Published online Oct 21, 2024. doi: 10.3748/wjg.v30.i39.4308
Revised: August 27, 2024
Accepted: September 19, 2024
Published online: October 21, 2024
Processing time: 84 Days and 0.6 Hours
A recently published article in the World Journal of Gastroenterology clarified that elafibranor, a dual peroxisome proliferator activated receptor α/δ (PPARα/δ) agonist, reduced inflammation and fibrosis in alcohol-associated liver disease (ALD). This letter aims to discuss the findings presented in that article. ALD is a global health problem, and no effective drugs has been approved by the Food and Drug Administration to cure it. Thus, finding targeted therapies is of great urgency. Herein, we focus on the pathogenesis of ALD and the role of PPARα/δ in its development. Consistent with the conclusion of the article of interest, we think that elafibranor may be a promising therapeutic option for ALD, due to the pivotal involvement of PPARα/δ in the pathogenesis of the disease. However, its treatment dose, timing, and side effects need to be further investigated in future studies.
Core Tip: Alcohol-associated liver disease (ALD) remains a significant global health challenge. Peroxisome proliferator-activated receptors α and δ (PPARα/δ) play a crucial role in the pathogenesis of ALD. Elafibranor, a dual PPARα/δ activator, shows promise as a potential therapeutic agent for ALD.
- Citation: Gao FQ, Zhu JQ, Feng XD. Novel intervention for alcohol-associated liver disease. World J Gastroenterol 2024; 30(39): 4308-4312
- URL: https://www.wjgnet.com/1007-9327/full/v30/i39/4308.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i39.4308
Alcohol-associated liver disease (ALD) represents a global health concern, accounting for about 47% of liver disease-related deaths worldwide[1]. ALD is caused by excessive alcohol consumption. Definitions of heavy drinking are established by the National Institute on Alcohol Abuse and Alcoholism and the Centers for Disease Control and Prevention, while criteria for low-risk drinking are formulated by the World Health Organization. As detailed in Table 1, for women, consumption of alcohol exceeding 56 g per day or 112 g per week is considered heavy drinking, while less than 20 g per day and 140 g per week is categorized as low-risk. For men, heavy drinking is defined as more than 70 g per day or 210 g per week, with low-risk thresholds set at less than 40 g per day and 280 g per week.
| Women | Men | |||
| Alcohol intake | Drinks | Alcohol intake | Drinks | |
| Heavy drinking (NIAAA and CDC) | ||||
| Per day | ≥ 56 g | ≥ 4 | ≥ 70 g | ≥ 5 |
| Per week | ≥ 112 g | ≥ 8 | ≥ 210 g | ≥ 15 |
| Low-risk drinking (WHO) | ||||
| Per day | < 20 g | - | < 40 g | - |
| Per week | < 140 g | - | < 280 g | - |
ALD includes a spectrum of conditions ranging from simple steatosis to steatohepatitis and finally to cirrhosis. In some cases, this progression culminates in hepatocellular carcinoma. Although it has severe public health implications, there are currently no therapies approved by the Food and Drug Administration for ALD treatment[2]. Consequently, it is crucial to investigate and develop therapies targeting the underlying mechanisms of ALD. The study by Koizumi et al[3] offers valuable insights for the development of effective treatment options for ALD[3].
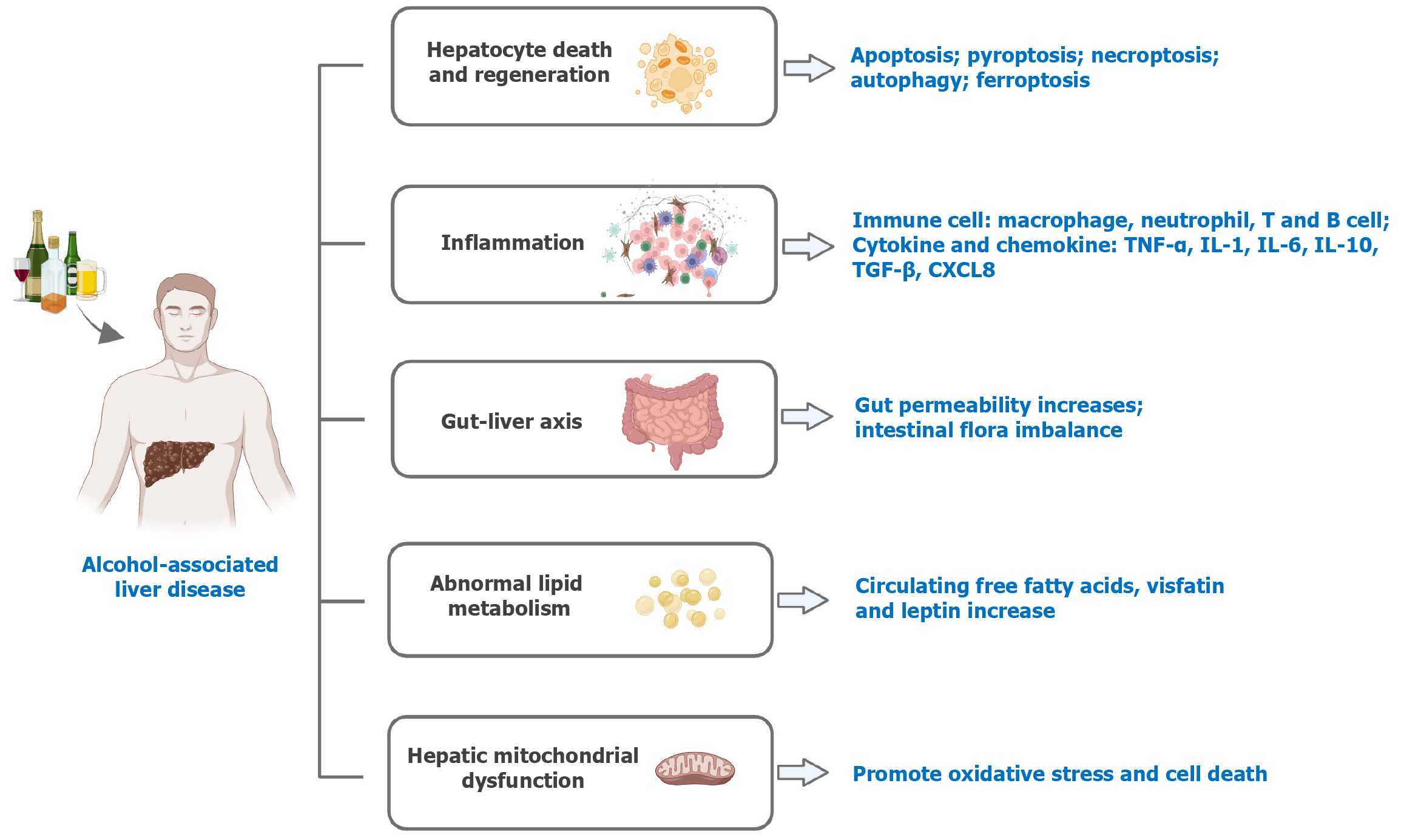
The potential pathogenesis of ALD is diverse[4], as depicted in Figure 1. Hepatocyte death and regeneration are key factors in the progression of ALD[5]. Programmed cell death mechanisms include apoptosis, pyroptosis, necroptosis, autophagy, and ferroptosis[6,7], all of which contribute to the disease process. Oxidative and endoplasmic reticulum stress driven by ethanol metabolism can trigger these cell death pathways in ALD.
Inflammatory response is crucial to the development of ALD. A complex interplay of immune cells and factors drive the inflammation associated with ALD. Macrophages and neutrophils significantly exacerbate ALD by producing inflammatory mediators and reactive oxygen species[8]. T cells are implicated in a profibrotic capacity[9]. And B cells promote the deposition of antibodies and the activation of the complement system, which contribute to liver damage[10]. Elevated levels of cytokines such as tumor necrosis factor-α, interleukin (IL)-1, IL-6, IL-10, IL-22, and transforming growth factor-β have been observed in ALD patients, exerting both protective and damaging effects on the disease[11]. Chemokines like CXCL8 and CCL20 play a critical role in attracting inflammatory cells to the liver, thus amplifying hepatic inflammation and fibrosis[12].
The gut-liver axis also plays a role in ALD. Chronic alcohol consumption increases gut permeability by downregulating junction proteins[13]. And then microbes translocate into the bloodstream. Alcohol also affects intestinal flora imbalance. It leads to a decrease in microbial diversity, an increase in pathogenic bacteria such as Candida, and a reduction in beneficial bacteria. These would affect the production of virulence factors such as cytolysin, further affecting the progression of ALD[14].
Long-term drinking can seriously damage adipose tissue function, resulting in metabolic dysregulation, thereby promoting the development of ALD[15]. Alcohol stimulates lipolysis in adipose tissue, leading to an increase in circulating free fatty acids. These acids play a crucial role in the development of hepatic steatosis and further trigger inflammatory pathways[16]. Moreover, long-term drinking elevates the secretion of adipose-derived hormones such as visfatin and leptin, which enhance fibrotic and inflammatory processes within the liver[15].
Other potential pathogeneses, such as mitochondrial functionality, also play a role. High-risk drinking negatively affects mitochondrial regeneration, and escalates oxidative stress, ultimately resulting in cell death[17].
Peroxisome proliferator-activated receptor α (PPARα), a ligand-activated transcription factor, is widely present in organs such as the liver, heart, and adipose tissue[18]. It can regulate glucolipid metabolism, inflammation responses, and cell death. PPARα modulates β-oxidation, lipid transport, as well as bile acid metabolism[19]. These metabolism activities are vital to ALD development. Loss of PPARα is linked to severe hepatic steatosis[20]. Regarding inflammation, another critical pathogenesis of ALD, PPARα mainly acts through trans-inhibition, that is, antagonizing regulatory factors such as AP-1 and STAT, thereby inhibiting the expression of pro-inflammatory genes[21]. Moreover, PPARα has been reported to induce autophagy[22], which also influences ALD progression. In addition, PPARα activation can reduce the levels of 4-hydroxynonenal, a lipid peroxide that contributes to ALD by inhibiting the activation of NF-κB[23].
Similar to PPARα, PPARδ is expressed and functions in multiple tissues. In the liver, it mitigates liver fibrosis by interfering the transformation of profibrogenic myofibroblasts[24]. Notably, PPARδ expression is found to be lower in patients with severe hepatic steatosis[25], suggesting its potential role in protecting against steatosis. And intestinal PPARδ can enhance mucosal defense capabilities and help prevent dysbiosis[26].
Elafibranor, bezafibrate, and pemafibrate are all PPAR agonists[27]. Both bezafibrate and pemafibrate are PPARα agonists used to treat hypertriglyceridemia. However, pemafibrate is a more selective PPARα agonist, offering greater specificity and fewer side effects compared to bezafibrate. Elafibranor, a dual agonist of PPARα/δ, exhibits superior efficacy in regulating glucose and lipid metabolism, as well as in reducing inflammation and fibrosis. Consequently, elafibranor shows greater potential in the treatment of alcoholic fatty liver disease. Although still an experimental drug[28], elafibranor’s promising effects justify further investigation.
It is well established that abstaining from alcohol can significantly benefit patients with ALD. However, these patients often struggle to quit drinking on their own. Alcohol cessation medications, such as disulfiram, naltrexone, and acamprosate, can be helpful in supporting their efforts. These medications, however, are primarily approved for use in alcohol use disorder (AUD)[29], and ALD patients do not always meet the criteria for AUD. Additionally, these medications can cause adverse effects like liver and kidney function damage. And unlike the PPAR activator elafibranor, these medications do not have the capacity to reverse the inflammation and fibrosis that have already developed in the liver.
Elafibranor is a dual agonist of PPARα and PPARδ. It has been found to have therapeutic effects on primary biliary cholangitis and non-alcoholic fatty liver disease[30,31], which share some pathogenic mechanisms with ALD. However, its efficacy in treating ALD has not yet been clarified. Given the beneficial roles of both PPARα and PPARδ in ALD, and the ability of elafibranor to activate these receptors, it is conceivable that elafibranor could significantly ameliorate ALD. Thus, investigating elafibranor’s impact on ALD presents an interesting area of research. The study conducted by Koizumi et al[3] has provided evidence that elafibranor effectively reduces liver steatosis, inflammation, and fibrosis in ALD.
ALD is a common disease with a poor prognosis. Finding targeted therapies for the disease can improve patient outcomes and their life quality. According to the research conducted by Koizumi et al[3], elafibranor represents a promising therapeutic candidate. However, further investigation into elafibranor’s application in ALD treatment is needed. This includes determining optimal therapeutic dosages and evaluating potential side effects specific to ALD.
| 1. | Lee BP, Witkiewitz K, Mellinger J, Anania FA, Bataller R, Cotter TG, Curtis B, Dasarathy S, DeMartini KS, Diamond I, Diazgranados N, DiMartini AF, Falk DE, Fernandez AC, German MN, Kamath PS, Kidwell KM, Leggio L, Litten R, Louvet A, Lucey MR, McCaul ME, Sanyal AJ, Singal AK, Sussman NL, Terrault NA, Thursz MR, Verna EC, Radaeva S, Nagy LE, Mitchell MC. Designing clinical trials to address alcohol use and alcohol-associated liver disease: an expert panel Consensus Statement. Nat Rev Gastroenterol Hepatol. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 2. | Bataller R, Arab JP, Shah VH. Alcohol-Associated Hepatitis. N Engl J Med. 2022;387:2436-2448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 135] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 3. | Koizumi A, Kaji K, Nishimura N, Asada S, Matsuda T, Tanaka M, Yorioka N, Tsuji Y, Kitagawa K, Sato S, Namisaki T, Akahane T, Yoshiji H. Effects of elafibranor on liver fibrosis and gut barrier function in a mouse model of alcohol-associated liver disease. World J Gastroenterol. 2024;30:3428-3446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 19] [Article Influence: 19.0] [Reference Citation Analysis (2)] |
| 4. | Mackowiak B, Fu Y, Maccioni L, Gao B. Alcohol-associated liver disease. J Clin Invest. 2024;134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 132] [Reference Citation Analysis (0)] |
| 5. | Nagy LE, Ding WX, Cresci G, Saikia P, Shah VH. Linking Pathogenic Mechanisms of Alcoholic Liver Disease With Clinical Phenotypes. Gastroenterology. 2016;150:1756-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 6. | Zheng M, Kanneganti TD. The regulation of the ZBP1-NLRP3 inflammasome and its implications in pyroptosis, apoptosis, and necroptosis (PANoptosis). Immunol Rev. 2020;297:26-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 334] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 7. | Stolz A, Ernst A, Dikic I. Cargo recognition and trafficking in selective autophagy. Nat Cell Biol. 2014;16:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 816] [Cited by in RCA: 940] [Article Influence: 85.5] [Reference Citation Analysis (0)] |
| 8. | Gao B, Ahmad MF, Nagy LE, Tsukamoto H. Inflammatory pathways in alcoholic steatohepatitis. J Hepatol. 2019;70:249-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 271] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 9. | Mehal W. Mechanisms of liver fibrosis in metabolic syndrome. eGastroenterology. 2023;1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Ahmadi AR, Song G, Gao T, Ma J, Han X, Hu M, Cameron AM, Wesson RN, Philosophe B, Ottmann S, King E, Gurakar A, Qi L, Peiffer B, Burdick J, Anders R, Zhou Z, Lu H, Feng D, Chen C, Qian J, Gao B, Zhu H, Sun Z. Discovery and characterization of cross-reactive intrahepatic antibodies in severe alcoholic hepatitis. eLife. 2023;12. [DOI] [Full Text] |
| 11. | Wang H, Mehal W, Nagy LE, Rotman Y. Immunological mechanisms and therapeutic targets of fatty liver diseases. Cell Mol Immunol. 2021;18:73-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 140] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 12. | Gao B, Xu M. Chemokines and alcoholic hepatitis: are chemokines good therapeutic targets? Gut. 2014;63:1683-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50:638-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 377] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 14. | Llopis M, Cassard AM, Wrzosek L, Boschat L, Bruneau A, Ferrere G, Puchois V, Martin JC, Lepage P, Le Roy T, Lefèvre L, Langelier B, Cailleux F, González-Castro AM, Rabot S, Gaudin F, Agostini H, Prévot S, Berrebi D, Ciocan D, Jousse C, Naveau S, Gérard P, Perlemuter G. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut. 2016;65:830-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 423] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 15. | Parker R, Kim SJ, Gao B. Alcohol, adipose tissue and liver disease: mechanistic links and clinical considerations. Nat Rev Gastroenterol Hepatol. 2018;15:50-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 134] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 16. | Wei X, Shi X, Zhong W, Zhao Y, Tang Y, Sun W, Yin X, Bogdanov B, Kim S, McClain C, Zhou Z, Zhang X. Chronic alcohol exposure disturbs lipid homeostasis at the adipose tissue-liver axis in mice: analysis of triacylglycerols using high-resolution mass spectrometry in combination with in vivo metabolite deuterium labeling. PLoS One. 2013;8:e55382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Nassir F, Ibdah JA. Role of mitochondria in alcoholic liver disease. World J Gastroenterol. 2014;20:2136-2142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 89] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 18. | Brown JD, Plutzky J. Peroxisome proliferator-activated receptors as transcriptional nodal points and therapeutic targets. Circulation. 2007;115:518-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 264] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 19. | Rotman Y, Sanyal AJ. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut. 2017;66:180-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 336] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 20. | Montagner A, Polizzi A, Fouché E, Ducheix S, Lippi Y, Lasserre F, Barquissau V, Régnier M, Lukowicz C, Benhamed F, Iroz A, Bertrand-Michel J, Al Saati T, Cano P, Mselli-Lakhal L, Mithieux G, Rajas F, Lagarrigue S, Pineau T, Loiseau N, Postic C, Langin D, Wahli W, Guillou H. Liver PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut. 2016;65:1202-1214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 397] [Cited by in RCA: 564] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 21. | Lefebvre P, Chinetti G, Fruchart JC, Staels B. Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J Clin Invest. 2006;116:571-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 747] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 22. | Luo R, Su LY, Li G, Yang J, Liu Q, Yang LX, Zhang DF, Zhou H, Xu M, Fan Y, Li J, Yao YG. Activation of PPARA-mediated autophagy reduces Alzheimer disease-like pathology and cognitive decline in a murine model. Autophagy. 2020;16:52-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 260] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 23. | Ding L, Wo L, Du Z, Tang L, Song Z, Dou X. Danshen protects against early-stage alcoholic liver disease in mice via inducing PPARα activation and subsequent 4-HNE degradation. PLoS One. 2017;12:e0186357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Li X, Chen Y, Wu S, He J, Lou L, Ye W, Wang J. microRNA-34a and microRNA-34c promote the activation of human hepatic stellate cells by targeting peroxisome proliferator-activated receptor γ. Mol Med Rep. 2015;11:1017-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Zarei M, Barroso E, Palomer X, Dai J, Rada P, Quesada-López T, Escolà-Gil JC, Cedó L, Zali MR, Molaei M, Dabiri R, Vázquez S, Pujol E, Valverde ÁM, Villarroya F, Liu Y, Wahli W, Vázquez-Carrera M. Hepatic regulation of VLDL receptor by PPARβ/δ and FGF21 modulates non-alcoholic fatty liver disease. Mol Metab. 2018;8:117-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 26. | Tomas J, Mulet C, Saffarian A, Cavin JB, Ducroc R, Regnault B, Kun Tan C, Duszka K, Burcelin R, Wahli W, Sansonetti PJ, Pédron T. High-fat diet modifies the PPAR-γ pathway leading to disruption of microbial and physiological ecosystem in murine small intestine. Proc Natl Acad Sci USA. 2016;113:E5934-E5943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 185] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 27. | Kamata S, Honda A, Kashiwagi N, Shimamura A, Yashiro S, Komori Y, Hosoda A, Akahoshi N, Ishii I. Different Coactivator Recruitment to Human PPARα/δ/γ Ligand-Binding Domains by Eight PPAR Agonists to Treat Nonalcoholic Fatty Liver Disease. Biomedicines. 2024;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Boeckmans J, Natale A, Rombaut M, Buyl K, Cami B, De Boe V, Heymans A, Rogiers V, De Kock J, Vanhaecke T, Rodrigues RM. Human hepatic in vitro models reveal distinct anti-NASH potencies of PPAR agonists. Cell Biol Toxicol. 2021;37:293-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 29. | Kranzler HR, Soyka M. Diagnosis and Pharmacotherapy of Alcohol Use Disorder: A Review. JAMA. 2018;320:815-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 397] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 30. | Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, Romero-Gomez M, Boursier J, Abdelmalek M, Caldwell S, Drenth J, Anstee QM, Hum D, Hanf R, Roudot A, Megnien S, Staels B, Sanyal A; GOLDEN-505 Investigator Study Group. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-α and -δ, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology. 2016;150:1147-1159.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 820] [Article Influence: 91.1] [Reference Citation Analysis (0)] |
| 31. | Kowdley KV, Bowlus CL, Levy C, Akarca US, Alvares-da-Silva MR, Andreone P, Arrese M, Corpechot C, Francque SM, Heneghan MA, Invernizzi P, Jones D, Kruger FC, Lawitz E, Mayo MJ, Shiffman ML, Swain MG, Valera JM, Vargas V, Vierling JM, Villamil A, Addy C, Dietrich J, Germain JM, Mazain S, Rafailovic D, Taddé B, Miller B, Shu J, Zein CO, Schattenberg JM; ELATIVE Study Investigators’ Group; ELATIVE Study Investigators' Group. Efficacy and Safety of Elafibranor in Primary Biliary Cholangitis. N Engl J Med. 2024;390:795-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 106] [Article Influence: 106.0] [Reference Citation Analysis (0)] |