Published online Jan 28, 2024. doi: 10.3748/wjg.v30.i4.308
Peer-review started: November 6, 2023
First decision: November 30, 2023
Revised: December 15, 2023
Accepted: January 10, 2024
Article in press: January 10, 2024
Published online: January 28, 2024
Processing time: 80 Days and 22.3 Hours
About 10%-31% of colorectal liver metastases (CRLM) patients would concomitantly show hepatic lymph node metastases (LNM), which was considered as sign of poor biological behavior and a relative contraindication for liver resection. Up to now, there’s still lack of reliable preoperative methods to assess the status of hepatic lymph nodes in patients with CRLM, except for pathology examination of lymph node after resection.
To compare the ability of mono-exponential, bi-exponential, and stretched-exponential diffusion-weighted imaging (DWI) models in distinguishing between benign and malignant hepatic lymph nodes in patients with CRLM who received neoadjuvant chemotherapy prior to surgery.
In this retrospective study, 97 CRLM patients with pathologically confirmed hepatic lymph node status underwent magnetic resonance imaging, including DWI with ten b values before and after chemotherapy. Various parameters, such as the apparent diffusion coefficient from the mono-exponential model, and the true diffusion coefficient, the pseudo-diffusion coefficient, and the perfusion fraction derived from the intravoxel incoherent motion model, along with distributed diffusion coefficient (DDC) and α from the stretched-exponential model (SEM), were measured. The parameters before and after chemotherapy were compared between positive and negative hepatic lymph node groups. A nomogram was constructed to predict the hepatic lymph node status. The reliability and agreement of the measurements were assessed using the coefficient of variation and intraclass correlation coefficient.
Multivariate analysis revealed that the pre-treatment DDC value and the short diameter of the largest lymph node after treatment were independent predictors of metastatic hepatic lymph nodes. A nomogram combining these two factors demonstrated excellent performance in distinguishing between benign and malignant lymph nodes in CRLM patients, with an area under the curve of 0.873. Furthermore, parameters from SEM showed substantial repeatability.
The developed nomogram, incorporating the pre-treatment DDC and the short axis of the largest lymph node, can be used to predict the presence of hepatic LNM in CRLM patients undergoing chemotherapy before surgery. This nomogram was proven to be more valuable, exhibiting superior diagnostic performance compared to quantitative parameters derived from multiple b values of DWI. The nomogram can serve as a preoperative assessment tool for determining the status of hepatic lymph nodes and aiding in the decision-making process for surgical treatment in CRLM patients.
Core Tip: This study compared the diagnostic effectiveness of mono-exponential, bi-exponential, and stretched exponential Diffusion-weighted magnetic resonance imaging in predicting hepatic lymph node metastases (LNM) in patients with colorectal liver metastases after chemotherapy. Our finding indicated that only the pre-treatment distributed diffusion coefficient value and the short diameter of the largest lymph node after treatment were independent predictors of hepatic LNM. We developed a nomogram incorporating these two factors to non-invasively and individually predict the status of hepatic lymph nodes, demonstrating significant potential in surgical planning and assessing high-risk patients.
- Citation: Zhu HB, Zhao B, Li XT, Zhang XY, Yao Q, Sun YS. Value of multiple models of diffusion-weighted imaging to predict hepatic lymph node metastases in colorectal liver metastases patients. World J Gastroenterol 2024; 30(4): 308-317
- URL: https://www.wjgnet.com/1007-9327/full/v30/i4/308.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i4.308
Colorectal carcinoma ranks as the most prevalent digestive tumors globally, with over 50% of patients developing colorectal liver metastases (CRLM) either at diagnosis (synchronous metastases) or during follow-up (metachronous metastases)[1]. Currently, the preferred approach in standard treatment guidelines involves perioperative chemotherapy combined with surgical resection, particularly when achieving complete resection with sufficient residual liver parenchyma is feasible[2,3]. Approximately 10%-31% of CRLM patients exhibit hepatic lymph node metastases (LNM), representing an adverse prognostic factor with significant impact on outcomes[4,5]. Surgery remains the sole potentially curative therapy if LNM are confined to the hepatic pedicle, although this procedure may be associated with potential postoperative complications, such as bleeding, lymphatic leakage, and ischemic bile duct stricture[6,7].
The gold standard for evaluating LNM still relies on histopathological assessment post-operation. Currently, there is inconsistency in the indications for lymphadenectomy in CRLM, partly due to the challenge of preoperatively predicting LNM. For instance, Grobmyer et al[8] examined 100 patients with hepatic lymph nodes undergoing resection for primary and metastatic hepatic malignancies. They found that both CT and intraoperative clinical palpation had a high negative predictive value (NPV = 95% and 99%, respectively) with a low positive predictive value (PPV = 30% and 39%, res-pectively). Similarly, Rau et al[9] discovered that a short diameter of lymph nodes larger than 15 mm and a morphologically round shape on computed tomography (CT) had a high NPV of 85% but a relatively low PPV of 43% for LNM. Intriguingly, up to 27% of patients with confirmed pathological LNM were not initially suspected using a combination of CT and intraoperative examination. Therefore, there is a crucial need for reliable predictors of LNM in CRLM before surgery to precisely guide individual decision-making and prevent overtreatment in low-risk patients.
Diffusion-weighted imaging (DWI) has undergone extensive investigation for its utility in cancer detection, treatment response assessment, and prognosis evaluation[10-12]. The apparent diffusion coefficient (ADC), derived from DWI, exhibits promising capabilities in distinguishing lymph nodes, providing a noninvasive assessment of the microscopic random Brownian motion of water molecules in biological tissues. For instance, Sumi et al[13] observed higher ADC values in metastatic lymph nodes compared to benign non-metastatic lymph nodes, whereas Abdel Razek et al[14] and Eiber et al[15] reported lower ADC values in metastatic lymph nodes. This inconsistency may arise from the mono-exponential decay formula used to calculate ADC values, assuming tissue homogeneity and water molecule movement with a Gaussian distribution. Intravoxel incoherent motion (IVIM) is a technique capable of potentially differentiating perfusion components from the pure diffusion of water molecules using a biexponential model. This model allows for the quantification of three parameters: The true diffusion coefficient (D), the pseudo-diffusion coefficient (D*), and the perfusion fraction (f). Consequently, parameters obtained from the IVIM model have demonstrated superior diagnostic performance compared to traditional ADC in differentiating hepatic lesions in previous studies[16,17]. More recently, Bennett et al[18] introduced the stretched-exponential model (SEM), providing an alternative approach to quantify intravoxel heterogeneity. The SEM employs two parameters: The distributed diffusion coefficient (DDC) and the intravoxel water diffusion heterogeneity (α). However, to date, there remains a paucity of studies comparing functional magnetic resonance imaging (MRI) parameters derived from different models to determine the status of hepatic lymph nodes in CRLM patients.
The objective of this study was to assess the diagnostic accuracy of three mathematical models of DWI in distinguishing between benign and malignant hepatic lymph nodes in CRLM patients who underwent chemotherapy prior to surgery.
This retrospective study protocol received approval from the Medical Ethics Committee of Beijing Cancer Hospital, and informed consent was waived.
CRLM patients with a pathologic diagnosis of hepatic lymph nodes in our hospital between January 2015 and January 2023 were included in this study. Patients had to undergo at least two cycles of neoadjuvant chemotherapy and undergo MRI examinations before neoadjuvant chemotherapy (pre-treatment point) and within 1 mo before surgery (post-treatment point). Exclusion criteria were: (1) Patients who underwent hepatectomy without hepatic lymph node resection; (2) Patients without measurable hepatic lymph nodes > 5 mm on the baseline MRI; and (3) Patients without multiple b-values of DWI sequence or insufficient quality of DWI for analysis. A total of 97 patients were enrolled in this study.
All patients underwent MRI examinations using a 1.5T MRI device (Signa Excite II; GE Healthcare, Milwaukee, WI, United States) equipped with an 8-channel phased array body coil. The imaging protocol included axial T2-weighted imaging (T2WI) with fat saturation, multiple b-values of DWI, and dynamic contrast-enhanced (DCE) MRI sequences. A respiratory-triggered single-shot echo planar imaging sequence was employed for DWI, with b-values of 0, 20, 50, 100, 200, 600, 800, 1000, 1200, and 1500 s/mm2, respectively. The DWI sequence parameters were: Repetition time (TR)/echo time (TE) = 3000/80; slice thickness = 6 mm; slice gap = 1 mm; matrix = 128 × 90. The total acquisition time for the DWI sequence was approximately 6 min and 19 s. The corresponding parameters for T2WI were: TR/TE = 12630/70 ms; slice thickness = 6 mm; slice gap = 1 mm; matrix = 228 × 224.
Images were independently analyzed by two radiologists (B.Z., with 6 years of experience, and H.B.Z with 12 years of experience), utilizing the FuncTool Software implemented in GE Workstation 4.6. The radiologists were blinded to clinical information, pathological results, and each other’s findings. To determine the regions of interest (ROI), the radiologists manually drew the ROI on the DWI image with a b-value of 800 s/mm2 at the maximum transverse diameter of the hepatic lymph node, avoiding areas containing adjacent vessels and artifacts. T2WI and DCE-MRI images served as references. Additionally, the mean value of parameters obtained from the two observers for each ROI was calculated for further analysis.
The signal intensity (SI) of each ROI was fitted using the following mathematical models, where S(b) is the SI at a particular b value, and S(0) is the SI with b = 0 s/mm2:
(1) ADC was calculated using the mono-exponential model:
S(b)/S(0) = exp(-b × ADC)
(2) Three parameters were calculated using biexponential IVIM model according to the following equation:
S(b)/S(0) = f × exp(-b × D*)+ (1-f) × (-b × D)
D: The true diffusion coefficient; D*: Pseudo-diffusion coefficient; f: The fraction of pseudo-diffusion.
(3) DDC and α were acquired from SEM using the following mathematical equation:
S(b)/S(0) = exp{-(b × DDC)}α
DDC: The distributed diffusion coefficient, characterizing the distribution of diffusion rates within a voxel; α: Ranging from 0 to 1, represents intravoxel diffusion heterogeneity.
Hepatic lymph nodes were delineated based on specific criteria, encompassing nodes along the hepatoduodenal ligament, which includes structures like the proper hepatic artery, portal vein, bile duct, and retro-pancreatic head. Nodes along the common hepatic artery and coeliac artery, covering the coeliac, common hepatic, and left gastric arteries, were also considered. Since hepatic lymph nodes were not routinely dissected, only suspected nodes on preoperative imaging and/or intraoperative examination were removed. Hematoxylin and eosin stained specimens of the surgically removed lymph nodes were examined by specialized pathologists, and all pathological results were obtained from final patho-logical reports.
Clinical information of CRLM patients was collected retrospectively, encompassing age, sex, location (left half colon vs right half colon), T and N stage of the primary tumor, synchronous or metachronous liver metastases, number of liver metastases (single vs multiple), RAS gene status (mutation type vs wild type), treatment response based on RECIST1.1 standard, disappearing lesions (identified when no visible lesion is observed on all imaging sequences after chemotherapy), and levels of carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9). Serum tumor markers were categorized into two groups: Those within normal limits and those exceeding normal limits, defined as 5 ng/mL for CEA and 40 ng/mL for CA19-9.
Continuous variables are presented as mean ± SD, while categorical variables are expressed as numbers and percentages. To compare characteristics between the two groups, independent-samples t/Mann-Whitney or chi-square tests were employed. To identify independent factors associated with hepatic LNM, multivariable logistic regression was conducted using a forward stepwise approach. The diagnostic performance of the predictive model was assessed using the receiver operating characteristic (ROC) curve, and the area under the ROC curve (AUC) with its 95% confidence interval (CI) was calculated. The model's cutoffs were determined using the maximum Youden’s method. Sensitivity, specificity, PPV and NPV were also computed to evaluate the model's performance. Inter-observer agreements of quantitative metrics were tested using intraclass correlation coefficients (ICC), with ICC > 0.75 indicating good agreement, 0.40 to 0.75 suggesting moderate agreement, and ≤ 0.40 indicating poor agreement. All statistical analyses were performed using SPSS 25.0 (IBM Corporation, Armonk, NY, United States). A two-sided P value less than 0.05 was considered statistically significant, indicating a significant difference or association between variables.
Among the 97 enrolled patients, 40 patients (41.2%; mean ± age = 57.53 ± 9.43 years) exhibited hepatic LNM, while the other 57 patients (58.8%; mean ± age = 52.91 ± 10.48 years) did not.
In univariate analysis, the short and long axes of the largest lymph node before treatment, short and long axes of the largest lymph node after treatment, pre-treatment D, pre-treatment DDC, post-treatment ADC, post-treatment DDC, and post-treatment α were found to be statistically significant with hepatic LNM (P < 0.05).
In multivariate analysis, only pre-treatment DDC (OR < 0.001; P = 0.002) and the short axis of the largest lymph node after treatment (OR = 1.509; P < 0.001) were identified as independent risk factors for the status of hepatic LNM. The detailed results of the univariate and multivariate analyses are presented in Table 1.
| Univariate analysis | Multivariate analysis | |||||
| Non-hepatic LNM (n = 57) | hepatic LNM HLN (n = 40) | P value | OR (95%CI) | P value | ||
| Gender | Male/female | 44/13 | 27/13 | 0.029a | ||
| Age | 52.91 ± 10.48 | 57.53 ± 9.43 | 0.054 | |||
| BMI | 24.63 ± 3.04 | 24.50 ± 3.04 | 0.842 | |||
| Primary location | Right/left-side | 15/42 | 6/34 | 0.944 | ||
| Differentiation | Low to moderate/High | 55/2 | 40/0 | 0.510 | ||
| T stage of primary tumor | T1+2/T3+4 | 3/54 | 4/36 | 0.650 | ||
| N stage of primary tumor | N0/N+ | 9/48 | 5/35 | 0.149 | ||
| Gene | RAS-wild/mutation | 38/19 | 28/12 | 0.729 | ||
| Simultaneous liver metastases | No/Yes | 13/44 | 12/28 | 0.425 | ||
| Distribution | Solitary/Bilateral | 20/37 | 15/25 | 0.808 | ||
| Number of CRLM | ≤ 3/> 3 | 17/40 | 15/25 | 0.429 | ||
| Size (mm) | 38.25 ± 27.69 | 37.88 ± 22.11 | 0.944 | |||
| RECIST | Response/Non-response | 33/24 | 21/19 | 0.599 | ||
| Disappearing lesion | No/Yes | 46/11 | 34/6 | 0.584 | ||
| pre-CEA | ≤ 5/> 5 ng/mL | 15/42 | 11/29 | 0.897 | ||
| pre-CA199 | ≤ 40/> 40 U/mL | 26/31 | 17/23 | 0.761 | ||
| post-CEA | ≤ 5/> 5 ng/mL | 27/30 | 17/23 | 0.635 | ||
| post-CA199 | ≤ 40/> 40 U/mL | 33/24 | 25/15 | 0.649 | ||
| Short axis of largest lymph node before treatment | mm | 7.39 ± 2.65 | 11.88 ± 5.35 | < 0.001a | ||
| Long axis of largest lymph node before treatment | mm | 14.25 ± 6.41 | 18.28 ± 7.28 | 0.005a | ||
| Pre-ADC | mm2/s | 1.54 ± 0.35 | 1.49 ± 0.30 | 0.394 | ||
| Pre-D | mm2/s | 1.21 ± 0.43 | 1.02 ± 0.25 | 0.005a | ||
| Pre-D* | mm2/s | 3.33 ± 2.37 | 2.70 ± 2.38 | 0.200 | ||
| Pre-f | 0.49 ± 0.17 | 0.45 ± 0.14 | 0.330 | |||
| Pre-DDC | mm2/s | 3.21 ± 1.69 | 2.01 ± 0.83 | < 0.001a | < 0.001 | 0.002a |
| Pre-α | 0.59 ± 0.17 | 0.62 ± 0.16 | 0.363 | |||
| Short axis of largest lymph node after treatment | mm | 6.74 ± 2.13 | 10.43 ± 3.62 | < 0.001a | 1.509 (1.235-1.845) | < 0.001a |
| Long axis of largest lymph node after treatment | mm | 13.46 ± 5.78 | 17.08 ± 6.82 | 0.006a | ||
| Post-ADC | mm2/s | 1.64 ± 0.32 | 1.45 ± 0.32 | 0.006a | ||
| Post-D | mm2/s | 1.35 ± 0.86 | 1.24 ± 0.78 | 0.529 | ||
| Post-D* | mm2/s | 3.69 ± 2.96 | 3.48 ± 3.38 | 0.751 | ||
| Post-f | 0.51 ± 0.18 | 0.52 ± 0.18 | 0.732 | |||
| Post-DDC | 3.46 ± 1.48 | 2.37 ± 0.91 | < 0.001a | |||
| Post-α | 0.61 ± 0.13 | 0.67 ± 0.13 | 0.035a | |||
Table 2 summarizes the results of ROC analysis for quantitative parameters from all three models for predicting hepatic LNM. Pre-DDC had the largest AUC (AUC = 0.770; 95%CI: 0.676-0.865), followed by post-DDC (AUC = 0.739; 95%CI: 0.641-0.838) and post-ADC (AUC = 0.664; 95%CI: 0.553-0.774). The sensitivity, specificity, PPV, NPV, and accuracy of pre-DDC for differentiating malignant and benign hepatic lymph nodes were 85.0%, 59.6%, 59.6%, 85.0%, and 70.1%, respectively, with an optimal cutoff value of 1.92 × 10-3 mm2/s.
| AUC | Cut off value | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | |
| Pre-ADC | 0.551 (0.436-0.666) | 1.70 | 32.5 | 82.5 | 56.5 | 63.5 | 61.5 |
| Pre-D | 0.648 (0.538-0.758) | 1.15 | 55.0 | 77.2 | 62.9 | 58.1 | 68.0 |
| Pre-D* | 0.592 (0.477-0.707) | 2.51 | 55.0 | 66.7 | 53.7 | 71.0 | 62.1 |
| Pre-f | 0.577 (0.462-0.692) | 3.98 | 70.0 | 47.5 | 48.3 | 64.3 | 56.7 |
| Pre-DDC | 0.770 (0.676-0.865) | 1.92 | 85.0 | 59.6 | 59.6 | 85.0 | 70.1 |
| Pre-α | 0.573 (0.456-0.689) | 0.59 | 62.5 | 59.6 | 52.1 | 69.4 | 60.8 |
| Post-ADC | 0.664 (0.553-0.774) | 1.46 | 75.0 | 52.6 | 52.6 | 75.0 | 61.9 |
| Post-D | 0.581 (0.447-0.681) | 1.21 | 50.0 | 70.2 | 54.1 | 66.7 | 62.1 |
| Post-D* | 0.558 (0.438-0.678) | 1.27 | 85.0 | 33.3 | 47.2 | 76.0 | 54.6 |
| Post-f | 0.521 (0.403-0.638) | 3.98 | 77.5 | 31.6 | 44.3 | 66.7 | 50.5 |
| Post-DDC | 0.739 (0.641-0.838) | 2.26 | 82.5 | 52.5 | 55.0 | 81.1 | 64.9 |
| Post-α | 0.623 (0.509-0.737) | 0.65 | 57.5 | 70.2 | 57.5 | 70.2 | 65.0 |
| Short axis of largest lymph node before treatment (mm) | 0.773 (0.674-0.872) | 12 | 50.0 | 94.7 | 87.0 | 73.0 | 76.3 |
| Short axis of largest lymph node after treatment (mm) | 0.811 (0.724-0.899) | 10 | 52.5 | 94.7 | 38.9 | 74.0 | 77.3 |
| Nomogram | 0.873 (0.803, 0.943) | 1.03 | 82.5 | 82.5 | 87.0 | 76.7 | 82.5 |
Furthermore, the short axis of the largest lymph node before and after treatment also exhibited good performance in predicting hepatic LNM. The highest accuracy (77.3%) was achieved at a cutoff value of 10 mm (the best cut off value = 9.5 mm) for the short axis of the largest hepatic lymph node after treatment, which had 52.5% sensitivity and 94.7% specificity for differentiating the status of hepatic lymph nodes.
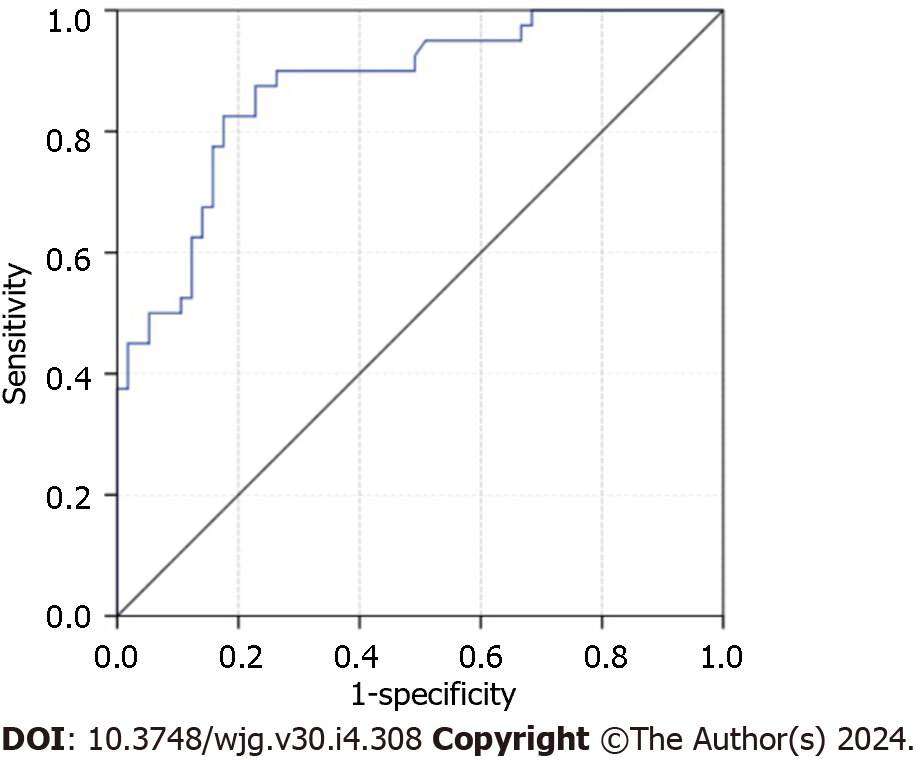
The nomogram, incorporating pre-treatment DDC and the short axis of the largest lymph node after treatment, exhibited effective performance in predicting hepatic LNM. The AUC of the nomogram was 0.873 (95%CI: 0.803-0.943) (Figure 1), with sensitivity, specificity, PPV, NPV, and accuracy at 82.5%, 82.5%, 87.0%, 76.7%, and 82.5%, respectively. The nomogram for predicting hepatic LNM is presented in Figure 2.
Moderate or good interobserver agreement was achieved for quantitative parameters (ICC range: 0.47-0.83). The ICCs of DDC before and after treatment were 0.52 and 0.81, respectively.
In this study, our goal was to assess the diagnostic potential of DWI parameters using three models to differentiate between benign hepatic lymph nodes and metastatic lymph nodes in patients with initially resectable CRLM. Our findings indicate that the DDC values obtained from the SEM were significantly lower in metastatic lymph nodes compared to non-metastatic lymph nodes, both before and after treatment. Notably, the baseline DDC value exhibited the highest accuracy for preoperative lymph node status diagnosis in CRLM patients, outperforming the accuracy of ADC from the mono-exponential model, as well as D, D*, and f from the IVIM model. Furthermore, there was substantial agreement between two independent readers in assessing DDC, suggesting that DDC, along with the short diameter of the largest lymph node, may serve as a reliable, non-invasive, and promising technique in clinical practice for distinguishing between metastatic and non-metastatic lymph nodes before surgery.
Our results show that the baseline DDC from the SEM demonstrated the highest diagnostic performance in distinguishing metastatic from benign hepatic lymph nodes, followed by post-DDC and post-ADC, although the differences among them were not statistically significant. The DDC value is considered a weighted sum of continuous distributions of ADCs and can offer more information on non-Gaussian distribution. These results can be attributed to increased cellularity, higher nucleus-to-cytoplasm ratios, and more limited extracellular space in malignant lymph nodes, leading to greater intravoxel diffusion heterogeneity[19,20]. Therefore, DDC may have a superior ability to differentiate between benign and malignant liver lesions with minimal overlap compared to ADC calculated from the mono-exponential model, consistent with previous studies on gliomas, ovarian cancer, bladder cancer, and hepatic lesions[21-24]. Additionally, our findings suggest that DDC values calculated from the SEM are more reliable than those from the mono-exponential and IVIM models, aligning with previous studies[25-27].
On the contrary, while quantitative parameters obtained from the IVIM model, except for post-f of benign hepatic lesions, were higher in malignant lymph nodes, the difference was not statistically significant. Several factors may contribute to these results. Firstly, the predictive value of the IVIM model for lymph node status has not been consistently supported in previous literature. For instance, in a study on rectal adenocarcinoma patients, Jia et al[28] found that the group with positive lymph nodes exhibited a significantly lower D* value and a higher f value. Conversely, another study on rectal cancer patients showed that the metastatic group had significantly lower D and D* values compared to the nonmetastatic group[29]. Various factors, such as the setting of b-values (especially b-values < 200 s/mm²), TR, and scan techniques, may influence the results of IVIM parameters. Secondly, the heterogeneity of hepatic lesions can impact the quantitative parameters of the IVIM model. Malignant lesions typically demonstrate more heterogeneity in terms of cellularity, vascularity, and perfusion compared to benign lesions. This inherent heterogeneity can lead to variations in the IVIM parameters, making it challenging to differentiate between benign and malignant lesions based solely on IVIM parameters. Additionally, the limited sample size in our study may introduce selection bias.
Our study also revealed that the short diameter of the largest lymph node after treatment was useful in predicting the status of hepatic lymph nodes in CRLM patients. This finding aligns with a previous study indicating tumor size as an independent predictor of lymph node metastases[30]. We identified the optimal diagnostic threshold for the short diameter of lymph nodes as 10 mm, with a sensitivity of 52.5%, specificity of 94.7%, and accuracy of 77.3%. The nomogram, combining DDC and the short diameter of the largest lymph node, can quantitatively evaluate lymph node metastases with enhanced diagnostic efficacy. The nomogram's diagnostic efficiency, with an AUC of 0.873, demonstrated superior performance compared to using either IVIM or SEM alone. Furthermore, the nomogram exhibited improved sensitivity, specificity, and accuracy. These results suggest that the nomogram can effectively prevent unnecessary lymph node dissection in CRLM patients.
The current study has several limitations. Firstly, it was a retrospective, single-center study with a relatively small sample size. Therefore, further studies with a larger sample size and external validation are needed to validate the findings. Secondly, there may be selection bias because we only included patients with clinically suspected lymph node metastasis who underwent surgical resection. This could potentially underestimate the severity of the condition, as most CRLM patients were excluded if they did not have clinically suspicious metastatic lymph nodes. Thirdly, there may be uncertainty regarding the alignment between the lymph node evaluated by the pathologist and the image slices where the DWI parameters were obtained. Additionally, the setting of b-values in DWI remains controversial. While using too many b-values would result in prolonged scan time, further research is required to determine the optimal number and interval of b-values for accurate assessment, considering the trade-off between scan time and accuracy. Lastly, the study did not analyze the relationship between the models and the survival outcome of the patients.
In conclusion, our results suggest that a nomogram incorporating the pre-DDC value calculated from SEM-DWI along with the short diameter of the largest lymph node after treatment may have the potential to predict lymph node metastasis noninvasively in CRLM patients after chemotherapy. This nomogram can be used for individualized, noninvasive high-risk assessment and surgical planning for CRLM patients with suspected metastatic hepatic lymph nodes, thereby reducing unnecessary surgical procedures and the occurrence of complications.
More than 50% of patients with colorectal cancer develop colorectal liver metastases (CRLM), and the presence of metastatic hepatic lymph nodes can greatly influence treatment decisions and patient outcomes. Precise preoperative prediction of hepatic lymph node status is beneficial for individualized treatment and reducing complications.
However, there is currently a lack of reliable radiological tools for predicting the presence of metastatic hepatic lymph nodes in CRLM prior to surgery.
The study aimed to assess the predictive ability of different diffusion-weighted imaging (DWI) models (mono-exponential, bi-exponential, and stretched-exponential) in distinguishing between benign and malignant hepatic lymph nodes in CRLM patients who underwent neoadjuvant chemotherapy.
A retrospective study was conducted involving 97 CRLM patients with pathologically confirmed hepatic lymph node status who underwent magnetic resonance imaging, including DWI with ten b values before and after chemotherapy. Various parameters, including apparent diffusion coefficient, the true diffusion coefficient, the pseudo-diffusion coefficient, the perfusion fraction, distributed diffusion coefficient (DDC), and α, derived from different DWI models, were measured and compared between positive and negative hepatic lymph node groups. A nomogram was constructed, and the reliability and agreement of the measurements were assessed using appropriate statistical analyses.
Multivariate analysis revealed that the pre-treatment DDC value and the short diameter of the largest lymph node after treatment were independent predictors of metastatic hepatic lymph nodes. A nomogram combining these factors demonstrated excellent performance in distinguishing between benign and malignant lymph nodes in CRLM patients, with area under the receiver operating characteristic curve of 0.873. Furthermore, parameters from the stretched-exponential model showed substantial repeatability.
The developed nomogram, incorporating the pre-treatment DDC and the short axis of the largest lymph node, can be utilized to predict the presence of hepatic lymph node metastases in CRLM patients who undergo chemotherapy prior to surgery. This nomogram was found to be more valuable than quantitative parameters derived from multiple b values of DWI, exhibiting superior diagnostic performance.
In the future, the nomogram can serve as a preoperative assessment tool for determining the status of hepatic lymph nodes and aiding in the decision-making process for surgical treatment in CRLM patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Koehler H, Germany; Padilla M, Latvia S-Editor: Fan JR L-Editor: A P-Editor: Chen YX
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20512] [Article Influence: 2051.2] [Reference Citation Analysis (20)] |
| 2. | Nieuwenhuizen S, Puijk RS, van den Bemd B, Aldrighetti L, Arntz M, van den Boezem PB, Bruynzeel AME, Burgmans MC, de Cobelli F, Coolsen MME, Dejong CHC, Derks S, Diederik A, van Duijvendijk P, Eker HH, Engelsman AF, Erdmann JI, Fütterer JJ, Geboers B, Groot G, Haasbeek CJA, Janssen JJ, de Jong KP, Kater GM, Kazemier G, Kruimer JWH, Leclercq WKG, van der Leij C, Manusama ER, Meier MAJ, van der Meijs BB, Melenhorst MCAM, Nielsen K, Nijkamp MW, Potters FH, Prevoo W, Rietema FJ, Ruarus AH, Ruiter SJS, Schouten EAC, Serafino GP, Sietses C, Swijnenburg RJ, Timmer FEF, Versteeg KS, Vink T, de Vries JJJ, de Wilt JHW, Zonderhuis BM, Scheffer HJ, van den Tol PMP, Meijerink MR. Resectability and Ablatability Criteria for the Treatment of Liver Only Colorectal Metastases: Multidisciplinary Consensus Document from the COLLISION Trial Group. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 3. | Morris VK, Kennedy EB, Baxter NN, Benson AB 3rd, Cercek A, Cho M, Ciombor KK, Cremolini C, Davis A, Deming DA, Fakih MG, Gholami S, Hong TS, Jaiyesimi I, Klute K, Lieu C, Sanoff H, Strickler JH, White S, Willis JA, Eng C. Treatment of Metastatic Colorectal Cancer: ASCO Guideline. J Clin Oncol. 2023;41:678-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 311] [Article Influence: 155.5] [Reference Citation Analysis (0)] |
| 4. | Liu W, Yan XL, Wang K, Bao Q, Sun Y, Xing BC. The outcome of liver resection and lymphadenectomy for hilar lymph node involvement in colorectal cancer liver metastases. Int J Colorectal Dis. 2014;29:737-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Elias D, Saric J, Jaeck D, Arnaud JP, Gayet B, Rivoire M, Lorimier G, Carles J, Lasser P. Prospective study of microscopic lymph node involvement of the hepatic pedicle during curative hepatectomy for colorectal metastases. Br J Surg. 1996;83:942-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Ercolani G, Grazi GL, Ravaioli M, Grigioni WF, Cescon M, Gardini A, Del Gaudio M, Cavallari A. The role of lymphadenectomy for liver tumors: further considerations on the appropriateness of treatment strategy. Ann Surg. 2004;239:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 119] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Ishizuka D, Shirai Y, Hatakeyama K. Ischemic biliary stricture due to lymph node dissection in the hepatoduodenal ligament. Hepatogastroenterology. 1998;45:2048-2050. [PubMed] |
| 8. | Grobmyer SR, Wang L, Gonen M, Fong Y, Klimstra D, D'Angelica M, DeMatteo RP, Schwartz L, Blumgart LH, Jarnagin WR. Perihepatic lymph node assessment in patients undergoing partial hepatectomy for malignancy. Ann Surg. 2006;244:260-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Rau C, Blanc B, Ronot M, Dokmak S, Aussilhou B, Faivre S, Vilgrain V, Paradis V, Belghiti J. Neither preoperative computed tomography nor intra-operative examination can predict metastatic lymph node in the hepatic pedicle in patients with colorectal liver metastasis. Ann Surg Oncol. 2012;19:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Ko CC, Yeh LR, Kuo YT, Chen JH. Imaging biomarkers for evaluating tumor response: RECIST and beyond. Biomark Res. 2021;9:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 11. | Messina C, Bignone R, Bruno A, Bruno F, Calandri M, Caruso D, Coppolino P, Robertis R, Gentili F, Grazzini I, Natella R, Scalise P, Barile A, Grassi R, Albano D. Diffusion-Weighted Imaging in Oncology: An Update. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 12. | Bonekamp S, Corona-Villalobos CP, Kamel IR. Oncologic applications of diffusion-weighted MRI in the body. J Magn Reson Imaging. 2012;35:257-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Sumi M, Sakihama N, Sumi T, Morikawa M, Uetani M, Kabasawa H, Shigeno K, Hayashi K, Takahashi H, Nakamura T. Discrimination of metastatic cervical lymph nodes with diffusion-weighted MR imaging in patients with head and neck cancer. AJNR Am J Neuroradiol. 2003;24:1627-1634. [PubMed] |
| 14. | Abdel Razek AA, Soliman NY, Elkhamary S, Alsharaway MK, Tawfik A. Role of diffusion-weighted MR imaging in cervical lymphadenopathy. Eur Radiol. 2006;16:1468-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 252] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 15. | Eiber M, Beer AJ, Holzapfel K, Tauber R, Ganter C, Weirich G, Krause BJ, Rummeny EJ, Gaa J. Preliminary results for characterization of pelvic lymph nodes in patients with prostate cancer by diffusion-weighted MR-imaging. Invest Radiol. 2010;45:15-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 16. | Zhou XJ, Gao Q, Abdullah O, Magin RL. Studies of anomalous diffusion in the human brain using fractional order calculus. Magn Reson Med. 2010;63:562-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | Ai Z, Han Q, Huang Z, Wu J, Xiang Z. The value of multiparametric histogram features based on intravoxel incoherent motion diffusion-weighted imaging (IVIM-DWI) for the differential diagnosis of liver lesions. Ann Transl Med. 2020;8:1128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Bennett KM, Schmainda KM, Bennett RT, Rowe DB, Lu H, Hyde JS. Characterization of continuously distributed cortical water diffusion rates with a stretched-exponential model. Magn Reson Med. 2003;50:727-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 340] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 19. | Liu C, Wang K, Li X, Zhang J, Ding J, Spuhler K, Duong T, Liang C, Huang C. Breast lesion characterization using whole-lesion histogram analysis with stretched-exponential diffusion model. J Magn Reson Imaging. 2018;47:1701-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Seo N, Chung YE, Park YN, Kim E, Hwang J, Kim MJ. Liver fibrosis: stretched exponential model outperforms mono-exponential and bi-exponential models of diffusion-weighted MRI. Eur Radiol. 2018;28:2812-2822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Bai Y, Lin Y, Tian J, Shi D, Cheng J, Haacke EM, Hong X, Ma B, Zhou J, Wang M. Grading of Gliomas by Using Monoexponential, Biexponential, and Stretched Exponential Diffusion-weighted MR Imaging and Diffusion Kurtosis MR Imaging. Radiology. 2016;278:496-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 172] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 22. | Wang F, Wang Y, Zhou Y, Liu C, Xie L, Zhou Z, Liang D, Shen Y, Yao Z, Liu J. Comparison between types I and II epithelial ovarian cancer using histogram analysis of monoexponential, biexponential, and stretched-exponential diffusion models. J Magn Reson Imaging. 2017;46:1797-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Wang Y, Hu D, Yu H, Shen Y, Tang H, Kamel IR, Li Z. Comparison of the Diagnostic Value of Monoexponential, Biexponential, and Stretched Exponential Diffusion-weighted MRI in Differentiating Tumor Stage and Histological Grade of Bladder Cancer. Acad Radiol. 2019;26:239-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Hu Y, Tang H, Li H, Li A, Li J, Hu D, Li Z, Kamel IR. Assessment of different mathematical models for diffusion-weighted imaging as quantitative biomarkers for differentiating benign from malignant solid hepatic lesions. Cancer Med. 2018;7:3501-3509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Li H, Liang L, Li A, Hu Y, Hu D, Li Z, Kamel IR. Monoexponential, biexponential, and stretched exponential diffusion-weighted imaging models: Quantitative biomarkers for differentiating renal clear cell carcinoma and minimal fat angiomyolipoma. J Magn Reson Imaging. 2017;46:240-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Kim HC, Seo N, Chung YE, Park MS, Choi JY, Kim MJ. Characterization of focal liver lesions using the stretched exponential model: comparison with monoexponential and biexponential diffusion-weighted magnetic resonance imaging. Eur Radiol. 2019;29:5111-5120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Jerome NP, Miyazaki K, Collins DJ, Orton MR, d'Arcy JA, Wallace T, Moreno L, Pearson AD, Marshall LV, Carceller F, Leach MO, Zacharoulis S, Koh DM. Repeatability of derived parameters from histograms following non-Gaussian diffusion modelling of diffusion-weighted imaging in a paediatric oncological cohort. Eur Radiol. 2017;27:345-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Jia H, Jiang X, Zhang K, Shang J, Zhang Y, Fang X, Gao F, Li N, Dong J. A Nomogram of Combining IVIM-DWI and MRI Radiomics From the Primary Lesion of Rectal Adenocarcinoma to Assess Nonenlarged Lymph Node Metastasis Preoperatively. J Magn Reson Imaging. 2022;56:658-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 29. | Wang C, Yu J, Lu M, Li Y, Shi H, Xu Q. Diagnostic Efficiency of Diffusion Sequences and a Clinical Nomogram for Detecting Lymph Node Metastases from Rectal Cancer. Acad Radiol. 2022;29:1287-1295. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 30. | Zhu HB, Xu D, Sun XF, Li XT, Zhang XY, Wang K, Xing BC, Sun YS. Prediction of hepatic lymph node metastases based on magnetic resonance imaging before and after preoperative chemotherapy in patients with colorectal liver metastases underwent surgical resection. Cancer Imaging. 2023;23:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |