Published online Dec 28, 2024. doi: 10.3748/wjg.v30.i48.5152
Revised: October 2, 2024
Accepted: November 11, 2024
Published online: December 28, 2024
Processing time: 161 Days and 22.8 Hours
For the treatment of gastritis, rebamipide, a mucoprotective agent, and nizatidine, a gastric acid suppressant, are commonly employed individually.
To compare the efficacy of Mucotra® SR (rebamipide 150 mg) and Axid® (nizatidine 150 mg) combination therapy with the sole administration of Axid® in managing erosive gastritis.
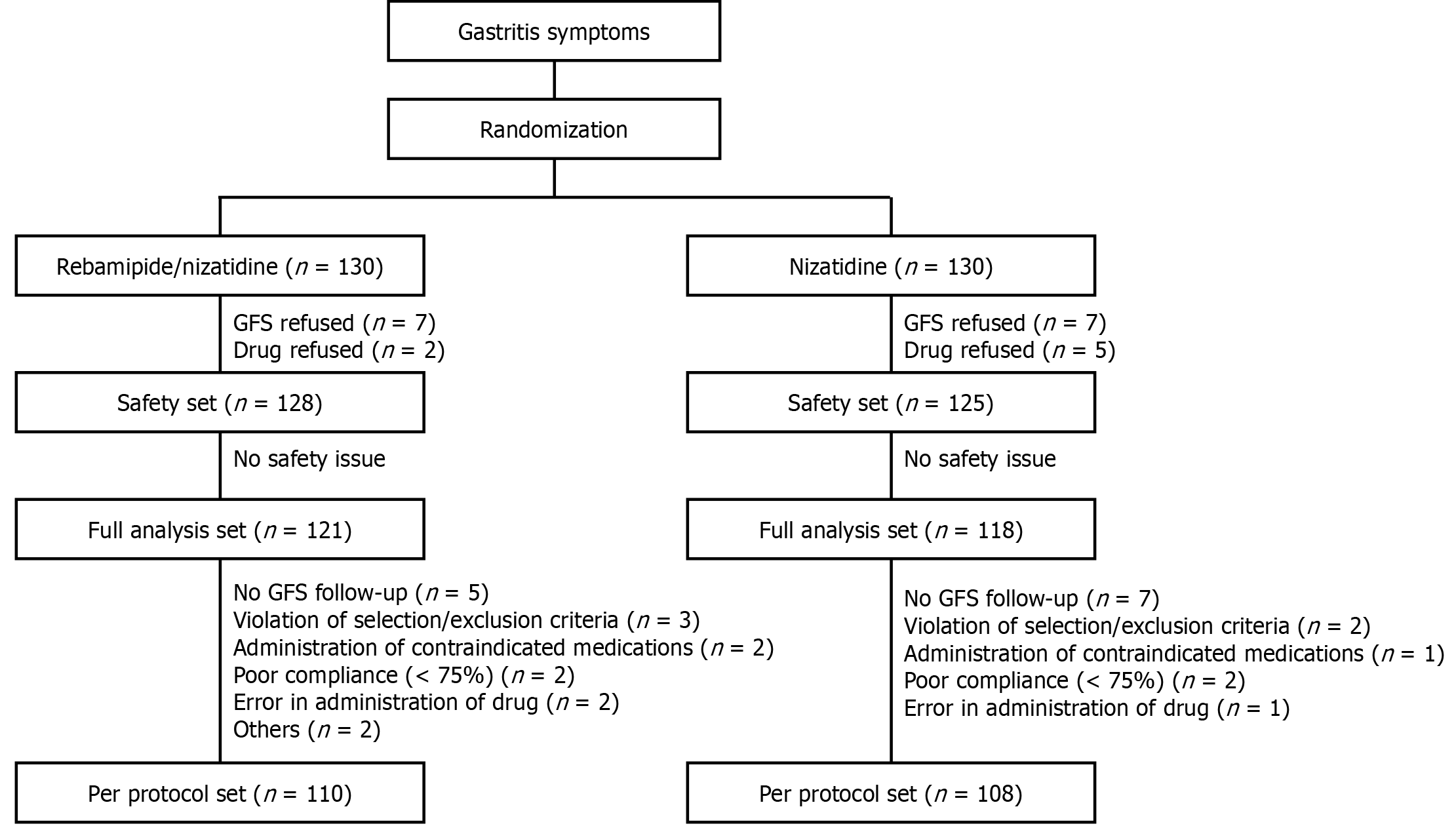
A total of 260 patients diagnosed with endoscopically confirmed erosive gastritis were enrolled in this open-label, multicenter, randomized, phase 4 clinical trial, allocating them into two groups: Rebamipide/nizatidine combination twice daily vs nizatidine twice daily for 2 weeks. The full-analysis set analysis encompassed 239 patients (rebamipide/nizatidine, n = 121; nizatidine, n = 118), while the per-protocol analysis included 218 patients (n = 110 vs 108). Post-treatment assessments comprised primary (erosion improvement rate) and secondary (erosion and edema cure rates, erythema improvement rates, hemorrhage, and gastrointestinal symptoms) endpoints. Furthermore, drug-related adverse effects were evaluated.
Primary efficacy assessment showed a statistically significant improvement rate in mucosal erosions in the combination group compared to the control group in the full-analysis set (rebamipide/nizatidine 62.0%, nizatidine 49.2%, P = 0.046), with a similar trend noted in the per-protocol analysis (62.7% vs 50.0%, P = 0.058). Both groups were effective in curing erosion and edema and improvement of bleeding, erythema, and gastrointestinal symptoms, whereas no inter-group differences were noted. When confined to the participants with gastritis symptoms, improvement of erosion was more optimal in the combination group (63.0% vs 49.5%, P = 0.046). No adverse events related to the drugs were observed.
Rebamipide/nizatidine combination is effective in treatment of erosive gastritis.
Core Tip: This multicenter, randomized phase 4 trial compared the efficacy and safety of rebamipide/nizatidine combination therapy vs nizatidine monotherapy in treating erosive gastritis. The study demonstrated that the combination therapy significantly improved mucosal erosion healing compared to monotherapy, highlighting the potential benefit of combining an acid suppressant with a mucoprotective agent. While both treatment groups showed positive outcomes in managing symptoms, no significant differences in adverse events were observed. These findings suggest that combination therapy may offer a more effective approach for patients with erosive gastritis.
- Citation: Kang D, Choi MG, Shim KN, Jung HK, Nam SJ, Park JH, Kim SG, Kim NH, Hong SJ, Jeon TJ, Chung JI, Lee HL, Lee JY, Kim TO, Lee CM, Kim SM, Kim JH, Kim JE, Moon JS, Kim HD, Lee WS, Park HJ. Efficacy and safety of rebamipide/nizatidine in patients with erosive gastritis: A randomized, multicenter, phase 4 study. World J Gastroenterol 2024; 30(48): 5152-5161
- URL: https://www.wjgnet.com/1007-9327/full/v30/i48/5152.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i48.5152
One of the most prevalent gastrointestinal diseases worldwide is gastritis. It presents in a variety of symptoms, including epigastric pain, dyspepsia, nausea, vomiting, acid reflux, abdominal bloating, poor appetite, soreness, and belching[1]. With the widespread accessibility of endoscopy in Korea, during endoscopic examinations, several patients are diagnosed with gastritis, which reveal erosion, edema, redness, and hemorrhages in the gastric mucosa. It is reported that the prevalence of endoscopy-proven gastritis is high, reaching up to 85.9%, with erosive gastritis accounting for 23.7%[2]. Given the array of symptoms and endoscopic findings associated with gastritis, a substantial portion of the population is afflicted by this condition[2]. There is no established definitive treatment for gastritis. Acid-suppression therapy, mucoprotective agent, or misoprostol may be effective for the treatment of erosive gastritis. These medications are primarily employed to manage symptoms and promote healing of a gastric lesion.
Gastric acid plays a pivotal role in the pathophysiology of gastritis. Consequently, the inhibition of gastric acid secretion may play a significant role in its treatment. Histamine 2 receptor antagonists (H2RA), proton pump inhibitors (PPI), and recently developed potassium-competitive acid blockers are extensively utilized or currently undergoing clinical trials for gastritis treatment. H2RA has been used to treat patients with gastritis and gastroesophageal reflux disease. Challenges associated with H2RA use, including tachyphylaxis and relatively lower efficacy despite its well-established safety profile, have been observed. However, compared to PPI, H2RA still has some advantages; it is relatively fast, safe, and convenient. Especially, nizatidine uniquely inhibits acetylcholinesterase, increasing acetylcholine concentration at the synapses of the cholinergic nervous system, thus activating gastrointestinal motility and stimulating saliva secretion[3-6].
For the treatment of gastritis, gastric mucoprotective agents can be used. Rebamipide, a commonly used mucoprotective agent, has shown its efficacy in the treatment of acute and chronic gastritis[7,8], as well as peptic ulcers[9]. Rebamipide promotes gastric mucosal healing through the stimulation of prostaglandin and mucous glycoprotein synthesis, inhibiting the production of reactive oxygen radicals and inflammatory cytokines and suppressing leukocyte activity[10]. Moreover, evidence has shown that the long term use of rebamipide has a good safety profile[11]. While these pharmacological agents are frequently administered for the management of gastritis, the therapeutic efficacy of monotherapy is often inadequate. A synergistic approach has been proposed to address these shortcomings, specifically the concomitant use of an acid suppressant and a mucoprotective agent, which could enhance the treatment outcomes for gastritis. Particularly, nizatidine and rebamipide, which are commonly prescribed for this condition, may exhibit augmented efficacy when used together. This combination therapy is anticipated to yield superior management of gastritis while retaining a safety profile that is consistent with the use of either nizatidine or rebamipide as monotherapy. Accordingly, a randomized, multicenter, phase 4 clinical trial was conducted to assess the comparative efficacy and safety of the rebamipide/nizatidine combination vs nizatidine alone, with particular emphasis on safety, endoscopic improvement, and alleviation of gastrointestinal symptoms in patients suffering from gastritis.
This study was a multicenter (23 medical centers), randomized, open-label, active-control, phase 4 clinical trial conducted in Korea from September 2021 to December 2022. Symptomatic patients aged 19 to 75 years with acute or chronic gastritis who had one or more gastric erosions on baseline esophagogastroduodenoscopy (EGD) and one or more subjective symptoms requiring medical treatment were enrolled. The exclusion criteria comprised patients with the following conditions: (1) Peptic ulcer in the active or healing stage; (2) Reflux esophagitis; (3) Inflammatory bowel diseases; (4) Cardiovascular and cerebrovascular diseases; (5) Zollinger-Ellison syndrome; (6) Previous history of esophageal or gastric surgery; (7) Gastrointestinal malignancies; (8) Known hypersensitivity to H2RAs; (9) Drug or alcohol abuse; (10) Significant hepatic, renal, neurologic, cardiovascular, pulmonary, endocrine, hemato-oncologic, or urologic impairment; (11) Cardiovascular or cerebrovascular event within 24 weeks; (12) Systemic bleeding tendency, coagulation disorder, or thrombotic disorder; (13) Clinically significant psychiatric disorder; (14) Currently taking corticosteroids, nonsteroidal anti-inflammatory drugs, aspirin, anti-thrombotic agents, bisphosphonates, antispasmodics, prokinetics, iron supplements, serotonin re-uptake inhibitors, or herbal medicines within 2 weeks of the administration of the investigational product and during the study period; (15) Abnormal laboratory test values upon screening (creatine clearance < 50 mL/minute or alanine aminotransferase and aspartate aminotransferase > 3 × the upper limit of normal); (16) Pregnancy or lactation; and (17) Nonuse of contraception during the childbearing age.
This trial was conducted following the principles of Good Clinical Practice and the Declaration of Helsinki. The study protocol was approved by the Institutional Review Board of each of the participating institutions including Seoul St. Mary’s Hospital (IRB number: KC21MSDV0559). Upon enrolment, written informed consent was obtained from all the participants. This trial was registered as a standard, randomized clinical trial (ClinicalTrials.gov: NCT05072938).
The treatment allocation list was created using a computer-generated randomization code with block randomization centrally and distributed to each institution via a web-based interactive response system. Participants underwent screening tests including blood tests, urinalysis, electrocardiography, and EGD. Thereafter, eligible participants were randomly assigned to have one of the following two medications for 2 weeks at a 1:1 ratio: (1) Test group (Axid® capsule, Daewoong Co. Ltd., nizatidine 150 mg and Mucotra® SR tablet, Daewoong Co. Ltd., rebamipide 150 mg twice daily); and (2) Control group (Axid® capsule, Daewoong Co. Ltd., nizatidine 150 mg twice daily).
After treatment initiation, patients underwent follow-up endoscopy at 2 weeks. Compliance was evaluated by the number of remaining tablets per drug type during the follow-up visit. If the drug compliance was ≥ 75%, the participants’ data were analyzed in the per-protocol manner. Those protocol are summarized in Figure 1. During EGD, acute gastritis was characterized by the presence of mucosal edema, redness, and hemorrhage. Conversely, chronic gastritis was identified by the presence of atrophy and metaplasia. The presence of Helicobacter pylori infection was identified via at least one of the following diagnostic methods: Histology, rapid urease test, or urea breath test.
Efficacy: Each patient underwent an EGD both before and 2 weeks after treatment initiation. Gastric erosion evaluation was conducted on a scale of 1 to 4, using the following scores: 1 indicating no visible erosion, 2 indicating one or two erosions, 3 indicating three to five erosions, and 4 indicating more than five erosions[12]. The improvement rate of erosions was the primary efficacy endpoint, defined as the percentage of patients exhibiting an erosion score improve
Prior to the commencement of the clinical trial, the principal investigators from the participating institutions engaged in discussions regarding the assessment of endoscopic findings, particularly concerning erosions. All EGD examinations were recorded and evaluated by the principal investigators to ascertain consistent assessments, who also verified the data in cases where sub-investigators conducted the EGD procedures. Recognizing the critical importance of accurately evaluating the presence of gastric erosions during the initial screening EGD for the study result reliability, independent investigators who were not involved in this study conducted a re-evaluation of the screening EGD images.
The secondary efficacy endpoints encompassed the healing rates of erosion and edema, the improvement rates of redness and hemorrhage, and the enhancement in subjective symptoms, all evaluated 2 weeks following treatment initiation. Edema was evaluated on a scale of 1-2; redness, 1-4; and hemorrhage, 1-5. Erosion and edema healing was defined as their complete disappearance[14,15], while the improvement of redness and hemorrhage was defined as a reduction of at least 50% from their initial scores during the follow-up EGD conducted 2 weeks after treatment initiation.
The subjective symptoms were self-reported and included epigastric pain, heartburn, epigastric discomfort, early satiety, postprandial fullness, intragastric pooling, upper abdominal bloating, nausea, vomiting, and excessive belching[13]. Each symptom was evaluated for intensity on a scale from 0 to 5 (0: No problem; 1: Slight problem; 2: Mild problem; 3: Moderate problem; 4: Severe problem; and 5: Very severe problem). Symptom frequency was rated on a scale of 0 to 4 (0: Absent; 1: 1 to 2 days per week; 2: 3 to 4 days per week; 3: 5 to 6 days per week; and 4: Daily). Symptom scores were calculated by summing the intensity and frequency scores for all symptoms, with a maximum possible score of 64. Subjective symptom improvement was defined as a reduction of at least 50% in the initial gastritis symptom scores[13]. Furthermore, exploratory endpoints included assessment of the improvement rate of erosions according to the Helicobacter pylori infection status (positive or negative), degree of erosion in the initial endoscopy, and self-reported symptoms.
Safety: Safety assessments consist of adverse events (AEs) and adverse drug reactions (ADRs), including any gastrointestinal symptoms and abnormalities in the electrocardiography, laboratory findings, or vital signs. During the study, all AEs reported regardless of their relationship with the investigational product, were recorded in detail in terms of the date of onset, duration, seriousness, severity, required treatment modification, causal relationship with the study medication, and outcome. ADRs were defined as AEs for which a causal relationship could not be ruled out. AEs and ADRs were recorded using the Medical Dictionary for Regulatory Activities version 25.0 and classified using System Organ Class and Preferred Term.
Sample size: The sample size was estimated with the assumption that the efficacy rates of rebamipide/nizatidine and nizatidine for the gastric erosions determined by EGD were 0.891 and 0.741, respectively. According to these threshold parameters, the total sample size was calculated as 130 patients in each group, with a power of 80% at a two-sided significance level of 0.05 and a drop rate of 20%.
The patient data underwent three distinct types of analyses: The safety set, the full-analysis set (FAS), and the per-protocol set (PPS). The FAS was used to conduct the efficacy assessments, which encompassed all participants who possessed data concerning the primary efficacy evaluation parameters following treatment with the investigational products. Subsequently, individuals identified as having no erosion on their baseline EGD were excluded from the FAS analysis after evaluation by independent investigators. This exclusion was due to the primary efficacy endpoint revolving around the improvement rate of erosions, contingent upon the number of erosions detected during EGD.
The safety set analysis, which mainly formed the basis for safety-related data, included all data from randomly assigned participants who had administered at least one dose of the investigational products post-randomization and had undergone at least one safety assessment follow-up. Conversely, the PPS analysis concentrated on participants from the FAS analysis who showed data signifying their clinical trial protocol compliance. Efficacy parameters were presented as frequency and proportion (with a 95% confidence interval) in each group. Pearson’s χ2 test and analysis of covariance were performed for categorical and continuous variables, respectively, to compare the efficacy of the two groups.
A total of 260 participants were included in the study, equally distributed to the combination group and the control group. In each group, 121 (98.46%) and 118 (96.15%) patients were included in FAS and 115 (88.46%) and 115 (88.46%) were included in PPS.
Table 1 illustrates the demographic and clinical characteristics of patients in the two groups. No differences between those groups were observed including age, sex, height, weight, body mass index, smoking status, alcohol consumption, and gastrointestinal symptom scores. The baseline endoscopic findings of the patients were also similar in the two groups (Table 2).
| Rebamipide/nizatidine (n = 130) | Nizatidine (n = 130) | Total (n = 260) | P value | |
| Age (year), mean ± SD | 45.96 ± 14.25 | 44.0 ± 13.54 | 44.98 ± 13.91 | 0.296 |
| Sex, male, n (%) | 41 (31.54) | 50 (38.46) | 91 (35.00) | 0.242 |
| Height (cm), mean ± SD | 163.13 ± 8.19 | 164.95 ± 7.24 | 164.04 ± 7.77 | 0.034 |
| Weight (cm), mean ± SD | 63.25 ± 13.65 | 63.48 ± 12.17 | 63.36 ± 12.91 | 0.530 |
| Body mass index (kg/m2), mean ± SD | 23.61 ± 3.76 | 23.21 ± 3.43 | 23.41 ± 3.60 | 0.528 |
| Smoking status, n (%) | 0.688 | |||
| No | 107 (82.31) | 104 (80.00) | 211 (81.15) | |
| Past smoker | 13 (10.00) | 12 (9.23) | 25 (9.62) | |
| Current smoker | 10 (7.69) | 14 (10.77) | 24 (9.23) | |
| Alcohol consumption, yes, n (%) | 0.494 | |||
| No | 53 (40.77) | 44 (33.85) | 97 (37.31) | |
| Past | 11 (8.46) | 11 (8.46) | 22 (8.46) | |
| Current | 66 (50.77) | 75 (57.69) | 141 (54.23) | |
| Concurrent disease, yes, n (%) | 25 (19.23) | 20 (15.38) | 45 (17.31) | 0.412 |
| Concomitant medication, yes, n (%) | 10 (7.69) | 15 (11.54) | 25 (9.62) | 0.293 |
| Gastrointestinal symptom scores, mean ± SD | ||||
| Total | 14.6 ± 10.0 | 15.0 ± 11.2 | 14.8 ± 10.6 | 0.758 |
| Severity | 8.5 ± 5.9 | 8.5 ± 6.5 | 8.5 ± 6.2 | 0.968 |
| Frequency | 6.1 ± 4.6 | 6.5 ± 5.0 | 6.3 ± 4.8 | 0.524 |
| Rebamipide/nizatidine (n = 121) | Nizatidine (n = 118) | P value | |
| Erosion | 0.784 | ||
| 1 (no erosion) | 0 (0) | 0 (0) | |
| 2 (1-2 erosions) | 42 (34.7) | 46 (39.0) | |
| 3 (3-5 erosions) | 43 (35.5) | 40 (33.9) | |
| 4 (> 5 erosions) | 36 (29.8) | 32 (27.1) | |
| Edema | 0.379 | ||
| 1 (none) | 86 (71.1) | 90 (76.9) | |
| 2 (pale/whiter and slightly accentuated hexagonal area gastric pattern) | 35 (28.9) | 27 (23.1) | |
| Redness | 0.906 | ||
| 1 (none) | 35 (28.9) | 31 (26.5) | |
| 2 (minimal but obvious change) | 74 (61.2) | 74 (63.2) | |
| 3 (conspicuous patchy discoloration) | 11 (9.1) | 10 (8.5) | |
| 4 (color change is beefy-red in intensity) | 1 (0.8) | 2 (1.7) | |
| Hemorrhage | 0.622 | ||
| 1 (none) | 86 (71.1) | 77 (65.8) | |
| 2 (1 hemorrhagic lesion) | 15 (12.4) | 17 (14.5) | |
| 3 (2-5 hemorrhagic lesions) | 15 (12.4) | 20 (17.1) | |
| 4 (> 6 hemorrhagic lesions) | 5 (4.1) | 3 (2.6) |
Throughout the treatment period, the drug compliance rates were 94.90% and 94.93% in the combination and nizatidine single groups, respectively, with no statistically significant difference. A total of four participants (1.54%), comprising two from the combination group (1.65%) and two from the control group (1.69%), failed to adhere to the prescribed drug administration schedule, defined as compliance below 75%, thereby constituting protocol violations.
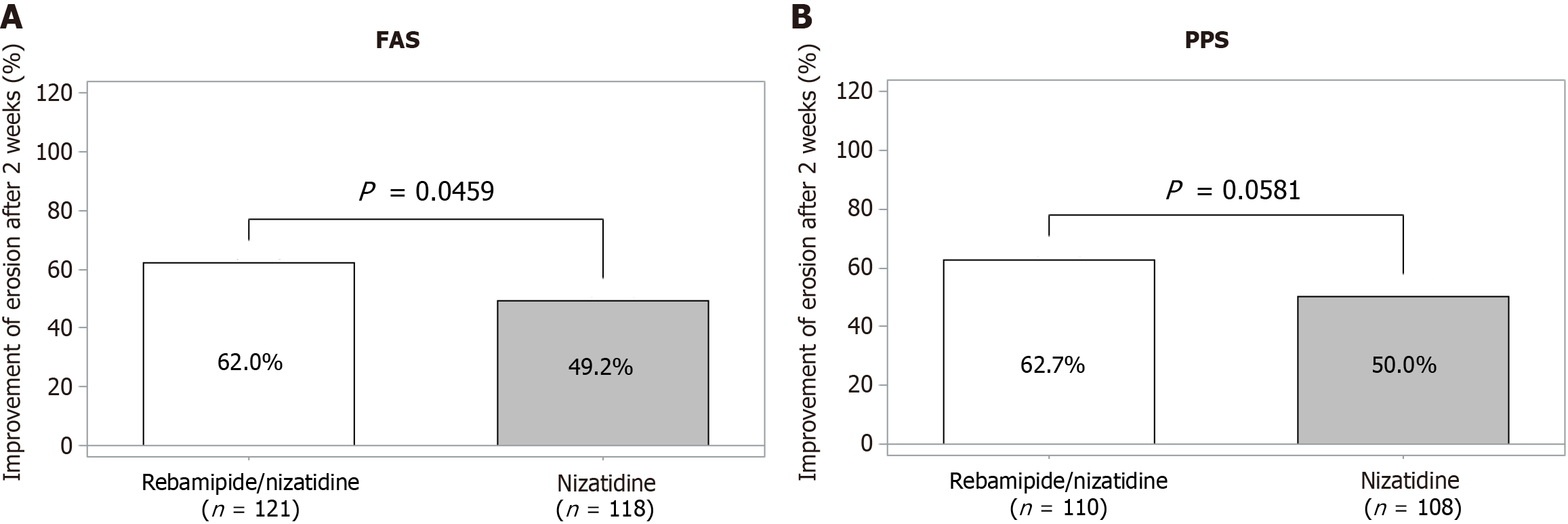
According to the FAS analysis, the rates of improvement of erosion after a 2-week treatment were 62.0% and 49.2% in the rebamipide/nizatidine combination and nizatidine single groups, respectively, and the combination group demonstrated a statistically significantly higher improvement rate (P = 0.046). The same trend was observed in PPS (62.73% and 50.00%, rebamipide/nizatidine group and nizatidine group, respectively) without statistical significance (P = 0.058) (Figure 2 and Table 3). For the sensitivity analysis, the efficacy was compared by including participants who underwent follow-up endoscopy 3 days later than scheduled days (five in the combination group and five in the control group). Results were similar to those observed in the per-protocol analysis, demonstrating an excellent trend (62.93% vs 50.44%, P = 0.057).
| Rebamipide/nizatidine | Nizatidine | P value | |
| FAS | |||
| Total number of patients | 121 | 118 | |
| Primary outcome | |||
| Erosion improvement | 75/121 (62.0) | 58/118 (49.2) | 0.046 |
| Secondary outcome | |||
| Erosion cure | 62/121 (51.2) | 50/118 (42.4) | 0.170 |
| Bleeding improvement | 26/35 (74.3) | 30/40 (75.0) | 0.943 |
| Erythema improvement | 38/86 (44.2) | 43/86 (50.0) | 0.445 |
| Edema cure | 22/35 (62.9) | 19/27 (70.4) | 0.535 |
| Symptom improvement | 74/121 (61.2) | 67/118 (56.8) | 0.492 |
| Erosion improvement in symptomatic subjects1 | 68/108 (63.0) | 54/109 (49.5) | 0.046 |
| PPS | |||
| Total number of patients | 110 | 108 | |
| Primary outcome | |||
| Erosion improvement | 69/110 (62.7) | 54/108 (50.0) | 0.058 |
| Secondary outcome | |||
| Erosion cure | 57/110 (51.8) | 47/108 (43.5) | 0.220 |
| Bleeding improvement | 24/33 (72.7) | 26/35 (74.3) | 0.884 |
| Erythema improvement | 33/79 (41.8) | 41/80 (51.2) | 0.231 |
| Edema cure | 20/31 (64.5) | 16/23 (69.6) | 0.697 |
| Symptom improvement | 71/110 (64.5) | 61/108 (56.5) | 0.223 |
| Erosion improvement in symptomatic subjects1 | 64/102 (62.8) | 51/104 (49.0) | 0.048 |
Both groups showed a notable endoscopic efficacy in achieving complete resolution of erosion (51.2% vs 42.4% for rebamipide/nizatidine vs nizatidine respectively, P = 0.170) and edema (62.9% vs 70.4%, P = 0.535), including improvement in bleeding (74.3% vs 75.0%, P = 0.943) and erythema (44.2% vs 50.0%, P = 0.445). Notably, no statistically significant inter-group disparities were discerned. Regarding patients’ symptomatic outcomes, approximately 60% of individuals in both cohorts experienced symptom amelioration, with no statistically significant inter-group variance noted (61.2% vs 56.8%, P = 0.492). Additionally, in patients exhibiting symptoms, the rebamipide/nizatidine group [63.0% (68/108)] revealed a significantly greater improvement in erosion compared to the nizatidine group [49.5% (54/109)] (P = 0.046) (Table 3).
The incidence rate of treatment-emergent AEs was 12.50% (16/128 cases, 22 events) in the test group and 14.40% (18/125 cases, 21 events) in the control group after drug administration, with no statistically significant difference between the groups regarding treatment-emergent AEs incidence (P = 0.7144). The ADR incidence rate was 2.34% (3/128 cases, four events) in the test group and 1.60% (2/125 cases, three events) in the control group, with no statistically significant difference between the groups regarding ADR incidence (P = 1.0000). No serious AEs occurred in the test group, while it was 0.80% (1/125 cases, one event) in the control group, with no statistically significant difference between the groups regarding serious AEs incidence (P = 0.4941). No severe ADRs were observed, and the incidence rates of AEs leading to discontinuation of administration were 1.56% (2/128 cases, two events) in the test group and 0.80% (1/125 cases, two events) in the control group, with no statistically significant difference between the groups regarding the incidence of AEs leading to the discontinuation of administration (P = 1.0000) (Table 4).
| Variables | Rebamipide/nizatidine (n = 128) | Nizatidine (n = 125) |
| Total | 3 (2.34) | 2 (1.60) |
| Gastrointestinal disorders | 3 (2.34) | 1 (0.80) |
| Diarrhea | 2 (1.56) | 0 |
| Dyspepsia | 0 | 1 (0.80) |
| Epigastric discomfort | 1 (0.78) | 0 |
| Nausea | 1 (0.78) | 0 |
| Immune system disorders | 0 | 1 (0.80) |
| Drug hypersensitivity | 0 | 1 (0.80) |
| Skin and subcutaneous tissue disorders | 0 | 1 (0.80) |
| Yellow skin | 0 | 1 (0.80) |
This clinical trial confirmed that rebamipide/nizatidine combination therapy for 2 weeks had a superior erosion healing effect compared to nizatidine monotherapy. The test group demonstrated a higher efficacy of 62.0% compared to 49.2% in the control group with statistical significance (P = 0.046) according to the FAS analysis. The PPS analysis results were similar to the FAS results [test group 62.73% (69/110 participants), control group 50.00%, P = 0.058]. Prior clinical investigations have indicated the efficacy of rebamipide on erosions ranging from 30% to 50% when administered in isolation, with comparable efficiency rates noted in the nizatidine monotherapy group in our study[8,13,16]. Hence, it is reasonable to infer that the combined rebamipide/nizatidine therapy surpasses the efficacy of either nizatidine or rebamipide alone.
Moreover, upon analyzing efficacy according to the presence of subjective symptoms, a statistically significant contrast emerged between the treatment groups regarding mucosal healing efficacy in participants experiencing such symptoms. These findings indicate that the concurrent administration of rebamipide and nizatidine may offer potential benefits in alleviating mucosal healing compared to the solitary use of nizatidine for patients diagnosed with acute and chronic gastritis manifesting subjective symptoms. Currently, gastritis treatment targets to control gastritis-associated symptoms such as epigastric pain, nausea/vomiting, and abdominal distension. Acid suppressants, gastrointestinal motility modulators, antacids or gastric mucoprotective agents are widely employed in real-world situations.
In controlling gastritis symptoms, acid suppressants such as PPIs or H2RAs are somewhat effective. Between these two options, PPIs are regarded as an effective treatment option. However, there are some weaknesses reported with PPIs, including a slow onset of action due to the pharmaceutical limitation of prodrugs, a diminished inhibitory effect on gastric acid secretion when administered after a meal, and difficulty in controlling nocturnal acid breakthrough. Moreover, oxyntic and enterochromaffin-like cell hyperplasia, including hypergastrinemia, can occur as a result of PPI use. Hence, H2RAs play a role in this situation: They are prompt, safe, and convenient.
Gastric mucoprotective agents, whether employed in isolation or in conjunction with acid suppressants, present a versatile therapeutic modality. Rebamipide, an amino acid derivative, has shown efficacy in bolstering the gastric mucosa’s defense mechanisms. Clinical reports suggest that it exerts inhibitory effects on reactive oxygen species and stimulates the production of prostaglandins and the prostaglandin EP4 receptor. Consequently, these actions contribute to the reduction of gastric acid secretion and the enhancement of mucus glycoprotein synthesis. Additionally, rebamipide facilitates the healing of ulcers and tissue regeneration via angiogenesis. Moreover, it activates endothelial growth factor and its receptor, fostering cell proliferation and reepithelialization[9].
Notably, single agent therapy with acid suppressants or mucoprotective agents has exhibited limited therapeutic effects in endoscopic gastritis. In this study, it was confirmed that combining acid suppressants and mucoprotective agents would be more effective in the treatment of gastritis. The rebamipide/nizatidine combination showed superior efficacy in ameliorating mucosal erosion. Among patients reporting subjective symptoms, this observed trend was consistent. It can be presumed that this is because, compared to the effects of a single agent on gastritis, a synergy was achieved via the attenuation of aggressive factors through the suppression of gastric acid secretion, promotion of protective factor production, and other numerous effects. According to these findings, the concurrent administration of combination therapy emerges as a highly favorable approach in the treatment of gastritis.
This study has some limitations. First, our study cohort comprised individuals of a singular ethnicity, specifically Koreans. Consequently, when extrapolated to a global context, the generalizability of the findings may vary. Nonetheless, given the widespread utilization of nizatidine and rebamipide[16] in other regions, particularly across Asia, it is plausible that similar outcomes may be anticipated. In the future, conducting studies on a population with more diverse ethnicities is expected to help analyze the effectiveness of the combination therapy. Second, gastritis was defined based on endoscopic observations, incorporating four principal indicators of inflammation: Erosion, edema, erythema, and hemorrhage. The primary endoscopic manifestation was erosion[1]. Erosion is reported to have a higher interobserver agreement among the endoscopic findings of gastritis and is therefore considered appropriate as a primary outcome parameter before and after treatment. Additionally, in this study, independent investigators meticulously reviewed key endoscopic images, thereby mitigating interobserver discrepancies and facilitating a consistent diagnosis of gastritis.
This study represents the first investigation into the efficacy and safety of H2RA and mucoprotective agent combination therapy for the treatment of gastritis. While previous studies have evaluated the individual effects of H2RAs and mucoprotective agents separately, our findings underscore the superiority of combining these agents over H2RA monotherapy. Moreover, the combination therapy showed an enhanced efficacy in participants experiencing gastritis symptoms, highlighting its efficacy in symptom management.
The rebamipide/nizatidine combination presents a promising novel option for the treatment of gastritis.
| 1. | Laine L, Cohen H, Sloane R, Marin-Sorensen M, Weinstein WM. Interobserver agreement and predictive value of endoscopic findings for H. pylori and gastritis in normal volunteers. Gastrointest Endosc. 1995;42:420-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Park HK, Kim N, Lee SW, Park JJ, Kim JI, Lee SY, Cha HM, Kim H, Park SH, Shim KN, Kim SE, Hong SJ, Chung IK, Baik GH, Kim HS, Kim S, Seong JK, Seo GS, Jee SR, Moon JS, Kim JW, Chung MG, Park SM, Nah BK, Nam SY, Seo KS, Ko BS, Jo YJ, Jang JY, Kim BG, Kim JW, Park KS, Park HS, Kim SY, Lim SH, Kim CH, Park MJ, Yim JY, Cho KR, Kim D, Park SJ, Song GA, Kim HJ, Kim SW, Im EH, Lee KS, Hyun DH, Kim SH, Kim SM, Shin JE, Park CG, Yang CH, Park SH, Jung HC, Chung IS. The distribution of endoscopic gastritis in 25,536 heath check-up subjects in Korea. Korean J Helicobacter Up Gastrointest Res. 2012;12:237-243. [RCA] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Kikuchi T, Hirano K, Genda T, Tsuzura H, Sato S, Kanemitsu Y, Narita Y, Iijima K, Ichida T. A study of the effects of saliva stimulation by nizatidine on dry mouth symptoms of primary biliary cirrhosis. World J Hepatol. 2013;5:90-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Nin T, Umemoto M, Negoro A, Miuchi S, Sakagami M. Nizatidine enhances salivary secretion in patients with dry mouth. Auris Nasus Larynx. 2008;35:224-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Parente F, Bianchi Porro G. Acid inhibitory characteristics of nizatidine in man: an overview. Scand J Gastroenterol Suppl. 1994;206:3-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Callaghan JT, Bergstrom RF, Rubin A, Chernish S, Crabtree R, Knadler MP, Obermeyer B, Offen WW, Schneck DW, Aronoff G. A pharmacokinetic profile of nizatidine in man. Scand J Gastroenterol Suppl. 1987;136:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Hou D, Yang M, Hu Z, Yang L. Effects of rebamipide for chronic atrophic gastritis: A protocol for systematic review and meta-analysis. Medicine (Baltimore). 2020;99:e20620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Kim GH, Lee HL, Joo MK, Park HJ, Jung SW, Lee OJ, Kim H, Chun HJ, Lee ST, Kim JW, Jeon HH, Chung IK, Kim HS, Lee DH, Kim KO, Lim YJ, Park SJ, Cho SJ, Kim BW, Ko KH, Jeon SW, Kim JG, Sung IK, Kim TN, Sung JK, Park JJ. Efficacy and Safety of Rebamipide versus Its New Formulation, AD-203, in Patients with Erosive Gastritis: A Randomized, Double-Blind, Active Control, Noninferiority, Multicenter, Phase 3 Study. Gut Liver. 2021;15:841-850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Arakawa T, Kobayashi K, Yoshikawa T, Tarnawski A. Rebamipide: overview of its mechanisms of action and efficacy in mucosal protection and ulcer healing. Dig Dis Sci. 1998;43:5S-13S. [PubMed] |
| 10. | Katz PO, Dunbar KB, Schnoll-Sussman FH, Greer KB, Yadlapati R, Spechler SJ. ACG Clinical Guideline for the Diagnosis and Management of Gastroesophageal Reflux Disease. Am J Gastroenterol. 2022;117:27-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 463] [Article Influence: 154.3] [Reference Citation Analysis (1)] |
| 11. | Jang E, Park M, Jeong JE, Lee JY, Kim MG. Frequently reported adverse events of rebamipide compared to other drugs for peptic ulcer and gastroesophageal reflux disease. Sci Rep. 2022;12:7839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Seol SY, Kim MH, Ryu JS, Choi MG, Shin DW, Ahn BO. DA-9601 for erosive gastritis: results of a double-blind placebo-controlled phase III clinical trial. World J Gastroenterol. 2004;10:2379-2382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Choi YJ, Lee DH, Choi MG, Lee SJ, Kim SK, Song GA, Rhee PL, Jung HY, Kang DH, Lee YC, Lee SH, Choi SC, Shim KN, Seol SY, Moon JS, Shin YW, Kim HS, Lee ST, Cho JW, Choi EK, Lee OY, Jang JS. Evaluation of the Efficacy and Safety of DA-9601 versus Its New Formulation, DA-5204, in Patients with Gastritis: Phase III, Randomized, Double-Blind, Non-Inferiority Study. J Korean Med Sci. 2017;32:1807-1813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Shim KN, Kim JI, Kim N, Kim SG, Jo YJ, Hong SJ, Shin JE, Kim GH, Park KS, Choi SC, Kwon JG, Kim JH, Kim HJ, Kim JW. The efficacy and safety of irsogladine maleate in nonsteroidal anti-inflammatory drug or aspirin-induced peptic ulcer and gastritis. Korean J Intern Med. 2019;34:1008-1021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Moon JS, Park SH, Park JJ, Lee SW, Lee DH, Lee YC, Jung HY, Kim JG, Lee OY, Kim JJ. Therapeutic Efficacy of Gliptide (Sulglycotide 200 mg): A Double Blinded, Randomized, Active Drug Comparative, Multicenter Study. Korean J Helicobacter Up Gastrointest Res. 2013;13:173. [DOI] [Full Text] |
| 16. | Jaafar MH, Safi SZ, Tan MP, Rampal S, Mahadeva S. Efficacy of Rebamipide in Organic and Functional Dyspepsia: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2018;63:1250-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |