Published online Dec 14, 2024. doi: 10.3748/wjg.v30.i46.4904
Revised: October 10, 2024
Accepted: November 4, 2024
Published online: December 14, 2024
Processing time: 79 Days and 8 Hours
Over the last decade, the treatment options for inflammatory bowel disease (IBD) have significantly progressed with the emergence of new medications designed to target various immune pathways and mitigate inflammation. Adalimumab (ADA) is a tumor necrosis factor alpha antagonist and stands as an effective treatment for IBD. In April 2021, the province of British Columbia implemented a mandatory non-medical switch policy of the ADA originator Humira® to ADA biosimilars. Biosimilars offer a potential cost-effective, safe, and efficacious alternative to the originator, yet there remains limited real-world evidence on long-term outcomes of ADA non-medical switching in IBD.
To assess the long-term outcomes of non-medical switching from the ADA originator Humira® to an ADA biosimilar among IBD patients.
A retrospective observational chart review study was conducted on IBD patients eligible for the provincially mandated non-medical switch to an ADA biosimilar. The primary outcome was treatment persistence at 30 months post-switch. Secondary outcomes included the proportion of and reasons for therapy alteration or ADA discontinuation, loss of response (LOR) rates, adverse events (AE), and clinical and biochemical remission status. Patients who remained on the originator throughout the switch period, through compassionate support or private pay, constituted the comparison group.
Patients in the originator (n = 43) and biosimilar switch (n = 228) groups displayed similar demographics and baseline disease characteristics. By the study endpoint of 30 months, there was no difference in the rate of treatment persistence in either group (n = 36, 83.7% originator group vs n = 201, 88.2% biosimilar group, P = 0.451). Treatment persistence demonstrated similar rates of discontinuation between both study groups (log-rank P = 0.543). There was a numerical but not statistically significant difference in rates of adverse outcomes between either group (39.5% originator vs 28.9% biosimilars, P = 0.206). This included comparable rates of LOR (27.9% vs 17.5%) or AE (11.6% vs 11.4%) between the originator and biosimilar cohorts, respectively. C-reactive protein and fecal calprotectin levels were similar one year pre- and post-switch.
These data support the long-term efficacy and safety of non-medical ADA switching in IBD and will help inform patients and physicians in jurisdictions currently undergoing biosimilar switching.
Core Tip: Although there have been several studies evaluating the short-term clinical outcomes of adalimumab (ADA) biosimilar switching, there has been a lack of data assessing the long-term outcomes, especially in Canada. Non-medical switching of the ADA originator to biosimilars did not result in significant differences in treatment persistence, loss of response, or adverse events compared to ADA originator continuation in inflammatory bowel disease (IBD). The findings from this real-world evidence study support the long-term efficacy and safety of non-medical ADA switching in IBD. These data will help inform patients and physicians in jurisdictions currently undergoing biosimilar switching and guide future biosimilar adoption and practice guidelines.
- Citation: Liu Chen Kiow J, Hoang T, Bedi HK, Majdzadeh Ardekani Z, Rosenfeld D, Reise-Filteau M, Bressler B, Leung Y, Rosenfeld G. Real-world experience and long-term outcomes of a mandatory non-medical switch of adalimumab originator to biosimilars in inflammatory bowel disease. World J Gastroenterol 2024; 30(46): 4904-4913
- URL: https://www.wjgnet.com/1007-9327/full/v30/i46/4904.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i46.4904
Inflammatory bowel disease (IBD), comprising Crohn’s disease (CD) and ulcerative colitis (UC), is a chronic autoimmune inflammatory disorder characterized by relapsing and remitting inflammation of the gastrointestinal tract that can lead to debilitating symptoms such as abdominal pain, diarrhea, anemia, weight loss, and fatigue[1]. The prevalence and incidence of IBD in Canada are among the highest in the world, with a reported prevalence of 735 per 100000 (95% PI 716-735) in 2018[2]. IBD is considered a lifelong condition with no cure. Current treatment options target symptom manage
Over the last decade, the therapeutic landscape of IBD has evolved considerably with the development of novel drugs targeting various immune pathways to mitigate inflammation[4]. Tumor necrosis factor alpha (TNF-α) antagonist therapies stand as effective therapies for the treatment of moderate-to-severe IBD. Adalimumab (ADA) is a recombinant human IgG1 monoclonal antibody that binds specifically to human TNF-α, a pro-inflammatory cytokine involved in the pathogenesis of IBD, inhibiting its interaction with p55 and p75 TNF-α receptors[5]. Approved by Health Canada in December 2007 for the treatment of CD and in November 2013 for the treatment of UC, the patent for the original biologic agent, Humira® (AbbVie), expired in 2018[6].
The expiration of Humira®’s patent led to the development of biosimilar drugs, which are pharmacokinetically similar to their originators[7]. Health Canada implements rigorous approval processes for biosimilars as for their originator molecules; however, in part, because fewer nonclinical and clinical studies are often required for the approval of biosimilars, manufacturers are able offer them at a lower cost[7]. With their presumed comparable efficacy and safety, biosimilars may offer a cost-effective alternative to originator biologics, addressing the significant healthcare expenditures associated with IBD medications[8]. In an effort to reduce costs, the Biosimilars Initiative of Canada, implemented by PharmaCare in May 2019, mandated the non-medical switch from originator biologics to biosimilars. This policy targeted a few anti-TNF agents including infliximab, an originator drug sold under the trade name Remicade®, and its Health Canada approved biosimilars (CTP-13 or SB2) which had become available in 2017[9]. Subsequently, a similar mandatory non-medical switch policy was implemented from Humira® to 5 approved ADA biosimilars (Amgevita, Hadlima, Hulio, Hyrimoz or Idacio) from April 7, 2021 to October 6, 2021. Stable patients on publicly funded Humira® were required to switch to a biosimilar.
There remains a lack of real-world evidence on the comparative safety and effectiveness of long-term biosimilar switching, especially in Canada. Canadian data on biosimilar switching are different compared to data from other regions globally due to unique Patient Support Programs (PSP) associated with the advanced therapies used to treat IBD. These PSP are tailored to each medication and play a fundamental role in patient care. When a patient switches to a different biologic or biosimilar, the accompanying PSP also changes accordingly. Therefore, certain variations in PSP dynamics can influence patient outcomes, contributing to potential differences observed in clinical outcomes among countries.
This study aimed to fill this gap by assessing the impact of non-medical ADA biosimilar switching on long-term clinical outcomes for IBD patients using patient level data from electronic health records, hospital administrative databases, and publicly funded insurance programs. Specifically, this study investigated treatment persistence, adverse events (AE), and loss of response (LOR) following the non-medical switch policy between Humira® and the ADA biosimilars. The findings from this study have the potential to inform policy decisions in other jurisdictions and guide future biosimilar adoption in IBD therapy.
This single-centre retrospective, observational study was conducted on patients at the IBD Centre of British Columbia (IBDCBC), a major IBD tertiary referral centre in Vancouver, Canada affiliated with St. Paul’s Hospital and the University of British Columbia. Eligible patients undergoing the switch from Humira® to a biosimilar between January 1, 2020 and April 31, 2023 were identified from the IBDCBC’s electronic medical record, the IBD Data Lake, and the biosimilar’s respective PSP. The IBD Data Lake is a novel data repository of all our IBD patients, utilizing machine learning and natural language processing techniques as tools to curate and retrieve relevant clinical data in real-time. Our research group at the IBDCBC developed the IBD Data Lake, which is currently undergoing rigorous validation in comparison to our electronic medical records and other established standards.
Patients were categorized into two groups: The ADA biosimilar group, subjected to a mandatory non-medical switch from Humira® to Amgevita, Hadlima, Hulio, Hyrimoz or Idacio, and the control/originator group, who continued with Humira®. Only patients receiving publicly funded Humira® were required to switch to a biosimilar, while others remained on Humira® and served as the control group. Although a waiver of consent was granted, informed consent was obtained from most participants to include their data in our IBD database. The study adhered to the STROBE Statement and was approved by the University of British Columbia Providence Health Care Research Ethics Board.
The study included patients, 18 years or older, diagnosed with IBD and receiving ADA treatment at the IBDCBC. Patients had an established diagnosis of CD or UC by a gastroenterologist specialized in IBD, based on standard clinical, endoscopic, and histological criteria. Patients must have been in symptomatic remission, and on ongoing Humira® therapy before switching to a biosimilar, receiving at least one dose of the biosimilar at the usual dosing interval during the mandated switch period of April 7 to October 6, 2021. Symptomatic remission was defined as partial mayo score < 2 for UC and Harvey-Bradshaw Index < 5 for CD[10,11]. Concurrent use of immunomodulators or corticosteroids was permitted.
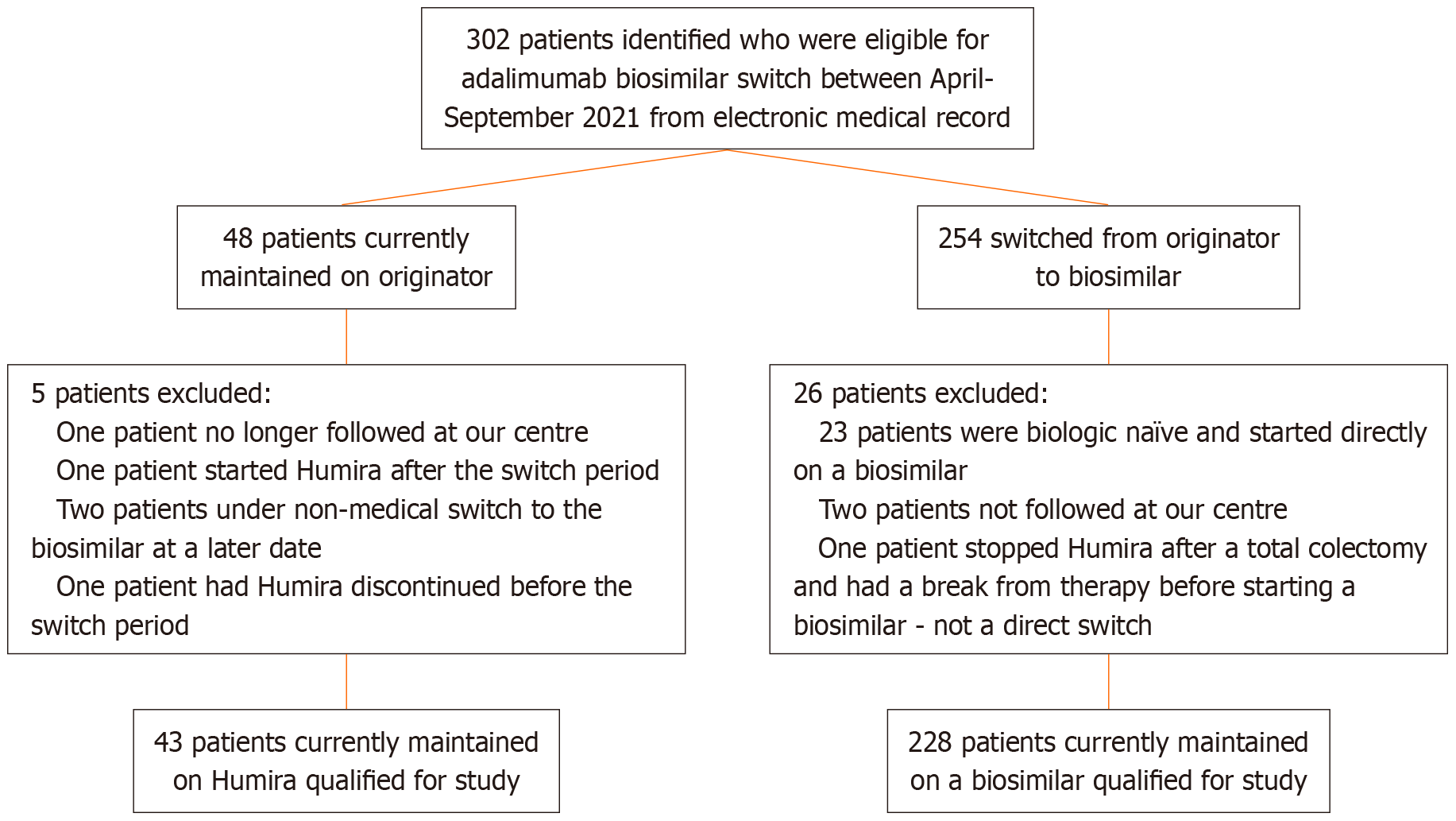
Exclusion criteria included inadequate follow-up to assess biosimilar continuation, discontinuation of ADA due to LOR or AE, refusal to receive a biosimilar, and patients initiating a biosimilar as initial therapy for their IBD.
Each participant was assigned a unique study identification number and information was extracted from the IBDCBC electronic medical record and IBD Data Lake. Collected data encompassed demographic features, smoking history, disease characteristics (IBD type, location, severity, behavior for CD, and extraintestinal manifestations), duration on Humira® pre-switch, endoscopy reports when available, CRP and fecal calprotectin levels pre and post switch, and concurrent use of 5-aminosalysilic acid, steroids, and immunomodulators (azathioprine and methotrexate). Post-switch clinical outcomes were also documented by AE, dose alterations, ADA discontinuation and reasons for discontinuation, initiation of immunomodulators or additional biologics, clinical remission status, as well as emergency department (ED) visits and hospitalizations related to biosimilar drug. Missing data were searched for by investigators. Participant data were collected up to 30 months post-switch when available.
The primary outcome of the study was the persistence of ADA in the biosimilar group at 30 months post-switch, compared to the control group, where persistence was assessed following July 2021, the mid-point of the switch period. Persistence of biologic treatment was defined as ongoing ADA injections noted in injection reports or clinical notes. Secondary outcomes included reasons for discontinuation, duration to ADA biosimilar discontinuation or therapy change, therapy modifications, clinical remission rates, ED/hospital admissions, and fecal calprotectin levels.
Categorical variables were expressed as proportions and analyzed using Pearson’s χ2 test or Fisher’s Exact Test. Continuous variables were expressed as means ± SD (or ± standard error of the mean) and analyzed via Mann-Whitney U test or Kruskall-Wallis ANOVA. Changes in CRP and FCP pre-and post-switch were assessed using paired sample t-tests. Treatment persistence was evaluated with Kaplan-Meier survival analyses and compared using pairwise two-sided log-rank tests. Multivariate Cox proportional hazards regression adjusted for pre-switch Humira® duration, IBD subtype, and concurrent immunomodulator use. P values < 0.05 indicated significance.
During the mandated ADA biosimilar switch period from April 7, 2021 to October 6, 2021, 271 patients satisfied inclusion criteria (Figure 1). The originator group consisted of 43 patients who remained on Humira® through private pay or compassionate coverage, while the 228 participated in the switch formed the biosimilars group. Both groups displayed comparable baseline characteristics, aside from age at time of switch (Table 1). Of the total study cohort, 227 patients had CD (83.8%) and 44 had UC (16.2%). This is reflected in the originator group which consisted of 35 (81.4%) CD and 8 (18.6%) UC patients, and the biosimilars group with 192 (84.2%) CD and 36 (15.8%) UC patients. Both cohorts had similar disease severity and phenotype, except a slight increase in age of diagnoses > 40 (A3) in the biosimilar switch group. The biosimilar group consisted of 27 (11.8%) on MSB11022 (Idacio), 63 (27.6%) on SB5 (Hadlima), 86 (37.7%) on FKB327 (Hulio), 48 (21.1%) on GP2017 (Hyrimoz), and 4 (1.74%) on ABP501 (Amgevita). The biosimilars group had a numerically shorter, but not statistically different pre-switch duration on ADA compared to the originator cohort (64.5 ± 40.3 vs 89.5 ± 206.0 months, P = 0.719).
| Adalimumab originator | Adalimumab biosimilar switch | P value | |
| Male gender | 20 (46.5) | 128 (56.1) | 0.249 |
| Age at switch (years ± SD) | 46.1 ± 15.3 | 40.6 ± 14.9 | < 0.001 |
| Smoking | 6 (14.0) | 19 (8.33) | 0.264 |
| Age at inflammatory bowel disease diagnosis (years ± SD) | 32.1 ± 15.7 | 26.3 ± 12.7 | 0.135 |
| Crohn’s disease | 35 (81.4) | 192 (84.2) | 0.654 |
| Age at diagnosis | |||
| A1 (≤ 16) | 4 (11.4) | 47 (24.5) | 0.122 |
| A2 (17-40) | 20 (57.1) | 120 (62.5) | 0.574 |
| A3 (> 40) | 11 (31.4) | 26 (13.5) | 0.011 |
| Location | |||
| L1 (ileal) | 12 (34.3) | 69 (35.9) | 1 |
| L2 (colonic) | 8 (22.9) | 41 (21.4) | 0.825 |
| L3 (ileocolonic) | 15 (42.9) | 78 (40.6) | 0.853 |
| L4 (isolated upper tract disease) | 1 (2.86) | 1 (0.52) | 0.285 |
| Behaviour | |||
| B1 (non-stricturing, non-penetrating) | 10 (28.6) | 70 (36.4) | 0.444 |
| B2 (stricturing) | 11 (31.4) | 53 (27.6) | 0.684 |
| B3 (penetrating) | 2 (5.71) | 38 (19.8) | 0.052 |
| Perianal disease modifier | 11 (31.4) | 65 (33.9) | 0.847 |
| Ulcerative colitis | 8 (18.6) | 36 (15.8) | |
| E1 (ulcerative proctitis) | 1 (12.5) | 1 (2.78) | 0.334 |
| E2 (left-sided colitis) | 1 (12.5) | 9 (25.0) | 0.659 |
| E3 (extensive colitis) | 6 (75.0) | 26 (72.2) | 1 |
| Extraintestinal manifestations | |||
| Skin | 3 (6.98) | 27 (11.8) | 0.437 |
| Joint | 6 (14.0) | 38 (16.7) | 0.823 |
| Eye | 4 (9.30) | 10 (4.38) | 0.249 |
| Primary sclerosing cholangitis | 0 | 1 (0.44) | 1 |
| Concurrent immunomodulator therapy (azathioprine/methotrexate) | 4 (9.30) | 39 (17.1) | 0.258 |
| Concurrent corticosteroid use | 3 (6.98) | 11 (4.82) | 0.473 |
| Duration on adalimumab prior to switch (months ± SD) | 89.5 ± 206.0 | 64.5 ± 40.3 | 0.719 |
| Pre-switch C-reactive protein (mg/L ± SD) | 2.21 ± 1.99 | 6.46 ± 22.0 | 0.359 |
| Pre-switch fecal calprotectin (mcg/g ± SD) | 215.6 ± 279.7 | 203.8 ± 413.6 | 0.972 |
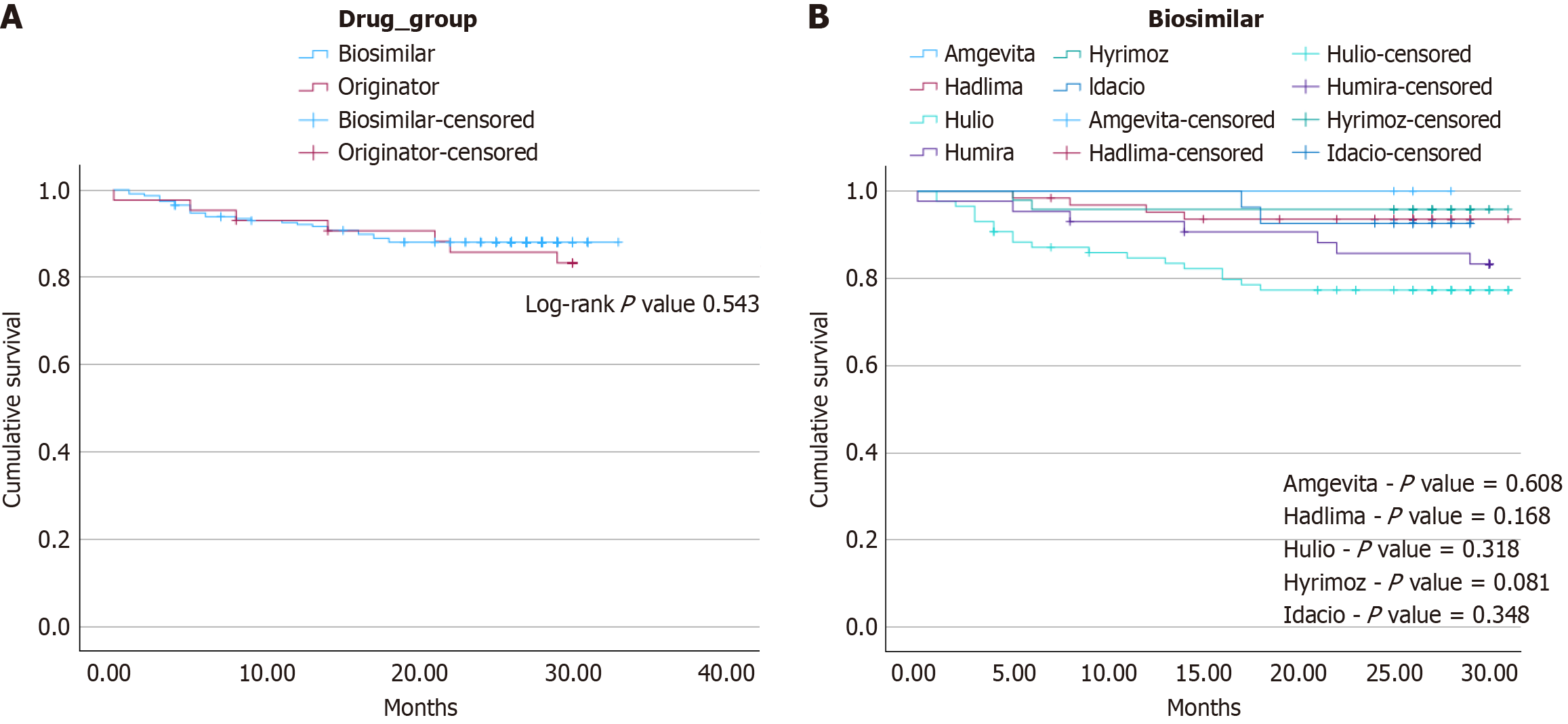
Data was collected up to an endpoint of January 30, 2024. Clinical outcomes were documented up to the first 30 months of follow-up post-switch. To mitigate possible confounders, Cox regression via proportional hazards model was performed to control for IBD type [hazard ratio (HR) = 0.984, confidence interval (CI): 0.40-2.43, P = 0.97), concurrent immunomodulator use (HR = 1.35, CI: 0.58-3.13, P = 0.49), concurrent corticosteroid use (HR = 1.71, CI: 0.50-5.80, P = 0.39) and pre-switch ADA duration (HR 1.0, CI: 0.99-1.01, P = 0.74), all of which showed no statistically significant effect. While the study endpoint was 30 months, the mean duration of ADA continuation following July 7, 2021 (middle of the switch period) was 26.5 ± 7.92 months in the originator group compared to the mean post-switch biosimilar duration of 24.4 ± 7.07 months in the comparator group. Overall, there was no statistically significant difference in treatment persistence by the study endpoint between each group (36, 83.7% originators vs 201, 88.2% switchers, P = 0.451). Treatment persistence assessed via Kaplan-Meier survival analysis again demonstrated similar rates of discontinuation between both study groups (Figure 2A, log-rank P value = 0.543). Specific reasons for discontinuation were categorized into LOR, AE, and other patient factors and are summarized in Table 2. A subgroup of the survival analysis with pair-wise comparisons of each individual biosimilar again showed no statistically significant differences in rates of treatment persistence by 30 months compared to the originator controls (Figure 2B). Patients who switched to Hyrimoz demonstrated more reliable treatment persistence by 30 months compared to the originator controls, though this difference was not statistically significant (log-rank P value = 0.081). Only Hulio demonstrated decreased treatment persistence by study endpoint, though this was not statistically significant (log-rank P value = 0.318). Table 3 shows similar post-switch biosimilar durations between all sub-groups, and outlines reasons for treatment discontinuation.
| Adalimumab originator (n = 43) | Adalimumab biosimilar switch (n = 228) | P value | |
| Duration on biosimilar after switch (months ± SD) | - | 24.4 ± 7.08 | - |
| Discontinuation of adalimumab | 7 (16.3) | 27 (11.8) | 0.451 |
| Reason for discontinuation | |||
| Loss of response | 2 (4.65) | 14 (6.11) | 1 |
| Adverse events | 2 (4.65) | 10 (4.39) | 1 |
| Other patient factors | 3 (6.98)1 | 3 (1.31)2 | 0.053 |
| Adalimumab originator (n = 43) | Idacio (MSB11022; | Hadlima (SB5; n = 63) | Hulio (FKB327; | Hyrimoz (GP2017; | Amgevita | P value | |
| Duration on biosimilar after switch (months ± SD) | - | 26.0 ± 2.83 | 24.9 ± 5.32 | 22.3 ± 9.52 | 26.5 ± 4.64 | 26.3 ± 1.26 | 0.131 |
| Discontinuation of adalimumab | 7 (16.3) | 2 (7.41) | 4 (6.35) | 19 (22.1) | 2 (4.17) | 0 | |
| Reason for discontinuation | |||||||
| Loss of response | 2 (4.65) | 1 (3.70) | 1 (1.59) | 12 (14.0) | 0 | 0 | |
| Adverse events | 2 (4.65) | 0 | 3 (4.76) | 5 (5.81) | 2 (4.17) | 0 | |
| Other patient factors | 3 (6.98) | 1 (3.70) | 0 | 2 (2.33) | 0 | 0 |
A subgroup analysis was performed on patients with CD and UC and summarized in Table 4. The 227 CD patients consisted of 192 switchers (84.6%) and 35 originators (15.4%). Within each subgroup, there was no difference in the treatment persistence between switchers and originators (CD: 88.5% vs 82.9% P = 0.399, UC: 86.1 vs 87.5% P = 1.0) respectively. There was additionally no difference in treatment persistence rates between CD or UC patients (87.7% vs 86.3% P = 0.276).
| Inflammatory bowel disease subtype | Treatment persistence by 30 months | Treatment discontinuation by 30 months | Total | P value | ||
| Ulcerative colitis | Drug group | Biosimilar | 31 (86.1) | 5 (13.9) | 36 | 1.0 |
| Originator | 7 (87.5) | 1 (12.5) | 8 | |||
| Total | 36 | 6 | 44 | |||
| Crohn’s disease | Drug group | Biosimilar | 170 (88.5) | 22 (11.5) | 192 | 0.400 |
| Originator | 29 (82.9) | 6 (17.1) | 35 | |||
| Total | 199 | 28 | 227 | |||
| Total | Drug group | Biosimilar | 201 (88.2) | 27 (11.8) | 228 | 0.451 |
| Originator | 36 (83.7) | 7 (16.3) | 43 | |||
| Total | 237 | 34 | 271 | |||
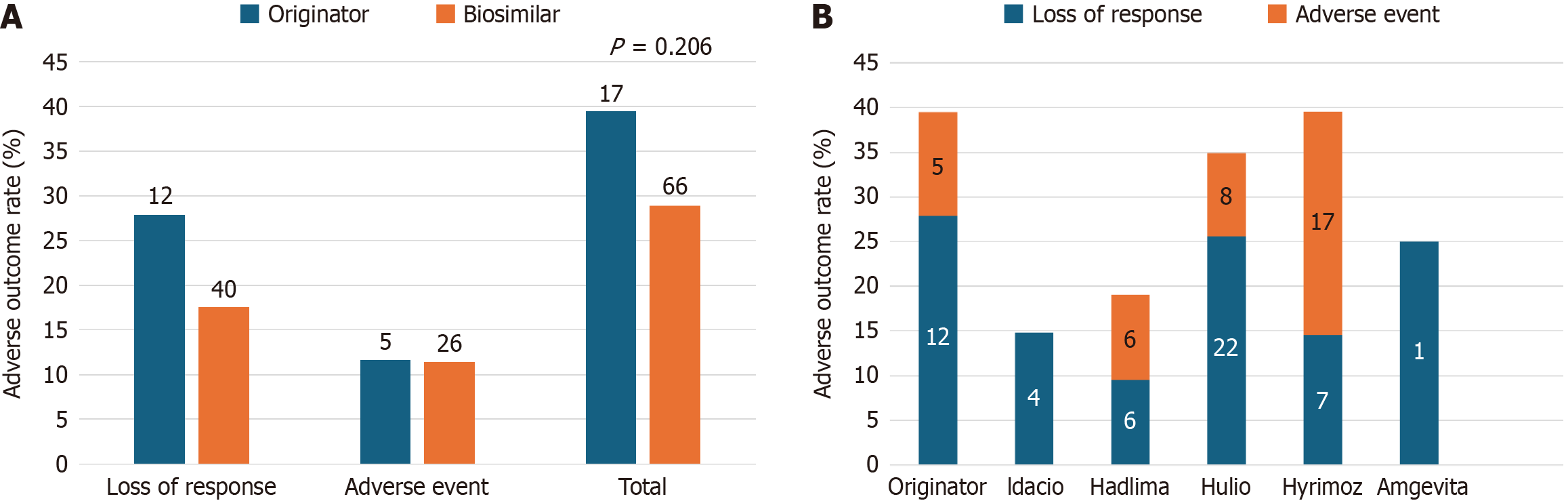
The two adverse outcomes of interest for this study were LOR and medication-related AE, regardless of whether the outcome resulted in treatment discontinuation. Adverse outcome rates for both groups are highlighted in Figure 3A, showing no statistically significant difference (n = 17, 39.5% originator vs n = 66, 28.9% biosimilars, P = 0.206). Together this led to five hospitalizations (11.6%) in the originator group and thirteen hospitalizations (5.7%) in the biosimilars group (P = 0.173). Similar adverse outcome rates separated by individual biosimilars are highlighted in Figure 3B.
In total, 12 patients (27.9%) in the control group developed LOR to Humira® following the mid-switch period which led to treatment discontinuation in two patients. One patient went on to total colectomy and another required an out-of-class biologic switch. Of the patients who did not cease treatment, two achieved disease response with dose escalation while the remainder are still pending further follow up by the study end point. Four originator patients (9.3%) presented to ED and required hospitalization specifically for disease flare secondary to LOR. In comparison, 40 patients (17.5%) in the biosimilar group developed symptoms consistent with LOR following biosimilar switching, leading to treatment cessation in 14 patients (6.1%). Of these patients, ten subsequently switched out of class, one patient switched back to the originator, two were treated with corticosteroids, and one had treatment cessation due to a new diagnosis of an appendiceal malignancy during management of flare symptoms requiring chemotherapy. Among the 26 patients who remained on treatment, disease re-capture was obtained in nine patients who underwent dose escalation, seven who began corticosteroids, and one who required immunomodulator re-initiation. The remaining nine patients are still pending further follow-up by study endpoint. Twelve switchers (5.26%) required ED visit and subsequent hospitalization for symptomatic flares secondary to LOR.
Five (11.6%) patients in the originator group developed AE (Figure 3A) leading to two treatment discontinuations (Table 2). One patient required treatment discontinuation for severe psoriasis requiring initiation of methotrexate and cessation of ADA. The second patient developed significant fatigue and was switched to vedolizumab. The remaining patients developed non-specific symptoms including polyarthalgias, mild pustular eruption, and loose bowel movements that were managed medically and they were continued on ADA. The biosimilars group had 26 patients (11.4 %) who developed AE (Figure 3A) leading to ten discontinuations (Table 2). Among these ten patients, five underwent an out-of-class switch and two switched to another biosimilar. The remaining three patients discontinued biologic therapy following their adverse event without clear plans to restart therapy by study endpoint. The remaining patients who did not discontinue treatment had mild non-specific symptoms that were again managed medically, and they remained on ADA. Only one originator patient presented to ED for generalized fatigue and irritable bowel-related symptoms thought to be secondary to ADA itself. No ED visits for treatment AE were documented in the biosimilars group.
This retrospective observational study using real-world data from a large tertiary IBD referral centre showed that switching from the ADA originator Humira® to one of the approved ADA biosimilars was not associated with treatment discontinuation or adverse outcomes in IBD patients on maintenance therapy. Notably, the rates of treatment persistence, LOR, and AE were not significantly different between IBD patients who stayed on Humira®vs those who switched to a biosimilar after 2.5 years of follow-up.
Evidence from previous studies support the safety and efficacy of switching between biosimilars and originator biologics in patients with immune-mediated inflammatory disorders. A recent meta-analysis which incorporated findings from 31 studies (5252 patients) comprising 44 distinct switch treatment periods for 21 different biosimilars showed that transitioning to a biosimilar did not alter the risk of treatment discontinuation, serious AE, or mortality for patients with immune-mediated inflammatory disorders[12]. Furthermore, several studies have been conducted to determine the outcomes of ADA biosimilar switching in IBD, with favourable experiences predominately from Europe. The ADA-SWITCH study was a large observational multicentre study in Spain that compared treatment persistence, clinical efficacy, and adverse outcomes between IBD patients who continued Humira® and those who transitioned to a biosimilar. After 13 months of follow-up in the biosimilar switch cohort, the incidence rate of ADA discontinuation, relapse, and AE were not significantly different from the originator cohort[13]. A prospective observational study investigating the impact on switching from an ADA biosimilar to another biosimilar, including multiple switching, showed that after 6 months, 82% of 61 patients achieved the primary outcome of switch success, defined as the absence of systemic corticosteroid use within that time period, non-discontinuation of biosimilar, and no need for dosage escalation[14]. Additionally, the short-term experience from a tertiary Czech IBD Centre found no disparity in disease activity, AE, and fecal calprotectin or CRP levels between patients on the ADA originator and those who switched to an ADA biosimilar after 10 weeks[15].
The reported treatment persistence in our study of 83.7% and 88.2% in the originator and biosimilar switch groups, respectively, is certainly comparable to the published literature on this topic. In fact, a recent systematic review on anti-TNF biosimilar switching, which included 11 real-world observational studies of adult IBD patients on ADA, revealed that out of the 86% of patients in clinical remission at the time of the ADA switch, 82% maintained remission at the 6-month follow-up[16]. We did not experience the nocebo effect, a well-known phenomenon reported in other studies involving IBD patients undergoing a biosimilar switch[17]. Interestingly, there was instead a numerical but not statistically significant difference in our reported rate of treatment persistence by 30 months, with the originator group having more discontinuations (16.3% vs 11.8%) and total adverse outcomes (39.5% vs 28.9%). This may be the result of our study being underpowered because of the limited number of patients in the originator group. Subgroup analyses were conducted by stratifying according to IBD type and biosimilar, revealing no difference in the rates of treatment persistence. This is anticipated since there is evidence to support that ADA biosimilars are equally effective and that switching, including multiples switches, from ADA biosimilar to biosimilar is safe and effective[14].
The main strength of our study is the relatively long follow-up period, which incorporates a control group who stayed on the ADA originator. This extended duration allows for a more comprehensive evaluation of the long-term outcomes of persistence, effectiveness, and safety of ADA biosimilar switching. We also used data from a Canadian IBD tertiary centre with extensive experience in managing one of the largest IBD cohorts in Canada. To address potential confounding factors, we conducted Cox regression using a proportional hazards model to adjust for several variables including type of IBD, concurrent use of immunomodulators and corticosteroids, as well as the duration of pre-switch ADA treatment. Moreover, it is important to acknowledge the unique context of the Canadian healthcare system, where the majority of biologic patients are covered by a public payer. Unlike much of the other published real-world data, our study involved a mandatory biosimilar switch rather than a voluntary one. It is also worth noting another distinction in our study is that it assessed a switch to all available ADA biosimilars, rather than focusing on just one. The specific PSP associated with the different biologics in Canada can significantly influence patient outcomes and contribute to differences in clinical data compared to other countries. For that reason, our study contributes novel insights to the existing evidence in this area. Finally, to the best of our knowledge, our study is unique as it is the first Canadian study to establish the long-term (over 2.5 years) efficacy and safety of non-medical ADA biosimilar switch in IBD, with an extended follow-up period to assess the durability and sustainability of treatment outcomes. Expectedly, our results are mostly consistent with previous ADA biosimilar switch studies in IBD evaluating short-term outcomes.
We acknowledge several limitations. This is a retrospective study and therefore we were not able to comment on endoscopic remission due to the insufficient endoscopic outcomes in these patients. Nonetheless, it's important to note that this limitation stems from the real-world nature of our study, wherein only a minority of patients in sustained symptomatic remission receiving maintenance ADA therapy underwent endoscopic examination during follow-up. Medical notes from IBD providers were used to determine clinical remission (partial Mayo score < 2 for UC and Harvey-Bradshaw index < 5 for CD), and we used the fecal calprotectin as an endoscopy surrogate marker, which corroborated the safety and efficacy of the switch. Although both groups had mostly similar baseline characteristics, the ADA originator group was slightly older at the time of the switch, which could be a possible confounding factor. Another potential limitation of our study pertains to the relatively small sample size of the originator group. These patients remained on the originator medication throughout the switch period due to compassionate support or private payment, factors over which we had no control. Our study may have lacked sufficient statistical power to make conclusive remarks regarding the observed numerical differences in treatment discontinuation and overall adverse outcomes, which interestingly favoured the biosimilar switch cohort. Given that the cohorts primarily included CD patients, the potential to generalize our results to UC may be weaker compared to that for CD. Since trough levels and antibodies against ADA were not easily accessible, we were unable to assess any pharmacokinetic disparities between the originator and biosimilars.
Taken together, non-medical switching of the ADA originator to an ADA biosimilar did not result in significant differences in treatment persistence, LOR, or AE compared to ADA originator continuation for patients with IBD. The findings from this real-world evidence study support the long-term efficacy and safety of non-medical ADA switching in IBD. This will help inform patients and physicians in jurisdictions currently undergoing biosimilar switching and guide future biosimilar adoption and practice guidelines in the ever-evolving world of IBD therapy.
| 1. | Benchimol EI, Bernstein CN, Bitton A, Murthy SK, Nguyen GC, Lee K, Cooke-Lauder J, Siddiq S, Windsor JW, Carroll MW, Coward S, El-Matary W, Griffiths AM, Jones JL, Kuenzig ME, Lee L, Mack DR, Mawani M, Otley AR, Singh H, Targownik LE, Weizman AV, Kaplan GG. The Impact of Inflammatory Bowel Disease in Canada 2018: A Scientific Report from the Canadian Gastro-Intestinal Epidemiology Consortium to Crohn's and Colitis Canada. J Can Assoc Gastroenterol. 2019;2:S1-S5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 2. | Coward S, Clement F, Benchimol EI, Bernstein CN, Avina-Zubieta JA, Bitton A, Carroll MW, Hazlewood G, Jacobson K, Jelinski S, Deardon R, Jones JL, Kuenzig ME, Leddin D, McBrien KA, Murthy SK, Nguyen GC, Otley AR, Panaccione R, Rezaie A, Rosenfeld G, Peña-Sánchez JN, Singh H, Targownik LE, Kaplan GG. Past and Future Burden of Inflammatory Bowel Diseases Based on Modeling of Population-Based Data. Gastroenterology. 2019;156:1345-1353.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 321] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 3. | Turner D, Ricciuto A, Lewis A, D'Amico F, Dhaliwal J, Griffiths AM, Bettenworth D, Sandborn WJ, Sands BE, Reinisch W, Schölmerich J, Bemelman W, Danese S, Mary JY, Rubin D, Colombel JF, Peyrin-Biroulet L, Dotan I, Abreu MT, Dignass A; International Organization for the Study of IBD. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology. 2021;160:1570-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 1632] [Article Influence: 408.0] [Reference Citation Analysis (1)] |
| 4. | Chudy-Onwugaje KO, Christian KE, Farraye FA, Cross RK. A State-of-the-Art Review of New and Emerging Therapies for the Treatment of IBD. Inflamm Bowel Dis. 2019;25:820-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 5. | Rawla P, Sunkara T, Raj JP. Role of biologics and biosimilars in inflammatory bowel disease: current trends and future perspectives. J Inflamm Res. 2018;11:215-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 6. | Fiorino G, Gilardi D, Correale C, Furfaro F, Roda G, Loy L, Argollo M, Allocca M, Peyrin-Biroulet L, Danese S. Biosimilars of adalimumab: the upcoming challenge in IBD. Expert Opin Biol Ther. 2019;19:1023-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Government of Canada. Biosimilar biologic drugs in Canada: Fact Sheet. [cited 1 December 2023]. Available from: https://www.canada.ca/en/health-canada/services/drugs-health-products/biologics-radiopharmaceuticals-genetic-therapies/applications-submissions/guidance-documents/fact-sheet-biosimilars.html. |
| 8. | van der Valk ME, Mangen MJ, Leenders M, Dijkstra G, van Bodegraven AA, Fidder HH, de Jong DJ, Pierik M, van der Woude CJ, Romberg-Camps MJ, Clemens CH, Jansen JM, Mahmmod N, van de Meeberg PC, van der Meulen-de Jong AE, Ponsioen CY, Bolwerk CJ, Vermeijden JR, Siersema PD, van Oijen MG, Oldenburg B; COIN study group and the Dutch Initiative on Crohn and Colitis. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFα therapy: results from the COIN study. Gut. 2014;63:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 418] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 9. | British Columbia. Biosimilars Initiative for health professionals. [cited 1 December 2023]. Available from: https://www2.gov.bc.ca/gov/content/health/practitioner-professional-resources/pharmacare/prescribers/biosimilars-initiative-health-professionals. |
| 10. | Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008;14:1660-1666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 733] [Cited by in RCA: 711] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 11. | Harvey RF, Bradshaw JM. A simple index of Crohn's-disease activity. Lancet. 1980;1:514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1940] [Cited by in RCA: 2188] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 12. | Herndon TM, Ausin C, Brahme NN, Schrieber SJ, Luo M, Andrada FC, Kim C, Sun W, Zhou L, Grosser S, Yim S, Ricci MS. Safety outcomes when switching between biosimilars and reference biologics: A systematic review and meta-analysis. PLoS One. 2023;18:e0292231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 13. | Casanova MJ, Nantes Ó, Varela P, Vela-González M, Rivero M, Sierra-Gabarda O, Riestra S, Barreiro-de Acosta M, Martín-Rodríguez MDM, Gargallo-Puyuelo CJ, Reygosa C, Muñoz R, de la Filia-Molina IG, Núñez-Ortiz A, Kolle L, Calafat M, Huguet JM, Iglesias-Flores E, Martínez-Pérez TJ, Bosch O, Duque-Alcorta JM, Frago-Larramona S, Van Domselaar M, González-Cosano VM, Bujanda L, Rubio S, Mancebo A, Castro B, García-López S, de Francisco R, Nieto-García L, Laredo V, Gutiérrez-Casbas A, Mesonero F, Leo-Carnerero E, Cañete F, Ruiz L, Gros B, Del Moral-Martínez M, Rodríguez C, Chaparro M, Gisbert JP. Real-world outcomes of switching from adalimumab originator to adalimumab biosimilar in patients with inflammatory bowel disease: The ADA-SWITCH study. Aliment Pharmacol Ther. 2023;58:60-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Ribaldone DG, Tribocco E, Rosso C, Armandi A, Vernero M, Bugianesi E, Astegiano M, Saracco GM, Caviglia GP. Switching from Biosimilar to Biosimilar Adalimumab, Including Multiple Switching, in Crohn's Disease: A Prospective Study. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Lukas M, Malickova K, Kolar M, Bortlik M, Vasatko M, Machkova N, Hruba V, Duricova D, Lukas M. Switching From Originator Adalimumab to the Biosimilar SB5 in Patients With Inflammatory Bowel Disease: Short-term Experience From a Single Tertiary Clinical Centre. J Crohns Colitis. 2020;14:915-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 16. | Meade S, Squirell E, Hoang TT, Chow J, Rosenfeld G. An Update on Anti-TNF Biosimilar Switching-Real-World Clinical Effectiveness and Safety. J Can Assoc Gastroenterol. 2024;7:30-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 17. | D'Amico F, Pouillon L, Argollo M, Hart A, Fiorino G, Vegni E, Radice S, Gilardi D, Fazio M, Leone S, Bonovas S, Magro F, Danese S, Peyrin-Biroulet L. Multidisciplinary management of the nocebo effect in biosimilar-treated IBD patients: Results of a workshop from the NOCE-BIO consensus group. Dig Liver Dis. 2020;52:138-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |