Published online Nov 21, 2024. doi: 10.3748/wjg.v30.i43.4660
Revised: September 29, 2024
Accepted: October 18, 2024
Published online: November 21, 2024
Processing time: 97 Days and 7.6 Hours
We discuss the article by Koizumi et al published in the World Journal of Gastroenterology. Our focus is on the therapeutic targets for fibrosis associated with alcohol-related liver disease (ALD) and the mechanism of action of elafibranor (EFN), a dual agonist of peroxisome proliferator-activated receptor α (PPARα) and peroxisome PPAR δ (PPARδ). EFN is currently in phase III clinical trials for the treatment of metabolic dysfunction-associated fatty liver disease and primary biliary cholangitis. ALD progresses from alcoholic fatty liver to alcoholic steatohepatitis (ASH), with chronic ASH eventually leading to fibrosis, cirrhosis, and, in some cases, hepatocellular carcinoma. The pathogenesis of ALD is driven by hepatic steatosis, oxidative stress, and acetaldehyde toxicity. Alcohol consump
Core Tip: Peroxisome proliferator-activated receptor (PPAR) can inhibit the pathological process of alcohol-related liver disease (ALD). However, the dual PPARα/δ agonist elafibranor has been studied mainly in metabolic dysfunction-associated steatohepatitis and primary cholangitis, and its role in ALD and its mechanism need to be further explored.
- Citation: Sun YQ, Wu Y, Li MR, Wei YY, Guo M, Zhang ZL. Elafibranor alleviates alcohol-related liver fibrosis by restoring intestinal barrier function. World J Gastroenterol 2024; 30(43): 4660-4668
- URL: https://www.wjgnet.com/1007-9327/full/v30/i43/4660.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i43.4660
In 2019, the World Health Organization estimated that 4.4% of diagnosed cancers and 401000 cancer-related deaths globally were attributable to alcohol consumption. Alcohol-related liver disease (ALD) is a significant global health crisis, accounting for approximately 20% of liver cancer-related deaths[1]. Approximately 90% of ingested alcohol is meta
The gut-liver axis describes the bidirectional relationship between the gut microbiota and the liver, integrating signals from diet, genetics, and environmental factors[6]. Studies have shown that ethanol metabolites, such as acetaldehyde, increase the permeability of Caco-2 cell monolayers, disrupting tight junctions and compromising gut barrier integrity[7]. Gut inflammation is another critical factor in gut barrier dysfunction. Pathological bacterial translocation and elevated plasma levels of gut-derived microbial products, such as lipopolysaccharides (LPS), increase with gut permeability[8]. LPS, a component of gram-negative bacterial outer membranes, is a potent trigger of inflammation and immune responses and has been identified as a key contributor to the development of ALD[9].
Elafibranor (EFN; GFT505), a dual PPARα/δ agonist, has progressed to phase III clinical trials for the treatment of metabolic dysfunction-associated fatty liver disease[10], and PPAR has been identified as a promising pharmacological target for chronic liver diseases, including ALD[11,12]. Could EFN be a viable treatment option for ALD? This possibility remains largely unexplored. EFN protects the liver by acting on several key pathways associated with nonalcoholic steatohepatitis (NASH) pathogenesis, reducing steatosis, improving liver function, and inhibiting the expression of proinflammatory and profibrogenic genes[13,14]. EFN may follow a similar pathway to mitigate the pathological progression of ALD. Li et al[15] suggest that restoring alcohol-impaired PPARα and PPARδ function could reduce the severity of ASH, potentially through alcohol-induced autophagy in adipose tissue.
ALD primarily results from the toxic effects of ethanol and its metabolite acetaldehyde. Acetaldehyde, a highly reactive compound, interferes with several hepatocyte functions, inducing endoplasmic reticulum (ER) stress, mitochondrial dysfunction, and tissue damage[16]. The oxidation of ethanol and acetaldehyde results in the production of large amounts of reduced NADH, which promotes fat synthesis and inhibits mitochondrial fatty acid oxidation, leading to the development of fatty liver[17].
Histological fibrosis staging is a critical prognostic factor in both compensated and decompensated ALD patients. Hepatic fibrosis is a dynamic process involving the excessive accumulation of extracellular matrix components, which is sustained by hepatic myofibroblasts[18]. Fibrosis is a major determinant of the prognosis of ALD patients. Alcoholic hepatic fibrosis is characterized by perivenular fibrosis and venous occlusion, whereas liver injury manifests through macrovesicular steatosis, lobular inflammation, hepatocellular swelling, and necrosis[17]. Some morphological features of ALD can also be observed in nonalcoholic fatty liver disease; however, widespread microvesicular steatosis, cholestasis, and hepatic venular occlusive injuries are more specific to ALD[1]. Acetaldehyde is a key mediator of alcoholic liver fibrosis, activating quiescent hepatic stellate cells (qHSCs), which lose their characteristic cytoplasmic lipid droplets (LDs) and transdifferentiate into activated myofibroblasts (aHSCs)[19]. It also stimulates the synthesis of transforming growth factor β1 (TGF-β1) in HSCs, with secreted TGF-β1 promoting the expression of α-smooth muscle actin and collagen type I[20]. In addition, ROS and endogenous liver LPS contribute to liver fibrosis[21]. The metabolism of alcohol in the liver follows two main pathways[22]. First, ethanol is metabolized to acetaldehyde by ADH, generating byproducts such as ROS, which cause lipid peroxidation and other toxic effects[23-25]. Second, the mitochondrial ethanol oxidizing system, which involves cytochrome P450 (CYP) enzymes, also metabolizes ethanol. Chronic alcohol consumption increases the expression of CYP2E1, which generates large amounts of ROS[26,27] during the metabolism of ethanol to acetaldehyde. Excess ROS can activate apoptotic and autophagic pathways[28], as well as lipid peroxidation[29], exacerbating liver injury. ROS also induce oxidative stress, including ER stress and mitochondrial damage, while amplifying the inflammatory effects of LPS[30]. Interestingly, LPS-induced ROS production is also exacerbated by alcohol[31], impairing intestinal barrier function and increasing permeability. This allows LPS to cross the intestinal barrier and enter the portal circulation, directly affecting the liver[3]. Elevated levels of LPS activate Kupffer cells, promoting hepatic injury[32,33], whereas increased intestinal permeability further increases LPS levels in the liver. Therefore, ROS and LPS act synergistically in the progression of liver disease, exacerbating liver injury[34].
Increasing attention is being given to the treatment of alcoholic liver fibrosis. Nataraj et al[35] reported that estrogens protect female mice from alcohol-induced liver fibrosis by promoting protein arginine N-methyltransferase 6 expression and inhibiting integrin genes. Wu et al[36] reported that cluster of differentiation 73 (CD73) may regulate autophagy in HSCs through the AMPK/AKT/mTOR signaling pathway, thereby exerting an antifibrotic effect on ALD. Koizumi et al[37] demonstrated that EFN reduces liver enlargement, steatosis, and necroinflammation in mice with ALD. Consistent with the reduction in steatosis, EFN significantly decreases hepatic triglyceride (TG) and free fatty acid (FFA) levels, confirming that EFN can mitigate alcohol-induced steatohepatitis in mice. This raises the following question: Can EFN also alleviate lipid metabolism disorders induced by the alcohol metabolite acetaldehyde? These results indicate that while EFN does not alter the expression of lipogenesis-related markers, it significantly increases the expression of lipolysis- and fatty acid oxidation-related markers.
FFAs are absorbed by hepatocytes and converted into TGs, which are stored in LDs alongside cholesterol. The regulatory and functional similarities between autophagy, lipolysis, and lysosomal lipid degradation suggest that autophagy may contribute to the degradation of LDs and TGs[38]. Interestingly, EFN can increase autophagy, promote lipolysis and reduce hepatocyte apoptosis. In vivo, EFN significantly improved alcohol-induced liver injury and inhibited the progression of liver fibrosis in mice. However, intriguingly, in vitro, EFN does not directly affect LX2 cells but appears to modulate HepG2 cell activity, autophagy, and antioxidative responses through PPARα activation, thereby playing an antifibrotic role in ALD[37].
PPARs are fatty acid-activated transcription factors belonging to the nuclear hormone receptor superfamily and play a key role in regulating energy metabolism. Three subtypes of PPARs have been identified: PPARα, PPARγ, and PPARβ/δ[39]. PPARα is involved in fatty acid transport, esterification, and oxidation, whereas PPARβ/δ, which is ubiquitously expressed, not only regulates fatty acid oxidation but also plays a role in glucose metabolism. Evidence suggests that PPARα has anti-inflammatory effects by directly acting on adipocytes. The proposed mechanism involves sirtuin 1 (SIRT1), an NAD (+)-dependent deacetylase that inhibits TNF-α-induced CD40 expression via SIRT1-dependent signaling pathways[40,41]. PPARα also appears to promote macrophage polarization toward an anti-inflammatory phenotype. The known PPAR agonists and their functions are outlined in Table 1. Li et al[42] reported that Huang Qin Tang regulates fatty acid metabolism and mediates M2 macrophage polarization through the FFAR4-AMPK-PPARα pathway. PPARβ/δ influences lipid metabolism by regulating adipokine expression[43] and improving insulin sensitivity[44]. It also reduces acute liver inflammation, as administering PPARβ/δ agonists protects mice from chemically induced hepatotoxicity and downregulates the expression of proinflammatory mediators, including TNF-α and human macrophage chemoattractant protein-1 (MCP-1), by inhibiting nuclear factor κB (NF-κB) activation[45].
| Agonists | Roles |
| PPARα agonists | |
| Bezafibrate | Bezafibrate promotes fatty acid β-oxidation and ketogenesis while reducing abnormal lipid metabolism, inflammation, oxidative stress, and insulin resistance[71]. It ameliorates steatosis, alters lipid composition, and increases mitochondrial mass in the liver[72] |
| Fenofibrate | Fenofibrate has been shown to improve hepatic steatosis and increase TFEB activation, leading to lower levels of LDL and VLDL. It also elevates HDL levels and decreases TG levels[73] |
| Ciprofibrate | Ciprofibrate stimulates the expression of the CETP gene and alters cholesterol flow through reverse cholesterol transport, facilitating plasma cholesterol removal via LDL[74] |
| PPARα/δ agonists | |
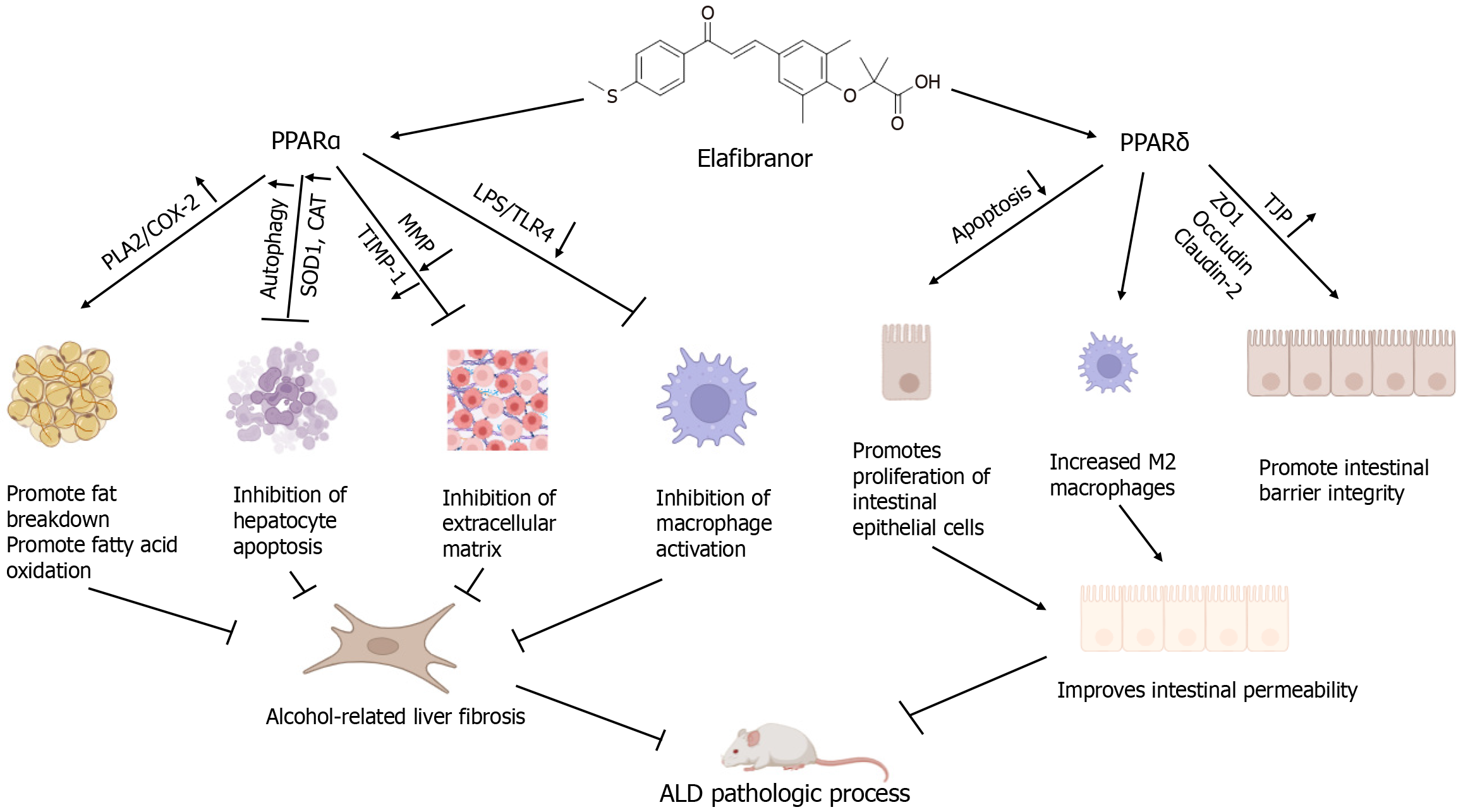
| Elafibranor | EFN promotes lipolysis and β-oxidation, enhances autophagy and antioxidant capacity, inhibits Kupffer cell-mediated inflammatory responses, and impairs the LPS and TLR4/NF-κB signaling pathways. Additionally, EFN improves intestinal barrier hyperpermeability by restoring tight junction proteins, enhancing autophagy, and inhibiting both apoptosis and inflammatory responses[37] |
| PPARγ agonists | |
| Rosiglitazone | Rosiglitazone increases the expression of adipogenic and energetic genes in adipose tissue, as well as defense genes in macrophages[71]. It is primarily used to treat type 2 diabetes, enhancing glucose utilization by cells, which subsequently lowers blood sugar levels[75] |
| Pioglitazone | Pioglitazone reduces glucose production in the liver, lowers triglyceride levels, and increases HDL, thus improving insulin sensitivity[76] |
Macrophages, crucial components of the innate immune system, are essential for immune defense, inflammation regulation, tissue remodeling, and homeostasis. Macrophages can be polarized into two phenotypes: M1-like (proinflammatory) and M2-like (anti-inflammatory) macrophages. M1 macrophages are primarily activated by LPS and interferon-gamma (IFN-γ), whereas M2 macrophages are induced by interleukin-4 (IL-4) and IL-13[46]. M1 macrophages secrete large amounts of proinflammatory cytokines, including IL-1β, inducible nitric oxide synthase, and TNF-α[47], whereas M2 macrophages primarily produce anti-inflammatory cytokines, such as IL-10 and Arg-1[47].
In the context of ALD, Kupffer cell activation plays a pivotal role, and monocyte macrophages are recruited to the liver, where they polarize into M1 or M2 phenotypes depending on the hepatic microenvironment[48]. Voican et al[49] reported reduced macrophage infiltration and increased M2 macrophage polarization in subcutaneous adipose tissue in ALD patients after alcohol withdrawal. Furthermore, M2 macrophages induce hepatocyte senescence via IL-6, mitigating alcohol-induced hepatocyte apoptosis and hepatic steatosis[50]. Thus, macrophage polarization, particularly toward the M2 phenotype, appears to play a key role in resolving ALD. Toll-like receptor 4 (TLR4), an innate immune receptor on macrophages, recognizes pathogen-associated molecular patterns and is the primary receptor for LPS[51]. During ALD progression, the LPS/TLR4 pathway is crucial for activating hepatic macrophages[52]. Increased intrahepatic LPS levels trigger the LPS/TLR4 pathway, leading to M1 macrophage polarization. However, EFN significantly inhibits the expression of LPS-binding protein, Tlr4, and its coreceptor Cd14, reducing the expression of the proinflammatory cytokine TNF-α (Tnfa) in the liver. EFN also decreases the expression of M1 macrophage markers such as Tnfa, Il1b, and Nos2 while increasing the expression of the M2 macrophage marker Arg in the gut of ALD mice. These findings suggest that EFN promotes autophagy in intestinal epithelial cells (IECs), increasing cell survival and maintaining intestinal barrier function[37]. Additionally, Hakeem et al[53] reported that in NASH, EFN reduces ileal inflammation by promoting M2 macrophage polarization, likely through the protective effects of PPARα against intestinal injury via IFN-γ inhibition. Briand et al[54] demonstrated that EFN significantly improves NASH induced by the HFCC/CDX diet (which consists of C57BL6/J mice fed a diet containing 60.00% high-fat, 1.25% cholesterol, and 0.50% cholic acid, along with 2.00% cyclodextrin in drinking water) by suppressing the innate immune system. This includes a reduction in Kupffer cells and natural killer T cells, thereby ameliorating the pathological progression of NASH. This raises the question of whether EFN could also inhibit the progression of ALD by promoting M2 macrophage polarization or by decreasing the overall number of immune cells in the ALD model.
The gut-liver axis refers to the dynamic relationship between the gut microbiota and the liver[55]. Approximately 70% of the liver’s blood supply comes from the intestine via the portal vein, where it carries products derived from food, the gut microbiota, and toxins. An early manifestation of impaired intestinal mucosal barrier function is increased permeability. The interface between the liver and microbiota forms the intestinal mucosal barrier, which separates the gut microbiota from host immune cells to maintain homeostasis. The integrity of the intestinal mucosa depends on several functional components: A protective mucin layer on the luminal surface of IECs and tight junction proteins (TJPs) between intestinal epithelial and immune cells[56,57]. Key TJPs include claudins, occludins, adhesion molecules, and zonula occludens (ZOs)[58]. Decreases in TJPs or alterations in IECs can result in increased mucosal permeability[59].
Alcohol intake increases the levels of acetaldehyde, a metabolite that damages tight junctions, leading to gut dysbiosis and increased intestinal permeability. This allows bacterial endotoxins, such as LPS, to enter the portal vein, where they activate TLRs on Kupffer cells and hepatocytes, promoting inflammation and fibrosis and exacerbating the pathophysiology of ALD[60]. In an ALD mouse model[37], increased expression of genes such as Ftcd and Sox9, which are associated with reduced TJPs and inflammation and inhibited epithelial cell proliferation, was observed, whereas the expression of Dhrs9, FoxM1, S100G, and Mgl2, which promote intestinal barrier function and have anti-inflammatory effects, decreased. EFN significantly mitigated these changes, effectively reducing the loss of TJPs, improving intestinal permeability, and restoring mucosal barrier integrity.
EFN has been shown to increase the expression of key TJPs, such as ZO-1 and occludin[37], thereby restoring intestinal barrier function. The intestinal epithelium, which is composed of a single layer of closely connected columnar epithelial cells, serves as the first line of defense for the intestinal mucosa. Increased permeability is closely linked with the apoptosis and autophagy of IECs[61,62]. Autophagy is crucial for cell survival under stress[15] and reinforces the epithelial barrier by preserving proteins such as ZO-1 and occludin[63,64]. While apoptosis is a natural protective mechanism, excessive apoptosis can hinder the repair and regeneration of IECs, leading to barrier dysfunction. Kim et al[65] reported that PPARα promotes intestinal tissue repair by inhibiting pigment epithelium-derived factor and encouraging intestinal stem cell proliferation, thereby supporting barrier integrity. Abdulqadir et al[66] demonstrated that PPARγ prevents the TNFα-induced increase in epithelial permeability by inhibiting the NF-κB p50/p65 signaling pathway and activating the myosin light chain kinase gene[66]. Similarly, Sohn et al[67] reported that PPARγ in Lactobacillus paracasei acts similarly to PPARδ. As a dual agonist of PPARα and PPARδ, the role of EFN in intestinal permeability and barrier function is significant. EFN has been reported to inhibit apoptosis in IECs by activating autophagy[15], which reduces the level of IL-1β/MCP-1 and restores the intestinal barrier. Koizumi et al[37] demon
In a recent issue of the World Journal of Gastroenterology, Koizumi et al[37] published an intriguing paper titled “Effects of elafibranor on liver fibrosis and gut barrier function in a mouse model of alcohol-associated liver disease”. This study addresses a critical question: How does EFN mitigate the pathological progression of ALD-related liver fibrosis?
EFN significantly alleviated hepatic steatosis, apoptosis, and liver fibrosis in the ALD mouse model. It primarily activates PPARα, promoting fat breakdown and β-oxidation in ethanol (EtOH)-stimulated HepG2 cells while enhancing autophagy and antioxidant capacity. Moreover, EFN inhibits Kupffer cell-mediated inflammatory responses, reducing liver exposure to LPS and downregulating the TLR4/NF-κB signaling pathway. EFN also improves intestinal barrier function by restoring TJPs, enhancing autophagy, and suppressing cell apoptosis and inflammatory responses. The protective effect of EFN on intestinal barrier integrity in EtOH-stimulated Caco-2 cells is largely mediated through PPARδ activation.
In contrast to a previously published article[15], which focused on a different aspect, Koizumi et al[37] emphasized the liver fibrosis process induced by ALD, a topic not highlighted by Li et al[15]. However, a question arises: While EFN significantly reduces liver fibrosis in the ALD mouse model, it does not notably affect LX2 cells. Could this suggest a novel therapeutic approach: Targeting ALD-related liver fibrosis through mechanisms that bypass LX2 cells? Additionally, given that HepG2 cells are derived from a hepatocellular carcinoma line, future research should focus on analyzing the effects of EFN on primary cultured liver cells.
The literature suggests that PPARα activation can influence IECs[68]. In Caco-2 cells, treatment with the PPARα agonist fenofibrate preserves barrier function, reduces junctional curling, and increases Claudin-1 expression after exposure to high glucose or inflammatory cytokines. However, Koizumi et al[37] revealed limited effects of PPARα on intestinal barrier function in Caco-2 cells treated with EFN, possibly due to differences in experimental models. A diagram illustrating the specific mechanisms of action of EFN in ALD mice is presented in Figure 1.
While compounds that activate multiple PPAR subtypes (dual agonists) or all PPARs (panagonists) may benefit lipid and glucose metabolism[69], the reduced target specificity of such agonists could lead to more adverse effects[70]. Therefore, although EFN shows promise in inhibiting the progression of ALD-related liver fibrosis, further investigations are needed to assess its potential side effects.
EFN, a dual agonist of PPARα and PPARδ, has demonstrated potential not only in treating metabolic disorders associated with hepatic steatosis and primary biliary cholangitis but also in inhibiting the development of EtOH + CCl4-induced liver fibrosis in ALD model mice. These findings suggest that EFN could be a promising therapeutic option for ALD. Furthermore, Koizumi et al[37] proposed the intriguing hypothesis that EFN may exert anti-ALD liver fibrosis effects without directly affecting LX2 cells, a notion that warrants further investigation and validation.
In conclusion, EFN holds promise as a therapeutic agent for ALD because of its dual activation of PPARα/δ, which enhances lipid metabolism, reduces inflammation, and strengthens gut barrier function. Additional clinical trials are essential to confirm EFN’s efficacy and safety in treating ALD and to explore its potential applications in other liver diseases.
| 1. | Caputo F, Lungaro L, Guarino M, Costanzini A, Caio G, Testino G, DE Giorgio R. Alcohol-related diseases: from metabolism to the main effect on the body. Minerva Med. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Linhart K, Bartsch H, Seitz HK. The role of reactive oxygen species (ROS) and cytochrome P-450 2E1 in the generation of carcinogenic etheno-DNA adducts. Redox Biol. 2014;3:56-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 136] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 3. | Seitz HK, Bataller R, Cortez-Pinto H, Gao B, Gual A, Lackner C, Mathurin P, Mueller S, Szabo G, Tsukamoto H. Alcoholic liver disease. Nat Rev Dis Primers. 2018;4:16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 807] [Article Influence: 115.3] [Reference Citation Analysis (0)] |
| 4. | Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer. 2007;7:599-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 745] [Cited by in RCA: 766] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 5. | Purohit V, Gao B, Song BJ. Molecular mechanisms of alcoholic fatty liver. Alcohol Clin Exp Res. 2009;33:191-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 245] [Article Influence: 15.3] [Reference Citation Analysis (1)] |
| 6. | Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol. 2020;72:558-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 1257] [Article Influence: 251.4] [Reference Citation Analysis (1)] |
| 7. | Rao RK. Acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Alcohol Clin Exp Res. 1998;22:1724-1730. [PubMed] |
| 8. | Hartmann P, Seebauer CT, Schnabl B. Alcoholic liver disease: the gut microbiome and liver cross talk. Alcohol Clin Exp Res. 2015;39:763-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 222] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 9. | Fadl AA, Sha J, Klimpel GR, Olano JP, Niesel DW, Chopra AK. Murein lipoprotein is a critical outer membrane component involved in Salmonella enterica serovar typhimurium systemic infection. Infect Immun. 2005;73:1081-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Zhang M, Barroso E, Ruart M, Peña L, Peyman M, Aguilar-Recarte D, Montori-Grau M, Rada P, Cugat C, Montironi C, Zarei M, Jurado-Aguilar J, Camins A, Balsinde J, Valverde ÁM, Wahli W, Palomer X, Vázquez-Carrera M. Elafibranor upregulates the EMT-inducer S100A4 via PPARβ/δ. Biomed Pharmacother. 2023;167:115623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 11. | Königshofer P, Brusilovskaya K, Petrenko O, Hofer BS, Schwabl P, Trauner M, Reiberger T. Nuclear receptors in liver fibrosis. Biochim Biophys Acta Mol Basis Dis. 2021;1867:166235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 12. | Tanaka N, Aoyama T, Kimura S, Gonzalez FJ. Targeting nuclear receptors for the treatment of fatty liver disease. Pharmacol Ther. 2017;179:142-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 169] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 13. | Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, Romero-Gomez M, Boursier J, Abdelmalek M, Caldwell S, Drenth J, Anstee QM, Hum D, Hanf R, Roudot A, Megnien S, Staels B, Sanyal A; GOLDEN-505 Investigator Study Group. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-α and -δ, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology. 2016;150:1147-1159.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 820] [Article Influence: 91.1] [Reference Citation Analysis (0)] |
| 14. | Staels B, Rubenstrunk A, Noel B, Rigou G, Delataille P, Millatt LJ, Baron M, Lucas A, Tailleux A, Hum DW, Ratziu V, Cariou B, Hanf R. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2013;58:1941-1952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 344] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 15. | Li TH, Yang YY, Huang CC, Liu CW, Tsai HC, Lin MW, Tsai CY, Huang SF, Wang YW, Lee TY, Huang YH, Hou MC, Lin HC. Elafibranor interrupts adipose dysfunction-mediated gut and liver injury in mice with alcoholic steatohepatitis. Clin Sci (Lond). 2019;133:531-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Jo SL, Baek IJ, Ko JW, Kwun HJ, Shin HJ, Hong EJ. Hepatic progesterone receptor membrane component 1 attenuates ethanol-induced liver injury by reducing acetaldehyde production and oxidative stress. Am J Physiol Gastrointest Liver Physiol. 2023;324:G442-G451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Lackner C, Tiniakos D. Fibrosis and alcohol-related liver disease. J Hepatol. 2019;70:294-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 200] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 18. | Parola M, Pinzani M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med. 2019;65:37-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 780] [Article Influence: 111.4] [Reference Citation Analysis (0)] |
| 19. | Lucantoni F, Martínez-Cerezuela A, Gruevska A, Moragrega ÁB, Víctor VM, Esplugues JV, Blas-García A, Apostolova N. Understanding the implication of autophagy in the activation of hepatic stellate cells in liver fibrosis: are we there yet? J Pathol. 2021;254:216-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 20. | Lestari N, Louisa M, Soetikno V, Suwana AG, Ramadhan PA, Akmal T, Arozal W. Alpha Mangostin Inhibits the Proliferation and Activation of Acetaldehyde Induced Hepatic Stellate Cells through TGF-β and ERK 1/2 Pathways. J Toxicol. 2018;2018:5360496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Ishida K, Kaji K, Sato S, Ogawa H, Takagi H, Takaya H, Kawaratani H, Moriya K, Namisaki T, Akahane T, Yoshiji H. Sulforaphane ameliorates ethanol plus carbon tetrachloride-induced liver fibrosis in mice through the Nrf2-mediated antioxidant response and acetaldehyde metabolization with inhibition of the LPS/TLR4 signaling pathway. J Nutr Biochem. 2021;89:108573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 22. | Ceni E, Mello T, Galli A. Pathogenesis of alcoholic liver disease: role of oxidative metabolism. World J Gastroenterol. 2014;20:17756-17772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 286] [Cited by in RCA: 354] [Article Influence: 32.2] [Reference Citation Analysis (5)] |
| 23. | de Groot H. Reactive oxygen species in tissue injury. Hepatogastroenterology. 1994;41:328-332. [PubMed] |
| 24. | Nakazawa H, Genka C, Fujishima M. Pathological aspects of active oxygens/free radicals. Jpn J Physiol. 1996;46:15-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Toyokuni S. Reactive oxygen species-induced molecular damage and its application in pathology. Pathol Int. 1999;49:91-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 370] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 26. | Seitz HK, Mueller S, Hellerbrand C, Liangpunsakul S. Effect of chronic alcohol consumption on the development and progression of non-alcoholic fatty liver disease (NAFLD). Hepatobiliary Surg Nutr. 2015;4:147-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 27. | Hrubec Z, Omenn GS. Evidence of genetic predisposition to alcoholic cirrhosis and psychosis: twin concordances for alcoholism and its biological end points by zygosity among male veterans. Alcohol Clin Exp Res. 1981;5:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 251] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Xing Y, Wei X, Liu Y, Wang MM, Sui Z, Wang X, Zhu W, Wu M, Lu C, Fei YH, Jiang Y, Zhang Y, Wang Y, Guo F, Cao JL, Qi J, Wang W. Autophagy inhibition mediated by MCOLN1/TRPML1 suppresses cancer metastasis via regulating a ROS-driven TP53/p53 pathway. Autophagy. 2022;18:1932-1954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 29. | Wang B, Wang Y, Zhang J, Hu C, Jiang J, Li Y, Peng Z. ROS-induced lipid peroxidation modulates cell death outcome: mechanisms behind apoptosis, autophagy, and ferroptosis. Arch Toxicol. 2023;97:1439-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 252] [Reference Citation Analysis (0)] |
| 30. | Salete-Granado D, Carbonell C, Puertas-Miranda D, Vega-Rodríguez VJ, García-Macia M, Herrero AB, Marcos M. Autophagy, Oxidative Stress, and Alcoholic Liver Disease: A Systematic Review and Potential Clinical Applications. Antioxidants (Basel). 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 31. | Yamada H, Arai T, Endo N, Yamashita K, Fukuda K, Sasada M, Uchiyama T. LPS-induced ROS generation and changes in glutathione level and their relation to the maturation of human monocyte-derived dendritic cells. Life Sci. 2006;78:926-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Mandrekar P, Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol. 2009;50:1258-1266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 396] [Cited by in RCA: 392] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 33. | Hines IN, Wheeler MD. Recent advances in alcoholic liver disease III. Role of the innate immune response in alcoholic hepatitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G310-G314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 112] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Zhang D, Liu Z, Bai F. Roles of Gut Microbiota in Alcoholic Liver Disease. Int J Gen Med. 2023;16:3735-3746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 35. | Nataraj K, Schonfeld M, Rodriguez A, Tikhanovich I. Protective role of 17β-estradiol in alcohol-associated liver fibrosis is mediated by suppression of integrin signaling. Hepatol Commun. 2024;8. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 36. | Wu X, Liu XQ, Liu ZN, Xia GQ, Zhu H, Zhang MD, Wu BM, Lv XW. CD73 aggravates alcohol-related liver fibrosis by promoting autophagy mediated activation of hepatic stellate cells through AMPK/AKT/mTOR signaling pathway. Int Immunopharmacol. 2022;113:109229. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 37. | Koizumi A, Kaji K, Nishimura N, Asada S, Matsuda T, Tanaka M, Yorioka N, Tsuji Y, Kitagawa K, Sato S, Namisaki T, Akahane T, Yoshiji H. Effects of elafibranor on liver fibrosis and gut barrier function in a mouse model of alcohol-associated liver disease. World J Gastroenterol. 2024;30:3428-3446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Reference Citation Analysis (2)] |
| 38. | Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131-1135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3239] [Cited by in RCA: 3105] [Article Influence: 194.1] [Reference Citation Analysis (0)] |
| 39. | Christofides A, Konstantinidou E, Jani C, Boussiotis VA. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metabolism. 2021;114:154338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 395] [Article Influence: 98.8] [Reference Citation Analysis (0)] |
| 40. | Wang L, Teng R, Di L, Rogers H, Wu H, Kopp JB, Noguchi CT. PPARα and Sirt1 mediate erythropoietin action in increasing metabolic activity and browning of white adipocytes to protect against obesity and metabolic disorders. Diabetes. 2013;62:4122-4131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 41. | Wang W, Lin Q, Lin R, Zhang J, Ren F, Zhang J, Ji M, Li Y. PPARα agonist fenofibrate attenuates TNF-α-induced CD40 expression in 3T3-L1 adipocytes via the SIRT1-dependent signaling pathway. Exp Cell Res. 2013;319:1523-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 42. | Li MY, Wu YZ, Qiu JG, Lei JX, Li MX, Xu N, Liu YH, Jin Z, Su ZR, Lee SM, Zheng XB, Xiao-Qi H. Huangqin Decoction ameliorates ulcerative colitis by regulating fatty acid metabolism to mediate macrophage polarization via activating FFAR4-AMPK-PPARα pathway. J Ethnopharmacol. 2023;311:116430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 43. | Choi KC, Lee SY, Yoo HJ, Ryu OH, Lee KW, Kim SM, Baik SH, Choi KM. Effect of PPAR-delta agonist on the expression of visfatin, adiponectin, and resistin in rat adipose tissue and 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2007;357:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Silva-Veiga FM, Rachid TL, de Oliveira L, Graus-Nunes F, Mandarim-de-Lacerda CA, Souza-Mello V. GW0742 (PPAR-beta agonist) attenuates hepatic endoplasmic reticulum stress by improving hepatic energy metabolism in high-fat diet fed mice. Mol Cell Endocrinol. 2018;474:227-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 45. | Shan W, Palkar PS, Murray IA, McDevitt EI, Kennett MJ, Kang BH, Isom HC, Perdew GH, Gonzalez FJ, Peters JM. Ligand activation of peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta) attenuates carbon tetrachloride hepatotoxicity by downregulating proinflammatory gene expression. Toxicol Sci. 2008;105:418-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 46. | Yunna C, Mengru H, Lei W, Weidong C. Macrophage M1/M2 polarization. Eur J Pharmacol. 2020;877:173090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 1424] [Article Influence: 284.8] [Reference Citation Analysis (0)] |
| 47. | Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi A, Afshari JT, Sahebkar A. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425-6440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1445] [Cited by in RCA: 3260] [Article Influence: 465.7] [Reference Citation Analysis (0)] |
| 48. | Li P, He K, Li J, Liu Z, Gong J. The role of Kupffer cells in hepatic diseases. Mol Immunol. 2017;85:222-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 164] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 49. | Voican CS, Njiké-Nakseu M, Boujedidi H, Barri-Ova N, Bouchet-Delbos L, Agostini H, Maitre S, Prévot S, Cassard-Doulcier AM, Naveau S, Perlemuter G. Alcohol withdrawal alleviates adipose tissue inflammation in patients with alcoholic liver disease. Liver Int. 2015;35:967-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 50. | Wan J, Benkdane M, Alons E, Lotersztajn S, Pavoine C. M2 kupffer cells promote hepatocyte senescence: an IL-6-dependent protective mechanism against alcoholic liver disease. Am J Pathol. 2014;184:1763-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 51. | Ciesielska A, Matyjek M, Kwiatkowska K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol Life Sci. 2021;78:1233-1261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 838] [Article Influence: 167.6] [Reference Citation Analysis (0)] |
| 52. | Olona A, Hateley C, Muralidharan S, Wenk MR, Torta F, Behmoaras J. Sphingolipid metabolism during Toll-like receptor 4 (TLR4)-mediated macrophage activation. Br J Pharmacol. 2021;178:4575-4587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 53. | Hakeem AN, Kamal MM, Tawfiq RA, Abdelrahman BA, Hammam OA, Elmazar MM, El-Khatib AS, Attia YM. Elafibranor modulates ileal macrophage polarization to restore intestinal integrity in NASH: Potential crosstalk between ileal IL-10/STAT3 and hepatic TLR4/NF-κB axes. Biomed Pharmacother. 2023;157:114050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 54. | Briand F, Heymes C, Bonada L, Angles T, Charpentier J, Branchereau M, Brousseau E, Quinsat M, Fazilleau N, Burcelin R, Sulpice T. A 3-week nonalcoholic steatohepatitis mouse model shows elafibranor benefits on hepatic inflammation and cell death. Clin Transl Sci. 2020;13:529-538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 55. | Pabst O, Hornef MW, Schaap FG, Cerovic V, Clavel T, Bruns T. Gut-liver axis: barriers and functional circuits. Nat Rev Gastroenterol Hepatol. 2023;20:447-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 152] [Reference Citation Analysis (0)] |
| 56. | Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146:1513-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 750] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 57. | Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology. 2015;148:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 548] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 58. | Buckley A, Turner JR. Cell Biology of Tight Junction Barrier Regulation and Mucosal Disease. Cold Spring Harb Perspect Biol. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 478] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 59. | Rawat M, Nighot M, Al-Sadi R, Gupta Y, Viszwapriya D, Yochum G, Koltun W, Ma TY. IL1B Increases Intestinal Tight Junction Permeability by Up-regulation of MIR200C-3p, Which Degrades Occludin mRNA. Gastroenterology. 2020;159:1375-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 155] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 60. | Dunagan M, Chaudhry K, Samak G, Rao RK. Acetaldehyde disrupts tight junctions in Caco-2 cell monolayers by a protein phosphatase 2A-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1356-G1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 61. | Armacki M, Trugenberger AK, Ellwanger AK, Eiseler T, Schwerdt C, Bettac L, Langgartner D, Azoitei N, Halbgebauer R, Groß R, Barth T, Lechel A, Walter BM, Kraus JM, Wiegreffe C, Grimm J, Scheffold A, Schneider MR, Peuker K, Zeißig S, Britsch S, Rose-John S, Vettorazzi S, Wolf E, Tannapfel A, Steinestel K, Reber SO, Walther P, Kestler HA, Radermacher P, Barth TF, Huber-Lang M, Kleger A, Seufferlein T. Thirty-eight-negative kinase 1 mediates trauma-induced intestinal injury and multi-organ failure. J Clin Invest. 2018;128:5056-5072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 62. | Subramanian S, Geng H, Tan XD. Cell death of intestinal epithelial cells in intestinal diseases. Sheng Li Xue Bao. 2020;72:308-324. [PubMed] |
| 63. | Huang L, Jiang Y, Sun Z, Gao Z, Wang J, Zhang D. Autophagy Strengthens Intestinal Mucosal Barrier by Attenuating Oxidative Stress in Severe Acute Pancreatitis. Dig Dis Sci. 2018;63:910-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 64. | Hu CA, Hou Y, Yi D, Qiu Y, Wu G, Kong X, Yin Y. Autophagy and tight junction proteins in the intestine and intestinal diseases. Anim Nutr. 2015;1:123-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 65. | Kim G, Chen Z, Li J, Luo J, Castro-Martinez F, Wisniewski J, Cui K, Wang Y, Sun J, Ren X, Crawford SE, Becerra SP, Zhu J, Liu T, Wang S, Zhao K, Wu C. Gut-liver axis calibrates intestinal stem cell fitness. Cell. 2024;187:914-930.e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 66. | Abdulqadir R, Al-Sadi R, Haque M, Gupta Y, Rawat M, Ma TY. Bifidobacterium bifidum Strain BB1 Inhibits Tumor Necrosis Factor-α-Induced Increase in Intestinal Epithelial Tight Junction Permeability via Toll-Like Receptor-2/Toll-Like Receptor-6 Receptor Complex-Dependent Stimulation of Peroxisome Proliferator-Activated Receptor γ and Suppression of NF-κB p65. Am J Pathol. 2024;194:1664-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 67. | Sohn W, Jun DW, Lee KN, Lee HL, Lee OY, Choi HS, Yoon BC. Lactobacillus paracasei Induces M2-Dominant Kupffer Cell Polarization in a Mouse Model of Nonalcoholic Steatohepatitis. Dig Dis Sci. 2015;60:3340-3350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 68. | Colin S, Briand O, Touche V, Wouters K, Baron M, Pattou F, Hanf R, Tailleux A, Chinetti G, Staels B, Lestavel S. Activation of intestinal peroxisome proliferator-activated receptor-α increases high-density lipoprotein production. Eur Heart J. 2013;34:2566-2574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 69. | Dubois V, Eeckhoute J, Lefebvre P, Staels B. Distinct but complementary contributions of PPAR isotypes to energy homeostasis. J Clin Invest. 2017;127:1202-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 278] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 70. | Cheng HS, Tan WR, Low ZS, Marvalim C, Lee JYH, Tan NS. Exploration and Development of PPAR Modulators in Health and Disease: An Update of Clinical Evidence. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 71. | Qiu YY, Zhang J, Zeng FY, Zhu YZ. Roles of the peroxisome proliferator-activated receptors (PPARs) in the pathogenesis of nonalcoholic fatty liver disease (NAFLD). Pharmacol Res. 2023;192:106786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 102] [Reference Citation Analysis (0)] |
| 72. | Franko A, Neschen S, Rozman J, Rathkolb B, Aichler M, Feuchtinger A, Brachthäuser L, Neff F, Kovarova M, Wolf E, Fuchs H, Häring HU, Peter A, Hrabě de Angelis M. Bezafibrate ameliorates diabetes via reduced steatosis and improved hepatic insulin sensitivity in diabetic TallyHo mice. Mol Metab. 2017;6:256-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 73. | Zhang D, Ma Y, Liu J, Wang D, Geng Z, Wen D, Chen H, Wang H, Li L, Zhu X, Wang X, Huang M, Zou C, Chen Y, Ma L. Fenofibrate improves hepatic steatosis, insulin resistance, and shapes the gut microbiome via TFEB-autophagy in NAFLD mice. Eur J Pharmacol. 2023;960:176159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 74. | Bighetti EJ, Patrício PR, Casquero AC, Berti JA, Oliveira HC. Ciprofibrate increases cholesteryl ester transfer protein gene expression and the indirect reverse cholesterol transport to the liver. Lipids Health Dis. 2009;8:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 75. | Grimaldi B, Kohan-Ghadr HR, Halari CD, Nandi P, Kingdom JC, Drewlo S. Rosiglitazone-Mediated Activation of PPARγ Restores HO1 Expression in the Human Preeclamptic Placenta. Hypertension. 2023;80:2386-2396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 76. | Hu Y, Tao R, Chen L, Xiong Y, Xue H, Hu L, Yan C, Xie X, Lin Z, Panayi AC, Mi B, Liu G. Exosomes derived from pioglitazone-pretreated MSCs accelerate diabetic wound healing through enhancing angiogenesis. J Nanobiotechnology. 2021;19:150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 182] [Article Influence: 45.5] [Reference Citation Analysis (0)] |