Published online Nov 28, 2024. doi: 10.3748/wjg.v30.i44.4725
Revised: September 27, 2024
Accepted: October 28, 2024
Published online: November 28, 2024
Processing time: 83 Days and 21.7 Hours
The long-term stability of hepatitis B surface antigen (HBsAg) seroclearance following peginterferon alpha (peg-IFN-α)-based therapy has not been extensively studied, leaving the full potential and limitations of this strategy unclear.
To assess HBsAg recurrence after seroclearance achieved by peg-IFN-α regimens.
This prospective, multicenter, observational study was conducted from November 2015 to June 2021 at three Chinese hospitals: The Second Affiliated Hospital of Xi’an Jiaotong University, Ankang Central Hospital, and The Affiliated Hospital of Yan’an University. Participants who achieved HBsAg seroclearance following peg-IFN-α-based treatments were monitored every 4-12 weeks post-treatment for hepatitis B virus (HBV) markers, HBV DNA, and liver function. The primary outcome was HBV recurrence, defined as the reemergence of HBsAg, HBV DNA, or both, at least twice within 4-8 weeks of follow-up.
In total, 121 patients who achieved HBsAg seroclearance were enrolled. After a median follow-up of 84.0 (48.0, 132.0) weeks, four subjects were lost to follow-up. HBsAg recurrence was detected in 16 patients. The cumulative HBsAg recurrence rate in the intention-to-treat population was 15.2%. Multivariate logistic regression analysis demonstrated that consolidation time < 12 weeks [odds ratio (OR) = 28.044, 95%CI: 4.525-173.791] and hepatitis B surface antibody disappearance during follow-up (OR = 46.445, 95%CI: 2.571-838.957) were strong predictors of HBsAg recurrence. HBV DNA positivity and decompensation of liver cirrhosis and hepatocellular carcinoma were not observed.
HBsAg seroclearance following peg-IFN-α treatment was durable over 84 weeks of follow-up with a cumulative recurrence rate of 15.2%.
Core Tip: Hepatitis B surface antigen seroclearance is an ideal target for peginterferon alpha (peg-IFN-α) treatment in patients with chronic hepatitis B and a focus of global research. However, there is limited information about the duration of seroclearance after peg-IFN-α withdrawal. This study examined follow-up observational data after peg-IFN-α withdrawal among patients who achieved functional cure at three centers. The longest follow-up duration was 252 weeks, representing one of the longest follow-up times currently available. We believe that this study offers valuable guidance and reference for the clinical use of peg-IFN-α, including the optimal timing of treatment cessation and the importance of continuous and standardized follow-up after treatment.
- Citation: Lu R, Zhang M, Liu ZH, Hao M, Tian Y, Li M, Wu FP, Wang WJ, Shi JJ, Zhang X, Jia XL, Jiang ZC, Li XM, Xu GH, Li YP, Dang SS. Recurrence and influencing factors of hepatitis B surface antigen seroclearance induced by peginterferon alpha-based regimens. World J Gastroenterol 2024; 30(44): 4725-4737
- URL: https://www.wjgnet.com/1007-9327/full/v30/i44/4725.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i44.4725
Hepatitis B virus (HBV) infection causes chronic, continuous, and progressive liver damage. Each year, approximately 650000 people worldwide die from HBV-related end-stage liver diseases, including liver failure, cirrhosis, and hepatocellular carcinoma (HCC)[1]. Nucleoside analogs (NAs), such as entecavir (ETV) and tenofovir (TDF), slow the progression of liver disease. Although long-term oral NA administration effectively maintains undetectable HBV DNA levels, patients often remain hepatitis B surface antigen (HBsAg)-positive, and HBV-related liver cirrhosis and HCC frequently occur. By contrast, the risk of end-stage liver disease is significantly reduced if HBsAg seroclearance is achieved, regardless of whether it is achieved spontaneously or induced by treatment. Serum HBsAg levels have been identified as a surrogate marker of transcriptionally active closed circular DNA (cccDNA) in the liver when serum HBV DNA is undetectable[2]. Lower HBsAg levels might indicate minimal cccDNA levels in the liver. Therefore, pursuing HBsAg seroclearance, which represents clinical cure (functional cure), is the optimal goal and a safe endpoint for antiviral therapy in patients with hepatitis B[1,3,4].
Compared with NAs, interferon alpha (IFN-α) displays greater efficacy in reducing serum HBsAg levels. In recent years, peginterferon alpha (peg-IFN-α) therapy alone or in combination with NAs has significantly improved the HBsAg seroclearance rate, highlighting the promise of these regimens.
However, the persistence of cccDNA and integrated HBV DNA in hepatocytes after chronic HBV infection are major challenges for antiviral therapy[5]. HBsAg seroclearance does not result in the complete elimination of cccDNA and integrated HBV DNA in the liver, leading to potential risks of serological reversion and disease progression. In this study, we observed the relapse of HBsAg seroclearance induced by peg-IFN-α and briefly discussed the possible factors related to recurrence, such as consolidation time and hepatitis B surface antibody (HBsAb) disappearance. Our findings will improve our understanding of the durability of the ‘clinically cured’ state and offer guidance for optimizing treatment strategies in chronic hepatitis B (CHB) management.
In this prospective, multicenter, observational study, patients were enrolled from November 2015 to June 2021 at The Second Affiliated Hospital of Xi’an Jiaotong University, Ankang Central Hospital, and The Affiliated Hospital of Yan’an University in China. Patients with CHB received either peg-IFN-α monotherapy or NA-containing regimens, and 121 patients achieved HBsAg seroclearance, defined as two or more consecutive negative HBsAg results with at least one repeated result after the end of treatment. Following the initial confirmation of HBsAg seroclearance, all patients continued peg-IFN-α therapy for several months as consolidation therapy. After the completion of consolidation therapy, all medications, including peg-IFN-α and NAs, were discontinued.
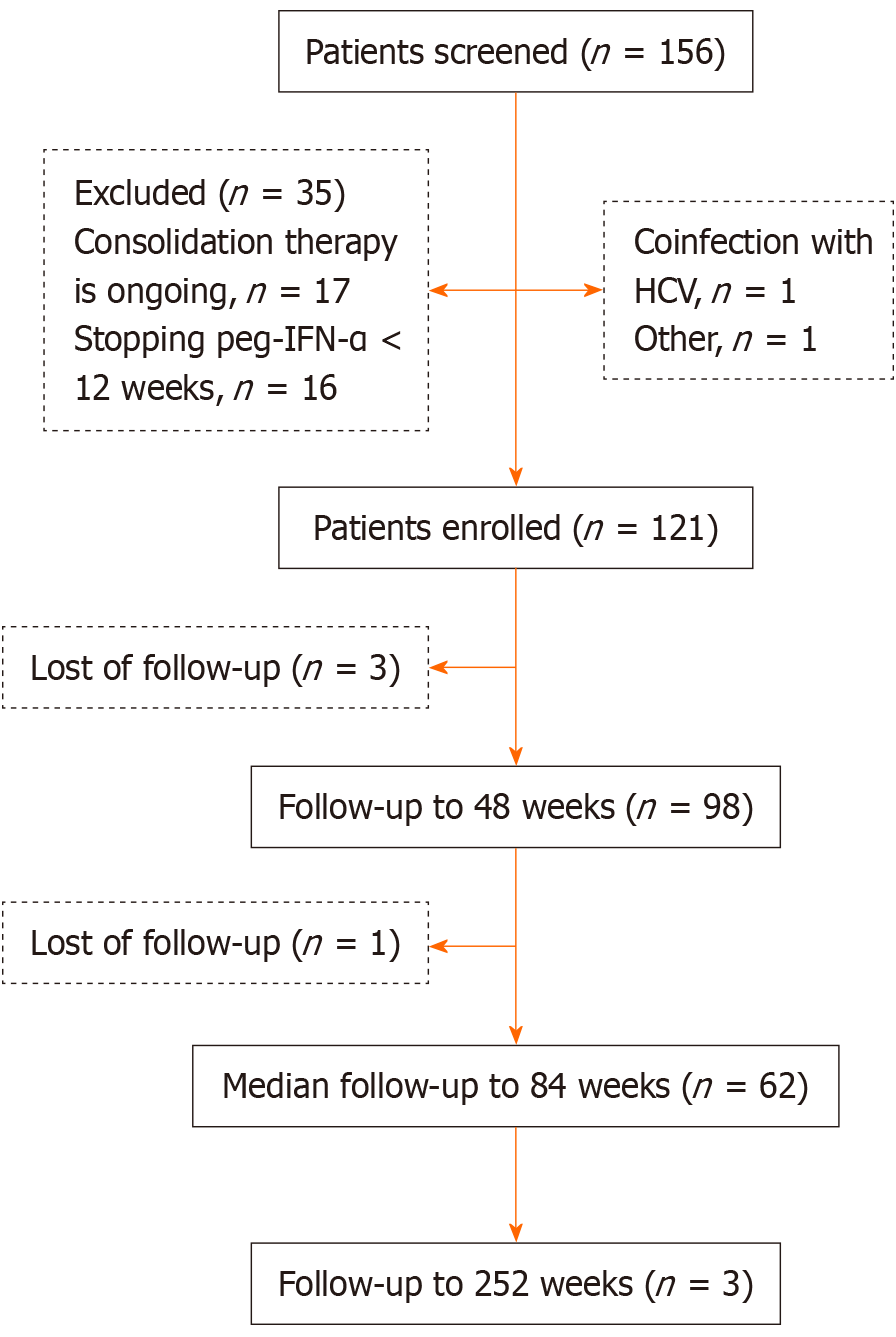
The key exclusion criteria were coinfection with human immunodeficiency virus or hepatitis C or D virus, evidence of HCC or liver decompensation, tumors in other systems, and the receipt of radiotherapy, chemotherapy, or immunomodulators during the follow-up period.
From the date of cessation of peg-IFN-α-based treatment, all subjects were evaluated for HBV markers, including HBsAg, HBsAb, HBV e antigen (HBeAg), HBV DNA, and liver function markers, every 4-12 weeks. Additionally, liver computed tomography (CT) was performed yearly during the follow-up period. In particular, all patients underwent at least 24 months of follow-up. The primary endpoint was HBV recurrence, whereas the secondary endpoints included the dynamic changes in HBsAb levels, alanine aminotransferase (ALT) flares, and the incidence of decompensation of liver cirrhosis and HCC.
In addition, general patient information was collected, including sex, age, and family history of HBV infection. In addition to serum HBV DNA quantification, serum HBsAg quantification, and determination of the HBeAg status at the initiation of peg-IFN-α administration, data on the treatment regimens, the durations of primary and consolidation treatment with peg-IFN-α, the types of peg-IFN-α, and the FibroScan value at the cessation of peg-IFN-α treatment were also collected.
‘HBV reversion’ was defined as the reappearance of HBsAg, HBV DNA, or both at least twice over an interval of 4-8 weeks during follow-up after treatment cessation. ‘Treatment time’ was defined as the duration from the initiation of peg-IFN-α treatment to the confirmation of HBsAg seroclearance. The duration from confirmed HBsAg seroclearance to the cessation of peg-IFN-α therapy was defined as the ‘consolidation time’. ‘HBsAb disappearance’ was defined as HBsAb positivity at the time of peg-IFN-α cessation and a subsequent decrease in HBsAb levels to < 10 mIU/mL during follow-up.
Serum HBV DNA was quantified using the automatic COBAS® AmpliPrep/COBAS® TaqMan 48 system and supporting reagents (Roche, Basel, Switzerland), which had a detection limit of 20 IU/mL. Serum HBV markers (HBsAg, HBsAb, HBeAg) were detected by chemiluminescence (Architect i2000 chemiluminescence immunoassay analyzer and its supporting reagents, Abbott, Chicago, IL, United States). The HBsAg detection limit was 0.05 IU/mL, HBsAg positivity was defined as a quantitative level exceeding 0.05 IU/mL, and HBsAb negativity was defined as a level lower than 10 mIU/mL. Serum ALT was assayed using an automatic biochemical analyzer (Roche) with an upper limit of normal of 50 IU/L in men and 40 IU/L in women. CT was performed using the LightSpeed VCT scanner (GE Healthcare, Chicago, IL, United Staets).
Statistical analysis was performed using the Statistical Package for the Social Sciences (version 18.0, IBM, Armonk, NY, United States). Measurement data with an approximately normal distribution were presented as the mean ± SD, and measurement data with a non-normal distribution were presented as the median and interquartile range. Student’s t-test, the Mann-Whitney U test, or repeated-measures analysis of variance was used for the intergroup comparisons of continuous variables as appropriate. Categorical variables were presented as counts and percentages and compared using the χ2 test or Fisher’s exact test. The Kaplan-Meier method was used to assess the cumulative recurrence rate. Logistic regression was used to analyze the variables related to recurrence. The receiver operating characteristic (ROC) curve was used to evaluate the HBsAb level predicting HBsAg recurrence. P < 0.05 was considered statistically significant.
In this study, 156 patients with CHB who achieved HBsAg seroclearance after peg-IFN-α treatment were screened. Of these, 35 patients were excluded for the following reasons: 17 patients were receiving consolidation therapy, 16 patients discontinued peg-IFN-α treatment before 24 weeks, one patient was coinfected with hepatitis C virus, and one patient refused to participate. Thus, 121 patients were enrolled in this study. The mean participant age was 35.6 ± 10.9 years, and 74 patients (61.2%) were male, giving a male-to-female ratio of 1.6:1. Additionally, 64 patients (52.9%) had family histories of HBV infection. Before starting peg-IFN-α therapy, the median HBsAg level was 85.6 (4.5, 362.4) IU/mL. Twenty patients (16.5%) were HBeAg-positive, and 37 patients (30.6%) had measurable HBV DNA levels. Concerning treatment, 41 patients (33.9%) received peg-IFN-α-2a, whereas 80 patients (66.1%) received peg-IFN-α-2b. All patients received individualized treatment regimens, including initial combination or sequential treatment with peg-IFN-α and NAs in 48 patients (39.7%; adefovir: 6, lamivudine: 1, ETV: 24, TDF: 16, and TDF alafenamide: 1) and peg-IFN-α alone in 73 inactive HBV carriers (60.3%). The median treatment duration before HBsAg seroclearance was 20.0 (12.0, 42.0) weeks, and the median consolidation treatment time was 12.0 (12.0, 24.0) weeks, giving a median total treatment time of 44.0 (28.0, 56.0) weeks. The median follow-up time in all patients was 84.0 (48.0, 132.0) weeks, and the longest follow-up time was 252 weeks. Four patients (3.3%) were lost to follow-up (Figure 1).
All patients were negative for HBeAg and HBV DNA at baseline. In total, 79 patients (65.3%) achieved HBsAg seroconversion with a baseline HBsAb level of 36.9 (9.6, 115.1) mIU/mL. The patient baseline characteristics are presented in Table 1.
| Characteristics | Total patients | Sustained HBsAg seroclearance (n = 105) | HBV reversion (n = 16) | P value |
| Age, years, mean ± SD | 35.6 ± 10.9 | 36.2 ± 11.2 | 31.8 ± 8.0 | 0.132 |
| Male | 74 (61.2) | 66 (62.9) | 8 (50.0) | 0.326 |
| Family history of HBV infection | 64 (52.9) | 57 (54.28) | 7 (43.8) | 0.432 |
| Characteristics before HBsAg seroclearance | ||||
| Therapy regimens | ||||
| IHCs treated with peg-IFN-α alone | 73 (60.3) | 66 (62.9) | 7 (43.8) | 0.342 |
| CHB treated with NA sequential combination with peg-IFN-α | 42 (34.7) | 34 (32.4) | 8 (50.0) | |
| CHB treated with NA de novo combination with peg-IFN-α | 6 (5.0) | 5 (4.7) | 1 (6.2) | |
| Peg-IFN-α types | ||||
| Peg-IFN-α-2a | 41 (33.9) | 34 (32.4) | 7 (43.8) | 0.371 |
| Peg-IFN-α-2b | 80 (66.1) | 71 (67.6) | 9 (56.3) | |
| HBsAg levels at the initiation of peg-IFN-α, log10 IU/mL, IQR | 85.6 (4.5, 362.4) | 71.4 (3.7, 289.4) | 497.4 (12.7, 1354.5) | 0.078 |
| HBsAb status | ||||
| HBsAb negative | 113 (93.4) | 98 (93.3) | 15 (93.8) | 1.000 |
| HBsAb positive | 8 (6.6) | 7 (6.7) | 1 (6.3) | |
| HBV DNA negative | 84 (69.4) | 75 (71.4) | 9 (56.3) | 0.250 |
| HBV DNA positive | 37 (30.6) | 30 (28.6) | 7 (43.8) | |
| HBeAg negative | 101 (83.5) | 87 (82.9) | 14 (87.5) | 1.000 |
| HBeAg positive | 20 (16.5) | 18 (17.1) | 2 (12.5) | |
| Treatment time before HBsAg loss, weeks, IQR | 20.0 (12.0, 42.0) | 20.0 (12.0, 38.0) | 40.0 (12.0, 57.0) | 0.212 |
| Consolidation time after HBsAg loss, weeks, IQR | 12.0 (12.0, 24.0) | 12.0 (12.0, 24.0) | 6.0 (0.0, 11.0) | < 0.001 |
| Total Duration of peg-IFN-α, weeks, IQR | 44.0 (28.0, 56.0) | 40.0 (28.0, 55.0) | 46.0 (29.0, 62.0) | 0.625 |
| Characteristics at the cessation of peg-IFN-α | ||||
| Patients with HBsAg seroconversion | 79 (65.3) | 74 (70.5) | 5 (31.3) | 0.074 |
| HBsAb levels, mIU/mL, IQR | 36.9 (9.6, 115.1) | 44.3 (9.9, 141.0) | 9.1 (3.9, 16.3) | < 0.001 |
| HBeAg negative | 121 (100.0) | 105 (100.0) | 16 (100.0) | - |
| HBV DNA negative | 121 (100.0) | 105 (100.0) | 16 (100.0) | - |
| ALT, IU/L, IQR | 38.0 (24.0, 64.0) | 38.0 (23.9, 60.0) | 40.0 (26.3, 96.0) | 0.218 |
| FibroScan value, kPa, mean ± SD | 5.7 ± 1.0 | 5.7 ± 1.0 | 6.0 ± 1.0 | 0.314 |
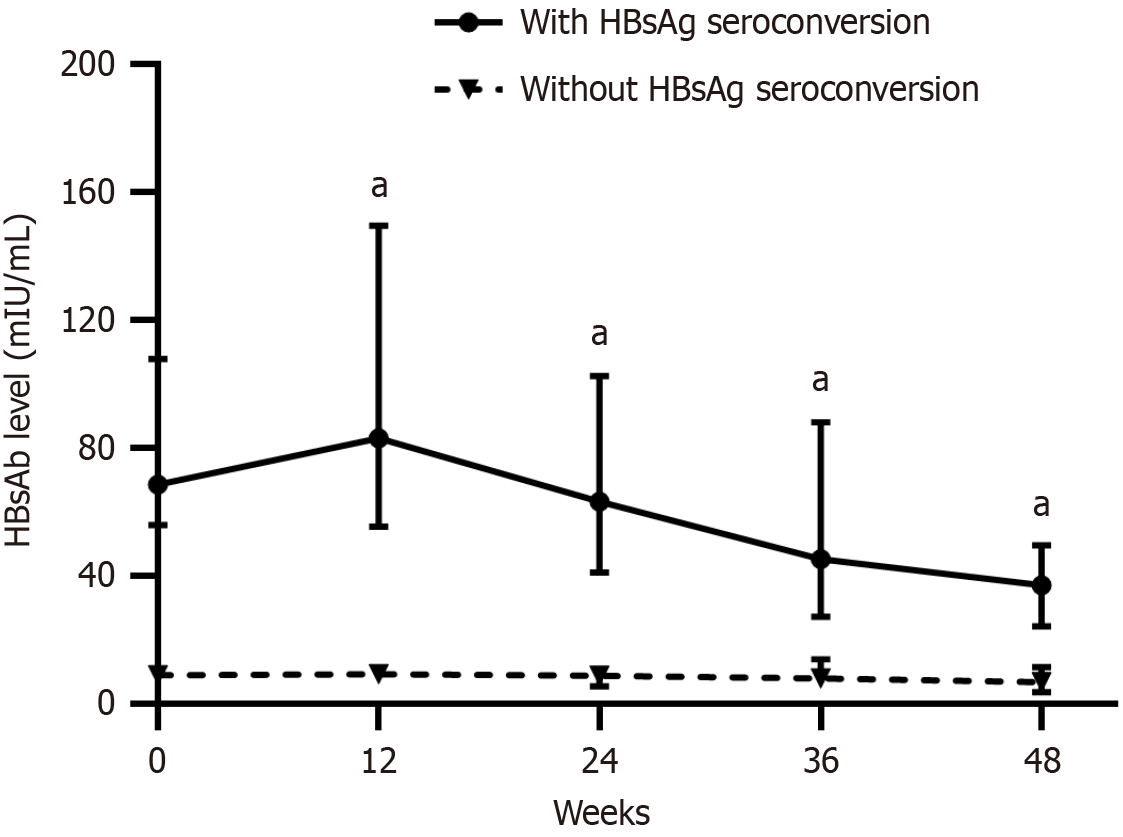
Among the 121 patients, 98 (81.0%) completed at least 48 weeks of follow-up. Because 93.8% of the patients experienced relapse within 48 weeks, we analyzed the dynamic changes in HBsAb levels from baseline to 48 weeks to ensure the accuracy of the outcomes. The patients were assigned to the HBsAg seroconversion or non-seroconversion group according to their baseline HBsAb status. The HBsAb level in the seroconversion group increased from 68.6 (36.9, 200.5) mIU/mL at baseline to 83.10 (37.0, 245.3) mIU/mL at week 12 and then gradually decreased to 63.3 (24.1, 200.3) mIU/mL at week 24, 45.3 (17.0, 101.4) mIU/mL at week 36, and 37.1 (14.2, 83.3) mIU/mL at week 48. In the non-seroconversion group, the HBsAb level increased from 9.0 (8.3, 9.7) mIU/mL at baseline to 9.2 (5.4, 14.2) mIU/mL at week 12 and then gradually decreased to 8.8 (5.2, 13.9) mIU/mL at week 24, 7.9 (4.5, 15.4) mIU/mL at week 36, and 6.7 (3.2, 21.7) mIU/mL at week 48 (Figure 2).
Further analysis illustrated that HBsAb levels in the HBsAg seroconversion group were all significantly higher than those in the non-seroconversion group at baseline, week 12, week 24, week 36, and week 48 (Z = -9.033, -7.327, -6.548, -5.649, and -4.974, respectively, all P < 0.001).
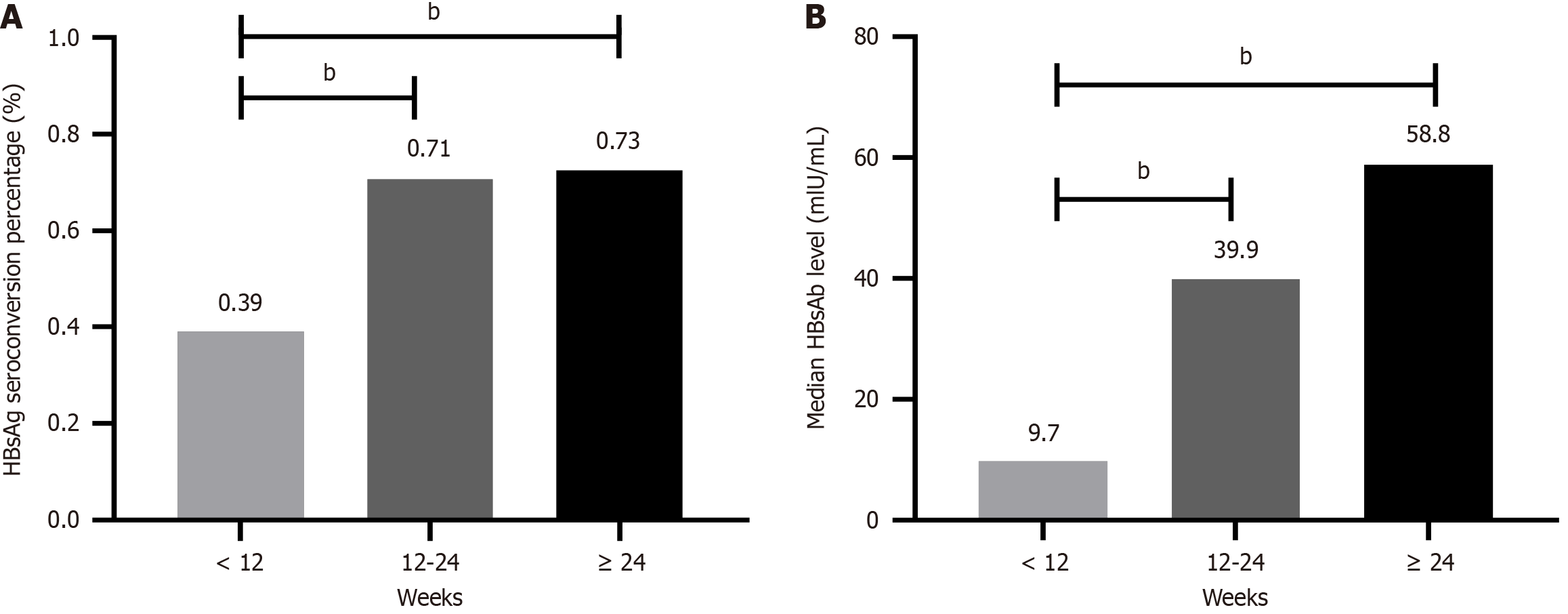
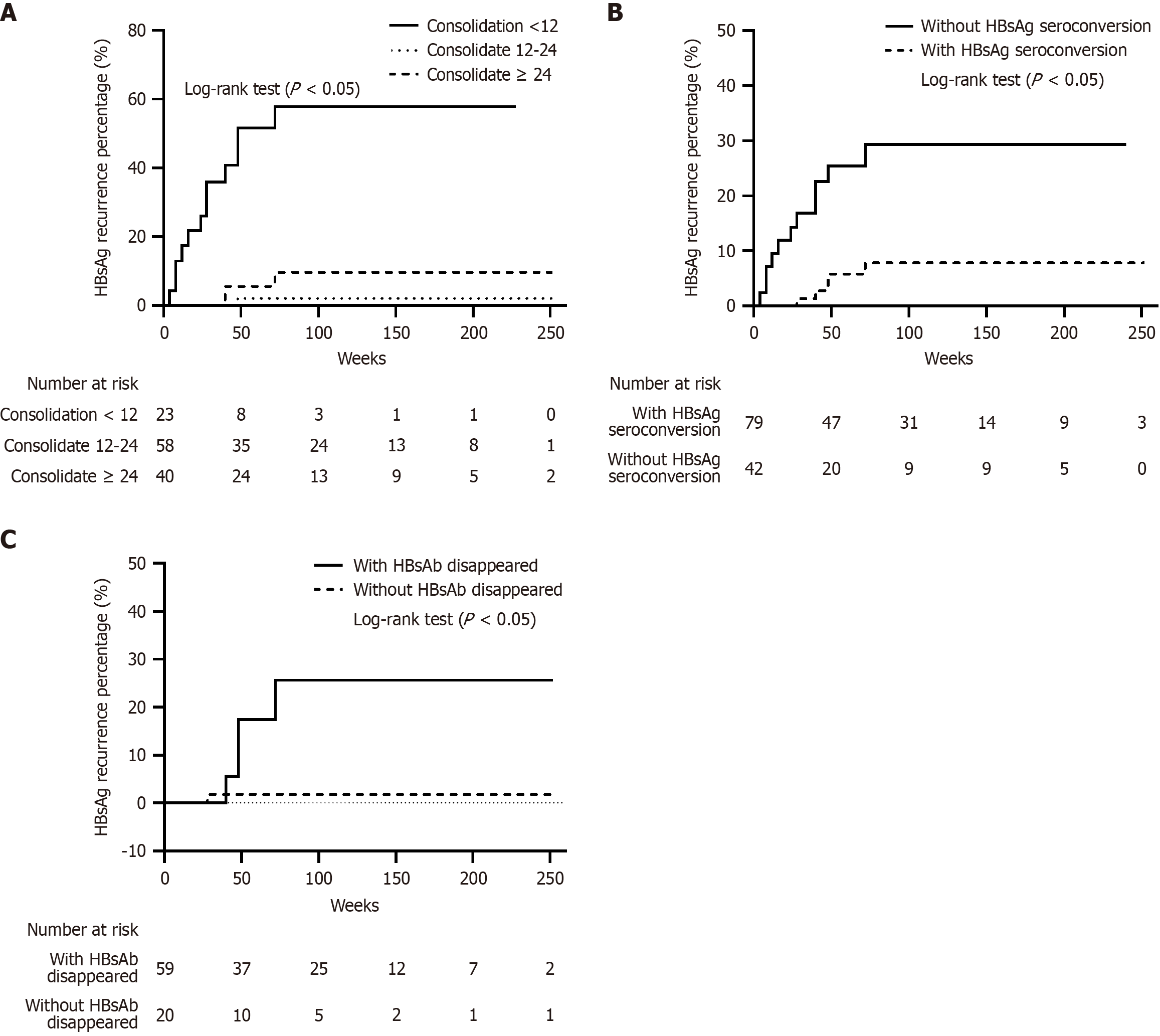
To compare the effects of different consolidation regimens on the baseline HBsAg seroconversion rate and HBsAb level, the consolidation treatment duration was categorized into three groups: < 12 weeks, 12-24 weeks, and ≥ 24 weeks. The HBsAg seroconversion rate for patients with treatment durations < 12 weeks was significantly lower than that in patients with treatment durations ≥ 12 weeks [39.1% (9/23) vs 71.4% (70/98), χ2 = 8.575, P = 0.003], whereas no difference was observed between the 12-24 weeks and ≥ 24 weeks groups [70.7% (41/58) vs 72.5% (29/40), χ2 = 0.038, P = 0.845;
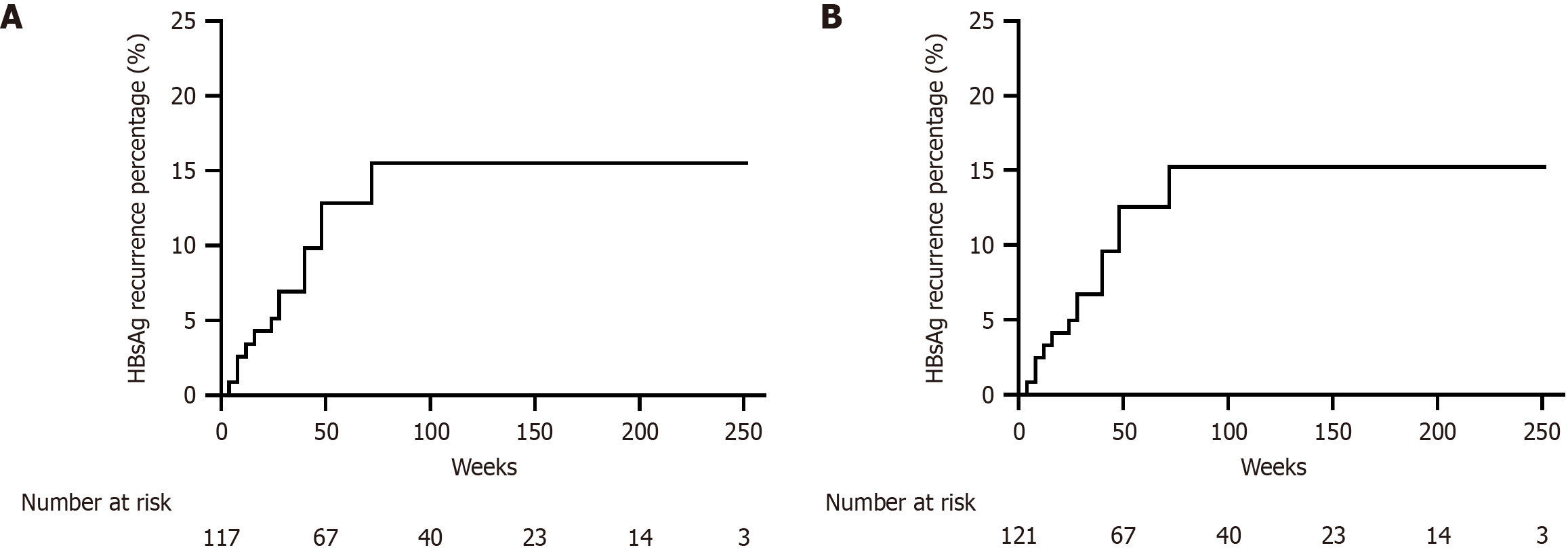
After a median follow-up time of 84.0 (48.0, 132.0) weeks, persistent HBsAg seroclearance was maintained in 105 patients (86.8%). The 72-week cumulative HBsAg recurrence rate was 15.5% in the per-protocol analysis (Figure 4A) and 15.2% (Figure 4B) in the intention-to-treat analysis. The median HBsAg level at relapse was 0.3 (0.1, 1.7) IU/mL. Of the 16 patients with recurrence, 14 (87.5%) experienced recurrence within 48 weeks after discontinuing peg-IFN-α therapy. In addition, all recurrences were limited to HBsAg recurrence, as HBV DNA recurrence was not observed.
The HBsAg recurrence rate was 10.8% (8/74) in male patients, vs 17.0% (8/47) in female patients (χ2 = 0.966, P = 0.326). Because of the large age span of the study population, which included a few patients younger than 20 years and some patients older than 60 years, the patients were divided into two age groups (< 35 and ≥ 35 years). Further stratified analysis revealed no significant difference in the HBsAg recurrence rate between these groups [17.5% (10/57) vs 9.4% (6/64), χ2 = 1.753, P = 0.185]. The liver FibroScan values were mostly distributed between 5.0 and 7.0 kPa, and thus, we divided patients into two groups (< 6 and ≥ 6 kPa). The HBsAg recurrence rate did not significantly differ between these groups [10.4% (8/77) vs 18.2% (8/44), χ2 = 1.482, P = 0.224].
Stratified analysis of the consolidation time illustrated that the HBsAg recurrence rate was significantly higher for consolidation time < 12 weeks than for consolidation time ≥ 12 weeks [52.2% (12/23) vs 4.1% (4/98), Z = 37.547, P < 0.001]. However, no difference was observed between the 12-24 weeks and ≥ 24 weeks groups [1.7% (1/58) vs 7.5% (3/40), Fisher’s exact test, P = 0.184; Figure 5A]. Patients without HBsAg seroconversion at baseline had a significantly higher HBsAg recurrence rate than those who achieved HBsAg seroconversion [26.3% (11/42) vs 6.3% (5/79), P = 0.002; Figure 5B]. In addition, the HBsAg recurrence rate in patients with HBsAb disappearance (4/20, 20%) during follow-up was significantly higher than that in patients without HBsAb disappearance (1/59, 1.7%, Fisher’s exact test, P = 0.013; Figure 5C).
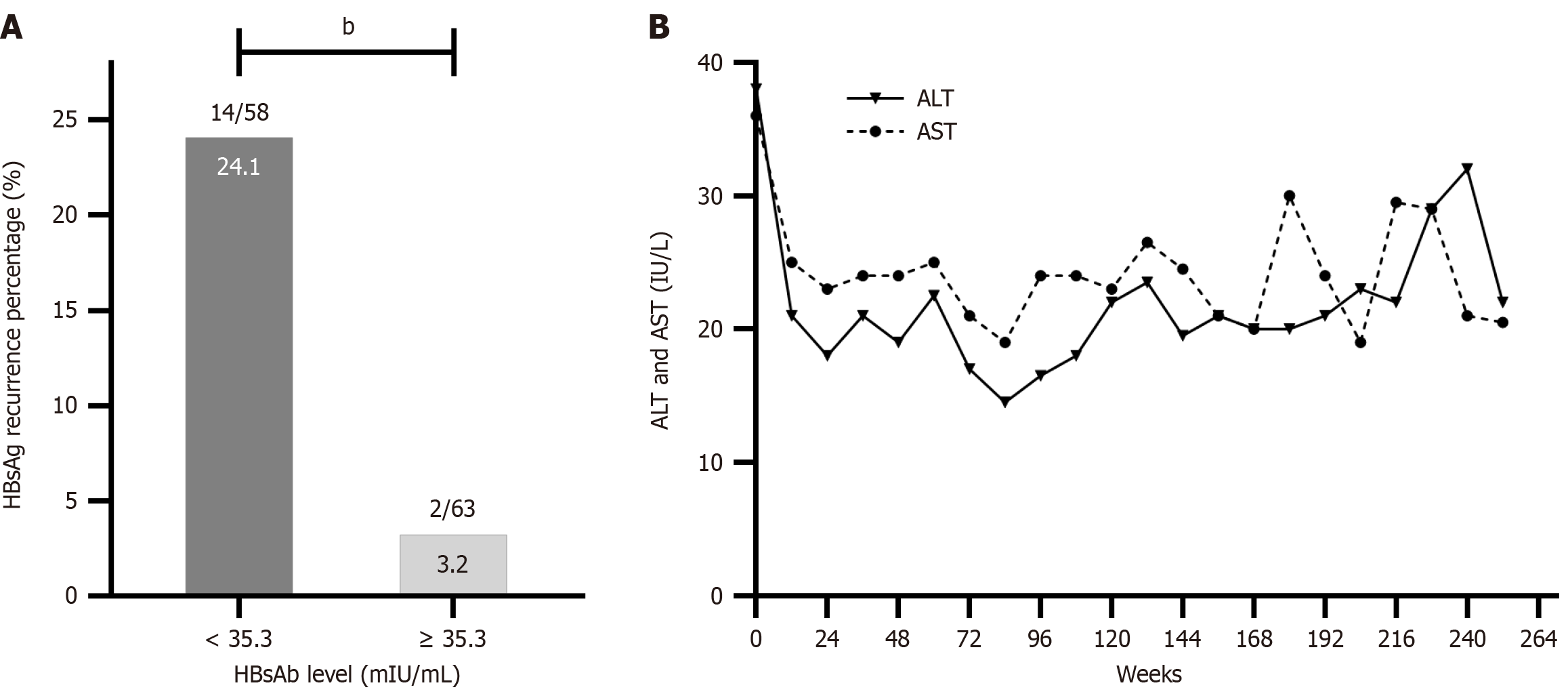
ROC curves were used to assess the effect of the baseline HBsAb level on HBsAg recurrence. The area under the ROC curve for the baseline HBsAb level was 0.836 with an optimal cutoff of 35.3 mIU/mL. The sensitivity and specificity were 0.581 and 0.875, respectively. Based on the optimal cutoff, the HBsAg recurrence rate was 24.1% (14/58) in patients with baseline HBsAb levels < 35.3 mIU/mL, compared with 3.2% (2/63) in patients with HBsAb levels ≥ 35.3 mIU/mL (χ2 = 11.566, P = 0.001; Figure 6A).
Univariate binary logistic regression illustrated that consolidation therapy for less than 12 weeks [odds ratio (OR) = 25.636, 95%CI: 7.039-93.356, P < 0.001], the absence of HBsAg seroconversion at baseline (OR = 5.252, 95%CI: 1.684-16.376, P = 0.004), HBsAb < 35.3 mIU/mL at baseline (OR = 9.705, 95%CI: 2.098-44.883, P = 0.004), and HBsAb disappearance during follow-up (OR = 14.500, 95%CI: 1.513-138.972, P = 0.020) were significantly associated with HBsAg recurrence. Other factors, such as sex, age (years), family history of HBV infection, FibroScan values (kPa), patient characteristics at the initiation of peg-IFN-α therapy (including HBsAg levels (IU/mL), HBsAb status, HBeAg status, and HBV DNA status), treatment regimens (IFN types and the use of monotherapy or combination therapy with NAs), total treatment duration, baseline ALT (IU/L) and aspartate aminotransferase levels (IU/L)) had no significant effect on the risk of HBsAg recurrence (Table 2).
| Characteristics | OR | 95%CI | P value |
| Age (years) | 0.962 | (0.916-1.012) | 0.133 |
| Gender | 1.692 | (0.588-4.870) | 0.329 |
| Family history of HBV infection | 0.655 | (0.227-1.890) | 0.434 |
| Characteristics at the initiation of peg-IFN-α | |||
| HBsAg level, IU/mL | 1.000 | (1.000-1.000) | 0.398 |
| HBsAb negative | 0.933 | (0.107-8.130) | 0.950 |
| HBV DNA positive | 0.514 | (0.176-1.506) | 0.225 |
| HBeAg positive | 1.448 | (0.302-6.934) | 0.643 |
| Peg-IFN-α-2a | 1.624 | (0.558-4.730) | 0.374 |
| Peg-IFN-α monotherapy | 0.588 | (0.224-1.546) | 0.282 |
| Treatment time of peg-IFN-α (weeks) | 1.014 | (0.992-1.035) | 0.217 |
| Total duration of peg-IFN-α (weeks) | 0.999 | (0.975-1.024) | 0.9570 |
| Consolidation time < 12 weeks | 25.636 | (7.039-93.356) | < 0.001 |
| Characteristics at the cessation of peg-IFN-α | |||
| Without HBsAg seroconversion | 5.252 | (1.684-16.376) | 0.004 |
| HBsAb, mIU/mL | 0.952 | (0.914-0.992) | 0.018 |
| HBsAb < 35.3 mIU/mL | 9.705 | (2.098-44.883) | 0.004 |
| HBsAb disappeared during the follow-up | 14.500 | (1.513-138.972) | 0.020 |
| ALT level at baseline, IU/L | 1.011 | (1.001-1.021) | 0.127 |
| FibroScan values at baseline (kPa) | 1.314 | (0.791-2.183) | 0.292 |
Multivariate logistic regression analysis was performed with adjustment for age, sex, and the type of peg-IFN-α. Consolidation time < 12 weeks (OR = 28.044, 95%CI: 4.525-173.791, P < 0.001) and HBsAb disappearance during follow-up (OR = 46.445, 95%CI: 2.571-838.957, P = 0.009) were confirmed as predictors of HBsAg recurrence (Table 3).
| Characteristics | OR | 95%CI | P value |
| Age (years) | 0.924 | (0.843-1.012) | 0.089 |
| Gender | 0.665 | (0.140-3.169) | 0.609 |
| Peg-IFN-α-2a | 0.759 | (0.165-3.485) | 0.723 |
| Without HBsAg seroconversion | 9.388 | (0.512-172.212) | 0.131 |
| HBsAb < 35.3 mIU/mL | 3.449 | (0.264-45.112) | 0.345 |
| Consolidation time < 12 weeks | 28.044 | (4.525-173.791) | < 0.001 |
| HBsAb disappeared during the follow-up | 46.445 | (2.571-838.957) | 0.009 |
One male patient experienced three ALT flares during 252 weeks of follow-up, and at week 60, he was diagnosed with fatty liver via CT and ultrasonography. ALT flares were not observed in the remaining patients throughout follow-up (Figure 6B).
Decompensation of liver cirrhosis or HCC was not observed in any patients during follow-up.
It was reported during a recent research meeting that clinical cure should be defined by undetectable HBV DNA and HBsAg levels for at least 6 consecutive months[6]. In fact, the number of patients who achieve HBsAg seroclearance remains limited, and few reports have described recurrence after HBsAg seroclearance, with most including small numbers of cases. This study addresses this gap by providing comprehensive real-world observational data on relapse after HBsAg seroclearance induced by peg-IFN-α. With a larger sample size and an extended follow-up period, our research offers significant insights into the durability of HBsAg seroclearance and the challenges in achieving a clinical cure in CHB treatment.
HBsAb production might reflect greater immune control of HBV infection by the host. A study found that extremely low HBV DNA levels could be detected in a small number of patients after HBsAg seroclearance. However, concomitant HBsAg seroconversion was the only significant factor found to reduce its occurrence[7]. HBsAb can eliminate circulating HBsAg through antibody-mediated mechanisms[8]. In clinical practice, HBsAg seroconversion is an important indicator of clinical cure. In this study, 65.3% patients experienced concomitant HBsAg seroconversion, consistent with the results of one previous study[9] but slightly lower than that in another study[10], whereas the current seroconversion rate was higher than the NA-induced and spontaneous HBsAg seroconversion rates reported by Wong et al[11], Kim et al[12], and Alawad et al[9]. This might be attributable to the immunomodulatory function of peg-IFN-α compared with NAs, leading to increased B cell[13] and NK cell activity[14] and thereby promoting HBsAg seroconversion. Meanwhile, our data illustrated that 12-24 weeks of peg-IFN-α consolidation therapy significantly improved both the baseline HBsAb level and baseline HBsAg seroconversion rate. However, extending the treatment to ≥ 24 weeks did not lead to further improve
The HBsAb level is related to the immune status of the body. During periods of severe immunosuppression, HBsAb levels naturally decline, sometimes rapidly[15]. However, we observed a significant increase in HBsAb levels during the early follow-up period (within 12 weeks) in this study. This interesting finding might result from a persistent nontherapeutic effect of peg-IFN-α. During weeks 12-48, HBsAb levels decreased to varying degrees, which could reflect weakened immune control of HBV[10].
The recurrence of HBsAg and HBV DNA can occur in patients with HBsAg seroclearance or seroconversion because of reduced immune control of HBV, especially in patients with immune disorders, tumors, or other diseases requiring immunosuppressive therapy or chemotherapy[16,17].
The phenomenon of HBV recurrence has been reported previously. In particular, the composite recurrence rate of HBsAg and HBV DNA 1.6 years after NA-induced HBsAg seroclearance was 10%, and the rate gradually increased over time[18]. In patients with HBsAg seroclearance induced by combined NA and IFN-α therapy, the cumulative HBsAg recurrence rate was 18% over a median follow-up of 96 weeks[19]. In a study by Wu et al[20] that assessed the durability of IFN-induced HBsAg seroclearance, 238 patients had a cumulative HBsAg recurrence rate of 9.66% during a median follow-up of 160 weeks. In another study of the durability of HBsAg seroclearance induced by peg-IFN-α in HBeAg-positive patients, the HBsAg recurrence rate after 48 weeks of follow-up was 8.2%[21]. Thus, our HBsAg recurrence rate was slightly higher than those in previous studies. One possible explanation is that HBsAg recurrence spontaneously resolved without treatment in some patients[18-20]. These patients appeared to commonly have extremely low HBsAg levels at the time of relapse. This phenomenon was defined as transient HBsAg elevation in some studies. In addition, the dynamic changes in HBsAg and HBsAb levels in 16 patients before and after relapse revealed an interesting phenomenon, with HBsAb levels rapidly declining in the period before HBsAg relapse. The rapid decline or even disappearance of HBsAb might indicate weakened immune protection in the host[10], resulting in the reactivation of cccDNA transcriptional activity.
The timing of HBsAg recurrence varies among different patients. In this study, relapse occurred within 4-72 weeks of follow-up, with 87.5% of the recurrences occurring within 48 weeks. In Wu et al’s study[20], recurrence peaked in week 52 after treatment cessation. These findings suggest that the first year after peg-IFN-α cessation carries the highest risk of relapse. Therefore, the durability of HBsAg seroclearance should be monitored for at least 1 year to ensure comprehensive assessment.
The HBV recurrence pattern is not limited to the recurrence of HBsAg, as it also includes HBV DNA recurrence. For HBeAg-negative patients, the 48-week cumulative HBsAg and HBV DNA recurrence rates were 12.79% and 2.33%, respectively[10]. In another study of HBeAg-positive patients, the 48-week cumulative HBsAg and HBV DNA recurrence rates were 8.2% and 3.9%, respectively[21]. For patients with HBV DNA recurrence, some researchers detected regional variation in the virus (including sD144A, sI126T, and sT140I) at the time in which the HBV DNA level exceeded 1 × 103 IU/mL[20]. In this study, all patients with relapse displayed HBsAg recurrence, and HBV DNA relapse was not observed. One possible explanation was that these patients received peg-IFN-α retreatment after a mild elevation in HBsAg levels.
Early identification of predictive factors related to recurrence can help guide the optimal timing of peg-IFN-α cessation, and this should be the focus of follow-up. In a recent study, patients with HBsAg seroclearance after 48 weeks of combined peg-IFN-α and NA therapy underwent 1-1.5 years of follow-up. During this period, seven patients experienced relapse, including five patients who did not receive IFN consolidation therapy[22].
IFN consolidation therapy is necessary to maintain clinical cure. Regarding the consolidation time, in previous studies, consolidation therapy for less than 12 weeks after HBsAg seroclearance was predictive of HBsAg relapse[10,21], consistent with the current results. We also found no significant difference in HBsAg recurrence rates between consolidation therapy durations of 12-24 and ≥ 24 weeks. This finding provides valuable guidance for clinical practice, suggesting that 12-24 weeks of consolidation therapy maximizes the HBsAg seroconversion rate and HBsAb level. In addition, this would help to avoid unnecessarily prolonged consolidation therapy, which can lead to increased side effects caused by peg-IFN-α.
In addition to the aforementioned factors, we found that the HBsAg recurrence rate was significantly higher in patients who did not achieve HBsAg seroconversion and in those with low baseline HBsAb levels. As determined by ROC curve analysis, the cutoff baseline HBsAb level predicting HBsAg recurrence was 35.3 mIU/mL, which was lower than that in a previous report[20]. One possible reason for this variability was the difference in sample sizes. HBsAb disappearance during follow-up was a strong predictor of HBsAg recurrence. These results highlight the important roles of HBsAb levels and dynamic changes in maintaining HBsAg persistence, reminding clinicians that a rapid decline in HBsAb levels during follow-up might be accompanied by the reappearance of HBsAg.
There was no significant association between the different treatment strategies and HBsAg recurrence, in line with prior results[20]. In addition, the virological data of the patients, including the HBV DNA level and status, HBeAg status, HBsAg level, family history of HBV infection, FibroScan value, and ALT level at the cessation of peg-IFN-α therapy, did not influence HBsAg recurrence, representing newly reported findings.
For both NA and peg-IFN-α treatment, the ultimate goal is to reduce the risk of disease progression, and thus, it is necessary to evaluate the safety of drug withdrawal. However, many studies confirmed that the risks of hepatitis recurrence, liver cirrhosis, and HCC are significantly reduced in patients with HBsAg seroclearance[9,20]. In a large retrospective study[23], the 5-year cumulative mortality rates of HCC and other liver diseases in patients with HBsAg seroclearance (either spontaneous or treatment-induced) were only 1.7% and 2.6%, respectively. The median follow-up was 9.6 years, and liver cirrhosis or HCC was not observed in 65 patients with spontaneous or treatment-induced HBsAg seroclearance[9]. The consistent results of this study further support this viewpoint. However, it must be stressed that our follow-up period might have been insufficient to capture all possible outcomes. HBV itself is neither directly nor acutely carcinogenic. The long-term coexistence of hepatocyte necrosis, regeneration, and liver inflammation in vivo might lead to abnormal repair and random genetic damage[24], eventually progressing to liver fibrosis, cirrhosis, and HCC. In fact, the interval between persistent HBV infection and complications typically ranges from years to decades. Second, our study did not directly compare patients with persistent HBsAg. In addition, it has been reported that patients with recurrent HBsAg have higher risks of long-term HCC and liver-related death[23]. Sixteen patients experienced HBsAg recurrence in this study, and their risks of future disease progression are unknown. This uncertainty illustrates that short-term follow-up results should not be used as the direct and only evidence of good outcomes for patients. Contiuous long-term follow-up is essential to ensure comprehensive evaluation.
The limitations of this study must be acknowledged. First, most patients had undetectable HBV DNA before IFN treatment, preventing us from assessing the influence of genotype on HBV recurrence. Second, our population was not sufficiently large because of the side effects and cost associated with treatment. In addition, the time points of patient inclusion were not consistent, leading to potential missing data. Future studies with larger sample sizes and longer follow-up are needed to verify the current results.
This study demonstrated that HBsAg seroclearance induced by peg-IFN-α treatment was durable, with a cumulative recurrence rate of 15.2% over an 84-week median follow-up period. Our findings confirmed that patients with CHB might significantly benefit from IFN therapy in the short and medium term, as disease progression was not observed during follow-up. Furthermore, the study highlighted the need to optimize the timing of peg-IFN-α withdrawal and emphasized the importance of continuous and standardized follow-up to ensure long-term treatment success. These insights provided valuable guidance to clinicians in managing CHB and optimizing antiviral treatment.
The authors thank the doctors at Ankang Central Hospital, The Affiliated Hospital of Yan’an University, and The Second Affiliated Hospital of Xi’an Jiaotong University for their assistance with patient enrollment and data collection.
| 1. | Chinese Society of Infectious Diseases; Chinese Medical Association; Chinese Society of Hepatology, Chinese Medical Association. [The guidelines of prevention and treatment for chronic hepatitis B (2019 version)]. Zhonghua Gan Zang Bing Za Zhi. 2019;27:938-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 111] [Reference Citation Analysis (0)] |
| 2. | Chan HL, Thompson A, Martinot-Peignoux M, Piratvisuth T, Cornberg M, Brunetto MR, Tillmann HL, Kao JH, Jia JD, Wedemeyer H, Locarnini S, Janssen HL, Marcellin P. Hepatitis B surface antigen quantification: why and how to use it in 2011 - a core group report. J Hepatol. 2011;55:1121-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 246] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 3. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 3790] [Article Influence: 473.8] [Reference Citation Analysis (1)] |
| 4. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 2834] [Article Influence: 404.9] [Reference Citation Analysis (0)] |
| 5. | Xia Y, Guo H. Hepatitis B virus cccDNA: Formation, regulation and therapeutic potential. Antiviral Res. 2020;180:104824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 131] [Article Influence: 26.2] [Reference Citation Analysis (1)] |
| 6. | Cornberg M, Lok AS, Terrault NA, Zoulim F; 2019 EASL-AASLD HBV Treatment Endpoints Conference Faculty. Guidance for design and endpoints of clinical trials in chronic hepatitis B - Report from the 2019 EASL-AASLD HBV Treatment Endpoints Conference. Hepatology. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 7. | Chu CM, Liaw YF. Prevalence of and risk factors for hepatitis B viremia after spontaneous hepatitis B surface antigen seroclearance in hepatitis B carriers. Clin Infect Dis. 2012;54:88-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Brunskole Hummel I, Zitzmann A, Erl M, Wenzel JJ, Jilg W. Characteristics of immune memory 10-15 years after primary hepatitis B vaccination. Vaccine. 2016;34:636-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Alawad AS, Auh S, Suarez D, Ghany MG. Durability of Spontaneous and Treatment-Related Loss of Hepatitis B s Antigen. Clin Gastroenterol Hepatol. 2020;18:700-709.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 10. | Li MH, Yi W, Zhang L, Lu Y, Lu HH, Shen G, Wu SL, Hao HX, Gao YJ, Chang M, Liu RY, Hu LP, Cao WH, Chen QQ, Li JN, Wan G, Xie Y. Predictors of sustained functional cure in hepatitis B envelope antigen-negative patients achieving hepatitis B surface antigen seroclearance with interferon-alpha-based therapy. J Viral Hepat. 2019;26 Suppl 1:32-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Wong RJ, Nguyen MT, Trinh HN, Chan C, Huynh A, Ly MT, Nguyen HA, Nguyen KK, Torres S, Yang J, Liu B, Garcia RT, Bhuket T, Baden R, Levitt B, da Silveira E, Gish RG. Hepatitis B surface antigen loss and sustained viral suppression in Asian chronic hepatitis B patients: A community-based real-world study. J Viral Hepat. 2017;24:1089-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Kim GA, Lim YS, An J, Lee D, Shim JH, Kim KM, Lee HC, Chung YH, Lee YS, Suh DJ. HBsAg seroclearance after nucleoside analogue therapy in patients with chronic hepatitis B: clinical outcomes and durability. Gut. 2014;63:1325-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 329] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 13. | Cao Z, Meng S, Zheng Y, Wang J, Wang R, Chen X. B cells were related to HBsAg seroconversion in inactive HBsAg carriers following peginterferon therapy. PLoS One. 2020;15:e0242559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Cao Z, Meng S, Zheng Y, Wang J, Wang R, Chen X. Contribution of NK cells to HBsAg seroconversion in inactive HBsAg carriers following pegylated IFN therapy. Innate Immun. 2020;26:601-608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 15. | Lin CC, Yong CC, Chen CL. Active vaccination to prevent de novo hepatitis B virus infection in liver transplantation. World J Gastroenterol. 2015;21:11112-11117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Kim EB, Kim DS, Park SJ, Park Y, Rho KH, Kim SJ. Hepatitis B virus reactivation in a surface antigen-negative and antibody-positive patient after rituximab plus CHOP chemotherapy. Cancer Res Treat. 2008;40:36-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Tavakolpour S, Alavian SM, Sali S. Hepatitis B Reactivation During Immunosuppressive Therapy or Cancer Chemotherapy, Management, and Prevention: A Comprehensive Review-Screened. Hepat Mon. 2016;16:e35810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Chi H, Wong D, Peng J, Cao J, Van Hees S, Vanwolleghem T, Qi X, Chen L, Feld JJ, de Knegt RJ, Hansen BE, Janssen HLA. Durability of Response After Hepatitis B Surface Antigen Seroclearance During Nucleos(t)ide Analogue Treatment in a Multiethnic Cohort of Chronic Hepatitis B Patients: Results After Treatment Cessation. Clin Infect Dis. 2017;65:680-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Lok AS, Zoulim F, Dusheiko G, Chan HLY, Buti M, Ghany MG, Gaggar A, Yang JC, Wu G, Flaherty JF, Subramanian GM, Locarnini S, Marcellin P. Durability of Hepatitis B Surface Antigen Loss With Nucleotide Analogue and Peginterferon Therapy in Patients With Chronic Hepatitis B. Hepatol Commun. 2020;4:8-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 20. | Wu Y, Liu Y, Lu J, Cao Z, Jin Y, Ma L, Geng N, Ren S, Zheng Y, Shen C, Chen X. Durability of Interferon-induced Hepatitis B Surface Antigen Seroclearance. Clin Gastroenterol Hepatol. 2020;18:514-516.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 21. | Li M, Sun F, Bi X, Lin Y, Yang L, Lu Y, Zhang L, Wan G, Yi W, Zhao L, Xie Y. Consolidation treatment needed for sustained HBsAg-negative response induced by interferon-alpha in HBeAg positive chronic hepatitis B patients. Virol Sin. 2022;37:390-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Marcellin P, Ahn SH, Ma X, Caruntu FA, Tak WY, Elkashab M, Chuang WL, Lim SG, Tabak F, Mehta R, Petersen J, Foster GR, Lou L, Martins EB, Dinh P, Lin L, Corsa A, Charuworn P, Subramanian GM, Reiser H, Reesink HW, Fung S, Strasser SI, Trinh H, Buti M, Gaeta GB, Hui AJ, Papatheodoridis G, Flisiak R, Chan HL; Study 149 Investigators. Combination of Tenofovir Disoproxil Fumarate and Peginterferon α-2a Increases Loss of Hepatitis B Surface Antigen in Patients With Chronic Hepatitis B. Gastroenterology. 2016;150:134-144.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 276] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 23. | Yip TC, Wong GL, Wong VW, Tse YK, Lui GC, Lam KL, Chan HL. Durability of hepatitis B surface antigen seroclearance in untreated and nucleos(t)ide analogue-treated patients. J Hepatol. 2017;S0168-8278(17)32332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 24. | Iannacone M, Guidotti LG. Immunobiology and pathogenesis of hepatitis B virus infection. Nat Rev Immunol. 2022;22:19-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 290] [Article Influence: 96.7] [Reference Citation Analysis (0)] |