Published online Feb 7, 2024. doi: 10.3748/wjg.v30.i5.429
Peer-review started: September 26, 2023
First decision: December 7, 2023
Revised: December 17, 2023
Accepted: January 12, 2024
Article in press: January 12, 2024
Published online: February 7, 2024
Processing time: 126 Days and 16.3 Hours
Pancreatitis and pancreatic cancer (PC) stand as the most worrisome ailments affecting the pancreas. Researchers have dedicated efforts to unraveling the mechanisms underlying these diseases, yet their true nature continues to elude their grasp. Within this realm, oxidative stress is often believed to play a causal and contributory role in the development of pancreatitis and PC. Excessive accumulation of reactive oxygen species (ROS) can cause oxidative stress, and the key enzyme responsible for inducing ROS production in cells is nicotinamide adenine dinucleotide phosphate hydrogen oxides (NOX). NOX contribute to pancreatic fibrosis and inflammation by generating ROS that injure acinar cells, activate pancreatic stellate cells, and mediate macrophage polarization. Excessive ROS production occurs during malignant transformation and pancreatic carcinogenesis, creating an oxidative microenvironment that can cause abnormal apoptosis, epithelial to mesenchymal transition and genomic instability. There
Core Tip: Nicotinamide adenine dinucleotide phosphate hydrogen oxides (NOX) plays a significant role in the development of pancreatitis and pancreatic cancer (PC) by contributing to pancreatic fibrosis and inflammation. It achieves this by generating reactive oxygen species, which damage acinar cells, activate pancreatic stellate cells, and induce macrophage polarization. Moreover, NOX promotes PC progression by interfering with abnormal cell apoptosis, initiating the epithelial to mesenchymal transition processes, and leading to cell genomic instability. A thorough understanding of NOX’s involvement in pancreatic diseases is crucial for comprehending the underlying mechanisms of pancreatitis and PC. This review provides a summary of NOX’s potential roles and mechanisms in pancreatic disorders, emphasizing areas that require further investigation.
- Citation: Bi YW, Li LS, Ru N, Zhang B, Lei X. Nicotinamide adenine dinucleotide phosphate oxidase in pancreatic diseases: Mechanisms and future perspectives. World J Gastroenterol 2024; 30(5): 429-439
- URL: https://www.wjgnet.com/1007-9327/full/v30/i5/429.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i5.429
Incidence of diseases of the pancreas, including acute and chronic pancreatitis (CP) and pancreatic cancer (PC) are rising globally[1-3]. Acute pancreatitis (AP) is the leading cause for gastrointestinal-disease related hospital admissions and is associated with significant morbidity, mortality and socioeconomic burden[4]. CP causes persistent pain, as well as exocrine and endocrine pancreatic insufficiency. It also poses a risk factor for the development of PC[5]. PC is the malignancies with an incidence/mortality ratio of as high as 94% and a 5-year survival rate of about 9%[6]. Although researchers have been dedicated to exploring these diseases, the precise pathogenesis remains unclear. Research indicates that aberrant redox homeostasis occurs in both pancreatitis and PC. Reactive oxygen species (ROS) exert oxidative stress on the pancreatic cells, deregulating the redox homeostasis and promoting inflammation and tumorigenesis by initiating an aberrant induction of signaling networks[7,8].
Nicotinamide adenine dinucleotide phosphate hydrogen oxidases (NOX) is indeed a primary source of cellular ROS. During the development of PC and pancreatitis, the levels of ROS in pancreatic tissue are significantly increased, the source of these ROS is related to dysregulation of NOX in pancreatic cells[9,10]. The dysregulation of NOX plays an important role in pancreatitis and PC. Therefore, to clarify the regulatory mechanism of NOX in pancreatic cells will be more conducive to understanding the pathological process of pancreatitis and PC. In a word, we will present the existing evidence regarding the role and the mechanism of NOX in both pancreatitis and PC.
AP occurs as a result of the abnormal activation of pancreatic enzymes, which leads to the digestion of the pancreas itself and surrounding organs[11]. It is primarily characterized by localized inflammation of the pancreas and can even cause systemic organ dysfunction. Acinar cell injury leading to premature activation of pancreatic enzymes is considered the primary factor in the initiation of AP[12]. The subsequent inflammation triggered by the necrosis of acinar cells plays a crucial role in the progression of the disease[13]. Among the immune cells responding to the released chemotactic factors from injured acinar cells during pancreatitis, macrophages are among the earliest[14]. Therefore, both acinar cells and macrophages play significant roles in the development of AP. As the disease worsens, AP can even cause multiple organ dysfunction, known as severe AP, which has a high mortality rate and attracts significant clinical attention[15]. Therefore, this section focuses on exploring the regulatory role of NOX in acinar cells, macrophages, and other organ failures associated with AP.
Pancreatic acinar cells are secretory cells that primarily synthesize, store and ultimately release digestive enzymes into the duodenum[16]. However, when exposed to harmful stimuli, acinar cells exhibit inflammatory characteristics by activating signaling transduction pathways associated with the expression of inflammatory mediators[17]. The injury or death of acinar cells can initiate inflammatory cascades, which is the main pathogenesis of AP.
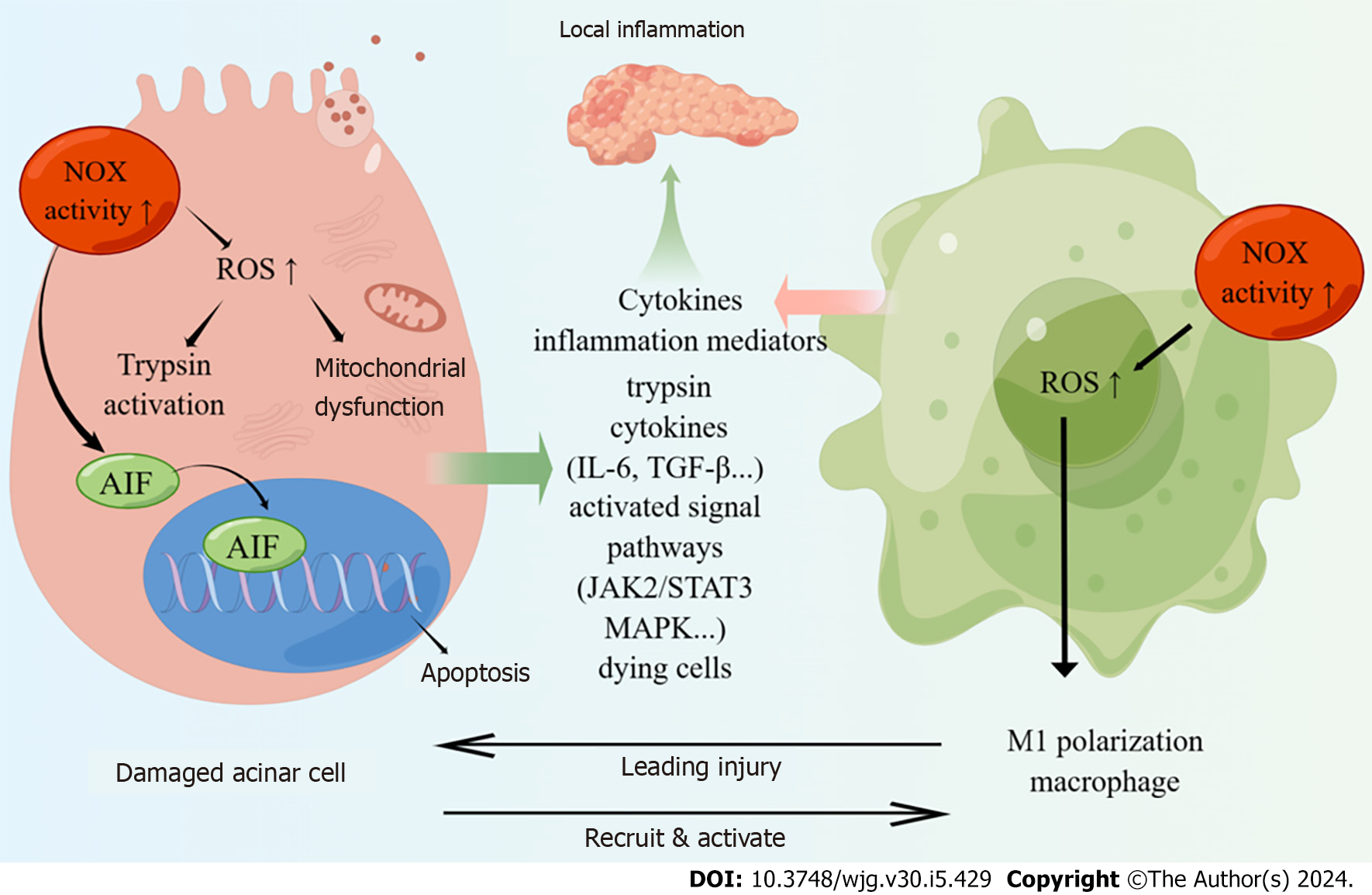
Pancreatic acinar cells constitutively express NOX subunits p67phox and p47phox in the cytosol, as well as NOX1 and p22phox in the membrane, which could be activated by cerulein[18]. Upon activation, a complex of the cytosolic subunits translocates to the membrane and facilitates NOX-dependent formation of superoxide and other secondary ROS. In the early stage of AP, the NOX activity of acinar cells is significantly upregulated, leading to the activation of downstream nuclear factor kappa-B (NF-κB) pathway and stimulation of interleukin (IL)-6 expression[18]. In addition to inducing AP, NOX can also participate in a series of inflammatory cascade reactions to promote the progression of AP.
NOX hyperactivity disrupts mitochondrial membrane potential, leading to ATP depletion and subsequent injury in pancreatic acinar cells[19]. The excessive production of ROS by NOX induced zymogen activation, mitochondrial dysfunction and cytokine expression, which further injury to pancreatic acinar cells[20,21]. And the use of the NOX1 inhibitor could suppress these responses and alleviate inflammation in alcoholic AP model. To further investigate the mechanism of NOX action on acinar cells in AP, Ju et al[22] discovered that NOX mediated the activation of Janus kinase (JAK)2/signal transducer and activator of transcription and mitogen-activated protein kinases (MAPKs) (ERK, JNK, p38) to induce the expression of transforming growth factor (TGF)-β1 in cerulein-stimulated pancreatic acinar cells, thereby facilitating the progress of AP. Furthermore, NOX is believed to be involved in acinar cell death. NOX upregulates IL-6 and mediates ROS-induced apoptosis in pancreatic acinar cells stimulated with the cholecystokinin analogue cerulein[23]. It is known that cerulein induced the expression of apoptosis-inducing factor (AIF) in pancreatic acinar cell. During the process of cell apoptosis, AIF relocates from the mitochondria to the cytoplasm, and subsequently enters the cell nucleus, resulting in the aggregation and fragmentation of nuclear DNA, ultimately inducing apoptosis in pancreatic acinar cells[24,25]. Previous studies have indicated that NOX activation might be the upstream events of AIF expression, leading to cerulein-induced apoptosis in pancreatic acinar cells[26].
Accumulating evidences shows that both the number and activation of macrophages play a crucial role in determining the severity of AP[27-29]. Damaged pancreatic acinar cells release cell contents including trypsin, zymogen granules, cytokines, cell-free DNA and other damage-related molecular patterns, which recruit and activate inflammatory macrophages[30]. Macrophages can be categorized into two main subtypes, M1 and M2, based on their stimuli and function in vitro[31]. M1 macrophages are responsible for producing cytokines and inflammatory mediators, which contribute to the amplification of local and systemic inflammation. As a result, they dominate the pro-inflammatory phase of AP. On the other hand, M2-like macrophages are prevalent during the process of pancreas repair/regeneration[32]. Therefore, M1 macrophages are dominated during the development of AP.
NOX-induced ROS production has a role in maintaining the polarization of M1 macrophage[33,34]. The involvement of NOX in mediating macrophage M1 polarization has been studied in various organs. For instance, NOX4 has been shown to induce macrophage M1 polarization following spinal cord injury[35]. In breast cancer, M1 macrophages exhibited significantly increased levels of ROS and mRNA expression of NOX2, NOX5, and CYBA (p22phox) compared to M2 macrophages[36]. Moreover, it is reported that NOX2 could mediate macrophage M1 polarization in traumatic brain injury through NF-κB pathway[37]. Regarding the pancreas, Han et al[38] discovered that NOX-mediated oxidative stress the polarization of M1 macrophages in the pancreas, thereby promoting the progression of AP via the activation of NF-κB and inflammasome pathways. Accordingly, NOX is capable of mediating the polarization of M1 polarization and contributing to the progression of AP. Further research is warranted to elucidate the underlying mechanisms by which NOX maintains M1 macrophages in the context of AP.
Despite the mild nature of AP in most patients, about 20%-30% experience a severe form that frequently results in dysfunction of one or multiple organs, requiring intensive care[39]. Moreover, recent studies have uncovered a link between NOX and organ dysfunction in AP, in addition to its role in inducing local inflammation in the pancreas.
Carrascal et al[40] showed that circulating exosomes involved in the progression of inflammation from the pancreas to distant organs leading to organ dysfunction in AP. Interestingly, these exosomes’ impact is dependent on NOX. Specifically, NOX is activated by proteins carried by exosomes, resulting in the production of free radicals and the promotion of an inflammatory response. Furthermore, NOX inhibitor pretreatment blocked the expression of IL-1β and tumour necrosis factor alpha mRNAs induced by exosomes obtained from patients with severe AP.
NOX is widely distributed and is participated in various pathological processes of different organs. Yang et al[41] showed that NOX regulate the activity of downstream p-AKT and glycogen synthase kinase (GSK)-3β by regulating ROS levels, thereby affecting the release of inflammatory mediators and regulating AP-related kidney injury. Jin et al[42] found NOX2 and NOX4 were upregulated in lung tissue of severe AP and NOX-mediated ROS could activate NACHT, LRR, and PYD domains-containing protein 3 inflammasome and NF-κB signaling and facilitate AP-associated lung injury. Wen et al[43] showed that hyperactivity of NOX underlies myocardial injury in severe AP by promoting ROS generation with increased oxidative stress and cardiomyocyte apoptosis via activating the MAPK pathway. Moreover, NOX is involved in the process of intestinal barrier damage in sever AP, which was associated with an increase in the systemic concentration of cytokines, oxidative stress and activated NF-κB and p38 MAPK expression[44].
In summary, NOX promotes the development of AP by causing acinar cell damage and inducing macrophage polarization into M1 type (Figure 1). Moreover, NOX also be involved in distant organ dysfunction in AP. While the specific mechanism of NOX act on acinar cell and macrophages needs further study, which help us to further elucidate the pathogenesis of AP.
CP manifests from a long-term inflammation, which results in a significant replacement of the parenchyma by extracellular matrix (ECM)-rich connective tissue (i.e., fibrosis) and permanent organ damage[45]. Notably, fibrosis is the hallmark histological feature of CP[46]. Fibrosis is a post-injury repair response in which tissue homeostasis is disrupted and fibrotic changes occur under the action of specific cytokines and a pro-oxidative environment, eventually leading to organ dysfunction[47]. The current clinical treatment of CP is limited to symptomatic treatment and management of complications. Thus, a better understanding of the mechanism underlying the pathogenesis of CP is necessary in order to develop more effective therapeutic options to attenuate the progression of the disease. Clarifying the mechanism of pancreatic fibrosis in CP and exploring therapeutic methods to delay or reverse pancreatic fibrosis are the basis to finding effective treatment for CP.
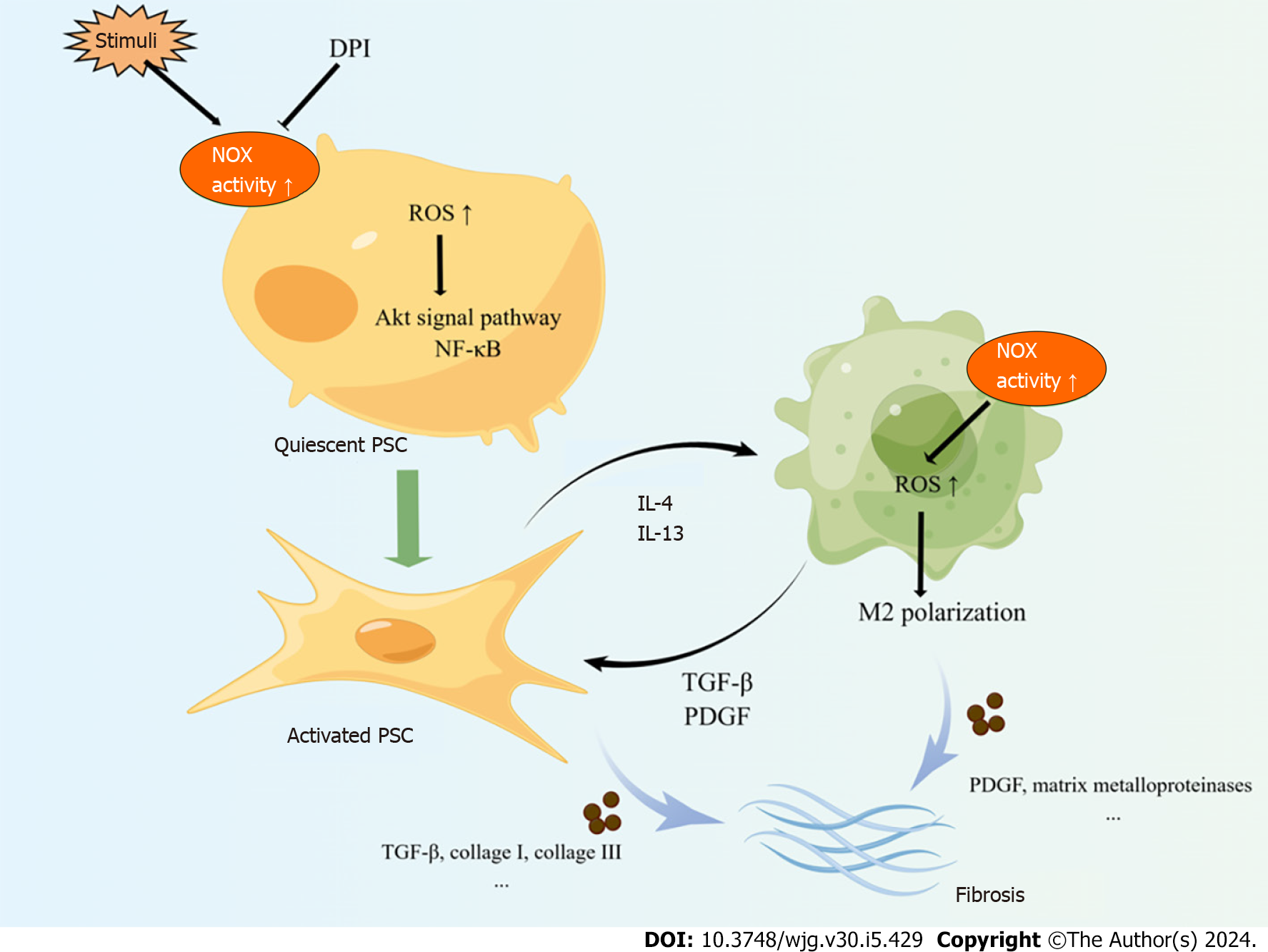
The activation of pancreatic stellate cells (PSCs) is the core to CP pathological processes. PSCs exist in two forms, the quiescent state and the activated state. Under physiological conditions, PSCs are in a quiescent state and secrete some growth-promoting cytokines to maintain the basic structure and function of the pancreas. When pancreas tissue damaged or in response to stimulation, PSCs are activated and transformed from their quiescent into myofibroblast-like phenotype, characterized by the disappearance of intracellular lipid droplets and the expression of α-smooth muscle actin (α-SMA) and ECM components such as type I collagen, type III collagen, and fibronectin[48]. PSCs express key components of NOX, p22phox, p47phox, NOX1, gp91phox/NOX2 and NOX4[49]; and NOX is recognized to be involved in PSCs activation.
Masamune et al[49] found that upregulating NOX activity in PSCs could induce PSCs activation and proliferation. Furthermore, diphenylene iodium (DPI) abolished ROS production in isolated PSCs and inhibited transformation of freshly isolated PSCs to a myofibroblast-like phenotype. NOX-mediated ROS in PSCs could accelerate fibrosis progression in CP. Xia et al[50] found Nox1-derived ROS in PSCs mediate the fibrotic process of CP by activating the downstream redox-sensitive signaling pathways AKT and NF-ĸB, up-regulating metalloproteases (MMP)-9 and Twist, and producing α-SMA and collagen I and III. However, limited research has been focused on exploring the mechanism of NOX promoting PSCs activation. More studies are needed in this topic.
In the pancreatic tissue of CP, M2 macrophages are the dominant type of macrophages[51]. These M2 macrophages secret cytokines including TGF-β, platelet-derived growth factor, IL-10 and various matrix metalloproteinases, which play a role in the progression of fibrosis and chronic inflammation in CP[52]. Furthermore, M2 macrophages can activate PSCs, and the cross-talk between activated PSCs and M2 macrophages initiates and sustains the fibrotic process in CP[53]. NOX-mediated ROS can act as second messengers playing an extremely important role in the regulation of macrophage polarization[54-56]. Previous studies showed NOX was involved in M2 polarization of macrophages. Reduced NOX2 expression improves the wound healing functions of M2 macrophages in degrading disulphide protein[57]. Furthermore, the interaction between M2 macrophages with apoptotic bodies triggers instability of NOX2 mRNAs through binding blockade of RNA-binding protein SYNCRIP to NOX2 3’ untranslated region. And this further defect the ROS production and leads to M2 macrophage polarization[58]. Mongue et al[59] found cardiomyocyte NOX4 modulated macrophage polarization toward M2 phenotype in myocardial injury mice model. Intervention of antioxidant butylated hydroxy anisole by inhibiting NOX-mediated O2− production blocked monocyte differentiation to M2 type[60]. These results suggest that NOX may play a role in regulating M2 polarization of macrophages in the pancreas of CP. Further studies are needed to investigate this relationship.
In summary, NOX is involved in the progression of CP (Figure 2). NOX promotes the activation of PSC in the fibrotic process of CP. Moreover, the application of NOX inhibitors in vitro effectively inhibits the activation of PSC. Additionally, several studies have shown that NOX induces M2 polarization of macrophages in other organs. It has been established that M2 macrophages promote the occurrence and development of CP. Therefore, further research is needed to investigate whether NOX also plays a regulatory role in the M2 polarization of macrophages in CP.
The global burden of PC has increased dramatically over the past few decades and is expected to continue to represent a leading cause of cancer-related mortality[61]. Although efforts are being made to explore the pathological process of PC, its specific etiology remains unclear. Furthermore, PC shows resistance to chemotherapy, and there is currently no effective clinical treatment available[62]. Therefore, elucidating the underlying mechanisms of PC and identifying potential therapeutic targets have been topics of great interest.
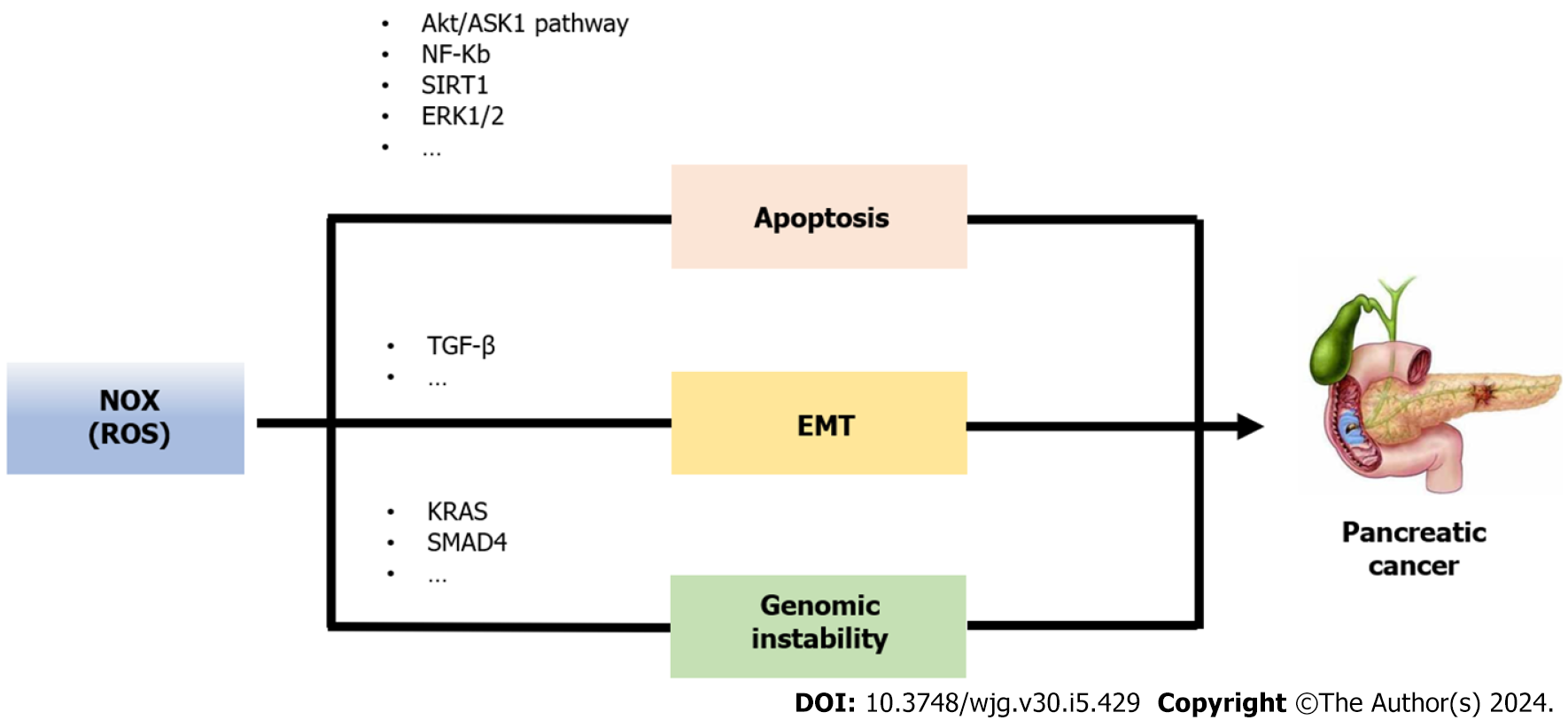
The oncogenic KRAS mutation is the major event in PC; it confers permanent activation of the KRAS protein, which acts as a molecular drive common phenotypes that expose specific vulnerabilities[63]. KRAS transformed PC cells have increased NOX activity and superoxide levels, as compared to parental cells[64,65]. Moreover, several reports have indicated that in human PC, expression of NOX family members is increased when compared to non-transformed pancreatic tissue[66-68]. KRAS gene mutations can lead cells to depart from common phenotypes and expose specific vulnerabilities. One example of such a phenotype is abnormal redox homeostasis, with excessive accumulation of ROS playing a crucial role in causing this aberrant redox homeostasis[69]. The ROS generated by KRAS, primarily relies on NOX production. ROS exerts oxidative stress on cells, which disrupts redox homeostasis and promotes tumor formation. This occurs due to an abnormal activation of signaling networks that initiate tumorigenesis[70]. NOX is a multi-subunit enzyme which is activated through the small GTPase Rac1[71,72]. Consequently, in PC cell lines, presence of oncogenic KRAS links to increased Rac1 activity and superoxide production; and KRAS-induced ROS production can be inhibited by downregulation of p47phox, the cytosolic regulatory subunit of NOX[73,74]. Therefore, there is a close correlation between NOX and the development of PC caused by the oncogenic KRAS gene mutation.
One reason why PC is highly aggressive and unresponsive to treatments is its resistance to apoptosis. ROS induce apoptosis indirectly through damage to DNA, proteins and lipids, or more directly through the activation of pro-apoptotic signaling cascades such as SAPK/JNK, ERK1/2, and p38 upon the induction of the MAPK pathways[75]. However, at high concentrations, ROS, especially as H2O2, can inhibit caspases, resulting in irreversible damage to cell components and leading to necrosis[76]. Conversely, in certain cases, NOX-produced ROS can trigger an anti-apoptotic effect by activating NF-κB or Akt/ASK1 transduction pathways[77].
Study have found that growth factors can induce the production of ROS by mediating NOX in PC cells, thus protecting the cells from apoptosis[72]. The oncosuppressor p53 gene plays a crucial role in the process of apoptosis in cancer cells. Research has found that NOX1 inhibits tumor cell apoptosis by regulating p53 deacetylation, suppressing its transcriptional activity, and activating the SIRT1 pathway[78]. Mochizuki et al[77] noted that ROS, generated by NOX4, transmits signals for cell survival through the AKT-ASK1 pathway. Furthermore, Lee et al[66] demonstrated that NOX4-generated ROS promote PC cell survival by inhibiting JAK2 dephosphorylation. Study has discovered that the application of a NOX inhibitor, Tyrosine, effectively inhibits cell proliferation of human and hamster PC cells by inhibiting the G1 phase of the cell cycle with cyclin D1 downregulation and inactivation of AKT-GSK3β and ERK1/2 signaling pathways[79]. Therefore, NOX could regulate PC cells from death.
The epithelial to mesenchymal transition (EMT) is a crucial mechanism by which tumor cells acquire motility and invasiveness[80]. More and more evidence indicates that EMT plays a vital role in the pathogenesis, invasion, metastasis, and drug resistance of PC[81,82]. It is worth noting that recently, it has been discovered that many important EMT regulators are sensitive to redox reactions, thereby being able to elucidate the molecular basis of EMT from a redox perspective[83].
NOX4, a subunit of NOX, has been implicated in the EMT process in PC[84]. NOX4 mRNA correlation with EMT gene expression such as collagen (COL1A2, COL3A1, COL5A2), MMP2, MMP9 and fibronectin (FN1)[85]. Additionally, studies have discovered that NOX4-derived ROS transmit TGF-β-triggered EMT signals through PTP1B in PC[86]. Furthermore, Witte et al[87] proposed that TGF-β1-induced EMT in PC cells is mediated through RAC1/NOX4/ROS/p38 MAPK cascade. More recent research has demonstrated that NOX4 caused inactivation of lysine demethylase 5A, leading to increased methylation modification of histone H3 and regulation of transcription of EMT-associated gene SNAIL1. Moreover, the deficiency of NOX4 has been shown to suppress hypoxia-induced EMT in PC cells[88].
Extensive reviews have investigated the impact of ROS on DNA damage. Cell exposure to chronic oxidative stress has been reported to elicit genomic instability. Moreover, there is evidence indicating elevated ROS levels in genomically unstable clones[89,90]. Although the precise function of NOX in cellular transformation remains unclear, several studies provide suggestive evidence for its role. NOX4 induces the production of ROS, which damages mitochondrial DNA and leads to mitochondrial dysfunction[91]. In addition to its known involvement in chromosomal instability, NOX1, NOX2, NOX4, and DUOX have been associated with the regulation of p53 transcription factor activity[92-94]. Moreover, p53 mutation can “transform” NOX4 from a protective and good prognostic indicator into a harmful one by promoting programs favorable to cancer progression, including EMT, cell migration, cell adhesion, and angiogenesis[85].
There are studies suggest a relationship between NOX and oncogene in PC. Ogrunc et al[95] demonstrated that NOX4 promotes the transformation of PC cells expressing oncogenes by generating mitogenic ROS. This transformation leads to a compromised DNA damage response and oncogene-induced cellular senescence bypass. Ju et al[96] identified that NOX4 as a critical factor that facilitates the interaction between KRAS activation and p16 inactivation, promoting the occurrence of PC.
In summary, NOX plays a crucial role in the progression of PC. NOX could regulate PC cells from death, promote the EMT process, and induce genomic instability (Figure 3). Furthermore, NOX is also involved in the key oncogenic process of abnormal redox homeostasis induced by the oncogene KRAS in PC. It is worth noting that that among the subunits of NOX, NOX4 has been extensively studied in relation to PC and has been found to promote PC through various mechanisms. Therefore, NOX4 may represent a potential therapeutic target for PC, but further research is needed to confirm this.
As we known, NOX is a membrane-bound multi-component enzyme complex. Different isoforms of NOX are distributed in different tissues, cells, and subcellular structures, exerting specific functions under physiological and pathological conditions. Although studies have demonstrated the significant role of NOX in pancreatitis and PC, the exact subunit of NOX responsible for these conditions remains unclear. NOX subunits express differently in acinar cells, PSCs and macrophages. Identifying the specific subunits participate in promoting pancreatic disorders progression help us better understand the pathogenesis of pancreatitis and PC. Further studies are needed to explore this topic.
Although numerous studies were conducted on the investigation of pancreatitis and PC, no effective methods of prevention and treatment have been developed. Since NOX play an important role in both pancreatitis and PC, it may be considered as a therapeutic target. Study showed inhibition of NOX by DPI suppresses apoptosis of pancreatic acinar cells by reducing the expression of apoptosis-associated genes and caspase-3 activity[97]. NOX2 inhibitor, GSK2795039, caused about 50% reduction in the level of serum amylase activity in AP mice[98]. Apocynin is a specific inhibitor of NOX. Recent studies proved that apocynin could prevent AP and AP-associated organs injury[41-44]. NOX1 knockout alleviate pancreatic fibrosis in CP mice[50]. In terms of PC, drug resistance is the main reason why chemotherapy drugs cannot achieve ideal treatment effects. It is worth noting that NOX is associated with chemotherapy resistance[99-101]. Recent breakthroughs in cancer treatment consisting of new combinations of existing medications. It reminds us that chemotherapy with NOX inhibitor may achieve better therapeutic effects in PC. More studies are needed to verify the therapeutic effect of NOX in pancreatic diseases.
Though they are distinct diseases of pancreas that pancreatitis is benign and PC is malignant, numerous studies indicate that pancreatitis is linked to PC[102,103]. The exact nature of this association is not fully elaborated. Aberrant redox homeostasis is the common features in the pathogenesis of pancreatitis and PC, which could be mediated by NOX. Therefore, further study may focus on the role of NOX in the transformation of pancreatitis and PC which help us clarify the complex relationship between them.
In conclusion, NOX plays a role in the occurrence and development of pancreatitis by regulating various types of pancreatic cells, such as acinar cells, PSCs, and macrophages. Additionally, it promotes PC progression by participating in abnormal cell apoptosis, triggering the EMT processes, and causing cell genomic instability. Understanding the role of NOX in pancreatic diseases is crucial for a gaining a deeper understanding of the underlying mechanisms of pancreatitis and PC. Further research is needed to uncover the specific functions of different subtypes within the NOX family in these diseases. Moreover, the development of NOX-specific inhibitors is necessary to validate the feasibility of targeting NOX as a treatment approach for pancreatic diseases.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen Z, China; Soni S, United States S-Editor: Wang JJ L-Editor: A P-Editor: Yu HG
| 1. | Iannuzzi JP, King JA, Leong JH, Quan J, Windsor JW, Tanyingoh D, Coward S, Forbes N, Heitman SJ, Shaheen AA, Swain M, Buie M, Underwood FE, Kaplan GG. Global Incidence of Acute Pancreatitis Is Increasing Over Time: A Systematic Review and Meta-Analysis. Gastroenterology. 2022;162:122-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 363] [Article Influence: 121.0] [Reference Citation Analysis (1)] |
| 2. | Beyer G, Habtezion A, Werner J, Lerch MM, Mayerle J. Chronic pancreatitis. Lancet. 2020;396:499-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 311] [Article Influence: 62.2] [Reference Citation Analysis (1)] |
| 3. | Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol. 2021;18:493-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 695] [Article Influence: 173.8] [Reference Citation Analysis (0)] |
| 4. | Peery AF, Crockett SD, Murphy CC, Jensen ET, Kim HP, Egberg MD, Lund JL, Moon AM, Pate V, Barnes EL, Schlusser CL, Baron TH, Shaheen NJ, Sandler RS. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2021. Gastroenterology. 2022;162:621-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 486] [Article Influence: 162.0] [Reference Citation Analysis (1)] |
| 5. | Le Cosquer G, Maulat C, Bournet B, Cordelier P, Buscail E, Buscail L. Pancreatic Cancer in Chronic Pancreatitis: Pathogenesis and Diagnostic Approach. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 6. | Chen X, Zeh HJ, Kang R, Kroemer G, Tang D. Cell death in pancreatic cancer: from pathogenesis to therapy. Nat Rev Gastroenterol Hepatol. 2021;18:804-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 220] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 7. | Badgley MA, Kremer DM, Maurer HC, DelGiorno KE, Lee HJ, Purohit V, Sagalovskiy IR, Ma A, Kapilian J, Firl CEM, Decker AR, Sastra SA, Palermo CF, Andrade LR, Sajjakulnukit P, Zhang L, Tolstyka ZP, Hirschhorn T, Lamb C, Liu T, Gu W, Seeley ES, Stone E, Georgiou G, Manor U, Iuga A, Wahl GM, Stockwell BR, Lyssiotis CA, Olive KP. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science. 2020;368:85-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 874] [Article Influence: 174.8] [Reference Citation Analysis (1)] |
| 8. | de Jesus DS, Bargi-Souza P, Cruzat V, Yechoor V, Carpinelli AR, Peliciari-Garcia RA. BMAL1 modulates ROS generation and insulin secretion in pancreatic β-cells: An effect possibly mediated via NOX2. Mol Cell Endocrinol. 2022;555:111725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011;21:103-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1739] [Cited by in RCA: 2667] [Article Influence: 177.8] [Reference Citation Analysis (0)] |
| 10. | Cao WL, Xiang XH, Chen K, Xu W, Xia SH. Potential role of NADPH oxidase in pathogenesis of pancreatitis. World J Gastrointest Pathophysiol. 2014;5:169-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 11. | Mederos MA, Reber HA, Girgis MD. Acute Pancreatitis: A Review. JAMA. 2021;325:382-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 499] [Article Influence: 124.8] [Reference Citation Analysis (1)] |
| 12. | Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, van Santvoort HC, Besselink MG. Acute pancreatitis. Lancet. 2020;396:726-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 585] [Article Influence: 117.0] [Reference Citation Analysis (0)] |
| 13. | Liu L, Zhang Y, Li X, Deng J. Microenvironment of pancreatic inflammation: calling for nanotechnology for diagnosis and treatment. J Nanobiotechnology. 2023;21:443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Stojanovic B, Jovanovic IP, Stojanovic MD, Jovanovic M, Vekic B, Milosevic B, Cvetkovic A, Spasic M, Stojanovic BS. The Emerging Roles of the Adaptive Immune Response in Acute Pancreatitis. Cells. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 15. | Baron TH, DiMaio CJ, Wang AY, Morgan KA. American Gastroenterological Association Clinical Practice Update: Management of Pancreatic Necrosis. Gastroenterology. 2020;158:67-75.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 418] [Article Influence: 83.6] [Reference Citation Analysis (2)] |
| 16. | Choi J, Oh TG, Jung HW, Park KY, Shin H, Jo T, Kang DS, Chanda D, Hong S, Kim J, Hwang H, Ji M, Jung M, Shoji T, Matsushima A, Kim P, Mun JY, Paik MJ, Cho SJ, Lee IK, Whitcomb DC, Greer P, Blobner B, Goodarzi MO, Pandol SJ, Rotter JI; North American Pancreatitis Study 2 (NAPS2) Consortium, Fan W, Bapat SP, Zheng Y, Liddle C, Yu RT, Atkins AR, Downes M, Yoshihara E, Evans RM, Suh JM. Estrogen-Related Receptor γ Maintains Pancreatic Acinar Cell Function and Identity by Regulating Cellular Metabolism. Gastroenterology. 2022;163:239-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Dios ID. Inflammatory role of the acinar cells during acute pancreatitis. World J Gastrointest Pharmacol Ther. 2010;1:15-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 18. | Yu JH, Lim JW, Kim H, Kim KH. NADPH oxidase mediates interleukin-6 expression in cerulein-stimulated pancreatic acinar cells. Int J Biochem Cell Biol. 2005;37:1458-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Gukovsky I, Pandol SJ, Gukovskaya AS. Organellar dysfunction in the pathogenesis of pancreatitis. Antioxid Redox Signal. 2011;15:2699-2710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Lee J, Lim JW, Kim H. Lycopene Inhibits Oxidative Stress-Mediated Inflammatory Responses in Ethanol/Palmitoleic Acid-Stimulated Pancreatic Acinar AR42J Cells. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Ku L, Lee J, Lim JW, Jin L, Seo JT, Kim H. Docosahexaenoic acid inhibits ethanol/palmitoleic acid-induced necroptosis in AR42J cells. J Physiol Pharmacol. 2020;71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Ju KD, Lim JW, Kim KH, Kim H. Potential role of NADPH oxidase-mediated activation of Jak2/Stat3 and mitogen-activated protein kinases and expression of TGF-β1 in the pathophysiology of acute pancreatitis. Inflamm Res. 2011;60:791-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Yu JH, Lim JW, Kim KH, Morio T, Kim H. NADPH oxidase and apoptosis in cerulein-stimulated pancreatic acinar AR42J cells. Free Radic Biol Med. 2005;39:590-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Wang X, Yang C, Chai J, Shi Y, Xue D. Mechanisms of AIF-mediated apoptotic DNA degradation in Caenorhabditis elegans. Science. 2002;298:1587-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 297] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 25. | Cregan SP, Dawson VL, Slack RS. Role of AIF in caspase-dependent and caspase-independent cell death. Oncogene. 2004;23:2785-2796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 423] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 26. | Yu JH, Kim KH, Kim H. Role of NADPH oxidase and calcium in cerulein-induced apoptosis: involvement of apoptosis-inducing factor. Ann N Y Acad Sci. 2006;1090:292-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Wu J, Zhang R, Hu G, Zhu HH, Gao WQ, Xue J. Carbon Monoxide Impairs CD11b(+)Ly-6C(hi) Monocyte Migration from the Blood to Inflamed Pancreas via Inhibition of the CCL2/CCR2 Axis. J Immunol. 2018;200:2104-2114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Sendler M, Weiss FU, Golchert J, Homuth G, van den Brandt C, Mahajan UM, Partecke LI, Döring P, Gukovsky I, Gukovskaya AS, Wagh PR, Lerch MM, Mayerle J. Cathepsin B-Mediated Activation of Trypsinogen in Endocytosing Macrophages Increases Severity of Pancreatitis in Mice. Gastroenterology. 2018;154:704-718.e10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 188] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 29. | Xue J, Habtezion A. Carbon monoxide-based therapy ameliorates acute pancreatitis via TLR4 inhibition. J Clin Invest. 2014;124:437-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 30. | Hu F, Lou N, Jiao J, Guo F, Xiang H, Shang D. Macrophages in pancreatitis: Mechanisms and therapeutic potential. Biomed Pharmacother. 2020;131:110693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 31. | Locati M, Curtale G, Mantovani A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu Rev Pathol. 2020;15:123-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1176] [Cited by in RCA: 1396] [Article Influence: 279.2] [Reference Citation Analysis (0)] |
| 32. | Wu J, Zhang L, Shi J, He R, Yang W, Habtezion A, Niu N, Lu P, Xue J. Macrophage phenotypic switch orchestrates the inflammation and repair/regeneration following acute pancreatitis injury. EBioMedicine. 2020;58:102920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 33. | Tan HY, Wang N, Li S, Hong M, Wang X, Feng Y. The Reactive Oxygen Species in Macrophage Polarization: Reflecting Its Dual Role in Progression and Treatment of Human Diseases. Oxid Med Cell Longev. 2016;2016:2795090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 442] [Cited by in RCA: 426] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 34. | Zheng W, Umitsu M, Jagan I, Tran CW, Ishiyama N, BeGora M, Araki K, Ohashi PS, Ikura M, Muthuswamy SK. An interaction between Scribble and the NADPH oxidase complex controls M1 macrophage polarization and function. Nat Cell Biol. 2016;18:1244-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Bermudez S, Khayrullina G, Zhao Y, Byrnes KR. NADPH oxidase isoform expression is temporally regulated and may contribute to microglial/macrophage polarization after spinal cord injury. Mol Cell Neurosci. 2016;77:53-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Griess B, Mir S, Datta K, Teoh-Fitzgerald M. Scavenging reactive oxygen species selectively inhibits M2 macrophage polarization and their pro-tumorigenic function in part, via Stat3 suppression. Free Radic Biol Med. 2020;147:48-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 37. | Wang J, Ma MW, Dhandapani KM, Brann DW. Regulatory role of NADPH oxidase 2 in the polarization dynamics and neurotoxicity of microglia/macrophages after traumatic brain injury. Free Radic Biol Med. 2017;113:119-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 38. | Han X, Ni J, Wu Z, Wu J, Li B, Ye X, Dai J, Chen C, Xue J, Wan R, Wen L, Wang X, Hu G. Myeloid-specific dopamine D(2) receptor signalling controls inflammation in acute pancreatitis via inhibiting M1 macrophage. Br J Pharmacol. 2020;177:2991-3008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 39. | Dellinger EP, Forsmark CE, Layer P, Lévy P, Maraví-Poma E, Petrov MS, Shimosegawa T, Siriwardena AK, Uomo G, Whitcomb DC, Windsor JA; Pancreatitis Across Nations Clinical Research and Education Alliance (PANCREA). Determinant-based classification of acute pancreatitis severity: an international multidisciplinary consultation. Ann Surg. 2012;256:875-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 342] [Article Influence: 26.3] [Reference Citation Analysis (1)] |
| 40. | Carrascal M, Areny-Balagueró A, de-Madaria E, Cárdenas-Jaén K, García-Rayado G, Rivera R, Martin Mateos RM, Pascual-Moreno I, Gironella M, Abian J, Closa D. Inflammatory capacity of exosomes released in the early stages of acute pancreatitis predicts the severity of the disease. J Pathol. 2022;256:83-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 41. | Yang X, Zhao K, Deng W, Zhao L, Jin H, Mei F, Zhou Y, Li M, Wang W. Apocynin Attenuates Acute Kidney Injury and Inflammation in Rats with Acute Hypertriglyceridemic Pancreatitis. Dig Dis Sci. 2020;65:1735-1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 42. | Jin HZ, Yang XJ, Zhao KL, Mei FC, Zhou Y, You YD, Wang WX. Apocynin alleviates lung injury by suppressing NLRP3 inflammasome activation and NF-κB signaling in acute pancreatitis. Int Immunopharmacol. 2019;75:105821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 43. | Wen Y, Liu R, Lin N, Luo H, Tang J, Huang Q, Sun H, Tang L. NADPH Oxidase Hyperactivity Contributes to Cardiac Dysfunction and Apoptosis in Rats with Severe Experimental Pancreatitis through ROS-Mediated MAPK Signaling Pathway. Oxid Med Cell Longev. 2019;2019:4578175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 44. | Deng W, Abliz A, Xu S, Sun R, Guo W, Shi Q, Yu J, Wang W. Severity of pancreatitisassociated intestinal mucosal barrier injury is reduced following treatment with the NADPH oxidase inhibitor apocynin. Mol Med Rep. 2016;14:3525-3534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Peery AF, Crockett SD, Murphy CC, Lund JL, Dellon ES, Williams JL, Jensen ET, Shaheen NJ, Barritt AS, Lieber SR, Kochar B, Barnes EL, Fan YC, Pate V, Galanko J, Baron TH, Sandler RS. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology. 2019;156:254-272.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 1082] [Article Influence: 180.3] [Reference Citation Analysis (1)] |
| 46. | Chang M, Chen W, Xia R, Peng Y, Niu P, Fan H. Pancreatic Stellate Cells and the Targeted Therapeutic Strategies in Chronic Pancreatitis. Molecules. 2023;28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 47. | Kleeff J, Whitcomb DC, Shimosegawa T, Esposito I, Lerch MM, Gress T, Mayerle J, Drewes AM, Rebours V, Akisik F, Muñoz JED, Neoptolemos JP. Chronic pancreatitis. Nat Rev Dis Primers. 2017;3:17060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 310] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 48. | Bynigeri RR, Jakkampudi A, Jangala R, Subramanyam C, Sasikala M, Rao GV, Reddy DN, Talukdar R. Pancreatic stellate cell: Pandora's box for pancreatic disease biology. World J Gastroenterol. 2017;23:382-405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 115] [Cited by in RCA: 136] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 49. | Masamune A, Watanabe T, Kikuta K, Satoh K, Shimosegawa T. NADPH oxidase plays a crucial role in the activation of pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2008;294:G99-G108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 50. | Xia D, Halder B, Godoy C, Chakraborty A, Singla B, Thomas E, Shuja JB, Kashif H, Miller L, Csanyi G, Sabbatini ME. NADPH oxidase 1 mediates caerulein-induced pancreatic fibrosis in chronic pancreatitis. Free Radic Biol Med. 2020;147:139-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 51. | Zeng XP, Wang LJ, Guo HL, He L, Bi YW, Xu ZL, Li ZS, Hu LH. Dasatinib ameliorates chronic pancreatitis induced by caerulein via anti-fibrotic and anti-inflammatory mechanism. Pharmacol Res. 2019;147:104357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 52. | Lin Y, Chen Y, Feng W, Hua R, Zhang J, Huo Y, Jiang H, Yin B, Yang X. Neddylation pathway alleviates chronic pancreatitis by reducing HIF1α-CCL5-dependent macrophage infiltration. Cell Death Dis. 2021;12:273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 53. | Xue J, Sharma V, Hsieh MH, Chawla A, Murali R, Pandol SJ, Habtezion A. Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis. Nat Commun. 2015;6:7158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 282] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 54. | Zhou J, Liu W, Zhao X, Xian Y, Wu W, Zhang X, Zhao N, Xu FJ, Wang C. Natural Melanin/Alginate Hydrogels Achieve Cardiac Repair through ROS Scavenging and Macrophage Polarization. Adv Sci (Weinh). 2021;8:e2100505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 152] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 55. | Chen Y, Wu G, Li M, Hesse M, Ma Y, Chen W, Huang H, Liu Y, Xu W, Tang Y, Zheng H, Li C, Lin Z, Chen G, Liao W, Liao Y, Bin J, Chen Y. LDHA-mediated metabolic reprogramming promoted cardiomyocyte proliferation by alleviating ROS and inducing M2 macrophage polarization. Redox Biol. 2022;56:102446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 63] [Reference Citation Analysis (0)] |
| 56. | Lu Y, Rong J, Lai Y, Tao L, Yuan X, Shu X. The Degree of Helicobacter pylori Infection Affects the State of Macrophage Polarization through Crosstalk between ROS and HIF-1α. Oxid Med Cell Longev. 2020;2020:5281795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 57. | Balce DR, Li B, Allan ER, Rybicka JM, Krohn RM, Yates RM. Alternative activation of macrophages by IL-4 enhances the proteolytic capacity of their phagosomes through synergistic mechanisms. Blood. 2011;118:4199-4208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 58. | Kuchler L, Giegerich AK, Sha LK, Knape T, Wong MS, Schröder K, Brandes RP, Heide H, Wittig I, Brüne B, von Knethen A. SYNCRIP-dependent Nox2 mRNA destabilization impairs ROS formation in M2-polarized macrophages. Antioxid Redox Signal. 2014;21:2483-2497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 59. | Mongue-Din H, Patel AS, Looi YH, Grieve DJ, Anilkumar N, Sirker A, Dong X, Brewer AC, Zhang M, Smith A, Shah AM. NADPH Oxidase-4 Driven Cardiac Macrophage Polarization Protects Against Myocardial Infarction-Induced Remodeling. JACC Basic Transl Sci. 2017;2:688-698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 60. | Zhang Y, Choksi S, Chen K, Pobezinskaya Y, Linnoila I, Liu ZG. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res. 2013;23:898-914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 416] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 61. | GBD 2017 Pancreatic Cancer Collaborators. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2019;4:934-947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 431] [Cited by in RCA: 439] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 62. | Cao D, Song Q, Li J, Jiang Y, Wang Z, Lu S. Opportunities and challenges in targeted therapy and immunotherapy for pancreatic cancer. Expert Rev Mol Med. 2021;23:e21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 63. | Buscail L, Bournet B, Cordelier P. Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2020;17:153-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 468] [Article Influence: 93.6] [Reference Citation Analysis (0)] |
| 64. | Du J, Nelson ES, Simons AL, Olney KE, Moser JC, Schrock HE, Wagner BA, Buettner GR, Smith BJ, Teoh ML, Tsao MS, Cullen JJ. Regulation of pancreatic cancer growth by superoxide. Mol Carcinog. 2013;52:555-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 65. | Wang P, Sun YC, Lu WH, Huang P, Hu Y. Selective killing of K-ras-transformed pancreatic cancer cells by targeting NAD(P)H oxidase. Chin J Cancer. 2015;34:166-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 66. | Lee JK, Edderkaoui M, Truong P, Ohno I, Jang KT, Berti A, Pandol SJ, Gukovskaya AS. NADPH oxidase promotes pancreatic cancer cell survival via inhibiting JAK2 dephosphorylation by tyrosine phosphatases. Gastroenterology. 2007;133:1637-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 67. | Lu W, Hu Y, Chen G, Chen Z, Zhang H, Wang F, Feng L, Pelicano H, Wang H, Keating MJ, Liu J, McKeehan W, Luo Y, Huang P. Novel role of NOX in supporting aerobic glycolysis in cancer cells with mitochondrial dysfunction and as a potential target for cancer therapy. PLoS Biol. 2012;10:e1001326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 68. | Wu Y, Lu J, Antony S, Juhasz A, Liu H, Jiang G, Meitzler JL, Hollingshead M, Haines DC, Butcher D, Roy K, Doroshow JH. Activation of TLR4 is required for the synergistic induction of dual oxidase 2 and dual oxidase A2 by IFN-γ and lipopolysaccharide in human pancreatic cancer cell lines. J Immunol. 2013;190:1859-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 69. | Hayes JD, Dinkova-Kostova AT, Tew KD. Oxidative Stress in Cancer. Cancer Cell. 2020;38:167-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1195] [Cited by in RCA: 1549] [Article Influence: 309.8] [Reference Citation Analysis (0)] |
| 70. | Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Semin Cell Dev Biol. 2018;80:50-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 1315] [Article Influence: 164.4] [Reference Citation Analysis (0)] |
| 71. | Pick E. Role of the Rho GTPase Rac in the activation of the phagocyte NADPH oxidase: outsourcing a key task. Small GTPases. 2014;5:e27952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 72. | Vaquero EC, Edderkaoui M, Pandol SJ, Gukovsky I, Gukovskaya AS. Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. J Biol Chem. 2004;279:34643-34654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 270] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 73. | Du J, Liu J, Smith BJ, Tsao MS, Cullen JJ. Role of Rac1-dependent NADPH oxidase in the growth of pancreatic cancer. Cancer Gene Ther. 2011;18:135-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 74. | Park MT, Kim MJ, Suh Y, Kim RK, Kim H, Lim EJ, Yoo KC, Lee GH, Kim YH, Hwang SG, Yi JM, Lee SJ. Novel signaling axis for ROS generation during K-Ras-induced cellular transformation. Cell Death Differ. 2014;21:1185-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 75. | Irani K. Oxidant signaling in vascular cell growth, death, and survival : a review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circ Res. 2000;87:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 563] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 76. | Blaser H, Dostert C, Mak TW, Brenner D. TNF and ROS Crosstalk in Inflammation. Trends Cell Biol. 2016;26:249-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 735] [Article Influence: 81.7] [Reference Citation Analysis (0)] |
| 77. | Mochizuki T, Furuta S, Mitsushita J, Shang WH, Ito M, Yokoo Y, Yamaura M, Ishizone S, Nakayama J, Konagai A, Hirose K, Kiyosawa K, Kamata T. Inhibition of NADPH oxidase 4 activates apoptosis via the AKT/apoptosis signal-regulating kinase 1 pathway in pancreatic cancer PANC-1 cells. Oncogene. 2006;25:3699-3707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 183] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 78. | Puca R, Nardinocchi L, Starace G, Rechavi G, Sacchi A, Givol D, D'Orazi G. Nox1 is involved in p53 deacetylation and suppression of its transcriptional activity and apoptosis. Free Radic Biol Med. 2010;48:1338-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 79. | Kato A, Naiki-Ito A, Nakazawa T, Hayashi K, Naitoh I, Miyabe K, Shimizu S, Kondo H, Nishi Y, Yoshida M, Umemura S, Hori Y, Mori T, Tsutsumi M, Kuno T, Suzuki S, Kato H, Ohara H, Joh T, Takahashi S. Chemopreventive effect of resveratrol and apocynin on pancreatic carcinogenesis via modulation of nuclear phosphorylated GSK3β and ERK1/2. Oncotarget. 2015;6:42963-42975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 80. | Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20:69-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1356] [Cited by in RCA: 2529] [Article Influence: 421.5] [Reference Citation Analysis (0)] |
| 81. | Alvarez MA, Freitas JP, Mazher Hussain S, Glazer ES. TGF-β Inhibitors in Metastatic Pancreatic Ductal Adenocarcinoma. J Gastrointest Cancer. 2019;50:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 82. | Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH, Leach SD, Stanger BZ. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1400] [Cited by in RCA: 1667] [Article Influence: 128.2] [Reference Citation Analysis (0)] |
| 83. | Jiang J, Wang K, Chen Y, Chen H, Nice EC, Huang C. Redox regulation in tumor cell epithelial-mesenchymal transition: molecular basis and therapeutic strategy. Signal Transduct Target Ther. 2017;2:17036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 84. | Bi Y, Lei X, Chai N, Linghu E. NOX4: a potential therapeutic target for pancreatic cancer and its mechanism. J Transl Med. 2021;19:515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 85. | Ma WF, Boudreau HE, Leto TL. Pan-Cancer Analysis Shows TP53 Mutations Modulate the Association of NOX4 with Genetic Programs of Cancer Progression and Clinical Outcome. Antioxidants (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 86. | Hiraga R, Kato M, Miyagawa S, Kamata T. Nox4-derived ROS signaling contributes to TGF-β-induced epithelial-mesenchymal transition in pancreatic cancer cells. Anticancer Res. 2013;33:4431-4438. [PubMed] |
| 87. | Witte D, Bartscht T, Kaufmann R, Pries R, Settmacher U, Lehnert H, Ungefroren H. TGF-β1-induced cell migration in pancreatic carcinoma cells is RAC1 and NOX4-dependent and requires RAC1 and NOX4-dependent activation of p38 MAPK. Oncol Rep. 2017;38:3693-3701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 88. | Li H, Peng C, Zhu C, Nie S, Qian X, Shi Z, Shi M, Liang Y, Ding X, Zhang S, Zhang B, Li X, Xu G, Lv Y, Wang L, Friess H, Kong B, Zou X, Shen S. Hypoxia promotes the metastasis of pancreatic cancer through regulating NOX4/KDM5A-mediated histone methylation modification changes in a HIF1A-independent manner. Clin Epigenetics. 2021;13:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 89. | Yang Y, Karakhanova S, Hartwig W, D'Haese JG, Philippov PP, Werner J, Bazhin AV. Mitochondria and Mitochondrial ROS in Cancer: Novel Targets for Anticancer Therapy. J Cell Physiol. 2016;231:2570-2581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 470] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 90. | Bonora M, Missiroli S, Perrone M, Fiorica F, Pinton P, Giorgi C. Mitochondrial Control of Genomic Instability in Cancer. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 91. | Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res. 2010;106:1253-1264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 434] [Cited by in RCA: 425] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 92. | Salmeen A, Park BO, Meyer T. The NADPH oxidases NOX4 and DUOX2 regulate cell cycle entry via a p53-dependent pathway. Oncogene. 2010;29:4473-4484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 93. | Eid AA, Ford BM, Block K, Kasinath BS, Gorin Y, Ghosh-Choudhury G, Barnes JL, Abboud HE. AMP-activated protein kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. J Biol Chem. 2010;285:37503-37512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 221] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 94. | Boudreau HE, Casterline BW, Burke DJ, Leto TL. Wild-type and mutant p53 differentially regulate NADPH oxidase 4 in TGF-β-mediated migration of human lung and breast epithelial cells. Br J Cancer. 2014;110:2569-2582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 95. | Ogrunc M, Di Micco R, Liontos M, Bombardelli L, Mione M, Fumagalli M, Gorgoulis VG, d'Adda di Fagagna F. Oncogene-induced reactive oxygen species fuel hyperproliferation and DNA damage response activation. Cell Death Differ. 2014;21:998-1012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 240] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 96. | Ju HQ, Ying H, Tian T, Ling J, Fu J, Lu Y, Wu M, Yang L, Achreja A, Chen G, Zhuang Z, Wang H, Nagrath D, Yao J, Hung MC, DePinho RA, Huang P, Xu RH, Chiao PJ. Mutant Kras- and p16-regulated NOX4 activation overcomes metabolic checkpoints in development of pancreatic ductal adenocarcinoma. Nat Commun. 2017;8:14437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 97. | Yu JH, Kim KH, Kim DG, Kim H. Diphenyleneiodonium suppresses apoptosis in cerulein-stimulated pancreatic acinar cells. Int J Biochem Cell Biol. 2007;39:2063-2075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 98. | Hirano K, Chen WS, Chueng AL, Dunne AA, Seredenina T, Filippova A, Ramachandran S, Bridges A, Chaudry L, Pettman G, Allan C, Duncan S, Lee KC, Lim J, Ma MT, Ong AB, Ye NY, Nasir S, Mulyanidewi S, Aw CC, Oon PP, Liao S, Li D, Johns DG, Miller ND, Davies CH, Browne ER, Matsuoka Y, Chen DW, Jaquet V, Rutter AR. Discovery of GSK2795039, a Novel Small Molecule NADPH Oxidase 2 Inhibitor. Antioxid Redox Signal. 2015;23:358-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 154] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 99. | Chang G, Chen L, Lin HM, Lin Y, Maranchie JK. Nox4 inhibition enhances the cytotoxicity of cisplatin in human renal cancer cells. J Exp Ther Oncol. 2012;10:9-18. [PubMed] |
| 100. | Yang WH, Huang Z, Wu J, Ding CC, Murphy SK, Chi JT. A TAZ-ANGPTL4-NOX2 Axis Regulates Ferroptotic Cell Death and Chemoresistance in Epithelial Ovarian Cancer. Mol Cancer Res. 2020;18:79-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 220] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 101. | Ju HQ, Gocho T, Aguilar M, Wu M, Zhuang ZN, Fu J, Yanaga K, Huang P, Chiao PJ. Mechanisms of Overcoming Intrinsic Resistance to Gemcitabine in Pancreatic Ductal Adenocarcinoma through the Redox Modulation. Mol Cancer Ther. 2015;14:788-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 102. | Sadr-Azodi O, Oskarsson V, Discacciati A, Videhult P, Askling J, Ekbom A. Pancreatic Cancer Following Acute Pancreatitis: A Population-based Matched Cohort Study. Am J Gastroenterol. 2018;113:1711-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 103. | Hao L, Zeng XP, Xin L, Wang D, Pan J, Bi YW, Ji JT, Du TT, Lin JH, Zhang D, Ye B, Zou WB, Chen H, Xie T, Li BR, Zheng ZH, Wang T, Guo HL, Liao Z, Li ZS, Hu LH. Incidence of and risk factors for pancreatic cancer in chronic pancreatitis: A cohort of 1656 patients. Dig Liver Dis. 2017;49:1249-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |