Published online Jan 28, 2024. doi: 10.3748/wjg.v30.i4.290
Peer-review started: October 5, 2023
First decision: December 6, 2023
Revised: December 19, 2023
Accepted: January 8, 2024
Article in press: January 8, 2024
Published online: January 28, 2024
Processing time: 112 Days and 13.1 Hours
Portal hypertension (PH) has traditionally been observed as a consequence of significant fibrosis and cirrhosis in advanced non-alcoholic fatty liver disease (NAFLD). However, recent studies have provided evidence that PH may develop in earlier stages of NAFLD, suggesting that there are additional pathogenetic mechanisms at work in addition to liver fibrosis. The early development of PH in NAFLD is associated with hepatocellular lipid accumulation and ballooning, leading to the compression of liver sinusoids. External compression and intra-luminal obstacles cause mechanical forces such as strain, shear stress and elevated hydrostatic pressure that in turn activate mechanotransduction pathways, resulting in endothelial dysfunction and the development of fibrosis. The spatial distribution of histological and functional changes in the periportal and perisinusoidal areas of the liver lobule are considered responsible for the pre-sinusoidal component of PH in patients with NAFLD. Thus, current diagnostic methods such as hepatic venous pressure gradient (HVPG) measurement tend to underestimate portal pressure (PP) in NAFLD patients, who might decompensate below the HVPG threshold of 10 mmHg, which is traditionally considered the most relevant indicator of clinically significant portal hypertension (CSPH). This creates further challenges in finding a reliable diagnostic method to stratify the prognostic risk in this population of patients. In theory, the measurement of the portal pressure gradient guided by endoscopic ultrasound might overcome the limitations of HVPG measurement by avoiding the influence of the pre-sinusoidal component, but more investigations are needed to test its clinical utility for this indication. Liver and spleen stiffness measurement in combination with platelet count is currently the best-validated non-invasive approach for diagnosing CSPH and varices needing treatment. Lifestyle change remains the cornerstone of the treatment of PH in NAFLD, together with correcting the components of metabolic syndrome, using nonselective beta blockers, whereas emerging candidate drugs require more robust confirmation from clinical trials.
Core Tip: Portal hypertension (PH) occurs in patients with cirrhosis, but in non-alcoholic fatty liver disease (NAFLD) it is sometimes observed in non-cirrhotic stages due to perisinusoidal fibrosis and damage to liver microcirculation. The severity of PH tends to be underestimated by hepatic venous pressure gradient (HVPG) measurement in NAFLD, potentially due to the presence of pre-sinusoidal component, and some patients decompensate at HVPG < 10 mmHg. Liver elastography needs further validation in obese patients as it might overestimate the severity of PH. While candidate drugs for PH are currently in development, lifestyle changes and modulation of metabolic derangements remain the mainstay of treatment.
- Citation: Madir A, Grgurevic I, Tsochatzis EA, Pinzani M. Portal hypertension in patients with nonalcoholic fatty liver disease: Current knowledge and challenges. World J Gastroenterol 2024; 30(4): 290-307
- URL: https://www.wjgnet.com/1007-9327/full/v30/i4/290.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i4.290
Portal hypertension (PH) plays a crucial prognostic role in chronic liver disease (CLD), including non-alcoholic fatty liver disease (NAFLD). PH develops during the evolution of CLD as a result of the increased accumulation of extracellular matrix in the liver, leading to elevated resistance to the portal blood flow, further aggravated by the distortion of the liver architecture and vascular network, which is caused by the formation of regenerative nodules. In addition to this static component, a reversible element of the heightened resistance derives from the contraction of hepatic stellate cells (HSCs) around the liver sinusoids, which is activated by the underlying pathogenetic process. This results in the development of PH at the sinusoidal level, which is typical for viral hepatitis and alcohol-related liver disease (ALD).
Current knowledge of the pathophysiology, diagnosis and treatment of PH relies predominantly on the data accumulated from the studies conducted regarding these two aetiologies of CLD. Considering the changing aetiological landscape of CLD, with NAFLD becoming the leading cause of liver-related morbidity, it is important to understand all aspects pertaining to the PH arising in the context of NAFLD. Based on recent reports, the development, diagnosis and prognosis of PH in NAFLD might not completely fit into the existing paradigms and rules established with chronic viral hepatitis and ALD. This article aims to describe the present understanding of the topic, as well as to highlight the unmet needs and controversial issues in the diagnosis and management of PH in patients with NAFLD.
Portal hypertension is defined as a clinical syndrome caused by elevated blood pressure in the portal venous system. Patients who suffer from advanced chronic liver disease (ACLD), especially cirrhosis, have an increased risk of developing PH[1,2]. Liver cirrhosis arises as the result of prolonged liver damage caused by various aetiological agents that finally lead to the replacement of the healthy parenchyma with fibrotic tissue, the formation of regenerative nodules and the distortion of the microarchitecture, including the liver vascular network[3,4]. In the portal tracts located at the periphery of the hepatic lobule (zone 1), terminal branches of both the hepatic artery and portal vein join into liver sinusoids and form a complex capillary network that drains into the centrilobular area of the central vein outflow (zone 3)[5,6]. Arteriolar inflow needs to be efficiently controlled to prevent damage and shear stress to liver sinusoids because of the very high arterial hydrostatic pressure, which is up to 40 times higher relative to that present in terminal branches of the portal vein[6-8]. Vasoregulatory changes in both intrahepatic and systemic circulation have an important role in the development and further aggravation of PH in individuals with cirrhosis. Hepatic causes of PH are essentially classified into three types according to the main location of the blood flow disturbance in the hepatic circulation: pre-sinusoidal, sinusoidal and post-sinusoidal[9,10]. Sinusoidal PH is the most common type, and it typically occurs in cirrhosis patients[11]. The principal causes of intrahepatic PH are depicted in Table 1.
| Pre-sinusoidal | Sinusoidal | Post-sinusoidal |
| Developmental abnormalities: | Fibrosis in the space of Disse: | Granulomatous phlebitis: |
| Adult polycystic liver disease | Metabolic cause: non-alcohol-associated fatty liver disease, Zellweger syndrome | Mycobacterium avium infection |
| Congenital hepatic fibrosis | Inflammatory cause: schistosomiasis, viral hepatitis B and C, chronic Q fever, cytomegalovirus | Mycobacterium intracellular infection |
| Arteriovenous fistulas | Induced by drugs or toxins: amiodarone, methotrexate, alcohol, vinyl chloride, copper | Sarcoidosis |
| Porto-sinusoidal vascular disease: | Early alcohol-associated liver disease (defenestration) | Primary vascular malignancies: |
| Idiopathic non-cirrhotic portal hypertension | Epithelioid haemangioendothelioma | |
| Angiosarcoma | ||
| Granulomatous liver disease: | Microvesicular steatosis hypertrophied hepatocytes | Phlebosclerosis of hepatic veins: |
| Schistosomiasis (bilharzia) | Alcohol-associated liver disease | |
| Mineral oil granuloma | Chronic radiation injury | |
| Sarcoidosis | Hypervitaminosis A | |
| Biliary diseases: | Infiltrative diseases: | Lipogranulomas: |
| Autoimmune cholangiopathy | Idiopathic myeloid metaplasia | Mineral oil granuloma |
| Primary sclerosing cholangitis | Gaucher disease | |
| Toxic biliary injury | Mastocytosis | |
| Biliary cholangitis | ||
| Neoplastic occlusion of the intrahepatic portal vein | Amyloid or light-chain deposition in the space of Disse | Sinusoidal obstruction syndrome |
| Acute hepatic injury | Budd-Chiari syndrome |
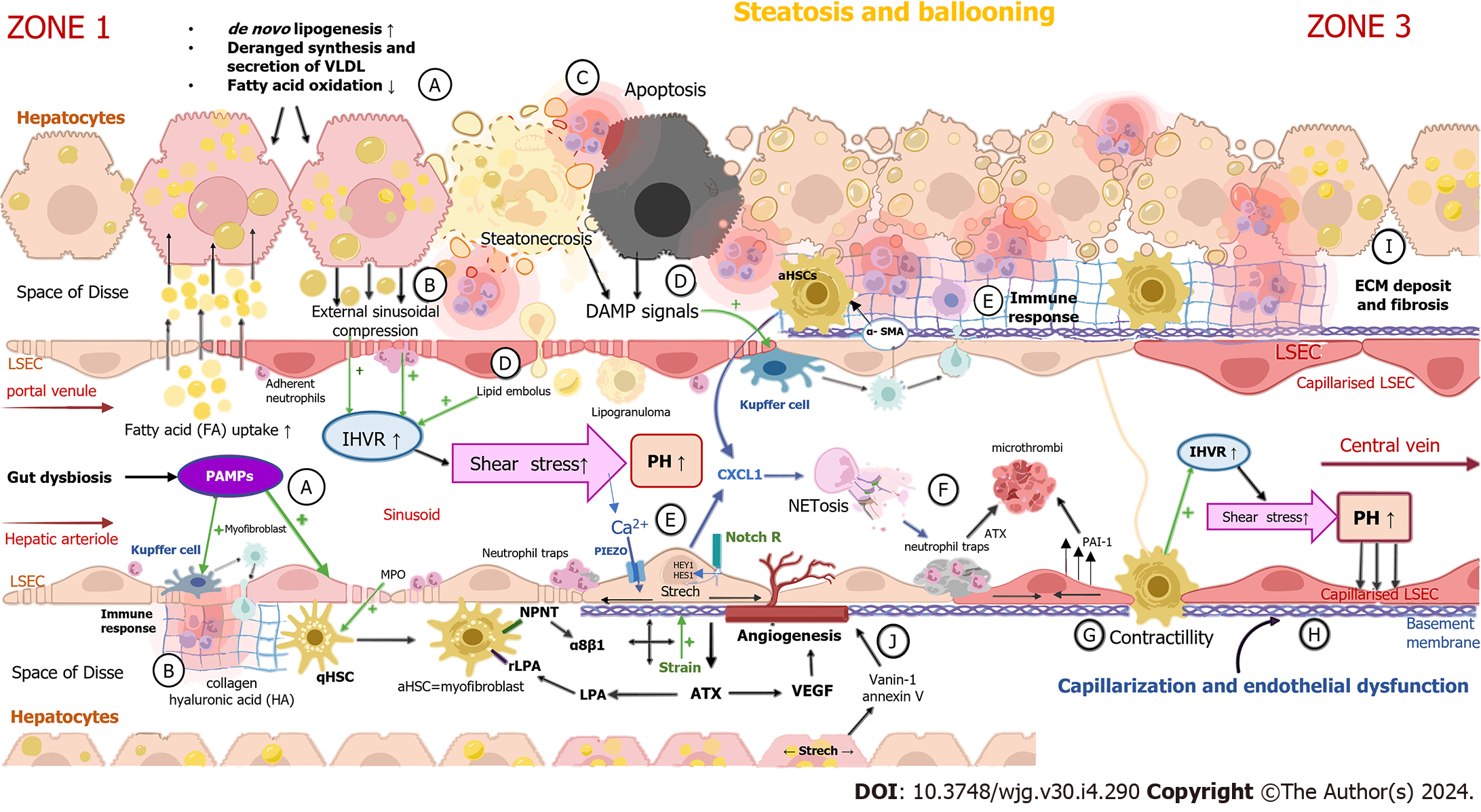
Hepatocyte ballooning occurs early in NAFLD pathogenesis because of the accumulation of cholesterol and fatty acids within the cytoplasm of hepatocytes[5,12]. Lipid-laden hepatocytes cause external sinusoidal compression, leading to increased intrahepatic vascular resistance (IHVR) and shear stress[5,13]. These sinusoids, which are deformed, tortuous and up to 50% narrower, are mostly located in the periportal region of hepatic lobules and impose a heightened resistance to portal blood flow before it enters the sinusoids[14,15]. Another structural change in NAFLD contributing to IHVR development is the formation of lipogranulomas commonly located near terminal hepatic venules, which are dispersed in portal tracts and the hepatic acinus[16,17]. Steatonecrosis, an event caused by the disintegration of hepatocytes due to excessive lipid accumulation[14,18], results in the liberation of lipid droplets which travel through the Disse space and the endothelium and fill the sinusoid as a sinusoidal lipid embolus[14].
The stretching of liver sinusoidal endothelial cells (LSECs) caused by the enlargement of hepatocytes activates Notch-dependent neutrophil chemotaxis[19]. Together with neutrophil chemotactic chemokines, which are produced by hepatocytes and HSCs, these signals have a crucial role in the recruitment of leukocytes and formation of neutrophil extracellular traps (NETs)[19], intraluminal web-like structures composed primarily of deoxyribonucleic acid (DNA)-histone complexes originating from neutrophils, which bind pathogens[20] and impose a barrier that leads to increased fluid shear stress at the level of sinusoids[21]. Thus, lipid accumulation in hepatocytes, with the consequent deformation of sinusoids, combined with the formation of lipogranulomas and NETs, as well as lipid emboli, contributes to sinusoid hypoperfusion[13,22,23], microvascular thrombosis[24] and the development of PH, with heightened presinusoidal resistance in NAFLD[13,25].
The principal mechanisms of portal hypertension development in NAFLD are illustrated in Figure 1.
The association between increased portal vein pressure (PVP) and steatosis has been observed in numerous animal experimental models. One of the oldest experiments confirming this connection was carried out almost 50 years ago. Donryu rats were fed a choline-deficient diet for eight to 38 weeks. Two thirds of the rats died during the feeding period and 27 developed a fatty liver (n = 7), some with fibrosis (n = 8) and others with cirrhosis (n = 12)[5,15]. The results showed a decrease in portal blood flow, an increase in PVP and a narrowing of sinusoids without visible abnormalities in the pre- and post-sinusoidal vessels. All these findings were detected in rats with steatosis without fibrosis, suggesting that steatosis alone is sufficient for the formation of PH[5,10]. In another experimental NAFLD model, obese male Zucker rats with high-grade hepatic steatosis without cirrhosis were studied in comparison with lean rats aged 25 to 30 weeks (n = 7 vs 7). Compared to the control animals, an increment in IHVR and reductions of 35% to 38% in the total hepatic blood flow and portal venous flow were observed[5,26]. Francque et al[27] conducted a similar study on male Wistar rats given a methionine-choline-deficient (MCD) diet (n = 30) while another group was fed a control diet (n = 30) for four weeks. The two groups were compared through in vivo haemodynamic measurements and in situ perfusion experiments, as well as vascular corrosion and liver tissue and serum analysis. In the MCD diet group, the histopathology showed severe steatosis without evidence of inflammation or fibrosis, and the portal pressure gradient was significantly elevated, indicating an increased intrahepatic resistance, while vascular corrosion casts demonstrated a replacement of the regular sinusoidal anatomy by a sinusoidal wall with a disorganized pattern, in addition to vascular extensions and multiple interconnections. An increase in the expression of vasoconstrictor molecules and enzymes [thromboxane synthase and endothelin-1 (ET-1)] was also registered[27].
Endothelial dysfunction is defined as the loss of various key functions of the endothelium[28,29], chiefly characterized by a lower response of LSECs to the endothelium-dependent vasodilator acetylcholine[30] and a decrease in the production and release of endothelium-driven vasodilatory factors such as nitric oxide (NO)[31,32]. In a normal liver, hepatocytes release low levels of vascular endothelial growth factor (VEGF), which helps LSECs to generate NO through a cytosolic calcium increase, leading to calmodulin binding and the activation of endothelial nitric oxide synthase (eNOS)[33,34]. To maintain physiological pressure in the sinusoids, shear stress induced by blood flow represents a constant stimulus of NO production in LSECs[35]. The first step in the development of endothelial dysfunction is the reduced production of NO[36] supplied by lessened protein kinase B (Akt)-dependent eNOS phosphorylation, causing diminished eNOS activity[30]. A very important molecule in the endothelial production of NO is insulin[37]. Insulin activates NO release through Akt via the Ca2+-independent pathway[37,38]. The disruption of insulin signaling observed in insulin resistance impairs the endothelial production of NO[38,39]. Decreased NO bioavailability[40] can also be generated by increased intra-cellular levels of reactive oxygen species[41] because of excessive lipid accumulation in the liver, endoplasmic reticular stress and mitochondrial dysfunction[10,42,43]. Elevated ROS concentrations reduce the amount of bioactive NO through direct chemical interactions, inducing the formation of toxic peroxynitrite[44]. The latter uncouples eNOS to become a dysfunctional superoxide-generating enzyme, which additionally contributes to vascular oxidative stress[44]. eNOS dysfunction is also caused by the formation of eNOS inhibitors[45] such as asymmetric dimethylarginine[46], a paracrine and a competitive inhibitor of eNOS. The reduced bioavailability of NO can lead to sinusoidal contraction through the activation of perisinusoidal HSCs, resulting in increased IHVR and the elevation of portal pressure[47]. Sinusoidal dysfunction and IHVR in NAFLD pathogenesis are represented in feedback loops and interactions between LSECs, hepatocytes, Kupffer cells, hepatic stellate cells and other immune system cells[10]. The chronology of changes in the structural and functional causes of IHVR is difficult to establish due to the complexity of cell-cell interactions[48].
In NAFLD-ballooned hepatocytes, activated HSCs and macrophages stimulate angiogenesis by producing a greater amount of VEGF as a response to shear stress, hypoxia and inflammation[5,49]. Liver steatosis induces hypoxia by both a mechanical pressure on sinusoids and an excessive lipid metabolism which increases oxygen consumption[49]. Increased VEGF levels in NAFLD promote angiogenesis (the formation of new blood vessels)[50] and qualitative changes in liver vessels called vascular remodeling[49,51]. Sinusoidal capillarization, an early morphological feature of endothelial dysfunction, is marked by a dedifferentiation of LSECs, as well as the formation of the basal membrane and loss of fenestration, and represents an example of qualitative vascular remodeling[49,52]. Both angiogenesis and sinusoidal capillarization contribute to the distortion of the normal liver vascular network, blood shunting with consequent tissue hypoxia and deranged metabolic exchange across the endothelial interface[53]. The triggers of sinusoidal capillarization have not been fully elucidated[54], but it is believed that capillarization occurs as a result of the exposure of LSECs to extreme lipid accumulation in parenchymal cells and a surplus amount of circulating lipids in the sinusoidal blood flow[30]. As a response to disproportionate lipid exposure, LSECs express lipid-induced adhesion molecules, integrins (vascular cell adhesion molecule 1, intercellular adhesion molecule, E-selectin and vascular adhesion protein 1), leading to the induction of the recruitment of leukocytes and their translocation into the liver parenchyma[55]. The excessive exposure of LSECs to lipids may cause mitochondrial dysfunction, DNA damage in hepatocytes, endoplasmic reticulum stress and cytoskeleton alterations[56]. A perfusion of hepatic sinusoids can also be aggravated by functional impairments, such as the contracting and swelling of LSECs in response to vasoactive mediators produced by ballooned hepatocytes, e.g., ET-1[23,57]. However, it is important to note that the main liver cells involved in controlling the sinusoidal diameter are perisinusoidal HSCs, also known as liver-specific pericytes[58,59]. Lipid-laden hepatocytes secrete microparticles that promote angiogenesis[60]. Examples of such molecules are vanin-1 and annexin V, which are isolated in the blood of perisinusoidal spaces and produced by stretched and/or compressed centrilobular hepatocytes[5,60]. Damage in the periportal vascular area may also play an important pathogenic role in NAFLD-dependent PH[5,10]. A high degree of steatosis or periportal fibrosis leads to a poor regulation of arteriolar inflow and creates shear stress in liver sinusoids, which are low-pressure, low-flow vascular channels linking the periportal area of portal inflow (zone 1) to the centrilobular area of central vein outflow (zone 3)[5]. Between zone 1 and zone 3, intralobular arterioles occasionally drain to sinusoids[61]. The influence of “arterial twigs” on sinusoidal flow has not been fully clarified, but they may represent zones of higher pressure[61]. A recently conducted study showed that splanchnic vasodilatation in NAFLD also contributes to the rise in portal pressure long before the development of cirrhosis[62]. Splanchnic vasodilatation and hyperdynamic circulation in NAFLD-dependent PH are characterized by low arterial responsiveness to a vasoconstrictor mediator, a rise in portal venous and mesenteric arterial blood flow and a decrease in main arterial blood pressure[62,63]. Numerous vasoactive mediators (calcitonin gen-related peptide, glucagon, NO, platelet-activating factor, atrial natriuretic peptide and adrenomedullin, as well as bile salts and endocannabinoids) are involved in the arteriolar vasodilatation in the visceral vascular bed that drains into the portal circulation[11,64] and results in an increase in portal inflow and pressure[11,64].
The mechanisms that are important in the development of endothelial dysfunction and capillarization are shown in Figure 2. To summarize the information about early pathophysiological changes in the development of PH in NAFLD, external compression and intraluminal obstacles (e.g., microthrombi, lipid emboli and neutrophil traps) caused by structural changes in NAFLD result in impaired sinusoidal blood flow and may contribute to the development of PH in early NAFLD. Mechanotransduction pathways activated by multiple mechanical forces such as strain, shear stress and hydrostatic pressure result in endothelial dysfunction and fibrosis development, contributing to the maintenance and progression of PH.
PH in NAFLD begins to develop as a result of IHVR and the de-differentiation of liver cells[35]. The initial site of IHVR formation is the hepatic sinusoid[65], while the distal segment of the preterminal portal venule serves as a sphincter for blood redistribution[66]. IHVR has two components, which are structural[67] and functional[68], characterized by extrasinusoidal and intrasinusoidal disturbances[35]. Total available space within the liver capsule in NAFLD becomes restricted as a result of lipid accumulation and hepatocellular swelling, leading to a volumetric squeeze and consequently to a reduction in the sinusoidal spaces and a drop in blood flow[15,69,70] Mechanical forces taking place in the sinusoidal microenvironment of NAFLD (such as increased hydrostatic pressure, strain and shear stress) cause the deformation of cellular structures such as caveolae and plasma membrane lipid rafts, and result in an excessive extracellular matrix (ECM) deposition in the perisinusoidal space of Disse as well as sinusoidal hypercontractility. They also modify the conductivity of ion channels, expose new protein-binding sites and change the activity of transmembrane receptors[35,71,72]. The increase in ECM stiffness that results from the cross-linking of ECM proteins and collagen[73,74] is detected by integrins, mechanosensitive transmembrane proteins that initiate key biological processes upon stretch-induced conformational changes[74,75]. These are also involved in the binding and recruitment of cytoskeleton linker proteins[76], the activation of the transforming growth factor-β (TGF-β) signaling pathway[77] and the conformational alteration of ion channels[78]. Structural changes caused by steatosis in connection with mechanical forces induced by ECM accumulation and haemodynamic changes, as described previously, lead to the compression and/or stretching of liver cells and stimulation of signaling pathways[35], including the contraction and relaxation of the actin filament of the hepatocyte cytoskeleton that result in increased intracellular tension[35,79,80]. Intracellular tension pulls ECM-bound integrins, which then organize into focal adhesions and, together with adaptor proteins, strengthen the ECM-cytoskeleton connection[35,79,80]. The tension generated in the cytoskeleton is transmitted through the linker of the nucleoskeleton and cytoskeleton complex[35,79,80]. The deformation of the nucleus, which is proportional to the stiffness of the ECM[81], affects the change in the gene expression by changing the permeability of the nuclear membrane and altering the rheology of chromatin[35]. This causes the translocation of transcription factors and co-factors[79] such as the yes-associated protein (YAP)/transcriptional coactivator with PDZ-binding motif (TAZ)[82]. The co-factors YAP/TAZ are mechanosensitive and can detect fluid shear stress, as well as increases in liver cell density and changes in ECM stiffness[83]. YAP/TAZ regulate the biological behavior of liver cells and the profibrotic response through an insufficiently elucidated mechanism[84,85], resulting in the further accumulation of fibrosis[86,87]. Other important transcription factors are myocardin-related transcription factor-A[88] and zyxin, part of the mechanosensing FA complex[89]. These factors translocate to the nucleus as a result of cell stretching and regulate the expression of genes related to inflammation, proliferation and cell apoptosis[35,76,89-91].
In conclusion, disrupted mechanical homeostasis in liver sinusoids is the key contributor to the pathogenesis of NAFLD, caused by intracellular lipid accumulation, enhanced ECM stiffness and altered functions in the contractile cytoskeleton that finally lead to further fibrosis accumulation and cellular contractility, representing the positive feedback loop mediated through the mechanotransduction pathways.
In NAFLD, excessive lipid accumulation triggers inflammation through cytokine secretion and immune cell infiltration[92]. The initiation of the inflammatory response leads to hepatocyte necrosis, apoptosis[93] and HSC activation[94,95] as the characteristic features of non-alcoholic steatohepatitis (NASH), in which a large amount of free fatty acids released from the injured hepatocytes, as well as damage-associated molecular patterns (DAMPs), are removed by Kupffer cells[96-98]. The latter release profibrogenic growth factors (TGF-β and platelet-derived growth factor)[99] that, in conjunction with ROS, pro-inflammatory cytokines (interleukin-6, interleukin-10 and tumor necrosis factor α)[100,101] and products of lipid peroxidation[102], along with endothelin and fibronectin produced by capillarized LSECs, result in HSC activation[5]. Activated HSCs transdifferentiate from the quiescent phenotype to proliferative, contractile and collagen-producing myofibroblasts[103]. These cause the synthesis of ECM through the production of collagen (types I, III and V) and hyaluronic acid[103]. In addition to the ECM products, myofibroblasts also synthesize α-smooth muscle actin[104], a hallmark of HSC activation[105], and release VEGF and chemokines such as macrophage colony-stimulating factor and monocyte chemoattractant protein-1 . Collagen is deposited in the space of Disse as an early phenomenon in NAFLD that generates the formation of perisinusoidal fibrosis and narrowing of the sinusoidal lumen[5]. Fibrosis in NAFLD develops in the pericellular space around the central veins and in the perisinusoidal space of zone 3[106], whereas the fibrosis pattern in other chronic liver diseases initially shows a portal instead of a pericentral distribution[107]. Due to the specific distribution of fibrosis in patients with NAFLD, PH may occur before the development of cirrhosis[107]. Increased ECM stiffness sends a positive feedback signal to HSCs, contributing to the further progression of liver fibrosis[35]. This results in the remodeling of the liver architecture and the formation of cirrhotic nodules with the additional distortion of hepatic microcirculation[108,109]. Both the structural component, represented by accumulated fibrosis with narrowed sinusoids and a distorted microvascular network, and the dynamic one resulting from endothelial dysfunction and myofibroblast contraction (with the latter considered responsible for 20%-30% of the IHVR) contribute to the rise in portal pressure[110,111].
In conclusion, the development of liver fibrosis has a fundamental influence on the advancement and further aggravation of PH, not only as the structural barrier to the intrahepatic blood flow, but also by inducing the secretion of local vasoactive mediators. This leads to vascular dysregulation and the functional deterioration of endothelial dysfunction that additionally aggravates IHVR[5,35].
Large-scale epidemiological investigations focused on the prevalence of PH among the NAFLD patients are lacking. However, several clinical studies have been conducted to investigate the relationship between the development and severity of PH and the histological and clinical features of NAFLD. In a cohort of 50 overweight patients who underwent transjugular liver biopsy (TJLB) coupled with HVPG measurements, PH (HVPG > 5 mmHg) was diagnosed in 14 (28%) of subjects and the only histological parameter that differed between them and those without PH was a higher grade of steatosis (P = 0.016). In the group with PH, only 21% of patients had advanced fibrosis/cirrhosis. The independent clinical predictors of PH were waist circumference (P = 0.008) and the homeostatic model assessment for insulin resistance (HOMA IR; P = 0.043)[112]. In a prospective cohort study that included 40 obese patients who underwent TJLB (30% with diabetes, 70% with NASH) and HVPG measurements, PH was found in eight (20%) patients, and none had cirrhosis. The presence of PH positively correlated with the proinflammatory blood cytokine profile as well as with microvascular changes in the form of sinusoidal dilatation, previously reported as an early histological change in severe steatosis even in the absence of advanced fibrosis[27,113]. In an observational investigation, in a cohort of 354 subjects with biopsy-confirmed NAFLD, 100 patients exhibited clinical signs of PH (the presence of at least one of esophageal varices (EV), portosystemic encephalopathy, splenomegaly or ascites). Among them, 77 had liver cirrhosis and 11 had bridging fibrosis (stage F3). However, signs of PH were also present even in 12 (12%) patients who had no or only mild fibrosis (stages F0-F2)[107]. PH was increasingly detected in patients at a more advanced stage of fibrosis (r = 0.48, P = 0.006). In the F0-F2 subgroup (n = 204), a comparison between those with PH (n = 12) and those without PH (n = 192) was made, and the only histological feature that was significantly different between the groups was a higher grade of liver steatosis in patients with PH (mean grade 2.3 ± 0.5 vs 1.9 ± 0.7, P = 0.03). This work provides evidence that even clinically significant PH may exist before liver fibrosis enters an advanced stage, which is classically considered the threshold for PH development, and this might be caused by fat overload leading to the progressive enlargement of hepatocytes and reduction of the sinusoidal lumen[10,107].
In another investigation, 14/89 (16%) patients with clinically significant portal hypertension (CSPH) diagnosed by HVPG measurement were found to not have cirrhosis, and seven had stages F0-F2 (five were diagnosed with NASH). All these patients had perisinusoidal fibrosis and 8/14 had hepatocyte ballooning[114]. Based on these results, it becomes clear that patients with NAFLD may have PH and even CSPH without cirrhosis. Somewhat different results came from a study that investigated the prevalence of PH in a cohort of 292 NAFLD patients with metabolic syndrome associated with a liver stiffness measurement (LSM) > 8 kPa and/or liver blood test abnormalities (alanine aminotransferase > upper limit of normal), with no prior liver decompensation events. These patients were referred for TJLB and HVPG measurements, and 75/292 had liver cirrhosis. Among the 217 non-cirrhotic patients, 36 had PH (only one had CSPH), and there was no difference in steatosis or inflammatory grade between the patients with and without PH[115]. The only patient who presented with CSPH in the non-cirrhotic group was a young woman with Alström syndrome, severe type 2 diabetes, arterial hypertension and obesity. To compare, in the group of 75 patients with cirrhosis, PH was present in 53 (71%), CSPH in 38 (51%) and severe PH in 29 (39%). Accordingly, whereas PH might appear even in a non-cirrhotic liver, severe PH was not observed in NAFLD patients in the absence of cirrhosis[115].
Portal hypertension and advanced cirrhosis, regardless of aetiology, are traditionally associated with splenomegaly[116]. Interestingly, the results of a recent retrospective study in a large population of patients with biopsy-proven NAFLD revealed a strong correlation between splenomegaly and increased body weight, whereas none between splenomegaly and the histological degree of the underlying disease could be confirmed[117]. Thus, splenomegaly might be considered a consequence of visceral lipid deposition in the spleen and not necessarily a sign of PH. This view is further supported by the results from some other investigations demonstrating an enlarged spleen size in people with NAFLD with no other signs of PH, as well as in otherwise healthy individuals with a higher body height and weight[118,119].
In terms of stratifying the risk of hepatic decompensation, the prognostic properties of the HVPG have mostly been derived from investigations conducted in patients with viral and alcoholic aetiologies of chronic liver disease, where they have demonstrated robust predictive values. The normal HVPG value is 1-5 mmHg, and values of 6-9 mmHg are considered subclinical PH, while an HVPG ≥10 mmHg represents CSPH, as from this threshold all major complications related to PH start to develop, including the formation EV, ascites accumulation and portal encephalopathy[120-123]. Esophageal varices bleed at an HVPG ≥ 12 mmHg, and the risk of death increases significantly in patients with an HVPG ≥ 16 mmHg[120,121,123]. Given the complexity of the histological presentation and pathogenesis of PH in NAFLD, the HVPG cut-off values that are used in other aetiologies might not be appropriate for this purpose in NAFLD. To further elucidate this issue, a multicentric cross-sectional study was conducted with a cohort of 548 patients with advanced NAFLD and 444 with advanced hepatitis C (aHCV), who underwent detailed PH evaluation including HVPG measurement, TJLB, gastroscopy and abdominal imaging. Advanced chronic liver disease was defined either clinically by the presence of PH (HVPG > 5 mmHg) or histologically by the presence of stage 3 or 4 of liver fibrosis, and the majority of patients had compensated ACLD (cACLD; 71%). The median HVPG was lower in patients with advanced non-alcoholic fatty liver disease (aNAFLD; 13 mmHg vs 15 mmHg), although the indicators of liver function were similar between them and the aHCV group, whereas decompensation rates were higher among aNAFLD patients (32% vs 25%, P = 0.019), suggesting that NAFLD patients decompensated at lower HVPG levels[124]. According to the classic HVPG thresholds, clinical decompensation appeared in both groups at an HVPG > 10 mmHg, while no signs were detected in aHCV patients with an HVPG < 10 mmHg. Interestingly, some NAFLD patients experienced decompensation even when the HVPG was < 10 mmHg[124]. Further insights into this issue were provided from a study that investigated the agreement between wedge hepatic vein pressure (WHVP) and portal pressure (PP) in patients with decompensated NASH cirrhosis (n = 40), as well as those with alcohol-related (n = 40) and HCV-related decompensated cirrhosis (n = 40). All the patients were treated with a transjugular intrahepatic portosystemic shunt and the results revealed an excellent correlation between WHVP and PP in those with alcohol-related or HCV-related cirrhosis (r = 0.92; P < 0.001; intraclass correlation coefficient (ICC) 0.96; P < 0.001) whereas it was only moderate in the NASH group (r = 0.61; P <0.001; ICC 0.74; P < 0.001). When the WHVP differed by > 10% from PP, this was regarded as a disagreement between the two, and this occurred more frequently in the NASH group (37.5% vs 14%; P = 0.003)[125], where WHVP tended to underestimate PP. Data from a simtuzumab trial revealed that 14% of patients with NASH cirrhosis and an HVPG < 10 mmHg developed liver decompensation during a median follow-up of 4.7 mo. Nevertheless, an HVPG ≥ 10 mmHg maintained its prognostic properties in terms of predicting the liver decompensation in the overall group of patients with NASH cirrhosis, in comparison to those who had an HVPG < 10 mmHg (hazard ratio 2.83; 95% confidence interval, 1.33-6.02; P = 0.007)[126].
Based on these studies, there is strong evidence for the underestimation of portal pressure in NAFLD patients by HVPG, probably due to the presence of a pre-sinusoidal component. In this line, portal inflammation and ductular reaction in the portal tracts were described in patients with advanced NASH[127,128], and it may be plausible that biliary injury contributes to increased presinusoidal pressure, and therefore favors PP underestimation. Whether periportal fibrosis and/or biliary injury may contribute to increase vascular tone and resistance to blood flow at the level of the portal venules remains to be elucidated.
The hepatic venous pressure gradient (HVPG) represents the gold standard for diagnosing and grading PH[2,121,123,129]. However, it is invasive, expensive and not widely available[130,131]. Among the most serious drawbacks of the HVPG is its inability to detect pre-sinusoidal PH, which obviously takes place in patients with NAFLD, and thus the HVPG might not reliably rule out CSPH in this group. These objective limitations of the HVPG have influenced the search for other methods to be invented and used for diagnosing PH.
Endoscopic ultrasound-guided portal pressure gradient (EUS-PPG) measurement represents a new diagnostic approach to direct PVP assessment. This new method is currently being tested in correlation to traditional HVPG measurement[10]. Under EUS guidance, a modified 25-gauge fine-needle aspiration needle connected to a digital manometer, a self-calibrating compact pressure transducer, is inserted through the liver parenchyma directly into a hepatic vein branch and the portal vein[10,132]. After three consecutive measurements, the mean value is calculated and recorded as the EUS-PPG. In theory, this method could overcome limitations from the HVPG as it measures EUS-PPG, and thus it might more reliably assess the PH grade even in the presence of a pre-sinusoidal component. The first EUS-guided portal vein puncture with portography and pressure measurement was performed on a pig model in 2004[133]. In further animal models, an excellent correlation between EUS-PPG and HVPG measurements (r = 0.99) was demonstrated[132]. In a human pilot study conducted in 28 patients with chronic liver disease, EUS-PPG demonstrated a 100% feasibility of accessing all targeted vessels, with no adverse events. In addition, the EUS-PPG results were highly correlated with the presence of clinical signs of PH (no HVPG measurements were available)[134]. Although it has exhibited promising results, this method needs to be further tested over a larger cohort of patients with different aetiologies of chronic liver disease. Moreover, the issue of how to validate its accuracy in patients with presinusoidal PH, in the absence of a gold diagnostic standard (because the HVPG might not be considered as such in this scenario), still remains. Another limitation of EUS-PPG is that sedation must be used to achieve reliable EUS-PPG measurements, but this heavily influences the hepato-portal haemodynamic and thus probably the results of EUS-PPG measurements as well. The direct measurement of portal pressure is also possible through a surgical approach, which is obviously not acceptable for wider clinical use[10,135].
In addition to the HVPG as an invasive assessment of PVP, numerous non-invasive diagnostic methods have been investigated, and some of them are currently utilized in clinical practice. Ultrasound-based methods have been the most frequently evaluated and are widely implemented in hepatology practices, as they are harmless, with no ionizing radiation, easy to use and supported by a large body of scientific evidence. By employing conventional ultrasound, it is possible to detect morphological signs of PH, such as splenomegaly, the presence of ascites or portosystemic collaterals, which is also achievable through other imaging methods such as computed tomography (CT) or magnetic resonance imaging (MRI). The last two are either ionizing (CT), or not that readily available (MRI). However, morphological signs of PH detected by imaging methods indicate the presence of CSPH, whereas the goal should be to detect the existence of CSPH as early as possible before the signs of the advanced stage develop. In this line, elastography represents one of the most promising candidates, and has become probably the most influential non-invasive diagnostic tool applied in everyday hepatology practice, including the assessment of PH. Most data have been accumulated with the use of transient elastography (TE), but significant evidence also exists for other ultrasound-based methods such as point shear wave elastography and two-dimensional shear wave elastography[136,137]. The pivotal study testing the diagnostic performance of TE for CSPH was published in 2021 and included an international cohort of 836 patients with CLD of mixed aetiology (including 248 with NAFLD), paired LSM and HVPG measurements and no history of liver decompensation. All patients had an LSM ≥ 10 kPa, and the overall prevalence of PH and CSPH was 83% and 59%, respectively. At the LSM cut-off ≥ 25 kPa, TE had a ≥ 90% positive predictive value (PPV) for ruling in CSPH in all aetiologies except for NAFLD, where only 77% of patients with an LSM over this threshold had CSPH according to the HVPG measurements. For non-obese NAFLD patients, the PPV of LSM over 25 kPa was better, with 91.7% of these patients having CSPH. The PPV for obese patients with NAFLD was lower, but the specificity was similar, and the reduced PPV was due to a lower prevalence of CSPH. For ruling CSPH out, a combination of LSM ≤ 15 kPa and platelet count ≥ 150 × 109/L had a ≥ 90% negative predictive value for all aetiologies of CLD including NAFLD, except for hepatitis B, due to the very low number of tested participants. In an attempt to improve the prediction of CSPH in NAFLD patients, the authors constructed a nomogram by using LSM, body mass index (BMI) and platelet count, and demonstrated that at a certain LSM the probability of CSPH is much lower in obese patients compared to their non-obese counterparts[138]. These results were considered by the Baveno VII consensus, which issued recommendations for the non-invasive evaluation of PH utilizing the cut-off values of LSM and platelet count as obtained in this work[139]. To validate these non-invasive criteria for diagnosing CSPH, a retrospective cohort study on 76 cACLD patients (23 with NAFLD) was conducted, and the results revealed that the LSM ≥ 25 kPa criterion had 88.9% specificity and 87.1% PPV for ruling in CSPH, whereas the LSM ≤ 15 kPa and Plt ≥ 150 criterion had 100% sensitivity and a negative predictive value (NPV) for ruling out CSPH. This paper also confirmed that with an increasing BMI, for any given level of platelet count, higher LSM values were needed for a certain probability of having CSPH[140]. According to the Baveno criteria, patients with platelet count > 150 × 109 cells/L and LSM < 20 kPa exhibit a very low risk of having high-risk varices and can safely avoid screening endoscopy[139,141].
A spleen stiffness measurement (SSM) that demonstrated high accuracy in classifying patients according to the presence of varices needing treatment (VNT) and CSPH might be helpful in borderline cases, as it reflects an increased resistance to portal blood flow, and the SSM is not affected by liver steatosis[142-145]. Accordingly, the Baveno VII consensus issued recommendations that an SSM > 50 kPa measured by TE might be applied to rule in CSPH, and an SSM < 21 kPa to exclude it in patients with viral hepatitis[139]. A combined approach in which two out of three criteria (LSM ≥ 25 kPa, SSM > 40 kPa and Plt < 150 × 109/L) were employed in cACLD patients to non-invasively identify those with CSPH correctly classified 88% of patients in a recent individual patient data meta-analysis[146]. Whereas the respective PPVs were 91% and 93% in the subgroups with obesity and a non-viral aetiology, the corresponding specificities were 71% and 85%. The combination of two criteria (LSM ≤ 15 kPa, SSM ≤ 40 kPa and Plt count ≥ 150 × 109/L) demonstrated a suboptimal NPV (67%) for ruling out CSPH in non-viral aetiology, whereas the NPV was 91% if SSM < 21 kPa was utilized instead. Whether the performance of these cut-offs is limited to patients with NAFLD remains to be further validated. Some promising initial results were published using contrast-enhanced ultrasound, specifically the subharmonic aided pressure estimation method[147], but these results require further validation.
Beside imaging methods, biomarker(s) from blood or stool would be welcome for early detection of PH in NAFLD patients, as this approach could potentially have wider applicability. Given that NAFLD is closely related to type 2 diabetes, postprandial blood glucose (PPG) has been studied as an important blood biomarker for assessing the progression of liver disease from steatosis to fibrosis[148-150]. Elevated PPG during NAFLD occurs prior to fibrosis, indicating a bidirectional relationship between postprandial dysfunction in NAFLD and fibrosis development[149,150]. The results of a recent study on a Chinese NAFLD population showed an independent association between elevated PPG and progression of liver fibrosis[148]. Whereas some investigations described distinctive metabolomic blood/stool signature of advanced fibrosis/cirrhosis in comparison to simple steatosis or mild fibrosis, they still have not revealed reliable single biomarker or biomarker combination specific for PH, and thus further research in this regard is warrantied[151,152].
Progression of liver cirrhosis and PH leads to the development of CSPH and its complications in the form of EV, ascites accumulation, portal encephalopathy and EV[9,11,153,154]. Esophago-gastro-duodenoscopy (EGD) represents the gold standard method for diagnosing and assessing the degree of EV, and it offers the possibility of treating EV at the same time[155,156]. However, EGD is an invasive procedure, associated with some risks, including damage to the gut wall, bleeding and perforation, and it is not very well accepted by some patients. Therefore, non-invasive approaches to the diagnosing of EV have been extensively investigated during the last decade. By using non-invasive methods, such as TE in the first place, liver disease is more frequently detected at an early stage, which significantly increases the number of unnecessary endoscopies[157]. Non-invasive Baveno VI (LSM < 20 kPa, Plt > 150 × 109/L) and expanded Baveno VI criteria (LSM < 25 kPa, Plt > 110 × 109/L) have proven to be efficient in ruling out high-risk VNT[141,158]. According to the original report, it was possible to safely avoid 40% of EGDs at the cost of missing only 1.6% of VNT by applying expanded Baveno VI criteria[159]. However, only a minority of patients included in these investigations had NAFLD, whereas the data referred mostly to patients with alcohol-related cirrhosis and viral cirrhosis. Therefore, NAFLD cirrhosis criteria were proposed by Petta et al[160] based on a multicentric international study that included 790 patients with compensated NAFLD cirrhosis who underwent EGD and LSM by TE no more than six months apart. Accordingly, the best performing criteria for ruling out VNT by utilizing an M probe were an LSM < 30 kPa and platelet count > 110000/mm3, whereas the corresponding values if an XL probe was employed were an LSM < 25 kPa and platelet count > 110000/mm3, the latter identical to the expanded Baveno VI criteria[160].
Based on these data, an algorithmic approach to ruling out VNT in patients with NAFLD cirrhosis was finally proposed: if LSM could be reliably assessed by an M probe, then Baveno VI criteria should be applied to non-obese subjects, and NAFLD cirrhosis criteria to obese ones. If the XL probe had to be used, again Baveno VI criteria should be applied to non-obese subjects, and NAFLD cirrhosis criteria (extended Baveno VI) to obese ones[160,161]. In a retrospective cohort study from China, the authors investigated the diagnostic performance of Baveno VI and extended the Baveno VI criteria for ruling out VNT in a cohort of 224 patients with biopsy- or clinically proven compensated NAFLD cirrhosis. It should be highlighted that 60.7% patients had coexisting hepatitis B, 15.6% hepatitis C and 8.9% alcohol-related chronic liver disease. The mean LSM was 18.1 ± 13.9 kPa and the authors did not declare which probe(s) they utilized. By employing Baveno VI and expanded Baveno VI criteria, it was possible to avoid endoscopy in 37.5% and 56.7% patients, with the respective risk of missing VNT in 1.19% and 3.15% patients[162]. Obviously, the dilemma regarding the steatosis influence on LSM is still an open issue, in terms of both its impact on the accuracy of non-invasive staging of liver fibrosis in NAFLD patients, as well as in assessing the severity and complications of PH[138,163,164]. Measuring spleen stiffness might also be helpful in this clinical scenario, as it was demonstrated that in patients who were outside Baveno VI criteria based on LSM and platelet count assessment, an SSM ≤ 46 kPa had a 98% NPV for ruling out VNT[165]. Accordingly, the Baveno VII consensus issued a recommendation that endoscopy could be safely avoided in patients who do not meet LSM/platelet criteria if their SSM is ≤ 40 kPa[139]. Again, this recommendation relies on the data obtained mostly from patients with viral hepatitis and thus should be further validated in those with NAFLD.
The numerous molecular and cellular pathophysiological processes that contribute to IHVR in patients with NAFLD represent potential therapeutic targets for PH[5,10,166] Nonselective beta blockers like carvedilol and propranolol are utilized for the prevention of clinical decompensation in patients with compensated cirrhosis and CSPH[167]. Statins have been demonstrated to stimulate the eNOS-NO-soluble guanylate cyclase (sGC)-cyclic guanosine monophosphate pathway with increased intrahepatic NO production, the upregulation of a transcription factor (Krüppel-like transcription factor) and the inhibition of the RhoA/Rho-associated coiled-coil-containing kinase pathway that is important for the development of LSEC capillarization and vasoconstrictive effects[166,168] Multikinase inhibitors (sorafenib) were investigated for attenuating pathological angiogenesis in the course of chronic liver disease[166,169,170]. Immunomodulatory drugs (thalidomide), caspase inhibitors (emricasan), antioxidative drugs, radical scavengers, cyclooxygenase inhibitors and antibiotics (rifaximin)[166] were used to reduce hepatic inflammation and bacterial translocation as important steps in preventing the progression of PH. Farnesoid X receptor agonists were demonstrated to decrease IHVR by stimulating eNOS[171] activity in a rat model of cirrhotic PH[172].
However, despite ongoing research efforts, there are still no specific agents approved for the treatment of PH caused by NAFLD[173]. Therefore, general guidelines for PH should be followed in patients with NAFLD, while lifestyle changes (reduction in caloric intake, weight loss and daily exercise) remain the mainstay of the treatment approach[10].
The development of clinically significant portal hypertension mostly occurs in patients with cirrhotic NAFLD. Despite this fact, multiple lines of evidence confirm the early elevation of portal vein pressure and onset of PH in non-cirrhotic NAFLD patients. Increased IHVR is the main cause of PH in NAFLD and arises because of perisinusoidal fibrosis and microcirculation damage. HVPG as an invasive diagnostic method underestimates portal pressure in patients with NAFLD and some patients develop liver decompensation below an HVPG of 10 mmHg, which is traditionally considered the threshold for CSPH. Obesity seems to reduce the diagnostic accuracy of LSM, leading to the overestimation of PH severity. Baveno VII criteria might be used for non-invasive ruling out, but they have suboptimal diagnostic performance for ruling in CSPH in obese NAFLD patients. Similarly, Baveno criteria are reliable for ruling out VNT in non-obese NAFLD patients, whereas in obese patients NAFLD cirrhosis criteria might work better. Recent advances in understanding the pathophysiological background of NAFLD and related PH have resulted in several candidate molecules and pathways that might serve as the targets for pharmacological compounds, but this is still an area of ongoing research, and currently we still lack specific drugs for PH in NAFLD. Nevertheless, it is unrealistic to expect that a single medication could reverse all pathological changes taking place along the complex pathways of PH development in NAFLD, and thus a combination of lifestyle changes, liver-targeted therapies and modulation of metabolic derangements would probably represent the solution to this problem.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: He F, China; Ji G, China S-Editor: Gong ZM L-Editor: A P-Editor: Chen YX
| 1. | Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1315] [Article Influence: 119.5] [Reference Citation Analysis (0)] |
| 2. | Kumar M, Sakhuja P, Kumar A, Manglik N, Choudhury A, Hissar S, Rastogi A, Sarin SK. Histological subclassification of cirrhosis based on histological-haemodynamic correlation. Aliment Pharmacol Ther. 2008;27:771-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Pinzani M, Rosselli M, Zuckermann M. Liver cirrhosis. Best Pract Res Clin Gastroenterol. 2011;25:281-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 164] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 4. | Sarin SK, Kapoor D. Non-cirrhotic portal fibrosis: current concepts and management. J Gastroenterol Hepatol. 2002;17:526-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Baffy G. Origins of Portal Hypertension in Nonalcoholic Fatty Liver Disease. Dig Dis Sci. 2018;63:563-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Vollmar B, Menger MD. The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev. 2009;89:1269-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 373] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 7. | NAKATA K, LEONG GF, BRAUER RW. Direct measurement of blood pressures in minute vessels of the liver. Am J Physiol. 1960;199:1181-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 58] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Oda M, Yokomori H, Han JY. Regulatory mechanisms of hepatic microcirculation. Clin Hemorheol Microcirc. 2003;29:167-182. [PubMed] |
| 9. | Bosch J, Iwakiri Y. The portal hypertension syndrome: etiology, classification, relevance, and animal models. Hepatol Int. 2018;12:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 10. | Ryou M, Stylopoulos N, Baffy G. Nonalcoholic fatty liver disease and portal hypertension. Explor Med. 2020;1:149-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Bosch J, García-Pagán JC. Complications of cirrhosis. I. Portal hypertension. J Hepatol. 2000;32:141-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 338] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 12. | Ijaz S, Yang W, Winslet MC, Seifalian AM. Impairment of hepatic microcirculation in fatty liver. Microcirculation. 2003;10:447-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 147] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Mitten EK, Portincasa P, Baffy G. Portal Hypertension in Nonalcoholic Fatty Liver Disease: Challenges and Paradigms. J Clin Transl Hepatol. 2023;11:1201-1211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Wisse E, Braet F, Shami GJ, Zapotoczny B, Vreuls C, Verhaegh P, Frederik P, Peters PJ, Olde Damink S, Koek G. Fat causes necrosis and inflammation in parenchymal cells in human steatotic liver. Histochem Cell Biol. 2022;157:27-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Wada K, Fujimoto K, Fujikawa Y, Shibayama Y, Mitsui H, Nakata K. Sinusoidal stenosis as the cause of portal hypertension in choline deficient diet induced fatty cirrhosis of the rat liver. Acta Pathol Jpn. 1974;24:207-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8233] [Article Influence: 411.7] [Reference Citation Analysis (5)] |
| 17. | Hübscher SG. Histological assessment of non-alcoholic fatty liver disease. Histopathology. 2006;49:450-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 202] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 18. | Wanless IR, Bargman JM, Oreopoulos DG, Vas SI. Subcapsular steatonecrosis in response to peritoneal insulin delivery: a clue to the pathogenesis of steatonecrosis in obesity. Mod Pathol. 1989;2:69-74. [PubMed] |
| 19. | Hilscher MB, Sehrawat T, Arab JP, Zeng Z, Gao J, Liu M, Kostallari E, Gao Y, Simonetto DA, Yaqoob U, Cao S, Revzin A, Beyder A, Wang RA, Kamath PS, Kubes P, Shah VH. Mechanical Stretch Increases Expression of CXCL1 in Liver Sinusoidal Endothelial Cells to Recruit Neutrophils, Generate Sinusoidal Microthombi, and Promote Portal Hypertension. Gastroenterology. 2019;157:193-209.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 169] [Article Influence: 28.2] [Reference Citation Analysis (1)] |
| 20. | Manda A, Pruchniak MP, Araźna M, Demkow UA. Neutrophil extracellular traps in physiology and pathology. Cent Eur J Immunol. 2014;39:116-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Mitten EK, Baffy G. Mechanotransduction in the pathogenesis of non-alcoholic fatty liver disease. J Hepatol. 2022;77:1642-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 22. | Yoshihara H, Hijioka T, Eguchi H, Fukui H, Goto M, Inoue A, Kawano S, Sato N, Kamada T. Hepatic microcirculatory disturbance in fatty liver as a cause of portal hypertension. J Gastroenterol Hepatol. 1989;4 Suppl 1:279-281. [PubMed] |
| 23. | McCuskey RS. Morphological mechanisms for regulating blood flow through hepatic sinusoids. Liver. 2000;20:3-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 142] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 24. | Hilscher MB, Shah VH. Neutrophil Extracellular Traps and Liver Disease. Semin Liver Dis. 2020;40:171-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 25. | Baffy G, Bosch J. Overlooked subclinical portal hypertension in non-cirrhotic NAFLD: Is it real and how to measure it? J Hepatol. 2022;76:458-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 26. | Sun CK, Zhang XY, Wheatley AM. Increased NAD(P)H fluorescence with decreased blood flow in the steatotic liver of the obese Zucker rat. Microvasc Res. 2003;66:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Francque S, Laleman W, Verbeke L, Van Steenkiste C, Casteleyn C, Kwanten W, Van Dyck C, D'Hondt M, Ramon A, Vermeulen W, De Winter B, Van Marck E, Van Marck V, Pelckmans P, Michielsen P. Increased intrahepatic resistance in severe steatosis: endothelial dysfunction, vasoconstrictor overproduction and altered microvascular architecture. Lab Invest. 2012;92:1428-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 28. | Galle J, Quaschning T, Seibold S, Wanner C. Endothelial dysfunction and inflammation: what is the link? Kidney Int Suppl. 2003;S45-S49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Vairappan B. Endothelial dysfunction in cirrhosis: Role of inflammation and oxidative stress. World J Hepatol. 2015;7:443-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 30. | Pasarín M, La Mura V, Gracia-Sancho J, García-Calderó H, Rodríguez-Vilarrupla A, García-Pagán JC, Bosch J, Abraldes JG. Sinusoidal endothelial dysfunction precedes inflammation and fibrosis in a model of NAFLD. PLoS One. 2012;7:e32785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 184] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 31. | Ganz P, Vita JA. Testing endothelial vasomotor function: nitric oxide, a multipotent molecule. Circulation. 2003;108:2049-2053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 172] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 32. | Gupta TK, Toruner M, Chung MK, Groszmann RJ. Endothelial dysfunction and decreased production of nitric oxide in the intrahepatic microcirculation of cirrhotic rats. Hepatology. 1998;28:926-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 261] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 33. | Papapetropoulos A, García-Cardeña G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest. 1997;100:3131-3139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 865] [Cited by in RCA: 894] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 34. | Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109-142. [PubMed] |
| 35. | Felli E, Selicean S, Guixé-Muntet S, Wang C, Bosch J, Berzigotti A, Gracia-Sancho J. Mechanobiology of portal hypertension. JHEP Rep. 2023;5:100869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 36. | Picchi A, Gao X, Belmadani S, Potter BJ, Focardi M, Chilian WM, Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ Res. 2006;99:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 264] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 37. | Montagnani M, Chen H, Barr VA, Quon MJ. Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser(1179). J Biol Chem. 2001;276:30392-30398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 427] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 38. | Duncan ER, Crossey PA, Walker S, Anilkumar N, Poston L, Douglas G, Ezzat VA, Wheatcroft SB, Shah AM, Kearney MT. Effect of endothelium-specific insulin resistance on endothelial function in vivo. Diabetes. 2008;57:3307-3314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 39. | Duncan ER, Walker SJ, Ezzat VA, Wheatcroft SB, Li JM, Shah AM, Kearney MT. Accelerated endothelial dysfunction in mild prediabetic insulin resistance: the early role of reactive oxygen species. Am J Physiol Endocrinol Metab. 2007;293:E1311-E1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 40. | Tyagi N, Sedoris KC, Steed M, Ovechkin AV, Moshal KS, Tyagi SC. Mechanisms of homocysteine-induced oxidative stress. Am J Physiol Heart Circ Physiol. 2005;289:H2649-H2656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 302] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 41. | Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24:R453-R462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3416] [Cited by in RCA: 4580] [Article Influence: 458.0] [Reference Citation Analysis (0)] |
| 42. | Clare K, Dillon JF, Brennan PN. Reactive Oxygen Species and Oxidative Stress in the Pathogenesis of MAFLD. J Clin Transl Hepatol. 2022;10:939-946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 77] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 43. | Parker KJ, Ormachea J, Drage MG, Kim H, Hah Z. The biomechanics of simple steatosis and steatohepatitis. Phys Med Biol. 2018;63:105013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 44. | Förstermann U. Nitric oxide and oxidative stress in vascular disease. Pflugers Arch. 2010;459:923-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 528] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 45. | Mookerjee RP, Mehta G, Balasubramaniyan V, Mohamed Fel Z, Davies N, Sharma V, Iwakiri Y, Jalan R. Hepatic dimethylarginine-dimethylaminohydrolase1 is reduced in cirrhosis and is a target for therapy in portal hypertension. J Hepatol. 2015;62:325-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 46. | Németh B, Kustán P, Németh Á, Lenkey Z, Cziráki A, Kiss I, Sulyok E, Ajtay Z. [Asymmetric dimethylarginine: predictor of cardiovascular diseases?]. Orv Hetil. 2016;157:483-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 47. | Fernandez M. Molecular pathophysiology of portal hypertension. Hepatology. 2015;61:1406-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 48. | Swain SM, Liddle RA. Piezo1 acts upstream of TRPV4 to induce pathological changes in endothelial cells due to shear stress. J Biol Chem. 2021;296:100171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 128] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 49. | Lei L, Ei Mourabit H, Housset C, Cadoret A, Lemoinne S. Role of Angiogenesis in the Pathogenesis of NAFLD. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 50. | Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3471] [Cited by in RCA: 4023] [Article Influence: 287.4] [Reference Citation Analysis (0)] |
| 51. | Poisson J, Lemoinne S, Boulanger C, Durand F, Moreau R, Valla D, Rautou PE. Liver sinusoidal endothelial cells: Physiology and role in liver diseases. J Hepatol. 2017;66:212-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 706] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 52. | Orlandi P, Solini A, Banchi M, Brunetto MR, Cioni D, Ghiadoni L, Bocci G. Antiangiogenic Drugs in NASH: Evidence of a Possible New Therapeutic Approach. Pharmaceuticals (Basel). 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 53. | Verhaegh P, Wisse E, de Munck T, Greve JW, Verheij J, Riedl R, Duimel H, Masclee A, Jonkers D, Koek G. Electron microscopic observations in perfusion-fixed human non-alcoholic fatty liver disease biopsies. Pathology. 2021;53:220-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 54. | Hammoutene A, Rautou PE. Role of liver sinusoidal endothelial cells in non-alcoholic fatty liver disease. J Hepatol. 2019;70:1278-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 239] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 55. | Nasiri-Ansari N, Androutsakos T, Flessa CM, Kyrou I, Siasos G, Randeva HS, Kassi E, Papavassiliou AG. Endothelial Cell Dysfunction and Nonalcoholic Fatty Liver Disease (NAFLD): A Concise Review. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 56. | Yuzefovych LV, Musiyenko SI, Wilson GL, Rachek LI. Mitochondrial DNA damage and dysfunction, and oxidative stress are associated with endoplasmic reticulum stress, protein degradation and apoptosis in high fat diet-induced insulin resistance mice. PLoS One. 2013;8:e54059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 207] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 57. | Reynaert H, Urbain D, Geerts A. Regulation of sinusoidal perfusion in portal hypertension. Anat Rec (Hoboken). 2008;291:693-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 58. | Pinzani M, Failli P, Ruocco C, Casini A, Milani S, Baldi E, Giotti A, Gentilini P. Fat-storing cells as liver-specific pericytes. Spatial dynamics of agonist-stimulated intracellular calcium transients. J Clin Invest. 1992;90:642-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 226] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 59. | Pinzani M. Hepatic stellate (ITO) cells: expanding roles for a liver-specific pericyte. J Hepatol. 1995;22:700-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 91] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 60. | Povero D, Eguchi A, Niesman IR, Andronikou N, de Mollerat du Jeu X, Mulya A, Berk M, Lazic M, Thapaliya S, Parola M, Patel HH, Feldstein AE. Lipid-induced toxicity stimulates hepatocytes to release angiogenic microparticles that require Vanin-1 for uptake by endothelial cells. Sci Signal. 2013;6:ra88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 165] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 61. | McCuskey RS. A dynamic and static study of hepatic arterioles and hepatic sphincters. Am J Anat. 1966;119:455-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 152] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 62. | Francque S, Wamutu S, Chatterjee S, Van Marck E, Herman A, Ramon A, Jung A, Vermeulen W, De Winter B, Pelckmans P, Michielsen P. Non-alcoholic steatohepatitis induces non-fibrosis-related portal hypertension associated with splanchnic vasodilation and signs of a hyperdynamic circulation in vitro and in vivo in a rat model. Liver Int. 2010;30:365-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 63. | Nababan SHH, Lesmana CRA. Portal Hypertension in Nonalcoholic Fatty Liver Disease: From Pathogenesis to Clinical Practice. J Clin Transl Hepatol. 2022;10:979-985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 64. | Iwakiri Y, Shah V, Rockey DC. Vascular pathobiology in chronic liver disease and cirrhosis - current status and future directions. J Hepatol. 2014;61:912-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 230] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 65. | Rockey DC, Weisiger RA. Endothelin induced contractility of stellate cells from normal and cirrhotic rat liver: implications for regulation of portal pressure and resistance. Hepatology. 1996;24:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 255] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 66. | Kaneda K, Ekataksin W, Sogawa M, Matsumura A, Cho A, Kawada N. Endothelin-1-induced vasoconstriction causes a significant increase in portal pressure of rat liver: localized constrictive effect on the distal segment of preterminal portal venules as revealed by light and electron microscopy and serial reconstruction. Hepatology. 1998;27:735-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 68] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 67. | Wanless IR. The Role of Vascular Injury and Congestion in the Pathogenesis of Cirrhosis: the Congestive Escalator and the Parenchymal Extinction Sequence. Curr Hepatol Rep. 2020;19:40-53. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 68. | García-Pagán JC, Gracia-Sancho J, Bosch J. Functional aspects on the pathophysiology of portal hypertension in cirrhosis. J Hepatol. 2012;57:458-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 207] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 69. | Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Semin Liver Dis. 2001;21:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 334] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 70. | Watson CJ, Calne RY, Padhani AR, Dixon AK. Surgical restraint in the management of liver trauma. Br J Surg. 1991;78:1071-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 71. | Chatterjee S. Endothelial Mechanotransduction, Redox Signaling and the Regulation of Vascular Inflammatory Pathways. Front Physiol. 2018;9:524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 126] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 72. | Tanaka K, Joshi D, Timalsina S, Schwartz MA. Early events in endothelial flow sensing. Cytoskeleton (Hoboken). 2021;78:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 73. | Deng B, Zhao Z, Kong W, Han C, Shen X, Zhou C. Biological role of matrix stiffness in tumor growth and treatment. J Transl Med. 2022;20:540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 123] [Reference Citation Analysis (0)] |
| 74. | Duscher D, Maan ZN, Wong VW, Rennert RC, Januszyk M, Rodrigues M, Hu M, Whitmore AJ, Whittam AJ, Longaker MT, Gurtner GC. Mechanotransduction and fibrosis. J Biomech. 2014;47:1997-2005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 147] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 75. | Martino F, Perestrelo AR, Vinarský V, Pagliari S, Forte G. Cellular Mechanotransduction: From Tension to Function. Front Physiol. 2018;9:824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 503] [Cited by in RCA: 617] [Article Influence: 88.1] [Reference Citation Analysis (0)] |
| 76. | Elosegui-Artola A, Oria R, Chen Y, Kosmalska A, Pérez-González C, Castro N, Zhu C, Trepat X, Roca-Cusachs P. Mechanical regulation of a molecular clutch defines force transmission and transduction in response to matrix rigidity. Nat Cell Biol. 2016;18:540-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 562] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 77. | Buscemi L, Ramonet D, Klingberg F, Formey A, Smith-Clerc J, Meister JJ, Hinz B. The single-molecule mechanics of the latent TGF-β1 complex. Curr Biol. 2011;21:2046-2054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 201] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 78. | Le Roux AL, Quiroga X, Walani N, Arroyo M, Roca-Cusachs P. The plasma membrane as a mechanochemical transducer. Philos Trans R Soc Lond B Biol Sci. 2019;374:20180221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 79. | Maurer M, Lammerding J. The Driving Force: Nuclear Mechanotransduction in Cellular Function, Fate, and Disease. Annu Rev Biomed Eng. 2019;21:443-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 174] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 80. | Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci U S A. 1997;94:849-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1282] [Cited by in RCA: 1127] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 81. | Gupta M, Sarangi BR, Deschamps J, Nematbakhsh Y, Callan-Jones A, Margadant F, Mège RM, Lim CT, Voituriez R, Ladoux B. Adaptive rheology and ordering of cell cytoskeleton govern matrix rigidity sensing. Nat Commun. 2015;6:7525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 183] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 82. | Elosegui-Artola A, Andreu I, Beedle AEM, Lezamiz A, Uroz M, Kosmalska AJ, Oria R, Kechagia JZ, Rico-Lastres P, Le Roux AL, Shanahan CM, Trepat X, Navajas D, Garcia-Manyes S, Roca-Cusachs P. Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell. 2017;171:1397-1410.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 943] [Article Influence: 117.9] [Reference Citation Analysis (0)] |
| 83. | Pocaterra A, Romani P, Dupont S. YAP/TAZ functions and their regulation at a glance. J Cell Sci. 2020;133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 246] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 84. | Yamashiro Y, Thang BQ, Ramirez K, Shin SJ, Kohata T, Ohata S, Nguyen TAV, Ohtsuki S, Nagayama K, Yanagisawa H. Matrix mechanotransduction mediated by thrombospondin-1/integrin/YAP in the vascular remodeling. Proc Natl Acad Sci U S A. 2020;117:9896-9905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 85. | Mannaerts I, Leite SB, Verhulst S, Claerhout S, Eysackers N, Thoen LF, Hoorens A, Reynaert H, Halder G, van Grunsven LA. The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. J Hepatol. 2015;63:679-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 309] [Article Influence: 30.9] [Reference Citation Analysis (1)] |
| 86. | Wang X, Zheng Z, Caviglia JM, Corey KE, Herfel TM, Cai B, Masia R, Chung RT, Lefkowitch JH, Schwabe RF, Tabas I. Hepatocyte TAZ/WWTR1 Promotes Inflammation and Fibrosis in Nonalcoholic Steatohepatitis. Cell Metab. 2016;24:848-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 320] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 87. | Qing J, Ren Y, Zhang Y, Yan M, Zhang H, Wu D, Ma Y, Chen Y, Huang X, Wu Q, Mazhar M, Wang L, Liu J, Ding BS, Cao Z. Dopamine receptor D2 antagonism normalizes profibrotic macrophage-endothelial crosstalk in non-alcoholic steatohepatitis. J Hepatol. 2022;76:394-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 88. | Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113:329-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1042] [Cited by in RCA: 1075] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 89. | Wójtowicz A, Babu SS, Li L, Gretz N, Hecker M, Cattaruzza M. Zyxin mediation of stretch-induced gene expression in human endothelial cells. Circ Res. 2010;107:898-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 90. | Huveneers S, Danen EH. Adhesion signaling - crosstalk between integrins, Src and Rho. J Cell Sci. 2009;122:1059-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 621] [Cited by in RCA: 662] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 91. | Kechagia JZ, Ivaska J, Roca-Cusachs P. Integrins as biomechanical sensors of the microenvironment. Nat Rev Mol Cell Biol. 2019;20:457-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 809] [Article Influence: 161.8] [Reference Citation Analysis (0)] |
| 92. | Musso G, Cassader M, Paschetta E, Gambino R. Bioactive Lipid Species and Metabolic Pathways in Progression and Resolution of Nonalcoholic Steatohepatitis. Gastroenterology. 2018;155:282-302.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 245] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 93. | Mihm S. Danger-Associated Molecular Patterns (DAMPs): Molecular Triggers for Sterile Inflammation in the Liver. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 156] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 94. | Heyens LJM, Busschots D, Koek GH, Robaeys G, Francque S. Liver Fibrosis in Non-alcoholic Fatty Liver Disease: From Liver Biopsy to Non-invasive Biomarkers in Diagnosis and Treatment. Front Med (Lausanne). 2021;8:615978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 120] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 95. | Affo S, Yu LX, Schwabe RF. The Role of Cancer-Associated Fibroblasts and Fibrosis in Liver Cancer. Annu Rev Pathol. 2017;12:153-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 511] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 96. | Guicciardi ME, Malhi H, Mott JL, Gores GJ. Apoptosis and necrosis in the liver. Compr Physiol. 2013;3:977-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 262] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 97. | Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem. 2006;281:12093-12101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 576] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 98. | Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver Int. 2006;26:1175-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 617] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 99. | Nguyen-Lefebvre AT, Horuzsko A. Kupffer Cell Metabolism and Function. J Enzymol Metab. 2015;1. [PubMed] |
| 100. | Tomita K, Tamiya G, Ando S, Ohsumi K, Chiyo T, Mizutani A, Kitamura N, Toda K, Kaneko T, Horie Y, Han JY, Kato S, Shimoda M, Oike Y, Tomizawa M, Makino S, Ohkura T, Saito H, Kumagai N, Nagata H, Ishii H, Hibi T. Tumour necrosis factor alpha signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut. 2006;55:415-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 352] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 101. | Van Herck MA, Weyler J, Kwanten WJ, Dirinck EL, De Winter BY, Francque SM, Vonghia L. The Differential Roles of T Cells in Non-alcoholic Fatty Liver Disease and Obesity. Front Immunol. 2019;10:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 184] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 102. | Slevin E, Baiocchi L, Wu N, Ekser B, Sato K, Lin E, Ceci L, Chen L, Lorenzo SR, Xu W, Kyritsi K, Meadows V, Zhou T, Kundu D, Han Y, Kennedy L, Glaser S, Francis H, Alpini G, Meng F. Kupffer Cells: Inflammation Pathways and Cell-Cell Interactions in Alcohol-Associated Liver Disease. Am J Pathol. 2020;190:2185-2193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 103. | Rojkind M, Giambrone MA, Biempica L. Collagen types in normal and cirrhotic liver. Gastroenterology. 1979;76:710-719. [PubMed] |
| 104. | Miao CG, Yang YY, He X, Huang C, Huang Y, Zhang L, Lv XW, Jin Y, Li J. Wnt signaling in liver fibrosis: progress, challenges and potential directions. Biochimie. 2013;95:2326-2335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 105. | Ramzy MM, Abdelghany HM, Zenhom NM, El-Tahawy NF. Effect of histone deacetylase inhibitor on epithelial-mesenchymal transition of liver fibrosis. IUBMB Life. 2018;70:511-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 106. | Orrego H, Blendis LM, Crossley IR, Medline A, Macdonald A, Ritchie S, Israel Y. Correlation of intrahepatic pressure with collagen in the Disse space and hepatomegaly in humans and in the rat. Gastroenterology. 1981;80:546-556. [PubMed] |
| 107. | Mendes FD, Suzuki A, Sanderson SO, Lindor KD, Angulo P. Prevalence and indicators of portal hypertension in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2012;10:1028-33.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 108. | Yeh MM, Brunt EM. Pathology of nonalcoholic fatty liver disease. Am J Clin Pathol. 2007;128:837-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 200] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 109. | Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2244] [Cited by in RCA: 2198] [Article Influence: 129.3] [Reference Citation Analysis (0)] |
| 110. | Shah V. Molecular mechanisms of increased intrahepatic resistance in portal hypertension. J Clin Gastroenterol. 2007;41 Suppl 3:S259-S261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 111. | Gracia-Sancho J, Marrone G, Fernández-Iglesias A. Hepatic microcirculation and mechanisms of portal hypertension. Nat Rev Gastroenterol Hepatol. 2019;16:221-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 112. | Francque S, Verrijken A, Mertens I, Hubens G, Van Marck E, Pelckmans P, Michielsen P, Van Gaal L. Visceral adiposity and insulin resistance are independent predictors of the presence of non-cirrhotic NAFLD-related portal hypertension. Int J Obes (Lond). 2011;35:270-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 113. | Vonghia L, Magrone T, Verrijken A, Michielsen P, Van Gaal L, Jirillo E, Francque S. Peripheral and Hepatic Vein Cytokine Levels in Correlation with Non-Alcoholic Fatty Liver Disease (NAFLD)-Related Metabolic, Histological, and Haemodynamic Features. PLoS One. 2015;10:e0143380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 114. | Rodrigues SG, Montani M, Guixé-Muntet S, De Gottardi A, Berzigotti A, Bosch J. Patients With Signs of Advanced Liver Disease and Clinically Significant Portal Hypertension Do Not Necessarily Have Cirrhosis. Clin Gastroenterol Hepatol. 2019;17:2101-2109.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 115. | Moga L, Laroyenne A, Larrue H, Bureau C, Rautou PE. Patients with NAFLD do not have severe portal hypertension in the absence of cirrhosis. J Hepatol. 2021;74:1269-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 116. | Berzigotti A, Seijo S, Arena U, Abraldes JG, Vizzutti F, García-Pagán JC, Pinzani M, Bosch J. Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology. 2013;144:102-111.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 392] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 117. | Cacciottolo TM, Kumar A, Godfrey EM, Davies SE, Allison M. Spleen Size Does Not Correlate With Histological Stage of Liver Disease in People With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2023;21:535-537.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 118. | Tsushima Y, Endo K. Spleen enlargement in patients with nonalcoholic fatty liver: correlation between degree of fatty infiltration in liver and size of spleen. Dig Dis Sci. 2000;45:196-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 119. | Chow KU, Luxembourg B, Seifried E, Bonig H. Spleen Size Is Significantly Influenced by Body Height and Sex: Establishment of Normal Values for Spleen Size at US with a Cohort of 1200 Healthy Individuals. Radiology. 2016;279:306-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 120. | Abraldes JG, Sarlieve P, Tandon P. Measurement of portal pressure. Clin Liver Dis. 2014;18:779-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 121. | Silva-Junior G, Baiges A, Turon F, Torres F, Hernández-Gea V, Bosch J, García-Pagán JC. The prognostic value of hepatic venous pressure gradient in patients with cirrhosis is highly dependent on the accuracy of the technique. Hepatology. 2015;62:1584-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 122. | Ripoll C, Groszmann R, Garcia-Tsao G, Grace N, Burroughs A, Planas R, Escorsell A, Garcia-Pagan JC, Makuch R, Patch D, Matloff DS, Bosch J; Portal Hypertension Collaborative Group. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 810] [Article Influence: 45.0] [Reference Citation Analysis (1)] |
| 123. | Bosch J, Abraldes JG, Berzigotti A, García-Pagan JC. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol. 2009;6:573-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 523] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 124. | Bassegoda O, Olivas P, Turco L, Mandorfer M, Serra-Burriel M, Tellez L, Kwanten W, Laroyenne A, Farcau O, Alvarado E, Moga L, Vuille-Lessard E, Fortea JI, Ibañez L, Tosetti G, Vanwolleghem T, Larrue H, Burgos-Santamaría D, Stefanescu H, Paternostro R, Cippitelli A, Lens S, Augustin S, Llop E, Laleman W, Trebicka J, Chang J, Masnou H, Zipprich A, Miceli F, Semmler G, Forns X, Primignani M, Bañares R, Puente A, Berzigotti A, Rautou PE, Villanueva C, Ginès P, Garcia-Pagan JC, Procopet B, Bureau C, Albillos A, Francque S, Reiberger T, Schepis F, Graupera I, Hernandez-Gea V. Decompensation in Advanced Nonalcoholic Fatty Liver Disease May Occur at Lower Hepatic Venous Pressure Gradient Levels Than in Patients With Viral Disease. Clin Gastroenterol Hepatol. 2022;20:2276-2286.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 125. | Ferrusquía-Acosta J, Bassegoda O, Turco L, Reverter E, Pellone M, Bianchini M, Pérez-Campuzano V, Ripoll E, García-Criado Á, Graupera I, García-Pagán JC, Schepis F, Senzolo M, Hernández-Gea V. Agreement between wedged hepatic venous pressure and portal pressure in non-alcoholic steatohepatitis-related cirrhosis. J Hepatol. 2021;74:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 126. | Sanyal AJ, Harrison SA, Ratziu V, Abdelmalek MF, Diehl AM, Caldwell S, Shiffman ML, Aguilar Schall R, Jia C, McColgan B, Djedjos CS, McHutchison JG, Subramanian GM, Myers RP, Younossi Z, Muir AJ, Afdhal NH, Bosch J, Goodman Z. The Natural History of Advanced Fibrosis Due to Nonalcoholic Steatohepatitis: Data From the Simtuzumab Trials. Hepatology. 2019;70:1913-1927. [PubMed] [DOI] [Full Text] |
| 127. | Gadd VL, Skoien R, Powell EE, Fagan KJ, Winterford C, Horsfall L, Irvine K, Clouston AD. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology. 2014;59:1393-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 344] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 128. | Bedossa P, Patel K. Biopsy and Noninvasive Methods to Assess Progression of Nonalcoholic Fatty Liver Disease. Gastroenterology. 2016;150:1811-1822.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 129. | PATON A, REYNOLDS TB, SHERLOCK S. Assessment of portal venous hypertension by catheterisation of hepatic vein. Lancet. 1953;1:918-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 62] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 130. | Bosch J, Garcia-Pagán JC, Berzigotti A, Abraldes JG. Measurement of portal pressure and its role in the management of chronic liver disease. Semin Liver Dis. 2006;26:348-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 142] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 131. | Groszmann RJ, Wongcharatrawee S. The hepatic venous pressure gradient: anything worth doing should be done right. Hepatology. 2004;39:280-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 390] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 132. | Huang JY, Samarasena JB, Tsujino T, Chang KJ. EUS-guided portal pressure gradient measurement with a novel 25-gauge needle device versus standard transjugular approach: a comparison animal study. Gastrointest Endosc. 2016;84:358-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 133. | Lai L, Poneros J, Santilli J, Brugge W. EUS-guided portal vein catheterization and pressure measurement in an animal model: a pilot study of feasibility. Gastrointest Endosc. 2004;59:280-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 134. | Huang JY, Samarasena JB, Tsujino T, Lee J, Hu KQ, McLaren CE, Chen WP, Chang KJ. EUS-guided portal pressure gradient measurement with a simple novel device: a human pilot study. Gastrointest Endosc. 2017;85:996-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 135. | Boyer TD, Triger DR, Horisawa M, Redeker AG, Reynolds TB. Direct transhepatic measurement of portal vein pressure using a thin needle. Comparison with wedged hepatic vein pressure. Gastroenterology. 1977;72:584-589. [PubMed] |
| 136. | Grgurevic I, Madir A, Trkulja V, Bozin T, Aralica G, Podrug K, Mikolaševic I, Tsochatzis E, O'Beirne J, Pinzani M. Assessment of clinically significant portal hypertension by two-dimensional shear wave elastography. Eur J Clin Invest. 2022;52:e13750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 137. | Thiele M, Hugger MB, Kim Y, Rautou PE, Elkrief L, Jansen C, Verlinden W, Allegretti G, Israelsen M, Stefanescu H, Piscaglia F, García-Pagán JC, Franque S, Berzigotti A, Castera L, Jeong WK, Trebicka J, Krag A. 2D shear wave liver elastography by Aixplorer to detect portal hypertension in cirrhosis: An individual patient data meta-analysis. Liver Int. 2020;40:1435-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (1)] |
| 138. | Pons M, Augustin S, Scheiner B, Guillaume M, Rosselli M, Rodrigues SG, Stefanescu H, Ma MM, Mandorfer M, Mergeay-Fabre M, Procopet B, Schwabl P, Ferlitsch A, Semmler G, Berzigotti A, Tsochatzis E, Bureau C, Reiberger T, Bosch J, Abraldes JG, Genescà J. Noninvasive Diagnosis of Portal Hypertension in Patients With Compensated Advanced Chronic Liver Disease. Am J Gastroenterol. 2021;116:723-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 161] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 139. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1482] [Article Influence: 494.0] [Reference Citation Analysis (2)] |
| 140. | Podrug K, Trkulja V, Zelenika M, Bokun T, Madir A, Kanizaj TF, O'Beirne J, Grgurevic I. Validation of the New Diagnostic Criteria for Clinically Significant Portal Hypertension by Platelets and Elastography. Dig Dis Sci. 2022;67:3327-3332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 141. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2291] [Article Influence: 229.1] [Reference Citation Analysis (3)] |
| 142. | Colecchia A, Montrone L, Scaioli E, Bacchi-Reggiani ML, Colli A, Casazza G, Schiumerini R, Turco L, Di Biase AR, Mazzella G, Marzi L, Arena U, Pinzani M, Festi D. Measurement of spleen stiffness to evaluate portal hypertension and the presence of esophageal varices in patients with HCV-related cirrhosis. Gastroenterology. 2012;143:646-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 373] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 143. | Colecchia A, Colli A, Casazza G, Mandolesi D, Schiumerini R, Reggiani LB, Marasco G, Taddia M, Lisotti A, Mazzella G, Di Biase AR, Golfieri R, Pinzani M, Festi D. Spleen stiffness measurement can predict clinical complications in compensated HCV-related cirrhosis: a prospective study. J Hepatol. 2014;60:1158-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 144. | Elkrief L, Rautou PE, Ronot M, Lambert S, Dioguardi Burgio M, Francoz C, Plessier A, Durand F, Valla D, Lebrec D, Vilgrain V, Castéra L. Prospective comparison of spleen and liver stiffness by using shear-wave and transient elastography for detection of portal hypertension in cirrhosis. Radiology. 2015;275:589-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 158] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 145. | Song J, Huang J, Huang H, Liu S, Luo Y. Performance of spleen stiffness measurement in prediction of clinical significant portal hypertension: A meta-analysis. Clin Res Hepatol Gastroenterol. 2018;42:216-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 146. | Dajti E, Ravaioli F, Zykus R, Rautou PE, Elkrief L, Grgurevic I, Stefanescu H, Hirooka M, Fraquelli M, Rosselli M, Chang PEJ, Piscaglia F, Reiberger T, Llop E, Mueller S, Marasco G, Berzigotti A, Colli A, Festi D, Colecchia A; Spleen Stiffness—IPD-MA Study Group. Accuracy of spleen stiffness measurement for the diagnosis of clinically significant portal hypertension in patients with compensated advanced chronic liver disease: a systematic review and individual patient data meta-analysis. Lancet Gastroenterol Hepatol. 2023;8:816-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 147. | Eisenbrey JR, Dave JK, Halldorsdottir VG, Merton DA, Miller C, Gonzalez JM, Machado P, Park S, Dianis S, Chalek CL, Kim CE, Baliff JP, Thomenius KE, Brown DB, Navarro V, Forsberg F. Chronic liver disease: noninvasive subharmonic aided pressure estimation of hepatic venous pressure gradient. Radiology. 2013;268:581-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 148. | Chang X, Bian H, Xia M, Zhu X, Sun X, Yang X, Gao J, Lin H, Yan H, Gao X. Postprandial glucose is correlated with an increasing risk of liver fibrosis in Chinese patients with nonalcoholic fatty liver disease. Diabetes Metab. 2022;48:101377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 149. | Grandt J, Jensen AH, Werge MP, Rashu EB, Møller A, Junker AE, Hobolth L, Mortensen C, Johansen CD, Vyberg M, Serizawa RR, Møller S, Gluud LL, Wewer Albrechtsen NJ. Postprandial dysfunction in fatty liver disease. Physiol Rep. 2023;11:e15653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 150. | Jesrani G, Gupta M, Kaur J, Kaur N, Lehl SS, Singh R. One-Hour Postload Plasma Glucose in Obese Indian Adults with Nonalcoholic Fatty Liver Disease: An Observational Study from North India. Indian J Endocrinol Metab. 2021;25:450-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 151. | Ganesan R, Gupta H, Jeong JJ, Sharma SP, Won SM, Oh KK, Yoon SJ, Kim DJ, Suk KT. A metabolomics approach to the validation of predictive metabolites and phenotypic expression in non-alcoholic fatty liver disease. Life Sci. 2023;322:121626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 152. | McGlinchey AJ, Govaere O, Geng D, Ratziu V, Allison M, Bousier J, Petta S, de Oliviera C, Bugianesi E, Schattenberg JM, Daly AK, Hyötyläinen T, Anstee QM, Orešič M. Metabolic signatures across the full spectrum of non-alcoholic fatty liver disease. JHEP Rep. 2022;4:100477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 153. | D'Amico G, Pasta L, Morabito A, D'Amico M, Caltagirone M, Malizia G, Tinè F, Giannuoli G, Traina M, Vizzini G, Politi F, Luca A, Virdone R, Licata A, Pagliaro L. Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Aliment Pharmacol Ther. 2014;39:1180-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 373] [Article Influence: 33.9] [Reference Citation Analysis (1)] |
| 154. | Wongcharatrawee S, Groszmann R. Hemodynamic assessment in clinical practice in portal hypertensive cirrhotics. Ann Gastroenterol. 2001;14:158-165. |
| 155. | North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N Engl J Med. 1988;319:983-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 996] [Cited by in RCA: 841] [Article Influence: 22.7] [Reference Citation Analysis (1)] |
| 156. | de Franchis R; Baveno V Faculty. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 1030] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 157. | Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1439] [Article Influence: 179.9] [Reference Citation Analysis (3)] |
| 158. | European Association for Study of Liver; Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1177] [Cited by in RCA: 1332] [Article Influence: 133.2] [Reference Citation Analysis (0)] |
| 159. | Augustin S, Pons M, Maurice JB, Bureau C, Stefanescu H, Ney M, Blasco H, Procopet B, Tsochatzis E, Westbrook RH, Bosch J, Berzigotti A, Abraldes JG, Genescà J. Expanding the Baveno VI criteria for the screening of varices in patients with compensated advanced chronic liver disease. Hepatology. 2017;66:1980-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 204] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 160. | Petta S, Sebastiani G, Bugianesi E, Viganò M, Wong VW, Berzigotti A, Fracanzani AL, Anstee QM, Marra F, Barbara M, Calvaruso V, Cammà C, Di Marco V, Craxì A, de Ledinghen V. Non-invasive prediction of esophageal varices by stiffness and platelet in non-alcoholic fatty liver disease cirrhosis. J Hepatol. 2018;69:878-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 110] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 161. | de Franchis R, Krag A. Ruling out esophageal varices in NAFLD cirrhosis: Can we do without endoscopy? J Hepatol. 2018;69:769-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 162. | Zheng KI, Liu C, Li J, Zhao L, Zheng MH, Wang F, Qi X. Validation of Baveno VI and expanded Baveno VI criteria to identify high-risk varices in patients with MAFLD-related compensated cirrhosis. J Hepatol. 2020;73:1571-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 163. | Petta S, Wong VW, Cammà C, Hiriart JB, Wong GL, Marra F, Vergniol J, Chan AW, Di Marco V, Merrouche W, Chan HL, Barbara M, Le-Bail B, Arena U, Craxì A, de Ledinghen V. Improved noninvasive prediction of liver fibrosis by liver stiffness measurement in patients with nonalcoholic fatty liver disease accounting for controlled attenuation parameter values. Hepatology. 2017;65:1145-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 175] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 164. | Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, Guha IN, Cobbold JF, Deeks JJ, Paradis V, Bedossa P, Newsome PN. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156:1717-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 965] [Article Influence: 160.8] [Reference Citation Analysis (0)] |
| 165. | Colecchia A, Ravaioli F, Marasco G, Colli A, Dajti E, Di Biase AR, Bacchi Reggiani ML, Berzigotti A, Pinzani M, Festi D. A combined model based on spleen stiffness measurement and Baveno VI criteria to rule out high-risk varices in advanced chronic liver disease. J Hepatol. 2018;69:308-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 139] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 166. | Schierwagen R, Klein S, Uschner F, Trebicka J. Novel Targets and Drug Development in Portal Hypertension. Curr Hepatol Rep. 2019;18:187-196. [DOI] [Full Text] |
| 167. | Reiberger T, Ulbrich G, Ferlitsch A, Payer BA, Schwabl P, Pinter M, Heinisch BB, Trauner M, Kramer L, Peck-Radosavljevic M; Vienna Hepatic Hemodynamic Lab. Carvedilol for primary prophylaxis of variceal bleeding in cirrhotic patients with haemodynamic non-response to propranolol. Gut. 2013;62:1634-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 230] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 168. | Brusilovskaya K, Königshofer P, Schwabl P, Reiberger T. Vascular Targets for the Treatment of Portal Hypertension. Semin Liver Dis. 2019;39:483-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 169. | Coriat R, Gouya H, Mir O, Ropert S, Vignaux O, Chaussade S, Sogni P, Pol S, Blanchet B, Legmann P, Goldwasser F. Reversible decrease of portal venous flow in cirrhotic patients: a positive side effect of sorafenib. PLoS One. 2011;6:e16978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 170. | Pinter M, Sieghart W, Reiberger T, Rohr-Udilova N, Ferlitsch A, Peck-Radosavljevic M. The effects of sorafenib on the portal hypertensive syndrome in patients with liver cirrhosis and hepatocellular carcinoma--a pilot study. Aliment Pharmacol Ther. 2012;35:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 171. | Li J, Kuruba R, Wilson A, Gao X, Zhang Y, Li S. Inhibition of endothelin-1-mediated contraction of hepatic stellate cells by FXR ligand. PLoS One. 2010;5:e13955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 172. | Verbeke L, Farre R, Trebicka J, Komuta M, Roskams T, Klein S, Elst IV, Windmolders P, Vanuytsel T, Nevens F, Laleman W. Obeticholic acid, a farnesoid X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats. Hepatology. 2014;59:2286-2298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 213] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 173. | Mantovani A, Dalbeni A. Treatments for NAFLD: State of Art. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 144] [Article Influence: 36.0] [Reference Citation Analysis (0)] |