TO THE EDITOR
Recently, the critical role of the gut microbiota in the pathophysiology of neurological diseases has received increasing attention in the field of neuroscience[1]. Extensive research has revealed that the gut microbiota is central to regulating various physiological and pathological processes and that a complex network of biological interactions exists between the brain and the gut[2]. This two-way communication system, the gut-brain axis, plays a vital role in maintaining homeostasis[3,4]. The central nervous system and the gastrointestinal tract are connected by a sophisticated communication network called the brain-gut-enteric microbiota axis. This axis includes the neuroendocrine, immune and autonomic nervous systems, as well as the enteric nervous system and the hypothalamo-pituitary-adrenal axis[5]. Various molecules (neuropeptides, neurohormones, cytokines, etc.) exchange information between these systems. Signals generated by the host shape the composition of the gut microbiota, while microbial metabolites can also influence brain function[5,6]. Antibiotic administration, fecal microbiota transplantation and germ-free animal models are experimental models used to understand the mechanisms of this interaction. Factors such as metabolites produced by gut microbiota, vagus nerve and gut hormones play an important role in brain-gut communication, and the capacity of gut microbiota to produce neuroactive substances further emphasizes the complexity of this axis[7].
The brain has long been thought to influence gut function, but the influence of signals from the gut on human mood, behavior and cognitive function has received more limited recognition. The idea that the gut and the brain work together to stay healthy and eliminate disease states, and that this interaction is mediated by the brain-gut axis, is becoming increasingly important. The gastrointestinal tract is defined as a complex structure that encompasses a large area that synthesizes hormones and is also an important part of the immune system[8,9]. The central nervous system and the gastrointestinal system mutually influence each other through mediators such as the neural pathways and the immune system. The gut microbiota exerts influence on the brain through molecules such as neurotransmitters and metabolic products[10]. These molecules are recognized by receptors on the cell surface and function through nerve endings, immunological cells or the microbiota-gut-brain axis. The enteric nervous system is an intrinsic system of complex neural networks in the intestines and is composed of motor and sensory neurons and neurotransmitters[11,12].
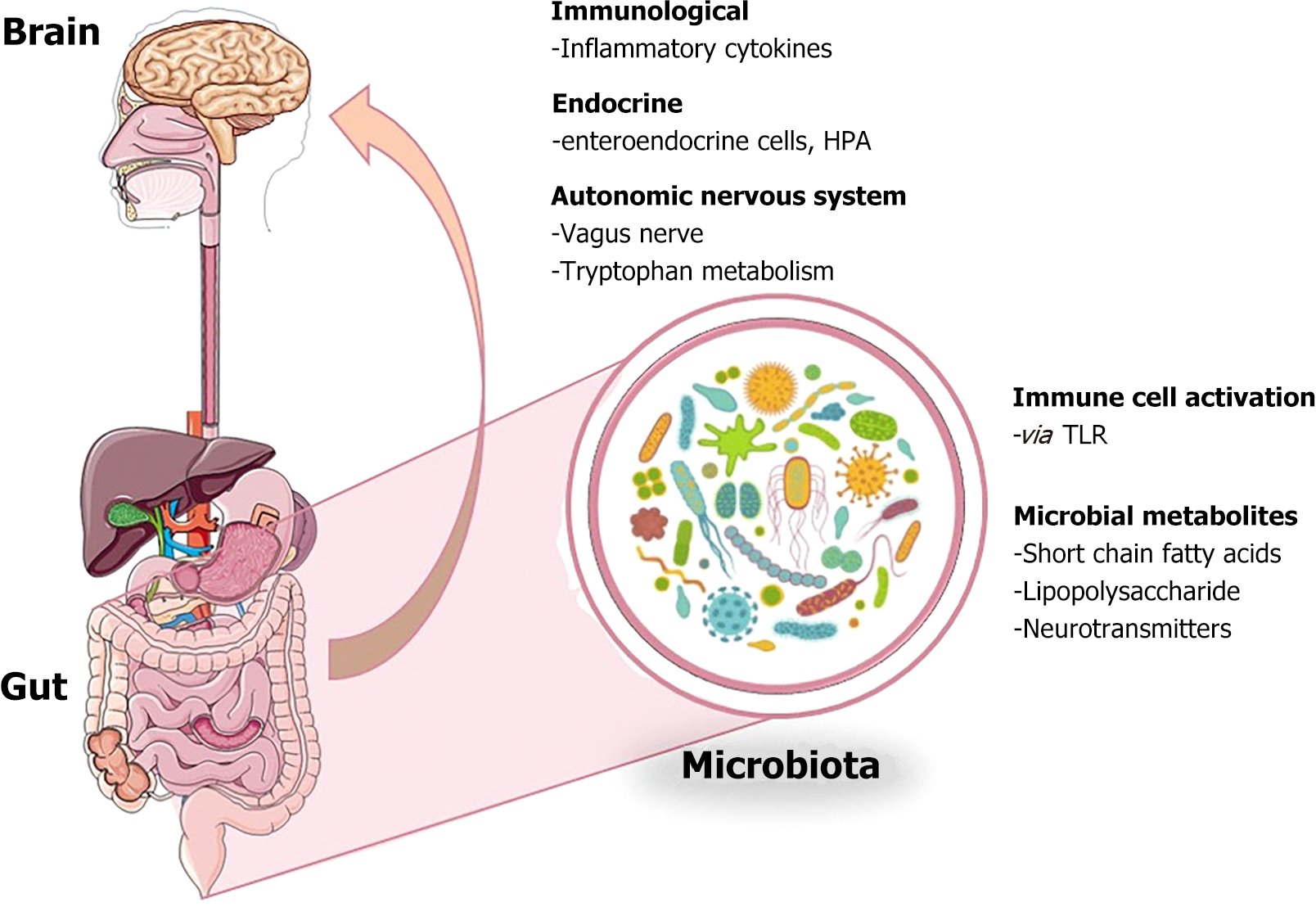
The complex relationships that compose the brain-gut-enteric microbiota axis between the central nervous system, the enteric nerve system, and the gut microbiota are depicted in Figure 1. Communication within this axis occurs through several pathways, including the neuroendocrine system, immune system, autonomic nervous system, and the hypothalamic-pituitary-adrenal axis. Signals from the brain, such as neuropeptides and stress-induced neuroendocrine molecules, regulate gut function and influence the composition of the gut microbiota. Conversely, microbial metabolites, neurotransmitters, and gut hormones produced by the microbiota can influence brain function, modulating mood, behavior, and cognition[13].
Figure 1 Gut-brain axis and its complex bi-directional communication network.
This description reflects the complexity and connectivity of the gut-brain axis, highlighting the interplay of neural, immune, and microbial signals in maintaining health and disease[14].
The central nervous system continuously receives and responds to chemical and neural signals from the gut. The hypothalamic-pituitary-adrenal axis and the sympathetic and parasympathetic branches of the autonomic nervous system play an important role in regulating gut function in this process. Factors such as stress can affect these regulatory mechanisms and trigger the release of neuroendocrine and neuronal molecules, which directly affect the function of the gut microbiota. Increased levels of norepinephrine after stress are also known to trigger the emergence of enteric pathogens[15,16]. The autonomic nervous system is another important pathway showing the influence of the central nervous system on the microbiota. This system is involved in the regulation of various functions such as intestinal motility, secretory functions and mucosal immune response. Changes in intestinal and gastric function following stressful situations affect the balance of the gut microbiota and regulate the enteric system[17-19]. Recent research, such as the study by Zhang et al[20] in the World Journal of Gastroenterology, has provided pivotal insights into the multifactorial nature of irritable bowel syndrome (IBS). This case-control study delves into the complex interplay between the neuroendocrine axis, gut microbiome, and inflammatory pathways and their collective role in the pathogenesis of IBS.
Microbiota-gut-brain axis
Numerous genetic connections have been found in the pathophysiology of IBS, with genes involved in immunological regulation, bile acid production, serotonin signaling, and epithelial barrier function being identified as key contributors. Particularly, there is a link between a higher risk of developing IBS and genes that code for cannabinoid receptors, glutamate receptor ionotropic delta-2 interacting protein, and endoplasmic reticulum protein retention receptor 1 endoplasmic reticulum protein retention receptor 2[21]. One notable study involving 110 IBS patients found a significant reduction in beneficial gut bacteria, including Bifidobacterium, Bacteroides, Methanobacteriales, and Prevotella, alongside an increase in harmful species such as Streptococcus spp., Enterococcus faecalis, Clostridium difficile, and Giardia duodenalis. These pathogenic organisms contribute to gut permeability and abdominal distension, triggering immune cell activation and the release of pro-inflammatory cytokines like interleukin (IL)-6, tumor necrosis factor-α, and IL-1. Additionally, stress exacerbates this dysbiosis by influencing the gut-brain axis, increasing levels of IL-8 and IL-6, which further activates the hypothalamic-pituitary-adrenal and hypothalamic-autonomic nervous system axes. This activation leads to elevated secretion of corticotropin-releasing factor, adrenocorticotropic hormone, and cortisol, all contributing to the exacerbation of IBS symptoms[22-24].
The recent case-control study by Zhang et al[20] supports these findings by demonstrating the significant dysregulation of the neuroendocrine axis in IBS patients, particularly the elevated levels of cortisol, serotonin, and neuropeptides. This study further highlights alterations in the gut microbiome, showing reduced diversity of beneficial bacteria such as Lactobacillus and Bifidobacterium, while pathogenic strains were more prevalent. These microbial imbalances were associated with heightened inflammatory responses, notably increased IL-6 and IL-8 levels, which correlated with gastrointestinal symptoms like abdominal pain and altered bowel habits[20].
Together, these findings suggest that the pathogenesis of IBS is multifactorial, involving genetic susceptibility, microbial dysbiosis, neuroendocrine dysfunction, and chronic inflammation. Addressing these interconnected pathways through therapeutic strategies targeting the neuroendocrine axis, restoring gut microbial balance, and reducing inflammation may provide a comprehensive approach to alleviating IBS symptoms and improving patient outcomes.
CONCLUSION
The study of IBS has developed meaningfully, with a growing focus on the complex interactions between the neuroendocrine axis, gut microbiome, and inflammatory responses. Zhang et al’s[20] research supports critical understanding into these complicated mechanisms, illustrating how dysregulation of the neuroendocrine system, microbial imbalances, and chronic inflammation collectively contribute to the pathogenesis of IBS. The findings from this case-control study underline the potential for novel therapeutic strategies that target these pathways, offering promising avenues to alleviate symptoms and improve the quality of life for IBS patients. Addressing IBS holistically by modulating the neuroendocrine axis, restoring a healthy balance of gut microbiota, and reducing inflammatory activity could mark a new era in personalized treatment approaches. As research continues to shed light on the genetic and environmental factors influencing IBS, it becomes increasingly clear that an integrated approach-combining microbiota modulation, stress management, and immune system regulation-holds the key to effectively managing this chronic condition. This study is a testament to the importance of continuing to explore these interconnected pathways, offering hope for better outcomes in IBS treatment.