Published online Oct 7, 2024. doi: 10.3748/wjg.v30.i37.4104
Revised: August 30, 2024
Accepted: September 13, 2024
Published online: October 7, 2024
Processing time: 121 Days and 0.3 Hours
Since the beginning of the coronavirus disease (COVID) 2019 pandemic, thou
Core Tip: More research is needed to address the specifics of a possible relationship between gut candidiasis and long coronavirus disease, in order to provide more robust knowledge on the topic, and consequently more appropriate management and treatment for affected patients. This, in turn, could lead to greater understanding and learning about the subject among all related healthcare professionals, with the potential to achieve better outcomes and improved quality of life and quality of care for the population.
- Citation: Bistagnino F, Pizzi D, Mantovani F, Antonino JR, Tovani-Palone MR. Long COVID and gut candidiasis: What is the existing relationship? World J Gastroenterol 2024; 30(37): 4104-4114
- URL: https://www.wjgnet.com/1007-9327/full/v30/i37/4104.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i37.4104
Long coronavirus disease (COVID) is a condition characterized by the emergence of new symptoms or the persistence of existing symptoms for at least two months, three months after the initial infection[1]. Although such a condition has initially been extensively studied, there are still many contradictions between the findings and methodologies of different related research articles[2,3]. Within this context and since the middle of the COVID-19 pandemic, important studies have been published in the literature reporting the occurrence of fungal infections among COVID-19 patients[4,5], including mucormycosis, and oral candidiasis[6]. On the other hand, evidence on a possible relationship between gut candidiasis and long COVID is still recent[7]. Indeed, a marked gastrointestinal (GI) fungal dysbiosis together with perturbation of the lung-gut axis has been observed in severe COVID-19 patients. This combined with neutrophilia and an exacerbated worsening of the inflammatory response, which can be implicated in the acute and chronic immunopathology of such a viral disease[7,8].
Furthermore, persistent changes in the immune system may also occur, resulting in a possible relationship with the occurrence of long COVID[7]. However, more targeted evidence is still scarce and the specific topic related to gut candidiasis has been the subject of little discussion. In response to this, in this article we discuss general aspects of long COVID, the inherent pathophysiology and current evidence of a potential relationship between this condition and gut candidiasis, in addition to providing recommendations for future research.
According to the literature, the term “long COVID” encompasses different nomenclatures, including chronic COVID-19 syndrome, late sequelae of COVID-19, long haul COVID, long-term COVID-19, post COVID syndrome, post-acute COVID-19, and post-acute sequelae of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Such a condition occurs in cases in which COVID-19 symptoms persist or emerge 3 months after the initial SARS-CoV-2 infection, lasting for at least 2 months without any other known cause[9-12].
In terms of epidemiology, the long COVID prevalence rates, as documented to date, have been variable between different countries. Although its real estimates are difficult to be appropriately measured due to different factors such as the attribution of different definitions for the condition and even variable follow-up times in the literature on the subject (especially before the introduction of the first 2021 World Health Organization definition of post-acute sequelae of SARS-CoV-2)[1,13], recent studies using standardized methodology confirm the existence of notable variations in the prevalence of long COVID between different regions of the world, reporting rates ranging from 51% in Asia, 44% in Europe, and 31% in North America[14].
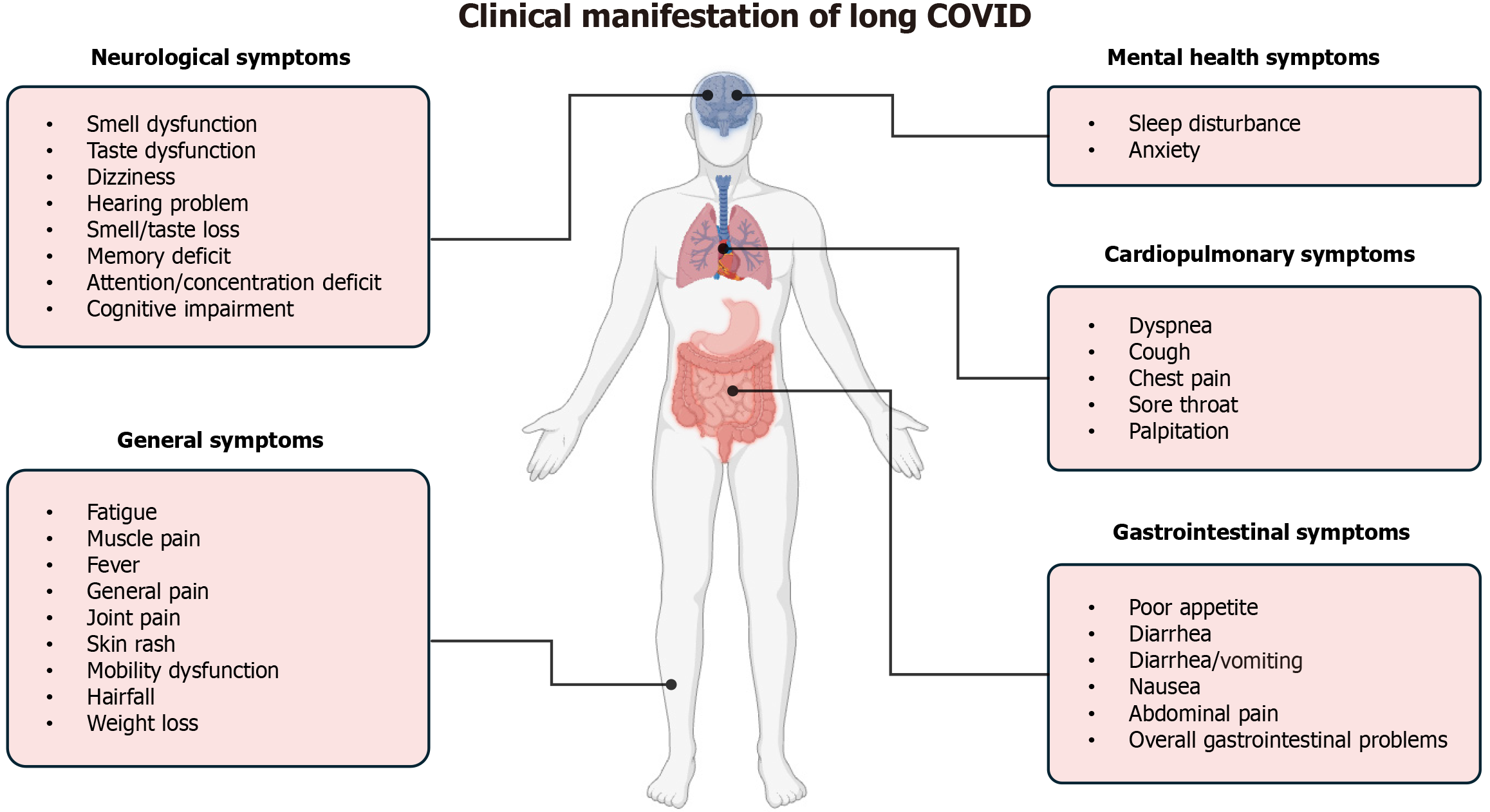
From this perspective, it is worth highlighting that the widespread prevalence of long COVID may also affect the burden and financial costs in healthcare systems. Indeed, the management of persistent symptoms involves substantial expenditures for patient care[15]. This, in turn, is related to the fact that different body systems can also be affected at this stage of the disease, including the respiratory, cardiovascular, neurological, GI, and musculoskeletal systems, each characterized by a wide range of clinical presentations, as represented in Figure 1[16-18]. Moreover, the prevalence of long COVID symptoms varies over different follow-up periods. In the first 6-9 months, symptoms such as cough, headache, loss of taste, and loss of smell are usually present, while after 12 months dyspnea, fatigue, myalgia, and sleep disorder are among the most reported symptoms[19].
An additional crucial point underscoring the severity of COVID-19 and the period following the initial infection concerns its link to a potential increased risk of also developing other systemic disorders and specific complications, including but not limited to acute myocardial infarction[20], diabetes[21], and arterial hypertension[22]. However, despite the significant heterogeneity of the clinical presentation of COVID-19 and related disorders, and the resulting diversity of treatment approaches[23], the majority of studies have focused on alleviating general symptoms and thus new and more standardized trials continue to be needed[24].
On the other hand, given the possible outcomes of the disease, recent evidence suggests that the administration of antiviral treatment may correlate with a decreased risk of developing long COVID and the need for hospitalization as well as in the occurrence of related deaths[25]. Even more impactful may be the adoption of broad preventive measures through vaccination against SARS-CoV-2 infection, which can undoubtedly lead to more favorable outcomes for both patients and healthcare systems compared to the exclusive use of medicines that only aim to reduce the severity of COVID-19 or long COVID[26,27]. Of great value in this context, this practice towards achieving satisfactory vaccination coverage rates should therefore continue to be encouraged by governments, health authorities and other key stakeholders around the world, in order to effectively mitigate both COVID-19 and its implications.
The several phenotypic manifestations of long COVID pose a significant challenge in the discovery of a univocal pathogenesis. In principle, the related symptoms have been attributed as resulting from organ damage in the acute phase of COVID-19; however, another plausible explanation is that certain precipitating factors may lead to a state of inflammation that gives rise to the symptoms seen in patients with long COVID[18]. Previous literature suggests that the potential mechanisms related to long COVID may be linked to a complex interplay of different pathophysiological factors[18,28,29].
A common link between these hypotheses seems to be the presence of elevated levels of interleukin (IL)-6 in affected patients. Yin et al[30] confirmed it in a cohort of long COVID patients, in which was found a significant increase in IL-6 levels compared to healthy individuals. A mean value of 20.92 pg/mL was observed for the pooled estimate in patients with long COVID, while the forest plot analysis revealed a significant difference of 9.75 pg/mL between the IL-6 levels in patients with long COVID and healthy individuals. Considering that IL-6 is a potent inflammatory cytokine, such findings provide an important basis for a better understanding of long COVID and other possible associated mechanisms[30]. Table 1 summarizes some of the relevant available evidence on the pathophysiology of long COVID published in the literature[18,28,31-41].
| Proposed etiology | Available evidence | Ref. |
| Immune dysregulation | The immune profiling of patients recovering from acute COVID-19 shows an up-regulation of immunological signaling molecules associated with inflammation, including ESR, CRP, TNF-α, IL-4, and IL-6 | Haunhorst et al[28] |
| Auto-immunity | Both latent autoimmunity and PolyA have been observed in a high percentage of patients (83% and 62% of cases, respectively). The delayed resolution resulting from low-grade inflammation is evidenced by elevated levels of IL-1β, IL-6, IL-8, and TNF-α. The detection of circulating ANA/ENAs further reinforces this hypothesis | Rojas et al[31], Son et al[32] |
| Viral antigen persistence | The SARS-CoV-2 NP has been detected in different organs and structures of the body of patients who recovered from COVID-19, such as the gallbladder, lymph nodes, colon, appendix, ileum, hemorrhoid, and liver. In some of these cases, the presence of the viral antigen was observed in all the tissues evaluated, which may therefore predispose to the occurrence of a possible widespread involvement of multiple organs and structures | Cheung et al[33] |
| Latent virus reactivation | Marked differences in viral reactivities against non-SARS-CoV-2 antigens have been detected in patients with long COVID. In this respect, different herpesvirus antigens, such as the EBV minor viral capsid antigen gp23, the EBV fusion-receptor component gp42, and the varicella zoster virus glycoprotein E can be found in elevated levels in these patients | Klein et al[34] |
| End-organ damage | The invasive potential of SARS-CoV-2 has been shown to cause damage to different organs. A study that included a sample of young adults, most of whom were free of risk factors for severe COVID-19, found that 66% of the subjects had at least one radiological abnormality in various organs, such as the lungs, liver, pancreas, among others, thus demonstrating the possibility of multisystemic involvement. Organ damage has also been reported in the nervous system (brainstem) | Yong[18], Dennis et al[35] |
| Endotheliopathy | The levels of different biomarkers of endothelial damage have been observed to be changed in patients with long COVID, including Ang-1, Ang-2, sP-selectin, sICAM-1, VEGF, and vWf, demonstrating an important correlation to the presence of related symptoms | Vassiliou et al[36] |
| Mitochondrial dysfunction | Increased levels of blood biomarkers together with mitochondrial damage and oxidative stress have been evidenced in cases of long COVID. For example, increased levels of F2-isoprostanes, malondialdehyde, with a respective reduction in the levels of antioxidants (coenzyme Q10). Results from genomic studies have provided additional evidence in this respect. Patients with COVID-19 may exhibit altered gene expression associated with both mitochondrial function and the cellular response to viral infections | Molnar et al[37] |
| Microbiome dysbiosis | In addition to reduced bacterial diversity, the SCFA-producing salutary commensal bacteria are also significantly reduced in patients with previous COVID-19 history. SCFAs play a key role in regulation of the immune system response | Zhang et al[38] |
| Metabolic dysregulation | Metabolomic and proteomic profiling of patients with long COVID can aid in determining whether the symptoms of long COVID may be attributed to metabolic dysregulation. In this connection, higher levels of lactate, pyruvate, and total triglycerides, and significantly lower Apo-A1 and A2 levels have been observed in patients with long COVID compared to healthy individuals | Berezhnoy et al[39] |
| Autonomic nervous system dysfunction | Different mechanisms, such as, direct tissue damage, immune dysregulation, hormonal disturbances, persistent low-grade infection, invasion of SARS-CoV-2 into the brain, and elevated levels of cytokines have been proposed as pathways with potential to lead to reduced levels of vagal activity in patients with COVID-19. Patients with long COVID presenting neurological symptoms have been shown to exhibit higher levels of NfL and glial fibrillary acidic protein, which may be related to persistent axonal damage in such patients | Giunta et al[40], Marchegiani et al[41] |
In addition to the propositions described above, in a recent systematic review published in 2024, Diar Bakerly et al[42] examined different proposed pathophysiological mechanisms for long COVID through a combination of the evidence found with known biological relationships. In this context, as most symptoms can be causally associated with multiple etiologies, comprehensive and personalized multidisciplinary care should be required, especially for more severe and long-lasting cases. On the other hand, the lack of a clear causal relationship between some symptoms and the proposed pathophysiological mechanisms still represents an important challenge for both appropriate patient care and understanding of the condition, thus leading to difficulties in accurate and early diagnosis, as well as in effective case management[42]. Notwithstanding the increasing efforts directed towards a better comprehension of the pathophysiology underlying long COVID, a general consensus on this subject has not yet been reached. In light of this, new and updated knowledge on the topic should be extremely important, aiming above all at more progress in both research and related care practice.
Among the various systems affected by COVID-19, the GI tract emerges as a significant site of involvement, with related symptoms showing a wide variation in prevalence among affected patients[43]. Such symptoms cover a broad spectrum, including but not limited to diarrhea, nausea, vomiting, and abdominal pain; however, with conflicting data on their relative frequency and relevance[44,45]. Notably, GI tract involvement has been shown to be a very relevant parameter in clinical diagnosis, since the presence of GI symptoms together with respiratory complaints is associated with a 70% increased likelihood of testing positive for SARS-CoV-2[46]. Furthermore, such symptoms may be correlated with increased risks of hospitalization and worse outcomes, thus highlighting their clinical importance[47,48].
A further important point in this regard is that the GI tract symptoms of COVID-19 are not only related to its acute phase, as they may persist in cases of long COVID[49,50] manifesting through different clinical presentations known among other names as post-infectious functional GI disorders (PI-FGID), which include both irritable bowel syndrome and dyspepsia[51]. PI-FGID commonly arises following acute GI infections, affecting approximately 1 in 10 adult patients[52]. Although the prevalence of these complications in adults after acute COVID-19 infection is potentially lower, given its primary respiratory nature, the scale of COVID-19 cases (estimated at 775 million globally) has implied an important impact in this respect. Therefore, even if slightly less than 10% of patients develop PI-FGIDs, the potential for an increased burden on healthcare systems, the economy, and society may be considerably significant[51]. On top of this, some studies suggest that such percentages may range between 10% and 25%[49], and other evidence indicates that these symptoms can persist for 2-3 years after SARS-CoV-2 infection in previously hospitalized COVID-19 survivors[48].
In an attempt to advance further, different mechanisms have been proposed to elucidate how long COVID may impact the GI tract, including angiotensin-converting enzyme II related pathways, prolonged GI inflammation, neurochemical alterations, abnormal intestinal mucosal permeability, and involvement of both central and peripheral nervous systems[50]. Interestingly, while SARS-CoV-2 can affect the GI tract via highly expressed angiotensin-converting enzyme II receptors[53,54], the persistence of the virus in cases of long COVID does not appear to induce increased inflammation or even direct damage[55,56], which however is not yet fully understood. In addition to this, most patients may clear SARS-CoV-2 from the GI tract within 6 months of initial infection. In this connection, it should not be expected that the occurrence of long COVID symptoms could be explained solely on the basis of persistence of the virus[49].
Instead, the prolonged GI symptoms could be attributed to dysbiosis in the resident microbiota, as a result of the inflammatory condition during the acute phase of COVID-19[57,58]. However, it is still unclear whether the prolonged dysbiosis observed in COVID-19 patients is directly caused by SARS-CoV-2 infection or whether it is a consequence of the prolonged use of antibiotics and/or corticosteroids by many of these patients, especially those with more severe symptoms. Indeed, it has been observed that the severity of GI symptoms due to COVID-19 may be more closely linked to the severity of the disease and, consequently, the burden of treatment, and not simply to the patient’s COVID-positive status[49,56,59].
Another potential explanation for the association between long COVID and GI dysbiosis may be the increased global prevalence of depression and anxiety following the pandemic[60]. It is well established in the literature that irritable bowel syndrome is related to mental health disorders[52,61,62], and additional evidence also suggests that the increased incidence of some FGIDs over time, including irritable bowel syndrome and functional dyspepsia, can be impacted by chronic stress and anxiety[63]. More specifically, feelings of sadness or anxiety after COVID-19, as well as pre-existing mental health symptoms may increase the risks of developing GI symptoms[49,64]. However, although the precise mechanism underlying the impact of long COVID on the GI tract remains unclear, the high prevalence of PI-FGID among COVID-19 patients underscores the urgent need to delve deeper into this relevant issue and subsequent complications.
Candida spp. are well-established as commensal microorganisms in the human GI tract[65]. Different studies report that systemic candidiasis often originates from Candida spp. dissemination from the GI tract[65-67]. Moreover, results of molecular typing demonstrate that such systemic infections are usually related to strains already resident in affected individuals[65,68]. Understanding the connection between gut colonization and systemic candidiasis is essential to achieving more targeted and effective advances in preventing the spread of the infection from the gut to the rest of the body. Due to the relevant significance of this relationship, it is crucial to explore the pathophysiology of gut candidiasis, the mechanisms leading to Candida overgrowth, as well as the associated clinical manifestations, in order to better identify the potential risk factors, signs and symptoms also in patients with long COVID.
The shift from a commensal microorganism to a pathogenic one is influenced by various factors, primarily including host-related aspects such as the digestive tract environment, intestinal mucosa integrity and permeability, genetics, overall health, the state of the microbiota (e.g., dysbiosis), in addition to the morphology of Candida albicans (for instance the transition from yeast to hyphal morphology)[66,69]. Well-known risk factors involved in Candida overgrowth include broad-spectrum antibiotic use, immune system suppression (e.g., in cases of human immunodeficiency virus infection, chemotherapy, and use of corticosteroids), changes in the gut pH and nutrient availability (e.g., due to use of proton pump inhibitors; consumption of high-sugar diet), mucosal barrier disruption (e.g., in cases of inflammatory bowel diseases), hyphal transformation and biofilm formation[66,69,70]. Some of these risk factors and their associated manifes
The clinical manifestation of C. albicans colonization is very heterogeneous and aspecific, which can often make it difficult to identify such a relationship. Among the possible clinical features are the occurrence of oral, esophageal, gastric, and intestinal candidiasis, in addition to other GI manifestations such as, gastric and intestinal ulceration, GI bleeding, and cases of diarrhea and constipation. Other related important features include abdominal pain and peritonitis. Furthermore, perianal itch, napkin dermatitis, and other relevant disorders, such as chronic “irritable bowel” syndrome, and auto-brewery syndrome, can also be observed in affected patients[70,74]. In light of the mechanisms mentioned in this section of the manuscript and of the predisposing factors underlying the development of GI candidiasis, it is possible to highlight a potential existence of important relationships with long COVID. In continuation of this discussion, we address below further insights into the evidence between long COVID and GI candidiasis.
After a careful search of the PubMed database combining the following terms “long COVID” and “candidiasis” with the Boolean operator AND, the scarcity of specialized literature on the subject becomes clear. This is in contrast to the availability of literature on acute COVID-19 and candidiasis, especially when considering the more severe cases[75,76].
Within the limited literature on the topic mentioned above, Kusakabe et al[7] published one study with highly relevant findings. In their important research, the authors found evidence of a potential relationship between the occurrence of gut candidiasis and long COVID from the activation of neutrophils and their progenitors involving severe COVID-19 cases. Such findings reveal the possibility of persistence for a prolonged period of elevated levels of anti-fungal antibodies–C. albicans immunoglobulin G-derived from severe COVID-19 cases along with the activation of antifungal immune path
In addition and not least within this discussion, the study conducted by Johansson et al[78] investigated the general and oral symptoms of acute and long COVID in 80- and 90-year-old Swedish COVID-19 survivors. In this connection, the authors highlighted within their discussion the possibility of an interplay with candidiasis, including oral candidiasis and COVID-19 with the potential to increase negative outcomes[78]. It is worth noting here that the systemic immune dysfunction resulting from COVID-19, which is related to gut dysbiosis, may also contribute to the exacerbation of oral candidiasis. This in turn suggests a predisposition to systemic implications, in addition to the exacerbation of Candida spp. promoted by SARS-CoV-2 infected salivary glands[79], both in children and adults, which could be even more intense in severe COVID-19 cases[80]. A further important point is that a growing body of evidence suggests that different factors such as dysbiosis, damage to the gut barrier, and immune dysfunction can lead to disseminated C. albicans infection, which may occur especially via the GI tract[66]. Based on this, one could hypothesize the potential involvement of more severe cases of long COVID in this occurrence.
While long COVID can develop following mild, moderate, or severe acute SARS-CoV-2 infection, post-COVID symptoms are especially common in severe cases and those requiring intensive care unit admission. Consequently, it is crucial to investigate the relationship between COVID-19 treatment in severe cases and the proliferation of Candida[81,82]. In light of this, and complementary to what has been previously discussed, SARS-CoV-2 infection is not the only agent capable of creating a favorable environment for Candida spp. proliferation in the GI tract. Some of the medicines that have been administered to patients with COVID-19 also represent an important concern in this regard, especially antibiotics and corticosteroids[83,84]. Their use has also been associated with the occurrence of post-COVID symptoms[85]. Different studies have highlighted the relationship between the incidence of digestive tract candidiasis and the use of antibiotics. Esophageal candidiasis tends to occur in patients with chronic diseases who have been treated with antibiotics[86], and a high prevalence of this type of fungal infection can be observed in children undergoing antibiotic treatment[87].
Another study involving neonates and conducted by Aliaga et al[88] demonstrated a reduced incidence of invasive candidiasis associated with decreased use of broad-spectrum antibacterial antibiotics. Furthermore, a murine model of candidiasis treated with tetracycline and prednisolone showed how the concomitant effect of C. albicans and antibiotics can lead to destruction of the mucous membrane, thus allowing the passage of the fungus across the mucosa and consequently into the systemic circulation[89]. In light of these findings, antibiotics may be a risk factor for the development of gut candidiasis, as well as for its dissemination and the possibility of developing a systemic fungal infection.
A further point of concern in this regard is the use of long-term steroids. It is known that their use, especially in the acute setting of COVID-19, is recommended in hospitalized patients requiring oxygen therapy in order to reduce the systemic inflammatory response[83]. However, in the case of long COVID this question still remains controversial with different studies suggesting eventual benefits of their administration to manage the condition in certain situations, but with important reservations, whether it is about the occurrence of side effects[90] or even the need for more studies to confirm the related findings[91]. Considering this, it is worth mentioning that there is robust evidence demonstrating the role of corticosteroids, both in the promotion of GI candidiasis and its translocation from the bloodstream[92,93].
Different cases are reported in the literature highlighting the occurrence of Candida infection in individuals who recently recovered from COVID-19 and underwent antibiotic and/or corticosteroids therapy. Among the 4 cases cited below, 3 were affected by severe COVID-19, in line with the previously mentioned reasoning. Candida infection in these cases can affect different regions of the body, including the eyes, oral cavity, central nervous system and often following a candidemia episode[94-96]. This, together with the fact that the GI tract can be considered as one of the main sources of Candida spp.[66], should raise the concern that at least part of the related manifestations is the result of a previous GI spread of fungi. As a result, further research and investigation aimed at early identification of susceptible patients may represent a key step towards preventing potentially life-threatening scenarios.
In line with this premise, one of the relevant related articles is a case reported by Gautam et al[97] describing a patient who was being treated with corticosteroids to mitigate lung fibrosis after severe COVID-19 infection. In this context of immune dysregulation due to a secondary hemophagocytic lymphohistiocytosis in association with a post-COVID-19 status, the use of corticosteroid treatment may also have facilitated the development of invasive candidiasis. From the evidence presented and in reaction to all the above in this section, it should also be a priority that additional and new investigations focus on studying the GI tract as a possible cluster for the spread of Candida spp. This in turn should be linked to the aim of understanding in more detail how to prevent the set of related changes, which may be elementary for reducing the burden of possible disseminated and invasive forms of fungal involvement in patients with long COVID. Although there are already relevant studies that suggest that the GI tract may in fact be the source of candidemia[98], the establishment of new insights concerning the topic and more specifically related to cases of long COVID is essential and key for more effective advances towards appropriate management of affected patients.
Continuing the previous reasoning, even if new and continued advances in long COVID research are ongoing, appropriate addressing of more specific topics possibly related to this condition, such as gut candidiasis, still needs further investigation. With this and other related needs in mind, we have listed some topics that require priority for further research in this field. Table 2 describes our main propositions.
| Proposals for future research involving |
| Studies on the burden of gut candidiasis in patients with long COVID |
| More in-depth experimental investigations into the related pathophysiological mechanisms |
| Randomized clinical studies aimed at evaluating potential new therapies |
| Microbiological investigations to determine with greater predictability the predominant resident microbiota in such conditions |
| Studies to establish the average duration of gut candidiasis related to long COVID |
| Impact of using new technologies in case management |
In order to achieve a better and broader understanding of the pathophysiological mechanisms related to long COVID and its potential systemic and local associations, further research is needed as discussed in the present manuscript. In this connection and based on a clinical perspective, the likely persistence and similarity of predisposing factors for gut candidiasis in patients with long COVID compared to the acute phase of COVID-19, including GI dysbiosis, immune dysfunction, and altered permeability of the intestinal mucosa[66,79], should not be neglected during case evaluation. This outcome may also be impacted by the already often vulnerable conditions of many of the patients as a result of the prolonged use of antibiotics and/or corticosteroids for other purposes, which may have the potential to contribute to further affecting the diversity of the resident microbiota[99].
Finally, in addition to providing a greater knowledge of long COVID and its complications, new related insights can also serve as a basis for the development of future treatment protocols and therapies for this condition. Not least, considering the lack of appropriate surveillance of cases of long COVID around the world, as well as its outcomes[100], knowing more about the associated signs and symptoms should play a key role in improving health planning and care as well as in developing more and better targeted research in the area. Indeed, the well-documented underreporting of cases of long COVID[100], coupled with the possibility of non-specific clinical presentation of GI candidiasis[101], raises additional concerns about the related burden that imminently continues to require further action.
Tovani-Palone MR thanks the Saveetha Institute of Medical and Technical Sciences for supporting this study.
| 1. | World Health Organization. A clinical case definition of post COVID-19 condition by a Delphi consensus. [cited 27 May 2024]. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1. |
| 2. | Carod-Artal FJ. Post-COVID-19 syndrome: epidemiology, diagnostic criteria and pathogenic mechanisms involved. Rev Neurol. 2021;72:384-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 3. | Nalbandian A, Desai AD, Wan EY. Post-COVID-19 Condition. Annu Rev Med. 2023;74:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 87] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 4. | Kozlova O, Burygina E, Khostelidi S, Shadrivova O, Saturnov A, Gusev D, Rysev A, Zavrazhnov A, Vashukova M, Pichugina G, Mitichkin M, Kovyrshin S, Bogomolova T, Borzova Y, Oganesyan E, Vasilyeva N, Klimko N; Working Group. Invasive Candidiasis in Adult Patients with COVID-19: Results of a Multicenter Study in St. Petersburg, Russia. J Fungi (Basel). 2023;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 5. | Adzic-Vukicevic T, Velickovic J, Radovanovic-Spurnic A, Velickovic D, Milenkovic S, Petrovic F, Micic J, Dragutinovic N. Fatal invasive candidiasis in COVID-19 patient with severe bleeding and extensively drug-resistant Klebsiella enterobacter. J Infect Dev Ctries. 2022;16:1025-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Nambiar M, Varma SR, Jaber M, Sreelatha SV, Thomas B, Nair AS. Mycotic infections-mucormycosis and oral candidiasis associated with Covid-19: a significant and challenging association. J Oral Microbiol. 2021;13:1967699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Kusakabe T, Lin WY, Cheong JG, Singh G, Ravishankar A, Yeung ST, Mesko M, DeCelie MB, Carriche G, Zhao Z, Rand S, Doron I, Putzel GG, Worgall S, Cushing M, Westblade L, Inghirami G, Parkhurst CN, Guo CJ, Schotsaert M, García-Sastre A, Josefowicz SZ, Salvatore M, Iliev ID. Fungal microbiota sustains lasting immune activation of neutrophils and their progenitors in severe COVID-19. Nat Immunol. 2023;24:1879-1889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 49] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 8. | Aishwarya S, Gunasekaran K. Meta-analysis of the microbial biomarkers in the gut-lung crosstalk in COVID-19, community-acquired pneumonia and Clostridium difficile infections. Lett Appl Microbiol. 2022;75:1293-1306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 9. | World Health Organization. Post COVID-19 condition (Long COVID). [cited 27 May 2024]. Available from: https://www.who.int/europe/news-room/fact-sheets/item/post-COVID-19-condition. |
| 10. | Raveendran AV, Jayadevan R, Sashidharan S. Long COVID: An overview. Diabetes Metab Syndr. 2021;15:869-875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 427] [Cited by in RCA: 539] [Article Influence: 134.8] [Reference Citation Analysis (0)] |
| 11. | Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV; WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102-e107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 1459] [Article Influence: 486.3] [Reference Citation Analysis (0)] |
| 12. | COVID-19 rapid guideline: managing the long-term effects of COVID-19. London: National Institute for Health and Care Excellence (NICE); 2020-Dec-18 . [PubMed] |
| 13. | Lippi G, Sanchis-Gomar F, Henry BM. COVID-19 and its long-term sequelae: what do we know in 2023? Pol Arch Intern Med. 2023;133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 59] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 14. | Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global Prevalence of Post-Coronavirus Disease 2019 (COVID-19) Condition or Long COVID: A Meta-Analysis and Systematic Review. J Infect Dis. 2022;226:1593-1607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 791] [Article Influence: 263.7] [Reference Citation Analysis (0)] |
| 15. | Rizvi A, Ziv Y, Crawford JM, Trindade AJ. Gastrointestinal and Hepatobiliary Symptoms and Disorders with Long (Chronic) COVID Infection. Gastroenterol Clin North Am. 2023;52:139-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 16. | Natarajan A, Shetty A, Delanerolle G, Zeng Y, Zhang Y, Raymont V, Rathod S, Halabi S, Elliot K, Shi JQ, Phiri P. A systematic review and meta-analysis of long COVID symptoms. Syst Rev. 2023;12:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 87] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 17. | Su S, Zhao Y, Zeng N, Liu X, Zheng Y, Sun J, Zhong Y, Wu S, Ni S, Gong Y, Zhang Z, Gao N, Yuan K, Yan W, Shi L, Ravindran AV, Kosten T, Shi J, Bao Y, Lu L. Epidemiology, clinical presentation, pathophysiology, and management of long COVID: an update. Mol Psychiatry. 2023;28:4056-4069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 47] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 18. | Yong SJ. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis (Lond). 2021;53:737-754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 541] [Cited by in RCA: 737] [Article Influence: 184.3] [Reference Citation Analysis (0)] |
| 19. | Alkodaymi MS, Omrani OA, Fawzy NA, Shaar BA, Almamlouk R, Riaz M, Obeidat M, Obeidat Y, Gerberi D, Taha RM, Kashour Z, Kashour T, Berbari EF, Alkattan K, Tleyjeh IM. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: a systematic review and meta-analysis. Clin Microbiol Infect. 2022;28:657-666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 316] [Article Influence: 105.3] [Reference Citation Analysis (0)] |
| 20. | Zuin M, Rigatelli G, Battisti V, Costola G, Roncon L, Bilato C. Increased risk of acute myocardial infarction after COVID-19 recovery: A systematic review and meta-analysis. Int J Cardiol. 2023;372:138-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 41] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 21. | Lai H, Yang M, Sun M, Pan B, Wang Q, Wang J, Tian J, Ding G, Yang K, Song X, Ge L. Risk of incident diabetes after COVID-19 infection: A systematic review and meta-analysis. Metabolism. 2022;137:155330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 22. | Zuin M, Rigatelli G, Bilato C, Pasquetto G, Mazza A. Risk of Incident New-Onset Arterial Hypertension After COVID-19 Recovery: A Systematic Review and Meta-analysis. High Blood Press Cardiovasc Prev. 2023;30:227-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Chee YJ, Fan BE, Young BE, Dalan R, Lye DC. Clinical trials on the pharmacological treatment of long COVID: A systematic review. J Med Virol. 2023;95:e28289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 24. | Fawzy NA, Abou Shaar B, Taha RM, Arabi TZ, Sabbah BN, Alkodaymi MS, Omrani OA, Makhzoum T, Almahfoudh NE, Al-Hammad QA, Hejazi W, Obeidat Y, Osman N, Al-Kattan KM, Berbari EF, Tleyjeh IM. A systematic review of trials currently investigating therapeutic modalities for post-acute COVID-19 syndrome and registered on WHO International Clinical Trials Platform. Clin Microbiol Infect. 2023;29:570-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 25. | Choi YJ, Seo YB, Seo JW, Lee J, Nham E, Seong H, Yoon JG, Noh JY, Cheong HJ, Kim WJ, Kim EJ, Song JY. Effectiveness of Antiviral Therapy on Long COVID: A Systematic Review and Meta-Analysis. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 26. | Ceban F, Kulzhabayeva D, Rodrigues NB, Di Vincenzo JD, Gill H, Subramaniapillai M, Lui LMW, Cao B, Mansur RB, Ho RC, Burke MJ, Rhee TG, Rosenblat JD, McIntyre RS. COVID-19 vaccination for the prevention and treatment of long COVID: A systematic review and meta-analysis. Brain Behav Immun. 2023;111:211-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 74] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 27. | Gao P, Liu J, Liu M. Effect of COVID-19 Vaccines on Reducing the Risk of Long COVID in the Real World: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 120] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 28. | Haunhorst S, Bloch W, Wagner H, Ellert C, Krüger K, Vilser DC, Finke K, Reuken P, Pletz MW, Stallmach A, Puta C. Long COVID: a narrative review of the clinical aftermaths of COVID-19 with a focus on the putative pathophysiology and aspects of physical activity. Oxf Open Immunol. 2022;3:iqac006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 29. | Altmann DM, Whettlock EM, Liu S, Arachchillage DJ, Boyton RJ. The immunology of long COVID. Nat Rev Immunol. 2023;23:618-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 203] [Article Influence: 101.5] [Reference Citation Analysis (0)] |
| 30. | Yin JX, Agbana YL, Sun ZS, Fei SW, Zhao HQ, Zhou XN, Chen JH, Kassegne K. Increased interleukin-6 is associated with long COVID-19: a systematic review and meta-analysis. Infect Dis Poverty. 2023;12:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 64] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 31. | Rojas M, Rodríguez Y, Acosta-Ampudia Y, Monsalve DM, Zhu C, Li QZ, Ramírez-Santana C, Anaya JM. Autoimmunity is a hallmark of post-COVID syndrome. J Transl Med. 2022;20:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 104] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 32. | Son K, Jamil R, Chowdhury A, Mukherjee M, Venegas C, Miyasaki K, Zhang K, Patel Z, Salter B, Yuen ACY, Lau KS, Cowbrough B, Radford K, Huang C, Kjarsgaard M, Dvorkin-Gheva A, Smith J, Li QZ, Waserman S, Ryerson CJ, Nair P, Ho T, Balakrishnan N, Nazy I, Bowdish DME, Svenningsen S, Carlsten C, Mukherjee M. Circulating anti-nuclear autoantibodies in COVID-19 survivors predict long COVID symptoms. Eur Respir J. 2023;61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 84] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 33. | Cheung CCL, Goh D, Lim X, Tien TZ, Lim JCT, Lee JN, Tan B, Tay ZEA, Wan WY, Chen EX, Nerurkar SN, Loong S, Cheow PC, Chan CY, Koh YX, Tan TT, Kalimuddin S, Tai WMD, Ng JL, Low JG, Yeong J, Lim KH. Residual SARS-CoV-2 viral antigens detected in GI and hepatic tissues from five recovered patients with COVID-19. Gut. 2022;71:226-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 129] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 34. | Klein J, Wood J, Jaycox JR, Dhodapkar RM, Lu P, Gehlhausen JR, Tabachnikova A, Greene K, Tabacof L, Malik AA, Silva Monteiro V, Silva J, Kamath K, Zhang M, Dhal A, Ott IM, Valle G, Peña-Hernández M, Mao T, Bhattacharjee B, Takahashi T, Lucas C, Song E, McCarthy D, Breyman E, Tosto-Mancuso J, Dai Y, Perotti E, Akduman K, Tzeng TJ, Xu L, Geraghty AC, Monje M, Yildirim I, Shon J, Medzhitov R, Lutchmansingh D, Possick JD, Kaminski N, Omer SB, Krumholz HM, Guan L, Dela Cruz CS, van Dijk D, Ring AM, Putrino D, Iwasaki A. Distinguishing features of long COVID identified through immune profiling. Nature. 2023;623:139-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 300] [Article Influence: 150.0] [Reference Citation Analysis (0)] |
| 35. | Dennis A, Wamil M, Alberts J, Oben J, Cuthbertson DJ, Wootton D, Crooks M, Gabbay M, Brady M, Hishmeh L, Attree E, Heightman M, Banerjee R, Banerjee A; COVERSCAN study investigators. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021;11:e048391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 270] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 36. | Vassiliou AG, Vrettou CS, Keskinidou C, Dimopoulou I, Kotanidou A, Orfanos SE. Endotheliopathy in Acute COVID-19 and Long COVID. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 37. | Molnar T, Lehoczki A, Fekete M, Varnai R, Zavori L, Erdo-Bonyar S, Simon D, Berki T, Csecsei P, Ezer E. Mitochondrial dysfunction in long COVID: mechanisms, consequences, and potential therapeutic approaches. Geroscience. 2024;46:5267-5286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 36] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 38. | Zhang D, Zhou Y, Ma Y, Chen P, Tang J, Yang B, Li H, Liang M, Xue Y, Liu Y, Zhang J, Wang X. Gut Microbiota Dysbiosis Correlates With Long COVID-19 at One-Year After Discharge. J Korean Med Sci. 2023;38:e120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 49] [Reference Citation Analysis (0)] |
| 39. | Berezhnoy G, Bissinger R, Liu A, Cannet C, Schäfer H, Kienzle K, Bitzer M, Häberle H, Göpel S, Trautwein C, Singh Y. Maintained imbalance of triglycerides, apolipoproteins, energy metabolites and cytokines in long-term COVID-19 syndrome patients. Front Immunol. 2023;14:1144224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 40. | Giunta S, Giordani C, De Luca M, Olivieri F. Long-COVID-19 autonomic dysfunction: An integrated view in the framework of inflammaging. Mech Ageing Dev. 2024;218:111915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 41. | Marchegiani F, Recchioni R, Marcheselli F, Di Rosa M, Sabbatinelli J, Matacchione G, Giuliani A, Ramini D, Stripoli P, Biscetti L, Pelliccioni G, Sarzani R, Spannella F, Cherubini A, Corsonello A, Procopio AD, Bonfigli AR, Bonafè M, Lattanzio F, Olivieri F. Association of admission serum levels of neurofilament light chain and in-hospital mortality in geriatric patients with COVID-19. J Neurol. 2023;270:37-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 42. | Diar Bakerly N, Smith N, Darbyshire JL, Kwon J, Bullock E, Baley S, Sivan M, Delaney B. Pathophysiological Mechanisms in Long COVID: A Mixed Method Systematic Review. Int J Environ Res Public Health. 2024;21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 43. | Wong SH, Lui RN, Sung JJ. Covid-19 and the digestive system. J Gastroenterol Hepatol. 2020;35:744-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 445] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 44. | Dong ZY, Xiang BJ, Jiang M, Sun MJ, Dai C. The Prevalence of Gastrointestinal Symptoms, Abnormal Liver Function, Digestive System Disease and Liver Disease in COVID-19 Infection: A Systematic Review and Meta-Analysis. J Clin Gastroenterol. 2021;55:67-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 45. | Wang JG, Cui HR, Tang HB, Deng XL. Gastrointestinal symptoms and fecal nucleic acid testing of children with 2019 coronavirus disease: a systematic review and meta-analysis. Sci Rep. 2020;10:17846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 46. | Nobel YR, Phipps M, Zucker J, Lebwohl B, Wang TC, Sobieszczyk ME, Freedberg DE. Gastrointestinal Symptoms and Coronavirus Disease 2019: A Case-Control Study From the United States. Gastroenterology. 2020;159:373-375.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 265] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 47. | Cholankeril G, Podboy A, Aivaliotis VI, Pham EA, Spencer SP, Kim D, Ahmed A. Association of Digestive Symptoms and Hospitalization in Patients With SARS-CoV-2 Infection. Am J Gastroenterol. 2020;115:1129-1132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 48. | Fernández-de-Las-Peñas C, Martín-Guerrero JD, Navarro-Pardo E, Torres-Macho J, Guijarro C, Pellicer-Valero OJ. Exploring the recovery curve for gastrointestinal symptoms from the acute COVID-19 phase to long-term post-COVID: The LONG-COVID-EXP-CM Multicenter Study. J Med Virol. 2022;94:2925-2927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 49. | Freedberg DE, Chang L. Gastrointestinal symptoms in COVID-19: the long and the short of it. Curr Opin Gastroenterol. 2022;38:555-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 50. | Ghoshal UC, Ghoshal U. Gastrointestinal involvement in post-acute Coronavirus disease (COVID)-19 syndrome. Curr Opin Infect Dis. 2023;36:366-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 51. | Siah KTH, Mahadeva S. Post-COVID-19 functional gastrointestinal disorders: Prepare for a GI aftershock. J Gastroenterol Hepatol. 2022;37:413-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Barbara G, Grover M, Bercik P, Corsetti M, Ghoshal UC, Ohman L, Rajilić-Stojanović M. Rome Foundation Working Team Report on Post-Infection Irritable Bowel Syndrome. Gastroenterology. 2019;156:46-58.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 164] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 53. | Qian Q, Fan L, Liu W, Li J, Yue J, Wang M, Ke X, Yin Y, Chen Q, Jiang C. Direct Evidence of Active SARS-CoV-2 Replication in the Intestine. Clin Infect Dis. 2021;73:361-366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 119] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 54. | Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3643] [Cited by in RCA: 4149] [Article Influence: 197.6] [Reference Citation Analysis (0)] |
| 55. | Stein SR, Ramelli SC, Grazioli A, Chung JY, Singh M, Yinda CK, Winkler CW, Sun J, Dickey JM, Ylaya K, Ko SH, Platt AP, Burbelo PD, Quezado M, Pittaluga S, Purcell M, Munster VJ, Belinky F, Ramos-Benitez MJ, Boritz EA, Lach IA, Herr DL, Rabin J, Saharia KK, Madathil RJ, Tabatabai A, Soherwardi S, McCurdy MT; NIH COVID-19 Autopsy Consortium, Peterson KE, Cohen JI, de Wit E, Vannella KM, Hewitt SM, Kleiner DE, Chertow DS. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature. 2022;612:758-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 531] [Article Influence: 177.0] [Reference Citation Analysis (0)] |
| 56. | Britton GJ, Chen-Liaw A, Cossarini F, Livanos AE, Spindler MP, Plitt T, Eggers J, Mogno I, Gonzalez-Reiche AS, Siu S, Tankelevich M, Grinspan LT, Dixon RE, Jha D, van de Guchte A, Khan Z, Martinez-Delgado G, Amanat F, Hoagland DA, tenOever BR, Dubinsky MC, Merad M, van Bakel H, Krammer F, Bongers G, Mehandru S, Faith JJ. Limited intestinal inflammation despite diarrhea, fecal viral RNA and SARS-CoV-2-specific IgA in patients with acute COVID-19. Sci Rep. 2021;11:13308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 57. | Silva Andrade B, Siqueira S, de Assis Soares WR, de Souza Rangel F, Santos NO, Dos Santos Freitas A, Ribeiro da Silveira P, Tiwari S, Alzahrani KJ, Góes-Neto A, Azevedo V, Ghosh P, Barh D. Long-COVID and Post-COVID Health Complications: An Up-to-Date Review on Clinical Conditions and Their Possible Molecular Mechanisms. Viruses. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 232] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 58. | Ye Q, Wang B, Zhang T, Xu J, Shang S. The mechanism and treatment of gastrointestinal symptoms in patients with COVID-19. Am J Physiol Gastrointest Liver Physiol. 2020;319:G245-G252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (2)] |
| 59. | Xu X, Zhang W, Guo M, Xiao C, Fu Z, Yu S, Jiang L, Wang S, Ling Y, Liu F, Tan Y, Chen S. Integrated analysis of gut microbiome and host immune responses in COVID-19. Front Med. 2022;16:263-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 60. | COVID-19 Mental Disorders Collaborators. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 2021;398:1700-1712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2518] [Cited by in RCA: 2707] [Article Influence: 676.8] [Reference Citation Analysis (0)] |
| 61. | Parker CH, Naliboff BD, Shih W, Presson AP, Videlock EJ, Mayer EA, Chang L. Negative Events During Adulthood Are Associated With Symptom Severity and Altered Stress Response in Patients With Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2019;17:2245-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 62. | Luscombe FA. Health-related quality of life and associated psychosocial factors in irritable bowel syndrome: a review. Qual Life Res. 2000;9:161-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 63. | Almario CV, Makaroff K, Alvarez G, Chey WD, Spiegel B. S496 Examining the Impact of the COVID-19 Pandemic on the Prevalence of Rome IV Functional Gastrointestinal Disorders. Am J Gastroenterol. 2021;116:S220-S221. [DOI] [Full Text] |
| 64. | Blackett JW, Wainberg M, Elkind MSV, Freedberg DE. Potential Long Coronavirus Disease 2019 Gastrointestinal Symptoms 6 Months After Coronavirus Infection Are Associated With Mental Health Symptoms. Gastroenterology. 2022;162:648-650.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 65. | Poulain D. Candida albicans, plasticity and pathogenesis. Crit Rev Microbiol. 2015;41:208-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 66. | Basmaciyan L, Bon F, Paradis T, Lapaquette P, Dalle F. "Candida Albicans Interactions With The Host: Crossing The Intestinal Epithelial Barrier". Tissue Barriers. 2019;7:1612661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 67. | Jawhara S. How Gut Bacterial Dysbiosis Can Promote Candida albicans Overgrowth during Colonic Inflammation. Microorganisms. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 68. | Voss A, Hollis RJ, Pfaller MA, Wenzel RP, Doebbeling BN. Investigation of the sequence of colonization and candidemia in nonneutropenic patients. J Clin Microbiol. 1994;32:975-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 153] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 69. | Yan L, Yang C, Tang J. Disruption of the intestinal mucosal barrier in Candida albicans infections. Microbiol Res. 2013;168:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 70. | Kennedy MJ. Regulation of Candida albicans populations in the gastrointestinal tract: mechanisms and significance in GI and systemic candidiasis. Curr Top Med Mycol. 1989;3:315-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 71. | Corey L, Beyrer C, Cohen MS, Michael NL, Bedford T, Rolland M. SARS-CoV-2 Variants in Patients with Immunosuppression. N Engl J Med. 2021;385:562-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 325] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 72. | Zhang F, Lau RI, Liu Q, Su Q, Chan FKL, Ng SC. Gut microbiota in COVID-19: key microbial changes, potential mechanisms and clinical applications. Nat Rev Gastroenterol Hepatol. 2023;20:323-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 141] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 73. | Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1688] [Cited by in RCA: 2167] [Article Influence: 1083.5] [Reference Citation Analysis (0)] |
| 74. | Cater RE 2nd. Chronic intestinal candidiasis as a possible etiological factor in the chronic fatigue syndrome. Med Hypotheses. 1995;44:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 75. | Segrelles-Calvo G, de S Araújo GR, Llopis-Pastor E, Carrillo J, Hernández-Hernández M, Rey L, Melean NR, Escribano I, Antón E, Zamarro C, García-Salmones M, Frases S. Candida spp. co-infection in COVID-19 patients with severe pneumonia: Prevalence study and associated risk factors. Respir Med. 2021;188:106619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 76. | Altinkaya Çavuş M, Sav H. Opportunistic Candida Infections in Critical COVID-19 Patients. Pol J Microbiol. 2022;71:411-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 77. | Zuo T, Zhan H, Zhang F, Liu Q, Tso EYK, Lui GCY, Chen N, Li A, Lu W, Chan FKL, Chan PKS, Ng SC. Alterations in Fecal Fungal Microbiome of Patients With COVID-19 During Time of Hospitalization until Discharge. Gastroenterology. 2020;159:1302-1310.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 251] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 78. | Johansson AK, Omar R, Lehmann S, Sannevik J, Mastrovito B, Johansson A. General and orofacial symptoms associated with acute and long COVID in 80- and 90-year-old Swedish COVID-19 survivors. J Dent. 2024;141:104824. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 79. | Kordalewska M, Perlin DS. Candida in COVID-19: Gut-Lung Axis, Dysbiosis, and Infections. Curr Fungal Infect Rep. 2023;17:263-280. [DOI] [Full Text] |
| 80. | Pisano M, Romano A, Di Palo MP, Baroni A, Serpico R, Contaldo M. Oral Candidiasis in Adult and Pediatric Patients with COVID-19. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 81. | Niebauer JH, Binder-Rodriguez C, Iscel A, Schedl S, Capelle C, Kahr M, Cadjo S, Schamilow S, Badr-Eslam R, Lichtenauer M, Toma A, Zoufaly A, Valenta R, Hoffmann S, Charwat-Resl S, Krestan C, Hitzl W, Wenisch C, Bonderman D. Cardiopulmonary Long-Term Sequelae in Patients after Severe COVID-19 Disease. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 82. | Blomberg B, Mohn KG, Brokstad KA, Zhou F, Linchausen DW, Hansen BA, Lartey S, Onyango TB, Kuwelker K, Sævik M, Bartsch H, Tøndel C, Kittang BR; Bergen COVID-19 Research Group, Cox RJ, Langeland N. Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021;27:1607-1613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 430] [Article Influence: 107.5] [Reference Citation Analysis (0)] |
| 83. | Coronavirus Disease 2019 (COVID-19) Treatment Guidelines [Internet]. Bethesda (MD): National Institutes of Health (US); 2021 Apr 21–2024 Feb 29. . [PubMed] |
| 84. | Granata G, Schiavone F, Pipitone G, Taglietti F, Petrosillo N. Antibiotics Use in COVID-19 Patients: A Systematic Literature Review. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 85. | Abdelhafiz AS, Ali A, Maaly AM, Mahgoub MA, Ziady HH, Sultan EA. Predictors of post-COVID symptoms in Egyptian patients: Drugs used in COVID-19 treatment are incriminated. PLoS One. 2022;17:e0266175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 86. | Chocarro Martínez A, Galindo Tobal F, Ruiz-Irastorza G, González López A, Alvarez Navia F, Ochoa Sangrador C, Martín Arribas MI. Risk factors for esophageal candidiasis. Eur J Clin Microbiol Infect Dis. 2000;19:96-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 87. | Ezeonu IM, Ntun NW, Ugwu KO. Intestinal candidiasis and antibiotic usage in children: case study of Nsukka, South Eastern Nigeria. Afr Health Sci. 2017;17:1178-1184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 88. | Aliaga S, Clark RH, Laughon M, Walsh TJ, Hope WW, Benjamin DK, Kaufman D, Arrieta A, Benjamin DK Jr, Smith PB. Changes in the incidence of candidiasis in neonatal intensive care units. Pediatrics. 2014;133:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 89. | Kobayashi-Sakamoto M, Tamai R, Isogai E, Kiyoura Y. Gastrointestinal colonisation and systemic spread of Candida albicans in mice treated with antibiotics and prednisolone. Microb Pathog. 2018;117:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 90. | Goel N, Goyal N, Nagaraja R, Kumar R. Systemic corticosteroids for management of 'long-COVID': an evaluation after 3 months of treatment. Monaldi Arch Chest Dis. 2021;92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 91. | Mainous AG 3rd, Rooks BJ, Orlando FA. The Impact of Initial COVID-19 Episode Inflammation Among Adults on Mortality Within 12 Months Post-hospital Discharge. Front Med (Lausanne). 2022;9:891375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 92. | Lionakis MS, Kontoyiannis DP. Glucocorticoids and invasive fungal infections. Lancet. 2003;362:1828-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 450] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 93. | Myerowitz RL. Gastrointestinal and disseminated candidiasis. An experimental model in the immunosuppressed rat. Arch Pathol Lab Med. 1981;105:138-143. [PubMed] |
| 94. | Miranda MA, Sousa SC, Montes VL. Post-COVID-19 neurocandidiasis. Neurol Sci. 2021;42:4419-4420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 95. | Bhagali R, Prabhudesai NP, Prabhudesai MN. Post COVID-19 opportunistic candida retinitis: A case report. Indian J Ophthalmol. 2021;69:987-989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 96. | Jawanda MK, Narula R, Gupta S, Sharma V, Sidhu SK, Kaur N. Mixed Infections (Mucormycosis, Actinomycosis and Candidiasis) Leading to Maxillary Osteomyelitis in a Diabetic Mellitus Patient in Post COVID Phase: First Case Report. Acta Medica (Hradec Kralove). 2021;64:218-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 97. | Gautam S, Sharma G, Singla S, Garg S. Case Report: Secondary Hemophagocytic Lymphohistiocytosis (sHLH) and Candida auris Fungemia in Post-acute COVID-19 Syndrome: A Clinical Challenge. Front Med (Lausanne). 2022;9:835421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 98. | Nucci M, Anaissie E. Revisiting the source of candidemia: skin or gut? Clin Infect Dis. 2001;33:1959-1967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 292] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 99. | Cruellas M, Yubero A, Zapata M, Galvez EM, Gascón M, Isla D, Lastra R, Martínez-Lostao L, Ocariz M, Pardo J, Ramírez A, Sesma A, Torres-Ramón I, Paño JR. How Could Antibiotics, Probiotics, and Corticoids Modify Microbiota and Its Influence in Cancer Immune Checkpoint Inhibitors: A Review. Infect Immun. 2021;89:e0066520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 100. | Wise J. Covid-19: Long covid cases are underreported in GP records, research suggests. BMJ. 2021;374:n1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 101. | Friedman M, Ramsay DB, Borum ML. An unusual case report of small bowel Candida overgrowth as a cause of diarrhea and review of the literature. Dig Dis Sci. 2007;52:679-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |