, Rees M. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer. 2006;94:982-999. [PubMed] |
| 19. | Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, Ciardiello F, D'Hoore A, Diaz-Rubio E, Douillard JY, Ducreux M, Falcone A, Grothey A, Gruenberger T, Haustermans K, Heinemann V, Hoff P, Köhne CH, Labianca R, Laurent-Puig P, Ma B, Maughan T, Muro K, Normanno N, Österlund P, Oyen WJ, Papamichael D, Pentheroudakis G, Pfeiffer P, Price TJ, Punt C, Ricke J, Roth A, Salazar R, Scheithauer W, Schmoll HJ, Tabernero J, Taïeb J, Tejpar S, Wasan H, Yoshino T, Zaanan A, Arnold D. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2286] [Cited by in RCA: 2435] [Article Influence: 270.6] [Reference Citation Analysis (31)] |
| 20. | Yoshino T, Arnold D, Taniguchi H, Pentheroudakis G, Yamazaki K, Xu RH, Kim TW, Ismail F, Tan IB, Yeh KH, Grothey A, Zhang S, Ahn JB, Mastura MY, Chong D, Chen LT, Kopetz S, Eguchi-Nakajima T, Ebi H, Ohtsu A, Cervantes A, Muro K, Tabernero J, Minami H, Ciardiello F, Douillard JY. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol. 2018;29:44-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 422] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 21. | Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309-18; discussion 318. [PubMed] |
| 22. | Roberts KJ, White A, Cockbain A, Hodson J, Hidalgo E, Toogood GJ, Lodge JP. Performance of prognostic scores in predicting long-term outcome following resection of colorectal liver metastases. Br J Surg. 2014;101:856-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Wimmer K, Schwarz C, Szabo C, Bodingbauer M, Tamandl D, Mittlböck M, Kaczirek K. Impact of Neoadjuvant Chemotherapy on Clinical Risk Scores and Survival in Patients with Colorectal Liver Metastases. Ann Surg Oncol. 2017;24:236-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda G, Angelino P, Bot BM, Morris JS, Simon IM, Gerster S, Fessler E, De Sousa E Melo F, Missiaglia E, Ramay H, Barras D, Homicsko K, Maru D, Manyam GC, Broom B, Boige V, Perez-Villamil B, Laderas T, Salazar R, Gray JW, Hanahan D, Tabernero J, Bernards R, Friend SH, Laurent-Puig P, Medema JP, Sadanandam A, Wessels L, Delorenzi M, Kopetz S, Vermeulen L, Tejpar S. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350-1356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3408] [Cited by in RCA: 3558] [Article Influence: 355.8] [Reference Citation Analysis (0)] |
| 25. | Leung U, Gönen M, Allen PJ, Kingham TP, DeMatteo RP, Jarnagin WR, D'Angelica MI. Colorectal Cancer Liver Metastases and Concurrent Extrahepatic Disease Treated With Resection. Ann Surg. 2017;265:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 26. | Zhang J, Deng J, Hu J, Zhong Q, Li J, Su M, Liu W, Lv M, Xu T, Lin D, Guo X. Safety and feasibility of neoadjuvant chemotherapy as a surgical bridge for acute left-sided malignant colorectal obstruction: a retrospective study. BMC Cancer. 2022;22:806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 27. | D'Angelica M, Ammori J, Gonen M, Klimstra DS, Low PS, Murphy L, Weiser MR, Paty PB, Fong Y, Dematteo RP, Allen P, Jarnagin WR, Shia J. Folate receptor-α expression in resectable hepatic colorectal cancer metastases: patterns and significance. Mod Pathol. 2011;24:1221-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Zhou Z, Han X, Sun D, Liang Z, Wu W, Ju H. A Comprehensive Prognostic Model for Colorectal Cancer Liver Metastasis Recurrence After Neoadjuvant Chemotherapy. Front Oncol. 2022;12:855915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 29. | Brudvik KW, Mise Y, Chung MH, Chun YS, Kopetz SE, Passot G, Conrad C, Maru DM, Aloia TA, Vauthey JN. RAS Mutation Predicts Positive Resection Margins and Narrower Resection Margins in Patients Undergoing Resection of Colorectal Liver Metastases. Ann Surg Oncol. 2016;23:2635-2643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 114] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 30. | Wang K, Liu W, Yan XL, Li J, Xing BC. Long-term postoperative survival prediction in patients with colorectal liver metastasis. Oncotarget. 2017;8:79927-79934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | Sasaki K, Morioka D, Conci S, Margonis GA, Sawada Y, Ruzzenente A, Kumamoto T, Iacono C, Andreatos N, Guglielmi A, Endo I, Pawlik TM. The Tumor Burden Score: A New "Metro-ticket" Prognostic Tool For Colorectal Liver Metastases Based on Tumor Size and Number of Tumors. Ann Surg. 2018;267:132-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 326] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 32. | Tsilimigras DI, Hyer JM, Bagante F, Guglielmi A, Ruzzenente A, Alexandrescu S, Poultsides G, Sasaki K, Aucejo F, Pawlik TM. Resection of Colorectal Liver Metastasis: Prognostic Impact of Tumor Burden vs KRAS Mutational Status. J Am Coll Surg. 2021;232:590-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 33. | Margonis GA, Sasaki K, Gholami S, Kim Y, Andreatos N, Rezaee N, Deshwar A, Buettner S, Allen PJ, Kingham TP, Pawlik TM, He J, Cameron JL, Jarnagin WR, Wolfgang CL, D'Angelica MI, Weiss MJ. Genetic And Morphological Evaluation (GAME) score for patients with colorectal liver metastases. Br J Surg. 2018;105:1210-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 130] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 34. | Wang HW, Wang LJ, Jin KM, Bao Q, Li J, Wang K, Xing BC. The prognostic impact of resection margin status varies according to the genetic and morphological evaluation (GAME) score for colorectal liver metastasis. J Surg Oncol. 2021;124:619-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 35. | Chen Y, Chang W, Ren L, Chen J, Tang W, Liu T, Jian M, Liu Y, Wei Y, Xu J. Comprehensive Evaluation of Relapse Risk (CERR) Score for Colorectal Liver Metastases: Development and Validation. Oncologist. 2020;25:e1031-e1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 36. | Chakedis J, Squires MH, Beal EW, Hughes T, Lewis H, Paredes A, Al-Mansour M, Sun S, Cloyd JM, Pawlik TM. Update on current problems in colorectal liver metastasis. Curr Probl Surg. 2017;54:554-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 37. | Li J, Yuan Y, Yang F, Wang Y, Zhu X, Wang Z, Zheng S, Wan D, He J, Wang J, Ba Y, Bai C, Bai L, Bai W, Bi F, Cai K, Cai M, Cai S, Chen G, Chen K, Chen L, Chen P, Chi P, Dai G, Deng Y, Ding K, Fan Q, Fang W, Fang X, Feng F, Fu C, Fu Q, Gu Y, He Y, Jia B, Jiang K, Lai M, Lan P, Li E, Li D, Li J, Li L, Li M, Li S, Li Y, Li Z, Liang X, Liang Z, Lin F, Lin G, Liu H, Liu J, Liu T, Liu Y, Pan H, Pan Z, Pei H, Qiu M, Qu X, Ren L, Shen Z, Sheng W, Song C, Song L, Sun J, Sun L, Sun Y, Tang Y, Tao M, Wang C, Wang H, Wang S, Wang X, Wu A, Wu N, Xia L, Xiao Y, Xing B, Xiong B, Xu J, Xu N, Xu R, Xu Z, Yang Y, Yao H, Ye Y, Yu Y, Yue J, Zhang J, Zhang S, Zhang W, Zhang Y, Zhang Z, Zhao L, Zhao R, Zhou F, Zhou J, Jin J, Gu J, Shen L. Expert consensus on multidisciplinary therapy of colorectal cancer with lung metastases (2019 edition). J Hematol Oncol. 2019;12:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 38. | Lucarini A, Garbarino GM, Orlandi P, Garofalo E, Bragaglia L, Laracca GG, Canali G, Pecoraro A, Mercantini P; Sant’Andrea GLAM collaborative group. From "Cure" to "Care": The Role of the MultiDisciplinary Team on Colorectal Cancer Patients' Satisfaction and Oncological Outcomes. J Multidiscip Healthc. 2022;15:1415-1426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Taylor C, Munro AJ, Glynne-Jones R, Griffith C, Trevatt P, Richards M, Ramirez AJ. Multidisciplinary team working in cancer: what is the evidence? BMJ. 2010;340:c951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 304] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 40. | Keller DS, Berho M, Perez RO, Wexner SD, Chand M. The multidisciplinary management of rectal cancer. Nat Rev Gastroenterol Hepatol. 2020;17:414-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 202] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 41. | Peng D, Cheng YX, Cheng Y. Improved Overall Survival of Colorectal Cancer under Multidisciplinary Team: A Meta-Analysis. Biomed Res Int. 2021;2021:5541613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 42. | Zhang Q, Zhou Y, Song L, Fang W, Qiu M, Gu Y, Yang Y, Zhang J, Liu J, Li J, Lu M, Gong T, Wang X, Li Y, Yang J, Ye Y, Shen L. China special issue on gastrointestinal tumors-Improved survival after multidisciplinary team decision for patients with advanced gastrointestinal cancer: A multicenter, noninterventional, controlled study. Int J Cancer. 2023;153:1885-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 43. | Rosander E, Holm T, Sjövall A, Hjern F, Weibull CE, Nordenvall C. Preoperative multidisciplinary team assessment is associated with improved survival in patients with locally advanced colon cancer; a nationwide cohort study in 3157 patients. Eur J Surg Oncol. 2021;47:2398-2404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 44. | Milana F, Famularo S, Luberto A, Rimassa L, Scorsetti M, Comito T, Pressiani T, Franzese C, Poretti D, Di Tommaso L, Personeni N, Rodari M, Pedicini V, Donadon M, Torzilli G. Multidisciplinary Tumor Board in the Management of Patients with Colorectal Liver Metastases: A Single-Center Review of 847 Patients. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 45. | De Greef K, Rolfo C, Russo A, Chapelle T, Bronte G, Passiglia F, Coelho A, Papadimitriou K, Peeters M. Multisciplinary management of patients with liver metastasis from colorectal cancer. World J Gastroenterol. 2016;22:7215-7225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 46. | Cheng X, Zhao F, Chen D, Yang P, Zhong W, Xu X, Wang W. Successful treatment of colorectal liver metastasis harboring intrahepatic cholangiocarcinoma: A case report. Medicine (Baltimore). 2018;97:e13751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 47. | Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, Giacchetti S, Paule B, Kunstlinger F, Ghémard O, Levi F, Bismuth H. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240:644-57; discussion 657. [PubMed] |
| 48. | Khoo E, O'Neill S, Brown E, Wigmore SJ, Harrison EM. Systematic review of systemic adjuvant, neoadjuvant and perioperative chemotherapy for resectable colorectal-liver metastases. HPB (Oxford). 2016;18:485-493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 49. | Sun H, Sun L, Ke X, Liu L, Li C, Jin B, Wang P, Jiang Z, Zhao H, Yang Z, Sun Y, Liu J, Wang Y, Sun M, Pang M, Wu B, Sang X, Xing B, Yang H, Huang P, Mao Y. Prediction of Clinical Precision Chemotherapy by Patient-Derived 3D Bioprinting Models of Colorectal Cancer and Its Liver Metastases. Adv Sci (Weinh). 2024;11:e2304460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 50. | Noda T, Takahashi H, Tei M, Nishida N, Hata T, Takeda Y, Ohue M, Wada H, Mizushima T, Asaoka T, Uemura M, Kobayashi S, Murata K, Satoh T, Doki Y, Eguchi H; Clinical Study Group of Osaka University (CSGO), Colorectal Group, and Hepato‐Biliary‐Pancreatic Group. Clinical outcomes of neoadjuvant chemotherapy for resectable colorectal liver metastasis with intermediate risk of postoperative recurrence: A multi-institutional retrospective study. Ann Gastroenterol Surg. 2023;7:479-490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 51. | Van Cutsem E, Oliveira J; ESMO Guidelines Working Group. Advanced colorectal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20 Suppl 4:61-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 52. | Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde CJ, Balmana J, Regula J, Nagtegaal ID, Beets-Tan RG, Arnold D, Ciardiello F, Hoff P, Kerr D, Köhne CH, Labianca R, Price T, Scheithauer W, Sobrero A, Tabernero J, Aderka D, Barroso S, Bodoky G, Douillard JY, El Ghazaly H, Gallardo J, Garin A, Glynne-Jones R, Jordan K, Meshcheryakov A, Papamichail D, Pfeiffer P, Souglakos I, Turhal S, Cervantes A. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol. 2012;23:2479-2516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1035] [Cited by in RCA: 1107] [Article Influence: 85.2] [Reference Citation Analysis (1)] |
| 53. | Cervantes A, Adam R, Roselló S, Arnold D, Normanno N, Taïeb J, Seligmann J, De Baere T, Osterlund P, Yoshino T, Martinelli E; ESMO Guidelines Committee. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:10-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 818] [Article Influence: 409.0] [Reference Citation Analysis (34)] |
| 54. | Primrose J, Falk S, Finch-Jones M, Valle J, O'Reilly D, Siriwardena A, Hornbuckle J, Peterson M, Rees M, Iveson T, Hickish T, Butler R, Stanton L, Dixon E, Little L, Bowers M, Pugh S, Garden OJ, Cunningham D, Maughan T, Bridgewater J. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial. Lancet Oncol. 2014;15:601-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 310] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 55. | Bridgewater JA, Pugh SA, Maishman T, Eminton Z, Mellor J, Whitehead A, Stanton L, Radford M, Corkhill A, Griffiths GO, Falk S, Valle JW, O'Reilly D, Siriwardena AK, Hornbuckle J, Rees M, Iveson TJ, Hickish T, Garden OJ, Cunningham D, Maughan TS, Primrose JN; New EPOC investigators. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis (New EPOC): long-term results of a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21:398-411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 160] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 56. | Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Mauer M, Tanis E, Van Cutsem E, Scheithauer W, Gruenberger T; EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und–tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD). Perioperative FOLFOX4 chemotherapy and surgery vs surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 921] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 57. | Padmanabhan C, Parikh A. Perioperative chemotherapy for resectable colorectal hepatic metastases-What does the EORTC 40983 trial update mean? Hepatobiliary Surg Nutr. 2015;4:80-83. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 58. | Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, Mulroy L, Lock M, Rodrigues GB, Yaremko BP, Schellenberg D, Ahmad B, Senthi S, Swaminath A, Kopek N, Liu M, Moore K, Currie S, Schlijper R, Bauman GS, Laba J, Qu XM, Warner A, Senan S. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J Clin Oncol. 2020;38:2830-2838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 309] [Cited by in RCA: 840] [Article Influence: 168.0] [Reference Citation Analysis (0)] |
| 59. | Robin TP, Olsen JR. SABR for metastasis-directed therapy - what we've learned and what's to come. Nat Rev Clin Oncol. 2020;17:593-594. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 60. | Katipally RR, Martinez CA, Pugh SA, Bridgewater JA, Primrose JN, Domingo E, Maughan TS, Talamonti MS, Posner MC, Weichselbaum RR, Pitroda SP; with the S:CORT Consortium. Integrated Clinical-Molecular Classification of Colorectal Liver Metastases: A Biomarker Analysis of the Phase 3 New EPOC Randomized Clinical Trial. JAMA Oncol. 2023;9:1245-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 61. | Hao Z, Parasramka S, Chen Q, Jacob A, Huang B, Mullett T, Benson AB. Neoadjuvant Versus Adjuvant Chemotherapy for Resectable Metastatic Colon Cancer in Non-academic and Academic Programs. Oncologist. 2023;28:48-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 62. | Su IH, Lund JL, Gaber CE, Sanoff HK, Strassle PD, Duchesneau ED. Regarding "Neoadjuvant Versus Adjuvant Chemotherapy for Resectable Metastatic Colon Cancer in Non-academic and Academic Programs". Oncologist. 2023;28:e588-e589. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 63. | Chakrabarti S, Grewal US, Vora KB, Parikh AR, Almader-Douglas D, Mahipal A, Sonbol MBB. Outcome of Patients With Early-Stage Mismatch Repair Deficient Colorectal Cancer Receiving Neoadjuvant Immunotherapy: A Systematic Review. JCO Precis Oncol. 2023;7:e2300182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 64. | Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, El Dika IH, Segal N, Shcherba M, Sugarman R, Stadler Z, Yaeger R, Smith JJ, Rousseau B, Argiles G, Patel M, Desai A, Saltz LB, Widmar M, Iyer K, Zhang J, Gianino N, Crane C, Romesser PB, Pappou EP, Paty P, Garcia-Aguilar J, Gonen M, Gollub M, Weiser MR, Schalper KA, Diaz LA Jr. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N Engl J Med. 2022;386:2363-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 989] [Cited by in RCA: 904] [Article Influence: 301.3] [Reference Citation Analysis (0)] |
| 65. | Xiao BY, Zhang X, Cao TY, Li DD, Jiang W, Kong LH, Tang JH, Han K, Zhang CZ, Mei WJ, Xiao J, Pan ZZ, Li YF, Zhang XS, Ding PR. Neoadjuvant Immunotherapy Leads to Major Response and Low Recurrence in Localized Mismatch Repair-Deficient Colorectal Cancer. J Natl Compr Canc Netw. 2023;21:60-66.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 66. | Hollebecque A, Chung HC, de Miguel MJ, Italiano A, Machiels JP, Lin CC, Dhani NC, Peeters M, Moreno V, Su WC, Chow KH, Galvao VR, Carlsen M, Yu D, Szpurka AM, Zhao Y, Schmidt SL, Gandhi L, Xu X, Bang YJ. Safety and Antitumor Activity of α-PD-L1 Antibody as Monotherapy or in Combination with α-TIM-3 Antibody in Patients with Microsatellite Instability-High/Mismatch Repair-Deficient Tumors. Clin Cancer Res. 2021;27:6393-6404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 67. | Famularo S, Milana F, Cimino M, Procopio F, Costa G, Galvanin J, Paoluzzi Tomada E, Bunino FM, Palmisano A, Donadon M, Torzilli G. Hepatectomy vs Chemotherapy for Resectable Colorectal Liver Metastases in Progression after Perioperative Chemotherapy: Expanding the Boundaries of the Curative Intent. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 68. | Viganò L, Capussotti L, Barroso E, Nuzzo G, Laurent C, Ijzermans JN, Gigot JF, Figueras J, Gruenberger T, Mirza DF, Elias D, Poston G, Letoublon C, Isoniemi H, Herrera J, Sousa FC, Pardo F, Lucidi V, Popescu I, Adam R. Progression while receiving preoperative chemotherapy should not be an absolute contraindication to liver resection for colorectal metastases. Ann Surg Oncol. 2012;19:2786-2796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 69. | Schwartz LH, Litière S, de Vries E, Ford R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J, Hayes W, Hodi FS, Hoekstra OS, Huang EP, Lin N, Liu Y, Therasse P, Wolchok JD, Seymour L. RECIST 1.1-Update and clarification: From the RECIST committee. Eur J Cancer. 2016;62:132-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 682] [Cited by in RCA: 1328] [Article Influence: 147.6] [Reference Citation Analysis (0)] |
| 70. | Lech G, Słotwiński R, Słodkowski M, Krasnodębski IW. Colorectal cancer tumour markers and biomarkers: Recent therapeutic advances. World J Gastroenterol. 2016;22:1745-1755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 234] [Cited by in RCA: 285] [Article Influence: 31.7] [Reference Citation Analysis (8)] |