Published online Nov 7, 2024. doi: 10.3748/wjg.v30.i41.4439
Revised: September 8, 2024
Accepted: September 23, 2024
Published online: November 7, 2024
Processing time: 145 Days and 19 Hours
Oral contrast-enhanced ultrasound (OCEUS) is widely used in the noninvasive diagnosis and screening of gastric cancer (GC) in China.
To investigate the clinical application of OCEUS in evaluating the preoperative T staging of gastric cancer.
OCEUS was performed before the operation, and standard ultrasound images were retained. The depth of infiltration of GC (T-stage) was evaluated according to the American Joint Committee on Cancer 8th edition of the tumor-node-metastasis staging criteria. Finally, with postoperative pathological staging as the gold standard reference, the sensitivity, specificity, negative predictive value, positive predictive value, and diagnostic value of OCEUS T staging were evalu
OCEUS achieved diagnostic accuracy rates of 76.6% (T1a), 69.6% (T1b), 62.7% (T2), 60.8% (T3), 88.0% (T4a), and 88.7% (T4b), with an average of 75.5%. Ultrasonic T staging sensitivity exceeded 62%, aside from T1b at 40.3%, while specificity was over 91%, except for T3 with 83.5%. The Youden index was above 60%, with T1b and T2 being exceptions. OCEUS T staging corresponded closely with pathology in T4b (kappa > 0.75) and moderately in T1a, T1b, T2, T3, and T4a (kappa 0.40-0.75), registering a concordance rate exceeding 84%.
OCEUS was effective, reliable, and accurate in diagnosing the preoperative T staging of GC. As a noninvasive diagnostic technique, OCEUS merits clinical popularization.
Core Tip: This study evaluated the efficacy of oral contrast-enhanced ultrasound (OCEUS) for preoperative T staging of gastric cancer, adhering to the American Joint Committee on Cancer 8th edition criteria. The results demonstrated the high diagnostic accuracy, reliability, and noninvasive advantages of OCEUS, supporting its use as a valuable tool for clinical staging and potentially improving treatment strategies for patients with gastric cancer.
- Citation: Liang Y, Jing WY, Song J, Wei QX, Cai ZQ, Li J, Wu P, Wang D, Ma Y. Clinical application of oral contrast-enhanced ultrasound in evaluating the preoperative T staging of gastric cancer. World J Gastroenterol 2024; 30(41): 4439-4448
- URL: https://www.wjgnet.com/1007-9327/full/v30/i41/4439.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i41.4439
Gastric cancer (GC) is one of the most common malignant tumors of the digestive tract. Recently published global cancer research data show that the incidence of GC ranked fifth in the world in 2020[1], and it is reported to be even higher in China, ranking fourth in incidence of malignant tumors and third in mortality rate, seriously threatening people's lives and health[2]. There are regional differences in the survival and prognosis of patients with GC at home and abroad. The reason for this discrepancy may be due to differences in the staging criteria for GC[3,4], which are considered highly ambiguous. The current international standard for GC staging is based on the Union for International Cancer Control and American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging system (8th edition)[5]. According to postoperative pathological observations of the depth of tumor infiltration into the gastric wall, tumors are classified into T1-T4 stages, and imaging T staging is based on pathological criteria[6].
Different imaging techniques, such as endoscopic ultrasonography (EUS), computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography/CT, have good diagnostic efficacy in judging the size and range of cancer, depth of invasion and lymph node metastasis[6,7], and they can accurately perform TNM staging of GC.
Oral contrast-enhanced ultrasound (OCEUS), a noninvasive, free from radiation hazards, and convenient screening method for GC, could be a beneficial supplement to T staging of GC[8]. OCEUS has been widely used in China for a long time, but outside of China, filling of the stomach cavity is still performed with drinking water when performing gastric ultrasound[9,10].
We explored the clinical value of OCEUS in preoperative T staging of GC to improve the acceptance and recognition of OCEUS worldwide.
A retrospective analysis of the medical records of GC patients was performed. All clinical data were completely anonymous, and the requirement for informed consent was waived by our ethics committee. In this study, the following inclusion criteria were used to determine GC cases: (1) GC confirmed by pathological results after surgical resection of the gastric mass; (2) Performance of preoperative OCEUS examination, with clear ultrasound images; (3) Postoperative pathological results clearly describing the depth of the mass infiltration; and (4) Accurate basic clinical information. The exclusion criteria were as follows: (1) Patients with GC confirmed by gastroscopic biopsy without surgery; (2) Patients with suspected GC by gastroscopic biopsy but confirmed nonGC by postoperative pathology; (3) Patients with poor image quality on OCEUS examination, which impacts the staging judgement; and (4) Patients with preoperative gastric tumors receiving radiotherapy, chemotherapy, endoscopic partial resection or other treatment.
Instruments: Color Doppler ultrasound diagnostic instruments such as Mindray DC-80, Philips EPIQ5, Samsung RS80A, Supersonic Aixplorer, Canon Aplio i800, convex array probes (frequency 3.5-5 MHz), and line array probes (frequency 7.5-15 MHz) were used.
Oral contrast agent for assistive display: The gastrointestinal oral contrast-assisted display agent was produced by China Yanbian Junyi Medical Technology Company Limited. The main ingredients are lotus root, coix seed, orange peel, rice, corn, and soybean.
A total of 25 g of gastrointestinal oral contrast assistant display agent was brewed with 500 mL boiling water and mixed thoroughly to achieve a paste. The subject fasted for 8-12 hours, followed by oral intake of the display agent. The clinician initiated ultrasound to perform continuous dynamic examinations of multiple sections. The order of scanning was as follows: Cardia; the gastric fundus; the anterior and posterior walls of the gastric body; the size curve of the gastric body; the gastric angle; the gastric antrum; the duodenal bulb; the descending part; the horizontal part; the perigastric and distant lymph nodes; the liver; the pancreas; the abdominal cavity; and the pelvic cavity. The examination positions were the left lateral position, supine position, and right lateral position. Multisection scanning was used to observe the structure of the gastric wall and the shape and peristalsis of the stomach. After the location of the lesion was determined, the thickness and the maximum upper and lower diameters of the lesion were measured, the standard images were retained, and the hierarchical structure of the gastric wall was examined by local magnification. All operations were performed by physicians with expertise in gastrointestinal ultrasound diagnostic procedures.
The OCEUS results and pathological results of GC patients were obtained from the Picture Archiving and Com
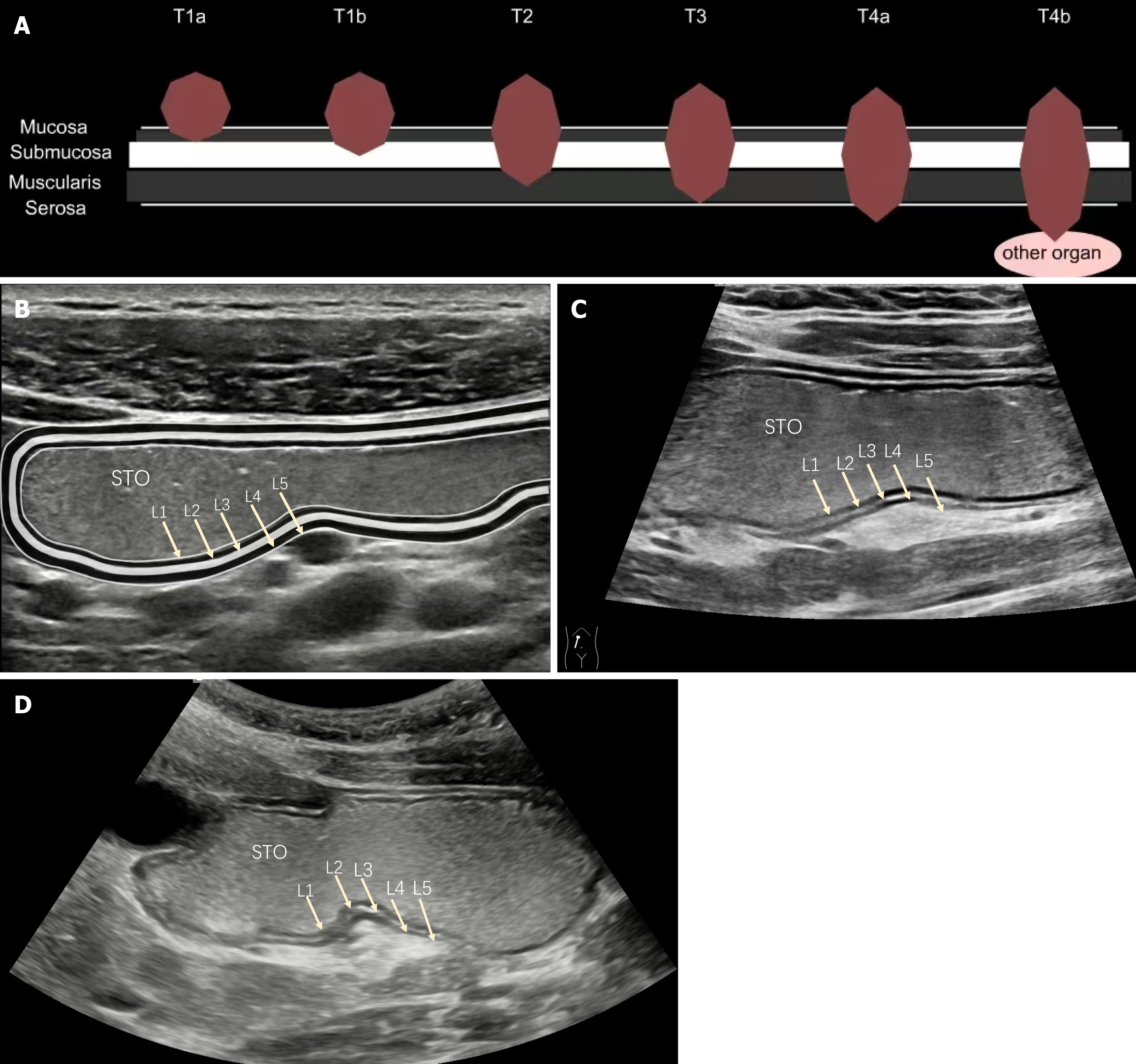
PT criteria for GC: PT was performed according to the diagnostic criteria of the AJCC TNM staging system (8th edition), as shown in Table 1. Ultrasonic T staging criteria for GC: According to the relevant literature[11,12], the ultrasound image of a normal gastric wall shows five layers represented by the parallel arrangement of three hyperechoic bands and two hypoechoic bands, indicating the following structures from inside to outside: The interface echo of the mucosal layer and gastric cavity (superficial mucosal layer), the interface echo of the mucosal lamina propria and muscular layer (deep mucosal layer), the interface echo of the muscularis mucosa and submucosa, the echo of the muscularis propria, and the echo of the serosal layer and external serosal tissue. T was performed according to the degree of infiltration of the gastric lesion revealed by OCEUS, as shown in Table 1.
| T stages | Pathological definition | Ref. ultrasonic imaging |
| T1a | The tumor invades the lamina propria or the mucosal muscularis layer | Layer 1 (superficial mucosal layer) continuity interrupted, Layer 2 (deep mucosal layer) low-echo thickening, Layer 3 (submucosa) remains continuous |
| T1b | The tumor invades the submucosa | Layer 3 (submucosa) high-echo continuity interrupted, muscularis propria layer and serosa layer is intact |
| T2 | The tumor invades the muscularis propria | Layer 4 (muscularis propria) low-echo invasion, with the outer layer retaining a smooth echo boundary |
| T3 | The tumor penetrates the subserosal connective tissue but does not invade the visceral peritoneum | Each layer structure completely disappears, but the outermost layer retains a smooth high-echo band (serosal layer) |
| T4a | The tumor invades the serosal membrane (visceral peritoneum) but not the adjacent structures/organs | Each layer structure completely disappears, and the high-echo band (serosal layer) disappears, or there is a clearly visible serosal layer high-echo line breakthrough with a burr sign or crab foot sign |
| T4b | Tumors invade the adjacent structures/organs | The whole layer is involved, and the echo boundary between the adjacent organ structure (aorta, pancreas, liver, etc.) disappears, and it adheres with the adjacent organs without relative movement |
All the study data were statistically analyzed and plotted using SPSS 26.0 and MedCalc software. The count data are expressed as the number of cases (n) or percentage (%), and comparisons between groups were performed using the χ2 test or Fisher’s exact probability method. The postoperative pathological results were used as the "gold standard"; the accuracy rate was expressed as a percentage when the sensitivity, specificity, positive predictive value, and negative predictive value of OCEUS examination for preoperative T staging of GC were determined. The following formula was applied: Sensitivity = {true positive (TP)/[TP + false negative (FN)]} × 100%, where TP refers to cases where the test correctly identifies the presence of the disease, and FN refers to cases where the test incorrectly identifies the absence of the disease when it is actually present; specificity = [true negative (TN)/{[false positive (FP) + TN]} × 100%, where TN refers to cases where the test correctly identifies the absence of the disease, and FP refers to cases where the test incorrectly identifies the presence of the disease when it is actually absent; positive predicted value (PPV) = [TP/(TP + FP)] × 100%; negative predicted value = [TN/(TN + FN)] × 100%; and diagnostic accuracy (DA) = [(TP + TN)/(TP + TN + FP + FN)] × 100%. The consistency was tested using the kappa method: 0.01-0.40 was considered poor consistency, 0.40-0.75 was considered moderate consistency, and 0.75-1.00 was considered good consistency. P < 0.05 (bilateral test) indicated that the difference was statistically significant.
A total of 1756 patients with suspected gastric tumors received surgical treatment at Sichuan Provincial People's Hospital from July 2018 to July 2022, of which 1387 patients were pathologically diagnosed with malignant gastric tumors and 369 with benign tumors. All patients were confirmed pathologically after surgical resection. Therefore, based on the exclusion criteria, there were: (1) 0 cases of GC confirmed by gastroscopic biopsy but not by surgery; (2) 89 cases of GC suspected by gastroscopic biopsy but not confirmed by postoperative pathology; (3) 256 cases with poor image quality of the OCEUS affecting the staging; and (4) 222 cases of GC receiving radiotherapy, chemotherapy, endoscopic partial resection, or other treatments before surgery. A total of 1387 cases of GC were confirmed via gastroscopy and surgery, and lesions were identified via ultrasound. A total of 318 cases of GC were not accurately diagnosed by ultrasound, including 186 cases (186/256) with poor image quality and 132 cases (132/909) with good image quality.
After all inappropriate cases were excluded, a total of 909 patients who met the above conditions were included in the study, including 636 males and 273 females aged 22 to 90 years (62.40 ± 11.14). Depending on the site and extent of the lesions, the cases were categorized mainly as gastric antrum cancer (226 cases, 24.8%) or multiple GC (347 cases, 38.1%). OCEUS revealed that the thickness diameter (depth of infiltration) of the gastric mass was 14.24 ± 6.45 mm, and the maximum upper and lower diameters were 50.49 ± 22.45 mm. All patients underwent OCEUS examination within one week before surgery. The clinical parameters of the patients with GC included in this study are shown in Table 2.
| Clinical parameters | Number of cases |
| Sex: Male/female | 636 (69.9)/273 (30.1) |
| Age (year) | 62.40 ± 11.14 |
| Ultrasound shows the thickness diameter of gastric mass (mm, mean ± SD) | 14.24 ± 6.45 |
| Ultrasound shows the maximum upper and lower diameter of gastric mass (mm, mean ± SD) | 50.49 ± 22.45 |
| Anatomical location of gastric masses | |
| Cardia & esophagogastric junction | 46 (5.0) |
| Stomach fundus | 42 (4.6) |
| Stomach body | 93 (10.2) |
| Gastric antral | 226 (24.8) |
| Pyloric canal | 18 (1.9) |
| Greater curvature of stomach | 51 (5.6) |
| Lesser curvature of stomach | 86 (9.4) |
| Partial overlapping lesions of stomach | 347 (38.1) |
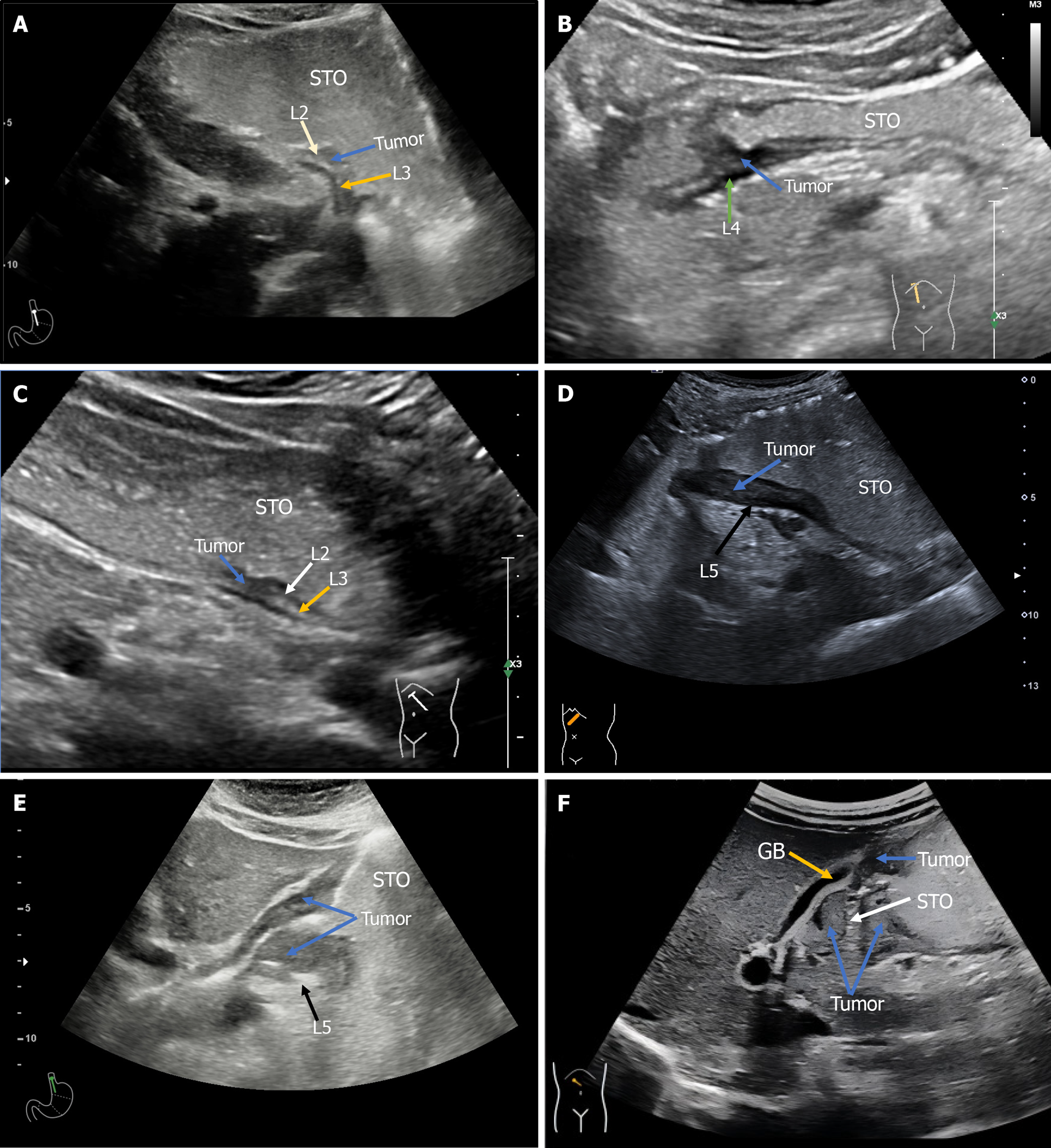
All patients were categorized with postoperative pT as the "golden standard". The pT-staging of GC included 32 cases of T1a, 57 cases of T1b, 94 cases of T2, 204 cases of T3, 416 cases of T4a, and 106 cases of T4b. Pathological T-staging confirmed that 79.8% (726/909) of the patients included in this study had T3, T4a, or T4b disease. According to the ultrasonic T-staging criteria, the T-staging results with OCEUS were as follows: 30 cases of T1a, 33 cases of T1b, 94 cases of T2, 296 cases of T3, 367 cases of T4a and 89 cases of T4b. The DA of OCEUS was 76.6% for T1a, 69.6% for T1b, 62.7% for T2, 60.8% for T3, 88.0% for T4a, 88.7% for T4b, and 75.5% overall. These findings indicated that most patients with advanced GC were included in this study and that OCEUS demonstrated high DA for the preoperative T staging of GC. The specific results are shown in Table 3, and a typical case chart of the OCEUS is shown in Figures 1 and 2.
| OCEUS T-stage | No. of cases | Pathological T-stage | Correct diagnosis rate (%) | |||||
| T1a | T1b | T2 | T3 | T4a | T4b | |||
| T1a | 30 | 23 | 7 | 0 | 0 | 0 | 0 | 76.66 |
| T1b | 33 | 4 | 23 | 6 | 0 | 0 | 0 | 69.69 |
| T2 | 94 | 5 | 19 | 59 | 8 | 3 | 0 | 62.76 |
| T3 | 296 | 0 | 7 | 22 | 180 | 82 | 5 | 60.81 |
| T4a | 367 | 0 | 1 | 7 | 14 | 323 | 22 | 88.01 |
| T4b | 89 | 0 | 0 | 0 | 2 | 8 | 79 | 88.76 |
| Summation | 909 | 32 | 57 | 94 | 204 | 416 | 106 | 75.57 |
The sensitivity of all ultrasound T-staging was greater than 62%, with the exception of the T1b stage (the sensitivity evaluation was 40.3%). The specificity of all ultrasound T-staging was greater than 91%, with the exception of the T3 stage (the specificity evaluation was 83.5%). The Youden index exceeded 50%, except for T1b, indicating that the OCEUS method was reasonably effective in identifying real patients.
The consistency rate of ultrasound T-staging was greater than 80%, although the consistency between ultrasound cT-staging and pathological pT-staging of T4b tumors was good (kappa value > 0.75). Other ultrasound T-staging and pathological pT-staging methods were moderately consistent (kappa value between 0.4 and 0.75), and the total kappa value was 0.66, indicating that OCEUS demonstrated good reliability in the preoperative T-staging of GC.
The PPV of ultrasound T-staging was also good, with the highest T4b of 88.7%, and the negative predictive value was generally high, with the lowest T4a of 82.8%, indicating that OCEUS had good benefits in the preoperative T-staging of GC, especially for T4 GC. The specific results are shown in Table 4.
| OCEUS T-stage | Validity | Reliability | Revenue | ||||
| Sensitivity | Specificity | Youden index | Coincidence rate | Kappa value | Positive predictive value | Negative predictive value | |
| T1a | 71.87 | 99.2 | 71.07 | 98.23 | 0.73 | 76.66 | 98.97 |
| T1b | 40.35 | 98.82 | 39.17 | 95.15 | 0.49 | 69.69 | 96.11 |
| T2 | 62.76 | 95.71 | 58.47 | 92.29 | 0.58 | 62.76 | 95.71 |
| T3 | 88.23 | 83.54 | 71.77 | 84.59 | 0.62 | 60.81 | 96.08 |
| T4a | 77.64 | 91.07 | 68.71 | 84.92 | 0.69 | 88.01 | 82.84 |
| T4b | 74.52 | 98.75 | 73.27 | 95.92 | 0.79 | 88.76 | 96.71 |
Accurate preoperative clinical staging of GC is of paramount importance in guiding treatment decisions and predicting patient outcomes. Commonly utilized imaging modalities for clinical staging include CT, MRI, and EUS. Notably, MRI has demonstrated the highest accuracy, approximately 82.9%, in T-staging, outperforming CT at 71.5% and EUS at approximately 75%[6,13]. A multicenter retrospective study conducted in the United States revealed that the clinical T-staging accuracy was only 46.2%, emphasizing the need to combine multiple imaging methods to achieve accurate preoperative staging[14].
OCEUS has become increasingly feasible in clinical practice owing to advancements in gastric window ultrasound contrast agents and improved ultrasound imaging equipment. Its clinical significance has been recognized, positioning OCEUS as a noninvasive, safe, and efficient imaging technique. The Guidelines for the Diagnosis and Treatment of GC 2022 Edition issued by the National Health Commission of the People's Republic of China clearly emphasize OCEUS as a valuable supplement to gastroscopy and other imaging examinations, expanding its application scope. OCEUS particularly benefits pediatric and elderly patients who are intolerant to gastroscopy, those with large esophageal obstructions, radiation-sensitive individuals, and patients with mobility challenges. Importantly, gastrointestinal oral ultrasound contrast agents, which are primarily composed of edible materials, have been shown to be safe and are approved for use in China. In this study, we observed that uniform filling of the gastric cavity with an echo contrast agent created a distinct echo area within the gastric cavity, facilitating a clear view of the five-layer structure of the gastric wall[11,15]. This view enabled precise assessment of tumor infiltration depth, surrounding organ invasion, and lymph node metastasis. OCEUS overcomes the common limitations of other imaging techniques, including reduced echogenicity and thickening of the gastric wall, indistinct display of the five-layer structure of the gastric wall, mucosal interruptions, ulcers, and mass formations.
OCEUS has proven valuable in determining the size, location, and depth of mass infiltration, thereby assisting in surgical planning and therapeutic decision-making. This study focused primarily on T-staging, given its pivotal role in treatment modality selection. The criteria for ultrasound T-staging rely on the depth of tumor infiltration into the gastric wall and the presence of serosal invasion or involvement of surrounding organs. Ultrasound demonstrated satisfactory accuracy, exceeding 60%, with an impressive 88.7% accuracy for T4b staging, culminating in an overall accuracy of 75.5%. The moderate consistency, as indicated by a kappa value of 0.66 compared with pathological T-staging, highlights its reliability. Notably, ultrasound T-staging exhibited adequate sensitivity and specificity for evaluating T1a, T3, T4a, and T4b tumors, surpassing 71%, with Youden index values exceeding 0.68. PPVs also yielded satisfactory results, con
In the context of ultrasonic T1a/T1b staging, the accuracy was not excellent. This limitation may be attributed to various factors, including the frequency and image quality of extracorporeal ultrasound, the size of the T1 lesions, and the inherent difficulty of imaging lesions located centrally or visualizing intraluminal lesions from an external perspective. Achieving high accuracy in the visualization of early GC infiltration depth remains a challenge. In contrast, the accuracy of T4a and T4b staging was excellent. This impressive accuracy is particularly important, as serosal invasion diagnosis remains challenging, especially with EUS, thus accentuating the contribution of OCEUS in advanced GC T-staging[17,18].
While ultrasound T2/T3 staging exhibited acceptable consistency with the pathological T-stage (kappa > 0.58), it demonstrated lower accuracy levels of 62.7% and 60.8%, along with corresponding PPVs. The challenges in this context stem from the hyperechoic nature of both subserosal connective tissue and the serosa in ultrasonic images. Distinguishing between these layers and accurately assessing infiltration depth when tumors reach the deep muscularis propria of the gastric wall present difficulties. Pathologists also face subjectivity in determining tumor proximity or penetration of the serosal layer[19], contributing to the potential between ultrasound and pathological T-staging. Additionally, GC-related inflammatory reactions in the serosal surface and perigastric area can obscure the visual clarity of the serosal layer, potentially leading to overstaging of T3 tumors[20]. Ultrasonic T4a/T4b staging for pathologically advanced GC, in contrast, yields clear and typical signs under OCEUS and pathological examination. With a substantial number of cases (522/909), this study achieved the highest DA and PPV, exceeding 88.0%. These findings underscore the suitability of ultrasonic T-staging for clinically evaluating advanced GC.
Compared with other traditional diagnostic methods for T-staging, OCEUS studies are limited in number, but the larger sample size examined in this study bolsters confidence in the results[8]. Clinically, EUS and CT remain the primary choices for the preoperative T-staging of GC, followed by MRI. Gastric contrast-enhanced ultrasound is less frequently utilized.EUS, with its combined ultrasound and endoscopy functions, has an accuracy of 75.0% for the T-staging of GC[13]. However, the invasiveness of EUS limits its utility in patients with endoscopic contraindications and those who are averse to invasive procedures. CT, especially multidetector CT, has significantly improved the accuracy of GC T-staging, achieving rates exceeding 90.0%[21,22]. Its superiority lies in detecting T4 tumors and distant metastases[23]. In recent years, MRI has also demonstrated value in the preoperative T-staging of GC, with sensitivity and specificity rates of 76% and 89%, respectively[24]. These modalities have enriched clinical evaluation and treatment approaches for GC. Moreover, in China, gastric double contrast-enhanced ultrasonography has been explored and has shown DA rates reaching 82.3% for T staging[12]. Recent meta-analyses have indicated that OCEUS excels in T1-T2 staging but has slightly reduced accuracy for T3-T4 stages, with an overall sensitivity and specificity of 94% and 91%, respectively[8,25]. In addition, five studies involving 536 patients were included in the OCEUS analysis[26], which yielded a combined sensitivity of 0.733, a combined specificity of 0.982 and an area under the curve of 0.93. These findings suggest that OCEUS can serve as a feasible supplementary tool for the clinical T-staging of GC.
In summary, multiple imaging techniques complement each other in assessing the depth of tumor invasion (T-staging) in GC, and OCEUS presents distinct clinical value in this regard. The European Federation of Societies for Ultrasound in Medicine and Biology and the Japan Society for Ultrasound in Medicine have both highlighted the utility of OCEUS in diagnosing and monitoring a spectrum of gastrointestinal diseases[27,28]. Their guidelines and consensus emphasize the practicality of OCEUS for conditions such as GC, gastric ulcers, gastrointestinal stromal tumors, and inflammatory bowel diseases. Additionally, physicians who lack experience in gastrointestinal ultrasound may benefit from standardized scanning, which could help reduce the incidence of missed diagnoses and improve the detection of lesions, thereby allowing more accurate assessments of the preoperative T staging of GC and greatly enhancing the clinical application value of OCEUS.
However, it is essential to acknowledge the limitations of OCEUS. First, the impact of physician expertise on diagnostic outcomes is a potential source of variability. OCEUS is strongly dependent on the operator, necessitating that the physician responsible for performing gastric filling ultrasound possesses a minimum of five years of experience in abdominal ultrasound. Furthermore, the physician is required to undergo a rigorous six-month standardized training program in OCEUS to ensure diagnostic consistency. Without meeting these criteria, inexperienced physicians may encounter difficulties in accurately assessing the preoperative T staging of GC. Second, our study focused primarily on patients with advanced GC cases, limiting the ability of OCEUS to assess early-stage tumors. Additionally, the exclusion of the preoperative N-staging evaluation is another limitation worth noting. Finally, the patient's own condition may also affect the judgment of the sonographer, such as abdominal flatulence, obesity, and the degree of cooperation of patients during the examination.
In summary, OCEUS has advantages in evaluating the preoperative T-staging of GC, with high DA. Additionally, the noninvasive nature, real-time dynamicity, repeatability, and ease of application of OCEUS improve its clinical application to clearly display lesions in the gastric cavity, gastric wall and perigastric area and observe the depth of GC infiltration into the gastric wall. This technique has high clinical practical value and merits clinical popularization.
We express our sincere gratitude to the experts from the Gastrointestinal Subspecialty Group of the Department of Ultrasound Medicine at Sichuan Provincial People's Hospital, as well as the valuable contributions from our pathology colleagues. Furthermore, we are thankful for the profound feedback from our colleagues and the editorial team, which played a significant role in enhancing this study.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64542] [Article Influence: 16135.5] [Reference Citation Analysis (176)] |
| 2. | Wang SM, Zheng RS, Zhang SW, Zeng HM, Chen R, Sun KX, Gu XY, Wei WW, He J. [Epidemiological characteristics of gastric cancer in China, 2015]. Zhonghua Liu Xing Bing Xue Za Zhi. 2019;40:1517-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 3. | Gunduz I, Acehan T, Alemdar A, Surel AA, Coskun N. Comparison of staging systems in gastric carcinoma. Int J Clin Pract. 2021;75:e14703. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Hu Y, Bao H, Jin H, Zhao J, Xu Y, Huang X, Liu S, Lu B. Performance evaluation of four prediction models for risk stratification in gastric cancer screening among a high-risk population in China. Gastric Cancer. 2021;24:1194-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Son T, Sun J, Choi S, Cho M, Kwon IG, Kim HI, Cheong JH, Choi SH, Noh SH, Woo Y, Fong Y, Park S, Hyung WJ. Multi-institutional validation of the 8th AJCC TNM staging system for gastric cancer: Analysis of survival data from high-volume Eastern centers and the SEER database. J Surg Oncol. 2019;120:676-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Seevaratnam R, Cardoso R, McGregor C, Lourenco L, Mahar A, Sutradhar R, Law C, Paszat L, Coburn N. How useful is preoperative imaging for tumor, node, metastasis (TNM) staging of gastric cancer? A meta-analysis. Gastric Cancer. 2012;15 Suppl 1:S3-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 183] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 7. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 2847] [Article Influence: 569.4] [Reference Citation Analysis (5)] |
| 8. | Xu L, Wang X, Wu W, Li Y. Diagnostic Accuracy of Double Contrast-Enhanced Ultrasonography in Clarifying Tumor Depth (T Stage) of Gastric Cancer: Meta-analysis. Ultrasound Med Biol. 2021;47:2483-2493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Liu Z, Guo J, Wang S, Zhao Y, Li J, Ren W, Tang S, Xie L, Huang Y, Sun S, Huang L. Evaluation of transabdominal ultrasound after oral administration of an echoic cellulose-based gastric ultrasound contrast agent for gastric cancer. BMC Cancer. 2015;15:932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Sato K, Saito H, Yashima K, Isomoto H, Hirooka Y. Transabdominal Ultrasonography for Assessing the Depth of Tumor Invasion in Gastric Cancer. Yonago Acta Med. 2017;60:154-161. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Li S, Huang P, Wang Z, Chen J, Xu H, Wang L, Zhang Y, Mo G, Zhu J, Cosgrove D. Preoperative T staging of advanced gastric cancer using double contrast-enhanced ultrasound. Ultraschall Med. 2012;33:E218-E224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Wang L, Liu Z, Kou H, He H, Zheng B, Zhou L, Yang Y. Double Contrast-Enhanced Ultrasonography in Preoperative T Staging of Gastric Cancer: A Comparison With Endoscopic Ultrasonography. Front Oncol. 2019;9:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Cardoso R, Coburn N, Seevaratnam R, Sutradhar R, Lourenco LG, Mahar A, Law C, Yong E, Tinmouth J. A systematic review and meta-analysis of the utility of EUS for preoperative staging for gastric cancer. Gastric Cancer. 2012;15 Suppl 1:S19-S26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 14. | Spolverato G, Ejaz A, Kim Y, Squires MH, Poultsides GA, Fields RC, Schmidt C, Weber SM, Votanopoulos K, Maithel SK, Pawlik TM. Use of endoscopic ultrasound in the preoperative staging of gastric cancer: a multi-institutional study of the US gastric cancer collaborative. J Am Coll Surg. 2015;220:48-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Yan C, Bao X, Shentu W, Chen J, Liu C, Ye Q, Wang L, Tan Y, Huang P. Preoperative Gross Classification of Gastric Adenocarcinoma: Comparison of Double Contrast-Enhanced Ultrasound and Multi-Detector Row CT. Ultrasound Med Biol. 2016;42:1431-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Yu T, Wang X, Zhao Z, Liu F, Liu X, Zhao Y, Luo Y. Prediction of T stage in gastric carcinoma by enhanced CT and oral contrast-enhanced ultrasonography. World J Surg Oncol. 2015;13:184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Xu HM, Wang PL, Gong YB. [Optimization of TNM staging and control of pathological quality in gastric cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2020;23:87-91. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Pyo JH, Ahn S, Lee H, Min BH, Lee JH, Shim SG, Choi MG, Lee JH, Sohn TS, Bae JM, Kim KM, Yeon S, Jung SH, Kim JJ, Kim S. Clinicopathological Features and Prognosis of Mixed-Type T1a Gastric Cancer Based on Lauren's Classification. Ann Surg Oncol. 2016;23:784-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Nanishi K, Shoda K, Kubota T, Kosuga T, Konishi H, Shiozaki A, Fujiwara H, Okamoto K, Otsuji E. Diagnostic accuracy of the gastric cancer T-category with respect to tumor localization. Langenbecks Arch Surg. 2020;405:787-796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Huo X, Yuan K, Shen Y, Li M, Wang Q, Xing L, Shi G. Clinical value of magnetic resonance imaging in preoperative T staging of gastric cancer and postoperative pathological diagnosis. Oncol Lett. 2014;8:275-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Makino T, Fujiwara Y, Takiguchi S, Tsuboyama T, Kim T, Nushijima Y, Yamasaki M, Miyata H, Nakajima K, Mori M, Doki Y. Preoperative T staging of gastric cancer by multi-detector row computed tomography. Surgery. 2011;149:672-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Gai QZ, Li XL, Li N, Li L, Meng Z, Chen AF. Clinical significance of multi-slice spiral CT, MRI combined with gastric contrast-enhanced ultrasonography in the diagnosis of T staging of gastric cancer. Clin Transl Oncol. 2021;23:2036-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Nie RC, Yuan SQ, Chen XJ, Chen S, Xu LP, Chen YM, Zhu BY, Sun XW, Zhou ZW, Chen YB. Endoscopic ultrasonography compared with multidetector computed tomography for the preoperative staging of gastric cancer: a meta-analysis. World J Surg Oncol. 2017;15:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Zhang Y, Yu J. The role of MRI in the diagnosis and treatment of gastric cancer. Diagn Interv Radiol. 2020;26:176-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 25. | Zhang X, Yao J, Zhang Y, Huang X, Wang W, Huang H. Updated Evaluation of the Diagnostic Performance of Double Contrast-Enhanced Ultrasonography in the Preoperative T Staging of Gastric Cancer: A Meta-Analysis and Systematic Review. Front Oncol. 2022;12:844390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 26. | Zhong Y, Xiao YY, Ye JY, Jian GL, Huang WJ. Diagnostic efficacy of contrast-enhanced gastric ultrasonography in staging gastric cancer: a meta-analysis. BMC Cancer. 2024;24:422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 27. | Maconi G, Nylund K, Ripolles T, Calabrese E, Dirks K, Dietrich CF, Hollerweger A, Sporea I, Saftoiu A, Maaser C, Hausken T, Higginson AP, Nürnberg D, Pallotta N, Romanini L, Serra C, Gilja OH. EFSUMB Recommendations and Clinical Guidelines for Intestinal Ultrasound (GIUS) in Inflammatory Bowel Diseases. Ultraschall Med. 2018;39:304-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 28. | Nishida M, Hasegawa Y, Hata J. Basic practices for gastrointestinal ultrasound. J Med Ultrason (2001). 2023;50:285-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |