Published online Jan 28, 2024. doi: 10.3748/wjg.v30.i4.367
Peer-review started: December 12, 2023
First decision: December 19, 2023
Revised: December 21, 2023
Accepted: January 3, 2024
Article in press: January 3, 2024
Published online: January 28, 2024
Processing time: 45 Days and 4.1 Hours
L-type calcium channels are the only protein channels sensitive to calcium channel blockers, and are expressed in various cancer types. The Cancer Genome Atlas database shows that the mRNA levels of multiple L-type calcium channel subunits in esophageal squamous cell carcinoma tumor tissue are significantly higher than those in normal esophageal epithelial tissue. Therefore, we hypothesized that amlodipine, a long-acting dihydropyridine L-type calcium channel blocker, may inhibit the occurrence and development of esophageal cancer (EC).
To investigate the inhibitory effects of amlodipine on EC through endoplasmic reticulum (ER) stress.
Cav1.3 protein expression levels in 50 pairs of EC tissues and corresponding paracancerous tissues were examined. Subsequently, the inhibitory effects of amlodipine on proliferation and migration of EC cells in vitro were detected using 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide and Transwell assays. In vivo experiments were performed using murine xenograft model. To elucidate the underlying mechanisms, in vitro cell studies were performed to confirm that ER stress plays a role in inhibition proliferation and migration of EC cells treated with amlodipine.
The expression level of Cav1.3 in esophageal carcinoma was 1.6 times higher than that in paracancerous tissues. Amlodipine treatment decreased the viability of esophageal carcinoma cells in a dose- and time-dependent manner. In vivo animal experiments also clearly indicated that amlodipine inhibited the growth of EC tumors in mice. Additionally, amlodipine reduces the migration of tumor cells by inhibiting epithelial-mesenchymal transition (EMT). Mechanistic studies have demonstrated that amlodipine induces ER stress-mediated apoptosis and suppresses EMT. Moreover, amlodipine-induced autophagy was characterized by an increase in autophagy lysosomes and the accumulation of light chain 3B protein. The combination of amlodipine with the ER stress inhibitor 4-phenylbutyric acid further confirmed the role of the ER stress response in amlodipine-induced apoptosis, EMT, and autophagy. Furthermore, blocking autophagy increases the ratio of apoptosis and migration.
Collectively, we demonstrate for the first time that amlodipine promotes apoptosis, induces autophagy, and inhibits migration through ER stress, thereby exerting anti-tumor effects in EC.
Core Tip: L-type calcium channel blockers have been shown to inhibit the growth of various tumors. We observed a higher expression of the L-type calcium channel Cav1.3 in esophageal cancer (EC) tissue than in paracancerous tissues. Subsequently, we confirmed that amlodipine inhibited the development of EC both in vivo and in vitro. Finally, we established that this inhibitory effect is related to the activation of endoplasmic reticulum stress.
- Citation: Chen YM, Yang WQ, Gu CW, Fan YY, Liu YZ, Zhao BS. Amlodipine inhibits the proliferation and migration of esophageal carcinoma cells through the induction of endoplasmic reticulum stress. World J Gastroenterol 2024; 30(4): 367-380
- URL: https://www.wjgnet.com/1007-9327/full/v30/i4/367.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i4.367
Esophageal cancer (EC) is a common malignant tumor of digestive system, the eighth of incidence and the sixth leading cause of cancer-related mortality in the world and consists of two subtypes: Esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC)[1]. Data from relevant organizations suggests that more than half of all EC cases worldwide are occur in China, with over 90% being squamous cell carcinoma subtypes[2]. Despite significant advances in medical technology and surgical techniques in recent years, patients with late-stage EC still face challenges regarding effective treatment. In China, the 5-year overall survival rate for EC is currently < 20%[3], highlighting the urgent need to identify new biological markers and therapeutic strategies for EC.
Voltage-gated calcium channels comprise a major group of cell membrane potential transducers, with L-type calcium channels being the only subtype. These channels are responsible for altering intracellular calcium ion transit, which triggers various physiological processes[4]. Conventional studies on L-type calcium channels have primarily focused on the excitation-contraction coupling of skeletal and cardiac muscles[5], regulation of hormone secretion from endocrine cells, and neurotransmitter release from nerve cells[6]. Previous research has shown that L-type calcium channels play a critical role in various biological processes, such as cell proliferation, differentiation, and migration[7]. Current research suggests that the expression levels of L-type calcium channels are closely related to potential biomarkers off certain types of cancer, such as those found in the prostate, breast, and uterine cervix[8]. Previous studies have found that amlodipine, a dihydropyridine L-type calcium channel blocker, significantly suppresses the proliferation of human colon carcinoma cells[9]. Similarly, amlodipine, another dihydropyridine L-type calcium channel blocker inhibited the growth of breast cancer[10], gastric cancer[11], and melanoma cells[12].
The endoplasmic reticulum (ER) is a fundamental organelle found in eukaryotic cells that fulfills several key functions such as protein synthesis and folding, calcium ion storage, and lipid and carbohydrate metabolism[13]. ER plays a vital role in maintaining the homeostasis of intracellular Ca2+ by acting as a major storage site. Disturbance of this balance results in the activation of the unfolded protein response (UPR) signaling pathway, which leads to the ER stress response. This leads to the expression of molecular chaperones within the ER, restoring normal cellular homeostasis[14]. In mammals, the UPR activation begins with three primary ER stress sensors: PKR-like ER kinase (PERK), inositol-requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6)[15]. These sensors remain inactive by binding to the chaperone glucose-regulated protein 78-kDa (GRP78) under normal conditions. However, when ER stress occurs, un/misfolded proteins bind to GRP78, leading to the dissociation of PERK, IRE1, and ATF6 from GRP78. This initiates downstream signaling molecules in the UPR pathway[16]. ER stress initiates multiple signals, including activation of PERK, which induces phosphorylation of the eukaryotic translation initiation factor-2 alpha subunit (eIF2a). This phosphorylation inhibits the synthesis of most proteins, reducing the accumulation of proteins in the ER, and promoting UPR-related protein expression, including ATF4, which induces the transcription of C/EBP homologous protein (CHOP)[17]. These responses either restore normal ER function or induce apoptosis and autophagy as self-protective mechanisms to maintain normal cell functions. When the ER stress response persists for an extended period without resolution, the UPR triggers specific cellular apoptosis programs designed to eliminate cells that have suffered significant damage. ER stress-induced apoptosis mainly involves two processes. One process activates signaling pathways, such as PERK/eIF2a/CHOP, where increased expression levels of the CHOP protein effectively suppress sustained increases in Bcl-2 levels. The ER stress response produces a molecule called caspase-12, which is another factor contributing to apoptosis in cells[18]. Other studies have also shown that ER stress may be closely related to the cellular autophagy process. Activation of PERK/eIF2a/CHOP signaling can lead to increased expression of the autophagy marker light chain 3 (LC3)[19]. ER stress is a common cellular response to anti-cancer treatments. Several studies have suggested that certain natural bioactive compounds induce prolonged ER stress and activate pro-death signaling pathways in different types of cancer[20]. However, some reports have indicated that ER stress plays a protective role against cisplatin resistance in lung cancer patients[21].
The Cancer Genome Atlas database showed that the mRNA levels of multiple L-type calcium channel subunits (such as CACNA1C and CACNA1D) in ESCC tumor tissues were significantly higher than those in normal controls. Although L-type calcium channel blockers such as amlodipine have been effective in blocking certain types of cancer, their effects on EC have not yet been reported. Therefore, in this study, we first hypothesized that amlodipine could effectively act on EC cells and then investigated the associated ER stress processes and UPR activation results in detail. We found that amlodipine inhibited the cell proliferation in vitro and in vivo, amlodipine also suppressed the migration of EC cells. Moreover, we demonstrated that one mechanism by which amlodipine plays an anti-EC role involves autophagic cell death initiated by the activation of ER stress signaling.
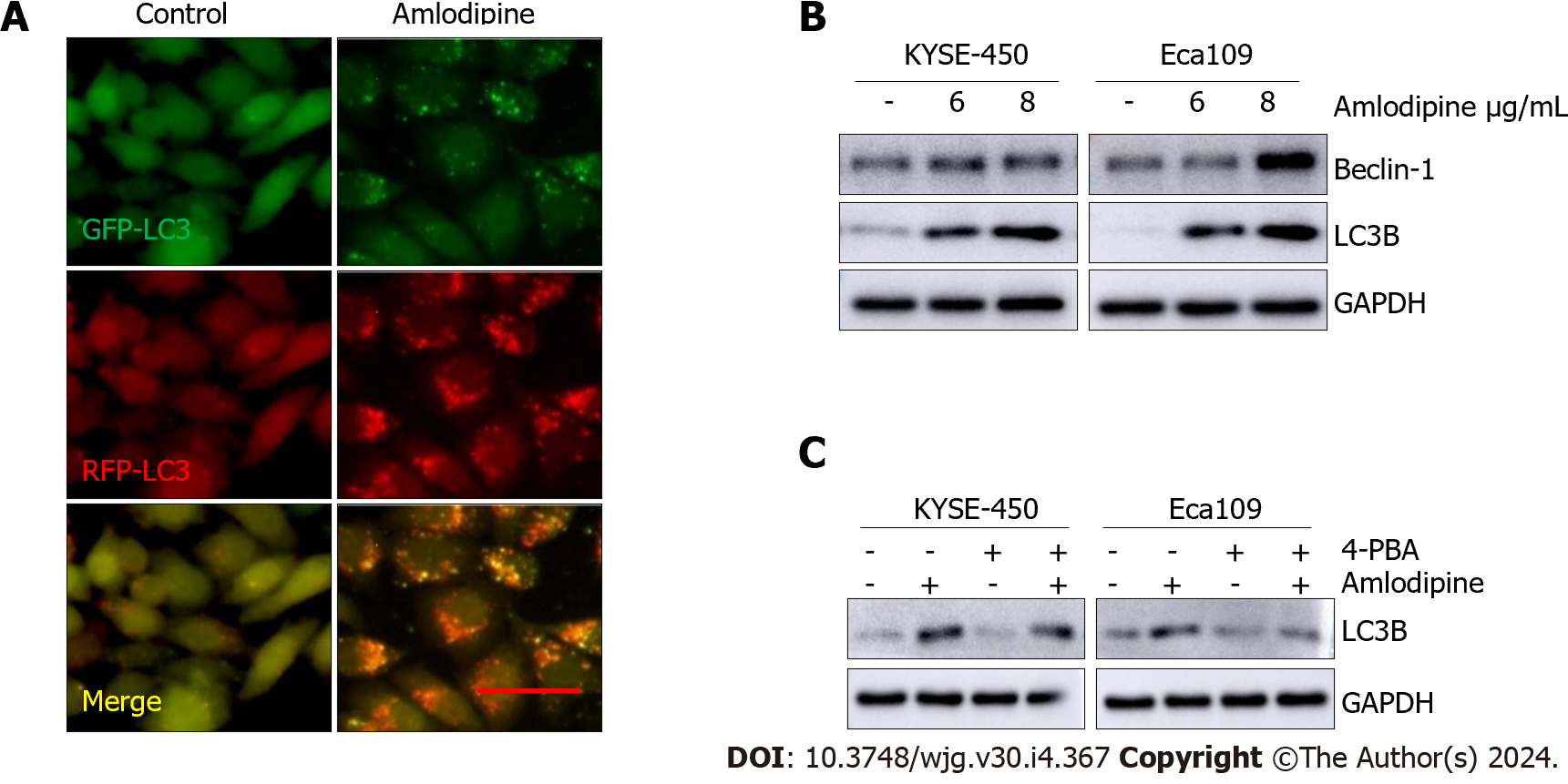
Amlodipine was obtained from the Shihuida Pharma Group (Jilin Province, China). 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl tetrazolium bromide (MTT) and the ER stress inhibitor 4-phenylbutyric acid (4-PBA) were purchased from Sigma-Aldrich (St. Louis, MO, United States). The autophagy inhibitor 3-methyladenine (3-MA) was ordered from APEXBIO (Houston, TX, United States). Annexin V-FITC/propidium iodide Apoptosis Detection Kit was procured from Beyotime Technology (Shanghai, China). The primary antibodies against Cav1.3, E-cadherin, N-cadherin, Vimentin, β-catenin, ATF6, CHOP, GRP78, p-eIF2α, Bax, Bcl-2, cytochrome C, cleaved-PARP, cleaved caspase 3, cleaved caspase 9, cleaved caspase 12, beclin-1, light chain 3 B (LC3B), GAPDH, and goat anti-rabbit secondary antibody were all purchased from Cell Signal Technologies (Danvers, MA, United States). Hanbio Biotechnology (Shanghai, China) supplied the mRFP-GFP-LC3 adenovirus particles. All the other chemicals were purchased from Boster (Wuhan, Hubei Province, China).
This study enrolled 50 patients diagnosed with ESCC based on pathological analyses. All patients had undergone surgery at The First Affiliated Hospital of Xinxiang Medical University between 2017 and 2018, and none had received neo-adjuvant radiotherapy or chemotherapy. The collected tissue specimens included both the tumor tissue and paired paracancerous esophageal mucous membranes obtained from 5 cm beyond the edge of the tumor tissue. All participants were required to sign informed consent forms, and the research plan was approved by the committee of The First Affiliated Hospital of Xinxiang Medical University before the commencement of the study.
Human EC cell lines were procured from Cobioer Biosciences (Nanjing, Jiangsu Province, China). These cells were cultured in PRMI-1640 medium supplemented with 10% heated fetal bovine serum (FBS; Biological Industries, Israel), maintaining a constant temperature of 37 °C in a 5% CO2 humidified incubator. Amlodipine was prepared in a PBS solution and diluted in the culture medium, with PBS serving as the control. Cells underwent various treatments as follows: amlodipine at concentrations ranging from 4 to 10 μg/mL, 4 μg/mL amlodipine treatment in the presence and absence of 3-MA, or 0.2 μM 4-PBA treatment 24 h prior to the application of 4 μg/mL amlodipine.
The KYSE-450, Eca109, SKGT-4 (seeded at 4 × 103 cells/well), and TE-1 cells (seeded at 5 × 103 cells/well) were plated overnight in 96-well plates. Subsequently, cells were subjected to treatment with various concentrations (4 μg/mL, 6 μg/mL, 8 μg/mL, and 10 μg/mL) and doses of amlodipine. Control and experimental groups were established for each concentration. After one, two, and three days of culture, 10 μL of 0.5 mg/mL MTT dye was added to each well. Following a further 4-h incubation, the resulting blue MTT formazan crystals were dissolved in 100 μL/well of DMSO. Absorbance was measured at 490 nm using a Multisken Spectrum microplate reader (Thermo Fisher Scientific, Carlsbad, CA, United States). OD values were used to calculate cell viability. The percentage of live cells was determined using the following formula: Percentage of live cells in each well = the average absorbance value per well from five tests divided by the average absorbance value from the control wells, multiplied by 100%. The percentage of live cells treated with each concentration was calculated as the average percentage of live cells obtained from five replicate experiments.
Cell migration was assessed using a Transwell assay. Specifically, 1 × 105 of KYSE-450 or Eca109 cell suspension in 200 μL serum-free medium was added directly to the upper chamber with 8 μm micro-pores (Corning Costar, Manassas, Virginia, United States). Subsequently, 600 μL of complete culture medium was added to the lower chamber of the insert. Amlodipine was administered at specified doses to both the upper and lower compartments of the insert. After the migration period, non-migrating cells on the upper side of the membrane were removed using a cotton swab. Cells that migrated to the underside of the Transwell were fixed in a 4% paraformaldehyde solution and stained with 0.1% crystal violet. Five randomly selected fields on each membrane were observed under a phase-contrast microscope (Nikon, Tokyo, Japan) to determine the number of migrated cells.
KYSE-450 or Eca109 cells at a density of 8 × 104 cells were seeded in 6-mm culture dishes with a complete culture medium. After a 12-h incubation period, cells were treated with amlodipine at concentrations of 6 μg/mL and 8 μg/mL. Cells were harvested after 48 h of treatment. Cell apoptosis was assessed using membrane-associated protein V-FETC or propidium iodide staining. This allowed for the detection of cells undergoing apoptosis. The apoptosis rate was calculated using the BD FACS Calibur™.
Autophagic flux was examined by infecting cells with the mRFP-GFP-LC3 adenovirus. Briefly, the mRFP-GFP-LC3 adenoviral infection was induced according to the manufacturer’s instructions. After 24 h of infection, the cells were transferred onto glass coverslips in 12-well plates at a density of 1 × 104 cells/well and incubated overnight. Subsequently, these cells were treated with 4 μg/mL of amlodipine for 24 h. Finally, the cells were meticulously observed under an Axio Observer A1 microscope (Carl Zeiss, Germany), and the images were acquired with a Nikon digital camera DS-U3 (Nikon, Tokyo, Japan).
RIPA lysis buffer supplemented with 1 mmol/L PMSF, 2 μg/mL aprotinin, and 100 μM leupeptin was used to lyse cells. Equal amounts of 30 μg protein extracts were separated using SDS-polyacrylamide gel and transferred to nitrocellulose membranes. After blocking with a 5% non-fat dry milk solution, the membranes were incubated at 4°C overnight with primary antibodies targeting various proteins, including Cav1.3, cyclin B1, p21, Bax, Bcl-2, cleaved-PARP, cytochrome C, E-cadherin, N-cadherin, Vimentin, β-catenin, ATF6, CHOP, GRP78, cleaved caspase-3, cleaved caspase-9, cleaved caspase-12, beclin-1, or LC3B. Subsequently, the samples were washed with PBS-T and incubated with a secondary antibody conjugated with horseradish peroxidase. Following three washes with PBS-T, the samples were treated with chemiluminescent substrates using an UltraSignal West Pico kit (Thermo Fisher Scientific, Waltham, MA, United States). Finally, all blots were analyzed using the AmershamTM Imager 600 System (GE Healthcare Bio-Sciences, Pittsburgh, PA, United States), and quantitative analysis was performed using ImageJ (Version 1.53c).
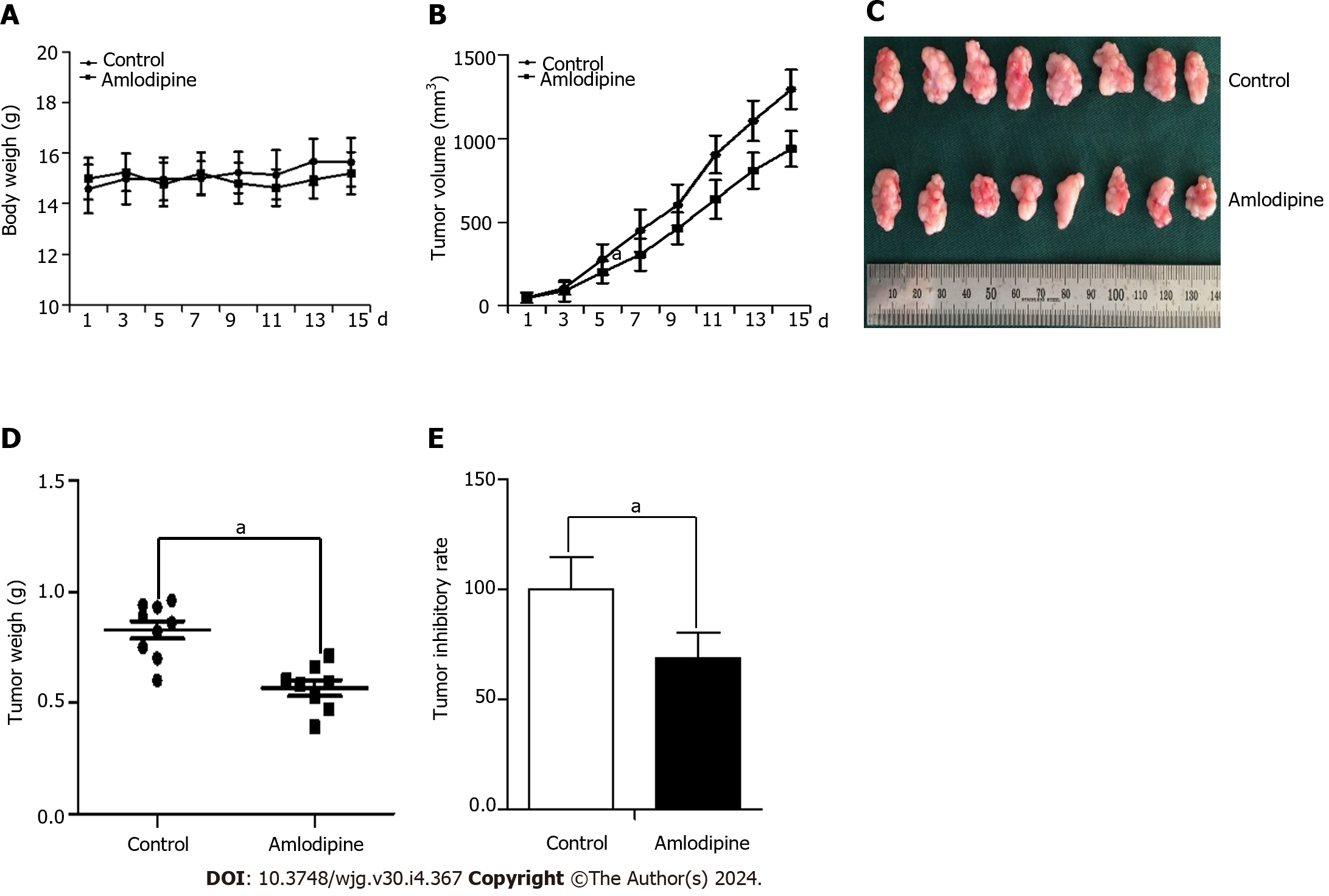
A total of 16 female BALB/c nude mice were procured from Beijing Vital River Laboratory Animal Technology (Beijing, China) and maintained under specific pathogen-free conditions (12-h light/dark cycle, 21 °C ± 2 °C, humidity 50% ± 10%). Mice were cared for in accordance with the guidelines approved by the Ethics Committee of the First Affiliated Hospital of Xinxiang Medical University. A tumor xenograft mouse model was successfully established by injecting 1 × 106 Eca109 cells into the left hind limb of BALB/c mice. Tumor size was calculated using the formula V = L × W2/2, where L and W represent the maximum and minimum diameters, respectively. Once the tumor volume reached approximately 50 mm3, the mice were randomly divided into two groups of eight mice each, and gavage administration was initiated. One group was treated with the vehicle control PBS, whereas the other group was treated with amlodipine at a concentration of 13 mg/kg per day for 15 d. Daily weight and tumor size were measured and recorded throughout the procedure. At the end of the 15-d treatment period with amlodipine, the experimental mice were euthanized, and the tumor was removed, weighed, and measured.
The data were analyzed using the SPSS 26 statistical software, and the mean and standard deviation were calculated. The Student’s t-test was used to assess the differences between two sets of data to determine statistical significance. For comparisons involving multiple groups, an analysis of variance (ANOVA) was conducted, followed by post hoc least significant difference testing, where appropriate. Statistical significance was set at P < 0.05.
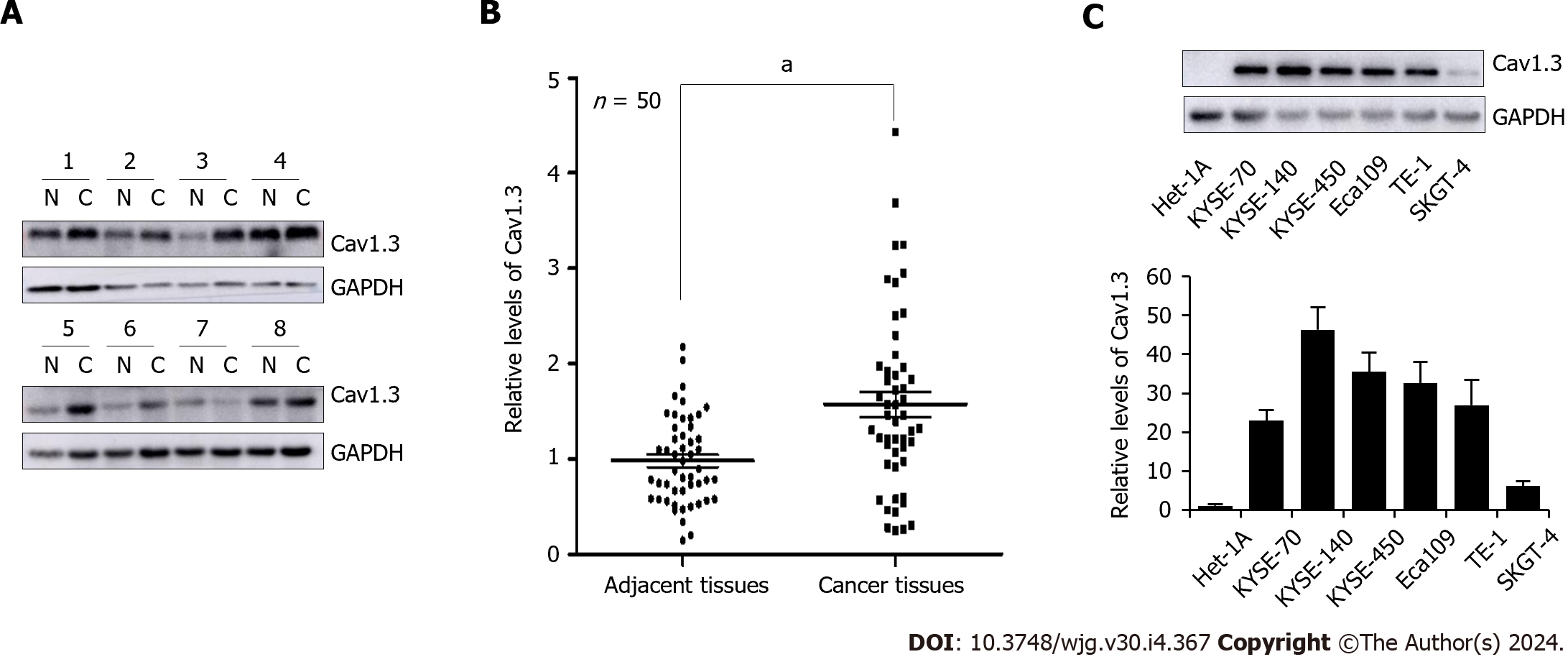
Evidence indicates that abnormal expression of L-type calcium channels is associated with cancer progression. To investigate the protein levels of Cav1.3, a crucial subunit of the L-type calcium ion channel, in patients with ESCC, tumor tissues and paired adjacent tissues from 50 patients with ESCC, whose clinical features are detailed in Table 1, were subjected to Western blot analysis. The representative results are shown in Figure 1A. Quantitative analysis revealed that the levels of Cav1.3 in ESCC tumor tissues were 1.60 times higher than those in the adjacent tissues (Figure 1B). Subsequent examination of Cav1.3 protein levels in various EC cell lines revealed that they were higher than those in normal human esophageal epithelial cells (Figure 1C). Consequently, we hypothesized that elevated levels of Cav1.3 promote the occurrence and development of ESCC and that L-type calcium ion channel blockers may play an inhibitory role against ESCC. Amlodipine, a third-generation dihydropyridine long-acting calcium channel blocker, is commonly used to treat cardiovascular diseases because of its minimal side effects. Therefore, we used amlodipine in follow-up experiments to explore the effect of L-type calcium channel blockers on progression of EC.
| Parameters | n | Cav1.3 expression | P value | |
| High, n = 28 | Low, n = 22 | |||
| Age | 0.833 | |||
| < 65 | 33 | 17 | 16 | |
| ≥ 65 | 17 | 11 | 6 | |
| Gender | 0.231 | |||
| Male | 34 | 21 | 13 | |
| Female | 16 | 7 | 9 | |
| Tumor location | 0.485 | |||
| Middle | 30 | 18 | 12 | |
| Lower | 20 | 10 | 10 | |
| Differentiation | 0.102 | |||
| Low | 16 | 8 | 8 | |
| Moderate | 21 | 12 | 9 | |
| High | 13 | 8 | 5 | |
| TNM staging | 0.435 | |||
| I | 10 | 6 | 4 | |
| II | 27 | 13 | 14 | |
| III | 13 | 9 | 4 | |
| Lymphatic metastasis | 0.854 | |||
| Yes | 22 | 12 | 10 | |
| No | 28 | 16 | 12 | |
| Hypertension | 0.284 | |||
| Yes | 14 | 7 | 7 | |
| No | 36 | 21 | 15 | |
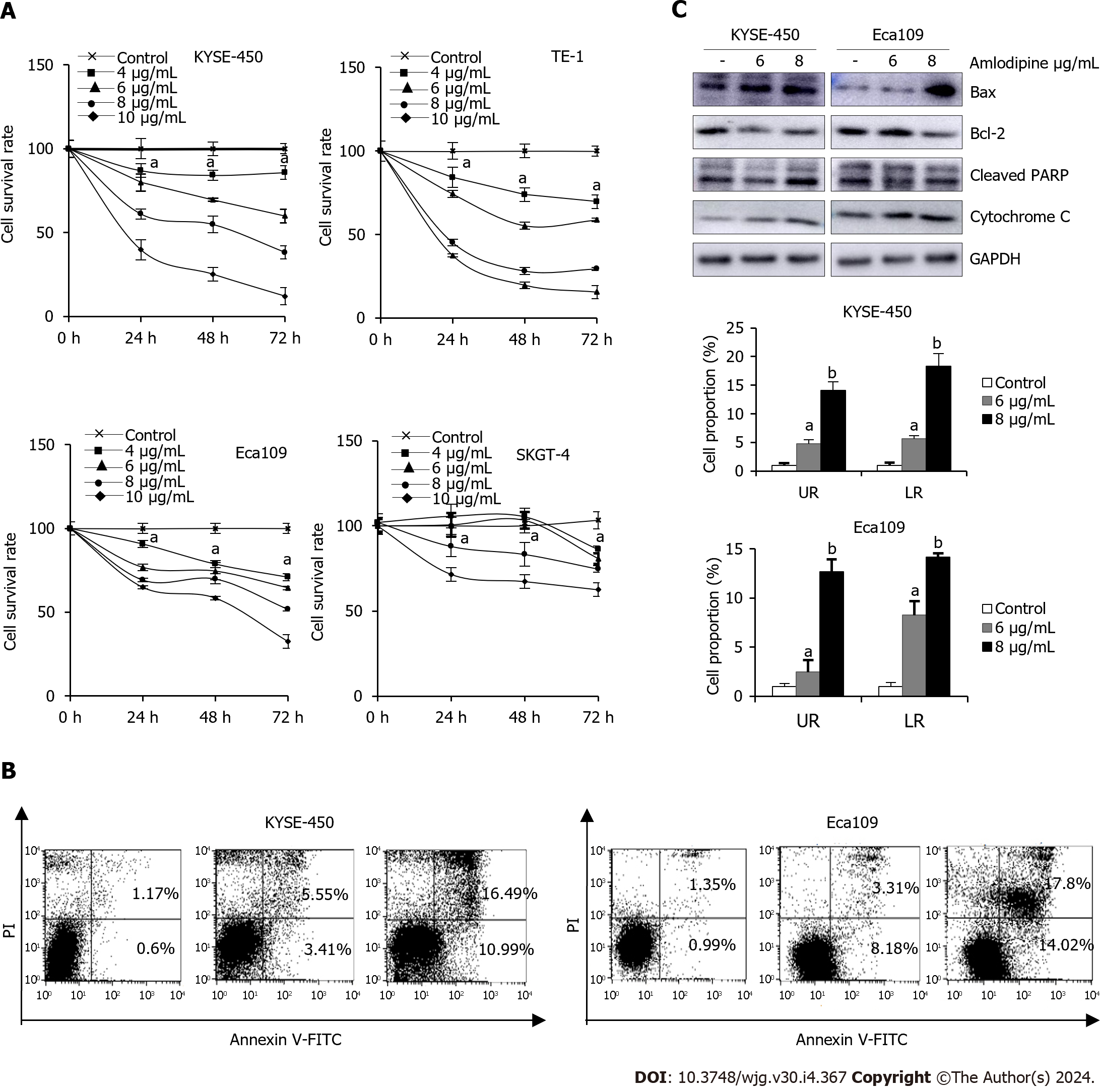
To investigate the anti-tumor effect of amlodipine on EC cells, an MTT assay was initially conducted to assess its effect on cell proliferation in both ESCC and EAC cells. Various concentrations of amlodipine (ranging from 4 μg/mL to 10 μg/mL) were applied to the cells, with different treatment durations ranging from 24 h to 72 h. Figure 2A illustrates the effects of amlodipine concentration and treatment duration on cell viability, which indicated a significant inhibitory effect on ESCC cell proliferation.
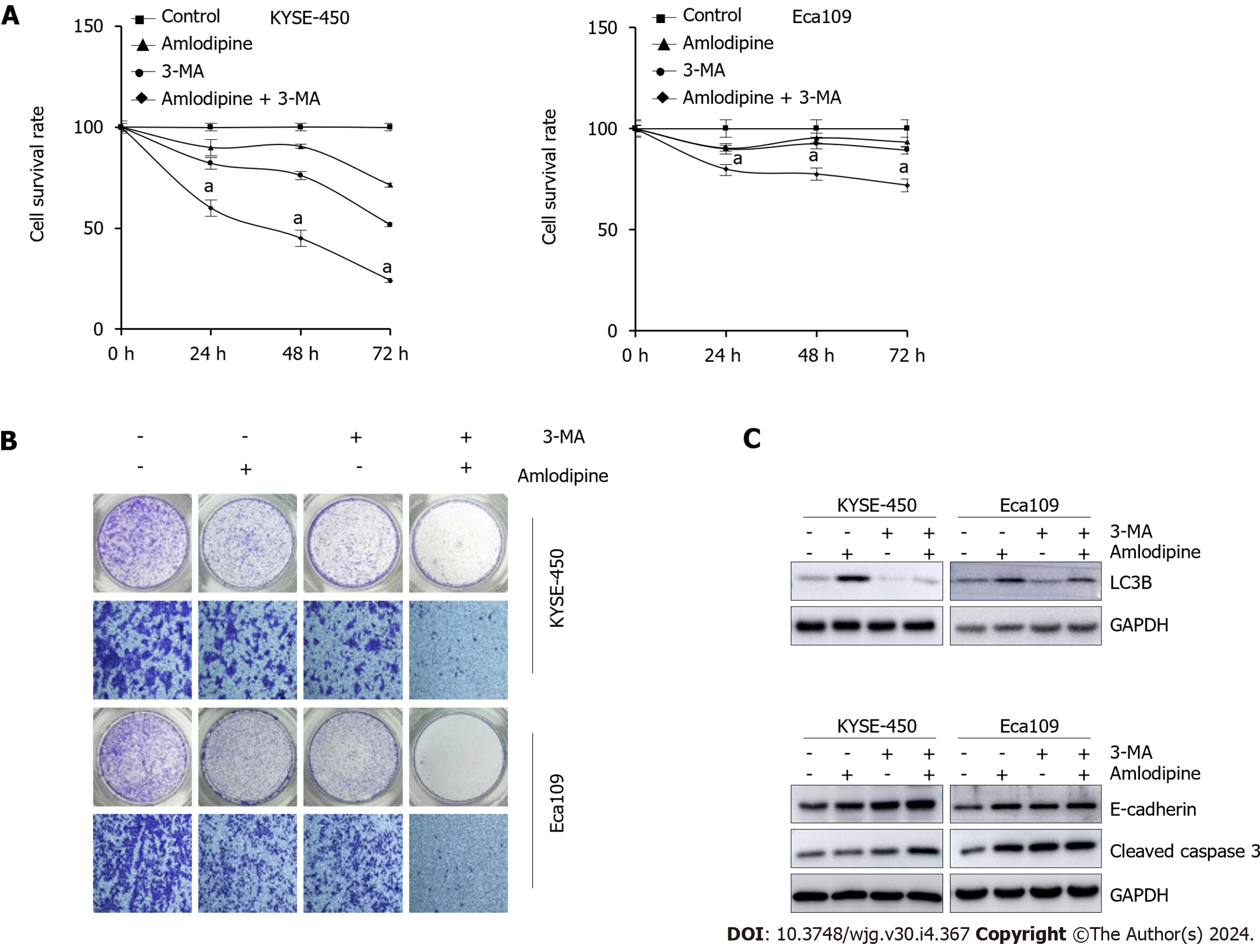
To explore the potential mechanisms underlying the inhibition of ESCC cell proliferation by amlodipine, its effects on apoptosis in KYSE-450 and Eca109 cells were investigated. After treatment with amlodipine for 48 h, flow cytometry revealed an increase in the apoptotic index (Figure 2B), establishing a correlation between the dose of amlodipine and the induction of apoptosis in KYSE-450 and Eca109 cells. To delve deeper into the molecular signals involved in amlodipine-induced apoptosis, we examined apoptosis-related proteins, specifically focusing on mitochondrial apoptosis markers, including Bax, Bcl-2, cytochrome C, and cleaved-PARP, by Western blotting (Figure 2C). The results indicated that amlodipine reduced the levels of Bcl-2 and induced the levels of Bax, cytochrome C, and cleaved-PARP. Overall, our findings suggest that the amlodipine-induced inhibition of ESCC cell viability is associated with the induction of mitochondrial apoptosis.
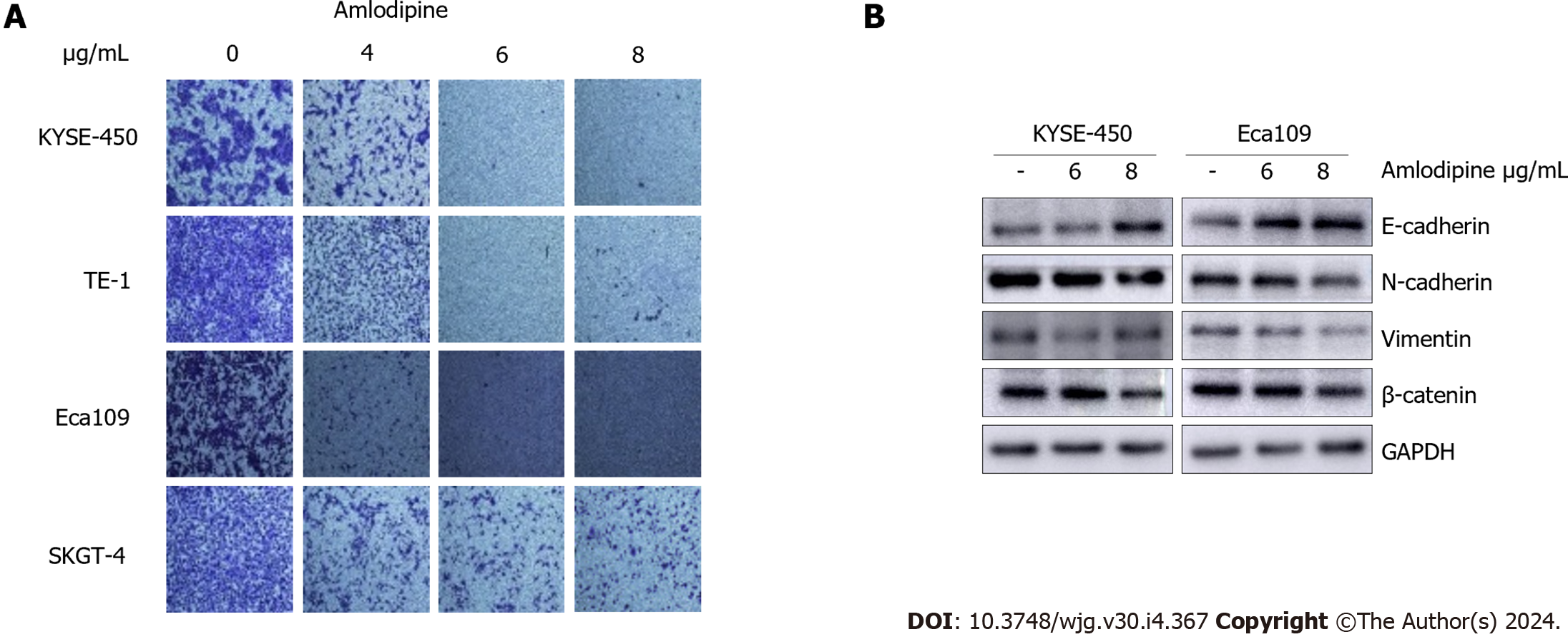
To identify the effect of amlodipine on EC cell migration, a Transwell assay was performed. Based on the MTT assay results described in Figure 1A, doses of amlodipine at 4 μg/mL, 6 μg/mL, and 8 μg/mL were selected, and cells were allowed to migrate for 24 h post-amlodipine treatment. Notably, the inhibitory effect of amlodipine on ESCC cell migration was more potent than that observed on EAC cells (Figure 3A), which was consistent with the cell proliferation data presented in Figure 1A. Cell migration involves multiple steps, including epithelial-mesenchymal transition (EMT). To clarify whether amlodipine inhibits EMT, the levels of EMT markers, including β-catenin, E-cadherin, N-cadherin, and Vimentin were measured. The results demonstrated that amlodipine significantly reduced the expression levels of β-catenin, Vimentin, and N-cadherin, while increasing the protein content of E-cadherin, as depicted in Figure 3B. These findings suggest that the inhibitory effect of amlodipine on EC cell migration is primarily due to its inhibitory effect on EMT.
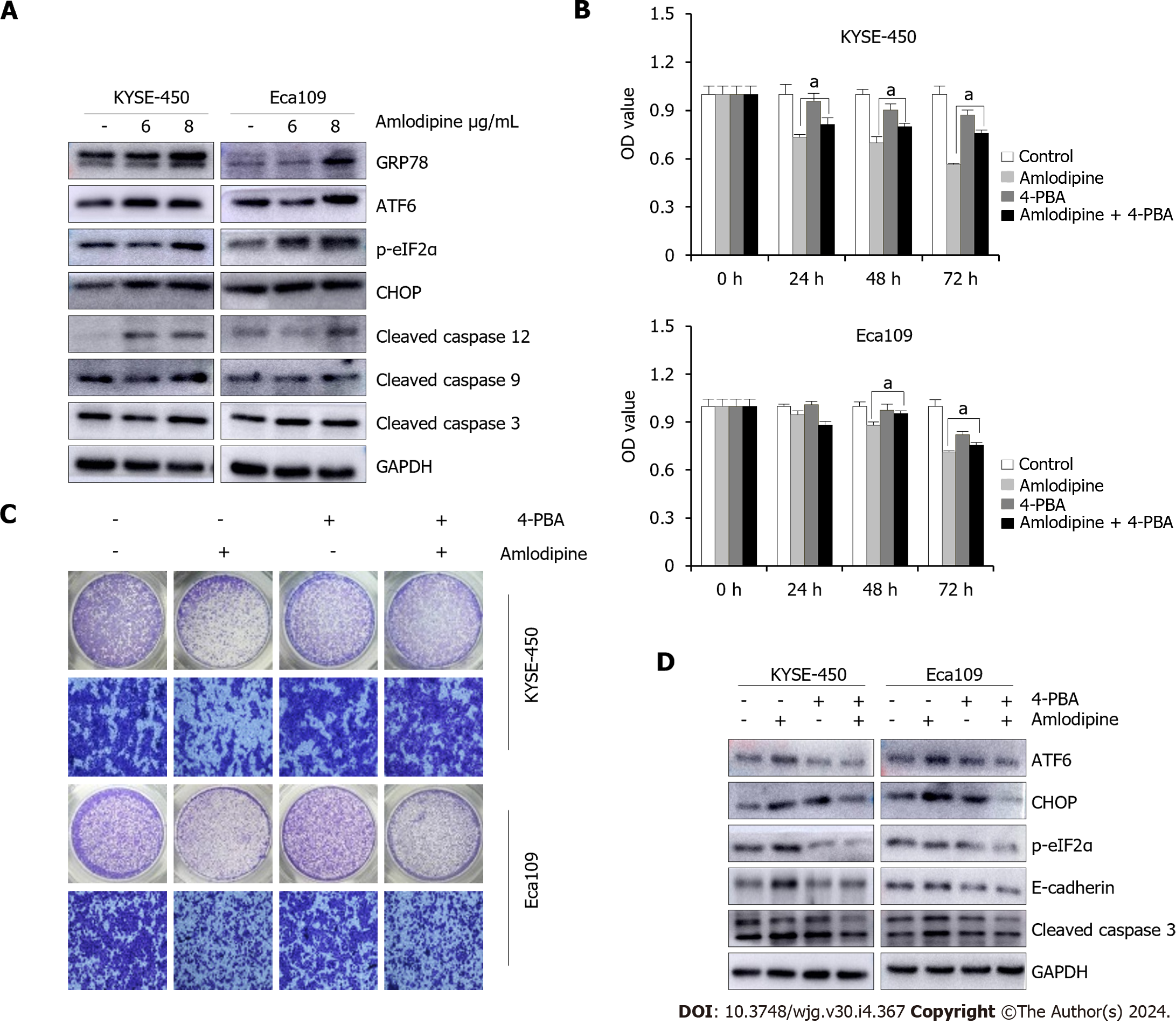
Given that amlodipine is an L-type calcium channel blocker that can induce changes in intracellular calcium homeostasis by triggering ER stress, the protein levels of ER stress molecules in patients after amlodipine treatment were systematically investigated. As shown in Figure 4A, the levels of UPR sensors, including GRP78, ATF-6, p-eIF2α, and CHOP, increased after amlodipine treatment in KYSE-450 and EC-109 cell lines, indicating that amlodipine induces ER stress. Studies have suggested that CHOP, which is elevated in response to excessive ER stress, activates the caspase cascade. Therefore, the effect of amlodipine on apoptosis-related caspase cleavage was investigated. Amlodipine upregulated the levels of cleaved caspase-12, cleaved caspase-9, and cleaved caspase-3 (Figure 4A). To further explore whether the effects of amlodipine on ESCC cell viability and migration were mediated by ER stress, the inhibitor 4-PBA was applied to ESCC cells before amlodipine treatment. Pretreatment with 4-PBA significantly blocked the amlodipine-induced inhibitory effects on ESCC cell viability (Figure 4B), and migration (Figure 4C) exhibiting a definite reversal effect on amlodipine-induced alterations in ATF-6, p-eIF2α, and CHOP proteins related to ER stress, cleaved caspase-3 proteins related to apoptosis, and E-cadherin proteins related to EMT (Figure 4D). Collectively, these results suggest that amlodipine reduces ESCC cell viability and migration by inducing ER stress.
The Ca2+ signaling pathway plays a critical role in various cellular activities, and its impact on autophagy can either facilitate or inhibit[22]. Given that the blockade of L-type calcium channels leads to an imbalance in intracellular calcium homeostasis, whether amlodipine promotes autophagy in ESCC cells was investigated. Autophagy dual-fluorescent LC3 adenoviruses were introduced into ESCC cells and alterations in autophagic flux in response to amlodipine treatment were examined. In control cells (left lane in Figure 5A), both green (GFP-LC3) and red (RFP-LC3) fluorescence were dispersed in the cytoplasm. However, after amlodipine treatment (right lane in Figure 5A), green fluorescent spots (autophagosomes) and red fluorescent spots (autophagolysosomes) were observed, suggesting that amlodipine promotes autophagy. Microtubule-associated protein 1 LC3B, an autophagosome membrane-type LC3 converted from cytoplasmic LC3-I, and beclin-1, a key regulator required for the initiation of autophagosome formation, were examined. The results demonstrated that amlodipine increased the levels of LC3B and beclin-1 in a dose-dependent manner (Figure 5B), further confirming the role of amlodipine in promoting autophagy. To investigate whether autophagy is mediated by amlodipine-induced ER stress, the cells were treated with an ER stress inhibitor 4-PBA. As shown in Figure 5C, 4-treatment reduced the amlodipine-induced upregulation of LC3B. These data suggest that amlodipine promotes autophagy via activation of ER stress in ESCC cells.
Autophagy is induced when ER stress occurs and serves as a crucial compensatory protective mechanism in cells. When autophagy is insufficient to counteract damaging factors, apoptosis can occur. Therefore, we investigated whether amlodipine-induced autophagy functions as a compensatory protective mechanism in ESCC cells and whether it promotes cell apoptosis. To verify this hypothesis, the autophagy inhibitor 3-MA was used. Compared to amlodipine treatment alone, the combination of 3-MA and amlodipine resulted in more pronounced inhibitory effects on cell viability and migration (Figure 6A and B), indicating that the inhibition of autophagy enhances amlodipine-induced apoptosis and migration. Western blot analysis revealed that the combination treatment blocked amlodipine-induced LC3B formation and promoted amlodipine-induced upregulation of E-cadherin, while cleaved caspase-3 decreased (Figure 6C). In summary, the results of these experiments demonstrate the efficacy of amlodipine and suggest that inhibiting autophagy increases amlodipine-induced cell death.
To further determine whether amlodipine exhibits an anti-tumor role against ESCC in vivo, a study was conducted in BALB/c nude mice, wherein these mice were implanted with Eca109 cells to establish a tumor xenograft model. The mice were then divided into the amlodipine treatment and vehicle groups. Compared to the vehicle group, the amlodipine group exhibited a dynamically significant reduction in tumor size without affecting body weight (Figure 7A and B). At the end of the experiment, the tumor tissues (Figure 7C) were photographed, and their average weight (Figure 7D) was calculated. Images and results showed that, compared to the vehicle group, the tumors had a smaller volume and reduced tumor weight in the amlodipine-treated group, further supporting the anti-tumor role of amlodipine in ESCC. The amlodipine-induced tumor inhibition rate was 30.95% (Figure 7E). Taken together, these data indicated that amlodipine inhibited EC growth in vivo.
Cytosolic free Ca2+ is a ubiquitous secondary messenger that controls various fundamental cellular processes, including muscle contraction, cell motility, neurotransmitter release, exocytosis, and endocytosis. It also has been well documented that cytosolic free Ca2+ plays essential roles in cell proliferation, migration, cell cycle control, and apoptosis, which are general features that malignant cells possess[23]. Intracellular Ca2+ homeostasis is regulated by calcium channels on the cell membrane, ER, and mitochondrial surface[24]. One type of calcium channel on the cell membrane is the voltage-gated calcium channel (VGCC), the activation of which causes Ca2+ influx into the cells. Previous research and available public datasets have demonstrated that L-type calcium channels, which constitute a major type of VGCCs, are functionally expressed in various cancer cells and tissue samples from diverse cancer types[8]. Therefore, the extent to which L-type calcium channels are expressed in ESCC is unclear. In this study, we measured the levels of Cav1.3, an essential subunit of the L-type calcium ion channel, in 50 cases of paired EC and adjacent tissues from patients with ESCC and discovered that the average level of Cav1.3 in cancerous tissue was 1.60 times higher than that seen in adjacent tissues. Higher levels of Cav1.3 were detected in both ESCC and EAC cells compared to those in the esophageal epithelial cell line Het-1A. These results suggest that L-type calcium channels possibly function as potential targets for anti-EC therapies.
Dihydropyridine calcium channel blockers are the best-known class of L-type calcium channel blockers and are widely used to treat hypertension, angina pectoris, and atherosclerosis[25]. Many studies have demonstrated that dihydro-pyridine calcium channel blockers effectively suppress cancer cell growth[26]. Amlodipine, a third-generation calcium antagonist, suppresses the proliferation and migration of various cancer cell types. For example, this drug can utilize ERK1 or ERK2, integrin-β1, Bcl-2, and other inhibitors of the proliferation of breast cancer cells in the body, resulting in the formation of colonies, which are vulnerable to invasion[10]. Amlodipine also suppresses the growth of gastric cancer stem cells and exerts an anti-tumor effect mainly by affecting the stability of Ca2+ concentrations within the cells[27].
When the intracellular Ca2+ concentration is out of balance, it often destroys Ca2+ homeostasis in the ER, consequently leading to ER stress, which maintains ER homeostasis and ensures cell survival via the UPR[14]. The UPR mainly includes three protein signal transduction pathways: PERK, IRE1, and ATF6. The UPR mechanism activated during ER stress reduces the accumulation of misfolded or unfolded proteins in the ER, thereby minimizing the damage caused by protein accumulation, restoring ER homeostasis, and ultimately promoting the survival of stressed cells. These effects are mediated through the activation of three signaling pathways described previously[16]. However, when persistent and severe ER stress occurs, the survival mode of UPR changes to a mode that promotes apoptosis. CHOP is a marker gene for apoptosis induced by ER stress[28]. ER stress-mediated apoptosis involves several cellular mechanisms, including the regulation of CHOP gene expression and the subsequent activation of downstream targets, such as members of the Bcl-2 protein family. Continuous activation of CHOP can lead to the downregulation of the anti-apoptotic protein Bcl-2, ultimately leading to the activation of mitochondrial apoptosis regulated by Bax[29]. Moreover, caspase-12 is also recognized as a key factor in ER stress-induced apoptosis. IRE1α, located on the ER membrane, can induce apoptosis during periods of ER stress. Activation of caspase-12 induced by ER stress leads to the cleavage and activation of caspase-9, which then activates a series of effector caspases and induces apoptosis[30]. This experiment demonstrates that the drug effect of amlodipine increases the content of ER stress-related proteins in ESCC cells, the most important ones being GRP78, ATF6 and p-eIF2α. This causes the process of Bcl-2 expression, regulated by CHOP signaling, to decrease. Consequently, the expression levels of both cytochrome c and the pro-apoptotic protein Bax in cells are increased. These phenomena provide strong evidence that amlodipine causes an ER stress response and apoptosis in the mitochondria. At the same time, we detected an increase in the expression of ER stress-specific apoptotic molecules, including cleaved caspase-12, whose expression was elevated. This further activated cleaved caspase-9 and cleaved caspase-3 to promote cell apoptosis.
Recent studies have found that ER stress/UPR are not only related to tumor survival and apoptosis, but are also related to tumor metastasis. Research has indicated that molecules associated with ER stress signaling can impact the EMT process in tumor cells, mainly by regulating the transcriptional changes of EMT-induced transcription factors[31]. For example, the activation of IRE1 α/XBP1 signal pathway can induce Snail transcription in breast cancer cells, promote EMT in these cells, and enhance their migration and invasion ability[32,33]. It has also been reported that the activation of ER stress inhibits the metastasis of gastric and lung cancers by inhibiting EMT[34,35]. This study provides valuable evidence that amlodipine inhibits EMT, which ultimately affects the migration of ESCC cells. To further confirm that amlodipine induces ER stress to promote apoptosis and inhibit EMT, we used 4-PBA, an inhibitor of ER stress, and observed the reversal of ER stress, apoptosis, and EMT-related protein functions. In in vivo experiments, we demonstrated that amlodipine has an anti-tumor effect, although its internal mechanism could not be clarified. These data suggested that the anti-cancer effects of amlodipine against ESCC cells may be attributed to the activation of ER stress.
The proteasome-regulated degradation system cannot handle excessive amounts of un/misfolded proteins during ER stress. Consequently, autophagy is induced to remove these proteins[36]. It has been proven that ER stress and autophagy induced by it are closely related to tumors and have double-edged sword effects[37]. Therefore, it is particularly important to utilize different conditions to clarify the effects and related mechanisms of autophagy in tumor cells, to provide a valuable basis for the development of treatments involving cellular autophagy. In this study, 4-PBA combined with amlodipine downregulated LC3B levels. The results of this study indicate that amlodipine-induced autophagy may be due to ER stress. To further elucidate the role of autophagy in amlodipine-induced ER stress, the autophagy inhibitor 3-MA was used. Amlodipine combined with 3-MA significantly enhanced amlodipine-induced cell death and inhibited cell migration. Amlodipine promotes autophagy through ER stress, and autophagy plays a role in protecting tumor cells, providing a basis for the combined application of amlodipine and autophagy inhibitors in clinical setting.
In conclusion, we have demonstrated for the first time that amlodipine inhibits cell proliferation in vitro and in vivo, promotes apoptosis and inhibits EMT through ER stress in EC. Moreover, amlodipine induces autophagy, which alleviates ER stress and may play a cytoprotective role. Our findings provide essential evidence supporting amlodipine, and the combined use of amlodipine with an autophagy inhibitor, as potential therapeutic options for patients with ESCC.
Esophageal cancer (EC) is the sixth most common tumor worldwide and has a poor prognosis. Although L-type calcium channel blockers have demonstrated efficacy in inhibiting the occurrence and development of various tumors, their impact on EC remains unclear.
Patients with EC have a poor prognosis owing to a lack of effective treatments and prognostic indicators. This study aimed to explore a novel approach for treating EC and improving patient survival rates.
To elucidate the mechanism by which the L-type calcium channel blocker, amlodipine, inhibits the proliferation and migration of EC cells, thereby offering a potential new avenue for the treatment of EC.
Western blot analysis was used to assess the expression of relevant proteins. Cell migration was evaluated using Transwell assays and apoptosis was measured using flow cytometry. The endoplasmic reticulum (ER) stress inhibitor 4-phenylbutyric acid was used to prevent ER stress. Transduction of EC cells with GFP-RFP-LC3 adenovirus confirmed that amlodipine-mediated ER stress functions through downstream autophagy. In addition, a murine xenograft model constructed using Eca109 cells was used to validate the anti-tumor effects of amlodipine in vivo.
This study revealed that the Cav1.3 level in EC tissues was higher than that in adjacent tissues. The L-type calcium channel blocker, amlodipine, inhibits the proliferation and migration of EC cells while promoting apoptosis. Mechanistic investigations have indicated that these effects are associated with the induction of cellular ER stress. Further studies using GFP-RFP-LC3 adenovirus-transfected cells treated with amlodipine demonstrated that autophagy, mediated by ER stress, played a protective role in this process. In addition, amlodipine inhibited EC cell growth and presented an antitumor effect in vivo.
L-type calcium channels are highly expressed in EC tissues. Both in vivo and in vitro experiments revealed that amlodipine inhibited the proliferation and migration of EC cells through ER stress.
The expression levels of L-type calcium channels in EC may serve as an index of prognosis, and the combination of amlodipine with autophagy inhibitors holds promise as a novel treatment approach. Future endeavors should involve large-sample multicenter clinical studies to further validate its reliability.
We thank Chen-Fang Qi (Intensive Care Unit, Cedars-Sinai Medical Center, 8700 Beverly Blvd, Los Angeles, CA 90048, United States) for providing language editorial assistance.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology & hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Suresh Kumar VC, United States S-Editor: Chen YL L-Editor: A P-Editor: Chen YX
| 1. | Dias F, Morais M, Teixeira AL, Medeiros R. Involving the microRNA Targetome in Esophageal-Cancer Development and Behavior. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 2. | Song Y, Li L, Ou Y, Gao Z, Li E, Li X, Zhang W, Wang J, Xu L, Zhou Y, Ma X, Liu L, Zhao Z, Huang X, Fan J, Dong L, Chen G, Ma L, Yang J, Chen L, He M, Li M, Zhuang X, Huang K, Qiu K, Yin G, Guo G, Feng Q, Chen P, Wu Z, Wu J, Zhao J, Luo L, Fu M, Xu B, Chen B, Li Y, Tong T, Wang M, Liu Z, Lin D, Zhang X, Yang H, Zhan Q. Identification of genomic alterations in oesophageal squamous cell cancer. Nature. 2014;509:91-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 855] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 3. | Lv J, Guo L, Liu JJ, Zhao HP, Zhang J, Wang JH. Alteration of the esophageal microbiota in Barrett's esophagus and esophageal adenocarcinoma. World J Gastroenterol. 2019;25:2149-2161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 85] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (2)] |
| 4. | Xu L, Sun L, Xie L, Mou S, Zhang D, Zhu J, Xu P. Advances in L-Type Calcium Channel Structures, Functions and Molecular Modeling. Curr Med Chem. 2021;28:514-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Ednie AR, Bennett ES. Intracellular O-linked glycosylation directly regulates cardiomyocyte L-type Ca(2+) channel activity and excitation-contraction coupling. Basic Res Cardiol. 2020;115:59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Dickerson MT, Dadi PK, Butterworth RB, Nakhe AY, Graff SM, Zaborska KE, Schaub CM, Jacobson DA. Tetraspanin-7 regulation of L-type voltage-dependent calcium channels controls pancreatic β-cell insulin secretion. J Physiol. 2020;598:4887-4905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Phan NN, Wang CY, Chen CF, Sun Z, Lai MD, Lin YC. Voltage-gated calcium channels: Novel targets for cancer therapy. Oncol Lett. 2017;14:2059-2074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 8. | Jacquemet G, Baghirov H, Georgiadou M, Sihto H, Peuhu E, Cettour-Janet P, He T, Perälä M, Kronqvist P, Joensuu H, Ivaska J. L-type calcium channels regulate filopodia stability and cancer cell invasion downstream of integrin signalling. Nat Commun. 2016;7:13297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 9. | Floersheim GL, Racine C. Calcium antagonist radioprotectors do not reduce radiotherapeutic efficacy in three human tumor xenografts. Strahlenther Onkol. 1995;171:403-407. [PubMed] |
| 10. | Alqudah MAY, Al-Samman R, Azaizeh M, Alzoubi KH. Amlodipine inhibits proliferation, invasion, and colony formation of breast cancer cells. Biomed Rep. 2022;16:50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Panneerpandian P, Rao DB, Ganesan K. Calcium channel blockers lercanidipine and amlodipine inhibit YY1/ERK/TGF-β mediated transcription and sensitize the gastric cancer cells to doxorubicin. Toxicol In Vitro. 2021;74:105152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Shaughnessy M, Lamuraglia G, Klebanov N, Ji Z, Rajadurai A, Kumar R, Flaherty K, Tsao H. Selective uveal melanoma inhibition with calcium channel blockade. Int J Oncol. 2019;55:1090-1096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Schwarz DS, Blower MD. The endoplasmic reticulum: structure, function and response to cellular signaling. Cell Mol Life Sci. 2016;73:79-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 949] [Cited by in RCA: 1073] [Article Influence: 119.2] [Reference Citation Analysis (0)] |
| 14. | Groenendyk J, Agellon LB, Michalak M. Calcium signaling and endoplasmic reticulum stress. Int Rev Cell Mol Biol. 2021;363:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 90] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 15. | Di Conza G, Ho PC. ER Stress Responses: An Emerging Modulator for Innate Immunity. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 16. | Zhu L, Zhou Q, He L, Chen L. Mitochondrial unfolded protein response: An emerging pathway in human diseases. Free Radic Biol Med. 2021;163:125-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 17. | Yin Y, Sun G, Li E, Kiselyov K, Sun D. ER stress and impaired autophagy flux in neuronal degeneration and brain injury. Ageing Res Rev. 2017;34:3-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 170] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 18. | Hetz C, Zhang K, Kaufman RJ. Mechanisms, regulation and functions of the unfolded protein response. Nat Rev Mol Cell Biol. 2020;21:421-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 1615] [Article Influence: 323.0] [Reference Citation Analysis (0)] |
| 19. | Lee WS, Yoo WH, Chae HJ. ER Stress and Autophagy. Curr Mol Med. 2015;15:735-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 152] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 20. | Limonta P, Moretti RM, Marzagalli M, Fontana F, Raimondi M, Montagnani Marelli M. Role of Endoplasmic Reticulum Stress in the Anticancer Activity of Natural Compounds. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 21. | Lai ST, Wang Y, Peng F. Astragaloside IV sensitizes non-small cell lung cancer cells to cisplatin by suppressing endoplasmic reticulum stress and autophagy. J Thorac Dis. 2020;12:3715-3724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Sukumaran P, Nascimento Da Conceicao V, Sun Y, Ahamad N, Saraiva LR, Selvaraj S, Singh BB. Calcium Signaling Regulates Autophagy and Apoptosis. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 141] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 23. | Patergnani S, Danese A, Bouhamida E, Aguiari G, Previati M, Pinton P, Giorgi C. Various Aspects of Calcium Signaling in the Regulation of Apoptosis, Autophagy, Cell Proliferation, and Cancer. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 223] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 24. | Ye L, Zeng Q, Ling M, Ma R, Chen H, Lin F, Li Z, Pan L. Inhibition of IP3R/Ca2+ Dysregulation Protects Mice From Ventilator-Induced Lung Injury via Endoplasmic Reticulum and Mitochondrial Pathways. Front Immunol. 2021;12:729094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 25. | Santa-Helena E, Cabrera DDC, D'Oca MGM, Scaini JLR, de Oliveira MWB, Werhli AV, Machado KDS, Gonçalves CAN, Nery LEM. Long-chain fatty dihydropyridines: Docking calcium channel studies and antihypertensive activity. Life Sci. 2020;259:118210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Wu L, Lin W, Liao Q, Wang H, Lin C, Tang L, Lian W, Chen Z, Li K, Xu L, Zhou R, Ding Y, Zhao L. Calcium Channel Blocker Nifedipine Suppresses Colorectal Cancer Progression and Immune Escape by Preventing NFAT2 Nuclear Translocation. Cell Rep. 2020;33:108582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Shiozaki A, Katsurahara K, Kudou M, Shimizu H, Kosuga T, Ito H, Arita T, Konishi H, Komatsu S, Kubota T, Fujiwara H, Okamoto K, Otsuji E. Amlodipine and Verapamil, Voltage-Gated Ca(2+) Channel Inhibitors, Suppressed the Growth of Gastric Cancer Stem Cells. Ann Surg Oncol. 2021;28:5400-5411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Delbrel E, Soumare A, Naguez A, Label R, Bernard O, Bruhat A, Fafournoux P, Tremblais G, Marchant D, Gille T, Bernaudin JF, Callard P, Kambouchner M, Martinod E, Valeyre D, Uzunhan Y, Planès C, Boncoeur E. HIF-1α triggers ER stress and CHOP-mediated apoptosis in alveolar epithelial cells, a key event in pulmonary fibrosis. Sci Rep. 2018;8:17939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 123] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 29. | Hu H, Tian M, Ding C, Yu S. The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection. Front Immunol. 2018;9:3083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 792] [Article Influence: 132.0] [Reference Citation Analysis (0)] |
| 30. | Imran M, Rauf A, Abu-Izneid T, Nadeem M, Shariati MA, Khan IA, Imran A, Orhan IE, Rizwan M, Atif M, Gondal TA, Mubarak MS. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed Pharmacother. 2019;112:108612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 648] [Cited by in RCA: 552] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 31. | Han CC, Wan FS. New Insights into the Role of Endoplasmic Reticulum Stress in Breast Cancer Metastasis. J Breast Cancer. 2018;21:354-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Li H, Chen X, Gao Y, Wu J, Zeng F, Song F. XBP1 induces snail expression to promote epithelial- to-mesenchymal transition and invasion of breast cancer cells. Cell Signal. 2015;27:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 33. | Cuevas EP, Eraso P, Mazón MJ, Santos V, Moreno-Bueno G, Cano A, Portillo F. LOXL2 drives epithelial-mesenchymal transition via activation of IRE1-XBP1 signalling pathway. Sci Rep. 2017;7:44988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 34. | Liu SH, Lee WJ, Lai DW, Wu SM, Liu CY, Tien HR, Chiu CS, Peng YC, Jan YJ, Chao TH, Pan HC, Sheu ML. Honokiol confers immunogenicity by dictating calreticulin exposure, activating ER stress and inhibiting epithelial-to-mesenchymal transition. Mol Oncol. 2015;9:834-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 35. | Markov AV, Odarenko KV, Sen'kova AV, Salomatina OV, Salakhutdinov NF, Zenkova MA. Cyano Enone-Bearing Triterpenoid Soloxolone Methyl Inhibits Epithelial-Mesenchymal Transition of Human Lung Adenocarcinoma Cells In Vitro and Metastasis of Murine Melanoma In Vivo. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Adacan K, Obakan-Yerlikaya P, Arisan ED, Coker-Gurkan A, Kaya RI, Palavan-Unsal N. Epibrassinolide-induced autophagy occurs in an Atg5-independent manner due to endoplasmic stress induction in MEF cells. Amino Acids. 2020;52:871-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Zhou B, Lu Q, Liu J, Fan L, Wang Y, Wei W, Wang H, Sun G. Melatonin Increases the Sensitivity of Hepatocellular Carcinoma to Sorafenib through the PERK-ATF4-Beclin1 Pathway. Int J Biol Sci. 2019;15:1905-1920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |