Published online Sep 21, 2024. doi: 10.3748/wjg.v30.i35.3985
Revised: August 22, 2024
Accepted: September 2, 2024
Published online: September 21, 2024
Processing time: 76 Days and 20.9 Hours
This study examines the complex relationships among the neuroendocrine axis, gut microbiome, inflammatory responses, and gastrointestinal symptoms in patients with irritable bowel syndrome (IBS). The findings provide new insights into the pathophysiology of IBS and suggest potential therapeutic targets for im
To investigate the interactions between the neuroendocrine axis, gut microbiome, inflammation, and gastrointestinal symptoms in patients with IBS.
Patients diagnosed with IBS between January 2022 and January 2023 were se
IBS patients exhibited significant dysregulation of the neuroendocrine axis, with altered levels of cortisol, serotonin, and neuropeptides compared to healthy controls. The gut microbiome of IBS patients showed reduced diversity and specific alterations in bacterial genera, including Bifidobacterium, Lactobacillus, and Faecalibacterium, which were associated with neuroendocrine disturbances. Additionally, elevated levels of inflammatory markers, such as C-reactive protein, interleukin-6, and tumor necrosis factor-α, were observed and correlated with the severity of gastrointestinal symptoms like abdominal pain, bloating, and altered bowel habits.
The findings suggest that targeting the neuroendocrine axis, gut microbiome, and inflammatory pathways may offer novel therapeutic strategies to alleviate symptoms and improve the quality of life in IBS patients.
Core Tip: The findings suggest that targeting the neuroendocrine axis, gut microbiome, and inflammatory pathways may offer novel therapeutic strategies to alleviate symptoms and improve the quality of life in irritable bowel syndrome patients.
- Citation: Zhang X, Jin WW, Wang HG. Correlation between the neuroendocrine axis, microbial species, inflammatory response, and gastrointestinal symptoms in irritable bowel syndrome. World J Gastroenterol 2024; 30(35): 3985-3995
- URL: https://www.wjgnet.com/1007-9327/full/v30/i35/3985.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i35.3985
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder characterized by symptoms such as diarrhea, constipation, and others that significantly affect the quality of life of affected individuals[1]. IBS has a certain prevalence globally, occurring in all age groups, but predominantly affects young and middle-aged adults, particularly those aged 20-40 years[2]. Statistics indicate that 10% to 15% of adults suffer from IBS, with a slightly higher prevalence in females compared than in males, possibly due to factors such as physiological characteristics, hormone levels, and lifestyle. Previous studies have revealed that lifestyle and dietary habits play a role in the onset and symptoms of IBS; factors such as stress, irregular eating patterns, excessive consumption of fats, and irritant foods may increase the risk of developing the condition or exacerbating symptoms[3]. Additionally, the prevalence of IBS varies among different regions, with higher rates observed in developed countries and lower rates in developing countries[4]. IBS is a multifactorial disorder influenced by genetic, biological, psychological, environmental, and dietary factors, among others, affecting its onset and symptom manifestation. Although the exact mechanisms of IBS remain unclear, research suggests that factors such as neuroendocrine regulation, dysbiosis of the intestinal microbiota, and inflammatory responses play crucial roles in its pathogenesis and development[5].
The neuroendocrine axis, an important component of the neuroendocrine network, is closely related to the gut-brain axis, and the two interact with each other. The functional activity of the neuroendocrine axis not only participates in the regulation of growth, development, and metabolism but also directly influences visceral sensation and gastrointestinal motility, such as promoting intestinal peristalsis and accelerating intestinal emptying processes[6]. The gut microbiota structure in patients with IBS often undergoes changes, including reduced probiotics, decreased beneficial bacteria, and increased pathogenic bacteria. This dysbiosis may lead to weakened intestinal barrier function and increased inflammatory responses, and consequently affect patients' gastrointestinal function and symptom manifestation[7]. The gut-brain axis involves interactions between the nervous, endocrine, and immune systems, with the gut microbiota playing a crucial role in this axis. Studies have shown that the brain regulates intestinal motility, secretion, and permeability through the autonomic nervous system and neurotransmitters, thereby affecting the structure and function of the gut microbiota and directly regulating microbial hormone production. Certain bacteria and their metabolites in the gut microbiota can synthesize neurotransmitters and neuropeptides, with over 90% of serotonin (5-HT) produced by enterochromaffin cells in the gut[8]. 5-HT induces IBS symptoms by increasing epithelial permeability, activating visceral hypersensitivity, stimulating immune cells and altering intestinal motility. Signal transduction between the gut microbiota and central nervous system involves neural, endocrine, and immune pathways[9]. Dysbiosis of the gut microbiota can lead to changes in neurotransmitters, hormones, and microbial metabolites, thereby activating the enteric nervous system and affecting central nervous system function[10]. At the same time, the central nervous system influences the composition of the gut microbiota through these pathways. It is currently believed that the disruption of symbiosis between the human host and the gut microbiota, along with the interaction between the gut microbiota and autonomic nervous system, are core factors contributing to the persistent presence of IBS symptoms[11]. Intestinal infections can affect gastrointestinal motility and secretion, potentially leading to damage to the intestinal mucosal barrier and increased permeability. In such situations, inflammatory immune cells such as mast cells may be activated, triggering the release of a large number of pro-inflammatory cytokines (such as IL-6 and IL-8). Conversely, the anti-inflammatory cytokine IL-10 can inhibit the activation of NK cells, TM cells, and macrophages[12]. Moreover, IBS has several phenotypes, namely, diarrhea predominant IBS (IBS-D), constipation predominant IBS (IBS-C), mixed-type IBS (IBS-M), and unspecified-type IBS (IBS-U)[13]. Patients with IBS-D typically experience frequent episodes of diarrhea, often accompanied by abdominal pain and an increased frequency of bowel movements. Up to 40% of patients with IBS have diarrhea as the predominant bowel symptom. These individuals may also report a heightened sense of urgency, feeling the need to evacuate their bowels. In contrast, individuals with constipation-predominant IBS-C typically present with constipation as the primary symptom. This may be accompanied by abdominal pain and decreased frequency of bowel movements. They often experience difficulties in passing stools and may feel as though their bowel movements are incomplete[14]. In contrast, patients with IBS-M experience both diarrhea and constipation, with alternating bowel habits or different types of bowel movements at different times. Finally, some patients may not fit into any of these categories or may experience varying primary symptoms at different times. These cases were classified as IBS-U cases[15]. In this study, we investigated the different features of these different phenotypes in patients.
Although IBS is considered a functional gastrointestinal disorder without organic pathology, molecular biology research has confirmed changes in intestinal mucosal epithelial permeability and low-level inflammation as fundamental pathological changes in IBS-D. These changes are closely related to intestinal nutrient content and microbial abundance, and the pathogenesis mainly involves genetic susceptibility, abnormal brain-gut interactions, visceral hypersensitivity, increased mucosal permeability, and alterations in the gut microbiota. Among these factors, changes in the gut microbiota are key links throughout the disease process. The most significant feature of IBS-C is disturbance of gastrointestinal motility, characterized by widespread onset and high reactivity[16]. This functional abnormality is not limited to the colon but often involves the entire gastrointestinal tract, with excessively strong motor responses to various physiological and non-physiological stimuli (such as certain gastrointestinal hormones, colonic distension, and diet), and tends to recur. Experimental research[17] has found that both slowed and accelerated colonic motility exist in many IBS patients, manifesting as abnormal reflexes of gastric and colonic functions to the same stimuli. Compared to healthy individuals, IBS patients are more sensitive to colonic motor responses.
In this study, we hypothesized that abnormal function of the neuroendocrine axis is closely associated with gas
The objective of this study was to explore the relationship between the neuroendocrine axis, intestinal microbiota, inflammatory responses, and gastrointestinal symptoms in IBS patients. By analyzing the clinical data, we aimed to reveal the interactions among these factors, providing new theoretical insights and clinical guidance for the treatment of IBS.
This study adhered to the Helsinki Declaration for conducting experiments. The study included patients who met the diagnostic criteria for IBS at our hospital from January 2022 to January of the following year as well as a healthy group of individuals who underwent routine health examinations during the same period at our hospital. After thorough screening based on the inclusion and exclusion criteria, a total of 80 patients were included in the patient group, and an equal number of 80 individuals were included in the healthy group. The IBS patients were further categorized based on their IBS phenotypes, including 36 cases of IBS-D, 24 cases of IBS-C, 15 cases of IBS-M, and 5 cases of IBS-U. Basic information, medical history, neuroendocrine-related indicators [such as cortisol (COR)], composition of intestinal microbiota (analyzed through stool samples), levels of inflammatory markers (interleukins, etc.), and gastrointestinal symptom assessment data were collected from all study subjects. Statistical methods were used to analyze the collected data and explore the correlation between the neuroendocrine axis, intestinal microbiota, inflammatory responses, and gastrointestinal symptoms.
Inclusion criteria: Patients who met the diagnostic criteria outlined in the Chinese Expert Consensus on IBS[18]; did not use medications affecting neuroendocrine function, such as central acting drugs (e.g., anti-anxiety medications, antidepressants, etc.), or corticosteroids in the past three months; and aged over 18 years, regardless of sex.
The exclusion criteria were as follows: History of gastrointestinal surgery; concurrent severe conditions such as malignancies, thyroid disorders, liver or kidney dysfunction; diagnosis of cancer, blood disorders, or autoimmune dis
Diagnostic criteria for IBS: Patients with recurrent abdominal pain, bloating, or discomfort associated with any two or more of the following: (1) Altered stool frequency; (2) Altered stool form (appearance); and (3) Altered stool passage (straining, urgency, incomplete evacuation). Symptoms should have been present for at least six months prior to diagnosis, with symptom onset at least three months prior to diagnosis.
After enrollment, fresh fecal specimens were obtained from the two study groups using sterile cotton swabs weighing 5 g. The specimens were evenly mixed with an anaerobic diluent and diluted to 10-9 using a 10-fold dilution method. The diluted samples were inoculated into different culture media, with three plates inoculated at each dilution level, and an anaerobic culture medium was used for incubation. The average colony count was calculated from the three plates, and the contents of Escherichia coli, Bifidobacterium, Lactobacillus, Enterococcus, and Bacteroides per gram of the sample were determined. Additionally, 5 mL of peripheral venous blood samples were collected on an empty stomach, left to stand at room temperature for 30 minutes, and then centrifuged at a speed of 3000 revolutions per minute for 10 minutes with a radius of 10 cm. Serum samples were obtained after centrifugation, and enzyme-linked immunosorbent assays were used to measure neuroendocrine axis indicators, including adrenocorticotropic hormone (ACTH), COR, and serotonin (5-HT), as well as inflammatory factors, such as interleukin IL-6, IL-8, and IL-10. In the patient group, ACTH levels were measured using chemiluminescence at three time points within 24 hours (8:00, 16:00, and 24:00), and COR levels were determined using a radioimmunoassay with a gamma counter.
Neuroendocrine axis measurement: ACTH levels: Blood samples were collected from participants and centrifuged to separate the serum. Serum ACTH levels were measured using the Immulite 2000 XPi Immunoassay System (Siemens Healthcare Diagnostics, Germany). This system utilizes a solid-phase, two-site chemiluminescent immunometric assay principle. The sample was incubated with alkaline phosphatase-labeled anti-ACTH antibody, and the resulting chemiluminescent reaction was measured by the system. The ACTH concentration was determined by comparing the sample's luminescence to a calibration curve. COR levels and 5-HT levels: Serum samples were collected and analyzed using the Cortisol ELISA Kit (Catalog #KGE008, R&D Systems, United States). The ELISA kit employs the competitive enzyme immunoassay technique. Samples and standards were added to the wells coated with a polyclonal antibody specific for cortisol. A cortisol-peroxidase conjugate was then added, and the resulting color change was measured using a microplate reader. The cortisol concentration in the samples was calculated by comparing the optical densities to a standard curve.
Intestinal microbiota analysis: Fecal samples were collected from participants and genomic DNA was extracted using a commercial stool DNA extraction kit. Quantitative PCR (qPCR) was performed on a QuantStudio 5 real-time PCR System (Thermo Fisher Scientific, United States) using specific primer sets targeting the 16S rRNA gene of Escherichia coli, Bifidobacterium, Lactobacillus, Enterococcus, and Bacteroides. The qPCR reaction mixture contained the extracted DNA, gene-specific primers, and a SYBR Green-based fluorescent detection dye. The amplification was monitored in real-time, and the bacterial levels were quantified by comparing the sample's cycle threshold (Ct) values to standard curves constructed using known concentrations of each bacterial species.
Inflammatory response measurement: Serum samples were analyzed using the HCYTMAG-60K Multiplex ELISA Kit (Millipore Sigma, United States) on a MAGPIX Multiplexing Instrument (Luminex Corporation, United States). The kit utilizes magnetic beads coated with capture antibodies specific for IL-6, IL-8, and IL-10. Samples and standards were incubated with the antibody-coated beads, followed by the addition of detection antibodies. The resulting complexes were then measured by the MAGPIX instrument, which uses fluorescent dyes to detect and quantify the analytes. The concentrations of IL-6, IL-8, and IL-10 were determined by comparing the sample's fluorescence intensities to standard curves.
Gastrointestinal symptoms: Diarrhea and constipation were assessed using standardized questionnaires completed by the participants. For diarrhea, participants reported the frequency, consistency, and volume of bowel movements over the monitoring period. For constipation, participants reported the frequency of bowel movements, difficulty with defecation, and feelings of incomplete evacuation. The severity of diarrhea and constipation symptoms were scored using validated clinical scales, such as the Bristol Stool Scale and the Rome III Diagnostic Criteria for Functional Gastrointestinal Disorders.
Neuroendocrine axis measurements: Blood samples were collected from participants at 3 time points: 8:00, 16:00, and 24:00. At each time point, 5 mL of venous blood was drawn into serum separator tubes. The blood samples were allowed to clot at room temperature for 30 minutes, then centrifuged at 3000 rpm for 10 minutes to separate the serum. The serum samples were aliquoted and stored at -80 °C until analysis. ACTH levels were measured using the Immulite 2000 XPi Immunoassay System, as described earlier. COR levels were measured using the Cortisol ELISA Kit, also as described previously. By collecting the blood samples at these 3 specific time points (morning, afternoon, and evening), the researchers were able to evaluate the diurnal rhythms and fluctuations in the neuroendocrine markers ACTH and cortisol for both the diarrhea and constipation patient groups.
Pearson correlation analysis was used to investigate the relationship between the neuroendocrine axis of IBS patients and the intestinal microbiota, inflammatory responses, and gastrointestinal symptoms. The data were organized and analyzed using SPSS 26.0. Graphical representations were created using GraphPad Prism version 8. Quantitative data are presented as the mean ± SD, and the t-test was used for intergroup comparisons. Count data are presented as n (%), and the χ2 test was used for intergroup comparisons. The Benjamini-Hochberg procedure, which controls the false discovery rate, was employed to adjust for multiple comparisons. Statistical significance was set at P < 0.05.
The patient group consisted of 80 patients, including 33 males and 47 females, with an age range of 22 to 55 years and a mean age of 34.96 ± 6.17 years. The body mass index (BMI) ranged from 19 to 24 kg/m2, with a mean of 22.19 ± 1.37 kg/m2. Their educational background included 18 high school graduates, 26 college graduates, and 36 undergraduate degree holders. The healthy control group also had 80 cases, including 35 males and 45 females, with an age range of 22 to 55 years and a mean age of 35.08 ± 6.12 years. The BMI ranged from 19 to 24 kg/m2, with a mean of 22.07 ± 1.44 kg/m2. Their educational background included 20 high school graduates, 27 college graduates, and 33 undergraduate degree holders. There were no significant differences in the clinical data between the two groups, indicating comparability (P > 0.05) (Table 1).
| Patient group | Healthy group | t/χ2 | P value | ||
| Number of cases | - | 80 | 80 | - | - |
| Gender | Male | 33 | 35 | 0.102 | 0.749 |
| Female | 47 | 45 | |||
| Age (years) | - | 22-55 | 22-55 | - | - |
| Mean | 34.96 ± 6.17 | 35.08 ± 6.12 | 0.124 | 0.902 | |
| BMI (kg/m2) | - | 19-24 | 19-24 | - | - |
| Mean | 22.19 ± 1.37 | 22.07 ± 1.44 | 0.540 | 0.590 | |
| Education levels | High school | 18 | 20 | 0.025 | 0.874 |
| Associate degree | 26 | 27 | |||
| Bachelor's degree | 36 | 33 |
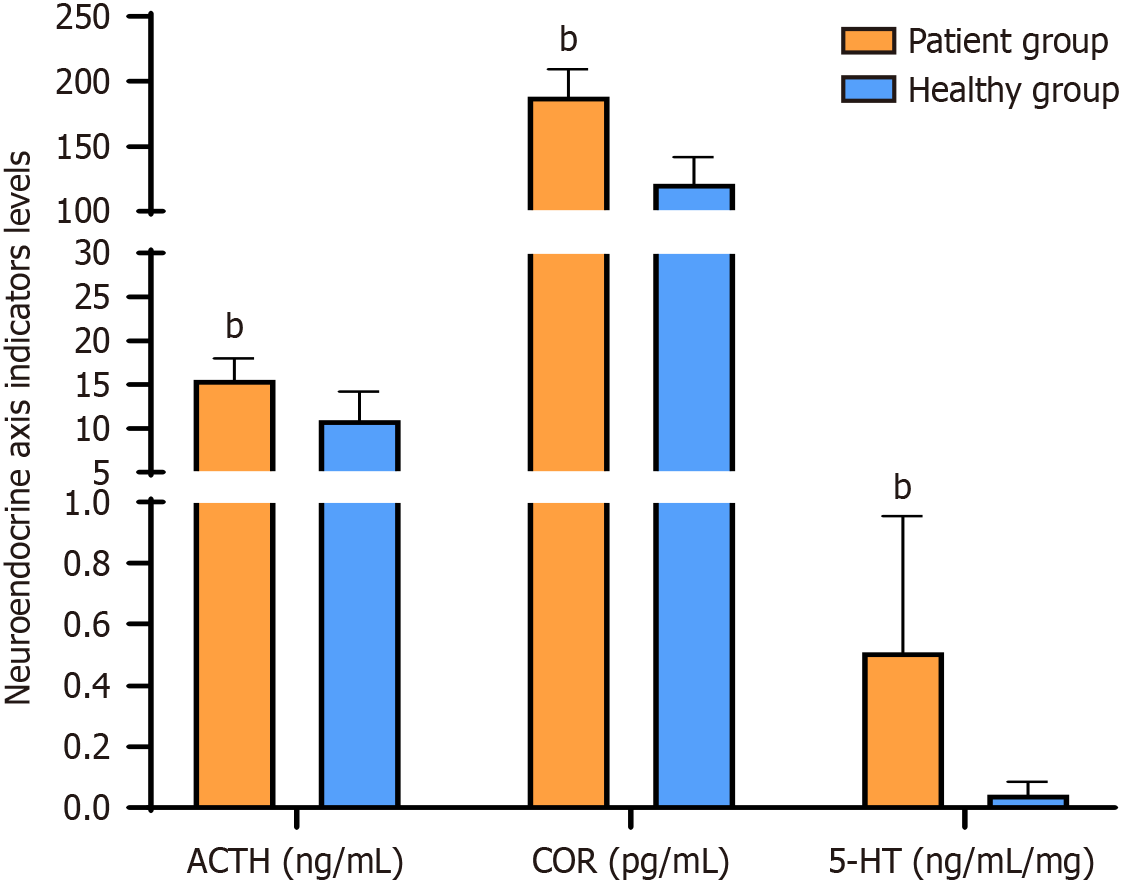
The levels of ACTH, COR, and 5-HT were significantly higher in the patient group than in the healthy group (P < 0.05) (Figure 1).
In terms of the comparison of ACTH, COR, and 5-HT levels among different IBS phenotypes, no significant difference in ACTH, COR, and 5-HT levels was identified between the four phenotypes of IBS (P > 0.05) (Table 2).
| IBS-D | IBS-C | IBS-M | IBS-U | F value | P value | |
| Number of cases | 36 | 24 | 15 | 5 | - | - |
| ACTH (ng/mL) | 16.45 ± 2.41 | 16.33 ± 2.39 | 17.48 ± 2.37 | 16.78 ± 2.54 | 1.315 | 0.274 |
| COR (pg/mL) | 191.37 ± 20.11 | 189.81 ± 20.45 | 190.23 ± 20.47 | 189.46 ± 20.73 | 3.552 | 0.338 |
| 5-HT (ng/mL/mg) | 0.52 ± 0.24 | 0.58 ± 0.34 | 0.54 ± 0.21 | 0.57 ± 0.246 | 0.452 | 0.638 |
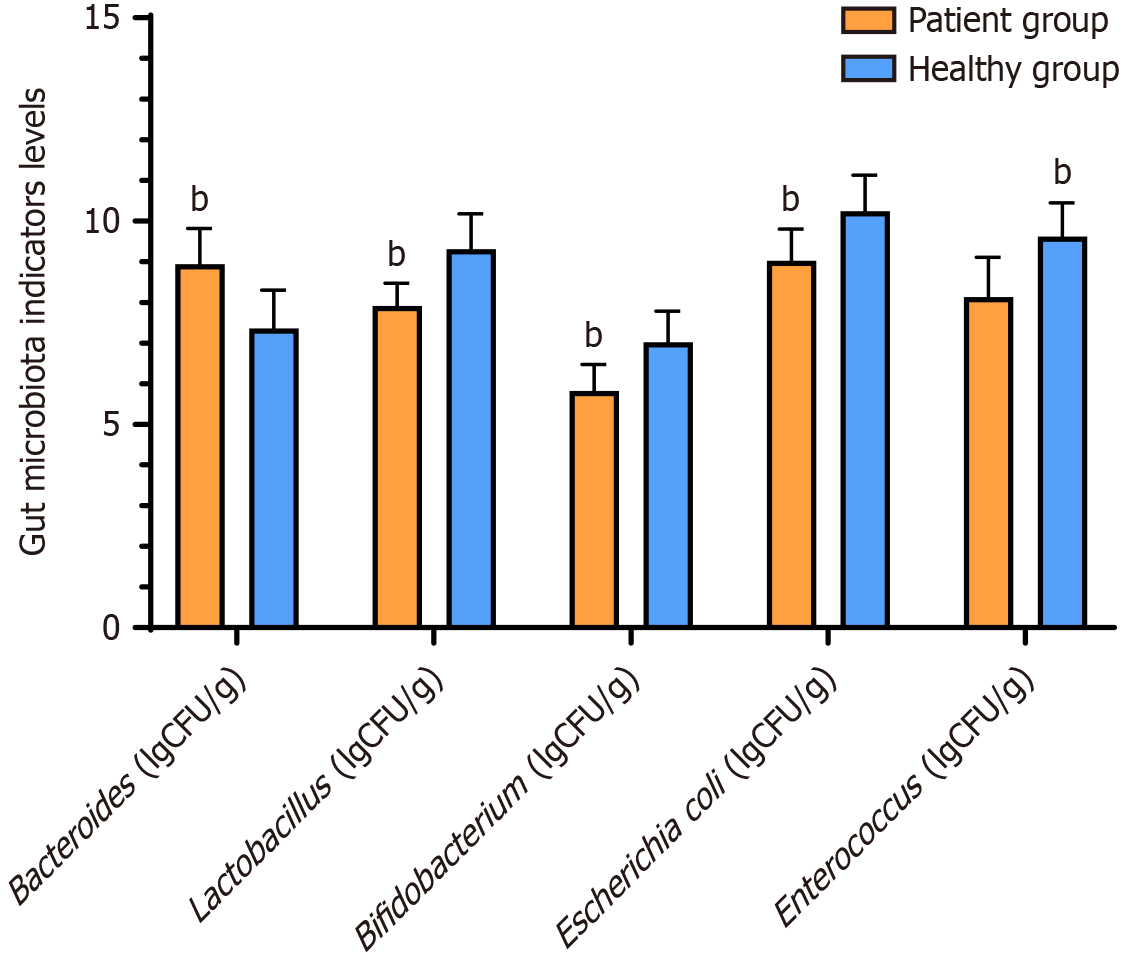
Bacteroides levels were significantly higher in the patient group than in the healthy group, whereas the levels of Bifidobacteria, Lactobacillus, Clostridium, and Bacteroides were significantly lower in the patient group than in the healthy group (P < 0.05) (Figure 2).
There was no significant difference in the levels of Bacteroides, Lactobacillus, Bifidobacterium, Escherichia coli, and Enterococcus among the four phenotypes of IBS with respect to gut microbiota indicators (P > 0.05) (Table 3).
| IBS-D | IBS-C | IBS-M | IBS-U | F | P value | |
| Number of cases | 36 | 24 | 15 | 5 | - | - |
| Bacteroides (lgCFU/g) | 8.55 ± 0.75 | 8.62 ± 0.82 | 8.61 ± 0.90 | 8.70 ± 0.95 | 0.719 | 0.491 |
| Lactobacillus (lgCFU/g) | 7.47 ± 0.62 | 7.38 ± 0.48 | 7.65 ± 0.71 | 7.22 ± 0.59 | 5.872 | 0.430 |
| Bifidobacterium (lgCFU/g) | 6.23 ± 0.71 | 6.17 ± 0.56 | 5.96 ± 0.62 | 6.08 ± 0.48 | 6.754 | 0.217 |
| Escherichia coli (lgCFU/g) | 8.45 ± 0.66 | 8.58 ± 0.91 | 8.23 ± 0.83 | 8.64 ± 0.72 | 2.519 | 0.846 |
| Enterococcus (lgCFU/g) | 8.75 ± 0.83 | 8.92 ± 0.74 | 8.85 ± 1.02 | 8.28 ± 0.91 | 3.719 | 0.290 |
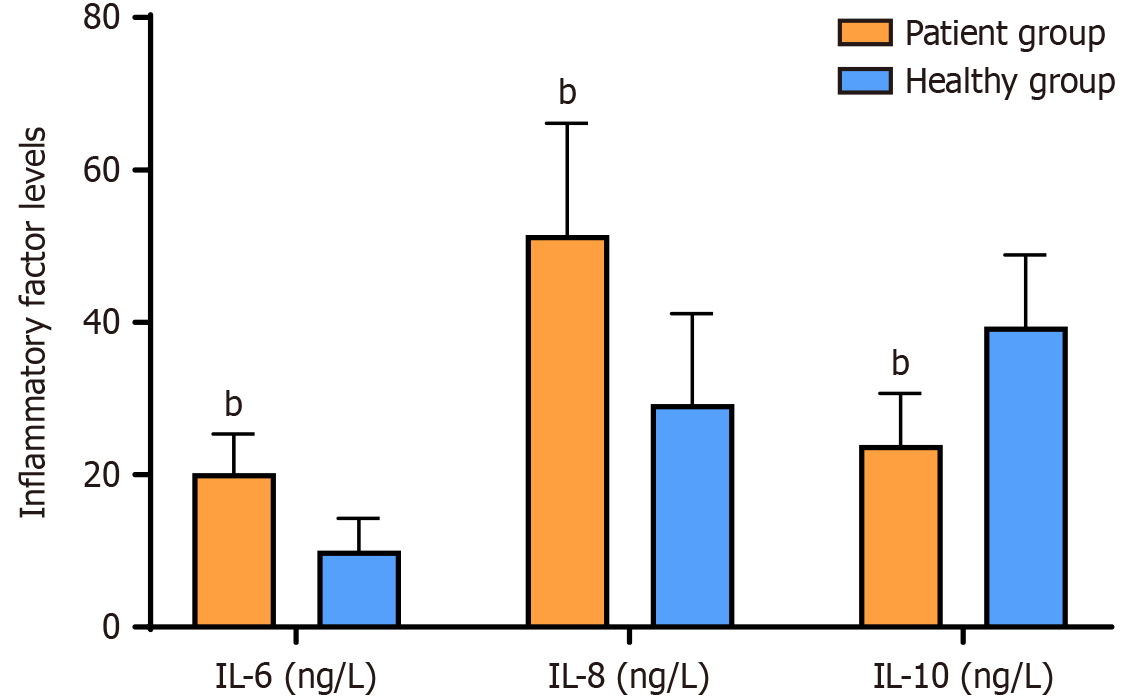
The levels of IL-6 and IL-8 were significantly higher in the patient group than in the healthy group, whereas the level of IL-10 was significantly lower in the patient group than in the healthy group (P < 0.05) (Figure 3). No significant difference was found between the two IBS phenotypes in terms of inflammatory factor levels (P > 0.05) (Table 4).
| IBS-D | IBS-C | IBS-M | IBS-U | F | P value | |
| Number of cases | 36 | 24 | 15 | 5 | - | - |
| IL-6 (ng/L) | 18.37 ± 4.76 | 22.05 ± 6.34 | 19.92 ± 4.98 | 20.68 ± 5.59 | 3.801 | 0.285 |
| IL-8 (ng/L) | 45.80 ± 12.56 | 59.23 ± 17.28 | 53.91 ± 15.03 | 49.77 ± 13.94 | 1.917 | 0.477 |
| IL-10 (ng/L) | 19.43 ± 7.82 | 28.15 ± 5.94 | 25.67 ± 6.32 | 22.08 ± 6.51 | 1.253 | 0.253 |
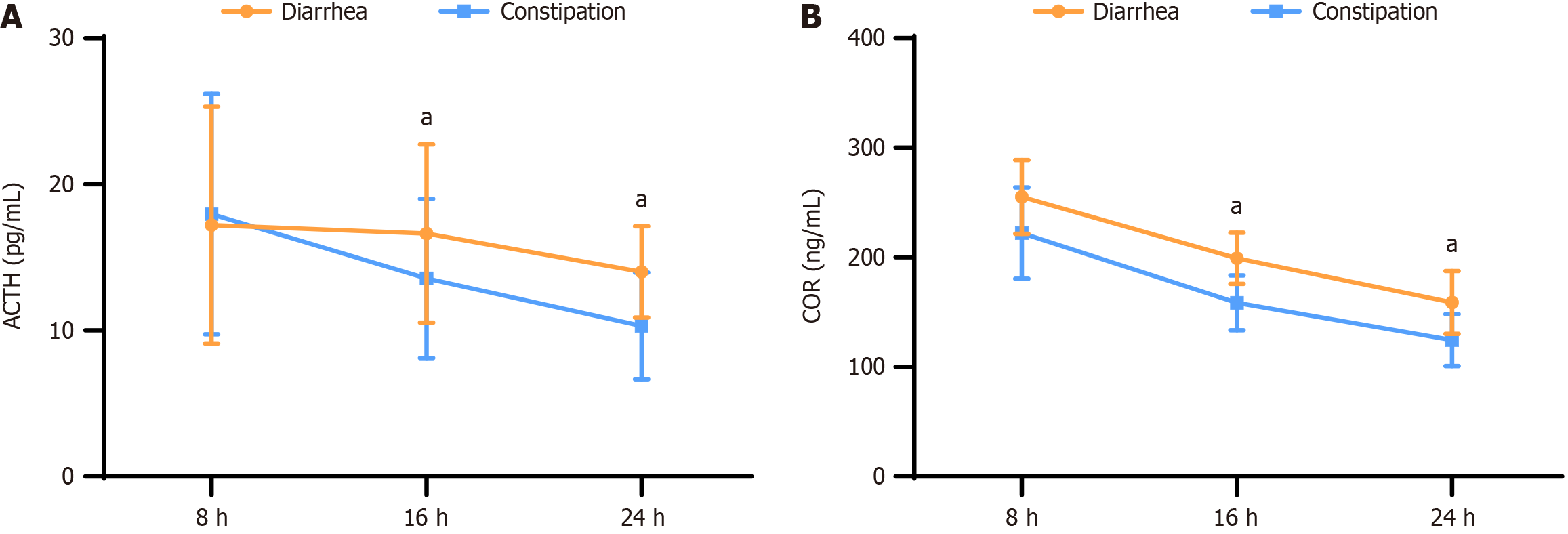
ACTH levels were consistently higher in patients with diarrhea than in those with constipation at all time points (8:00, 16:00, and 24:00). At 16:00 and 24:00, the mean ACTH levels in diarrhea patients were 16.65 pg/mL and 14.01 pg/mL, respectively, whereas in constipation patients, they were 13.56 pg/mL and 10.32 pg/mL, respectively. These differences were statistically significant (P < 0.05) between the two groups.
Similarly, for COR levels with diarrhea demonstrated elevated COR levels compared to patients with constipation at all time points. At 8:00, 16:00, and 24:00, the mean COR levels in diarrhea patients were 255.11 ng/mL, 199 ng/mL, and 158.89 ng/mL, respectively, whereas in constipation patients, they were 222.12 ng/mL, 158.56 ng/mL, and 124.65 ng/mL, respectively. These differences were also statistically significant (P < 0.05) between the two groups (Figure 4).
Pearson correlation analysis revealed a positive correlation between the neuroendocrine axis of IBS patients and Escherichia coli, IL-6, IL-8, and IL-10, and a statistically significant negative correlation with Lactobacillus, Bifidobacterium, Clostridium, and Bacteroides (P < 0.05) (Table 5).
| ACTH | COR | |||
| r | P value | r | P value | |
| Bacteroides | 0.378 | < 0.001 | 0.392 | < 0.001 |
| Lactobacillus | -0.359 | < 0.001 | -0.336 | < 0.001 |
| Bifidobacterium | -0.315 | < 0.001 | -0.348 | < 0.001 |
| Clostridium | -0.333 | < 0.001 | -0.329 | < 0.001 |
| Fusobacterium | -0.362 | < 0.001 | -0.347 | < 0.001 |
| IL-6 | 0.482 | < 0.001 | 0.392 | < 0.001 |
| IL-8 | 0.513 | < 0.001 | 0.496 | < 0.001 |
| IL-10 | 0.396 | < 0.001 | 0.423 | < 0.001 |
IBS is a functional disorder of the gastrointestinal tract characterized by clinical manifestations such as abdominal pain, bloating, and changes in bowel habits and/or stool consistency. This disease lacks obvious structural and biochemical abnormalities in the gastrointestinal tract[19,20].
This study aimed to explore the relationship between the neuroendocrine axis, gut microbiota, inflammatory response, and gastrointestinal symptoms in patients with IBS. By analyzing the clinical data, we aimed to reveal the interactions between these factors and provide new theoretical foundations and clinical guidance for IBS treatment. Our study found that some patients with IBS exhibited abnormalities in the neuroendocrine axis, characterized by elevated cortisol levels or abnormal adrenaline secretion. This finding is consistent with previous research suggesting that neuroendocrine regulation may be closely related to the pathogenesis and development of IBS[21]. Furthermore, research[22] has indicated that persistent stress can lead to chronic overactivity or suppression of the neuroendocrine axis and autonomic nervous system, leading to gastrointestinal motility disorders and changes in visceral sensitivity, thereby triggering or exacerbating IBS symptoms. Our study found that patients with IBS had increased levels of serum inflammatory markers, which is consistent with previous findings[23]. Research[23] has found that IBS patients have abnormal levels of IL-6 and IL-10), indicating an increase in the inflammatory response. This situation is closely related to the gastrointestinal symptoms experienced by patients, and may be an important reason for symptoms such as pain and diarrhea.
The results of this study show that the degree of neuroendocrine axis dysfunction in diarrhea-predominant IBS patients is more severe than that in constipation-predominant IBS patients. This may be because, when there is worsening dysfunction of the neuroendocrine axis, the intestinal barrier function is more severely compromised, leading to excessive colonic motility and an increased likelihood of diarrhea symptoms[24]. Our study found that patients with diarrhea had significantly higher ACTH levels at 16:00 and 24:00, and higher ACTH and COR levels at 8:00, 16:00, and 24:00 compared to patients with constipation. These results indicate that the secretion of ACTH and COR is significantly influenced by diurnal rhythms. Diurnal rhythms are an internal adaptation mechanism of organisms to environmental changes (such as light) and regulate various physiological functions, including the activity of the neuroendocrine axis. These fluctuations may explain the time-dependent nature of gastrointestinal symptoms and the differences in symptoms between patient groups at different times. As stress hormones, ACTH and COR are likely associated with gastrointestinal motility and dysfunction. The elevated levels of ACTH and COR in diarrhea patients may reflect a stronger response to stress, as diarrhea is often linked to acute or chronic stress, which could activate the hypothalamic-pituitary-adrenal (HPA) axis, leading to increased secretion of ACTH and COR. Furthermore, diarrhea patients may have different physiological mechanisms in gastrointestinal motility and secretion, which could impact the activity of the HPA axis, especially under conditions of gut inflammation and imbalance caused by diarrhea. Additionally, the secretion of ACTH and COR follows a diurnal rhythm, and the diurnal rhythm of diarrhea patients may differ from that of constipation patients. This implies that diarrhea patients may experience more pronounced physiological or psychological stress at specific times, leading to greater fluctuations in hormone levels. Changes in gastrointestinal function could also directly or indirectly affect hormone secretion, for example, by altering the metabolism or clearance mechanisms of hormones in the body. Future research should explore the role of diurnal rhythms in the neuroendocrine axis in different gastrointestinal symptom patients more deeply, and consider these rhythms as potential therapeutic targets to improve the management of related symptoms[25].
Based on the above results, it can be inferred that there is a correlation between neuroendocrine axis dysfunction and dysbiosis of the gut microbiota, inflammation response, and gastrointestinal symptoms. This is also reflected in the results of this study, where Pearson correlation analysis revealed a positive correlation between the neuroendocrine axis and intestinal bacteria, such as Bacteroides, IL-6, IL-8, and IL-10, and a negative correlation with Bifidobacterium, Lactobacillus, Clostridium, and Enterobacter. Therefore, in the treatment of IBS, in addition to symptomatic treatment, interventions targeting neuroendocrine function regulation, adjustment of gut microbiota structure, and reduction of inflammation levels should be emphasized to improve treatment efficacy and patient quality of life. Furthermore, in this study, no significant difference was observed between the different IBS phenotypes in terms of neuroendocrine axis indicators, gut microbiota indicators, and inflammatory factor levels. A previous study[26] compared the gut microbiota of patients with IBS and healthy individuals. Although there were differences in the overall diversity of microbiomes, no significant variations were found in specific bacterial genera between the two groups. This suggests that the composition of gut bacteria may not differ significantly between IBS patients and healthy controls. Another study[27] emphasized the complexity of IBS and its genetic underpinnings. Despite extensive research efforts using various methodologies, including family and genome-wide association studies, specific genetic variants associated with IBS remain elusive. Challenges include the lack of standardized cohorts and the infancy of epigenetic and pharmacogenetic approaches. Initiatives such as the GENIEUR network aim to overcome these hurdles and improve the future research in this area.
The present study provides valuable insights into the multifaceted mechanisms underlying IBS by elucidating the intricate relationships between the neuroendocrine axis, gut microbiome, and inflammatory responses. Despite the results achieved in this study, several limitations should be noted. Firstly, the sample size is relatively small, particularly for certain IBS subtypes (e.g., the IBS-U group, which had only 5 cases). This limits the ability to detect potential differences between subtypes and may result in a higher degree of homogeneity in the findings, potentially obscuring subtle differences between subtypes. Additionally, as a single-center study, further multi-center studies with larger sample sizes are necessary to enhance the generalizability and reliability of the results. Secondly, the use of self-reported questionnaires in this study may introduce reporting bias. Although efforts were made by the research team to minimize such bias, the subjective nature of self-reporting could still affect the accuracy of the data. Finally, this study did not evaluate emotional disorders, such as anxiety and depression, which are closely related to neuroendocrine axis dysfunction in IBS. This may result in the omission of important pathophysiological factors. Therefore, further research is needed to explore the specific mechanisms and interrelationships between the neuroendocrine axis, gut microbiota, and inflammatory responses[28].
This study identified a close relationship between neuroendocrine axis dysfunction, dysbiosis of gut microbiota, enhanced inflammatory response, and increased gastrointestinal symptoms in patients with IBS. This suggests that neuroendocrine regulation, balance of gut microbiota, inflammatory response, and other factors may collectively contribute to the onset and development of IBS. Therefore, a comprehensive treatment targeting these factors may represent an effective therapeutic strategy for improving the symptoms and quality of life of patients.
In clinical practice, these findings provide new theoretical foundations and clinical guidelines for IBS treatment. First, by modulating neuroendocrine function, it may be possible to regulate intestinal motility, improve mucosal permeability, and alleviate gastrointestinal symptoms in patients. Second, by adjusting the structure of the gut microbiota, increasing the content of beneficial bacteria, and reducing the number of pathogenic bacteria, it may be possible to enhance intestinal mucosal barrier function, decrease the inflammatory response, and alleviate symptoms in patients. Additionally, it may be possible to reduce pain and discomfort in patients by alleviating the inflammatory response, thereby improving their quality of life.
| 1. | Bonetto S, Fagoonee S, Battaglia E, Grassini M, Saracco GM, Pellicano R. Recent advances in the treatment of irritable bowel syndrome. Pol Arch Intern Med. 2021;131:709-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 2. | Sebastián Domingo JJ. Irritable bowel syndrome. Med Clin (Barc). 2022;158:76-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 3. | Borghini R, Donato G, Alvaro D, Picarelli A. New insights in IBS-like disorders: Pandora's box has been opened; a review. Gastroenterol Hepatol Bed Bench. 2017;10:79-89. [PubMed] |
| 4. | Staudacher HM, Black CJ, Teasdale SB, Mikocka-Walus A, Keefer L. Irritable bowel syndrome and mental health comorbidity - approach to multidisciplinary management. Nat Rev Gastroenterol Hepatol. 2023;20:582-596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 88] [Reference Citation Analysis (0)] |
| 5. | Zuloaga DG, Lafrican JJ, Zuloaga KL. Androgen regulation of behavioral stress responses and the hypothalamic-pituitary-adrenal axis. Horm Behav. 2024;162:105528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 6. | Dandi Ε, Spandou E, Dalla C, Tata DA. Τhe neuroprotective role of environmental enrichment against behavioral, morphological, neuroendocrine and molecular changes following chronic unpredictable mild stress: A systematic review. Eur J Neurosci. 2023;58:3003-3025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 7. | Xiao L, Liu Q, Luo M, Xiong L. Gut Microbiota-Derived Metabolites in Irritable Bowel Syndrome. Front Cell Infect Microbiol. 2021;11:729346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 92] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 8. | Canakis A, Haroon M, Weber HC. Irritable bowel syndrome and gut microbiota. Curr Opin Endocrinol Diabetes Obes. 2020;27:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 9. | Chen M, Ruan G, Chen L, Ying S, Li G, Xu F, Xiao Z, Tian Y, Lv L, Ping Y, Cheng Y, Wei Y. Neurotransmitter and Intestinal Interactions: Focus on the Microbiota-Gut-Brain Axis in Irritable Bowel Syndrome. Front Endocrinol (Lausanne). 2022;13:817100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 10. | Staudacher HM, Scholz M, Lomer MC, Ralph FS, Irving PM, Lindsay JO, Fava F, Tuohy K, Whelan K. Gut microbiota associations with diet in irritable bowel syndrome and the effect of low FODMAP diet and probiotics. Clin Nutr. 2021;40:1861-1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 11. | Ng QX, Soh AYS, Loke W, Lim DY, Yeo WS. The role of inflammation in irritable bowel syndrome (IBS). J Inflamm Res. 2018;11:345-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 216] [Article Influence: 30.9] [Reference Citation Analysis (2)] |
| 12. | Taghaddos D, Saqib Z, Bai X, Bercik P, Collins SM. Post-infectious ibs following Clostridioides difficile infection; role of microbiota and implications for treatment. Dig Liver Dis. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 13. | Sood R, Law GR, Ford AC. Diagnosis of IBS: symptoms, symptom-based criteria, biomarkers or 'psychomarkers'? Nat Rev Gastroenterol Hepatol. 2014;11:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Su Q, Tun HM, Liu Q, Yeoh YK, Mak JWY, Chan FK, Ng SC. Gut microbiome signatures reflect different subtypes of irritable bowel syndrome. Gut Microbes. 2023;15:2157697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 52] [Reference Citation Analysis (0)] |
| 15. | Altomare A, Di Rosa C, Imperia E, Emerenziani S, Cicala M, Guarino MPL. Diarrhea Predominant-Irritable Bowel Syndrome (IBS-D): Effects of Different Nutritional Patterns on Intestinal Dysbiosis and Symptoms. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 16. | Makker J, Chilimuri S, Bella JN. Genetic epidemiology of irritable bowel syndrome. World J Gastroenterol. 2015;21:11353-11361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 17. | Camilleri M. Management Options for Irritable Bowel Syndrome. Mayo Clin Proc. 2018;93:1858-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 18. | Song YR, Liang XN, Li CY, Zhang XL. [Interpretation on Chinese Expert Consensus of Irritable Bowel Syndrome in 2020]. Linchuang Huicui. 2021;49:1151-1155. [DOI] [Full Text] |
| 19. | Chey WD, Eswaran S, Kurlander J. JAMA patient page. Irritable bowel syndrome. JAMA. 2015;313:982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Saha L. Irritable bowel syndrome: pathogenesis, diagnosis, treatment, and evidence-based medicine. World J Gastroenterol. 2014;20:6759-6773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 318] [Cited by in RCA: 295] [Article Influence: 26.8] [Reference Citation Analysis (14)] |
| 21. | Sun Z, Wang X, Feng S, Xie C, Xing Y, Guo L, Zhao J, Ji C. A review of neuroendocrine immune system abnormalities in IBS based on the brain-gut axis and research progress of acupuncture intervention. Front Neurosci. 2023;17:934341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 22. | Schaper SJ, Stengel A. Emotional stress responsivity of patients with IBS - a systematic review. J Psychosom Res. 2022;153:110694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Hua MC, Lai MW, Kuo ML, Yao TC, Huang JL, Chen SM. Decreased interleukin-10 secretion by peripheral blood mononuclear cells in children with irritable bowel syndrome. J Pediatr Gastroenterol Nutr. 2011;52:376-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Cremonini F, Lembo A. IBS with constipation, functional constipation, painful and non-painful constipation: e Pluribus…Plures? Am J Gastroenterol. 2014;109:885-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Di Rosa C, Altomare A, Terrigno V, Carbone F, Tack J, Cicala M, Guarino MPL. Constipation-Predominant Irritable Bowel Syndrome (IBS-C): Effects of Different Nutritional Patterns on Intestinal Dysbiosis and Symptoms. Nutrients. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 26. | Ramic J, Milovac I, Mavija Z, Lojo-kadric N, Hadzic M, Vidovic S, Niesler B, Dovrolis N, Gazouli M, Pojskic N, Pojskic L. Fecal Microbiome Diversity in Irritable Bowel Syndrome (IBS) Clinical Subtypes. Arch Clin Biomed Res. 2021;5. [DOI] [Full Text] |
| 27. | Gazouli M, Wouters MM, Kapur-Pojskić L, Bengtson MB, Friedman E, Nikčević G, Demetriou CA, Mulak A, Santos J, Niesler B. Lessons learned--resolving the enigma of genetic factors in IBS. Nat Rev Gastroenterol Hepatol. 2016;13:77-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 28. | Nakashima M, Suga N, Ikeda Y, Yoshikawa S, Matsuda S. Relevant MicroRNAs of MMPs and TIMPs with Certain Gut Microbiota Could Be Involved in the Invasiveness and Metastasis of Malignant Tumors. Innov Discov. 2024;1:10. [DOI] [Full Text] |