Published online Oct 28, 2024. doi: 10.3748/wjg.v30.i40.4367
Revised: September 7, 2024
Accepted: September 23, 2024
Published online: October 28, 2024
Processing time: 68 Days and 21.9 Hours
Patients with human epidermal growth factor receptor 2 (HER2)-positive advanced gastric cancer have poor outcomes. Trastuzumab combined with chemotherapy is the first-line standard treatment for HER2-positive advanced gastric cancer. Inetetamab is a novel anti-HER2 drug, and its efficacy and safety in gastric cancer have not yet been reported.
To evaluate the efficacy and safety of the S-1 plus oxaliplatin (SOX) regimen combined with inetetamab as a first-line treatment for HER2-positive advanced gastric cancer.
Thirty-eight patients with HER2-positive advanced gastric cancer or gastroesophageal junction adenocarcinoma were randomly divided into two groups: One group received inetetamab combined with the SOX regimen, and the other group received trastuzumab combined with the SOX regimen. After 4-6 cycles, patients with stable disease received maintenance therapy. The primary endpoints were progression-free survival (PFS) and overall survival (OS), and the secondary endpoints were the objective response rate, disease control rate, and adverse events (AEs).
Thirty-seven patients completed the trial, with 18 patients in the inetetamab group and 19 patients in the trastuzumab group. In the inetetamab group, the median PFS was 8.5 months, whereas it was 7.3 months in the trastuzumab group (P = 0.046); this difference was significant. The median OS in the inetetamab group vs the trastuzumab group was 15.4 months vs 14.3 months (P = 0. 33), and the objective response rate was 50% vs 42% (P = 0.63), respectively; these differences were not significant. Common AEs included leukopenia, thrombocytopenia, nausea, and vomiting. The incidence rates of grade ≥ 3 AEs were 56% in the inetetamab group and 47% in the trastuzumab group (P = 0.63), with no significant difference.
In the first-line treatment of HER2-positive advanced gastric cancer, inetetamab and trastuzumab showed comparable efficacy. The inetetamab group showed superior PFS, and both groups had good safety.
Core Tip: Trastuzumab combined with the S-1 plus oxaliplatin (SOX) regimen is the standard first-line treatment for human epidermal growth factor receptor 2-positive advanced gastric cancer. This study demonstrated that the efficacy of inetetamab combined with the SOX regimen is similar to that of trastuzumab combined with the SOX regimen, and the inetetamab group exhibited superior progression-free survival without any additional adverse events.
- Citation: Kong Y, Dong Q, Jin P, Li MY, Ma L, Yi QJ, Miao YE, Liu HY, Liu JG. Inetetamab combined with S-1 and oxaliplatin as first-line treatment for human epidermal growth factor receptor 2-positive gastric cancer. World J Gastroenterol 2024; 30(40): 4367-4375
- URL: https://www.wjgnet.com/1007-9327/full/v30/i40/4367.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i40.4367
Gastric cancer is a common malignant tumor of the gastrointestinal tract. In 2020, there were more than 1 million new cases of gastric cancer worldwide, with 769000 related deaths, thus making gastric cancer the fifth most common cancer and the fourth leading cause of death. In China, these burdens are greater than the global burdens, with the morbidity and mortality of gastric cancer both ranking third among all countries in the world[1]. The overall prognosis of advanced gastric cancer patients is poor, the 5-year survival rate is low, and the prognosis is poor[2]. Fluorouracil, platinum and taxanes are the main chemotherapeutic drugs used for the treatment of advanced gastric cancer, and the S-1 plus oxaliplatin (SOX) regimen is gradually becoming the preferred first-line chemotherapy because of its simplicity[3]. However, the efficacy of traditional chemotherapeutic drugs has entered a bottleneck period. Due to the consistent use of immune checkpoint inhibitors, targeted antivascular drugs, and antibody drug conjugates for the treatment of gastric cancer, the prognosis of human epidermal growth factor receptor 2 (HER2)-positive advanced gastric cancer has greatly improved[4-7]. However, there has been little progress with respect to targeted drugs, as most targeted drugs are limited by failure. In the TOGA study[8], treatment with trastuzumab yielded positive results in HER2-positive advanced gastric cancer patients in China. Based on the results of the TOGA study, the combination of the anti-HER2 drug trastuzumab and other agents has become the recommended first-line standard treatment in domestic and international guidelines. Studies have shown that the percentage of Chinese patients with HER2-positive advanced gastric cancer is approximately 12%-13%[9,10]. Given the large patient population, the only first-line macromolecular antibody-targeted drug is trastuzumab, and treatment options are limited. Owing to the global drug economy, drug availability, and drug resistance, the application of trastuzumab has not improved the survival of Chinese patients with gastric cancer. Therefore, this problem urgently needs to be solved.
Inetetamab is a novel anti-HER2 mAb that was independently developed in China. It has stronger antibody-dependent cell-mediated cytotoxicity (ADCC) through Fc region modification and process optimization[11]. The HOPES breast cancer study confirmed that this stronger ADCC effect can translate into survival benefits; therefore, inetetamab was approved for the treatment of HER2-positive metastatic breast cancer[12]. In vitro test results have shown that inetetamab, which has the same anti-HER2 effect as trastuzumab but has a stronger ADCC effect, may exert effective antitumor effects on HER2-positive lung cancer and gastric cancer[13,14]. However, there are currently no clinical reports on the use of inetetamab in combination with chemotherapy for gastric cancer. Therefore, the aim of this study was to explore the efficacy and safety of inetetamab combined with the SOX regimen vs trastuzumab combined with the SOX regimen in the treatment of patients with advanced gastric cancer. Importantly, additional treatment options for patients with HER2-positive advanced gastric cancer are needed.
Study subjects: From September 2021 to September 2023, 38 HER2-positive patients with postoperative recurrence or newly treated metastatic advanced gastric cancer (confirmed by pathological tissue examination) admitted to the Second Affiliated Hospital of Shandong First Medical University were randomly divided into an inetetamab combined with SOX group and a trastuzumab combined with SOX group, with 19 patients in each group. In the inetetamab group, 1 patient refused further treatment after only one cycle of chemotherapy; this patient voluntarily withdrew from the study. Therefore, there were 18 patients (14 males and 4 females) in the inetetamab group, ranging in age from 48 to 80 years, with an average age of 64.28 ± 9.19 years. The tumor-node-metastasis stages of the patients in the inetetamab group were as follows: 3 patients had stage III disease, 15 patients had stage IV disease, and 7 patients had a history of surgery. There were 19 patients (13 males and 6 females) in the trastuzumab group, ranging in age from 53 to 79 years, with an average age of 66.47 ± 6.64 years. The tumor-node-metastasis stages of the patients in the trastuzumab group were as follows: 2 had stage III disease, 17 had stage IV disease, and 5 had a history of surgery. The general information of the patients in the two groups was comparable (P > 0.05), as shown in Table 1. This study was approved by the hospital ethics committee, and the patients and their families provided informed consent (Ethics Approval Number: 2021-001). This study is registered on the China Clinical Trials Center website (registration number: ChiCTR2100051574).
| Clinical characteristics | Inetetamab group (n = 18) | Trastuzumab group (n = 19) | χ2/t value | P value |
| Sex, n (%) | 0.073 | 0.787 | ||
| Male | 14 (77.8) | 13 (68.4) | ||
| Female | 4 (22.2) | 6 (31.6) | ||
| Age, mean ± SD | 64.28 ± 9.19 | 66.47 ± 6.64 | 0.836 | 0.068 |
| Clinical stage, n (%) | 0.004 | 0.948 | ||
| Stage III | 3 (16.7) | 2 (10.5) | ||
| Stage IV | 15 (83.3) | 17 (89.5) | ||
| HER2 status, n (%) | 0.140 | 0.709 | ||
| IHC3+ | 16 (88.9) | 15 (78.9) | ||
| IHC2+, FISH+ | 2 (11.1) | 4 (21.1) | ||
| Surgical history, n (%) | 0.667 | 0.414 | ||
| Yes | 7 (38.9) | 5 (26.3) | ||
| No | 11 (61.1) | 14 (73.7) | ||
| ECOG score, n (%) | 0.016 | 0.898 | ||
| 0 | 11 (61.1) | 12 (63.2) | ||
| 1 | 7 (38.9) | 7 (36.8) | ||
| Primary site, n (%) | 0.000 | 1.000 | ||
| Stomach | 14 (77.8) | 15 (78.9) | ||
| Cardia | 44 (22.2) | 4 (21.1) | ||
| Liver metastasis, n (%) | 0.222 | 0.638 | ||
| Yes | 10 (55.6) | 12 (63.2) | ||
| No | 8 (44.4) | 7 (36.8) | ||
| Smoking history, n (%) | 0.703 | 0.402 | ||
| Yes | 7 (38.9) | 10 (52.6) | ||
| No | 11 (61.1) | 9 (47.4) | ||
| Drinking history, n (%) | 1.337 | 0.248 | ||
| Yes | 7 (38.9) | 11 (57.9) | ||
| No | 11 (61.1) | 8 (42.1) | ||
| Comorbidities, n (%) | 0.000 | 1.000 | ||
| Yes | 44 (22.2) | 4 (21.1) | ||
| No | 14 (77.8) | 15 (78.9) |
The inclusion criteria were as follows: (1) Patients with pathologically, histologically or cytologically confirmed postoperative recurrence or newly treated metastatic gastric or gastroesophageal junction site adenocarcinoma who had not previously received anti-HER2 therapy; (2) IHC HER2 3+ or HER2 2+ and FISH+; (3) At least 1 measurable target lesion that could be continuously monitored by computed tomography/magnetic resonance imaging; (4) Eastern Cooperative Oncology Group score between 0 and 1 point and expected survival > 3 months; (5) > 6 months from the last dose of adjuvant chemotherapy therapy, if administered; and (6) Normal major organ (liver, kidney, and heart) function.
The exclusion criteria were as follows: (1) Patients with a clear tendency toward gastrointestinal bleeding; (2) Patients with mental disorders and those who had difficulty taking medication or were unable to take medication; (3) Patients with an Eastern Cooperative Oncology Group score ≥ 2 points, with many underlying diseases, and poor tolerance to systemic chemotherapy; (4) Patients with hematopoietic system diseases; (5) Patients with primary malignant tumors in multiple locations; (6) Patients with central nervous system metastasis; and (7) Patients with symptomatic ascites greater than 3 cm in size requiring clinical treatment intervention.
Participants were randomly allocated to one of the two treatment arms using a computer-generated randomization sequence. Block randomization with block sizes of 4 was utilized to maintain balance between the intervention group (receiving inetetamab combined with the SOX regimen) and the control group (receiving trastuzumab combined with the SOX regimen). Allocation was not concealed from the investigators or participants.
The inetetamab group was treated with inetetamab (8 mg/kg for the first dose; 6 mg/kg for the maintenance dose) combined with the SOX regimen. The standard chemotherapy regimen was as follows: S-1: 40-60 mg/m2, once in the morning and once in the evening. The dose was calculated based on body surface area (BSA): BSA < 1.25 m2, 40 mg/m2; BSA 1.25-1.5 m2, 50 mg/time; and BSA > 1.5-2.0 m2, 60 mg/time. The drug was orally administered after meals for days 1-14. For oxaliplatin, 130 mg/m2 was administered by intravenous drip on the first day, and 1 cycle included 21 days. Trastuzumab (8 mg/kg for the first dose; 6 mg/kg for the maintenance dose) combined with the SOX regimen was used in the trastuzumab group; the specific doses of the SOX regimen were the same as those used in the inetetamab group. At least 2 cycles of chemotherapy were administered to the patients in each group following the prescribed regimen. After 4-6 cycles of chemotherapy, if a patient’s condition stabilized, he or she was administered maintenance treatment. In the inetetamab group, inetetamab + S-1 was used as maintenance therapy, and in the trastuzumab group, trastuzumab + S-1 was used as maintenance therapy. Treatment was continued until disease progression or toxicity was observed.
The target lesions were measured at baseline (before chemotherapy), and the patients underwent imaging every two chemotherapy cycles to evaluate treatment efficacy. The efficacy of chemotherapy was evaluated according to the Response Evaluation Criteria in Solid Tumors 1.1 standard. Complete response (CR), was defined as the disappearance of all measured target lesions for more than 4 weeks. Partial response (PR) was defined as a decrease in the sum of the target lesion diameter by ≥ 30% for at least 4 weeks compared with the baseline value. Progressive disease (PD) was defined as a relative increase of ≥ 20% compared with the minimum of the sum of the diameters of all the target lesions, an increase ≥ 5 mm in the absolute value of the sum of the diameters or the appearance of new lesions. Stable disease (SD) indicated that the degree of target lesion reduction was between that of PR and that of PD. CR + PR/total number of samples = objective effective rate (ORR), and CR + PR + SD/total number of samples = disease control rate (DCR). If a patient’s condition suddenly worsened during treatment, a computed tomography scan was immediately conducted. Adverse events (AEs) were recorded from the first chemotherapy session to progression, death, loss to follow-up, or the end of follow-up. Progression-free survival (PFS) was defined as the time from the start of chemotherapy to disease progression or death from any cause. Overall survival (OS) was the time from the start of chemotherapy to death from any cause (survival status).
The AEs observed during chemotherapy were evaluated according to the evaluation criteria for adverse drug events (CTCAE 5.0) developed in the United States and were recorded in detail. Adverse reactions were graded on a scale from 0-5.
SPSS 27.0 software was used for statistical analysis. The measurement data fit a normal distribution and are expressed as the mean ± SD; intergroup comparisons were performed via t tests. Count data are expressed as rates (%), and intergroup comparisons were performed via the χ2 test. A P value < 0.05 indicated that a difference was significant. PFS and OS were analyzed. The Kaplan-Meier method was used to construct survival curves, and the log-rank test was used to compare survival between the two groups.
A total of 38 patients were enrolled; 1 patient withdrew, and thus, 37 patients completed the survival follow-up. The efficacy of the combination therapy was evaluated according to the computed tomography/magnetic resonance imaging results. There were 18 patients in the inetetamab group. After 6 weeks of treatment, 0 patients achieved CR, 9 patients achieved PR, 7 patients achieved SD, and 2 patients achieved PD. The ORR was 50%, and the DCR was 88.9%. Similarly, for the 19 patients in the trastuzumab group, after 6 weeks of treatment, the efficacy evaluation revealed 0 patients with a CR, 8 patients with a PR, 10 patients with SD, and 1 patient with PD. The ORR was 42%, and the DCR was 94.7%. We further compared the ORR and DCR between the two groups after 6 weeks of treatment, and the results revealed that the differences between the groups were not significant (χ2 = 0.232, P = 0.63 and χ2 = 0.002, P = 0.961).
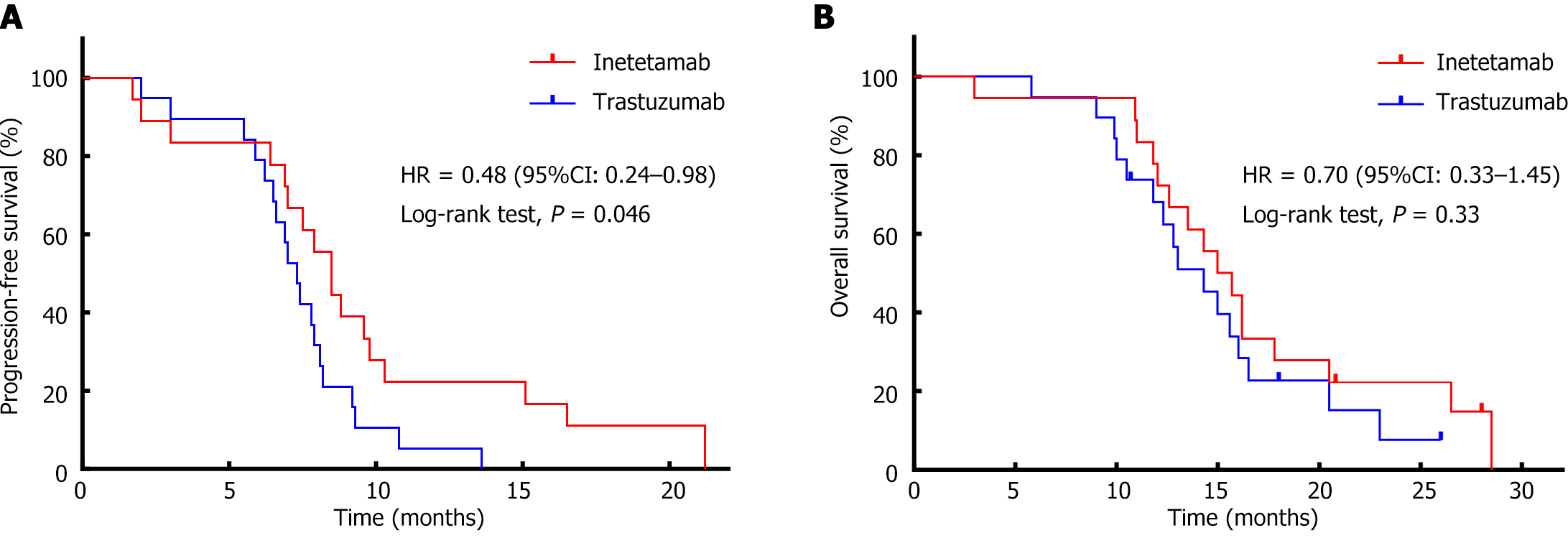
Among the 37 patients for whom efficacy was evaluated, the median PFS in the inetetamab group was 8.5 months [95% confidence interval (CI): 7.26-9.74], and the median PFS in the trastuzumab group was 7.3 months (95%CI: 6.59-8.01); this difference was significant (P = 0.046) (Figure 1A, PFS survival curve). The median OS in the inetetamab group was 15.4 months (95%CI: 12.09-17.91), and the median OS in the trastuzumab group was 14.3 months (95%CI: 11.33-17.27); this difference was not significant (Figure 1B, OS survival curve). In this study, inetetamab combined with the SOX regimen was found to be superior to trastuzumab combined with the SOX regimen for the first-line treatment of advanced gastric cancer, and the OS data supported this conclusion.
The AEs experienced by patients in the two groups were mainly hematological toxicity, such as leukopenia, thrombocytopenia, a reduction in hemoglobin, and liver function damage, and nonhematological toxicity, such as nausea, vomiting, diarrhea, anorexia, fatigue, and numbness of the hands and feet. Most of the AEs were grade 1 or 2. The incidences of cardiotoxicity, allergic reactions, and fever were low in both groups. Among the 37 patients, 4 patients in the inetetamab group had grade 3 or above leukopenia, of whom 1 patient had grade IV leukopenia. In the trastuzumab group, there were 3 patients with grade 3 or above leukopenia, with no patients with grade 4 AEs. The incidence of AEs ≥ grade 3 and above was 56% in the inetetamab group, and the incidence of AEs ≥ grade 3 and above in the trastuzumab group was 47% (χ2 = 0.232, P = 0.63); however, these differences were not significant. The specific event rates for each type of AE are detailed in Table 2. There were no dose adjustments or discontinuations due to AEs. No grade 5 toxicity occurred in any patient.
| AEs | Inetetamab group (n = 18) | Trastuzumab group (n = 19) | ||
| All AEs | ≥ Grade 3 AE | All AEs | ≥ Grade 3 AE | |
| Leukopenia | 15 (83) | 4 (22) | 15 (79) | 3 (16) |
| Reduction in hemoglobin | 11 (61) | 0 | 10 (53) | 1 (5) |
| Thrombocytopenia | 4 (22) | 1 (6) | 7 (37) | 1 (5) |
| Nausea | 15 (83) | 4 (22) | 16 (84) | 2 (11) |
| Vomiting | 7 (39) | 0 | 5 (26) | 1 (5) |
| Diarrhea | 2 (11) | 0 | 2 (11) | 0 |
| Anorexia | 11 (61) | 1 (6) | 6 (32) | 1 (5) |
| Abnormal liver function | 3 (17) | 0 | 3 (16) | 0 |
| Cardiotoxicity | 1 (6) | 0 | 2 (11) | 0 |
| Numbness in hands and feet | 3 (17) | 0 | 2 (11) | 0 |
| Allergic reaction | 1 (6) | 0 | 0 | 0 |
| Fever | 0 | 0 | 2 (11) | 0 |
In recent years, the incidence and mortality rates of gastric cancer have decreased slightly in Western countries. However, in China, rates are increasing; furthermore, the incidence and mortality rates of gastric cancer are higher than the global average. Most patients with gastric cancer are diagnosed at an advanced stage and are unable to undergo radical surgery, and the average natural survival time of patients with advanced gastric cancer is less than six months[15]. Even after radical surgical resection, patients are at risk of recurrence and metastasis. HER2-positive gastric cancer is closely related to recurrence and metastasis and is associated with a poor prognosis. Although anti-HER2 therapy has been shown to have excellent efficacy in the treatment of patients with HER2-positive advanced breast cancer, its efficacy in the treatment of patients with HER2-positive gastric cancer is limited. This may be related to the heterogeneity of gastric cancer itself. The TOGA study was undoubtedly a landmark study. The introduction of trastuzumab increased the median OS of patients in the combination therapy group from 11.1 to 13.8 months, an effect that was significantly greater than that observed in patients in the chemotherapy alone group. Based on the results of the TOGA study, domestic and international guidelines generally recommend screening newly diagnosed patients to identify those who are HER2 positive at the time of diagnosis. Anti-HER2 therapy combined with chemotherapy is recommended for HER2-positive gastric cancer patients, and chemotherapy alone is recommended for HER2-negative gastric cancer patients[8]. A prospective, real-world EVIDENCE study in China[16] further confirmed the safety and efficacy of trastuzumab in the Chinese population. The combination of trastuzumab and the XELOX regimen significantly improved the efficacy rate and survival benefit of first-line treatment. Although trastuzumab has played a dominant role in the first-line standard treatment of patients with HER2-positive advanced gastric cancer for more than a decade, the treatment needs of patients in China are still unmet due to the need for multiple drugs, poor availability, and inconsistent efficacy.
The results of this study revealed that for the inetetamab group vs the trastuzumab group, the median PFS was 8.5 months vs 7.3 months (P = 0.046), the median OS was 15.4 vs 14.3 months (P = 0.33), the ORR was 50% vs 42% (P = 0.63), and the DCR was 88.9% vs 94.7% (P = 0.961), respectively. There were no significant differences in OS, ORR, or DCR between the groups. In this study, the PFS in the trastuzumab group was slightly greater than that reported in the TOGA study and slightly lower than that reported in the EVIDENCE Study I cohort; however, the PFS was generally consistent with the results of the TOGA and EVIDENCE studies[8,16]. Both of these findings showed consistent benefits of trastuzumab as a first-line treatment for advanced HER2-positive gastric cancer. In this study, the OS in the trastuzumab group was 14.3 months, which was greater than the OS of 13.8 months reported in the TOGA study and the OS of 12.6 months reported for the Chinese cohort in the TOGA study but significantly lower than the OS of 22.3 months reported for the EVIDENCE Study I cohort. These differences may be related to the complexity and heterogeneity of gastric cancer itself. Second, the real-world EVIDENCE study included more varied chemotherapy regimens for first-line treatment and was not limited by the model used in this study. The third important reason for these differences is that during the follow-up period, fewer than half of the 37 patients included in this study received second-line and later-line treatment, which may have severely shortened the OS rate observed in this study. The effect of trastuzumab in this study was comparable to that reported in previous studies[17,18]. This consistency reflects the reliability of the results of this study. In addition, there was no significant difference in the incidence of AEs between the two groups. Therefore, we can conclude that, compared with trastuzumab combined with chemotherapy, inetetamab combined with chemotherapy has the same OS benefit and better PFS benefit without causing new AEs, thus providing a new first-line treatment option for patients with HER2-positive advanced gastric cancer.
Inetetamab is a recombinant humanized monoclonal antibody and is the first novel anti-HER2 monoclonal antibody that was independently developed in China. It retains the same Fab fragment as trastuzumab and is able to bind to the human HER2 protein, thereby inhibiting the overexpression of HER2 in cells and preventing proliferation; additionally, amino acid modifications in the Fc region improve binding to Fcγ receptors on immune cells, thereby activating immune cells, facilitating the release of various effector factors, and effectively killing cancer cells. Therefore, inetetamab has a powerful ADCC effect, and this stronger ADCC effect indicates a stronger antitumor effect. In vitro studies have shown that the ADCC effect of inetetamab is approximately 11.1% greater than that of trastuzumab[11], findings that are consistent with the results of the HOPES study[12]. As demonstrated by our study results, this stronger ADCC effect of inetetamab could translate into a survival benefit.
In recent years, for the first-line treatment of HER2-positive advanced gastric cancer, in addition to anti-HER2 macromolecular mAbs, immunotherapy has been the main focus of related research. A phase II clinical study showed that patients with HER2-positive advanced gastric cancer treated with chemotherapy and targeted therapy combined with immunotherapy as first-line treatment achieved a very high remission rate and survival benefit[19]. The results of the KEYNOTE-811 study are also very interesting[4]. A total of 698 newly treated HER2-positive advanced gastric cancer patients were enrolled in the study. The results of the first interim analysis of the study revealed that, compared with trastuzumab alone, the addition of pembrolizumab increased the objective remission rate (74.4% vs 51.9%, P = 0.00006). The results of the third interim analysis[20] also revealed that, compared with the placebo, pembrolizumab combined with pembrolizumab led to significant improvement in PFS (10.0 months vs 8.1 months, P = 0.0002). Although the median OS was prolonged (20.0 months vs 16.8 months), it did not reach the expected standard. We look forward to the final analysis results. Based on the encouraging results of the early interim analyses, in 2021, the Food and Drug Administration approved, through the accelerated approval process, the combination of pembrolizumab and trastuzumab + chemotherapy for the first-line treatment of patients with advanced HER2-positive gastric cancer. However, in China, the Chinese Society of Clinical Oncology guidelines defined this regimen only as a stage III recommendation. It remains unclear whether the combination treatment can translate into an OS benefit, as indicated by the higher objective remission rate and PFS in the combination group. For most gastric cancer patients in China, the addition of immunotherapy will likely impose a greater economic burden and lead to adverse immune events. Therefore, when choosing treatment options in clinical practice, the cost-effectiveness and feasibility of treatment need to be carefully considered.
There have been many failed attempts at first-line treatments for HER2-positive advanced gastric cancer. For example, in the JACOB study, an investigation of the dual-target combination of pertuzumab plus trastuzumab revealed that dual-target combination therapy plus chemotherapy did not significantly improve patient survival[21]; although the OS in the experimental group was greater (by 3.3 months) than that in the placebo group, the difference was not significant. A phase III study of lapatinib combined with capecitabine revealed that lapatinib combined with the XELOX regimen was an effective treatment for HER2-positive advanced gastric cancer[22]; however, the study endpoint was not reached, and lapatinib alone caused more side effects. Moreover, gastric cancer is a highly heterogeneous disease. Our future research will involve identifying more effective biomarkers other than HER2 and selecting appropriate target populations.
Novel antibody-drug conjugates have also been considered effective in the targeted treatment of HER2-positive gastric cancer. The representative drugs are disitamab vedotin and DS 8201 (T-DXd). In the C008 study[6], the antitumor activity of disitamab vedotin was observed in patients with both low and high HER2 expression. A phase II clinical trial revealed that the OS of patients treated with DS 8201 was longer[7] than that of patients treated with standard chemotherapy. In addition, the small molecule tyrosine kinase inhibitor pyrotinib was effective in the late-line treatment of patients with HER2-positive advanced gastric cancer[23].
In recent years, there have been many advances in drug development and research on the treatment of HER2-positive advanced gastric cancer, but most of these studies have focused on second-line treatment and thereafter. In terms of first-line treatment, only the trastuzumab-based TOGA study and the KEYNTOE-811 study reported positive results. Therefore, there are many clinical deficiencies with respect to first-line treatments for HER2-positive advanced gastric cancer, with few drugs available for treatment, and the efficacy of these treatments is limited. Although the combination of first-line chemotherapy and targeted immune checkpoint inhibitors has yielded positive results, in the real world, the implementation of this regimen is limited by its high price and increased incidence of AEs. The results of this study revealed that the efficacy of first-line inetetamab combination chemotherapy was not inferior to that of standard trastuzumab combination chemotherapy, that there was even a trend toward improvement, and that the adverse reactions were controllable. More importantly, as an innovative drug, inetetamab is expected to provide more benefits to more patients in China. This combination chemotherapy provides a new option for the treatment of HER2-positive advanced gastric cancer patients. Although the results of our study indicated that inetetamab was effective in the first-line treatment of HER2-positive gastric cancer, the study was limited by its single-center nature and small sample size. In the future, additional clinical studies with larger sample sizes are needed to further verify the effectiveness of inetetamab. Even though inetetamab has shown promise, it remains crucial to continue exploring other treatment options to provide diverse therapeutic options for patients with gastric cancer.
In conclusion, the efficacy of inetetamab combined with the SOX regimen as a first-line treatment for HER2-positive advanced gastric cancer is comparable to that of trastuzumab plus SOX. The study demonstrated that the inetetamab group had a significantly longer median PFS than the trastuzumab group (8.5 months vs 7.3 months, P = 0.046), although there were no significant differences in median OS or ORR between the two groups. Common AEs included leukopenia, thrombocytopenia, nausea, and vomiting, with similar rates of ≥ 3 grade AEs in both groups (56% vs 47%, P = 0.63). These findings suggest that inetetamab could be a valuable addition to first-line treatment options for HER2-positive gastric cancer; however, caution and rigorous clinical evaluation are needed before it can be adopted as a standard treatment. Additionally, continued exploration of other therapeutic options is necessary to provide diverse choices for patients with gastric cancer. Further validation through larger phase III trials is essential to confirm these results and to provide robust evidence for its long-term efficacy and safety.
The authors would like to express their gratitude to the scholars who participated in this study for their invaluable contributions.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64627] [Article Influence: 16156.8] [Reference Citation Analysis (176)] |
| 2. | Wöll E, Amann A, Eisterer W, Gerger A, Grünberger B, Rumpold H, Weiss L, Winder T, Greil R, Prager GW. Treatment Algorithm for Patients With Gastric Adenocarcinoma: Austrian Consensus on Systemic Therapy - An Update. Anticancer Res. 2023;43:2889-2897. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Lee KW, Chung IJ, Ryu MH, Park YI, Nam BH, Oh HS, Lee KH, Han HS, Seo BG, Jo JC, Lee HR, Kim JW, Park SR, Cho SH, Kang YK; SOPP study investigators. Multicenter phase III trial of S-1 and cisplatin versus S-1 and oxaliplatin combination chemotherapy for first-line treatment of advanced gastric cancer (SOPP trial). Gastric Cancer. 2021;24:156-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 4. | Chung HC, Bang YJ, S Fuchs C, Qin SK, Satoh T, Shitara K, Tabernero J, Van Cutsem E, Alsina M, Cao ZA, Lu J, Bhagia P, Shih CS, Janjigian YY. First-line pembrolizumab/placebo plus trastuzumab and chemotherapy in HER2-positive advanced gastric cancer: KEYNOTE-811. Future Oncol. 2021;17:491-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 5. | Su B, Huang T, Jin Y, Yin H, Qiu H, Yuan X. Apatinib exhibits synergistic effect with pyrotinib and reverses acquired pyrotinib resistance in HER2-positive gastric cancer via stem cell factor/c-kit signaling and its downstream pathways. Gastric Cancer. 2021;24:352-367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | Peng Z, Liu T, Wei J, Wang A, He Y, Yang L, Zhang X, Fan N, Luo S, Li Z, Gu K, Lu J, Xu J, Fan Q, Xu R, Zhang L, Li E, Sun Y, Yu G, Bai C, Liu Y, Zeng J, Ying J, Liang X, Xu N, Gao C, Shu Y, Ma D, Dai G, Li S, Deng T, Cui Y, Fang J, Ba Y, Shen L. Efficacy and safety of a novel anti-HER2 therapeutic antibody RC48 in patients with HER2-overexpressing, locally advanced or metastatic gastric or gastroesophageal junction cancer: a single-arm phase II study. Cancer Commun (Lond). 2021;41:1173-1182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 7. | Shitara K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, Chung HC, Kawakami H, Yabusaki H, Lee J, Saito K, Kawaguchi Y, Kamio T, Kojima A, Sugihara M, Yamaguchi K; DESTINY-Gastric01 Investigators. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N Engl J Med. 2020;382:2419-2430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 915] [Cited by in RCA: 828] [Article Influence: 165.6] [Reference Citation Analysis (0)] |
| 8. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5324] [Article Influence: 354.9] [Reference Citation Analysis (3)] |
| 9. | Sheng WQ, Huang D, Ying JM, Lu N, Wu HM, Liu YH, Liu JP, Bu H, Zhou XY, Du X. HER2 status in gastric cancers: a retrospective analysis from four Chinese representative clinical centers and assessment of its prognostic significance. Ann Oncol. 2013;24:2360-2364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (1)] |
| 10. | Huang D, Lu N, Fan Q, Sheng W, Bu H, Jin X, Li G, Liu Y, Li X, Sun W, Zhang H, Li X, Zhou Z, Yan M, Wang X, Sha W, Ji J, Cheng X, Zhou Z, Xu J, Du X. HER2 status in gastric and gastroesophageal junction cancer assessed by local and central laboratories: Chinese results of the HER-EAGLE study. PLoS One. 2013;8:e80290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Wang X, Liu P, Li FH, Tan Q. [Evaluation of critical quality attributes of an anti-HER2 humanized monoclonal antibody drug]. Zhongguo Yaoxue Zazhi. 2015;50:1054-1061. |
| 12. | Bian L, Xu BH, Di LJ, Wang T, Wang XJ, Jiao SC, Yang JL, Tong ZS, Liu J, Feng JF, Liu DG, Yu QT, Liu YP, Ma Y, Yu H, Jiang ZF. [Phase Ⅲ randomized controlled, multicenter, prospective study of recombinant anti-HER2 humanized monoclonal antibody (Cipterbin) combined with vinorelbine in patients with HER2 positive metastatic breast cancer: the HOPES Study]. Zhonghua Yi Xue Za Zhi. 2020;100:2351-2357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Cui J, He Y, Zhu F, Gong W, Zuo R, Wang Y, Luo Y, Chen L, Wang C, Huo G, Lu H, Liu Z, Chen P, Guo H. Inetetamab, a novel anti-HER2 monoclonal antibody, exhibits potent synergistic anticancer effects with cisplatin by inducing pyroptosis in lung adenocarcinoma. Int J Biol Sci. 2023;19:4061-4081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 14. | Deng L, Zhao L, Liu L, Huang H. Systemic investigation of inetetamab in combination with small molecules to treat HER2-overexpressing breast and gastric cancers. Open Life Sci. 2023;18:20220535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 15. | Alberts SR, Cervantes A, van de Velde CJ. Gastric cancer: epidemiology, pathology and treatment. Ann Oncol. 2003;14 Suppl 2:ii31-ii36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 266] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 16. | Qin S, Ji J, Xu RH, Wang W, Tang Y, Bi F, Li J, Wang K, Xu JM, Fan Q, Su W, Shen L. Treatment Patterns and Outcomes in Chinese Patients with Gastric Cancer by HER2 Status: A Noninterventional Registry Study (EVIDENCE). Oncologist. 2021;26:e1567-e1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Takahari D, Chin K, Ishizuka N, Takashima A, Minashi K, Kadowaki S, Nishina T, Nakajima TE, Amagai K, Machida N, Goto M, Taku K, Wakatsuki T, Shoji H, Hironaka S, Boku N, Yamaguchi K. Multicenter phase II study of trastuzumab with S-1 plus oxaliplatin for chemotherapy-naïve, HER2-positive advanced gastric cancer. Gastric Cancer. 2019;22:1238-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 18. | Yuki S, Shinozaki K, Kashiwada T, Kusumoto T, Iwatsuki M, Satake H, Kobayashi K, Esaki T, Nakashima Y, Kawanaka H, Emi Y, Komatsu Y, Shimokawa M, Makiyama A, Saeki H, Oki E, Baba H, Mori M. Multicenter phase II study of SOX plus trastuzumab for patients with HER2(+) metastatic or recurrent gastric cancer: KSCC/HGCSG/CCOG/PerSeUS 1501B. Cancer Chemother Pharmacol. 2020;85:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Janjigian YY, Maron SB, Chatila WK, Millang B, Chavan SS, Alterman C, Chou JF, Segal MF, Simmons MZ, Momtaz P, Shcherba M, Ku GY, Zervoudakis A, Won ES, Kelsen DP, Ilson DH, Nagy RJ, Lanman RB, Ptashkin RN, Donoghue MTA, Capanu M, Taylor BS, Solit DB, Schultz N, Hechtman JF. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21:821-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 265] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 20. | Janjigian YY, Kawazoe A, Bai Y, Xu J, Lonardi S, Metges JP, Yanez P, Wyrwicz LS, Shen L, Ostapenko Y, Bilici M, Chung HC, Shitara K, Qin SK, Van Cutsem E, Tabernero J, Li K, Shih CS, Bhagia P, Rha SY; KEYNOTE-811 Investigators. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. Lancet. 2023;402:2197-2208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 192] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 21. | Tabernero J, Hoff PM, Shen L, Ohtsu A, Shah MA, Cheng K, Song C, Wu H, Eng-Wong J, Kim K, Kang YK. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018;19:1372-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 357] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 22. | Hecht JR, Bang YJ, Qin SK, Chung HC, Xu JM, Park JO, Jeziorski K, Shparyk Y, Hoff PM, Sobrero A, Salman P, Li J, Protsenko SA, Wainberg ZA, Buyse M, Afenjar K, Houé V, Garcia A, Kaneko T, Huang Y, Khan-Wasti S, Santillana S, Press MF, Slamon D. Lapatinib in Combination With Capecitabine Plus Oxaliplatin in Human Epidermal Growth Factor Receptor 2-Positive Advanced or Metastatic Gastric, Esophageal, or Gastroesophageal Adenocarcinoma: TRIO-013/LOGiC--A Randomized Phase III Trial. J Clin Oncol. 2016;34:443-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 477] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 23. | Chen Z, Xu Y, Gong J, Kou F, Zhang M, Tian T, Zhang X, Zhang C, Li J, Li Z, Lai Y, Zou J, Zhu X, Gao J, Shen L. Pyrotinib combined with CDK4/6 inhibitor in HER2-positive metastatic gastric cancer: A promising strategy from AVATAR mouse to patients. Clin Transl Med. 2020;10:e148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |