Published online Nov 7, 2024. doi: 10.3748/wjg.v30.i41.4509
Revised: September 24, 2024
Accepted: October 8, 2024
Published online: November 7, 2024
Processing time: 66 Days and 14.2 Hours
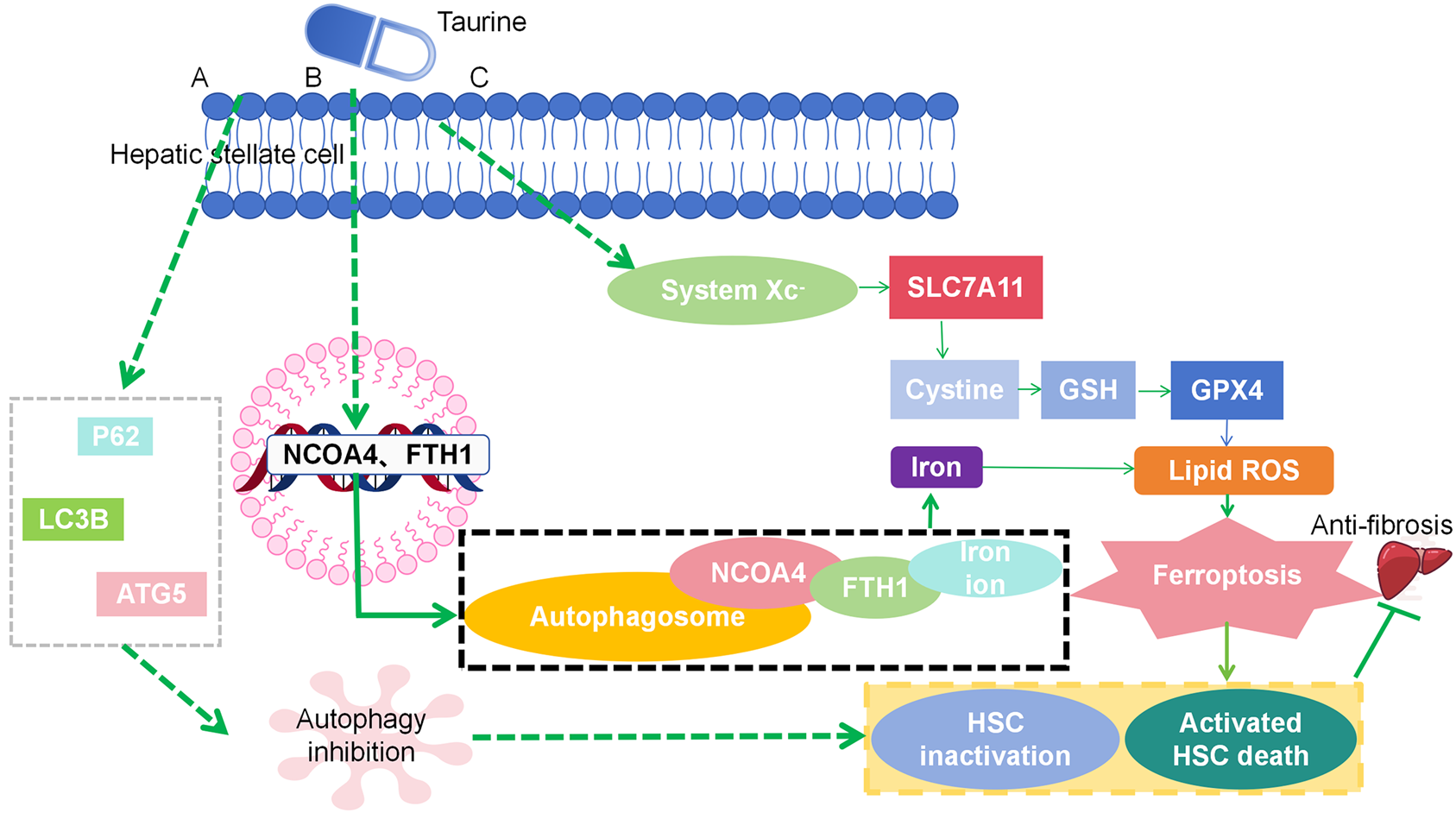
We summarize the mechanism by which taurine (Tau) inhibits autophagy and induces iron apoptosis in hepatic stellate cells. Tau interacts with autophagy regulates multifunctional proteins, microtubule-associated protein 1 light chain 3 Beta, and autophagy-related gene 5 to inhibit autophagy, binds to ferritin heavy chain 1 and nuclear receptor coactivator 4 to trigger ferritin autophagy, and interacts with glutathione peroxidase 4 to promote iron apoptosis. There is a solid rationale for developing Tau-based therapies targeting autophagy and ferroptosis regulation. From a pharmaceutical point of view, there are certain requirements for Tau protein delivery systems, such as loading efficiency, stability, and targeting. Nanomaterials should also contain a hydrophilic motif similar to Tau to optimize loading efficiency. Since Tau is a hydrophilic molecule with high water solubility, liposomes, micelles, and amphiphilic polymer nanoparticles may represent a superior choice. The nanostructure of the liposome includes a water region and a lipid membrane to sequester hydrophilic and hydrophobic drugs, respectively, whereas Tau is expected to be loaded into the water region. In addition, a representative method of actively targeting hematopoietic stem cells is introduced. A Tau-based method for the treatment of liver fibrosis is proposed based on the formulation of common liposomes (lecithin plus cholesterol).
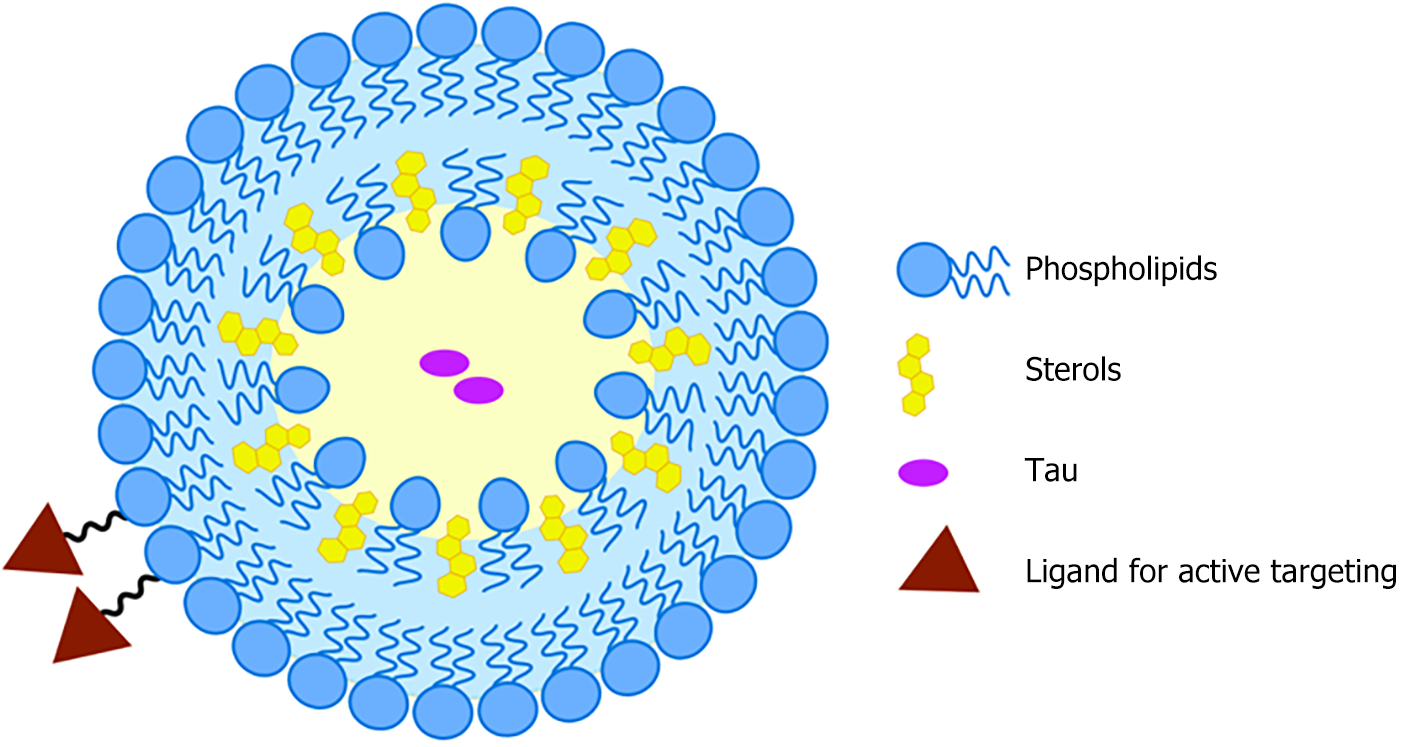
Core Tip: Nanoparticle delivery systems are effective for delivering taurine (Tau). The nanoarchitecture of liposomes includes a water zone and a lipid membrane to accommodate hydrophilic and hydrophobic drugs, respectively. Tau is a hydrophilic molecule with high water solubility, and it is expected to be loaded into the water zone. In theory, hepatic stellate cells (HSCs) may be targeted by Tau-incorporated liposomes via two mechanisms: Passive targeting and active targeting. Active targeting is more robust as it takes advantage of the unique features of the lesion site. Based on a formulation for common liposomes (lecithin plus cholesterol), Tau-based therapeutics was proposed to treat liver fibrosis as follows: A targetable liposome was fabricated through the combination of common lecithin and cholesterol, along with modified lecithin or cholesterol with HSC targetability. Tau was dispersed into the water zone of this liposomal system.
- Citation: Zhang XJ, Jiang XY, Ma YL, Huang FY, Huang ZW. Encapsulating taurine into liposomes: A promising therapeutic for liver fibrosis. World J Gastroenterol 2024; 30(41): 4509-4513
- URL: https://www.wjgnet.com/1007-9327/full/v30/i41/4509.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i41.4509
We are pleased to provide a critique of the research article, which was published in the World Journal of Gastroenterology[1]. The authors describe the use of taurine (Tau) as a bioactive compound to inhibit autophagy and activate the ferroptosis pathway in hepatic stellate cells (HSCs) as a potential therapy for liver fibrosis. The effect of Tau on attenuating the extracellular matrix in liver fibrosis was confirmed. A series of biochemical and pharmacological tests were performed to assess the hallmarks of autophagy [e.g., microtubule-associated protein 1 light chain 3 beta (LC3B), Beclin 1, and autophagy regulates multifunctional proteins (p62)] and ferroptosis (e.g., reactive oxygen species, glutathione, and malondialdehyde). A pioneer molecular docking experiment between Tau and ferritin heavy chain 1 (FTH1) or nuclear receptor coactivator 4 (NCOA4) was performed to demonstrate the presence of ferritinophagy, which represents crosstalk between autophagy and ferroptosis.
The article summarizes autophagy inhibition and ferroptosis induction mechanisms of Tau in HSCs, as shown in Figure 1. Briefly, Tau interacts with p62, LC3B, and autophagy-related gene 5 to inhibit autophagy, binds to FTH1 and NCOA4 to trigger ferritinophagy, and interacts with glutathione peroxidase 4 to promote ferroptosis.
In addition to this article, similar results were reported in other studies. It was well documented that Tau regulates cellular autophagy[2] and ferroptosis[3] caused by various factors. This suggests that developing Tau-based therapeutics based on autophagy and ferroptosis regulation is on a relatively firm ground.
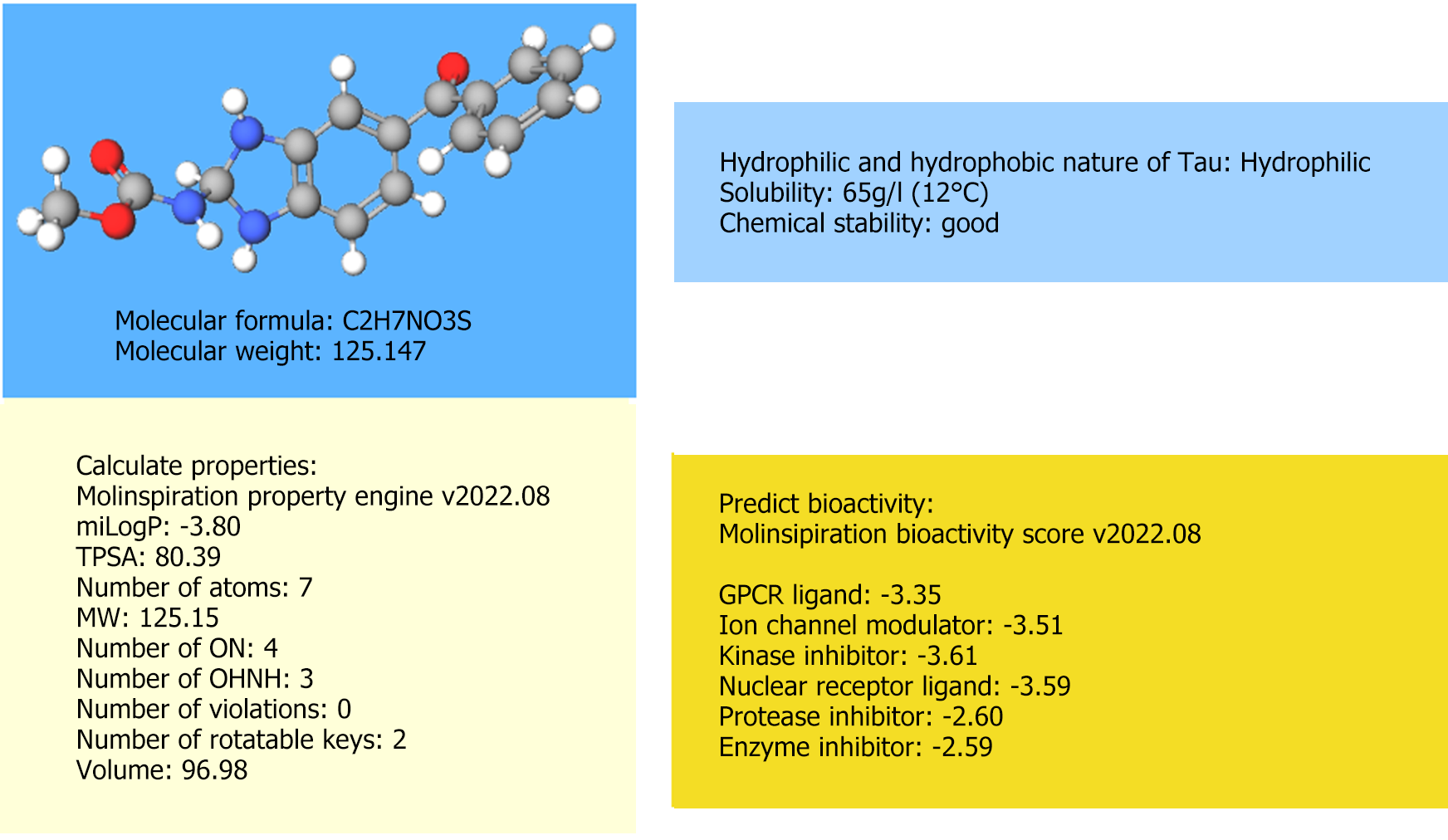
Nevertheless, there is still a long journey toward the real-world application of Tau-based therapeutics for liver fibrosis. The major pending issue that must be addressed is the design of an appropriate delivery system for Tau. From the perspective of pharmaceutics, an ideal Tau delivery system should have satisfactory loading efficiency, stability, and targetability. The molecular data for Tau, which was not provided in the original article, should be examined to design such a system. Based on the online molecule database (e.g., molinspiration cheminformatics, https://www.molin
The delivery systems that are suitable for Tau must be considered, which are based on the information shown in Figure 2. To ensure loading efficiency, stability, and targetability, nanoparticle delivery systems (NDS) are appropriate choices, as they have been extensively reported to exhibit these properties[6]. As shown in Figure 2, the chemical stability of Tau is relatively high. Thus, NDS design considerations to improve stability may be partially exempt. However, it is advisable to avoid NDS with unsatisfactory stability, such as nanocrystals[7], exosomes[8], and DNA origamis[9]. Furthermore, Tau is a hydrophilic molecule with a high water solubility. Based on the rationale of “like dissolves like”, the nanomaterial should contain motifs with similar hydrophilicity as Tau to ensure an acceptable loading efficiency. NDS is primarily composed of hydrophobic nanomaterials, such as solid lipid nanoparticles and nanostructured lipid carriers, which are less likely to achieve this goal. From this perspective, liposomes[10], micelles, and polymeric nanoparticles with amphiphilic properties (containing both hydrophilic and hydrophobic blocks) may be considered as better alternatives. Because liposomes are biocompatible vehicles with clinical translation potential as evidenced by the licensed products (Doxil, Arikayce, etc.), they stand out as most promising candidates for the Tau delivery system. The liposome nanoarchitecture involves a water zone and a lipid membrane to accommodate hydrophilic and hydrophobic drugs, respectively, and Tau is expected to be loaded into the water zone.
The targetability consideration has not been fulfilled. As demonstrated in the original article, HSCs are prime targets for liver fibrosis, and targeted therapy toward HSCs is emphasized herein. In theory, two processes: (1) Passive targeting and (2) Active targeting, are developed to target HSCs by Tau-incorporated liposomes. Passive targeting refers to taking advantage of the liver sinusoidal fenestrae diameter and rendering a < 100 nm particle size for the liposomes. For active targeting, the ligands of the abundantly expressed receptors on HSCs, including collagen type VI receptor, mannose 6-phosphate receptor, platelet-derived growth factor receptor-β, and retinol-binding protein, are tagged onto the liposome surface[11]. Active targeting is considered more robust as it takes advantage of the unique features of the lesion site while employing size-dependent passive targeting resulting in the accumulation at other sites with similar size-dependent endocytosis tendencies. For example, given the same < 100 nm particle size for the passive targeting of HSCs, this size condition may also be used for lymph node[12] targeting and enhanced permeability and retention effect-based tumor targeting[13]. In the liver, liposomes < 100 nm in size can also impact normal hepatocytes adjacent to HSCs[14]. This may lead to unexpected toxicity issues; therefore, we recommend adopting active targeting strategies.
The representative modification methods for the active targeting of HSCs are presented herein[1]. The cyclic Arg-Gly-Asp (cRGD) peptide can be employed to target the collagen type VI receptor[15]. Mannose 6-phosphate human serum albumin (M6P-HSA) can be used to target the mannose 6-phosphate receptor[16]. The cyclic peptide cysteine-serine-arginine-asparagine-leucine-isoleucine-aspartic acid-cysteine (CSRNLIDC), which has high selectivity, may be used to target the platelet-derived growth factor receptor-β[17]. Vitamin A is an ideal ligand to target retinol-binding protein[18]. Through click chemistry techniques, these ligands can be covalently bound with lecithin or cholesterol, which are the major components of liposomes[18]. Subsequently, the targeting liposomes can be produced by thin-film hydration, solvent evaporation, or other standard methods.
Based on the formulation of common liposomes (lecithin plus cholesterol), Tau-based therapeutics to treat liver fibrosis were proposed as follows: A targetable liposome was fabricated by combining lecithin and cholesterol, along with modified lecithin or cholesterol with HSCs targetability (i.e., cRGD, M6P-HSA, CSRNLIDC, and vitamin A decorated ones). Tau was dispersed into the water zone of this liposomal system. A schematic illustration of this process is shown in Figure 3. We advocate for the science community to validate the feasibility of the above design.
It is expected that this work could provide insight into the design and development of Tau-based therapeutics for liver fibrosis management and increase our understanding of liver fibrosis-targeting vehicles. We are optimistic that relevant studies or Tau-encapsulated vehicles products will be available in the near future.
| 1. | Li S, Ren QJ, Xie CH, Cui Y, Xu LT, Wang YD, Li S, Liang XQ, Wen B, Liang MK, Zhao XF. Taurine attenuates activation of hepatic stellate cells by inhibiting autophagy and inducing ferroptosis. World J Gastroenterol. 2024;30:2143-2154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (3)] |
| 2. | Sun Y, Dai S, Tao J, Li Y, He Z, Liu Q, Zhao J, Deng Y, Kang J, Zhang X, Yang S, Liu Y. Taurine suppresses ROS-dependent autophagy via activating Akt/mTOR signaling pathway in calcium oxalate crystals-induced renal tubular epithelial cell injury. Aging (Albany NY). 2020;12:17353-17366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Zhang X, Zhao L, Ying K, Xu J, Huang Y, Zhu R, Ding Y, Cai W, Wu X, Miao D, Xu Q, Zeng Y, Yu F. TUG1 protects against ferroptosis of hepatic stellate cells by upregulating PDK4-mediated glycolysis. Chem Biol Interact. 2023;383:110673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 4. | Wu D, Song L, Zhu C, Zhang X, Guo H, Yang C. Solubility of taurine and its application for the crystallization process improvement. J Mol Liq. 2017;241:326-333. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Saidi B, Warthesen J. Analysis and Heat Stability of Taurine in Milk. J Dairy Sci. 1990;73:1700-1706. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Oh JY, Yang G, Choi E, Ryu JH. Mesoporous silica nanoparticle-supported nanocarriers with enhanced drug loading, encapsulation stability, and targeting efficiency. Biomater Sci. 2022;10:1448-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Li J, Wang Z, Zhang H, Gao J, Zheng A. Progress in the development of stabilization strategies for nanocrystal preparations. Drug Deliv. 2021;28:19-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 8. | Gao Y, Yuan Z, Yuan X, Wan Z, Yu Y, Zhan Q, Zhao Y, Han J, Huang J, Xiong C, Cai Q. Bioinspired porous microspheres for sustained hypoxic exosomes release and vascularized bone regeneration. Bioact Mater. 2022;14:377-388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 9. | Khoshouei A, Kempf G, Mykhailiuk V, Griessing JM, Honemann MN, Kater L, Cavadini S, Dietz H. Designing Rigid DNA Origami Templates for Molecular Visualization Using Cryo-EM. Nano Lett. 2024;24:5031-5038. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Nsairat H, Ibrahim AA, Jaber AM, Abdelghany S, Atwan R, Shalan N, Abdelnabi H, Odeh F, El-Tanani M, Alshaer W. Liposome bilayer stability: emphasis on cholesterol and its alternatives. J Liposome Res. 2024;34:178-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 26] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 11. | Böttger R, Pauli G, Chao PH, Al Fayez N, Hohenwarter L, Li SD. Lipid-based nanoparticle technologies for liver targeting. Adv Drug Deliv Rev. 2020;154-155:79-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 12. | Mao Y, Liu J, Shi T, Chen G, Wang S. A Novel Self-Assembly Nanocrystal as Lymph Node-Targeting Delivery System: Higher Activity of Lymph Node Targeting and Longer Efficacy Against Lymphatic Metastasis. AAPS PharmSciTech. 2019;20:292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Sharifi M, Cho WC, Ansariesfahani A, Tarharoudi R, Malekisarvar H, Sari S, Bloukh SH, Edis Z, Amin M, Gleghorn JP, Hagen TLMT, Falahati M. An Updated Review on EPR-Based Solid Tumor Targeting Nanocarriers for Cancer Treatment. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 66] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 14. | Giraudi PJ, Becerra VJ, Marin V, Chavez-Tapia NC, Tiribelli C, Rosso N. The importance of the interaction between hepatocyte and hepatic stellate cells in fibrogenesis induced by fatty accumulation. Exp Mol Pathol. 2015;98:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Beljaars L, Molema G, Schuppan D, Geerts A, De Bleser PJ, Weert B, Meijer DK, Poelstra K. Successful targeting to rat hepatic stellate cells using albumin modified with cyclic peptides that recognize the collagen type VI receptor. J Biol Chem. 2000;275:12743-12751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 114] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Adrian JE, Kamps JA, Poelstra K, Scherphof GL, Meijer DK, Kaneda Y. Delivery of viral vectors to hepatic stellate cells in fibrotic livers using HVJ envelopes fused with targeted liposomes. J Drug Target. 2007;15:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Jia Z, Gong Y, Pi Y, Liu X, Gao L, Kang L, Wang J, Yang F, Tang J, Lu W, Li Q, Zhang W, Yan Z, Yu L. pPB Peptide-Mediated siRNA-Loaded Stable Nucleic Acid Lipid Nanoparticles on Targeting Therapy of Hepatic Fibrosis. Mol Pharm. 2018;15:53-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Sato Y, Murase K, Kato J, Kobune M, Sato T, Kawano Y, Takimoto R, Takada K, Miyanishi K, Matsunaga T, Takayama T, Niitsu Y. Resolution of liver cirrhosis using vitamin A-coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat Biotechnol. 2008;26:431-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 465] [Article Influence: 27.4] [Reference Citation Analysis (0)] |