Published online Oct 21, 2024. doi: 10.3748/wjg.v30.i39.4260
Revised: September 17, 2024
Accepted: September 23, 2024
Published online: October 21, 2024
Processing time: 52 Days and 18.5 Hours
In this editorial we comment on the article by Pacheco et al published in a recent issue of the World Journal of Gastroenterology. We focus specifically on the burden of illness associated with perianal fistulizing Crohn’s disease (PFCD) and the diagnostic and therapeutic challenges in the management of this condition. Evol
Core Tip: Perianal fistulizing Crohn’s disease (PFCD) is a challenging phenotype of inflammatory bowel disease. Treatment strategies for the management of this condition should focus on a patient-centred approach to care. Many gaps remain in the literature surrounding PFCD, especially regarding surveillance for anorectal malignancies. Available evidence related to PFCD should be leveraged to form the foundation of ongoing research in this field.
- Citation: Swaminathan A, Sparrow MP. Perianal Crohn’s disease: Still more questions than answers. World J Gastroenterol 2024; 30(39): 4260-4266
- URL: https://www.wjgnet.com/1007-9327/full/v30/i39/4260.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i39.4260
Perianal fistulizing Crohn’s disease (PFCD) represents a debilitating form of Crohn’s disease (CD) that is associated with symptoms of perianal pain, discharge and incontinence[1,2]. PFCD usually presents as fistulizing lesions in the perianal region in the presence or absence of an abscess[1]. A recent review by Pacheco et al[3] published in the World Journal of Gastroenterology has highlighted some of the challenges of managing this condition. However, to appreciate the full context of this disease, it is important to consider the epidemiology and classification of PFCD, and burden of illness.
In population-based inception cohorts, PFCD has been observed in 1 in 5 individuals with CD by 10 years of their index diagnosis of inflammatory bowel disease (IBD)[2]. Approximately 4%-5% of those with PFCD can present with this condition prior to the detection of luminal CD[2].
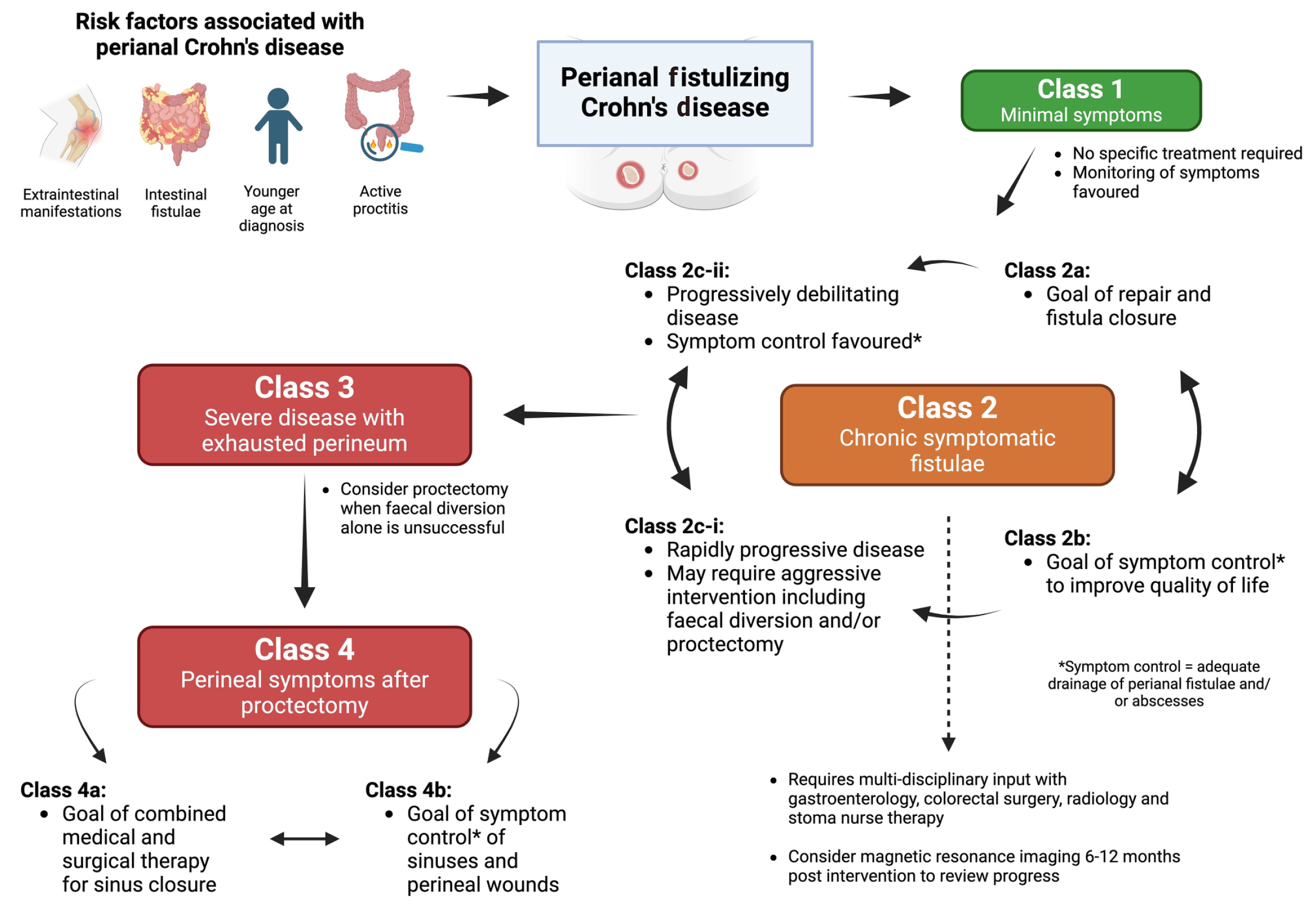
The presence of PFCD has been associated with a more pervasive course of IBD[4-7]. Risk factors associated with the development of PFCD include a younger age at diagnosis, the presence of proctitis, and a history of intestinal fistulae or extra-intestinal manifestations of IBD (Figure 1)[1,2,8]. Individuals with PFCD also appear to have a more severe phenotype of IBD with higher rates of progression to fibrostenotic complications, increased need for therapeutic interventions such as advanced biologic therapies, hospitalisations, surgeries, and increased risk for anal and rectal malignancies[9-12]. Approximately 30% to 50% of individuals with PFCD require faecal diversion to manage their disease[13-15]. This is often considered a temporary option and can improve symptoms in up to 50% of patients[15]. However, the restoration of bowel continuity is achieved in only 25% of individuals[15]. These rates are highest when biologic agents are used pre and post diversion, and in the absence of proctitis[15].
Individuals with PFCD also report a significant impact on interpersonal relationships, psychological morbidity and work-place productivity due to perianal symptoms[5-7,16]. However, the impact of PFCD alone on the burden of illness severity has not been easily extracted from those living with IBD in general[17,18]. Despite this, individuals with PFCD may have unique symptomatology that has a significant impact on their quality of life (QoL) and requires consideration when making therapeutic decisions. Patients with PFCD have been reported to have worse QoL compared to those with CD without perianal disease which is largely driven by symptoms of faecal incontinence and perianal pain and discharge[19].
The pathophysiology of PFCD is complex and our understanding of the mechanisms that trigger and perpetuate the development of this disease are still developing[1]. In PFCD, it is believed that immune dysregulation contributes to the production of tumour necrosis factor (TNF) by inflammatory cells, transforming growth factor β and interleukin (IL) 13 produced by fibroblasts, resulting in epithelial-to-mesenchymal transition and tissue remodelling[1]. This process results in the differentiation of epithelial cells that gain an ability to migrate and penetrate adjacent tissues[20]. Consequently, given the complexity surrounding the pathophysiology of PFCD, historic classification systems to define this illness have relied upon the anatomical distributions of perianal fistulas[4,21].
The challenges of using such classification systems alone, such as those described in the review by Pacheco et al[3], are that they do not incorporate the impact of this illness on a patient. Up to 13% of individuals with PFCD can have asymptomatic fistulae[22] and there is no proven benefit in escalation of medical or surgical therapy in the management of these individuals[23]. Additionally, the insights gained from the PISA-I[3] and PISA-II[24,25] trials have highlighted that surgical treatments need to be aligned with patient goals rather than be based purely on the presence of simple or complex fistulas as suggested by Pacheco et al[3]. Such strategies are likely to yield the greatest long term benefit as they marry the risks of surgical intervention (such as faecal incontinence) with potential benefits (reduction in perianal pain and discharge) in the context of a patient’s burden of illness (Figure 1)[24].
It is in response to these challenges that a new classification system (not described in the review by Pacheco et al[3]) has been recently developed which may provide an improved framework for decision making in the management of PFCD[23]. The TOpClass classification has been devised following a systematic review of the literature and consensus agreement of clinicians (gastroenterologists, colorectal surgeons and radiologists) with expertise in the management of PFCD (summarized in Figure 1)[23]. This system describes a continuum of illness from where there is minimal disease burden (Class 1) which requires observation alone, to a more pervasive phenotype where there is refractory disease despite aggressive medical and surgical interventions where the goals of care need to be aligned with a patient’s wishes and may include the repair of symptomatic sinuses or wounds (Class 4a) or symptom control with adequate drainage of new lesions (Class 4b) in a “burnt out” perineum[23]. The TOpClass classifications of PFCD described in between these extreme ends of the spectrum (Figure 1) include the presence of chronic symptomatic fistulae (Class 2a and Class 2b) which can lead to a more aggressive phenotype with destruction of the perineum that necessitates consideration of faecal diversion (Class 2c-i and Class 2c-ii) and protectomy (Class 3) in refractory cases[23,26]. Regardless of the phase of illness, this system reiterates the importance of a multi-disciplinary approach to decision making in PFCD as each therapeutic choice involves a delicate balance between symptom/QoL improvement and risks of surgical and medical intervention. Thus, the TOpClass system allows for more nuanced decision making which incorporates a patient’s views of their illness compared to a traditional focus on anatomical classification of PFCD alone. The utility of incorporating this disease classification system in day-to-day clinical practice, therapeutic trials, and patient-acceptability of this approach remains to be determined[26].
Treatment paradigms for the management of luminal IBD have focused heavily on “treating to target” in recent years[27]. This strategy requires proactive monitoring of gut inflammation using biomarkers such as faecal calprotectin which act as surrogate treatment targets[27]. However, there are no existing robust biomarkers of PFCD activity and practical guidance for monitoring this disease is limited and also not described in detail by Pacheco et al[3]. Radiological assessment (and identification of a fibrotic fistula tract) using magnetic resonance imaging (MRI) of the pelvis was associated with long lasting closure of fistula tracts in the PISA-II trial[24]. Thus, assessment of PFCD using MRI 6 to 12 months after the initiation of a medical or surgical treatment option could be considered (Figure 1)[26]. However, the utility of proactive monitoring of PFCD remains unclear.
Surgical management of PFCD ranges from the drainage of perianal abscesses and insertion of setons, to faecal diversion and proctectomy, as discussed in the review by Pacheco et al[3]. Although newer surgical techniques continue to evolve[28,29], the key findings from the ACCENT II trial (A CD clinical trial evaluating infliximab in a new long-term treatment regimen in patients with fistulizing CD) forms the foundations of modern treatment paradigms in PFCD[30]. The benefits of the anti-TNF agent infliximab in achieving fistula closure are well established[31], however, the importance of timing of anti-TNF induction (< 6 weeks or > 6 weeks) surrounding fistula surgery remains unclear with conflicting reports in the literature[32,33]. This is particularly relevant in the absence of luminal disease where a diagnosis of CD remains uncertain.
The utility of proactive monitoring and dose intensification in achieving improved fistula healing in PFCD remains unclear. Retrospective analyses have highlighted higher rates of fistula closure with higher infliximab trough levels at week 14 following induction[34,35]. Although higher serum trough levels are anticipated to be required to effectively manage PFCD compared to isolated luminal CD, optimal thresholds vary in the literature (ranging from 7.2 mg/L to 10.1 mg/L)[34,35]. It is hoped that these uncertainties will be further clarified in a currently recruiting study aiming to answer this question (PROACTIVE)[36].
The evidence for medical therapies in PFCD mainly surrounds the use of antibiotics and anti-TNF agents (either used alone or in combination)[31,37]. The utility of newer biologic and small-molecule agents (that are now approved for the treatment of luminal IBD) in PFCD remains unclear[31]. There is a relative paucity of data to inform clinicians and patients regarding second line medical therapies following the failure of anti-TNF agents. Although vedolizumab (anti-integrin) and ustekinumab (IL-12/23 inhibitor) appear to show some benefit in the management of PFCD[31,38,39], ustekinumab has been associated with a greater level of persistence at 1 year[40]. Although early analyses of IL-23/p19 inhibitors (such as risankizumab, mirikizumab and guselkumab) and janus kinase inhibitors (upadacitinib) have shown some promise in the management of PFCD, these data are yet to be fully presented[41,42].
The use of mesenchymal stem cell treatment in PFCD has gain significant interest in recent years and is described in the review by Pacheco et al[3] as a potential treatment mechanism to revolutionize the management of PFCD. Clinical trials of cell-based therapies have focused thus far on darvadstrocel (DVS), as used in the ADMIRE-CD phase 3 randomized control trial[43]. The promising findings from this study which are summarized by Pacheco et al[3] were predominately in a European cohort where DVS was well tolerated and effective (compared to placebo) in maintaining fistula closure[43]. However, these efficacy results were not replicated in a more recently recruited North American cohort[43,44]. Thus, the future of cell-based therapies (in particular DVS) in PFCD remains uncertain.
The presence of PFCD has been associated with an increased risk for anal and rectal malignancy[12,45]. A previous meta-analysis of intestinal cancers in CD has estimated the incidence of cancers arising from CD associated fistula to be 0.2/1000 patient-years[46]. Previous population studies have also shown that risk of anorectal cancer to be up to 4-fold higher in those with PFCD compared to those with non-perianal CD[10]. The aetiology of anorectal cancers includes both squamous cell carcinoma of the anus and colonic/rectal adenocarcinoma[45]. Features associated with a higher risk for the development of anorectal malignancy in those with PFCD include a history of smoking, younger age at diagnosis of PFCD, persistent proctitis, stricturing phenotype, history of human immunodeficiency virus and human papillomavirus (HPV), and receptive anal intercourse[45]. Thus, the presence of these factors should prompt clinicians to consider the risk for development of anorectal cancer in individuals with PFCD. However, there is a paucity of data to recommend optimal screening strategies (including the ideal diagnostic modality) for PFCD-associated cancer.
In their review, Pacheco et al[3] have proposed an opportunistic evaluation for anorectal malignancy in those with PFCD as part of routine bowel cancer screening programs. A workup involving anoscopy and anal cytology with HPV testing to triage those who may benefit from referral for high-resolution anoscopy with biopsies of suspicious lesions has been suggested. Given the relatively low overall incidence of anorectal cancer in this group, it would seem reasonable to leverage existing screening platforms (such as national bowel cancer screening programs that utilise sigmoidoscopy/colonoscopy) to improve early detection of these cancers. However, incorporating additional risk factors (as discussed above) into such a surveillance program[3] should be considered to refine an approach for the early detection of anorectal malignancy in those with PFCD. Additionally, the rates of cancer detection, patient-acceptability and cost-benefit analyses of such programs should be considered.
PFCD is a challenging and complex form of CD that often results in a significant illness burden. There remain diagnostic uncertainties and challenges in formulating an optimal disease classification system that incorporates patient values to form a framework for making treatment decisions. Despite the significant evolution of medical therapies for the management of IBD in recent years, more investigation is required on the utility of these treatments for those with PFCD. Additionally, there is a gap in the literature surrounding our understanding of PFCD-associated cancer and optimal strategies to screen for this. Although there have been significant advances in the diagnosis and management of PFCD in the biologic era, these remaining questions can help shape future trials to improve the long-term outcomes for individuals suffering from this debilitating illness.
| 1. | Adegbola SO, Dibley L, Sahnan K, Wade T, Verjee A, Sawyer R, Mannick S, McCluskey D, Yassin N, Phillips RKS, Tozer PJ, Norton C, Hart AL. Burden of disease and adaptation to life in patients with Crohn's perianal fistula: a qualitative exploration. Health Qual Life Outcomes. 2020;18:370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 2. | Anandabaskaran S, Hanna L, Iqbal N, Constable L, Tozer P, Hart A. Where Are We and Where to Next?-The Future of Perianal Crohn's Disease Management. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 3. | Pacheco T, Monteiro S, Barros L, Silva J. Perianal disease in inflammatory bowel disease: Broadening treatment and surveillance strategies for anal cancer. World J Gastroenterol. 2024;30:3373-3385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (2)] |
| 4. | Atia O, Asayag N, Focht G, Lujan R, Ledder O, Greenfeld S, Kariv R, Dotan I, Gabay H, Balicer R, Haklai Z, Nevo D, Turner D. Perianal Crohn's Disease Is Associated With Poor Disease Outcome: A Nationwide Study From the epiIIRN Cohort. Clin Gastroenterol Hepatol. 2022;20:e484-e495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Attauabi M, Burisch J, Seidelin JB. Efficacy of ustekinumab for active perianal fistulizing Crohn's disease: a systematic review and meta-analysis of the current literature. Scand J Gastroenterol. 2021;56:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Beaugerie L, Carrat F, Nahon S, Zeitoun JD, Sabaté JM, Peyrin-Biroulet L, Colombel JF, Allez M, Fléjou JF, Kirchgesner J, Svrcek M; Cancers et Surrisque Associé aux Maladies Inflammatoires Intestinales En France Study Group. High Risk of Anal and Rectal Cancer in Patients With Anal and/or Perianal Crohn's Disease. Clin Gastroenterol Hepatol. 2018;16:892-899.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 7. | Bouguen G, Siproudhis L, Gizard E, Wallenhorst T, Billioud V, Bretagne JF, Bigard MA, Peyrin-Biroulet L. Long-term outcome of perianal fistulizing Crohn's disease treated with infliximab. Clin Gastroenterol Hepatol. 2013;11:975-81.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 145] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 8. | Brochard C, Rabilloud ML, Hamonic S, Bajeux E, Pagenault M, Dabadie A, Gerfaud A, Viel JF, Tron I, Robaszkiewicz M, Bretagne JF, Siproudhis L, Bouguen G; Groupe ABERMAD. Natural History of Perianal Crohn's Disease: Long-term Follow-up of a Population-Based Cohort. Clin Gastroenterol Hepatol. 2022;20:e102-e110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 9. | Colombel JF, Irving P, Rieder F, Panaccione R, Schwartz D, Hayashi R, Zhu X, Lacerda AP, Dubcenco E, Marced E, Hecht P, Feng T, Berg S, Reinisch W. P491 Efficacy and safety of upadacitinib for the treatment of fistulas and fissures in patients with Crohn’s disease. J Crohns Colitis. 2023;17:i620-i623. [DOI] [Full Text] |
| 10. | Dewint P, Hansen BE, Verhey E, Oldenburg B, Hommes DW, Pierik M, Ponsioen CI, van Dullemen HM, Russel M, van Bodegraven AA, van der Woude CJ. Adalimumab combined with ciprofloxacin is superior to adalimumab monotherapy in perianal fistula closure in Crohn's disease: a randomised, double-blind, placebo controlled trial (ADAFI). Gut. 2014;63:292-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 172] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 11. | Gao X, Fujii T, Ye BD, Chou JW, Sugimoto K, Cao Q, Kligys K, Murakoshi K, Teng D, Zhang Y, Nakase H. Efficacy and safety of risankizumab for Crohn's disease in patients from Asian countries: a post hoc subanalysis of the global phase 3 ADVANCE, MOTIVATE, and FORTIFY studies. J Gastroenterol Hepatol. 2024;39:55-65. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | García-Olmo D, Gómez-Barrera M, de la Portilla F. Surgical management of complex perianal fistula revisited in a systematic review: a critical view of available scientific evidence. BMC Surg. 2023;23:29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 13. | Geldof J, Iqbal N, LeBlanc JF, Anandabaskaran S, Sawyer R, Buskens C, Bemelman W, Gecse K, Lundby L, Lightner AL, Danese S, Spinelli A, Carvello M, Faiz O, Warusavitarne J, Lung P, De Looze D, D'Hoore A, Vermeire S, Hart A, Tozer P. Classifying perianal fistulising Crohn's disease: an expert consensus to guide decision-making in daily practice and clinical trials. Lancet Gastroenterol Hepatol. 2022;7:576-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 14. | Gonczi L, Lakatos L, Golovics PA, Angyal D, Balogh F, Ilias A, Pandur T, David G, Erdelyi Z, Szita I, Lakatos PL. Burden of perianal disease in Crohn's disease: Accelerating medical therapy and high rates of perianal surgery over the last four decades - Results from a population-based study over four decades. Aliment Pharmacol Ther. 2024;59:656-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Gu B, De Gregorio M, Pipicella JL, Vande Casteele N, Andrews JM, Begun J, Connell W, D'Souza B, Gholamrezaei A, Hart A, Liew D, Radford-Smith G, Rimola J, Sutherland T, Toong C, Woods R, Wu Y, Xuan W, Williams AJ, Ng W, Ding NS, Connor S. Prospective randomised controlled trial of adults with perianal fistulising Crohn's disease and optimised therapeutic infliximab levels: PROACTIVE trial study protocol. BMJ Open. 2021;11:e043921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Hanna LN, Anandabaskaran S, Iqbal N, Geldof J, LeBlanc JF, Dige A, Lundby L, Vermeire S, D'Hoore A, Verstockt B, Bislenghi G, De Looze D, Lobaton T, Van de Putte D, Spinelli A, Carvello M, Danese S, Buskens CJ, Gecse K, Hompes R, Becker M, van der Bilt J, Bemelman W, Sebastian S, Moran G, Lightner AL, Wong SY, Colombel JF, Cohen BL, Holubar S, Ding NS, Behrenbruch C, Sahnan K, Misra R, Lung P, Hart A, Tozer P. Perianal Fistulizing Crohn's Disease: Utilizing the TOpClass Classification in Clinical Practice to Provide Targeted Individualized Care. Clin Gastroenterol Hepatol. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 17. | Jew M, Meserve J, Eisenstein S, Jairath V, McCurdy J, Singh S. Temporary Faecal Diversion for Refractory Perianal and/or Distal Colonic Crohn's Disease in the Biologic Era: An Updated Systematic Review with Meta-analysis. J Crohns Colitis. 2024;18:375-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Jiang J, Cazzetta SE, Athavale A, Kuharic M, Fan T, Silber A, Abilash V, Hadker N, Sharpe E, Nazarey PP. Observational Burden of Illness Study in Patients With Crohn's Disease With and Without Perianal Fistulas in the United States. Gastro Hep Adv. 2023;2:1066-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Kim PH, Park SH, Jin K, Ye BD, Yoon YS, Lee JS, Kim HJ, Kim AY, Yu CS, Yang SK. Supplementary Anal Imaging by Magnetic Resonance Enterography in Patients with Crohn's Disease Not Suspected of Having Perianal Fistulas. Clin Gastroenterol Hepatol. 2020;18:415-423.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Laukoetter MG, Mennigen R, Hannig CM, Osada N, Rijcken E, Vowinkel T, Krieglstein CF, Senninger N, Anthoni C, Bruewer M. Intestinal cancer risk in Crohn's disease: a meta-analysis. J Gastrointest Surg. 2011;15:576-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 21. | Mahadev S, Young JM, Selby W, Solomon MJ. Quality of life in perianal Crohn's disease: what do patients consider important? Dis Colon Rectum. 2011;54:579-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 22. | Mahadev S, Young JM, Selby W, Solomon MJ. Self-reported depressive symptoms and suicidal feelings in perianal Crohn's disease. Colorectal Dis. 2012;14:331-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Newman KL, Johnson LA, Stidham RW, Higgins PDR. Vedolizumab more likely to be discontinued than ustekinumab in anti-TNF-experienced patients with fistulizing Crohn's disease. Therap Adv Gastroenterol. 2023;16:17562848221148254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 24. | Panés J, García-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, Dignass A, Nachury M, Ferrante M, Kazemi-Shirazi L, Grimaud JC, de la Portilla F, Goldin E, Richard MP, Leselbaum A, Danese S; ADMIRE CD Study Group Collaborators. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn's disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016;388:1281-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 730] [Article Influence: 81.1] [Reference Citation Analysis (1)] |
| 25. | Panés J, Rimola J. Perianal fistulizing Crohn's disease: pathogenesis, diagnosis and therapy. Nat Rev Gastroenterol Hepatol. 2017;14:652-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 196] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 26. | Papamichael K, Vande Casteele N, Jeyarajah J, Jairath V, Osterman MT, Cheifetz AS. Higher Postinduction Infliximab Concentrations Are Associated With Improved Clinical Outcomes in Fistulizing Crohn's Disease: An ACCENT-II Post Hoc Analysis. Am J Gastroenterol. 2021;116:1007-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 27. | Parks AG, Gordon PH, Hardcastle JD. A classification of fistula-in-ano. Br J Surg. 1976;63:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1072] [Cited by in RCA: 925] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 28. | Plasencia A, Bahna H. Diverting Ostomy: For Whom, When, What, Where, and Why. Clin Colon Rectal Surg. 2019;32:171-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Meima-van Praag EM, Becker MAJ, van Rijn KL, Wasmann KATGM, Stoker J, D'Haens GRAM, Ponsioen CY, Gecse KB, Dijkgraaf MGW, Spinelli A, Danese S, Bemelman WA, Buskens CJ. Short-term anti-TNF therapy with surgical closure versus anti-TNF therapy alone for Crohn's perianal fistulas (PISA-II): long-term outcomes of an international, multicentre patient preference, randomised controlled trial. EClinicalMedicine. 2023;61:102045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 30. | Meima-van Praag EM, van Rijn KL, Wasmann KATGM, Snijder HJ, Stoker J, D'Haens GR, Gecse KB, Gerhards MF, Jansen JM, Dijkgraaf MGW, van der Bilt JDW, Mundt MW, Spinelli A, Danese S, Bemelman WA, Buskens CJ. Short-term anti-TNF therapy with surgical closure versus anti-TNF therapy in the treatment of perianal fistulas in Crohn's disease (PISA-II): a patient preference randomised trial. Lancet Gastroenterol Hepatol. 2022;7:617-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 31. | Sands BE, Anderson FH, Bernstein CN, Chey WY, Feagan BG, Fedorak RN, Kamm MA, Korzenik JR, Lashner BA, Onken JE, Rachmilewitz D, Rutgeerts P, Wild G, Wolf DC, Marsters PA, Travers SB, Blank MA, van Deventer SJ. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med. 2004;350:876-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1581] [Cited by in RCA: 1549] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 32. | Scharl M, Frei S, Pesch T, Kellermeier S, Arikkat J, Frei P, Fried M, Weber A, Jehle E, Rühl A, Rogler G. Interleukin-13 and transforming growth factor β synergise in the pathogenesis of human intestinal fistulae. Gut. 2013;62:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 33. | Schwartz DA, Peyrin-Biroulet L, Lasch K, Adsul S, Danese S. Efficacy and Safety of 2 Vedolizumab Intravenous Regimens for Perianal Fistulizing Crohn's Disease: ENTERPRISE Study. Clin Gastroenterol Hepatol. 2022;20:1059-1067.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 34. | Serclova Z, Garcia-olmo D, Chen ST, Wexner S, Panés J, Wu C, Fleshner P, Zhang B, Colombel JF, Song M, Mckay C, Nazarey P, Wright E, Raffals L. OP18 Efficacy and safety of darvadstrocel treatment in patients with complex perianal fistulas and Crohn’s Disease: results from the global ADMIRE-CD II phase 3 study. J Crohns Colitis. 2024;18:i34-i35. [DOI] [Full Text] |
| 35. | Shehab M, Alrashed F, Heron V, Restellini S, Bessissow T. Comparative Efficacy of Biologic Therapies for Inducing Response and Remission in Fistulizing Crohn's Disease: Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Inflamm Bowel Dis. 2023;29:367-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 36. | Singh S, Ding NS, Mathis KL, Dulai PS, Farrell AM, Pemberton JH, Hart AL, Sandborn WJ, Loftus EV Jr. Systematic review with meta-analysis: faecal diversion for management of perianal Crohn's disease. Aliment Pharmacol Ther. 2015;42:783-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 138] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 37. | Spinelli A, Yanai H, Girardi P, Milicevic S, Carvello M, Maroli A, Avedano L. The Impact of Crohn's Perianal Fistula on Quality of Life: Results of an International Patient Survey. Crohns Colitis 360. 2023;5:otad036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 38. | Swaminathan A, Fan D, Borichevsky GM, Mules TC, Hirschfeld E, Frampton CM, Day AS, Siegel CA, Gearry RB. The disease severity index for inflammatory bowel disease is associated with psychological symptoms and quality of life, and predicts a more complicated disease course. Aliment Pharmacol Ther. 2022;56:664-674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 39. | Swaminathan A, Fulforth JM, Frampton CM, Borichevsky GM, Mules TC, Kilpatrick K, Choukour M, Fields P, Ramkissoon R, Helms E, Hanauer SB, Leong RW, Peyrin-Biroulet L, Siegel CA, Gearry RB. The Disease Severity Index for Inflammatory Bowel Disease Is a Valid Instrument that Predicts Complicated Disease. Inflamm Bowel Dis. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 40. | Tarrant KM, Barclay ML, Frampton CM, Gearry RB. Perianal disease predicts changes in Crohn's disease phenotype-results of a population-based study of inflammatory bowel disease phenotype. Am J Gastroenterol. 2008;103:3082-3093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 184] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 41. | Tsai L, McCurdy JD, Ma C, Jairath V, Singh S. Epidemiology and Natural History of Perianal Crohn's Disease: A Systematic Review and Meta-Analysis of Population-Based Cohorts. Inflamm Bowel Dis. 2022;28:1477-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (1)] |
| 42. | Turner D, Ricciuto A, Lewis A, D'Amico F, Dhaliwal J, Griffiths AM, Bettenworth D, Sandborn WJ, Sands BE, Reinisch W, Schölmerich J, Bemelman W, Danese S, Mary JY, Rubin D, Colombel JF, Peyrin-Biroulet L, Dotan I, Abreu MT, Dignass A; International Organization for the Study of IBD. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology. 2021;160:1570-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 1595] [Article Influence: 398.8] [Reference Citation Analysis (1)] |
| 43. | Wasmann KA, de Groof EJ, Stellingwerf ME, D'Haens GR, Ponsioen CY, Gecse KB, Dijkgraaf MGW, Gerhards MF, Jansen JM, Pronk A, van Tuyl SAC, Zimmerman DDE, Bruin KF, Spinelli A, Danese S, van der Bilt JDW, Mundt MW, Bemelman WA, Buskens CJ. Treatment of Perianal Fistulas in Crohn's Disease, Seton Versus Anti-TNF Versus Surgical Closure Following Anti-TNF [PISA]: A Randomised Controlled Trial. J Crohns Colitis. 2020;14:1049-1056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 44. | Wong SY, Rowan C, Brockmans ED, Law CCY, Giselbrecht E, Ang C, Khaitov S, Sachar D, Polydorides AD, Winata LS, Verstockt B, Spinelli A, Rubin DT, Deepak P, McGovern DPB, McDonald BD, Lung P, Lundby L, Lightner AL, Holubar SD, Hanna L, Hamarth C, Geldof J, Dige A, Cohen BL, Carvello M, Bonifacio C, Bislenghi G, Behrenbruch C, Ballard DH, Altinmakas E, Sebastian S, Tozer P, Hart A, Colombel JF. Perianal Fistulizing Crohn's Disease-Associated Anorectal and Fistula Cancers: Systematic Review and Expert Consensus. Clin Gastroenterol Hepatol. 2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 45. | Yarur AJ, Kanagala V, Stein DJ, Czul F, Quintero MA, Agrawal D, Patel A, Best K, Fox C, Idstein K, Abreu MT. Higher infliximab trough levels are associated with perianal fistula healing in patients with Crohn's disease. Aliment Pharmacol Ther. 2017;45:933-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 231] [Article Influence: 28.9] [Reference Citation Analysis (1)] |
| 46. | Zhu P, Sun JF, Gu YF, Chen HJ, Xu MM, Li YR, Yang BL. Combined therapy with early initiation of infliximab following drainage of perianal fistulising Crohn's disease: a retrospective cohort study. BMC Gastroenterol. 2022;22:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |