Published online Feb 7, 2024. doi: 10.3748/wjg.v30.i5.471
Peer-review started: October 7, 2023
First decision: December 8, 2023
Revised: December 17, 2023
Accepted: January 12, 2024
Article in press: January 12, 2024
Published online: February 7, 2024
Processing time: 115 Days and 20.7 Hours
Primary sclerosing cholangitis (PSC) is characterized by chronic inflammation and it predisposes to cholangiocarcinoma due to lack of effective treatment options. Recombinant adeno-associated virus (rAAV) provides a promising platform for gene therapy on such kinds of diseases. A microRNA (miRNA) let-7a has been reported to be associated with the progress of PSC but the potential therapeutic implication of inhibition of let-7a on PSC has not been evaluated.
To investigate the therapeutic effects of inhibition of a miRNA let-7a transferred by recombinant adeno-associated virus 8 (rAAV8) on a xenobiotic-induced mouse model of sclerosing cholangitis.
A xenobiotic-induced mouse model of sclerosing cholangitis was induced by 0.1% 3,5-Diethoxycarbonyl-1,4-Dihydrocollidine (DDC) feeding for 2 wk or 6 wk. A single dose of rAAV8-mediated anti-let-7a-5p sponges or scramble control was injected in vivo into mice onset of DDC feeding. Upon sacrifice, the liver and the serum were collected from each mouse. The hepatobiliary injuries, hepatic inflammation and fibrosis were evaluated. The targets of let-7a-5p and downstream molecule NF-κB were detected using Western blot.
rAAV8-mediated anti-let-7a-5p sponges can depress the expression of let-7a-5p in mice after DDC feeding for 2 wk or 6 wk. The reduced expression of let-7a-5p can alleviate hepato-biliary injuries indicated by serum markers, and prevent the proliferation of cholangiocytes and biliary fibrosis. Furthermore, inhibition of let-7a mediated by rAAV8 can increase the expression of potential target molecules such as suppressor of cytokine signaling 1 and Dectin1, which consequently inhibit of NF-κB-mediated hepatic inflammation.
Our study demonstrates that a rAAV8 vector designed for liver-specific inhibition of let-7a-5p can potently ameliorate symptoms in a xenobiotic-induced mouse model of sclerosing cholangitis, which provides a possible clinical translation of PSC of human.
Core Tip: Primary sclerosing cholangitis (PSC) have a high risk of cholangiocarcinoma with a lack of effective treatment options. Then the present study aimed to investigate the therapeutic effects of inhibition of a microRNA let-7a transferred by recombinant adeno-associated virus 8 (rAAV8) on a 3,5-Diethoxycarbonyl-1,4-Dihydrocollidine-induced mouse model of sclerosing cholangitis. And the results of our study demonstrates that a rAAV8 vector designed for liver-specific inhibition of let-7a-5p can potently ameliorate symptoms in a xenobiotic-induced mouse model of sclerosing cholangitis, which provides a possible clinical human clinical translation of PSC.
- Citation: Hua H, Zhao QQ, Kalagbor MN, Yu GZ, Liu M, Bian ZR, Zhang BB, Yu Q, Xu YH, Tang RX, Zheng KY, Yan C. Recombinant adeno-associated virus 8-mediated inhibition of microRNA let-7a ameliorates sclerosing cholangitis in a clinically relevant mouse model. World J Gastroenterol 2024; 30(5): 471-484
- URL: https://www.wjgnet.com/1007-9327/full/v30/i5/471.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i5.471
Primary sclerosing cholangitis (PSC) is an idiopathic cholestatic disease and it affects intrahepatic and/or extrahepatic bile ducts characterized by chronic inflammation, ductal stricture, cholestasis, and fibrosis[1,2]. PSC patients can progress to liver cirrhosis and have a great risk of cholangiocarcinoma with liver failure at the end stage. PSC has been considered an orphan disease since the prevalence of PSC is up to 16.2 per 100000 population with variable distribution due to different regions[3-5]. Although the occurrence of PSC is rare, it showed significant morbidity and mortality in PSC patients as there is a very limited medical option to interfere with the course of PSC, and liver transplant is the exclusively therapeutic option at the end-stage of the disease[1,6]. MicroRNA (miRNA) represents a kind of small non-coding RNA with 18-23 nucleotide length. The miRNAs widely participate in various processes in physiological and disease conditions by regulating of expression mRNAs at the post-transcriptional level. Although the etiology and pathogenesis of PSC are complex and largely unknown, increasing data demonstrate that the dysregulation of miRNA can also contribute to the pathogenesis of PSC[7-9]. These aberrant miRNAs have been reported to be involved in almost all aspects of diseases including inflammation, hyperplasia, fibrosis, and malignancy of cholangiocytes in patients or mouse models of PSC[10,11]. Of these miRNAs, the let-7a family is one of the latest identified miRNAs that are dysregulated in cholestasis[12-16], suggesting possible medical targets of let-7a on cholestatic diseases. However, the therapeutic effect of targeting let-7a in cholestasis is yet to be determined.
Recombinant adeno-associated virus (rAAVs) provide a promising vehicle for gene therapy as they can efficiently transfer genes to the target tissue with low immunogenicity, low toxicity, and long persistence[17-23]. rAAV8 is one serotype that can transduce hepatocytes and cholangiocytes with high efficiency and specificity due to its liver tropism and has been implicated in many liver diseases including cholangiopathies[19,24-28]. Increasing data showed that rAAVs also can deliver miRNAs (mimics, precursor, or its antisense) to play the significant therapeutic effects of miRNAs on many other liver diseases[29-32], but there are rare therapeutic data about miRNAs delivered by rAAVs on cholangiopathies. Given the aberrance and importance of let-7a in cholangiopathies especially in PSC, we hypothesize that inhibition of let-7a delivered by rAAV8 has potential therapeutic effects on PSC. To support our hypothesis, we used a well-established mouse model of PSC- a mouse model of sclerosing cholangitis via 0.1% 3,5-Diethoxycarbonyl-1,4-Dihydrocollidine (DDC) feeding, following treatment with a single dose injection of rAAV8-delivered inhibitors (anti-senses of let-7a sponges) of let-7a for 2 wk or 6 wk. Our data showed that we have successfully developed a possible therapeutic strategy for PSC based on the administration of an AAV8 vector designed for liver-specific inhibition of let-7a. This strategy could markedly ameliorate biliary injuries by the decreased proliferation of cholangiocytes, biliary inflammation, and fibrosis in the mice induced by 0.1% DDC feeding for 2 wk or 6 wk. Furthermore, the therapeutic effects may be associated with the inhibition of NF-κB-mediated hepatic inflammation. The present study demonstrates that the rAAV-mediated miRNAs strategy provides a promising therapeutic opportunity for this debilitating and life-threatening disease.
Female C57BL/6J mice without specific pathogen, 6-wk old, 18-25 g in weight, were raised in a clean-grade animal house, free to eat and drink, and the animal lab environment as the following specifications: temperature range from 20 °C to 26 °C, humidity range from 40% to 70%, and 12 h/12 h light/dark. Tail vein injection and other operations were conducted in the animal barrier experimental operation room of the Experimental Animal Center of Xuzhou Medical University [SYXK (Su) 2016-0028]. Animal care and all experiments in this study were carried out following the guidelines of the National Laboratory Animal Center and were given humanitarian care according to the 3R principle of laboratory animals. The main procedures and protocols were approved by the Animal Care and Use Committee of Xuzhou Medical University (license IACUC: 201801w003).
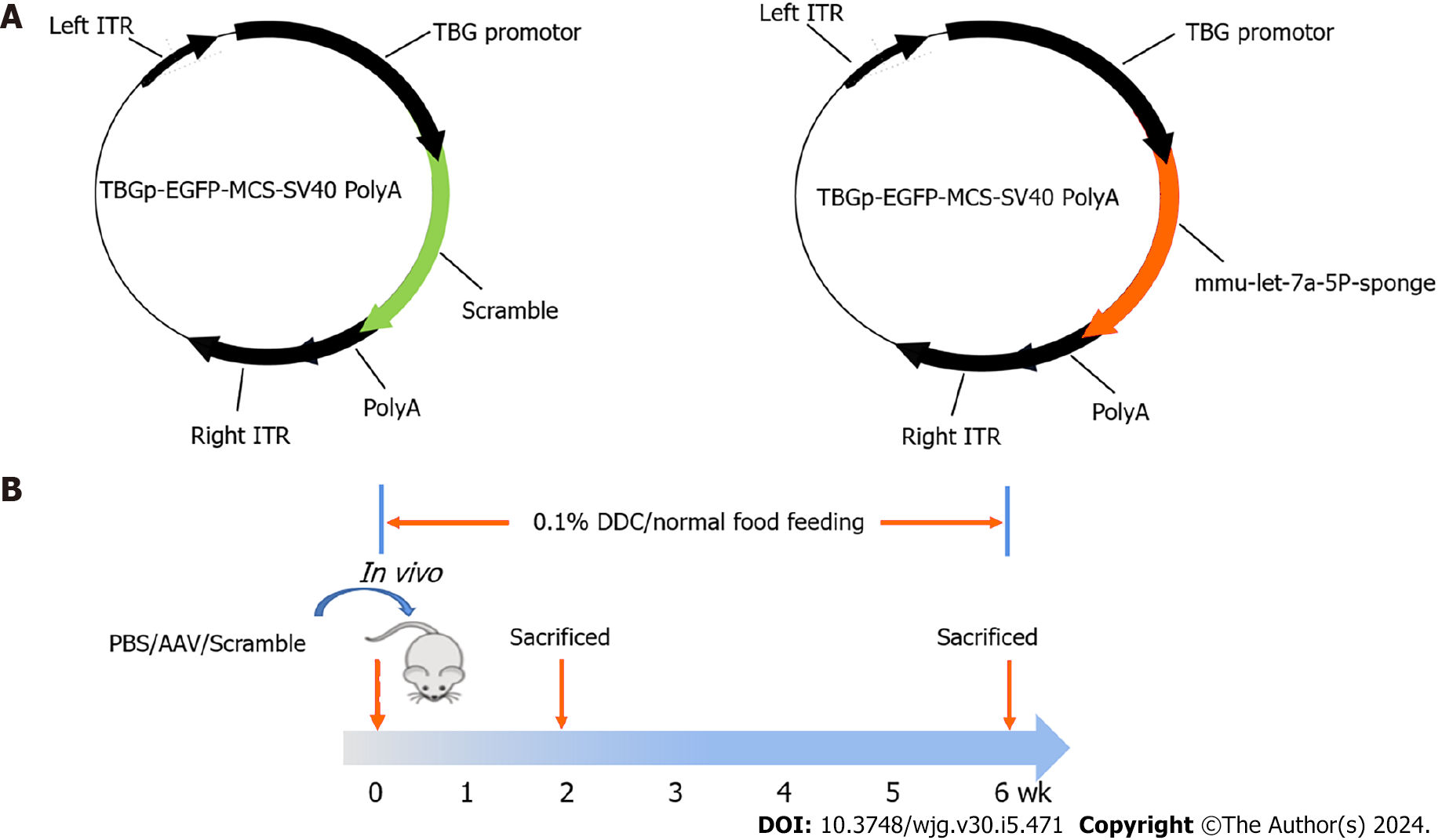
A sclerosing cholangitis mouse model was induced by 0.1% DDC feeding for 2 wk or 6 wk. Specifically, 24 mice were fed DDC food while the other 12 mice were fed chow food as a control. The adeno-associated virus (AAV) labeled with EGFP for targeting knocking down the expression of let-7a-5p in the liver was purchased from Genechem (Shanghai, China). The mice in the two DDC-feeding groups were intravenously injected with 100 μL 4 × 1012 vector genomes (VG)/mL AAV that overexpressed mmu-let-7a-5P-sponges or empty vehicles (4 × 1011 vg per mice). And the 12 normal control mice were injected with the same volume of PBS (LPS-free) in which the AAV original liquid was diluted. Following, half of the mice from each group were sacrificed by euthanasia in 2 wk, while the half of rest were sacrificed in 6 wk. The livers and sera from each mouse were harvested for further experiments. The inhibitor sponges of mmu-let-7a-5P are ACCACACAAgacCTACCTCCcttcACCACACAAgacCTACCTCCcttcACCACACAAgacCTACCTCCatccgtaAC CACACAAgacCTACCTCCcttcACCACACAAgacCTACCTCC, and the specific molding method is shown as Figure 1.
Total RNAs from liver tissues were extracted using the TRIZOL reagent (Invitrogen, Carlsbad, CA, United States), following the manufacturer’s instructions, and then reverse transcribed into cDNA using the miRcute Plus miRNA First-Strand cDNA Kit (TIANGEN Biotech, Beijing, China) or FastKing RT Kit (With gDNase) (TIANGEN Biotech, Beijing, China). Then, qPCR was performed using the SYBR Green Master Mix (TransGen Biotech, Beijing, China) and run on a StepOne Plus Real-Time PCR System (Roche Applied Science, Mannheim, Germany). The relative expression levels of miRNAs or mRNAs were normalized to U6 small nuclear RNA (snRNA) or β-actin following the 2-ΔΔCt comparative method. The sequences of the primers used in this study were optimized as Table 1.
| Target | Oligonucleotide sequence (5’-3’) | |
| Forward primer | Reverse primer | |
| let-7a-5p | GGAGGTAGTTCGTTGTGTGGT | |
| U6 | ATGGGTCGAAGTCGTAGCC | TTCTCGGCGTCTTCTTTCTCG |
| Gfp | TGCTTCAGCCGCTACCC | AGTTCACCTTGATGCCGTTC |
| β-actin | AACTCCATCATGAAGTGTGA | CTGCGGCTTCTATTGGGGAC |
| Tnfa | CTTGTTGCCTCCTCTTTTGCTTA | GACTTCAGCACTCAAGACATCC |
| Il6 | TCACAGAAGGAGTGGCTAAGGACC | ACGCACTAGGTTTGCCGAGTAGAT |
| Acta2 | TTCATCGGGATGGAGTCTGCTGG | TCGGTCGGCAATGCCAGGGT |
| Tgfb | CCACCTGCAAGACCATCGAC | CTGGCGAGCCTTAGTTTGGAC |
| Ccl2 | TTAAAAACCTGGATCGGAACCAA | GCATTAGCTTCAGATTTACGGGT |
The mouse serum was collected and transported to the Laboratory Department of the Affiliated Hospital of Xuzhou Medical University at low temperature to detect the activities of bile duct injury-related enzymes [alanine aminotransferase (ALT), total bilirubin (BILT), and total bile acid (TBA)].
For histological analysis, liver tissues were excised and fixed with 4% paraformaldehyde for 24 h at least. Thereafter, the fixed tissues were embedded in paraffin, sliced to a thickness of 4 μm, and routinely stained with hematoxylin and eosin (H&E) and Sirius red staining according to the recommendation of the manufacturer (Shanghai Xinfan Biotechnology, Shanghai, China), after sealing the slides with neutral adhesive, the pathological changes of stained histological sections were observed by a microscope (Olympus, Japan). The staining pictures were quantitatively analyzed with Image J, 6 pictures zoomed in 100 × were selected for each mouse for quantitative analysis, and the average value was taken as the quantitative value of that mouse, n = 6.
The liver tissue was deparaffinized, hydrated, and heated in citric acid buffer at 95 °C for 15 min and blocked with 5% BSA for 30 min, and then incubated overnight with primary anti-CK19 (1:500, ab52625, Abcam, Cambridge, United States) and then incubated with secondary antibodies for 30 min at room temperature. After washing with PBS, DAB (1:20, ZSGB-BIO, Beijing, China) as an enzyme substrate was added. Six high-power fields (× 100 magnifications, Olympus, Japan) were randomly selected from each mouse staining section and the percentage of CK19 positive area was calculated by ImageJ software (NIH, United States).
The slides with liver tissues were fixed in 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, and then blocked with 5% BSA. Slides were incubated with the indicated primary antibodies anti-GFP (1:100; Abcam, Cambridge, MA, United Kingdom) overnight at 4 °C and then incubated with fluorescence-labeled secondary antibodies for 1 h at room temperature. Nuclei were stained using DAPI for 10 min. Six images zoomed in 100 × per mouse were captured under a fluorescence microscope (AX70, Olympus, Japan), and the average signal value from the 6 images was used to represent the value from one mouse liver. Ten fields from each slide were randomly selected, and the positive color area was measured quantitatively using Image-Pro Plus 6.0 software.
Hepatic hydroxyproline (Hyp) content was determined using a commercially available kit according to the manufa
Liver homogenates were harvested and washed in cold PBS twice and then were treated with the lysis buffer (Beyotime, Shanghai, China) on ice for 30 min. The lysate was collected into microtubes and centrifuged for 15 min at 12000 rpm at 4 °C. Protein samples (20 mg) were denatured with the 5 × SDS loading buffer at 100 °C for 5 min then were segregated on a 10% SDS polyacrylamide gel electrophoresis and transferred onto 0.2 um nitrocellulose membranes. After 60 min of blocking with 5% fat-free milk, membranes were incubated with suppressor of cytokine signaling 1 (SOCS1) (1:1000; Cell Signaling Technology, United States), Dectin-1 (1:1000; Abclonal, Wuhan, Hubei Province, China), P-p65 (1:1000; Cell Signaling Technology, United States) and GAPDH antibody (1:2000; Abmart, Shanghai, China) overnight at 4 °C. After washing with TBST 3 times, blots were incubated with the anti-rabbit secondary antibody (1:5000; Abclonal, Wuhan, China) for 1 h. After washing, immunoreactive protein bands were detected by using enhanced chemiluminescence reagents (Bio-Rad, California, United States). Band intensities were normalized to GAPDH and analyzed using ImageLab software.
All data are presented as means ± SEM. The statistical analysis was performed with the use of the software package SPSS version 19.0 (SPSS Inc, Chicago, United States). One-way ANOVA analysis was used for the comparison of differences among more than two groups, which was followed by the Least Significant Difference test unless otherwise stated. In the case of the comparison of two groups, the differences were evaluated using a two-tailed Student’s t-test, a value of P < 0.05 was considered significant.
Since the expression of let-7a-5p is increased in both experimental obstructive cholestasis and patients[12], we investigated the potential therapeutic effects of an inhibitor of let-7a-5p delivered by highly hepatotropic rAAV8 on a mouse model of PSC at 2 wk and 6 wk. To address this, we generated rAAV8 expression plasmids containing either the scramble control (anti-SCR) or anti-let-7a-5p sponges under the control of a liver-specific thyroxine-binding globulin (TBG) promoter (Figure 1A) and injected rAAV8-anti-let-7a-5p (anti-let-7a-5p, 4 × 1011 vg/mouse) or rAAV8-scramble (anti-SCR, 4 × 1011 vg/mouse) with a single dose at the beginning of DDC feeding for 2 wk and 6 wk, respectively (n = 6 per group, Figure 1B).
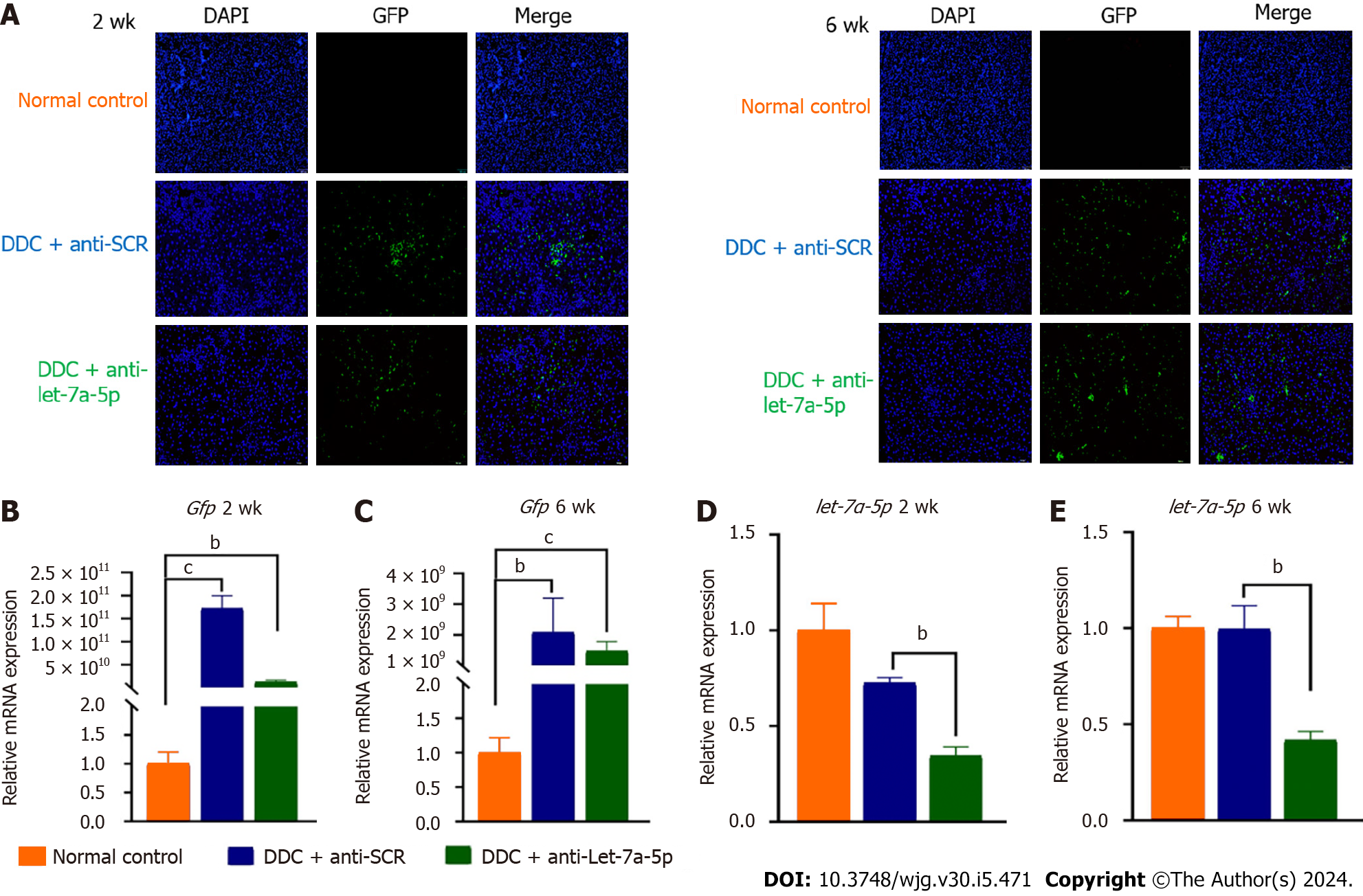
After 2 or 6 wk for DDC feeding, we found that the GFP-flagged rAAVs were extensively observed in the livers of mice injected with rAAVs anti-SCR control or anti-let-7a-5p sponges (Figure 2A-C), and the relative expression of GFP indicating the amounts of virus in the liver were significantly increased both in anti-SCR and inhibitor mice at 2 wk or 6 wk, although it seems that the titer of recombinant adenovirus has dropped from 2 wk to 6 wk. We further evaluated the inhibition efficiency of let-7a-5p using anti-let-7a-5p sponges, it was found that anti-let-7a-5p sponges can significantly depress approximately 50% (at 2 wk) and approximately 60% (at 6 wk) of let-7a-5p in the liver of rAAV8-anti-let-7a-5p-injected mice, compared with anti-SCR mice (Figure 2D and E).
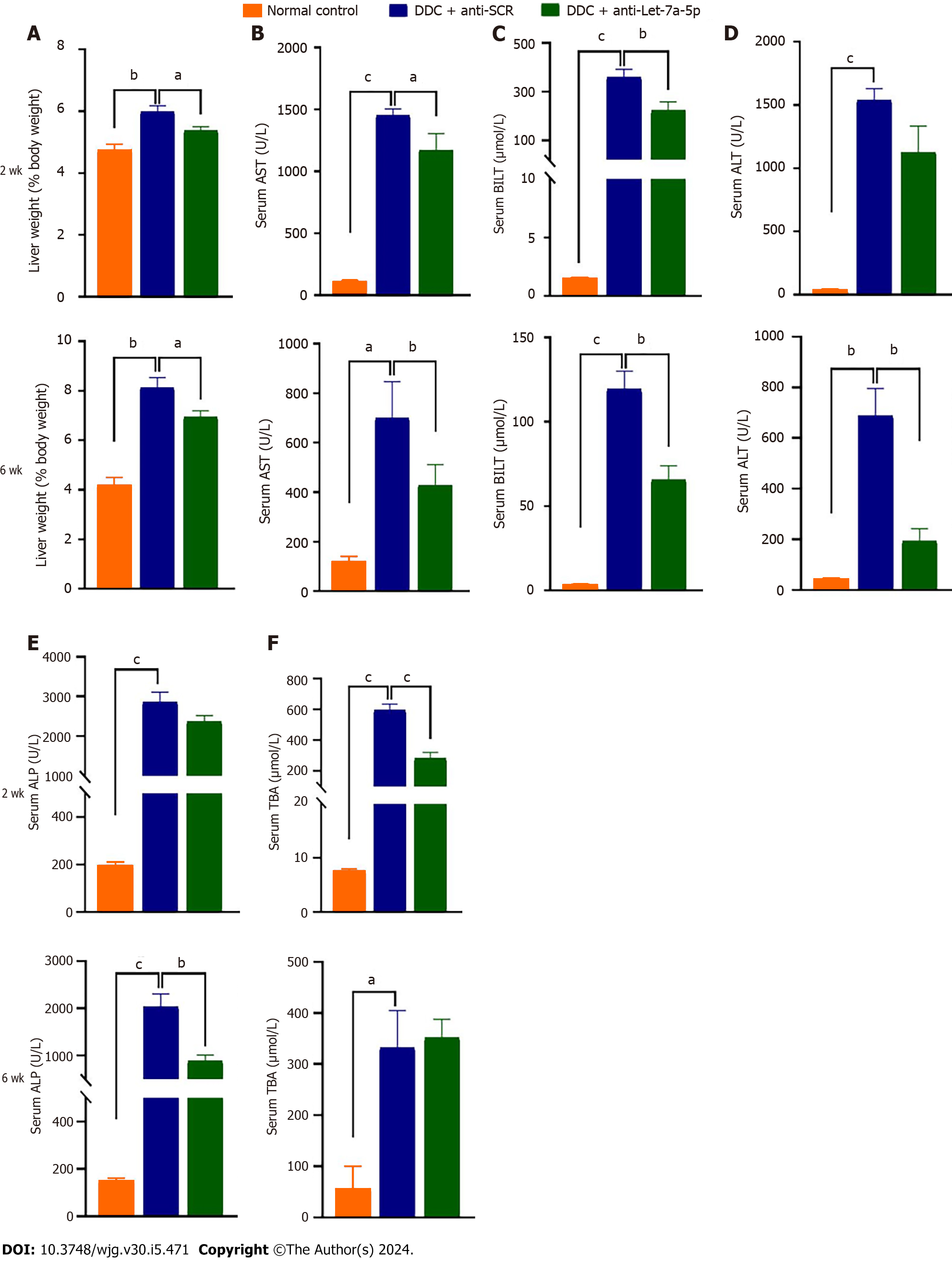
Next, we evaluated the amelioratory effects of let-7a-5p on hepato-biliary injuries caused by DDC (Figure 3). We found that DDC feeding for 2 wk or 6 wk both can significantly increase the ratio of liver weight to body weight which may indicate hepatomegaly, but let-7a-5p inhibitor depressed the ratio, compared with anti-SCR control at 2 wk or 6 wk (P < 0.05; Figure 3A). In addition, let-7a-5p inhibitor delivered by rAAVs can also reduce the levels of aspartate aminotransferase and BILT that were increased due to hepatic-biliary damages induced by DDC feeding both at 2 wk and 6 wk (P < 0.05, Figure 3B and C). At 2 wk, although there was no statistical significance of ALT and alkaline phosphatase (ALP) between anti-let-7a-5p mice and anti-SCR control mice, there are decreasing trends of ALT and ALP activities in anti-let-7a-5p mice than those anti-SCR control mice [P > 0.05, Figure 3D and E (upper panel)]. However, after 6 wk, there are significant decreases of ALT and ALP in the sera of anti-let-7a-5p mice, compared with anti-SCR control [P < 0.05, Figure 3D and E (nether panner)]. Taken together, let-7a-5p inhibitor delivered by rAAV8 ameliorates the hepato-biliary injuries caused by DDC feeding.
To assess the potential side effects of rAAV delivering anti-let-7a-5p sponges or anti-SCR on kidney function, we also detected serum urea, creatinine, and uric acid in those administrated mice both at 2 wk and 6 wk. Compared with normal control mice, we didn’t find any increases in these indexes after injection of rAAV8, suggesting that rAAV8 injection can’t induce kidney injuries (Supplementary Figure 1).
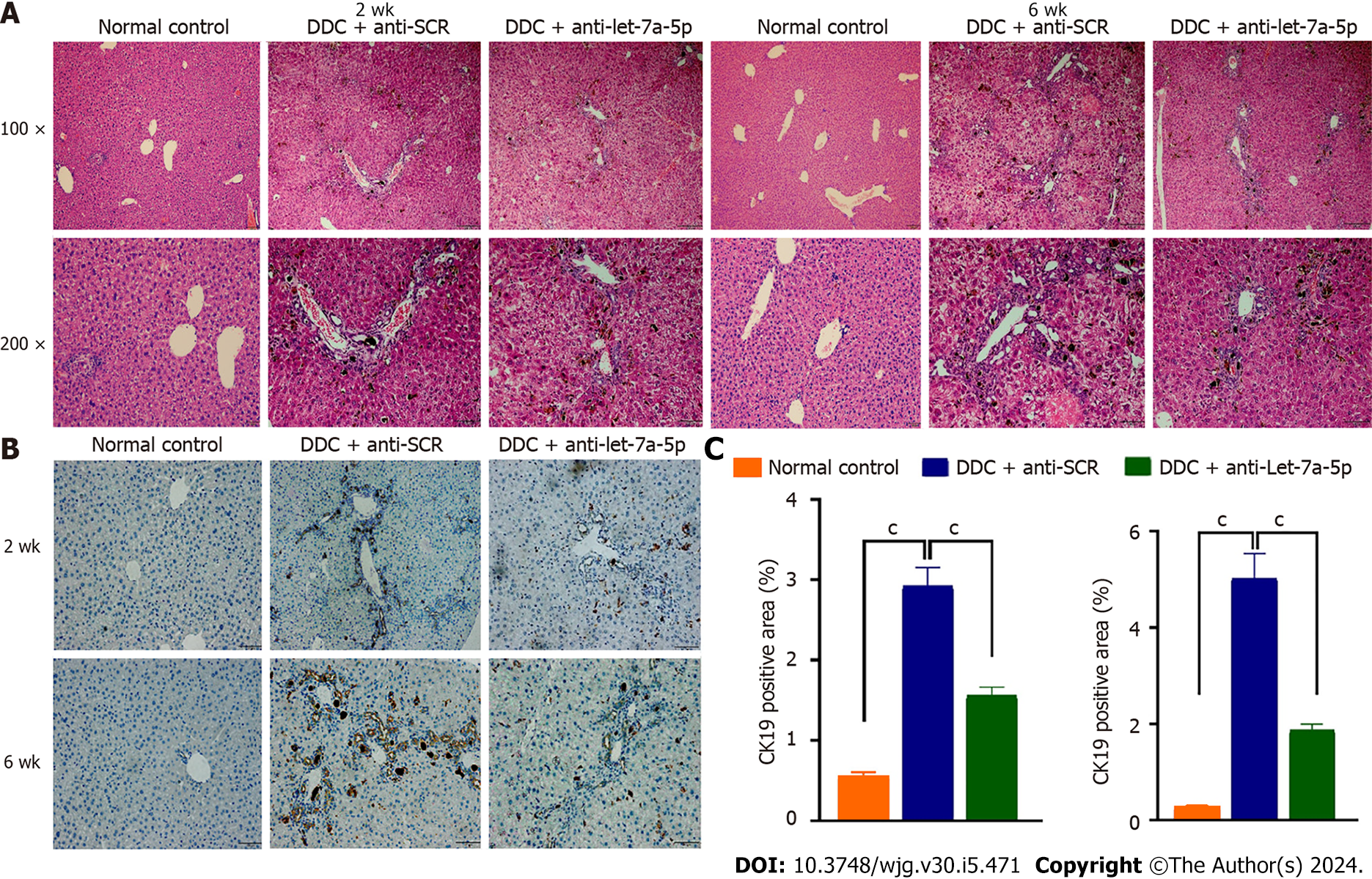
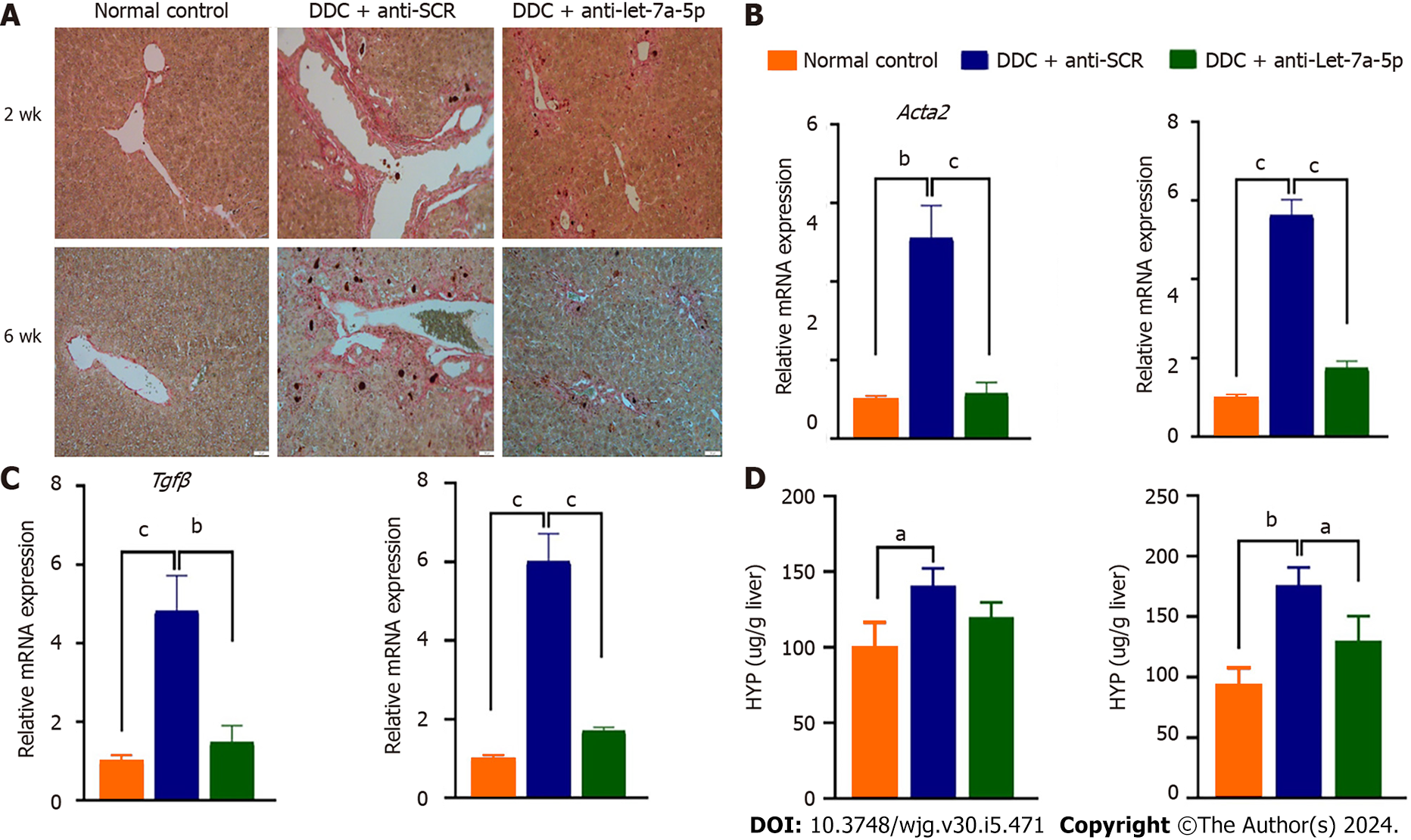
H&E staining showed DDC feeding causes a moderated ductular reaction at 2 wk, and DRs became severe with the extended time of DDC feeding at 6 wk (Figure 4A), but rAAV8-let-7a-5p inhibitor injected mice showed a significant amelioration of the ductular reaction both at 2 wk and 6 wk, compared with anti-SCR control mice (Figure 4A). We also stained the CK19-specific marker for cholangiocytes in the liver, we found that the percentage of positive CK19 cells was dramatically increased after DDC feeding, compared with normal control mice, but anti-let-7a-5p mediated by rAAV8 significantly decreased the percent of positive CK19 cholangiocytes both at 2 wk and 6 wk, compared with anti-SCR control mice (P < 0.05, Figure 4B and C).
Sirus-red staining showed there are massive “strip shape” collagen fibers deposition around bile ducts after DDC feeding for 2 wk and 6 wk, however, after treatment with rAAV8 mediated anti-let-7a-5p for 2 or 6 wk, the deposition of these collagen fibers was significantly decreased (Figure 5A). Furthermore, we detected other fibrotic biomarkers such as Acta2 (encoding α-SMA) and Tgfb using qPCR, after 2 or 6 wk of the feeding of DDC, we found that the relative expression of Acta2 and Tgfb was significantly higher in the DDC feeding mice than those in normal control mice without DDC feeding, but the mice injected with let-7a-5p inhibitor delivered by rAAV8 showed a noteworthy drop (almost five times drop) in Acta2 and Tgfb expression, compared with anti-SCR control mice when they were fed with DDC for 2 or 6 wk (P < 0.001, Figure 5B and C). We also detected Hyp-another liver fibrosis marker, it was shown that there was no significant difference in anti-SCR control mice and anti-let-7a-5p mice at 2 wk for DDC feeding, but after 6 wk of treatment, the content of Hyp was significantly decreased in anti-let-7a-5p mice, compared with anti-SCR control mice (P < 0.05, Figure 5D). Taken together, rAAV8 delivered let-7a-5p inhibition abates liver fibrosis in experimental sclerosing cholangitis.
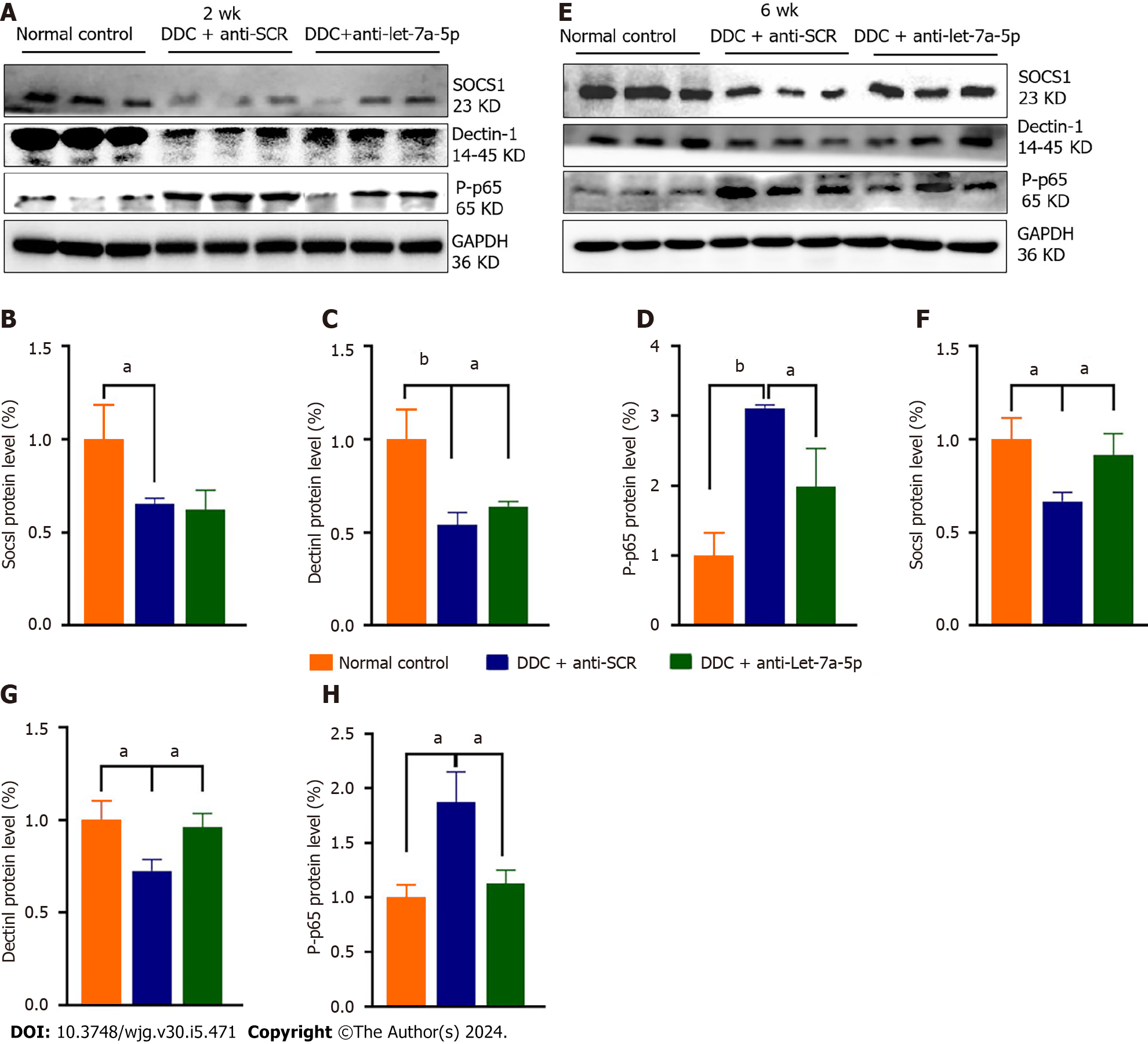
Our previous and other studies demonstrated that let-7a-5p can potently regulate immune responses to depression NF-κB-mediated pro-inflammation by targeting several molecules such as SOCS1 and Dectin-1[33]. Therefore, we detected hepatic expression of SOCS1, Dectin-1, and P-p65 in the DDC feeding mice that were injected with rAAV8 mediated anti-SCR control or anti-let-7a-5p. Regarding to 2 wk’ DDC feeding, there was no significant differences in the expression of SOCS1 between anti-SCR and anti-let-7a-5p group (P > 0.05, Figure 6A and B), but compared with rAAV8 mediated anti-SCR control group, the expression of Dectin-1 in rAAV8 mediated anti-let-7a-5p mice were significantly increased (P < 0.05, Figure 6A and C), and the level of downstream inflammatory transfactor P-p65 of NF-κB were remarkably decreased (P < 0.05, Figure 6A and D); For 6 wk DDC feeding, anti-let-7a-5p can both depressed the expression of SOCS1 (P < 0.05, Figure 6E and F) and Dectin-1 (P < 0.05, Figure 6E and G), which induced the lower level of P-p65 of NF-κB (P < 0.05, Figure 6E and H).
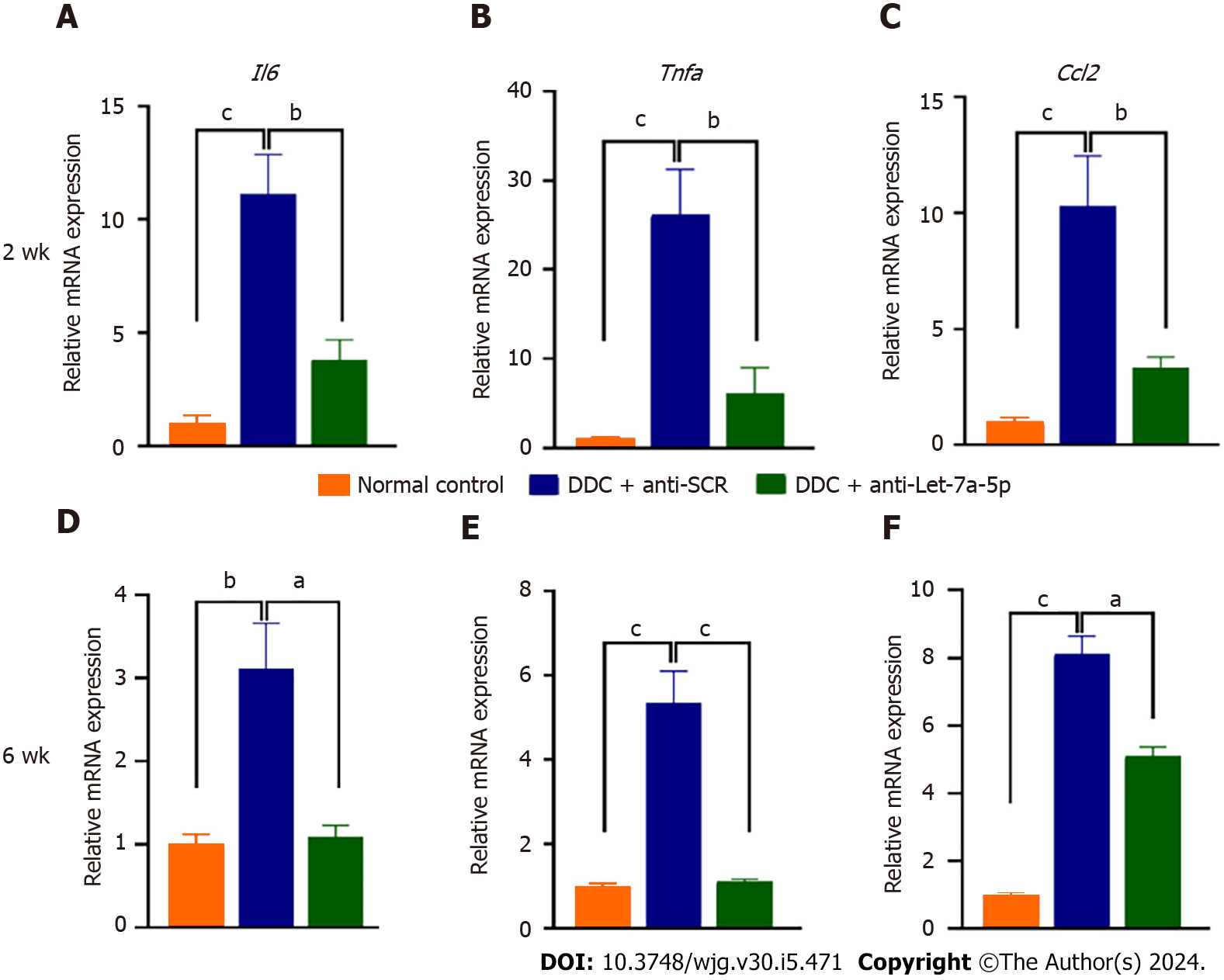
Sequentially, we further detected the pro-inflammatory cytokines such as IL-6, TNF-α, and CCl2. At 2 wk, DDC feeding induced high levels of Il6, Tnfa, and Ccl2 mRNA production, but rAAV8-mediated let-7a-5p inhibitor depressed the production of these cytokines, compared with an anti-SCR group (P < 0.01, Figure 7A-C, upper panel). The production of these cytokines in the mice at 6 wk after DDC feeding seems to be declined compared with those in the mice with DDC feeding for 2 wk, but they were still higher than those in the normal control mice (at least more than 3 times; P < 0.001, Figure 7A-C, nether panel); furthermore, the production of these cytokines were also significantly decreased in rAAV8 mediated anti-let-7a-5p mice, compared with anti-SCR group mice when they were feeding DDC for 6 wk (P < 0.01, Figure 7D-F, D for II6, E for Tnfa, and F for Ccl2 nether panel). Taken together, our data demonstrated that anti-let-7a-5p delivered by rAAV8 ameliorate experimental sclerosing cholangitis by targeting at Dectin-1 or SOCS1 -negatively regulated NF-κB.
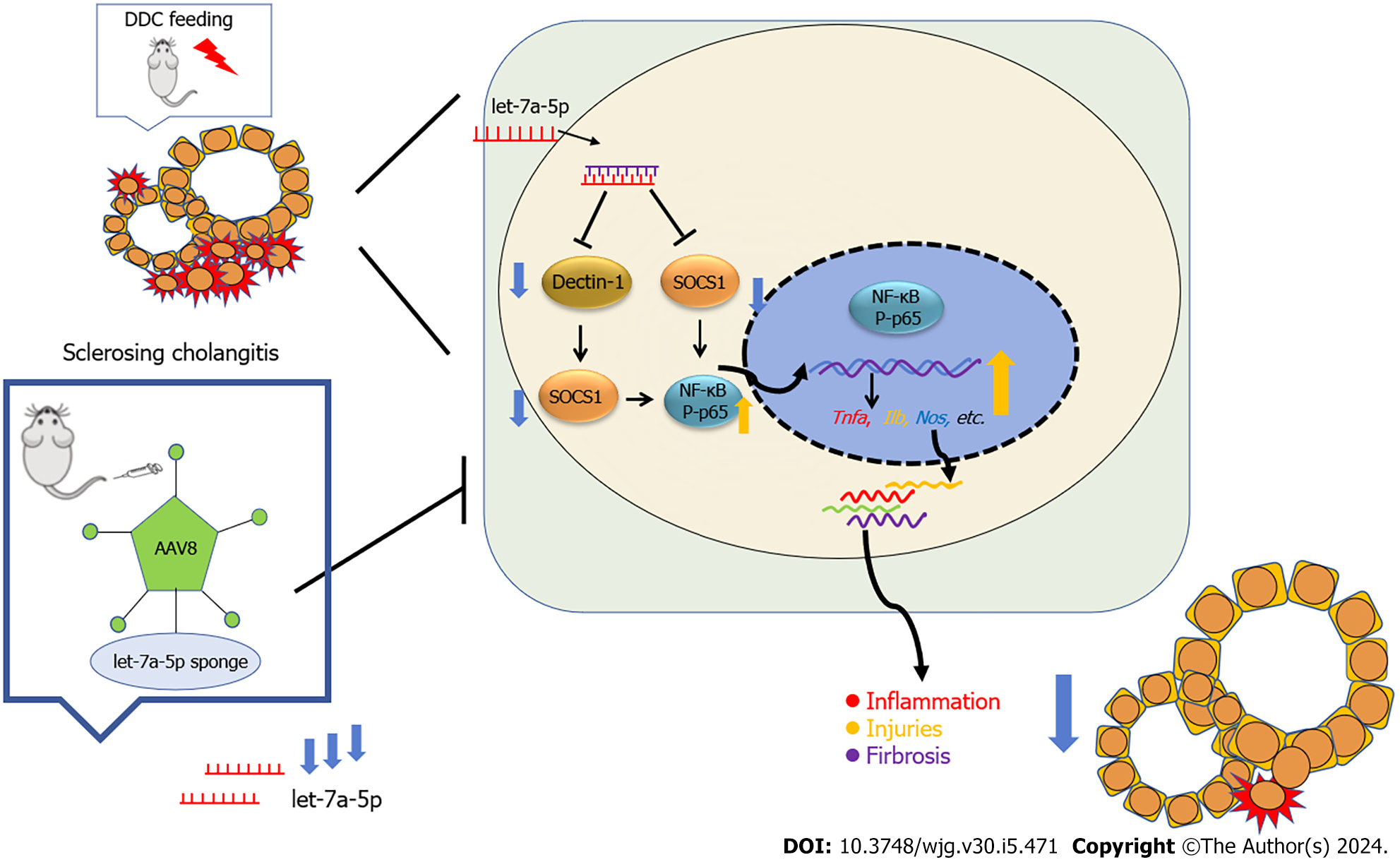
PSC is a rare but life-threatening chronic disease and there is no effective medical option to cure it except liver transplantation. DDC-fed (0.1%) mice are a xenobiotic- induced mouse model of cholangiopathy. It can induce reactive cholangiocytes, biliary inflammation, pericholangitis, periductal fibrosis, and ultimately portal-portal bridging affecting large bile ducts, which more closely resembles human sclerosing cholangitis and biliary fibrosis[34,35]. In the present study, using this experimental model of mice, we found that we demonstrate that a single dose of rAAV8-mediated inhibition of let-7a can protect mice from hepatic (biliary) injuries, biliary inflammation, proliferation, and fibrosis in experimental sclerosing cholangitis for 2 wk and 6 wk, suggesting that inhibition let-7a delivered by rAAV8 provides a potential therapeutic strategy for sclerosing cholangitis (Figure 8).
rAAV-based therapeutic has proved as the most effective strategy for gene therapy because rAAV8 can provide an effective, safe, and long-lasting vehicle to deliver genes (including miRNAs) to the targeted organs[36,37]. Currently, rAAV has been implicated in clinical trials in some human diseases such as cystic fibrosis, and Parkinson’s disease, and Glybera-the commercial drug based on rAAV has been applied in the treatment of lipoprotein lipase deficiency[38-43]. rAAV8 with TBG promoters is a liver-tropism serotype that has been widely used to deliver genes targeting hepatocytes with high transduction efficiency, low immunogenicity, and toxicity to the cell. In our present study, we also found that systematic injection of rAAV8 has no side effects on other organs such as the kidney (Supplementary Figure 1), sug
Increasing evidence demonstrates that miRNAs play critical roles in cholangiopathies[6]. Let-7 family is the first identified miRNA and increasing data suggested that let-7a plays pro-inflammatory or anti-inflammatory roles by inhibition of several targets[46-50]. Of these signaling pathways that let-7 is involved in, the TLR/NF-κB signaling pathway that is critical to the induction of pro-inflammatory cytokines can be also well-regulated by let-7. For example, let-7a targeted the SOCS1-the feedback inhibitor of NF-κB to facilitate the transcriptional activity of NF-κB and subsequent production of pro-inflammatory cytokines[51]. Similarly, let-7a has been reported to target A20-another inhibitor of the NF-κB pathway, leading to the increased TNF, IL-1β, and nitrite during Mycobacterium tuberculosis infection[52]. In agreement with these observations, we found that the pro-inflammatory cytokines such as TNF, IL-6, and CCl2 were decreased after the treatment with anti-let-7a delivered by rAAV8, which may be associated with the increased SOCS1 expression and the increased activities of NF-κB due to the loss of inhibitor effects of let-7a.
Many miRNAs have been demonstrated to participate in cholestasis[53-55]. Of these miRNAs, let-7a-5p is a recently identified miRNA that is dysregulated in animal models of cholangiopathies (such as BDL and Mdr2 KO mice) and patients with BA or PSC. Intriguing, it seems that let-7a-5p showed multiple contradictory functions in the different contexts of cholangiopathies due to targeting different genes. For example, let-7a decreased cholangiocytes in BDL mice and inhibits the secretin produced by cholangiocytes and S cells by targeting NGF, leading to a repression of the proliferation of cholangiocytes[13]. Similarly, in another study, the expression of let-7a was decreased in the liver of Mdr2 KO mice or human PSC, which probably increase downstream targets of let-7a (such as the NF-κB, IL-13, and NR1H4) and accelerate the severity of disease[14]. However, in another study, an increased let-7a-5p inhibited BCC2/Abcc2 (also known as MRP2) expression in obstructive cholestasis; the latter is critical for biliary excretion of conjugated bilirubin and the deficiency of BCC2 lead to obstructive cholestasis[12]. In our present study, we found that the inhibition of let-7a delivered by rAAV8 showed therapeutic effects on sclerosing cholangitis induced by DDC feeding at early (2 wk) or chronic stage (6 wk). The expression pattern of let-7a is discrepant in different cell types and the role of let-7a seems cell type-dependent in the different contexts of cholangiopathies. Glaser et al[13] found that the more than 15-fold increased expression of let-7a in hepatocytes, but deceased in cholangiocytes in the context of BDL mice. This may partly account for the diverse roles of let-7a in different cholangiopathies.
In summary, rAAV8 mediated let-7a-5p inhibitor provides powerful therapeutical effects on the DDC-induced sclerosing cholangitis, which can prevent hepato-biliary injuries, ductal reaction, and fibrosis by interfering with pro-inflammatory cytokines production. Our present study provides a possible clinical human clinical translation of PSC using rAAV8 systems to manipulate the expression of let-7a-5p. Other mechanisms by which therapeutic implication of inhibition of let-7a in DDC-induced sclerosing cholangitis are not excluded and further investigations are warranted.
Primary sclerosing cholangitis (PSC) which may progress to cholangiocarcinoma is an idiopathic cholestatic disease and there is a very limited medical option to interfere with the course of PSC. Recombinant adeno-associated virus (rAAV) provides a prospective platform for gene therapy on such kinds of diseases. A microRNA (miRNA) let-7a has been reported to be associated with the progress of PSC but the potential therapeutic implication of inhibition of let-7a on PSC has not been evaluated.
To investigate the potential function and mechanisms of miRNA let-7a-5p transferred by rAAV8 in animal model of PSC.
To study the therapeutic effects of inhibition of let-7a-5p transferred by rAAV8 on a 3,5-Diethoxycarbonyl-1,4-Dihydrocollidine (DDC)-induced mouse model of sclerosing cholangitis.
A mouse model of sclerosing cholangitis was induced by 0.1% DDC feeding for 2 wk or 6 wk, and rAAV8-mediated anti-let-7a-5p sponges or scramble control was injected in vivo onset of DDC feeding. After sacrifice of the mice, the liver and the serum were collected from each mouse. The hepatobiliary injuries, hepatic inflammation was evaluated. The targets of let-7a-5p and downstream molecule NF-κB were detected using Western blot.
The reduced expression of let-7a-5p can alleviate hepato-biliary injuries indicated by serum markers, and prevent the proliferation of cholangiocytes and biliary fibrosis. Furthermore, inhibition of let-7a mediated by rAAV8 can increase the expression of potential target molecules such as SOCS1 and Dectin1, which consequently inhibition of NF-κB-mediated hepatic inflammation.
Our findings suggested that a rAAV8 vector designed for liver-specific inhibition of let-7a-5p can potently ameliorate symptoms in a xenobiotic-induced mouse model of sclerosing cholangitis, which provides a possible therapeutic strategy for PSC.
The present study demonstrates that the rAAV-mediated miRNAs strategy may provide a promising therapeutic opportunity for this debilitating and life-threatening disease.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kotlyarov S, Russia S-Editor: Chen YL L-Editor: A P-Editor: Yu HG
| 1. | Dyson JK, Beuers U, Jones DEJ, Lohse AW, Hudson M. Primary sclerosing cholangitis. Lancet. 2018;391:2547-2559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 285] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 2. | Li ZJ, Gou HZ, Zhang YL, Song XJ, Zhang L. Role of intestinal flora in primary sclerosing cholangitis and its potential therapeutic value. World J Gastroenterol. 2022;28:6213-6229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 3. | Villard C, Friis-Liby I, Rorsman F, Said K, Warnqvist A, Cornillet M, Kechagias S, Nyhlin N, Werner M, Janczewska I, Hagström T, Nilsson E, Bergquist A. Prospective surveillance for cholangiocarcinoma in unselected individuals with primary sclerosing cholangitis. J Hepatol. 2023;78:604-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 4. | Hu C, Iyer RK, Juran BD, McCauley BM, Atkinson EJ, Eaton JE, Ali AH, Lazaridis KN. Predicting cholangiocarcinoma in primary sclerosing cholangitis: using artificial intelligence, clinical and laboratory data. BMC Gastroenterol. 2023;23:129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Tan N, Lubel J, Kemp W, Roberts S, Majeed A. Current Therapeutics in Primary Sclerosing Cholangitis. J Clin Transl Hepatol. 2023;11:1267-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Tanaka A. IgG4-Related Sclerosing Cholangitis and Primary Sclerosing Cholangitis. Gut Liver. 2019;13:300-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | Olaizola P, Lee-Law PY, Arbelaiz A, Lapitz A, Perugorria MJ, Bujanda L, Banales JM. MicroRNAs and extracellular vesicles in cholangiopathies. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1293-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Povero D, Tameda M, Eguchi A, Ren W, Kim J, Myers R, Goodman ZD, Harrison SA, Sanyal AJ, Bosch J, Ohno-Machado L, Feldstein AE. Protein and miRNA profile of circulating extracellular vesicles in patients with primary sclerosing cholangitis. Sci Rep. 2022;12:3027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Huang C, Xing X, Xiang X, Fan X, Men R, Ye T, Yang L. MicroRNAs in autoimmune liver diseases: from diagnosis to potential therapeutic targets. Biomed Pharmacother. 2020;130:110558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Kennedy LL, Meng F, Venter JK, Zhou T, Karstens WA, Hargrove LA, Wu N, Kyritsi K, Greene J, Invernizzi P, Bernuzzi F, Glaser SS, Francis HL, Alpini G. Knockout of microRNA-21 reduces biliary hyperplasia and liver fibrosis in cholestatic bile duct ligated mice. Lab Invest. 2016;96:1256-1267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Song CW, Qiu W, Zhou XQ, Feng XC, Chen WS. Elevated hepatic MDR3/ABCB4 is directly mediated by MiR-378a-5p in human obstructive cholestasis. Eur Rev Med Pharmacol Sci. 2019;23:2539-2547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Balasubramaniyan N, Devereaux MW, Orlicky DJ, Sokol RJ, Suchy FJ. Up-regulation of miR-let7a-5p Leads to Decreased Expression of ABCC2 in Obstructive Cholestasis. Hepatol Commun. 2019;3:1674-1686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Glaser S, Meng F, Han Y, Onori P, Chow BK, Francis H, Venter J, McDaniel K, Marzioni M, Invernizzi P, Ueno Y, Lai JM, Huang L, Standeford H, Alvaro D, Gaudio E, Franchitto A, Alpini G. Secretin stimulates biliary cell proliferation by regulating expression of microRNA 125b and microRNA let7a in mice. Gastroenterology 2014; 146: 1795-808. e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 14. | McDaniel K, Wu N, Zhou T, Huang L, Sato K, Venter J, Ceci L, Chen D, Ramos-Lorenzo S, Invernizzi P, Bernuzzi F, Wu C, Francis H, Glaser S, Alpini G, Meng F. Amelioration of Ductular Reaction by Stem Cell Derived Extracellular Vesicles in MDR2 Knockout Mice via Lethal-7 microRNA. Hepatology. 2019;69:2562-2578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Xiao Y, Liu R, Li X, Gurley EC, Hylemon PB, Lu Y, Zhou H, Cai W. Long Noncoding RNA H19 Contributes to Cholangiocyte Proliferation and Cholestatic Liver Fibrosis in Biliary Atresia. Hepatology. 2019;70:1658-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 16. | Zhang L, Yang Z, Huang W, Wu J. H19 potentiates let-7 family expression through reducing PTBP1 binding to their precursors in cholestasis. Cell Death Dis. 2019;10:168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Ronzitti G, Gross DA, Mingozzi F. Human Immune Responses to Adeno-Associated Virus (AAV) Vectors. Front Immunol. 2020;11:670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 220] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 18. | Arjomandnejad M, Dasgupta I, Flotte TR, Keeler AM. Immunogenicity of Recombinant Adeno-Associated Virus (AAV) Vectors for Gene Transfer. BioDrugs. 2023;37:311-329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 62] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 19. | Pupo A, Fernández A, Low SH, François A, Suárez-Amarán L, Samulski RJ. AAV vectors: The Rubik's cube of human gene therapy. Mol Ther. 2022;30:3515-3541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 178] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 20. | Pan XY, You HM, Wang L, Bi YH, Yang Y, Meng HW, Meng XM, Ma TT, Huang C, Li J. Methylation of RCAN1.4 mediated by DNMT1 and DNMT3b enhances hepatic stellate cell activation and liver fibrogenesis through Calcineurin/NFAT3 signaling. Theranostics. 2019;9:4308-4323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Chai S, Wakefield L, Norgard M, Li B, Enicks D, Marks DL, Grompe M. Strong ubiquitous micro-promoters for recombinant adeno-associated viral vectors. Mol Ther Methods Clin Dev. 2023;29:504-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Mücke MM, Fong S, Foster GR, Lillicrap D, Miesbach W, Zeuzem S. Adeno-associated viruses for gene therapy - clinical implications and liver-related complications, a guide for hepatologists. J Hepatol. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 23. | Meumann N, Cabanes-Creus M, Ertelt M, Navarro RG, Lucifora J, Yuan Q, Nien-Huber K, Abdelrahman A, Vu XK, Zhang L, Franke AC, Schmithals C, Piiper A, Vogt A, Gonzalez-Carmona M, Frueh JT, Ullrich E, Meuleman P, Talbot SR, Odenthal M, Ott M, Seifried E, Schoeder CT, Schwäble J, Lisowski L, Büning H. Adeno-associated virus serotype 2 capsid variants for improved liver-directed gene therapy. Hepatology. 2023;77:802-815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 24. | Lee S, Zhou P, Whyte S, Shin S. Adeno-Associated Virus Serotype 8-Mediated Genetic Labeling of Cholangiocytes in the Neonatal Murine Liver. Pharmaceutics. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Siew SM, Cunningham SC, Zhu E, Tay SS, Venuti E, Bolitho C, Alexander IE. Prevention of Cholestatic Liver Disease and Reduced Tumorigenicity in a Murine Model of PFIC Type 3 Using Hybrid AAV-piggyBac Gene Therapy. Hepatology. 2019;70:2047-2061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Weber ND, Odriozola L, Martínez-García J, Ferrer V, Douar A, Bénichou B, González-Aseguinolaza G, Smerdou C. Gene therapy for progressive familial intrahepatic cholestasis type 3 in a clinically relevant mouse model. Nat Commun. 2019;10:5694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Zhao L, Yang Z, Zheng M, Shi L, Gu M, Liu G, Miao F, Chang Y, Huang F, Tang N. Recombinant adeno-associated virus 8 vector in gene therapy: Opportunities and challenges. Genes Dis. 2024;11:283-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 28. | Yu H, Zhou L, Loong JHC, Lam KH, Wong TL, Ng KY, Tong M, Ma VWS, Wang Y, Zhang X, Lee TK, Yun JP, Yu J, Ma S. SERPINA12 promotes the tumorigenic capacity of HCC stem cells through hyperactivation of AKT/β-catenin signaling. Hepatology. 2023;78:1711-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 29. | Kang J, Huang L, Zheng W, Luo J, Zhang X, Song Y, Liu A. Promoter CAG is more efficient than hepatocytetargeting TBG for transgene expression via rAAV8 in liver tissues. Mol Med Rep. 2022;25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 30. | He X, Wang Y, Fan X, Lei N, Tian Y, Zhang D, Pan W. A schistosome miRNA promotes host hepatic fibrosis by targeting transforming growth factor beta receptor III. J Hepatol. 2020;72:519-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 31. | Bala S, Zhuang Y, Nagesh PT, Catalano D, Zivny A, Wang Y, Xie J, Gao G, Szabo G. Therapeutic inhibition of miR-155 attenuates liver fibrosis via STAT3 signaling. Mol Ther Nucleic Acids. 2023;33:413-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 32. | Jiang LF, Yang M, Meng HW, Jia PC, Du CL, Liu JY, Lv XW, Cheng-Huang, Li J. The effect of hepatic stellate cell derived-IL-11 on hepatocyte injury in hepatic fibrosis. Life Sci. 2023;330:121974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 33. | Yan C, Zhou QY, Wu J, Xu N, Du Y, Li J, Liu JX, Koda S, Zhang BB, Yu Q, Yang HM, Li XY, Zhang B, Xu YH, Chen JX, Wu Z, Zhu XQ, Tang RX, Zheng KY. Csi-let-7a-5p delivered by extracellular vesicles from a liver fluke activates M1-like macrophages and exacerbates biliary injuries. Proc Natl Acad Sci U S A. 2021;118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Zhang J, Lyu Z, Li B, You Z, Cui N, Li Y, Huang B, Chen R, Chen Y, Peng Y, Fang J, Wang Q, Miao Q, Tang R, Gershwin ME, Lian M, Xiao X, Ma X. P4HA2 induces hepatic ductular reaction and biliary fibrosis in chronic cholestatic liver diseases. Hepatology. 2023;78:10-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 35. | Shen Y, Jiang B, Zhang C, Wu Q, Li L, Jiang P. Combined Inhibition of the TGF-β1/Smad Pathway by Prevotella copri and Lactobacillus murinus to Reduce Inflammation and Fibrosis in Primary Sclerosing Cholangitis. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 36. | Kleven MD, Gomes MM, Wortham AM, Enns CA, Kahl CA. Ultrafiltered recombinant AAV8 vector can be safely administered in vivo and efficiently transduces liver. PLoS One. 2018;13:e0194728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Sen D, Gadkari RA, Sudha G, Gabriel N, Kumar YS, Selot R, Samuel R, Rajalingam S, Ramya V, Nair SC, Srinivasan N, Srivastava A, Jayandharan GR. Targeted modifications in adeno-associated virus serotype 8 capsid improves its hepatic gene transfer efficiency in vivo. Hum Gene Ther Methods. 2013;24:104-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Lopes-Pacheco M, Kitoko JZ, Morales MM, Petrs-Silva H, Rocco PRM. Self-complementary and tyrosine-mutant rAAV vectors enhance transduction in cystic fibrosis bronchial epithelial cells. Exp Cell Res. 2018;372:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Yan Z, Vorhies K, Feng Z, Park SY, Choi SH, Zhang Y, Winter M, Sun X, Engelhardt JF. Recombinant Adeno-Associated Virus-Mediated Editing of the G551D Cystic Fibrosis Transmembrane Conductance Regulator Mutation in Ferret Airway Basal Cells. Hum Gene Ther. 2022;33:1023-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Tang Y, Yan Z, Engelhardt JF. Viral Vectors, Animal Models, and Cellular Targets for Gene Therapy of Cystic Fibrosis Lung Disease. Hum Gene Ther. 2020;31:524-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 41. | Nakamura Y, Arawaka S, Sato H, Sasaki A, Shigekiyo T, Takahata K, Tsunekawa H, Kato T. Monoamine Oxidase-B Inhibition Facilitates α-Synuclein Secretion In Vitro and Delays Its Aggregation in rAAV-Based Rat Models of Parkinson's Disease. J Neurosci. 2021;41:7479-7491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Croft CL, Cruz PE, Ryu DH, Ceballos-Diaz C, Strang KH, Woody BM, Lin WL, Deture M, Rodríguez-Lebrón E, Dickson DW, Chakrabarty P, Levites Y, Giasson BI, Golde TE. rAAV-based brain slice culture models of Alzheimer's and Parkinson's disease inclusion pathologies. J Exp Med. 2019;216:539-555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 43. | Kok CY, MacLean LM, Ho JC, Lisowski L, Kizana E. Potential Applications for Targeted Gene Therapy to Protect Against Anthracycline Cardiotoxicity: JACC: CardioOncology Primer. JACC CardioOncol. 2021;3:650-662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 44. | Greig JA, Limberis MP, Bell P, Chen SJ, Calcedo R, Rader DJ, Wilson JM. Non-Clinical Study Examining AAV8.TBG.hLDLR Vector-Associated Toxicity in Chow-Fed Wild-Type and LDLR(+/-) Rhesus Macaques. Hum Gene Ther Clin Dev. 2017;28:39-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 45. | Mak KY, Chin R, Cunningham SC, Habib MR, Torresi J, Sharland AF, Alexander IE, Angus PW, Herath CB. ACE2 Therapy Using Adeno-associated Viral Vector Inhibits Liver Fibrosis in Mice. Mol Ther. 2015;23:1434-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 46. | Jiang S. Recent findings regarding let-7 in immunity. Cancer Lett. 2018;434:130-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 47. | Gilles ME, Slack FJ. Let-7 microRNA as a potential therapeutic target with implications for immunotherapy. Expert Opin Ther Targets. 2018;22:929-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 48. | Jin S, Zeng X, Fang J, Lin J, Chan SY, Erzurum SC, Cheng F. A network-based approach to uncover microRNA-mediated disease comorbidities and potential pathobiological implications. NPJ Syst Biol Appl. 2019;5:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 49. | Li K, Yan G, Huang H, Zheng M, Ma K, Cui X, Lu D, Zheng L, Zhu B, Cheng J, Zhao J. Anti-inflammatory and immunomodulatory effects of the extracellular vesicles derived from human umbilical cord mesenchymal stem cells on osteoarthritis via M2 macrophages. J Nanobiotechnology. 2022;20:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 119] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 50. | Lin D, Chen H, Xiong J, Zhang J, Hu Z, Gao J, Gao B, Zhang S, Chen J, Cao H, Li Z, Lin B, Gao Z. Mesenchymal stem cells exosomal let-7a-5p improve autophagic flux and alleviate liver injury in acute-on-chronic liver failure by promoting nuclear expression of TFEB. Cell Death Dis. 2022;13:865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 51. | Meng F, Glaser SS, Francis H, DeMorrow S, Han Y, Passarini JD, Stokes A, Cleary JP, Liu X, Venter J, Kumar P, Priester S, Hubble L, Staloch D, Sharma J, Liu CG, Alpini G. Functional analysis of microRNAs in human hepatocellular cancer stem cells. J Cell Mol Med. 2012;16:160-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 52. | Kumar M, Sahu SK, Kumar R, Subuddhi A, Maji RK, Jana K, Gupta P, Raffetseder J, Lerm M, Ghosh Z, van Loo G, Beyaert R, Gupta UD, Kundu M, Basu J. MicroRNA let-7 modulates the immune response to Mycobacterium tuberculosis infection via control of A20, an inhibitor of the NF-κB pathway. Cell Host Microbe. 2015;17:345-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 206] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 53. | Kumstel S, Janssen-Peters H, Abdelrahman A, Tang G, Xiao K, Ernst N, Wendt EHU, Palme R, Seume N, Vollmar B, Thum T, Zechner D. MicroRNAs as systemic biomarkers to assess distress in animal models for gastrointestinal diseases. Sci Rep. 2020;10:16931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Rodrigues PM, Perugorria MJ, Santos-Laso A, Bujanda L, Beuers U, Banales JM. Primary biliary cholangitis: A tale of epigenetically-induced secretory failure? J Hepatol. 2018;69:1371-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 55. | Tadokoro T, Morishita A, Masaki T. Diagnosis and Therapeutic Management of Liver Fibrosis by MicroRNA. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |