TO THE EDITOR
Liver cancer, with its high incidence and mortality rates, remains a formidable challenge[1]. Despite significant strides in diagnostic and therapeutic methods, the prognosis for liver cancer patients is often poor due to rapid disease progression, chemoresistance, and frequent recurrence[2]. It is imperative to delve into the cellular and molecular mechanisms underlying liver cancer to drive the development of novel and more efficacious treatments. The wingless/integrated (Wnt) signaling pathway and M2 tumor-associated macrophages (TAMs) are pivotal in liver cancer pathogenesis, and have garnered considerable research interest. Investigating these pathways may reveal innovative targets and strategies, offering hope for improved therapies and patient outcomes[3,4].
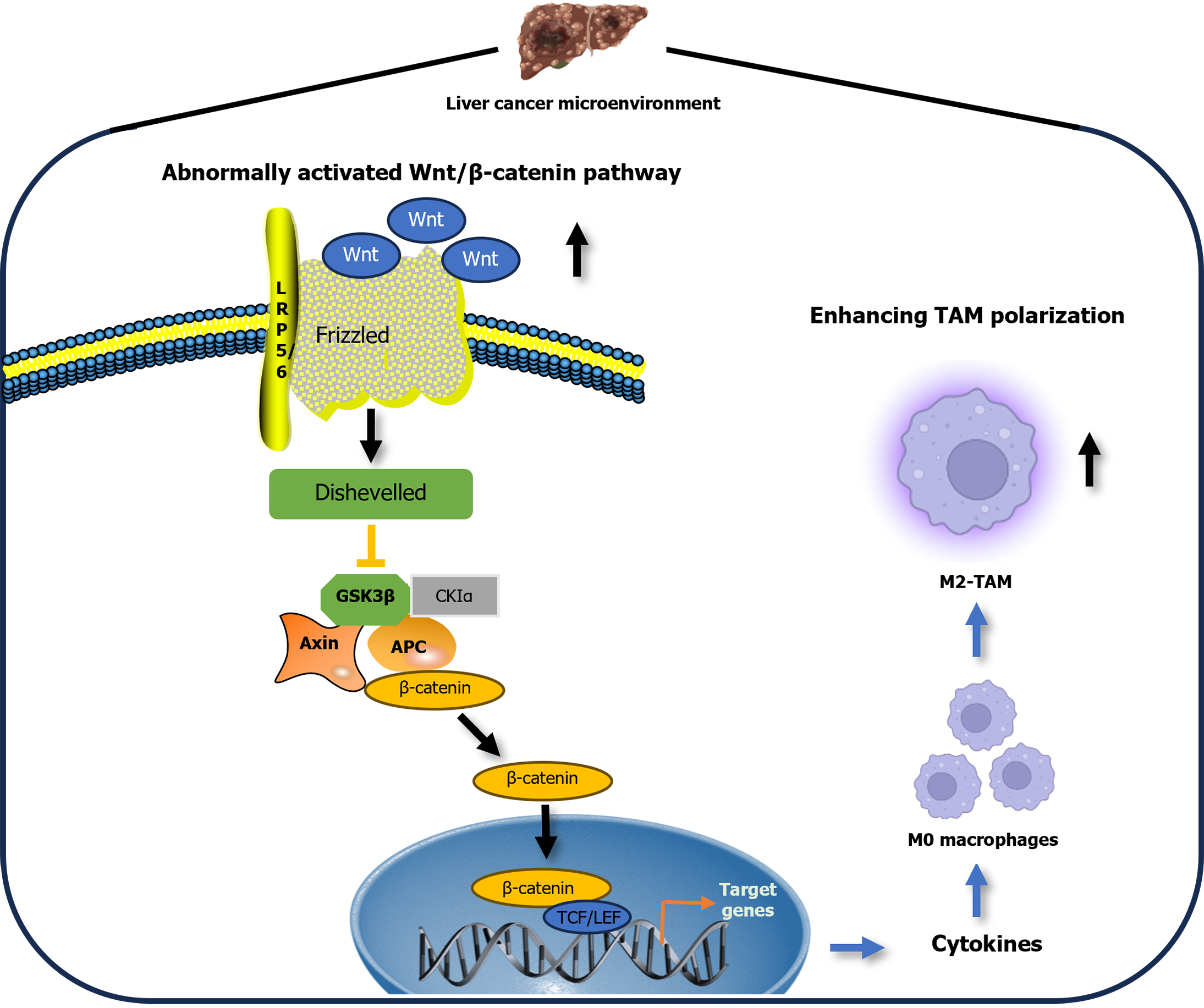
Wnt ligands, a class of secreted glycoproteins, initiate a cascade of interactions upon binding to the Frizzled receptor, culminating in the formation of a complex with the lipoprotein receptor-related protein 5/6 receptor on the cell membrane[5]. This interaction is a cornerstone of the Wnt signaling pathway, particularly the canonical Wnt/β-catenin pathway. Activation of this pathway triggers axis inhibition protein, which normally degrades β-catenin, to bind to its receptor, thereby disrupting the β-catenin destruction complex. As a result, stabilized β-catenin translocates to the nucleus and partners with T-cell factor to regulate gene expression. The dynamic movement of β-catenin between the cytoplasm and nucleus is a hallmark of Wnt/β-catenin pathway activation[6]. This pathway plays a pivotal role in maintaining liver equilibrium, fostering liver regeneration and driving tumorigenesis. Under normal conditions, β-catenin is anchored to the membrane in hepatocytes, with pathway activation localized to pericentral hepatocytes[7]. However, in liver cancer, aberrant activation of the Wnt/β-catenin pathway disrupts cellular responses and factors downstream, leading to a breakdown in bodily homeostasis[7].
Macrophages are essential components of the immune system, playing a crucial role in maintaining health through their diverse functions[8]. For example, macrophages recognize pathogens and damaged cells, initiating inflammatory signaling transduction and producing proinflammatory mediators. In the later stages of inflammation, macrophages clear cellular debris, restore tissue homeostasis, and promote injury repair; inflammation-induced proteases activate the coagulation pathway, and macrophages optimize injury repair by regulating this pathway; toll-like receptor 4 and protease-activated receptor 2 on the surface of macrophages play key roles in regulating inflammatory signaling and the repair process[8]. Macrophages can adapt to their environment, particularly within the tumor microenvironment (TME)[9]. M1 macrophages are typically associated with antitumor activities, while M2 macrophages, especially those known as TAMs, often promote tumor growth and metastasis. M2-TAMs, in particular, are implicated in aiding the proliferation and spread of hepatocarcinoma cells, utilizing pathways such as Wnt/β-catenin[10]. Understanding the dynamic role of these cells in the TME is crucial for developing targeted cancer therapies.
Research has shown that the Wnt/β-catenin signaling pathway plays a crucial role in the differentiation of monocytes into macrophages, particularly in the M2 polarization state[10]. This pathway is not only pivotal in normal physiological processes but also in pathological conditions such as tumor development. Tumor cells can manipulate this signaling by secreting Wnt ligands, thereby promoting an environment conducive to tumor growth. Consequently, targeting the Wnt/β-catenin pathway in liver tumor cells presents a promising avenue for modulating the tumor immune microenvironment and potentially curbing liver tumor progression. Aberrant activation of Wnt signaling acts as a catalyst for M2-TAM polarization, suggesting that its inhibition could serve as a strategic therapeutic intervention[11].
TARGETING WNT SIGNALING PATHWAY IS A THERAPEUTIC METHOD IN LIVER CANCER
Abnormal activation of the Wnt signaling pathway has been shown to promote the polarization of M2-TAMs, which is significant in liver cancer as it facilitates the growth and migration of hepatocarcinoma cells. The aforementioned phenomenon is attributed to their role as promoters of M2-TAMs, which can help liver cell growth and is frequently associated with an unfavorable prognosis. Conversely, inhibition of the Wnt signaling pathway may trigger inhibition of hepatocellular carcinoma (HCC) growth. The study by Huang et al[12], which was published in the recent World Journal of Gastroenterology, demonstrated the role of abnormally activated Wnt signaling pathway as a promoter of M2-TAM polarization, leading to tumor progression, whereas Calculus bovis (CB) inhibits M2-TAM polarization via Wnt/β-catenin pathway modulation to suppress liver cancer[12].
Tian et al[13] discovered that the long noncoding RNA LINC00662 is upregulated in HCC, and high levels of LINC00662 are associated with a lower survival rate in HCC patients. LINC00662 activates the Wnt/β-catenin signaling pathway in HCC cells by inducing secretion of WNT3A, thereby promoting the proliferation, cell cycle progression, and invasion of tumor cells, while inhibiting apoptosis in HCC cells. Importantly, through the secretion of WNT3A, LINC00662 activates the Wnt/β-catenin signaling pathway in macrophages in a paracrine manner, further promoting the polarization of M2 macrophages. In other words, by activating the Wnt/β-catenin signaling pathway and the polarization of M2-TAMs, LINC00662 significantly promotes the growth and metastasis of HCC tumors. This study was similar to that of Huang et al[12], which included both in vivo and in vitro studies.
Another study about the role of Wnt and M2-TAMs in HCC was by Jiang et al[14]. They demonstrated Wnt2b played an important regulatory role in the functions of TAMs in the TME. Specifically, polarization-promoting factors derived from HCC cells upregulated expression of Wnt2b in macrophages, which promoted polarization of TAMs to M2-like macrophages by activating Wnt2b/β-catenin/c-Myc signaling. This study also referred to both in vivo and in vitro studies.
As a potential target for liver cancer therapy, Wnt may play an important regulatory role in the functions of TAMs in the TME. In the recent study of Huang et al[12], they also focused on the significant role of CB, incorporating results from network pharmacology and multiomics experiments. The study provided a multifaceted analysis and confirmation of the comprehensive impact of CB on the TME, particularly on TAMs. They shed light on the critical role of CB in the fight against liver cancer, underscoring its distinctive ability to inhibit the differentiation of M2-type TAMs through modulation of the Wnt signaling pathway. Huang et al’s meticulous research investigated the intricate workings of CB, identifying its active components, cellular targets, and the impacted pathways[12]. Their research emphasized the vital role of CB in regulating macrophage plasticity and the interaction with the Wnt/β-catenin pathway within the TME, implying the role of abnormally activated Wnt signaling pathway as an M2-TAM polarization promoter (Figure 1). In contrast, inhibiting abnormal activation of the Wnt signaling pathway led to suppression of hepatocarcinoma cell growth and progression. These discoveries provide a foundation for the development of novel CB-based therapies aimed at preventing M2-TAM polarization and disrupting the Wnt/β-catenin pathway, thereby hindering the progression of liver cancer.
Figure 1 Under the tumor microenvironment, abnormally activated wingless/integrated signaling pathway in cancer cells can be interpreted as an M2-tumor-associated macrophage polarization mediator.
This figure was created with the assistance of Biorender. Wnt: Wingless/integrated; TAM: Tumor-associated macrophage; LRP5/6: Lipoprotein receptor-related proteins 5 and 6; GSK: Glycogen synthase kinase; CKIα: Casein kinase Ialpha; APC: Adnomatous polyposis coli; TCF: T-cell factor; LEF: Lymphoid enhancer-binding factor.
Wnt signaling pathway inhibitors have shown potential in clinical trials, such as LGK974[15], CWP232291[16], and PRI-724[17]. They all possess specificity and clinical application potential, but they also have side effects. For example, LGK974 is a small molecule inhibitor that specifically targets the porcupine enzyme in the Wnt signaling pathway, preventing the secretion of Wnt ligands[15]. It has shown potential in various cancers, especially in tumors where the Wnt signaling pathway is abnormally activated. Its side effects include fatigue, nausea and diarrhea[15,16]. CWP232291 is a small molecule inhibitor that targets the β-catenin/T-cell factor complex, blocking downstream transcriptional activity of the Wnt signaling pathway. It has shown potential in acute myeloid leukemia and multiple myeloma treatments[16]. Its side effects include gastrointestinal discomfort[16]. PRI-724 targets the β-catenin/CBP complex, blocking the transcriptional coactivation of the Wnt signaling pathway[17]. It shows clinical potential in pancreatic and colorectal cancers therapy but often accompanied by some side effects such as hypokalemia[17]. Therefore, the use of inhibitors of the Wnt signaling pathway in cancer clinical management requires more research to determine their long-term safety and efficacy and balance their beneficial effects in life saving strategies.
In the TME, besides the classical Wnt signaling pathway, there are many other signaling pathways that can influence the polarization of M2-TAMs, such as the phosphatidylinositol 3-kinase/protein kinase B[18], signal transducer and activator of transcription 3[19], transforming growth factor-beta[20] and nuclear factor kappa B[21] signaling pathways. These pathways play important roles in regulating the polarization of M2-TAMs and are potential therapeutic targets. By modulating these pathways, it is possible to influence tumor progression and treatment outcomes.
Huang et al’s research had several strengths; for example: Possible mechanisms include TAM polarity alteration and Wnt signaling pathways; utilizing traditional Chinese medicine to explore its therapeutic effects on cancer; analysis of active components revealed multiple potential ingredients such as lithocholic acid; exploration of signaling pathways through bioinformatics; and linking these pathways to uncover the potential of musk to influence TAM polarity alteration[12]. However, there are aspects of Huang et al’s research that warrant further investigation[12]: (1) As suggested by the authors, the active components identified through ingredient analysis that influence TAM polarity alteration can be tested and screened using in vitro cultures. This includes the identified active ingredients in the macrophage culture medium, and detecting the expression of M2 macrophage surface markers (such as CD206 and arginase1) to understand their impact on macrophage polarization; (2) In addition to the Wnt signaling pathway, are there other potential pathways for TAM polarity alteration, particularly those related to musk components that have been analyzed, such as the farnesoid X receptor (FXR) pathway? Exploring additional pathways could provide valuable insights. Utilize databases to predict potential targets of musk components and construct a component-target-disease network. Molecular docking simulations can be used to model the interactions between musk components and key proteins in other signaling pathways such as FXR, assessing binding affinity. In vitro experiments can use HepG2 cells and TAMs to test the effects of musk components on FXR or other signaling pathways. Western blotting can be used to detect expression changes in pathway-related proteins; (3) Conducting in vivo experiments is crucial as macrophages can adapt their characteristics to changing environments. Evaluating the therapeutic effects of musk treatment on TAM polarity alteration requires extracting macrophages from within the tumor. Administration of active substances or pathway inhibitors in vivo to observe changes in TAM polarization and their effects on tumor growth; (4) Functional testing should include assessing serological type 1 T helper/type 2 T helper inflammatory factors, as well as studying the impact on other relevant cells, such as T cells, in nude mice to understand their changes. The process includes the use of flow cytometry: Single-cell suspensions are extracted from the spleen, lymph nodes, or tumor tissues of nude mice, and T-cell surface markers are labeled with fluorescently tagged antibodies. The number and subpopulation distribution of T cells after administration of TAM or signal pathway inhibitors are analyzed using a flow cytometer to assess the impact of the drug on T cells. Additionally, in vitro polarized TAMs are cocultured with T cells to understand the inhibitory effect on T cell activation; (5) When considering treatment strategies, it is important to evaluate the effects on both the tumor and immune cells. For instance, investigating the combined administration of 5-fluorouracil to inhibit tumor growth along with TAM polarity alteration inhibitors could provide insights into the potential benefits of synergistic therapy; and (6) Incorporating precision medicine approaches, such as organoid research, into the exploration of the effects of traditional Chinese medicine would enhance the research. For instance, by cultivating liver organoids from mice with liver cancer and then adding traditional Chinese medicine to the organoid culture medium, one could observe changes in the morphology and quantity of the organoids or conduct immunofluorescence detection to examine the expression of organoid markers.
Macrophages are common sentinel cells that can identify and process foreign substances, thereby initiating immune responses. Kupffer cells are the resident macrophages in the liver and play a key role in the immune response and tumor progression[22]. The primary role of Kupffer cells in immunity is reflected in their ability to clear pathogens and cellular debris from the blood through phagocytosis, as well as in antigen presentation and regulation of immune responses[23]. Kupffer cells can engulf and clear tumor cells, especially during the early stages of tumor liver metastasis. However, in some cases, Kupffer cells may promote tumor growth and metastasis, particularly when they are reprogrammed by the TME into TAMs[24]. In the context of another organ, such as the lungs, alveolar macrophages are specific to the pulmonary region and play roles in pathogen recognition, inflammatory response, and inflammation regulation during early respiratory infections to prevent acute lung injury[25]. In the later stages, alveolar macrophages may also be reprogrammed by the TME to promote tumor growth and metastasis. Due to the dual role of macrophages in tumor development, they have become potential therapeutic targets, and regulating their function can affect the progression of the tumor[25].
CONCLUSION
New opportunities for liver cancer management could be opened up via the deep understanding of Wnt signaling and macrophages theory, suggesting the role of abnormally activated Wnt signaling pathway as an M2-TAM polarization promoter, accompanied by the utilization of Wnt inhibitors as therapeutic methods. Huang et al’s study had several strengths, such as utilizing traditional Chinese medicine to explore its therapeutic effects on cancer, including potential roles in TAM polarity alteration[12]. However, there were also areas that warrant further investigation, such as investigating more signaling pathways to enrich the content of the article. The clinical significance of the study primarily lies in: (1) Inhibiting the polarization of M2-TAMs reduces protumoral factors within the TME, thereby suppressing tumor growth and metastasis; (2) It provides potential therapeutic targets, namely the Wnt/β-catenin signaling pathway; and (3) It demonstrates the potential of traditional Chinese medicine components in modern medicine, promoting the research and application of integrating Chinese and western medicine in treating tumors.
Transforming basic research findings into clinical treatment strategies requires close collaboration among basic researchers, clinicians and pharmaceutical developers to jointly advance the translation of research findings. In practical application, molecular targets discovered in basic research need to be validated in preclinical models to ensure their critical role in disease. Based on validated targets, small molecule drugs, antibodies or other therapeutic methods are developed and evaluated for efficacy and safety before clinical trials are conducted. After a new drug is marketed, it must be monitored in actual clinical practice to assess its long-term effects and potential side effects.