INTRODUCTION
In 1901, Opie[1] first directed attention to the occurrence of pancreatitis caused by gallstone blockage of the pancreatic duct. Generally, biliopancreatic obstruction during gallstone migration into or passage through the ampulla of Vater triggers gallstone pancreatitis (GSP)[2-4]. Stones with a diameter up to 5 mm are important predisposing factors for this disease[5], and it has been reported that the risk of patients with at least one gallstone < 5 mm in diameter is increased more than four-folds[6]. GSP is a rare disease. One study reported that 89 (3.4%) of 2583 residents of Rochester, Minnesota, with gallstones diagnosed between 1950 and 1970 developed acute pancreatitis[7]. Another study reported that 191 (3.4%) of 5663 patients who received surgical treatment for gallstones at Ogaki Municipal Hospital, Japan, between 1976 and 2003 were diagnosed with GSP[8]. Lowenfels et al[9] noted that the risk of biliary pancreatitis development in patients with asymptomatic gallstones is not likely > 2% during a 20- or 30-year period. Patients with GSP have highly elevated serum transaminase and pancreatic enzyme levels, and the former could reflect microscopic acute inflammatory hepatocyte necrosis caused by sudden blockage of the ampulla of Vater by the migrating bile duct stones[10]. Thus, elevated liver enzyme levels during acute attacks indicate acute bile duct obstruction by impacted stones and are useful for predicting the gallstone etiology with acute pancreatitis[11-13]. Alanine transaminase (ALT) is the most clinically useful parameter for diagnosing GSP[14]. A prospective study conducted by Anderson et al[15] demonstrated that higher the ALT level, the higher the chance of a biliary cause, and that the combination of positive abdominal ultrasound (US) results for gallstones and elevated ALT levels almost completely confirms the diagnosis of GSP. Therefore, the diagnosis of GSP is established by a history of abdominal pain and increases in serum pancreatic and liver enzyme levels. Small stones impacted at the ampulla of Vater cause an increase in biliary pressure, which produces dilatation of the bile duct and, in some cases, subsequent relief of the obstruction[16]. Because of the passage of stones, most patients with GSP have mild disease with hyperamylasemia alone or interstitial edematous pancreatitis (IP), and exhibit rapid objective improvement with rapid decreases in serum hepatic and pancreatic enzyme levels.
Severe GSP refractory to maximum conservative therapy has wide clinical variations, and its pathophysiology remains controversial. In particular, there is no consensus on the events that follow persistent ampullary stone impaction with biliopancreatic obstruction and its effect on the course of GSP[17]. Furthermore, the pathogenesis of necrotizing pancreatitis (NP), which leads to early and late single or multiple organ failure[18], remains controversial. Investigations of humans must rely on findings from autopsies or laparotomies performed early during the rapid course of the disease. In 1901, Opie[1,19] proposed the obstruction and common channel theories to explain the etiology of gallstone-induced IP and NP, respectively. In 1909, Opie and Meakins[20] reported reflux of the duodenal contents as the cause of NP based on the autopsy findings of a patient without gallstones. In 1993, Isogai et al[21] identified two categories of severe GSP, biliary and pancreatic, based on a retrospective and observational study of 73 patients with severe GSP who underwent emergency laparotomies between 1976 and 1989, which were common practices at the time. The present study aimed to review a series of autopsy findings on which Opie based his proposed theories of obstruction, the common channel, and duodenal reflux, to describe the two types of severe GSP, and to clarify the pathophysiology of severe disease from clinicopathological and historical points of view.
THEORIES ON THE PATHOGENESIS OF SEVERE GSP
Obstruction theory
In 1901, based on the autopsy findings of a 47-year-old man with a history of jaundice lasting approximately 3 wk accompanied by abdominal pain and fever, Opie[1] proposed pancreatic duct obstruction as the cause of IP with fat necrosis (FN). The patient suddenly experienced severe vomiting accompanied by intense abdominal pain, followed by a gradual increase in abdominal distention. On the third day after admission, he experienced slight jaundice and was irrational at times, and his temperature increased to a maximum of 104°F. He underwent emergency surgery based on the diagnosis of suppurative pancreatitis, but he died 4 h after the procedure. Autopsy revealed a large abscess and FN foci in the lesser peritoneal cavity. The hepatic, cystic, and common bile ducts were dilated. The gallbladder was distended and contained more than 100 calculi with diameters varying from 0.5-1 cm. In the terminal common bile duct, a calculus with a 7-mm diameter that was lodged approximately 1.5 cm from the duodenal orifice, where the bile and main pancreatic ducts ran parallel to each other, that was separated by a thin interposed septum was observed. The pancreas was enlarged and the interstitial tissue was hemorrhagic; however, the glandular tissue was mostly well-preserved. Histologically, many acini were widely dilated, their cells were flat, and the lumen was distended and contained secretion products. Opie speculated that the stone might have compressed and occluded the main pancreatic duct, and that the obstructed secretion of pancreatic juice might have been forced backward into the pancreatic parenchyma and surrounding tissue, thus making contact with the fat cells and resulting in IP and localized pancreatic FN.
Common channel theory
In 1901, although the patient’s sex, age, and clinical course were missing, Opie[19] proposed a continuous closed channel as the mechanism of NP based on autopsy findings. An autopsy revealed a moderate amount of serosanguineous fluid in the peritoneal cavity with disseminated FN. The pancreas was gangrenous. The duodenal papilla was prominent. A small calculus with a 3-mm diameter was lodged at the duodenal apex, thereby occluding the orifice. Careful examination revealed that the impacted stone was so small that the orifices of the common bile and pancreatic ducts were not obstructed, and both ducts were converted into a continuous closed channel 10 mm in length. The pancreatic duct, which passes through the pancreatic head, resembled the common bile duct because it was stained with bile. Opie[19] speculated that bile reflux into the pancreas could repeatedly occur when any slight pressure differences in the bile and pancreatic ducts were overcome by gallbladder contraction; therefore, pancreatic enzymes were activated, producing NP.
Duodenal reflux theory
In 1909, based on the autopsy findings of a 55-year-old man with no gallstones, Opie and Meakins[20] suggested reflux of duodenal contents into the pancreas as the cause of NP. The patient reported sudden and severe epigastric pain followed by vomiting. Two days later, he experienced shock and died 6 h after admission to the hospital. During the autopsy, almost the entire gland of the body and tail and the greater part of the pancreatic head were necrotic. There were several characteristic findings. First, the seat of the pancreatic necrosis was localized to the portion drained by the duct of Santorini and the posterior and lower parts of the pancreatic head, which are in contact with the Wirsung duct, had a normal appearance. Second, the duct of Santorini, which is usually a small accessory duct, was much larger than the Wirsung duct, and ran through the entire length of the pancreas, which was the chief outlet of the gland. Third, the orifice of the duct of Santorini was relatively wide, thus permitting the use of a probe with a diameter of approximately 2 mm. They speculated that duodenal contents with enterokinase might reflux into the pancreatic duct through the patulous orifice of the duct of Santorini, which is unable to prevent regurgitation when the pressure within the duodenum is increased by vomiting, thereby producing NP. Considering the duodenal reflux theory in relation to GSP, it is possible that the evacuation of impacted ampullary stones damages the sphincter, which permits the reflux of duodenal contents into the pancreas[22], thereby causing NP.
TWO CATEGORIES OF SEVERE GSP
Isogai et al[21] identified two categories of severe GSP. One is the biliary type, which comprises life-threatening acute cholangitis and minimal pancreatic lesions or IP caused by the persistent impact of stones at the ampulla of Vater with biliopancreatic obstruction. The other is the pancreatic type, which comprises NP, but is uncomplicated by acute biliary tract disease.
Severe biliary-type GSP
Based on Opie’s[1] obstruction theory, and the hepatic histopathological changes that occur with acute bile duct obstruction caused by impacted bile duct stones[10,23], the pathogenesis of severe biliary-type GSP may be hypothesized as follows: (1)Pancreatic duct obstruction causes minimal pancreatic lesions or IP; (2) Persistent obstruction of the bile duct leads to acute cholangitis; and (3) The severity of pathological changes in the liver and biliary tract is more significant than that in the pancreas.
Severe pancreatic-type GSP
Based on Opie’s[19,20] common channel and duodenal reflux theories, the hypothesis regarding the pathogenesis of severe pancreatic-type GSP is as follows: (1) The reflux of bile or possible duodenal contents into the pancreas causes NP through the activation of pancreatic enzymes; (2) All stones that settle in the narrow duodenal orifice and allow bile reflux into the pancreas are expelled into the duodenum early during the course of disease; (3) Sphincter Oddi incompetence caused by the evacuation of impacted larger stones at the ampullary site permits reflux of duodenal contents into the pancreas, leading to NP; and (4) Virtually all stones responsible for NP pass into the duodenum and are lost or have been evacuated into the duodenum, providing no evidence of their former impaction, with acute hepatobiliary inflammation resolving rapidly.
In 2005, Isogai et al[8] conducted another clinical study to investigate the relationship between pancreatitis severity and biliary pathology of 183 consecutive patients with GSP during 1976 to 2002. Of the 183 study patients, 95 (52%) had severe disease and underwent emergency laparotomy (86 patients) or endoscopic retrograde cholangiopancreatography (ERCP; 9 patients) during acute attacks. Of the 95 patients with severe disease, 43 (45%), 23 (24%), and 29 (31%) had impacted ampullary stones, mobile bile duct stones without impaction, and gallbladder stones alone, respectively. Of the 43 patients with impacted ampullary stones, 14 (33%) and 6 (14%) had the Charcot triad and Reynolds pentad, respectively. Of the 23 patients with floating bile duct stones, 9 (40%) had purulent bile in the bile duct. All 43 patients with impacted ampullary stones and all 23 patients except one with floating bile duct stones had minimal or mild pancreatitis, without the features of severe pancreatitis defined according to the Atlanta classification[24,25]. Of the 95 patients with severe disease, 16 (17%) had NP, which was defined either as a macroscopically dark pancreas or as non-enhanced pancreatic parenchyma on contrast-enhanced CT. None of the 16 patients with NP had stones impacted at the ampulla, and all 16 patients, except one with mobile bile duct stones, retained uniformly small stones in the gallbladder alone; any one of those stones might have caused fatal diversion of bile or duodenal contents into the pancreatic duct, providing no evidence of their former impaction. Of the 95 patients with severe disease, 8 (8%) died. Of these eight fatalities, four patients without NP had impacted ampullary stones (two of those four patients died of acute obstructive cholangitis), and four patients with NP died of multiple organ failure[8]. The afore-mentioned study results supported the hypothesis of the pathogenesis of biliary and pancreatic types of severe disease.
PATHOGENESIS OF SEVERE BILIARY-TYPE GSP
The autopsy findings of the 47-year-old patient on which Opie[1] based the proposed obstruction theory showed that many pancreatic acini were widely dilated; their cells were flat and the lumen was distended and contained products of secretion. Intrapancreatic duct pressure was increased by pancreatic duct obstruction. The main pancreatitis-inducing factors include fatty acids, alcohol, bile acids, and physical pressure[26]. Increased pressure in the pancreatic duct is thought to be a key factor in the development of GSP[27]. Swain et al[27] showed that higher and prolonged elevation of the pancreatic duct pressure caused by pancreatic duct ligation in mice activated the calcium-permeable ion channel Piezo 1 in the pancreatic acinar cell, thus causing prolonged elevation of intracellular calcium levels, mitochondrial depolarization, and intracellular trypsin activation, ultimately leading to cell death. However, the clinical picture of the majority of patients with biliopancreatic obstruction caused by stones impacted at the ampulla is more often dominated by life-threatening acute cholangitis and septicemia than by acute pancreatitis[8,21,22,28]. Experimental and clinical investigations have questioned the relationship between the duration of pancreatic obstruction and severity of pancreatitis. In 1901, Opie[1] noted that pancreatic duct obstruction in humans and pancreatic duct ligation in animals do not cause hemorrhage or hemorrhagic inflammation. In 1964, McCutcheon[29] reported that pancreatic duct obstruction had little association with the etiology of pancreatitis, demonstrating that no patient with recurrent pancreatitis who underwent pancreatic duct ligation developed pancreatitis during the immediate postoperative period. In 1997, Arendt et al[30] used a rabbit model mimicking gallstone impaction in the human choledochoduodenal junction with biliopancreatic obstruction, and reported that pancreatic duct obstruction without a patent duct of Santorini produced pancreatic edema without acinar necrosis, whereas a patent duct of Santorini prevented the development of pancreatic edema caused by pancreatic duct obstruction. In 2006, Acosta et al[31] conducted a prospective randomized clinical trial involving patients with GSP and persistent ampullary obstruction but uncomplicated severe cholangitis and compared the efficacy of ERCP with or without endoscopic sphincterotomy (ES) between the study group (initial conservative treatment and ERCP with or without ES within 48 h if obstruction persisted for 24 h or more) and control group (conservative treatment with or without selective ERCP with or without ES after 48 h in the case of associated persistent jaundice or cholangitis). The study results showed that pancreatic phlegmon consisting of a pancreatic inflammatory mass, peripancreatic fluid, and FN was the most common pancreatic pathology, and that pancreatic phlegmon was identified only in control group patients with persistent obstruction for more than 48 h. The term “pancreatic phlegmon” does not appear in the Atlanta classification of acute pancreatitis[24,25], but it is likely consistent with IP.
Acosta et al[17] noted that stones > 3 mm in diameter completely filled the ampulla and prevented pancreatic reflux. However, whether pancreatic duct obstruction without bile reflux causes NP in humans is unknown[32]. The author believes that the stones impacted at the ampulla with biliopancreatic obstruction in the absence of bile reflux probably resulted in clinically minimal pancreatic lesions or IP with or without FN because of the absence of activation of the proteolytic pancreatic proenzymes. Regarding FN caused by pancreatic duct obstruction, the pathogenesis is explained as follows: When the pancreatic duct is obstructed, the pancreatic juice containing fat-splitting enzyme lipase, one of the few pancreatic enzymes that does not need to be activated[33], is forced back into the pancreatic parenchyma, thus penetrating the surrounding tissue and making contact with the fat cells, and the fat is split by the fat-splitting enzyme into fatty acid and glycerin. The insoluble fatty acids subsequently unite with calcium salts and develop into FN, whereas soluble glycerin is absorbed and carried away[1].
In contrast, bacterial cholangiovenous reflux develops if bile duct obstruction persists, thus leading to acute cholangitis. To understand the pathogenesis of acute cholangitis, it is necessary to understand the pathological changes that occur in the liver when the bile duct is obstructed by the impacted stones. As previously noted, patients with GSP have elevated serum transaminase levels. Hepatic histopathological changes in patients with GSP have been reported to be acute inflammatory hepatocyte necrosis and acute cholangitis caused by sudden blockage of the ampulla of Vater because of impacted bile duct stones[10]. These acute hepatic injuries are consistent with those identified in patients with severe biliary colic, marked elevation of serum transaminase levels similar to those with hepatitis, and only a mild increase in serum bilirubin, which, in 1991, Isogai et al[23] referred to “gallstone hepatitis” as a new clinical entity. This “medical” enzyme pattern in patients with gallstone hepatitis and GSP who have “surgical” pain is paradoxical and counterintuitive[34]. Mohamed et al[35] performed a systematic review and meta-analysis and concluded that marked or extreme transaminase elevations and minimal increases in alkaline phosphatase and bilirubin should prompt clinicians to evaluate and manage biliary obstruction. Culture results of common bile duct aspirate during emergency surgery were positive for 72% of patients with gallstone hepatitis, with Escherichia coli being the most frequent pathogen, followed by Klebsiella pneumoniae and Streptococcus faecalis[23]. A rapid increase in the bile duct pressure[13] and mechanical insufficiency of lymph circulation caused by a combination of bile stasis and inflammation[36] have been reported to cause liver cell necrosis. However, the mechanism of hepatocyte necrosis in gallstone hepatitis remains unclear.
The hepatocytes have tight junctional complexes that form a seal between the lumen of the bile canaliculus and hepatic intercellular space, which play the role of a canaliculi-sinusoidal barrier[37], and discontinuities in the junctional meshwork, which provide a direct pathway between the lumen of the bile canaliculus and intercellular space[38]. Elevation of liver enzyme levels is a serological reflection of microscopic hepatocyte necrosis, indicating disruption to the barrier. Bacterial cholangiovenous reflux occurs through damaged tight junctional complexes and also directly through disrupted liver cells[39] if biliopancreatic obstruction persists and the pressure in the bile canaliculus further increases, leading to acute cholangitis.
Conventionally, the cause of death of the 47-year-old patient whose autopsy findings were the basis for the obstruction theory proposed by Opie[1] was considered to be severe pancreatitis; however, this probably was not the actual cause. The patient likely died of acute obstructive cholangitis, which is a distinct clinical syndrome of overwhelming sepsis characterized by complete bile duct obstruction proposed by Reynolds and Dargan[40] in 1959. The patient had mental confusion and possible shock in addition to Charcot’s triad, abdominal pain, fever, and jaundice, on the day of surgery, and died during a fulminate postoperative course. Opie[1] did not mention the cause of his death, which might have been unavoidable because, at that time, the clinical concept of acute obstructive cholangitis did not exist; furthermore, even the terms “acute cholangitis” and “Charcot’s triad” did not appear in Opie’s[1,19,20] original articles. However, Opie[1] seemed to have considered a possible pathology other than pancreatic lesions that determined the disease severity, noting that individuals such as the autopsy patient, who were usually in fairly good health and perhaps, had a history of gallstone attacks suddenly experienced epigastric pain, accompanied by vomiting and followed by collapse and death, usually within 48 h. During autopsy, gallstones were found lodged in the common bile duct near its orifice, which might have caused a fatal attack. Opie’s[1] clinical scenario seems typical of acute obstructive cholangitis in patients with GSP with stones persistently impacted at the ampulla of Vater and biliopancreatic obstruction. This severe biliary-type GSP is not widely appreciated by general clinicians, possibly because either longer or permanent stone impaction may lead to severe pancreatitis. In 2023, Zhang et al[41] defined obstructive severe acute biliary pancreatitis as persistent single or multiple organ failure (> 48 h) according to the revised Atlanta Classification[25], with the cause of severe pancreatitis being biliary obstruction confirmed by imaging, which may be consistent with severe biliary-type GSP.
As mentioned previously, the real danger for biliary type patients is acute cholangitis that is refractory to supportive therapy and life-threatening. The clinical diagnosis of acute cholangitis has long been based on the clinical findings of Charcot’s triad. However, because distinction of the inflammatory response caused by acute pancreatitis from that caused by acute cholangitis may be difficult, reliance on Charcot’s triad is insufficient[42]. No standard criteria for the diagnosis of acute cholangitis are available when all of the components of Charcot’s triad were not present. However, in 2007, the first diagnostic and severity assessment criteria for acute cholangitis were presented, and a definite diagnosis was made if clinical manifestation, objective laboratory data including liver function test results, and imaging findings that support the evidence of inflammation and biliary obstruction were observed[43]. In 2013, the diagnostic criteria for acute cholangitis were revised and termed the Tokyo Guideline 2013, and the thresholds of numerous variables were accurately and reliably set as follows: Body temperature: > 38°C; white blood cell count: < 4 or > 10 × 1000/μL; C-reactive protein level: ≥ 1 mg/dL; jaundice (total bilirubin): ≥ 2 mg/dL; and liver function levels, such as alkaline phosphatase, γ-glutamyltransferase, aspartate aminotransferase, and ALT, > 1.5-times the upper limit of the normal values[44].
PATHOGENESIS OF SEVERE PANCREATIC-TYPE GSP
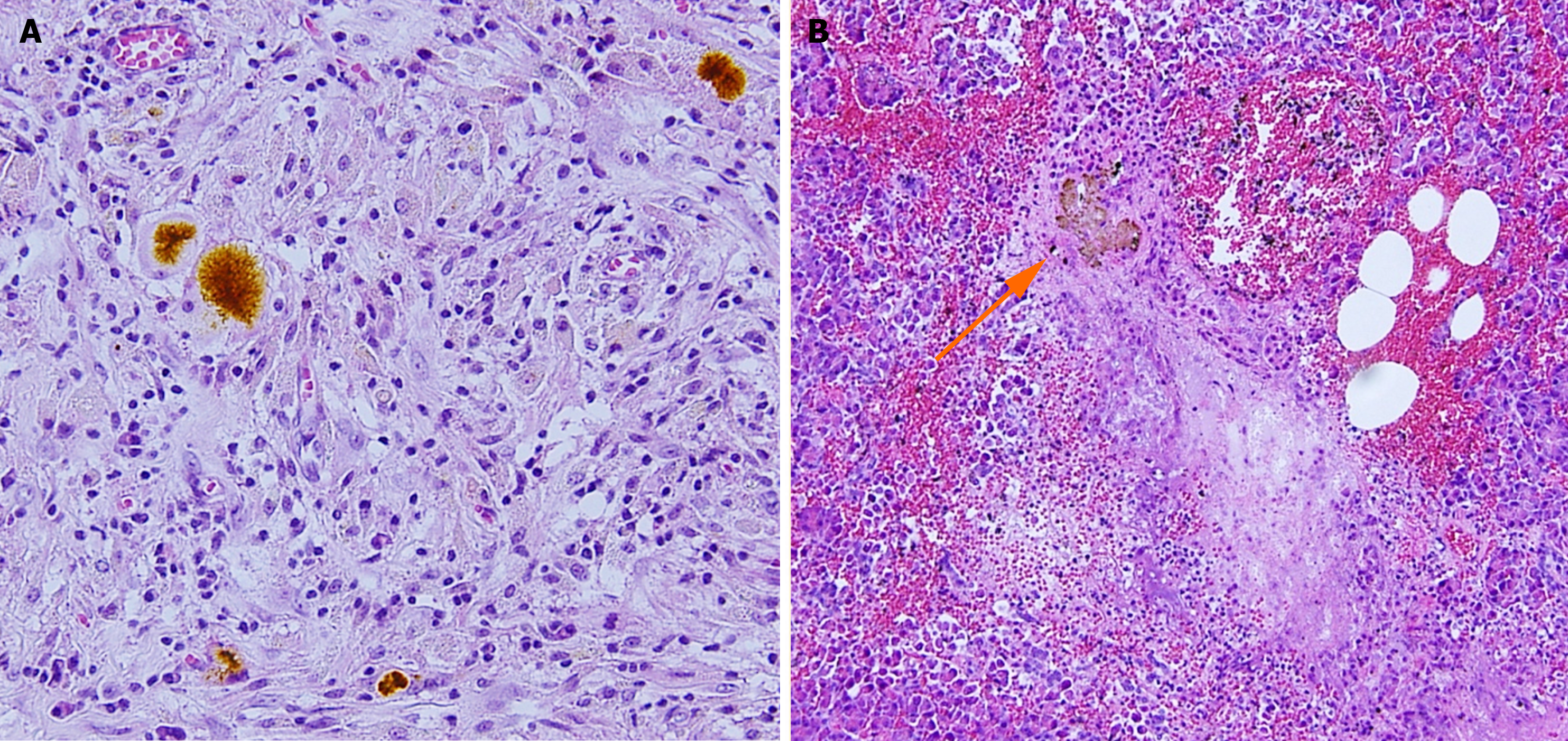
Regarding the reflux of bile as the cause of NP, animal models have shown that protease activation is highly dependent on calcium release[45], with bile acids inducing calcium-releasing signals and contributing to pancreatic cell damage[46]. One factor that disputes the common channel theory is the pressure within the pancreatic duct that is higher than that observed in the bile duct that does not allow for retrograde flow from the biliary system to the pancreas[47]. Experimental studies have suggested that after the entry of pancreatic juice into the biliary tree, the following a series of events occurs, thus producing NP: Activation of trypsinogen; reduction of mucin density by trypsin; enhancement of injurious actions and reduction of viscosity by mucin-free bile salts; and a diffusion of dangerous bile-trypsin mixture into the pancreas during a time when intra-abdominal pressure increases[48,49]. Clinically, however, questions regarding the evidence of bile reflux into the pancreatic duct and the presence of impacted stones have prevented wide acceptance of the common channel theory. In 2020, Isogai et al[50] reported a case of NP in which bile refluxed into the pancreas was histologically demonstrated in biopsy specimens of the pancreas collected during emergency surgery for GSP (Figure 1), thereby proving Opie’s long-speculated common channel theory. However, regarding the case studied by Isogai et al[50], intraoperative cholangiography showed no bile duct stones. Moreover, impacted stones are seldom found and remain a mystery.
Figure 1 Histological evidence of bile reflux into the pancreas in a patient with gallstone necrotizing pancreatitis.
A: Bile pigments among the inflammatory granulomatous tissue; B: Concretion of bile pigment-like material (arrow), periductal necrosis with neutrophil infiltration, and bleeding. Citation: Isogai M, Kaneoka Y, Iwata Y. Histological evidence of bile reflux in necrotizing pancreatitis: A case report. Med Case Rep Study Protoc 2020; 1: e0016. Copyright ©The Author 2020. Published by Wolters Kluwer Health Inc[50].
With the introduction of endoscopic US to investigate the bile duct, biliary sludge and biliary microlithiasis have become widely recognized as causes of acute pancreatitis[51]. Żorniak et al[51] proposed consensus definitions of biliary sludge, microlithiasis, and stones as hyperechoic material without acoustic shadowing, calculi ≤ 5 mm with acoustic shadowing, and calculi > 5 mm in the biliary tract and gallbladder, respectively; they showed no difference in the severity of pancreatitis between sludge-induced, microlithiasis-induced, and gallstone-induced pancreatitis. Recently, Hofstrand et al[52] reported a case of NP during the postpartum period that presented with clinical symptoms characteristic of severe pancreatitis, without bile duct stones, with gallbladder sludge on contrast-enhanced CT and magnetic resonance cholangiopancreatography, and with highly elevated liver and pancreatic enzymes in the blood. They concluded that the most likely etiology of NP was microlithiasis, which is difficult to detect in the bile duct using imaging techniques. After dissecting the biliary pancreatic junction of autopsy specimens, Kelly[53] reported that the mean diameter of the duodenal orifice was 2.5 mm and the mean length of the ampulla of Vater was 5 mm. It is likely that very small stones of such size, 3 mm in Opie’s[19] case, that are lodged at the duodenal orifice and convert the bile and pancreatic ducts into a common channel were either missed, even when surgery or traditional imaging studies were performed within 48 h of admission before sufficient time was allowed for stone passage[53], or expelled and evacuated after short-term occlusion of the duodenal orifice, thereby providing no evidence of their former impaction. Hernández and Lerch[54] noted that migrating stones induce functional stenosis at the sphincter of Oddi and a common channel can arise.
DiMagno et al[55] classified the pancreatic and bile duct entries as a common channel, an interposed septum, or separate opening categories; furthermore, they noted that a well-defined ampulla as a common channel that formed an ampullary structure and a long common channel > 3 mm without an ampullary structure are likely to have bile reflux into the pancreatic duct. However, common channels that allow bile reflux through impacted stones at the duodenal orifice are not universally present in patients with gallstones. McCutcheon[56] favored the reflux of duodenal contents rather than bile as a rational and simple explanation for the activation of pancreatic enzymes and subsequent pathological changes. Experimentally, he demonstrated that duodenopancreatic reflux occurred readily at physiological pressure if the papillary mucosal folds of the pancreatic duct that prevent reflux were damaged[29]. Clinically, the damage caused by the passage of stones possibly leads to insufficiency of the sphincter of Oddi, that is, loss of resistance to flow between the duodenum and pancreatic duct[57], and permits reflux of the duodenal contents into the pancreas, causing NP[22]. A case of NP for which intraoperative cholangioscopy was performed during emergency surgery and showed no stones in the bile duct but an injured and gaping sphincter has been reported elsewhere[8]. After surgery, stool samples were screened for gallstones, and a stone that was approximately 4 mm in diameter and grossly similar to that in the gallbladder was found[8], indicating possible reflux of the duodenal contents into the pancreas as the cause of NP. However, it is difficult to histologically prove that reflux is the cause of NP because the duodenal contents have no pigment to indicate their presence, and duodenal reflux has not been scientifically or clinically well-supported[28].
Thus, if the reflux of bile or duodenal content is the cause of NP, then the offending calculus will be expelled early during the disease course or pass into the duodenum and become lost. Genetic control has been explored as a possible determinant of pancreatitis severity[58]; however, the exact event responsible for progressive pancreatic inflammation after stone passage into the duodenum may be multifactorial and remains to be determined[22].
PATHOPHYSIOLOGY AND MANAGEMENT OF SEVERE GSP
Severe GSP may be a hybrid disease[59], with pathology polarized between acute cholangitis and NP, each of which has a distinct etiology and is inversely related to the presence or absence of impacted ampullary stones with biliopancreatic obstruction. When the status of the stones and the presence or absence of biliopancreatic obstruction without bile reflux are determined, the clinical course and outcome can be predicted.
Severe biliary-type GSP should not be misdiagnosed as severe pancreas-type GSP. If an impacted ampullary stone is detected using imaging modalities including a high-quality evaluation of the biliary system with endoscopic US[60] or magnetic resonance cholangiopancreatography[61], an accurate diagnosis of acute cholangitis can be made, or ongoing biliary obstruction can be strongly predicted by serum total bilirubin > 4mg/dL[62,63], then ERCP with stone removal through the duodenal papilla is indicated[63-65], for severe biliary-type GSP.
When an impacted ampullary stone with biliopancreatic obstruction is ruled out, contrast-enhanced CT is a safe and accurate method of identifying NP[66]. Kovalska et al[67] noted that during the early stages of NP, the pathological changes were impaired microcirculation caused by intravascular microthrombosis with endothelial desquamation and sludge. Thus, after the first week of disease, a non-enhancing area of the pancreatic parenchyma on contrast-enhanced CT should be considered pancreatic necrosis[25]. The CT severity index[68], when calculated after the first week of disease, has been reported to show the highest diagnostic and predictive accuracies[18]. Song et al[69] reported the usefulness of contrast-enhanced CT features at the time of admission, such as the presence of peripancreatic fluid and heterogeneous pancreatic parenchyma enhancement, for predicting the progression to NP in patients initially diagnosed with IP. Generally, however, it is difficult to determine the final severity of pancreatitis early during the disease course of patients with predicted severe pancreatic-type GSP, and maximum intensive care treatment is mandatory. A recent multicenter randomized controlled trial showed that urgent ERCP with ES within 24 h after presentation did not reduce major complications or mortality of patients with predicted severe GSP and without cholangitis[70].
FUTURE PERSPECTIVES
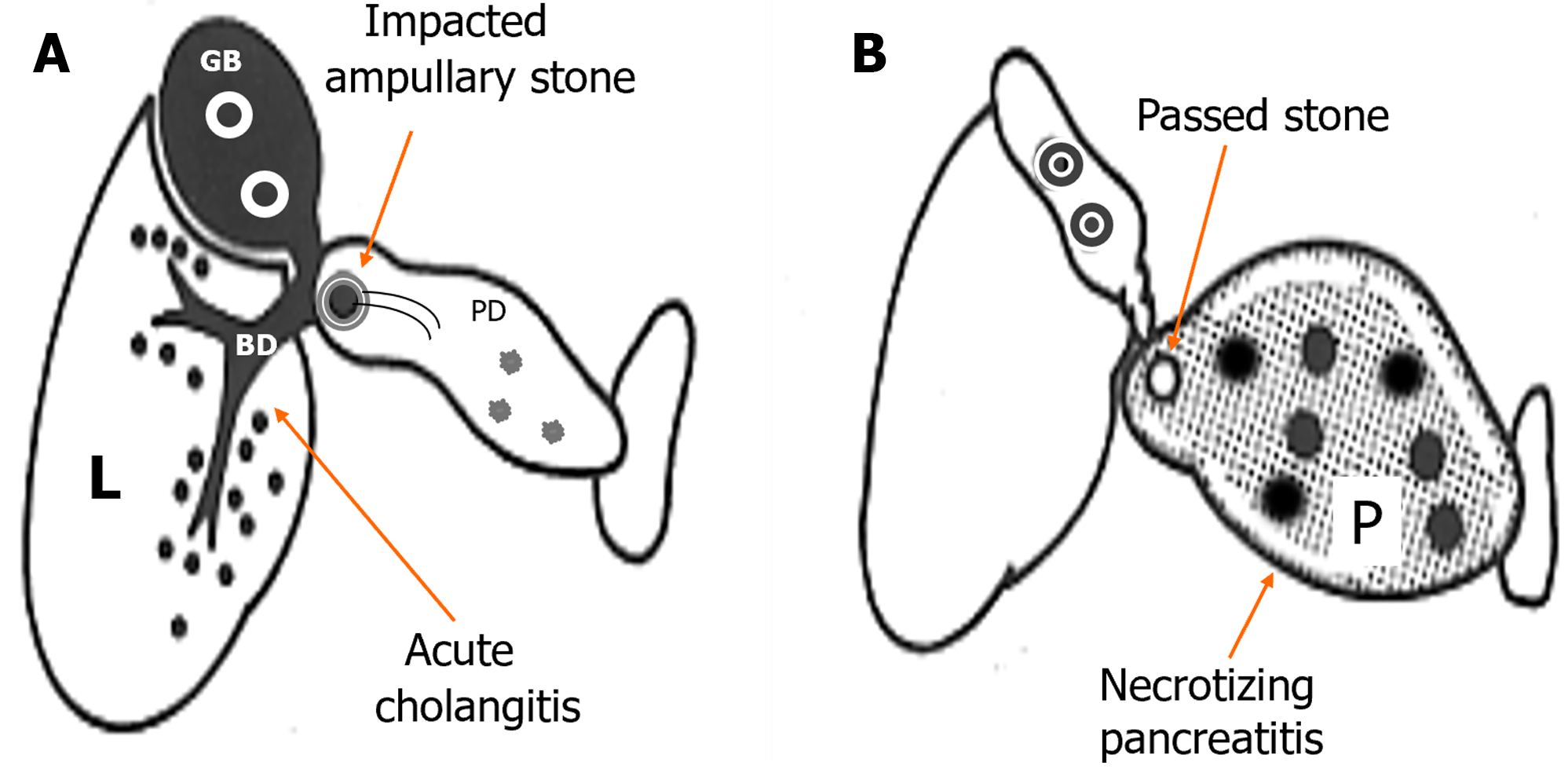
Conventionally, clinicians have focused less attention on the hepatobiliary diseases with GSP that may be unavoidable because the terminology of GSP refers to “pancreatitis” alone, without “hepatitis.” This can be explained by the lower availability of liver enzyme testing during the early 1960s, when Howard and Ehrlich[71] proposed the term “GSP “as a distinct clinical entity in 1962. In 1955, Wroblewski and LaDue[72] first reported increases in serum glutamic-oxaloacetic transaminase levels associated with liver disease that occurred as a result of the release of enzymes from damaged liver tissue. To provide a better understanding of the nature of GSP (i.e., gallstone-induced acute hepatitis and pancreatitis[73]) and direct the clinician’s attention to hepato-biliary-pancreatic lesions that occur in both the liver and pancreas, in 2021, Isogai[74] devised the term “gallstone hepatopancreatitis” in place of GSP. At the same time, the terms “gallstone cholangiopancreatitis” and “gallstone NP” were proposed for the biliary and pancreatic types of severe disease, respectively, with the former emphasizing that acute cholangitis is not a comorbid disease; instead, it is an essential condition that outweighs pancreatic lesions (Figure 2)[74].
Figure 2 Subdivision of severe gallstone pancreatitis into gallstone cholangiopancreatitis and gallstone necrotizing pancreatitis.
A: Gallstone cholangiopancreatitis with persistent ampullary stone impaction and ascending acute cholangitis complicated with minimal or mild pancreatic inflammation caused by biliopancreatic obstruction. B: Gallstone necrotizing pancreatitis caused by the reflux of bile or possible duodenal contents into the pancreas, not complicated by acute biliary tract disease caused by the passage of stones. L: Liver; BD: Bile duct; GB: Gallbladder; PD: Pancreatic duct; P: Pancreas. Citation: Isogai M. Proposal of the term “gallstone cholangiopancreatitis” to specify gallstone pancreatitis that needs urgent endoscopic retrograde cholangiopancreatography. World J Gastrointest Endosc 2021; 13: 451-459. Copyright ©The Author(s) 2021. Published by Baishideng Publishing Group Inc[74].
Gallstones represent the main cause of acute pancreatitis globally[75,76], with a contributory rate twice that of alcohol[75]. Thus, clinicians are expected to encounter GSP more often, and awareness of the etiology and pathogenesis of severe disease is mandatory.
CONCLUSION
Severe GSP may be a hybrid disease with pathology polarized between acute cholangitis and NP in which the severity of hepatobiliary and pancreatic lesions is inversely related to the presence or absence of impacted ampullary stones with biliopancreatic obstruction. When the status of the stones is determined, the clinical course and outcome can be predicted.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Japanes Society for Abdominal Emergency Medicine, 501-756-1830; Japan Surgical Association, 5292.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chow WK, Taiwan; Jovanovic P, Bosnia S-Editor: Lin C L-Editor: A P-Editor: Cai YX