Published online Dec 14, 2024. doi: 10.3748/wjg.v30.i46.4937
Revised: October 22, 2024
Accepted: October 30, 2024
Published online: December 14, 2024
Processing time: 62 Days and 6.2 Hours
Chronic biliary disease, including cholangitis and cholecystitis, is attributed to ascending infection by intestinal bacteria. Development of a mouse model for bile duct inflammation is imperative for the advancement of novel therapeutic approaches. Current models fail to replicate the harmful bacterial influx to the biliary tract observed in humans and spread of inflammation to the liver. There
To establish a cholecystoduodenal anastomosis model capable of mimicking the mechanisms of ascending infection and inflammation observed in human biliary diseases.
We established a mouse biliary disease model by directly connecting the gallbladder and duodenum, enabling ascending infection into the biliary tract without traversing the sphincter of Oddi.
In the cholecystoduodenal anastomosis mouse model, we observed impaired epithelial structure, wall thickening, and macrophage recruitment in the gallbladder. Despite the absence of postoperative antibiotics, we detected no changes in serum proinflammatory cytokine levels, indicating no systemic inflammation. Moreover, patency between the gallbladder and duodenum was confirmed via common bile duct ligation. Injection of patient-derived pathogenic bacteria into bile duct-ligated mice led to ascending infection, which significantly increased proinflammatory cytokine mRNA expression in the liver, duodenum, and ileum. These results indicate that our mouse model exhibited a direct connection between the gallbladder and duodenum, leading to ascending infection and closely mimicking the clinical features of biliary diseases observed in humans.
The cholecystoduodenal anastomosis mouse model is an effective chronic biliary disease model with significant relevance in the development of microbiome-based therapies for the prevention and treatment of biliary disease.
Core Tip: This study introduces a novel cholecystoduodenal anastomosis mouse model to replicate the ascending infection and inflammation observed in human biliary diseases without the use of antibiotics. The model demonstrates localized biliary inflammation and microbial dysbiosis, particularly involving Escherichia coli and Enterococcus, closely mimicking human conditions. This model offers a valuable platform for investigating microbiome-based therapies aimed at preventing and treating chronic biliary diseases.
- Citation: Jang Y, Kim JY, Han SY, Park A, Baek SJ, Lee G, Kang J, Ryu H, Kim SH. Establishment of a chronic biliary disease mouse model with cholecystoduodenal anastomosis for intestinal microbiome preservation. World J Gastroenterol 2024; 30(46): 4937-4946
- URL: https://www.wjgnet.com/1007-9327/full/v30/i46/4937.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i46.4937
Gallbladder cancer originates from epithelial cells lining the gallbladder that surround the bile ducts[1]. In South Korea, the mortality rate for gallbladder cancer accounts for approximately 20% of all cancers, making it the most common cancer worldwide[2]. Chronic biliary disease can be a causative factor for gallbladder cancer[3]. Influx of bacterial strains from the intestine is the leading cause of biliary diseases, including cholecystitis[4]. The sphincter of Oddi is a muscular, ring-shaped structure located between the common bile duct and duodenum. It regulates the passage of pancreatic secretions and bile into the duodenum[5]. Cholecystitis can occur when the sphincter of Oddi becomes lax, allowing for ascending infection with strains that induce inflammation, leading to chronic inflammatory conditions[5]. Additionally, it can develop in individuals who have undergone endoscopic retrograde cholangiopancreatography (ERCP)[6,7]. To date, cholecystitis has only been treated surgically[8] and cholangitis is treated with ERCP[9], which may increase the risk of cholecystitis or surgical intervention. Therefore, to develop therapies for biliary diseases, development and optimization of animal models that can faithfully replicate biliary tract inflammation in humans is crucial.
The term "microbiome", referring to microorganisms residing within the body, has gained increasing attention in the field of medicine due to its growing significance in the onset and treatment of various diseases, including neurological[10,11] and gastrointestinal[12,13] disorders. Microbial dysbiosis in the gallbladder is associated with gallbladder diseases[14,15]. Dysbiosis is induced by bacteria, such as Escherichia coli (E. coli) and Klebsiella, leading to decreased beta diversity[14,15]. Consequently, there is a pressing need to develop animal models to study the microbiome in biliary diseases. Currently employed models for gallbladder cancer involve ligating the cystic duct or promoting the formation of gallstones[16-18]; however, they fail to replicate the harmful bacterial influx observed in humans.
As previously reported, a single hepatic cell comprises a network of ducts, portal veins, and arterial connections. Therefore, cholecystitis has been reported to potentially lead to the propagation of inflammation in hepatic cells through adjacent ducts and arteries[19]. Current reports on animal models of cholecystitis include an acute model in which the cystic duct is ligated by suturing, followed by assessment of the gallbladder condition[16]. Additionally, there are models in which inflammation increases owing to the blockade of the cystic duct caused by the formation of gallstones[17], and scavenger receptor A knockout mice, which have acute cholecystitis-like phenotypes after cecal ligation and puncture as well as hypercholesterolemia[20]. In case of cholangitis, the dominant-negative TGF-βRII mouse model exhibits defects in the TGF-β signaling pathway, resulting in biliary inflammation and liver fibrosis[21]. However, current models fail to replicate the bacterial influx from the intestine to the biliary tract observed in humans and the spread of inflammation through the biliary tract to the liver. In this study, we present a model that allows for the influx of intestinal bacteria through a direct connection between the duodenum and gallbladder and not through the sphincter barrier, while excluding external factors, and enables the chronic confirmation of local inflammation in the biliary tract.
In terms of the microbiome, our biliary disease mouse model did not involve the use of antibiotics. This not only avoids influencing the composition of the microbiome population but also serves as a crucial model for the development of therapeutic approaches for biliary disease and biliary cancer. In this study, we aimed to address the limitations associated with microbiome alterations caused by antibiotic use, using a novel model of gallbladder inflammation. We aimed to establish a foundation for future therapeutic development and optimize an animal model of gallbladder inflammation using the gallbladder-duodenum anastomosis method.
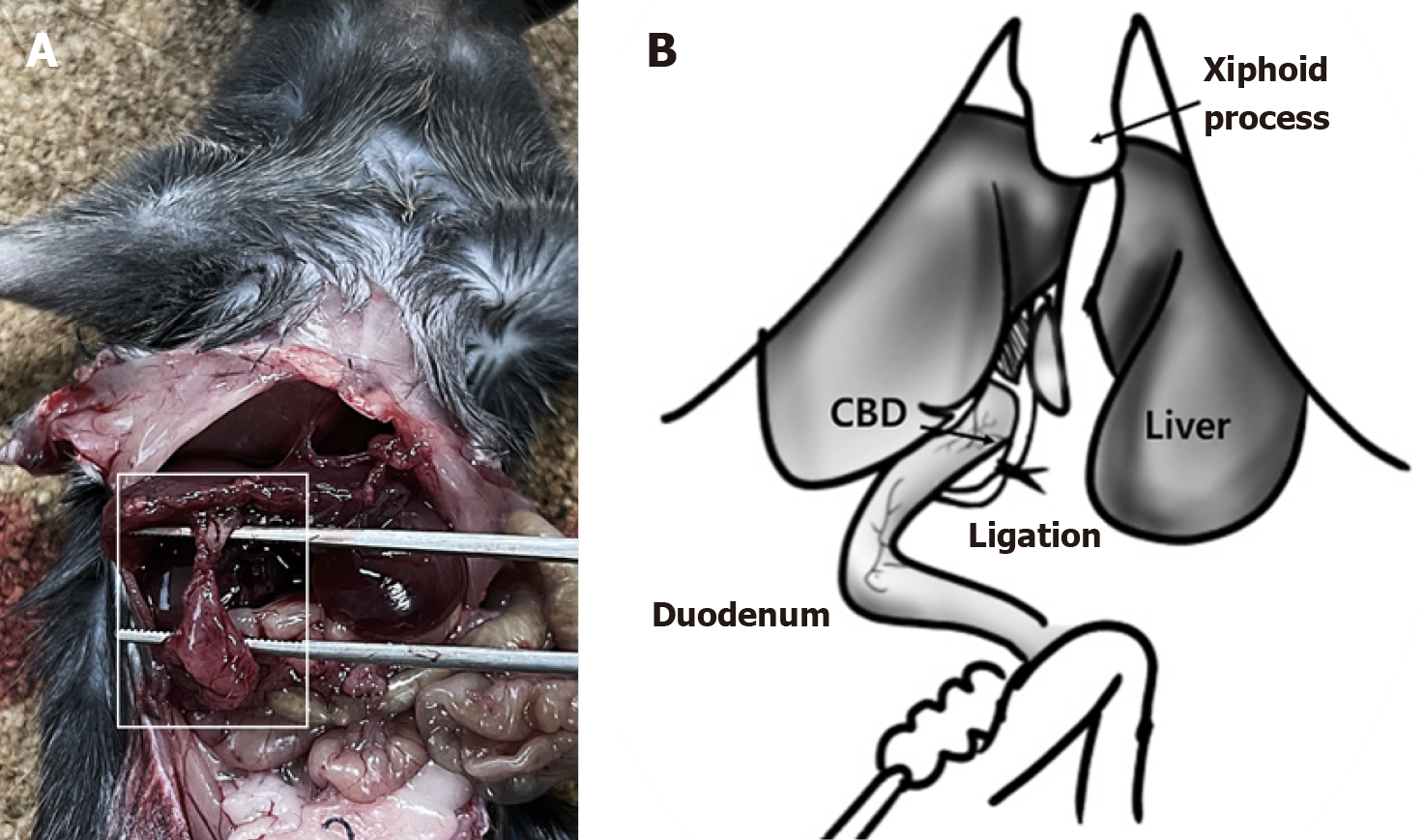
C57BL/6 mice (7-week-old, male) were maintained at 22 °C, and light conditions were adjusted to a 12 hours light-dark cycle. Animal experiments were approved by the Institutional Animal Care and Use Committee of Chungnam National University (approval number, CNUH-2023-IA0103-00; approval date, November 20, 2023). To establish a cholecystoduodenal anastomosis mouse model, we completely removed the abdominal hair of C57BL/6 male mice using clippers and razors and induced and maintained general anesthesia with a 5% mixture of sevoflurane and oxygen (n = 30, each group). An aqueous solution containing povidone iodine (75 mg/mL) was applied to the skin, followed by a 5 cm skin incision from the xiphoid process to the upper bladder. The xiphoid process was tagged using 3-0 black silk, pulled, and tagged symmetrically 1.5 cm apart. Subsequently, the common bile duct was ligated using 8-0 prolene. Sequentially, the gallbladder was separated from the liver, allowing the fundus to bend toward the duodenum. The bottom part of the gallbladder was trimmed to form a circular cross-section. A 2 mm linear incision was made at the first bend of the duodenum using microscissors. Using 8-0 prolene, we connected the gallbladder to the duodenum at the 12 o'clock (also referred to as 0°), 5 o'clock (or 150°), and 9 o'clock (or 270°) positions in an interrupted manner and then closed the skin in a continuous manner using 3-0 nylon.
Serum level of tumor necrosis factor-α (TNF-α), interleukin (IL)-6 and IL-1β (n = 7, each group) was measured using ELISA kit (Abcam, Cambridge, United Kingdom) We confirmed cytokine expression using serum obtained from co-cultures of liver organoids and fibroblasts. Media was collected and centrifuged at 1500 rpm at 4 °C for 10 minutes, then the supernatant was collected and stored at -80 °C. The cytokines analyzed included IL-6, IL-10, IFN-γ and TNF-α, which were detected using ELISA kit (R&D Systems, MN, United States). The assays were conducted in accordance with the manufacturer's instructions.
Gallbladder of cholecystoduodenal anastomosis and control mice were isolated and fixed with 40 g/L paraformaldehyde, then paraffin embedded. 5-μm sections were cut and after deparaffinization, stained with hematoxylin for 5 minutes. Then, sections were washed with running water 5 minutes, stained with eosin for 2 minutes. Serial washing with ethanol and xylene was proceeded. Images were acquired with a light microscope (Olympus, Tokyo, Japan). For CD68 immunohistochemical staining, sections were deparaffinized and blocked for 30 minutes with protein blocking buffer (DAKO, Glostrup, Denmark). After incubation with anti-CD68 antibody (Abcam, MA, United States), sections were washed with TBS/T buffer and incubated with DAB. Intracellular CD68 (n = 10 slides/ condition) was visualized using a light microscope (Olympus, Tokyo, Japan).
Total RNA of mice liver, duodenum and ileum was isolated using Trizol reagent (Invitrogen, MA, United States). Total RNA of mice bile was isolated using RNeasy Mini Kit (Qiagen, Hilden, Germany) according to manufacturer’s instruction. cDNA was synthesized using ReverTra Ace-α-TM (Toyobo, Osaka, Japan), according to the manufacturer's instructions. cDNA, forward and reverse primers (10 pmole each), 2X SYBR Master Mix (Appliedbiosystems, MA, United States), and distilled water (DW) were prepared for mixture. mRNA expression levels were determined using a QuantStudio5 (Applied biosystems, MA, United States). The expression level of each gene was normalized to GAPDH and represented as a relative expression and mean ± SEM (error bars). Primers used in this study: TNF-α, 5′-CAG AGG GCC TGT ACC TCA TC-3′ (forward) and 5′-GGA AGA CCC CTC CCA GAT AG-3′ (reverse); Il-1β, 5′-CAG GCA GGC AGT ATC ACT CA-3′ (forward) and 5′-TGT CCT CAT CCT GGA AGG TC-3′ (reverse); IL-6, 5′-ATA TTA GAG TCT CAA CCC CCA ATA A-3′ (forward) and 5′-GAG CTT CTC TTT CGT TCC CG-3′ (reverse); E.coli, 5′-CAT GCC GCG TGT ATG AAG AA-3′ (forward) and 5′-CGG GTA ACG TCA ATG AGC AAA-3′ (reverse); EIEC (ipaH) 5′-ACC ATG CTC GCA GAG AAA CT-3′ (forward) and 5′-TAC GCT TCA GTA CAG CAT GC-3′ (reverse); Enterococcus 5′-CGG AGA CTA CAC AAT TTG TTT TT-3′ (forward) and 5′-CGG TTG GGT TTT GAT CCT T-3′ (reverse).
Statistical analyses were performed using Prism version 8 (GraphPad, CA, United States). Data are presented as the mean ± SE of the mean. The significance of differences between the control and experimental groups was analyzed using a one-tailed student's t-test. Statistical significance was set at P < 0.05.
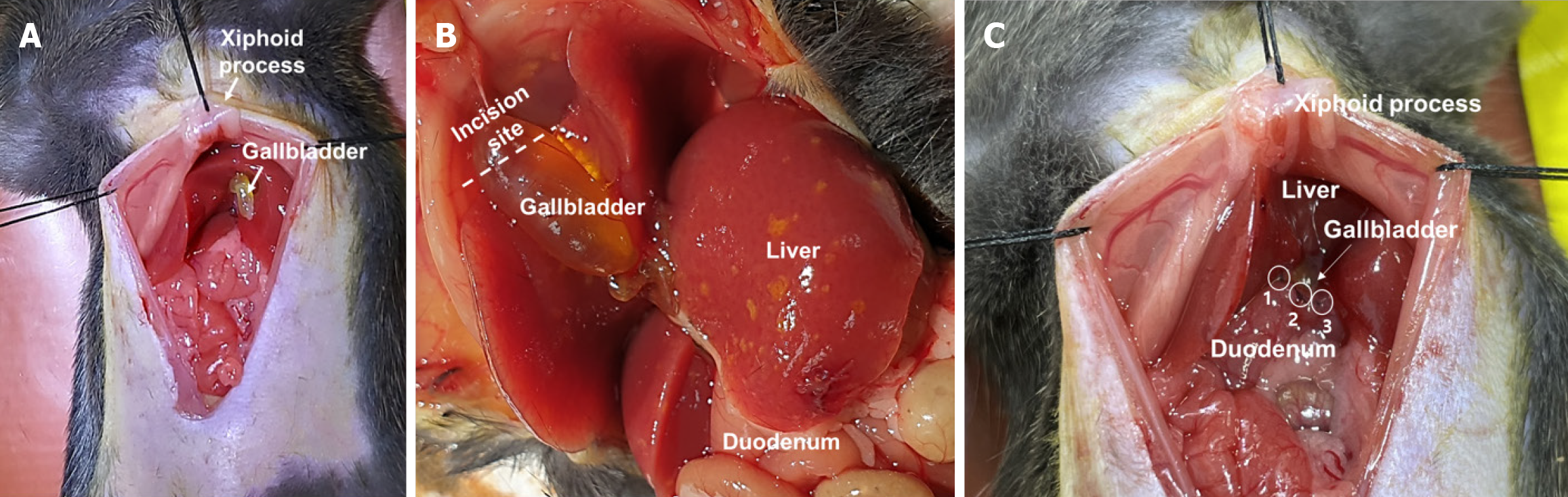
In this study, we developed a novel model of gallbladder inflammation by directly anastomosing the gallbladder and duodenum (Figure 1). First, a 5-cm skin incision was made from the xiphoid process to the upper bladder under anesthesia with a 5% mixture of sevoflurane and oxygen. Subsequently, the common bile duct was obstructed. In cases where ligation of the gallbladder and duodenum fails, this procedure is essential for maintaining patency between the gallbladder and duodenum to ensure survival because the absence of a pathway for bile discharge can lead to mortality. The fundus portion of the gallbladder was then separated from the liver, and the bottom of the gallbladder was cross-sectioned. The section of the gallbladder must be circular, and the first bend of the duodenum must be cut linearly by approximately 2 mm. Continuous suturing during surgery (Supplementary Figure 1) often results in gallbladder rupture, leading to bile leakage, intraperitoneal infection, and subsequent mortality. We employed an interrupted suturing technique to mitigate this risk. Therefore, the gallbladder was connected to the duodenum at three positions (0°, 150°, and 270°) (Figure 1C). Thus, we developed the first cholecystoduodenal anastomosis mouse model without antibiotics.
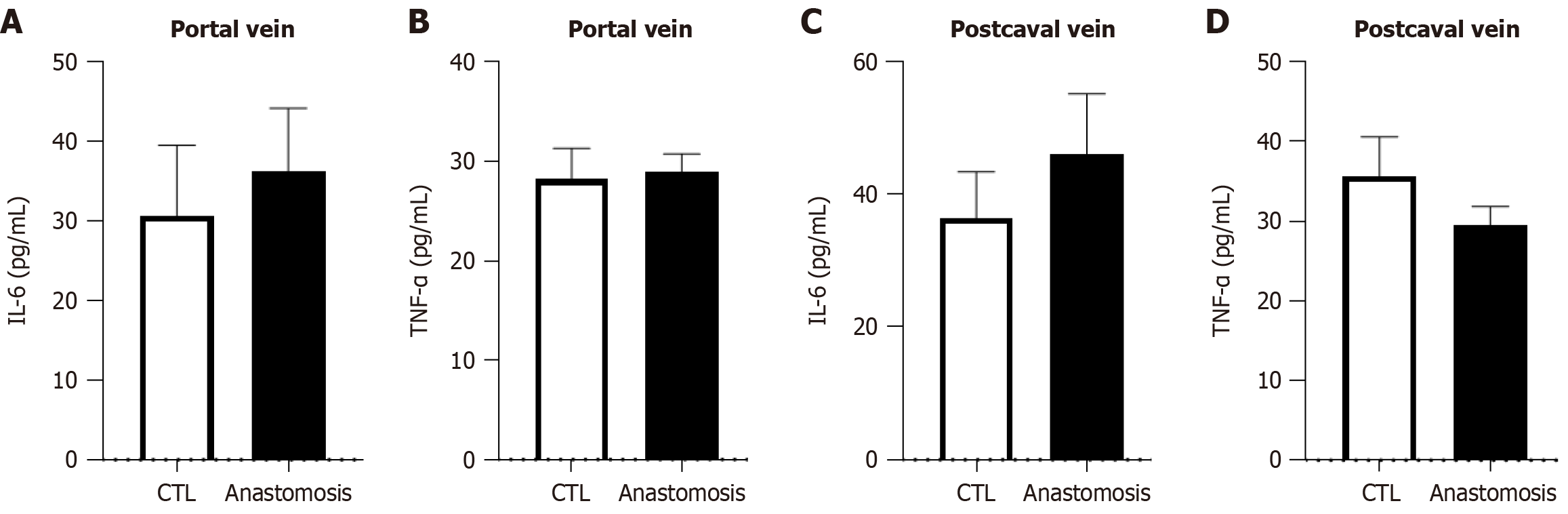
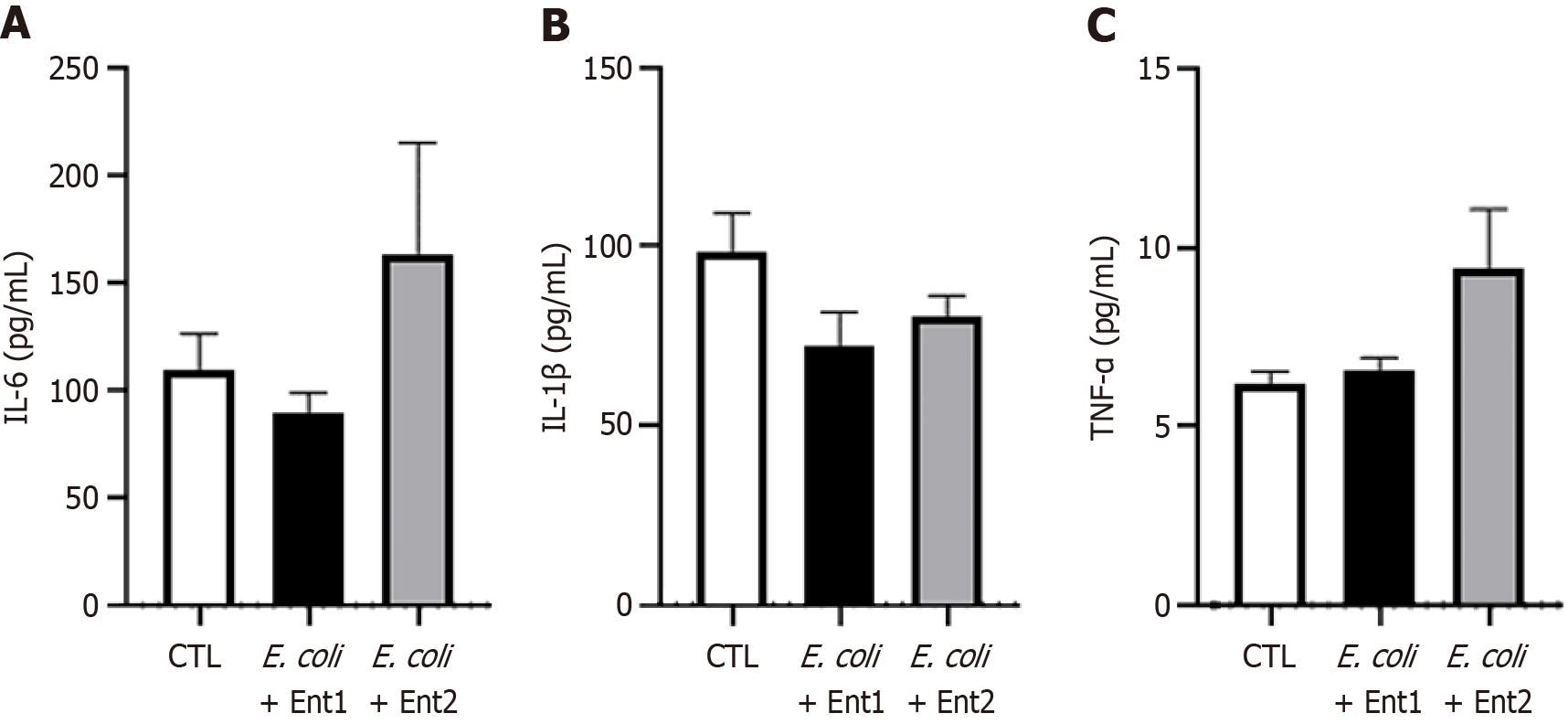
We evaluated the establishment of the cholecystoduodenal anastomosis model on postoperative day 14. We observed a connection between the gallbladder and duodenum without bile acid leakage (Figure 2A). Furthermore, we performed common bile duct ligation to validate patency between the gallbladder and duodenum (Figure 2B). Failure to connect the gallbladder to the duodenum would render no route for bile acid emission, leading to the death of mice. In patients with chronic biliary disease, systemic inflammation is not observed; however, localized inflammation of the biliary tract increases[22]. As chronic biliary disease progresses, changes in the inflammatory state can be observed using histological staining. Increased expression of inflammatory cytokines in the bloodstream serves as a crucial factor in predicting postsurgical outcomes and is a necessary parameter for evaluating the validity of the cholecystoduodenal anastomosis mouse model. After surgery that connected the gallbladder and duodenum, we observed no significant differences in the concentrations of proinflammatory cytokines (IL-6 and TNF-α) in blood samples collected from the portal vein and postcaval vein, as measured using enzyme-linked immunosorbent assay (Figure 3). Thus, there was no increase in systemic inflammatory responses due to intra-abdominal inflammation even without the use of antibiotics after surgery. Consequently, we propose that this animal model may serve as an important resource for future microbiome studies.
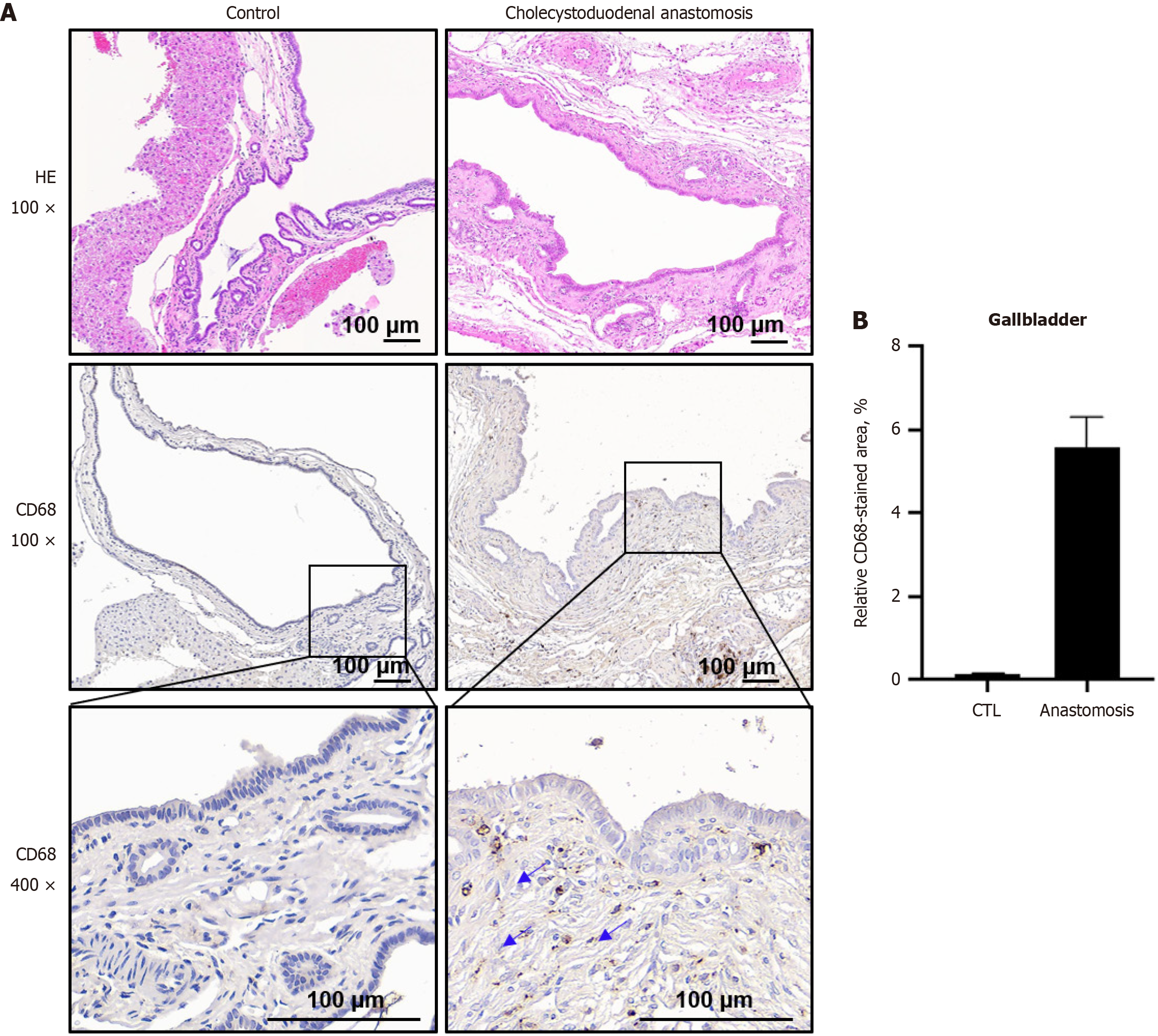
Next, we aimed to confirm biliary disease via immunohistochemical analysis of the gallbladder tissues. The histological features of biliary disease and normal gallbladder tissues were assessed using hematoxylin and eosin staining. In the normal gallbladder epithelium, simple columnar epithelial cells and submucosa remained intact (Figure 4). In contrast, gallbladders of cholecystoduodenal anastomosis mice displayed impaired epithelial structures, characterized by thickening of the epithelial wall and extension of the mucosal folds, compared with control mice (Figure 4), which is indicative of inflammation. Subsequently, immunohistochemistry (IHC) for the pro-inflammatory marker CD68 was performed on gallbladder tissues from both control and cholecystoduodenal anastomosis mice (Figure 4A). We observed a significant increase in CD68-positive cells in the gallbladders of cholecystoduodenal anastomosis mice (Figure 4A and B), suggesting an influx of macrophages and an associated inflammatory response. These findings are consistent with the histopathological features observed in patients with gallbladder inflammation in clinical settings. Therefore, our cholecystoduodenal anastomosis mouse model appeared to induce localized inflammation within the gallbladder without triggering systemic inflammation, even in the absence of antibiotic treatment. Moreover, this model did not disrupt the overall composition of the gut microbiome. Hence, we propose that this model is a valuable platform for evaluating the effectiveness of probiotics in managing gut microbiome imbalance and improving chronic biliary disease.
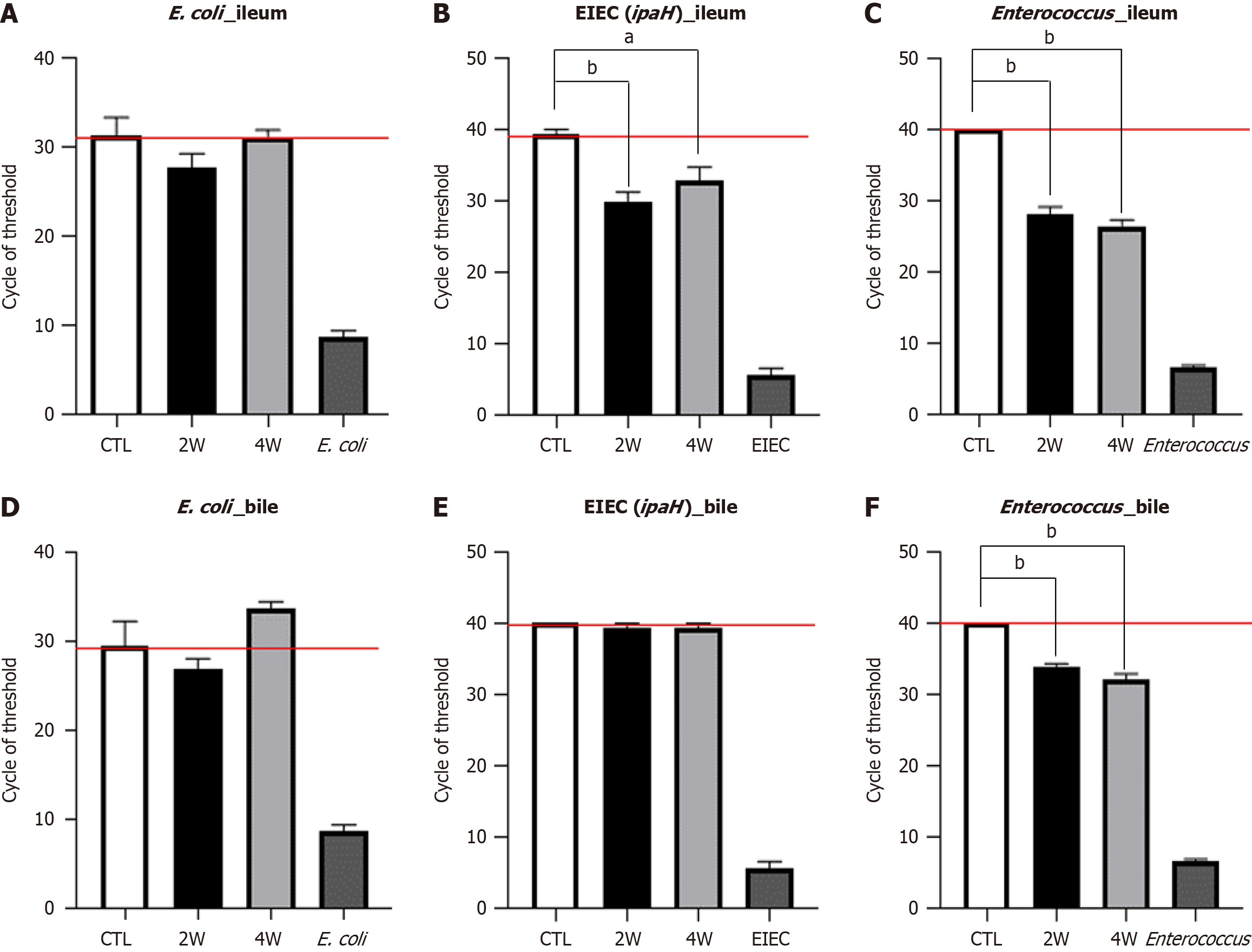
We ligated the common bile duct and confirmed patency between the gallbladder and duodenum. We then injected harmful bacteria originating from humans, including Enterococcus and E. coli, into cholecystoduodenal anastomosis mice. Two months after surgery, the Enterococcus and E. coli mixture was injected once daily via oral gavage for 14 days. Bacterial gene expression was confirmed using quantitative reverse transcription-polymerase chain reaction. The expression of the E. coli-specific 16S rRNA gene was comparable in the ileum and bile after bacterial injection (Figure 5A-D). In contrast, the expression of the EIEC-specific ipaH gene increased in the ileum but remained unchanged in the bile (Figure 5B and E).
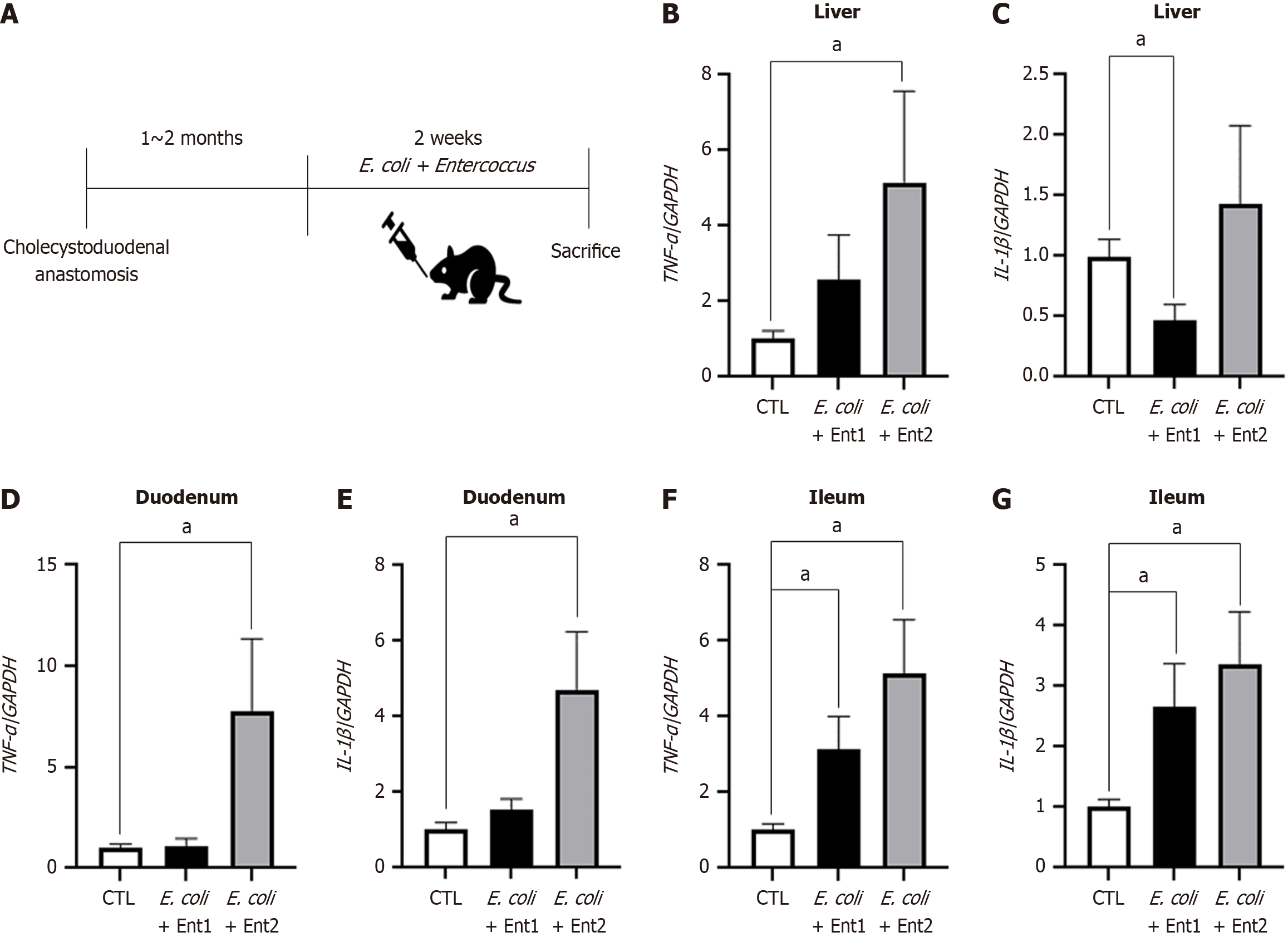
Additionally, Enterococcus-specific 16S rRNA gene expression was significantly elevated in both the ileum and bile of cholecystoduodenal anastomosis mice, with a cycle threshold lower than that in control mice (Figure 5C and F). These results suggested that oral bacterial injection into cholecystoduodenal anastomosis mice causes an ascending infection owing to the direct connection between the gallbladder and duodenum. This condition results in enhanced expression of proinflammatory cytokines in the liver, duodenum, and ileum. We observed that the mRNA expression levels of proinflammatory cytokines, including TNF-α and IL-1β, were induced by bacteria-induced ascending infection (Figure 6A) in the liver, duodenum, and ileum (Figure 6B-G). In terms of systemic inflammation, serum levels of TNF-α, IL-6, and IL-1β were comparable between the bacteria-injected and control groups (Figure 7).
Chronic biliary disease is known to be a potential precursor of gallbladder cancer[3]. Gallbladder cancer accounts for 20% of cancer-related deaths in South Koreans[2]. Chronic biliary disease can be triggered by an influx of bacteria from the intestine[4]. Given that surgical intervention remains the sole treatment option for both biliary disease including cholecystitis and cholangitis and gallbladder cancer[23,24], the development of a biliary tract inflammation mouse model is imperative for the advancement of novel therapeutic approaches. In the current study, we established a cholecystoduodenal anastomosis mouse model for biliary disease by inducing ascending infection from the duodenum.
To create the cholecystoduodenal anastomosis mouse model, we performed interrupted sutures on the fundus of the gallbladder and the first part of the duodenum in three directions (0°, 150°, and 270°) using a monofilament. The cholecystoduodenal anastomosis mice survived without antibiotics, and patency between the gallbladder and duodenum was confirmed by ligation of the common bile duct. We observed that these mice had biliary disease with impaired epithelial structure and infiltrated immune cells, as confirmed via IHC using the CD68 antibody and hematoxylin and eosin staining (Figure 4). In addition, we observed that the serum levels of inflammatory cytokines, including TNF-α, IL-6, and IL-1β, remained unchanged. This indicated that cholecystoduodenal anastomosis mice did not exhibit systemic inflammation, as confirmed using serum enzyme-linked immunosorbent assay (Figure 3). These results suggested that our cholecystoduodenal anastomosis mice exhibited inflammatory traits similar to those observed clinically in patients with biliary disease.
In chronic biliary disease, dysbiosis resulting from the influx of harmful gut bacteria, such as E. coli and Klebsiella, and a reduction in beta diversity have been observed[14,15]. Therefore, we orally injected cholecystoduodenal anastomosis mice with E. coli and patient-derived Enterococcus for 14 days, 2 months after surgery. We observed that the serum levels of proinflammatory cytokines, including TNF-α, IL-6, and IL-1β, in bacteria-injected cholecystoduodenal anastomosis mice were comparable to those in control mice. TNF-α and IL-1β mRNA expression levels in the liver tissue were 4.8 folds and 20.1 folds increased in bacteria-injected mice, respectively (Figure 6). In addition, TNF-α and IL-1β mRNA levels in the duodenum and ileum were significantly increased (Figure 6). We found that the injection of bacteria induced ascending infection in cholecystoduodenal anastomosis mice because of the direct connection between the gallbladder and duodenum (Figure 7). Further analysis is required to determine whether probiotics can ameliorate dysbiosis and alleviate biliary disease. We hypothesize that in our cholecystoduodenal anastomosis mouse model designed to induce ascending infection, correcting dysbiosis through probiotic intake may reduce biliary tract inflammation.
The establishment of a gallbladder inflammation model, without the use of antibiotics, enabled microbiome research. The cholecystoduodenal anastomosis mouse model has broad applications in the development of therapies and diagnostic techniques that utilize the microbiome, particularly in biliary diseases.
The cholecystoduodenal anastomosis mouse model mimics human biliary disease, including localized inflammation from ascending infection, and serves as a valuable tool for studying microbiome-based therapies.
We thank to Professor Seok-Hwan Kim for his support and supervision of this study.
| 1. | Ebata T, Ercolani G, Alvaro D, Ribero D, Di Tommaso L, Valle JW. Current Status on Cholangiocarcinoma and Gallbladder Cancer. Liver Cancer. 2016;6:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 2. | Wi Y, Woo H, Won YJ, Jang JY, Shin A. Trends in Gallbladder Cancer Incidence and Survival in Korea. Cancer Res Treat. 2018;50:1444-1451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Kanthan R, Senger JL, Ahmed S, Kanthan SC. Gallbladder Cancer in the 21st Century. J Oncol. 2015;2015:967472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 189] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 4. | Abril AG, Villa TG, Sánchez-Pérez Á, Notario V, Carrera M. The Role of the Gallbladder, the Intestinal Barrier and the Gut Microbiota in the Development of Food Allergies and Other Disorders. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Behar J, Corazziari E, Guelrud M, Hogan W, Sherman S, Toouli J. Functional gallbladder and sphincter of oddi disorders. Gastroenterology. 2006;130:1498-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 178] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 6. | Ting PH, Luo JC, Lee KC, Chen TS, Huang YH, Hou MC, Lee FY. Post endoscopic retrograde cholangiopancreatography cholecystitis: The incidence and risk factors analysis. J Chin Med Assoc. 2020;83:733-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Khan R, Osman H, Lee S, Chen YI, Singh A, Hookey L, Arya N, Causada Calo N, Grover SC, Tepox-Padrón A, Bass S, Cole M, Lei Y, Li S, Mohamed R, Turbide C, Koury HF, Chau M, Howarth M, Cartwright S, Heitman SJ, Forbes N. Post-ERCP cholecystitis: Incidence, characteristics, and outcomes from a prospective multicenter biliary endoscopy registry. Gastrointest Endosc. 2024;99:633-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Lee SO, Yim SK. [Management of Acute Cholecystitis]. Korean J Gastroenterol. 2018;71:264-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Mohammad Alizadeh AH. Cholangitis: Diagnosis, Treatment and Prognosis. J Clin Transl Hepatol. 2017;5:404-413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Michaelis L, Berg L, Maier L. Confounder or Confederate? The Interactions Between Drugs and the Gut Microbiome in Psychiatric and Neurological Diseases. Biol Psychiatry. 2024;95:361-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Liu C, Yang SY, Wang L, Zhou F. The gut microbiome: implications for neurogenesis and neurological diseases. Neural Regen Res. 2022;17:53-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 12. | Gall A, Fero J, McCoy C, Claywell BC, Sanchez CA, Blount PL, Li X, Vaughan TL, Matsen FA, Reid BJ, Salama NR. Bacterial Composition of the Human Upper Gastrointestinal Tract Microbiome Is Dynamic and Associated with Genomic Instability in a Barrett's Esophagus Cohort. PLoS One. 2015;10:e0129055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 13. | Crnčević N, Hukić M, Deumić S, Selimagić A, Dozić A, Gavrankapetanović I, Klepo D, Avdić M. Gastrointestinal Tract Microbiome Effect and Role in Disease Development. Diseases. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Liu J, Yan Q, Luo F, Shang D, Wu D, Zhang H, Shang X, Kang X, Abdo M, Liu B, Ma Y, Xin Y. Acute cholecystitis associated with infection of Enterobacteriaceae from gut microbiota. Clin Microbiol Infect. 2015;21:851.e1-851.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Choi SJ, Kim Y, Jeon J, Gwak HJ, Kim M, Kang K, Kim Y, Jeong J, Jung YK, Lee KG, Choi HS, Jung DH, Lee SG, Lee Y, Shin SJ, Jang K, Rho M, Choi D. Association of Microbial Dysbiosis with Gallbladder Diseases Identified by Bile Microbiome Profiling. J Korean Med Sci. 2021;36:e189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Kjaer DW, Mortensen FV, Møller JK, Hamilton-Dutoit SJ, Funch-Jensen P. Internal gallbladder drainage prevents development of acute cholecystitis in a pig model: a randomized study. Ann Surg Innov Res. 2010;4:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Wang TY, Portincasa P, Liu M, Tso P, Wang DQ. Mouse models of gallstone disease. Curr Opin Gastroenterol. 2018;34:59-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Ding L, Jiang J, Cheng L, Wang Y, Zhang W, Li D, Xu Z, Jiang J, Gao L, Li Z. Oral Administration of Nanoiron Sulfide Supernatant for the Treatment of Gallbladder Stones with Chronic Cholecystitis. ACS Appl Bio Mater. 2021;4:3773-3785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Gong J, Tu W, Liu J, Tian D. Hepatocytes: A key role in liver inflammation. Front Immunol. 2022;13:1083780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 20. | Drummond R, Song D, Hawisher D, Wolf PL, Vazquez DE, Nino DF, Coimbra R, Cauvi DM, De Maio A. Acute acalculous cholecystitis-like phenotype in scavenger receptor A knock-out mice. J Surg Res. 2012;174:344-351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Wang X, Wei Y, Yang Y, Yang Y, Li H, Li Y, Zhang F, Wang L. Animal models of primary biliary cholangitis: status and challenges. Cell Biosci. 2023;13:214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 22. | Tanaka A. New Therapies on the Horizon for Primary Biliary Cholangitis. Drugs. 2024;84:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Wang J, Li Z, Chen LL, Zhao JB, Wu JL, Leng ZW. Comparing robotic and open surgical techniques in gallbladder cancer management: a detailed systematic review and meta-analysis. J Robot Surg. 2024;18:111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 24. | Krell RW, Wei AC. Gallbladder cancer: surgical management. Chin Clin Oncol. 2019;8:36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |