Published online Sep 7, 2024. doi: 10.3748/wjg.v30.i33.3791
Revised: August 10, 2024
Accepted: August 16, 2024
Published online: September 7, 2024
Processing time: 167 Days and 15.2 Hours
In this editorial, we comment on the article published in the recent issue of the World Journal of Gastroenterology. Acute liver failure (ALF) is a fatal disease that causes uncontrolled massive hepatocyte death and rapid loss of liver function. Ferroptosis and pyroptosis, cell death forms that can be initiated or blocked concurrently, can play significant roles in developing inflammation and various malignancies. However, their roles in ALF remain unclear. The article discovered the positive feedback between ferroptosis and pyroptosis in the progression of ALF, and revealed that the silent information regulator sirtuin 1 (SIRT1) inhibits both pathways through p53, dramatically reducing inflammation and protecting hepatocytes. This suggests the potential use of SIRT1 and its downstream molecules as therapeutics for ALF. Thus, we will discuss the role of ferroptosis and pyroptosis in ALF and the crosstalk between these cell death mechanisms. Additionally, we address potential treatments that could alleviate ALF by simul
Core Tip: Acute liver failure (ALF) is a life-threatening disease characterized by uncontrolled death of hepatocytes. Ferroptosis and pyroptosis are two recently discovered types of cell death that can occur simultaneously. However, their roles in ALF remain unclear. The findings show that these two cell death pathways work together to advance ALF and suggest that silent information regulator sirtuin 1 (SIRT1) and its downstream molecules could be potential therapeutics for ALF. Therefore, we will discuss the roles and crosstalk of ferroptosis and pyroptosis in ALF. Activation of SIRT1 and suppression of both cell death pathways may offer new insights into therapeutic targets for ALF.
- Citation: Xing ZY, Zhang CJ, Liu LJ. Targeting both ferroptosis and pyroptosis may represent potential therapies for acute liver failure. World J Gastroenterol 2024; 30(33): 3791-3798
- URL: https://www.wjgnet.com/1007-9327/full/v30/i33/3791.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i33.3791
Acute liver failure (ALF) is an acute clinical syndrome that occurs in patients without a history of liver disease and is life-threatening[1]. In developed countries, there are approximately 1–6 cases per million individuals. Although the incidence is rare, ALF causes a mortality rate as high as 30%[2,3]. The most prevalent causes of ALF are hepatic toxicity induced by medications or poisons, acute viral hepatitis, autoimmune and metabolic disorders, and unexplained cryptogenic liver failure[1,3]. ALF can lead to severe complications, including coagulopathy, elevated transaminases, hepatic encephalopathy, and multi-organ failure. This is often attributed to a series of severe proinflammatory states triggered by extensive hepatocyte damage, resulting in DNA damage, oxidative stress, and an accompanying inflammatory factor storm[4-6].
The main event in ALF is the excessive and uncontrolled death of hepatocytes by apoptosis, necroptosis, and necrosis[5,7,8]. Further study has found that various types of cell death are related to liver diseases such as ALF, including ferroptosis and pyroptosis[9]. These cell death pathways can coexist in a pathological environment, and several shared overlapping mechanisms can be used as “backup” death strategies to maintain biological balance within the organism when the death induction threshold is reached, mediating various immune effects and inflammatory responses[10].
Dixon et al[11] originally described ferroptosis, an iron-dependent regulated cell death, in 2012. It is characterized by iron metabolism disorder and an accumulation of excessive intracellular lipid peroxides, which lead to redox imbalance and ultimately cell death[12].
When iron metabolism is disrupted, excess iron can be released from iron storage proteins into the cytoplasm or other organelles. Free iron can react with intracellular hydrogen peroxide or other oxidants to cause lipid peroxidation of polyunsaturated fatty acids in the cell membrane via the Fenton reaction, which produces a variety of reactive oxygen species (ROS) and lipid peroxidation radicals[13,14]. System Xc- is a key regulatory component of ferroptosis, consisting of solute carrier family 7 member 11 (SLC7A11) and solute carrier family 3 member 2[15], whose antioxidant system typically protects cells from oxidative stress[16]. However, during ferroptosis, the antioxidant system may fail to efficiently neutralize the produced ROS, resulting in the cell’s incapacity to withstand lipid peroxidation[17].
The p53 gene is essential in the cellular response to a variety of stressors, including DNA damage, hypoxia, nutrient starvation, and oncogene activation[18]. P53 can also regulate ferroptosis by directly acting on the SLC7A11 promoter to reduce its expression, thereby reducing extracellular cystine intake, decreasing glutathione synthesis, lowering glutathione peroxidase 4 (GPX4) activity, increasing lipid peroxide levels, and ultimately leading to ferroptosis[19]. P53 can also recruit the deubiquitinase ubiquitin-specific peptidase 7 to the histone H2B monoubiquitination modification (H2Bub1) in the promoter region of SLC7A11, reducing H2Bub1 on the SLC7A11 gene, resulting in lower SLC7A11 protein levels and ferroptosis[20]. However, in addition to promoting ferroptosis, p53 may also inhibit it by regulating the localization and activity of dipeptidyl peptidase 4 and other pathways, thereby promoting cell survival[21]. Therefore, p53 may exhibit a “dual role” in the regulation of ferroptosis.
Ferroptosis is thought to play a substantial role in hepatocyte death in ALF[22-24]. One study reported that the ferroptosis inhibitor ferrostatin-1 significantly prevented hepatotoxicity and lipid peroxidation in mice with aceta
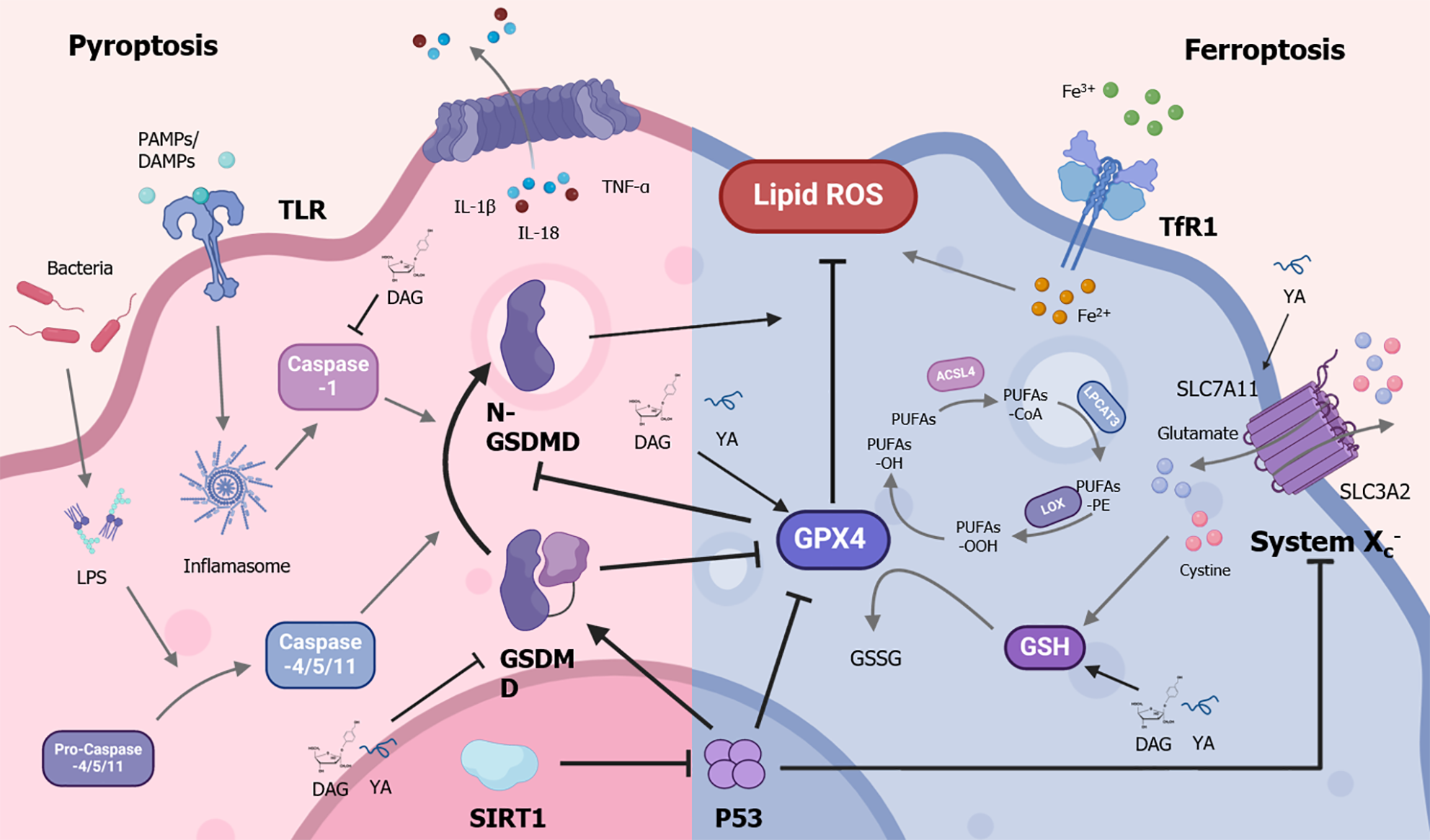
Pyroptosis is a type of immunogenic cell death mediated by caspases during microbial infections[35]. It helps the body eliminate invading pathogens. The gasdermin D (GSDMD) protein is the central driver of pyroptosis. When a cell receives signals such as pathogen-associated molecular patterns, damage-associated molecular patterns, and lipopolysaccharide (LPS) via classical or non-classical pathways, caspases-1 and caspases-4/5/11 are recruited and activated. The activated caspases perform proteolytic activity, leading to the formation of the active N-terminal fragment of GSDMD (GSDMD-N)[35]. It also promotes the cleavage of interleukin-1beta precursor and interleukin-18 precursor to produce mature cytokines[36,37]. Subsequently, GSDMD-N binds to acidic phospholipids on the cell membrane, forming oligomerized death-inducing pores and increasing intracellular osmotic pressure, leading to cell swelling and rupture[38-40]. It leads to the release of interleukin-1beta (IL-1β), interleukin (IL)-18, tumor necrosis factor-alpha (TNFα), ATP, and other substances into the extracellular space, attracting immune cells to the injury site and mediating the inflammatory immune response[37,41,42].
Previous studies have indicated that pyroptosis plays a crucial role in liver diseases. ALF patients’ liver tissue exhibits elevated levels of molecules associated with pyroptosis, including GSDMD-N, caspases-1/4, IL-1β, IL-18, and TNFα. In vitro research has shown that reducing GSDMD can lower MCP1/CCR2 protein levels, thereby decreasing neutrophil-mediated immune injury in the liver. In mouse models, deletion of the GSDMD gene effectively reduces liver inflammatory injury and increases survival rates in mice with D-Galn/LPS-induced ALF[43]. Moreover, several studies have shown that the use of pyroptosis inhibitors such as VX-765[44], GSDMD inhibitor necrosulfonamide[45], GSK3β inhibitor TDZD-8[46], limonin[47], 3,4-dihydroxyphenylethanol glycoside (DAG)[48], and tyrosine-alanine (YA)[49], can alleviate liver cell pyroptosis, reduce oxidative stress and inflammation, and improve liver injury. However, the regulatory mechanisms remain unclear. Additionally, p53-induced pyroptosis has been reported in several studies[50-53], but it is rarely reported in the liver.
Given that pyroptosis and ferroptosis are often simultaneously inhibited or encouraged in tissue injury or cancers[54-56], researchers have explored their relationship. Some studies have demonstrated a mutual regulatory link between ferroptosis and pyroptosis. For example, a deficiency of GPX4 in bone marrow cells might increase GSDMD production via caspase-1/11, resulting in pyroptosis[57]. In the diabetic retinopathy model, the ferroptosis inhibitor Ferr-1 can reduce GSDMD expression, thereby inhibiting pyroptosis and improving retinal tissue damage[58]. Chlorpyrifos promotes GSDMD cleavage and increases intracellular ROS levels, which in turn enhances p53-mediated ferroptosis[59]. The Stat3/p53/nuclear factor-E2-related factor 2 axis regulates both ferroptosis and pyroptosis in colorectal cancer cells[60]. These findings indicate that there may be crosstalk between ferroptosis and pyroptosis in diseases.
ALF is characterized by excessive death of hepatocytes. Therefore, crosstalk and co-activation of multiple death pathways are likely important mechanisms. However, there is currently little research on the combined role of ferroptosis and pyroptosis in ALF. A report revealed that treatment with YA and DAG can simultaneously reduce ferroptosis and pyroptosis in an ALF mouse model, thereby protecting the liver from damage and reducing mouse mortality[48,49]. Zhou et al[34] found that both ferroptosis and pyroptosis are triggered in the liver tissue of ALF patients. Inhibiting ferroptosis or pyroptosis protected mice from LPS/D-GalN-induced ALF. Furthermore, in the LPS/D-GalN-induced ALF mouse model, inhibiting GPX4 promoted ferroptosis and increased the expression of the pyroptosis marker GSDMD. Supplementation with GPX4 inhibited both ferroptosis and pyroptosis. It was found that the absence of GSDMD reduces not only pyroptosis but also ferroptosis in GSDMD knockout mice[34]. As a result, ferroptosis has a positive feedback regulatory effect on pyroptosis (Figure 1). Further identification of critical molecules or drugs that target the ferroptosis and pyroptosis processes may reveal potential strategies for ALF prevention and treatment.
SIRT1 is a class of nicotinamide adenine dinucleotide (+)-dependent histone deacetylases that regulate the deacetylation of histones and other proteins. The targets include p53, forkhead box class O1/3/4, heat shock factor1, hypoxia-inducible factor 1alpha, nuclear factor kappa B[61,62]. SIRT1 regulates a variety of activities associated with anti-aging and oxidative stress, including apoptosis, autophagy, mitochondrial function, DNA damage repair, metabolism, and inflammation[62,63]. SIRT1 down-regulation correlates with cell aging and increased inflammatory factors, such as IL6, IL8, and IL1B/IL-1β[64]. A recent study found that activating SIRT1 could inhibit ferroptosis induced by excessive iron through autophagy in foam cells, providing a new therapeutic target for atherosclerosis[65].
SIRT1 also plays a role in ALF (Table 1)[66-73]. Several studies have shown that the SIRT1 signal in ALF reduces cellular oxidative stress and inhibits hepatocyte death[66-68,74,75]. Further studies revealed that the p53 signaling pathway is involved in the process[69,70,76]. SIRT1 can deacetylate p53, promote autophagy, inhibit oxidative stress, and reduce inflammatory responses[70].
| Article title | Core tips | Ref. |
| Sirtuin 1 attenuates ALF by reducing reactive oxygen species via hypoxia-inducible factor 1α | Resveratrol, a SIRT1 activator, deacetylates hypoxia-inducible factor 1alpha and inhibits its activity, reducing ALF caused by hypoxia, reactive oxygen species, and apoptosis | Cao et al[66] |
| Short-term fasting attenuates lipopolysaccharide/D-galactosamine-induced ALF through SIRT1-autophagy signaling in mice | Short-term dietary restriction activates the SIRT1 signaling pathway, regulates autophagy, and reduces hepatocytes apoptosis in ALF | Long et al[67] |
| Fusobacterium nucleatum promotes the development of ALF by inhibiting the NAD+ salvage metabolic pathway | Fusobacterium nucleatum suppresses NAD+ and the SIRT1/adenosine monophosphate activated protein kinase signaling pathway, causing liver damage in ALF | Cao et al[68] |
| Hepato-protective effect of resveratrol against acetaminophen-induced liver injury is associated with inhibition of CYP-mediated bioactivation and regulation of SIRT1-p53 signaling pathways | Resveratrol helps to heal liver impairment caused by APAP by blocking CYP-mediated APAP bioactivation and regulating SIRT1, p53, cyclin D1, and proliferating cell nuclear antigen | Wang et al[69] |
| Apigenin prevents acetaminophen-induced liver injury by activating the SIRT1 pathway | Apigenin prevents acetaminophen-induced liver injury by regulating the SIRT1-p53 axis, which promotes autophagy and reduces the inflammatory response and oxidative stress caused by acetaminophen | Zhao et al[70] |
| Sirtuin-activating compounds alleviate D-galactosamine/lipopolysaccharide-induced hepatotoxicity in rats: Involvement of sirtuin 1 and heme oxygenase 1 | Quercetin and SRT1720 can activate SIRT1 protein and inhibit HO-1 expression, reducing liver damage caused by D-galactosamine/lipopolysaccharide in rats | Kemelo et al[71] |
| The sirtuin 1 activator SRT1720 alleviated endotoxin-induced fulminant hepatitis in mice | SRT1720, a SIRT1 activator, reduces fulminant hepatitis caused by lipopolysaccharide/D-Gal, potentially through inhibiting tumor necrosis factor-alpha production and activating the apoptotic cascade | Zhou et al[72] |
| Evaluation of the reparative effect of SIN in an acetaminophen-induced liver injury model | SIN successfully treats acetaminophen-induced liver injury by restoring SIRT1 levels, lowering oxidative stress, and repairing cell damage | Kayalı et al[73] |
Zhou et al[34] discovered that SIRT1 expression was reduced in human ALF liver tissue. Using SIRT1 activators or overexpressing SIRT1 could inhibit both ferroptosis events (reduced iron deposition and ROS activity, decreased expression of Acyl-CoA synthetase long-chain family 4, and increased expression of SLC7A11 and GPX4) and the expression of the pyroptosis marker GSDMD, thereby alleviating acute liver injury. In LPS/D-GalN-induced in vitro and in vivo models, the deactivation of SIRT1 increased ferroptosis and pyroptosis, exacerbating liver injury. Further research revealed that the inhibition of ferroptosis and pyroptosis by SIRT1 may depend on p53 deacetylation[34]. These results suggest that SIRT1 may serve as a molecular target to suppress multiple death processes and as a potential treatment for ALF.
Classical SIRT1 activators have been discovered, including natural ones like resveratrol, and synthetic compounds derived from the core structure of imidazole (1,2-b) thiazole, such as SRT1720 and SRT2104[77]. There have been no known clinical investigations on ALF treatment using SIRT1 activators. However, SIRT1 activators have shown an excellent safety profile and positive therapeutic effects on several diseases. Clinical trial results indicate that the natural SIRT1 activator resveratrol is effective in treating Alzheimer’s disease[78], obesity and metabolic disorders[79], and polycystic ovary syndrome[80]. The synthetic SIRT1 activator SRT2104 has been demonstrated to ameliorate sepsis[81], psoriasis[82], and blood lipid profiles in older adults[83]. Although natural drugs have some cytotoxicity, synthetic SIRT1 activators address these concerns. However, current medication development remains challenging, and clinical investigations have shown that orally administered SRT2104 has an absolute bioavailability of only 14% (NCT00937872), which is inadequate. Therefore, it may be necessary to develop appropriate drug delivery techniques, modify pharmacochemical structures, or use combination therapy. Kemelo et al[71] reported that quercetin (a natural polyphenol) and SRT1720 showed the ability to improve disease in a rat ALF model, providing evidence that combination therapy may be effective in ALF treatment. In addition, further clinical research will advance the application of SIRT1 activators in ALF treatment.
Various forms of cell death, such as apoptosis, necroptosis, necrosis, ferroptosis, and pyroptosis, have been implicated in ALF. However, the crosstalk between them remains unclear. Zhou et al[34] proved that the positive feedback between ferroptosis and pyroptosis plays an important role in hepatocyte mortality in ALF. Mutual stimulation of these cell death pathways may significantly reduce the survival rates of ALF patients. Thus, identifying targets that control the activation of these cell death pathways, as well as medicines that directly suppress these cell death pathways, may provide novel therapeutic strategies to treat ALF. SIRT1 could act as a treatment for ALF, influencing both ferroptosis and pyroptosis. However, it is unclear if SIRT1 can influence other death pathways, such as apoptosis, necroptosis, and necrosis, all of which play essential roles in ALF. Molecular targets capable of effectively controlling multiple cell death pathways have not been reported either. In addition, the efficacy of SIRT1 activation in the clinical treatment of ALF has not been reported. Therefore, addressing these concerns may provide therapeutic targets for treating ALF.
| 1. | Stravitz RT, Fontana RJ, Karvellas C, Durkalski V, McGuire B, Rule JA, Tujios S, Lee WM; Acute Liver Failure Study Group. Future directions in acute liver failure. Hepatology. 2023;78:1266-1289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 47] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 2. | Stravitz RT, Lee WM. Acute liver failure. Lancet. 2019;394:869-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 574] [Article Influence: 95.7] [Reference Citation Analysis (0)] |
| 3. | Shingina A, Mukhtar N, Wakim-Fleming J, Alqahtani S, Wong RJ, Limketkai BN, Larson AM, Grant L. Acute Liver Failure Guidelines. Am J Gastroenterol. 2023;118:1128-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 81] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 4. | Brenner C, Galluzzi L, Kepp O, Kroemer G. Decoding cell death signals in liver inflammation. J Hepatol. 2013;59:583-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 777] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 5. | Luedde T, Kaplowitz N, Schwabe RF. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology. 2014;147:765-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 575] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 6. | Vasques F, Cavazza A, Bernal W. Acute liver failure. Curr Opin Crit Care. 2022;28:198-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 7. | Schwabe RF, Luedde T. Apoptosis and necroptosis in the liver: a matter of life and death. Nat Rev Gastroenterol Hepatol. 2018;15:738-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 435] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 8. | Shojaie L, Iorga A, Dara L. Cell Death in Liver Diseases: A Review. Int J Mol Sci. 2020;21:9682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 197] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 9. | Aizawa S, Brar G, Tsukamoto H. Cell Death and Liver Disease. Gut Liver. 2020;14:20-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 10. | Gautheron J, Gores GJ, Rodrigues CMP. Lytic cell death in metabolic liver disease. J Hepatol. 2020;73:394-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 155] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 11. | Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4711] [Cited by in RCA: 11776] [Article Influence: 905.8] [Reference Citation Analysis (1)] |
| 12. | Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, Annicchiarico-Petruzzelli M, Antonov AV, Arama E, Baehrecke EH, Barlev NA, Bazan NG, Bernassola F, Bertrand MJM, Bianchi K, Blagosklonny MV, Blomgren K, Borner C, Boya P, Brenner C, Campanella M, Candi E, Carmona-Gutierrez D, Cecconi F, Chan FK, Chandel NS, Cheng EH, Chipuk JE, Cidlowski JA, Ciechanover A, Cohen GM, Conrad M, Cubillos-Ruiz JR, Czabotar PE, D'Angiolella V, Dawson TM, Dawson VL, De Laurenzi V, De Maria R, Debatin KM, DeBerardinis RJ, Deshmukh M, Di Daniele N, Di Virgilio F, Dixit VM, Dixon SJ, Duckett CS, Dynlacht BD, El-Deiry WS, Elrod JW, Fimia GM, Fulda S, García-Sáez AJ, Garg AD, Garrido C, Gavathiotis E, Golstein P, Gottlieb E, Green DR, Greene LA, Gronemeyer H, Gross A, Hajnoczky G, Hardwick JM, Harris IS, Hengartner MO, Hetz C, Ichijo H, Jäättelä M, Joseph B, Jost PJ, Juin PP, Kaiser WJ, Karin M, Kaufmann T, Kepp O, Kimchi A, Kitsis RN, Klionsky DJ, Knight RA, Kumar S, Lee SW, Lemasters JJ, Levine B, Linkermann A, Lipton SA, Lockshin RA, López-Otín C, Lowe SW, Luedde T, Lugli E, MacFarlane M, Madeo F, Malewicz M, Malorni W, Manic G, Marine JC, Martin SJ, Martinou JC, Medema JP, Mehlen P, Meier P, Melino S, Miao EA, Molkentin JD, Moll UM, Muñoz-Pinedo C, Nagata S, Nuñez G, Oberst A, Oren M, Overholtzer M, Pagano M, Panaretakis T, Pasparakis M, Penninger JM, Pereira DM, Pervaiz S, Peter ME, Piacentini M, Pinton P, Prehn JHM, Puthalakath H, Rabinovich GA, Rehm M, Rizzuto R, Rodrigues CMP, Rubinsztein DC, Rudel T, Ryan KM, Sayan E, Scorrano L, Shao F, Shi Y, Silke J, Simon HU, Sistigu A, Stockwell BR, Strasser A, Szabadkai G, Tait SWG, Tang D, Tavernarakis N, Thorburn A, Tsujimoto Y, Turk B, Vanden Berghe T, Vandenabeele P, Vander Heiden MG, Villunger A, Virgin HW, Vousden KH, Vucic D, Wagner EF, Walczak H, Wallach D, Wang Y, Wells JA, Wood W, Yuan J, Zakeri Z, Zhivotovsky B, Zitvogel L, Melino G, Kroemer G. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486-541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3672] [Cited by in RCA: 4526] [Article Influence: 646.6] [Reference Citation Analysis (0)] |
| 13. | Lei P, Bai T, Sun Y. Mechanisms of Ferroptosis and Relations With Regulated Cell Death: A Review. Front Physiol. 2019;10:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 403] [Cited by in RCA: 387] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 14. | Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31:107-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1891] [Cited by in RCA: 2451] [Article Influence: 612.8] [Reference Citation Analysis (0)] |
| 15. | Lewerenz J, Hewett SJ, Huang Y, Lambros M, Gout PW, Kalivas PW, Massie A, Smolders I, Methner A, Pergande M, Smith SB, Ganapathy V, Maher P. The cystine/glutamate antiporter system x(c)(-) in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxid Redox Signal. 2013;18:522-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 756] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 16. | Mou Y, Wang J, Wu J, He D, Zhang C, Duan C, Li B. Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol. 2019;12:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 392] [Cited by in RCA: 1249] [Article Influence: 208.2] [Reference Citation Analysis (0)] |
| 17. | Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22:266-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2184] [Cited by in RCA: 4264] [Article Influence: 1066.0] [Reference Citation Analysis (0)] |
| 18. | Kastenhuber ER, Lowe SW. Putting p53 in Context. Cell. 2017;170:1062-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 936] [Cited by in RCA: 1401] [Article Influence: 175.1] [Reference Citation Analysis (0)] |
| 19. | Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, Baer R, Gu W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1173] [Cited by in RCA: 2405] [Article Influence: 240.5] [Reference Citation Analysis (0)] |
| 20. | Wang Y, Yang L, Zhang X, Cui W, Liu Y, Sun QR, He Q, Zhao S, Zhang GA, Wang Y, Chen S. Epigenetic regulation of ferroptosis by H2B monoubiquitination and p53. EMBO Rep. 2019;20:e47563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 165] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 21. | Kang R, Kroemer G, Tang D. The tumor suppressor protein p53 and the ferroptosis network. Free Radic Biol Med. 2019;133:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 499] [Article Influence: 83.2] [Reference Citation Analysis (0)] |
| 22. | Mancardi D, Mezzanotte M, Arrigo E, Barinotti A, Roetto A. Iron Overload, Oxidative Stress, and Ferroptosis in the Failing Heart and Liver. Antioxidants (Basel). 2021;10:1864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 93] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 23. | Guo G, Yang W, Sun C, Wang X. Dissecting the potential role of ferroptosis in liver diseases: an updated review. Free Radic Res. 2023;57:282-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Lu Y, Hu J, Chen L, Li S, Yuan M, Tian X, Cao P, Qiu Z. Ferroptosis as an emerging therapeutic target in liver diseases. Front Pharmacol. 2023;14:1196287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 25. | Yamada N, Karasawa T, Kimura H, Watanabe S, Komada T, Kamata R, Sampilvanjil A, Ito J, Nakagawa K, Kuwata H, Hara S, Mizuta K, Sakuma Y, Sata N, Takahashi M. Ferroptosis driven by radical oxidation of n-6 polyunsaturated fatty acids mediates acetaminophen-induced acute liver failure. Cell Death Dis. 2020;11:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 237] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 26. | Lőrincz T, Jemnitz K, Kardon T, Mandl J, Szarka A. Ferroptosis is Involved in Acetaminophen Induced Cell Death. Pathol Oncol Res. 2015;21:1115-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 161] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 27. | Schnellmann JG, Pumford NR, Kusewitt DF, Bucci TJ, Hinson JA. Deferoxamine delays the development of the hepatotoxicity of acetaminophen in mice. Toxicol Lett. 1999;106:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Niu B, Lei X, Xu Q, Ju Y, Xu D, Mao L, Li J, Zheng Y, Sun N, Zhang X, Mao Y, Li X. Protecting mitochondria via inhibiting VDAC1 oligomerization alleviates ferroptosis in acetaminophen-induced acute liver injury. Cell Biol Toxicol. 2022;38:505-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 114] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 29. | Liu J, Huang C, Liu J, Meng C, Gu Q, Du X, Yan M, Yu Y, Liu F, Xia C. Nrf2 and its dependent autophagy activation cooperatively counteract ferroptosis to alleviate acute liver injury. Pharmacol Res. 2023;187:106563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 50] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 30. | Wang M, Liu CY, Wang T, Yu HM, Ouyang SH, Wu YP, Gong HB, Ma XH, Jiao GL, Fu LL, Wu QS, Kurihara H, Li YF, Shen T, He RR. (+)-Clausenamide protects against drug-induced liver injury by inhibiting hepatocyte ferroptosis. Cell Death Dis. 2020;11:781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 31. | Zhong X, Fan XG, Chen R. Repurposing Niclosamide as a Therapeutic Drug against Acute Liver Failure by Suppressing Ferroptosis. Pharmaceutics. 2023;15:1950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 32. | Shi P, Zhu W, Fu J, Liang A, Zheng T, Wen Z, Wu X, Peng Y, Yuan S, Wu X. Avicularin alleviates acute liver failure by regulation of the TLR4/MyD88/NF-κB and Nrf2/HO-1/GPX4 pathways to reduce inflammation and ferroptosis. J Cell Mol Med. 2023;27:3326-3338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 33. | Wang Y, Chen Q, Shi C, Jiao F, Gong Z. Mechanism of glycyrrhizin on ferroptosis during acute liver failure by inhibiting oxidative stress. Mol Med Rep. 2019;20:4081-4090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 34. | Zhou XN, Zhang Q, Peng H, Qin YJ, Liu YH, Wang L, Cheng ML, Luo XH, Li H. Silent information regulator sirtuin 1 ameliorates acute liver failure via the p53/glutathione peroxidase 4/gasdermin D axis. World J Gastroenterol. 2024;30:1588-1608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (1)] |
| 35. | Gao W, Wang X, Zhou Y, Wang X, Yu Y. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct Target Ther. 2022;7:196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 574] [Article Influence: 191.3] [Reference Citation Analysis (0)] |
| 36. | Rathinam VA, Fitzgerald KA. Inflammasome Complexes: Emerging Mechanisms and Effector Functions. Cell. 2016;165:792-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 831] [Article Influence: 103.9] [Reference Citation Analysis (0)] |
| 37. | Wu J, Lin S, Wan B, Velani B, Zhu Y. Pyroptosis in Liver Disease: New Insights into Disease Mechanisms. Aging Dis. 2019;10:1094-1108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 38. | Aglietti RA, Estevez A, Gupta A, Ramirez MG, Liu PS, Kayagaki N, Ciferri C, Dixit VM, Dueber EC. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc Natl Acad Sci USA. 2016;113:7858-7863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 714] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 39. | Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, Lieberman J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2315] [Cited by in RCA: 2356] [Article Influence: 261.8] [Reference Citation Analysis (0)] |
| 40. | Sborgi L, Rühl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H, Farady CJ, Müller DJ, Broz P, Hiller S. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016;35:1766-1778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 551] [Cited by in RCA: 897] [Article Influence: 99.7] [Reference Citation Analysis (0)] |
| 41. | Yu P, Zhang X, Liu N, Tang L, Peng C, Chen X. Pyroptosis: mechanisms and diseases. Signal Transduct Target Ther. 2021;6:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 996] [Cited by in RCA: 1398] [Article Influence: 349.5] [Reference Citation Analysis (0)] |
| 42. | Wei X, Xie F, Zhou X, Wu Y, Yan H, Liu T, Huang J, Wang F, Zhou F, Zhang L. Role of pyroptosis in inflammation and cancer. Cell Mol Immunol. 2022;19:971-992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 390] [Article Influence: 130.0] [Reference Citation Analysis (0)] |
| 43. | Li H, Zhao XK, Cheng YJ, Zhang Q, Wu J, Lu S, Zhang W, Liu Y, Zhou MY, Wang Y, Yang J, Cheng ML. Gasdermin D-mediated hepatocyte pyroptosis expands inflammatory responses that aggravate acute liver failure by upregulating monocyte chemotactic protein 1/CC chemokine receptor-2 to recruit macrophages. World J Gastroenterol. 2019;25:6527-6540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 44. | Jiao M, Wang J, Liu W, Zhao X, Qin Y, Zhang C, Yin H, Zhao C. VX-765 inhibits pyroptosis and reduces inflammation to prevent acute liver failure by upregulating PPARα expression. Ann Hepatol. 2023;28:101082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 45. | Wu YL, Ou WJ, Zhong M, Lin S, Zhu YY. Gasdermin D Inhibitor Necrosulfonamide Alleviates Lipopolysaccharide/D-galactosamine-induced Acute Liver Failure in Mice. J Clin Transl Hepatol. 2022;10:1148-1154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 46. | Zhang D, Shi C, Zhang Q, Wang Y, Guo J, Gong Z. Inhibition of GSK3β activity alleviates acute liver failure via suppressing multiple programmed cell death. J Inflamm (Lond). 2023;20:24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 47. | Yang R, Yu H, Chen J, Zhu J, Song C, Zhou L, Sun Y, Zhang Q. Limonin Attenuates LPS-Induced Hepatotoxicity by Inhibiting Pyroptosis via NLRP3/Gasdermin D Signaling Pathway. J Agric Food Chem. 2021;69:982-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 48. | Liu T, Yang L, Gao H, Zhuo Y, Tu Z, Wang Y, Xun J, Zhang Q, Zhang L, Wang X. 3,4-dihydroxyphenylethyl alcohol glycoside reduces acetaminophen-induced acute liver failure in mice by inhibiting hepatocyte ferroptosis and pyroptosis. PeerJ. 2022;10:e13082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 49. | Siregar AS, Nyiramana MM, Kim EJ, Cho SB, Woo MS, Lee DK, Hong SG, Han J, Kang SS, Kim DR, Choi YJ, Kang D. Oyster-Derived Tyr-Ala (YA) Peptide Prevents Lipopolysaccharide/D-Galactosamine-Induced Acute Liver Failure by Suppressing Inflammatory, Apoptotic, Ferroptotic, and Pyroptotic Signals. Mar Drugs. 2021;19:614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 50. | Ranjan A, Iwakuma T. Non-Canonical Cell Death Induced by p53. Int J Mol Sci. 2016;17:2068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 51. | Saha G, Chiranjivi AK, Khamar BM, Prerna K, Kumar M, Dubey VK. BLIMP-1 Mediated Downregulation of TAK1 and p53 Molecules Is Crucial in the Pathogenesis of Kala-Azar. Front Cell Infect Microbiol. 2020;10:594431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 52. | Yuliana ND, Tuarita MZ, Khatib A, Laila F, Sukarno S. GC-MS metabolomics revealed protocatechuic acid as a cytotoxic and apoptosis-inducing compound from black rice brans. Food Sci Biotechnol. 2020;29:825-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 53. | Zhu A, Cheng C, Lin S, Hong Z, Shi Z, Deng H, Zhang G. Silence of linc00023 inhibits pyroptosis and promotes cell proliferation via regulating p53. Gene. 2023;882:147628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 54. | Tang R, Xu J, Zhang B, Liu J, Liang C, Hua J, Meng Q, Yu X, Shi S. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J Hematol Oncol. 2020;13:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 879] [Cited by in RCA: 853] [Article Influence: 170.6] [Reference Citation Analysis (0)] |
| 55. | Cao Z, Qin H, Huang Y, Zhao Y, Chen Z, Hu J, Gao Q. Crosstalk of pyroptosis, ferroptosis, and mitochondrial aldehyde dehydrogenase 2-related mechanisms in sepsis-induced lung injury in a mouse model. Bioengineered. 2022;13:4810-4820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 56. | Wan S, Zhang G, Liu R, Abbas MN, Cui H. Pyroptosis, ferroptosis, and autophagy cross-talk in glioblastoma opens up new avenues for glioblastoma treatment. Cell Commun Signal. 2023;21:115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 57. | Kang R, Zeng L, Zhu S, Xie Y, Liu J, Wen Q, Cao L, Xie M, Ran Q, Kroemer G, Wang H, Billiar TR, Jiang J, Tang D. Lipid Peroxidation Drives Gasdermin D-Mediated Pyroptosis in Lethal Polymicrobial Sepsis. Cell Host Microbe. 2018;24:97-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 465] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 58. | Shao J, Bai Z, Zhang L, Zhang F. Ferrostatin-1 alleviates tissue and cell damage in diabetic retinopathy by improving the antioxidant capacity of the Xc(-)-GPX4 system. Cell Death Discov. 2022;8:426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 59. | Han C, Sheng J, Pei H, Sheng Y, Wang J, Zhou X, Li W, Cao C, Yang Y. Environmental toxin chlorpyrifos induces liver injury by activating P53-mediated ferroptosis via GSDMD-mtROS. Ecotoxicol Environ Saf. 2023;257:114938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 60. | Wu Y, Pi D, Zhou S, Yi Z, Dong Y, Wang W, Ye H, Chen Y, Zuo Q, Ouyang M. Ginsenoside Rh3 induces pyroptosis and ferroptosis through the Stat3/p53/NRF2 axis in colorectal cancer cells. Acta Biochim Biophys Sin (Shanghai). 2023;55:587-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 61. | Sauve AA, Wolberger C, Schramm VL, Boeke JD. The biochemistry of sirtuins. Annu Rev Biochem. 2006;75:435-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 589] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 62. | Yang Y, Liu Y, Wang Y, Chao Y, Zhang J, Jia Y, Tie J, Hu D. Regulation of SIRT1 and Its Roles in Inflammation. Front Immunol. 2022;13:831168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 288] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 63. | Singh CK, Chhabra G, Ndiaye MA, Garcia-Peterson LM, Mack NJ, Ahmad N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid Redox Signal. 2018;28:643-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 583] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 64. | Wang L, Xu C, Johansen T, Berger SL, Dou Z. SIRT1 - a new mammalian substrate of nuclear autophagy. Autophagy. 2021;17:593-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 65. | Su G, Yang W, Wang S, Geng C, Guan X. SIRT1-autophagy axis inhibits excess iron-induced ferroptosis of foam cells and subsequently increases IL-1Β and IL-18. Biochem Biophys Res Commun. 2021;561:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 66. | Cao P, Chen Q, Shi CX, Wang LW, Gong ZJ. Sirtuin1 attenuates acute liver failure by reducing reactive oxygen species via hypoxia inducible factor 1α. World J Gastroenterol. 2022;28:1798-1813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 67. | Long B, Tao H, Tong S, Wang X, Yin W. Short-term fasting attenuates lipopolysaccharide/D-galactosamine-induced acute liver failure through Sirt1-autophagy signaling in mice. MedComm (2020). 2023;4:e412. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 68. | Cao P, Chen Q, Shi C, Wang L, Gong Z. Fusobacterium nucleatum promotes the development of acute liver failure by inhibiting the NAD(+) salvage metabolic pathway. Gut Pathog. 2022;14:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 69. | Wang Y, Jiang Y, Fan X, Tan H, Zeng H, Wang Y, Chen P, Huang M, Bi H. Hepato-protective effect of resveratrol against acetaminophen-induced liver injury is associated with inhibition of CYP-mediated bioactivation and regulation of SIRT1-p53 signaling pathways. Toxicol Lett. 2015;236:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 70. | Zhao L, Zhang J, Hu C, Wang T, Lu J, Wu C, Chen L, Jin M, Ji G, Cao Q, Jiang Y. Apigenin Prevents Acetaminophen-Induced Liver Injury by Activating the SIRT1 Pathway. Front Pharmacol. 2020;11:514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 71. | Kemelo MK, Kutinová Canová N, Horinek A, Farghali H. Sirtuin-activating compounds (STACs) alleviate D-galactosamine/lipopolysaccharide-induced hepatotoxicity in rats: involvement of sirtuin 1 and heme oxygenase 1. Physiol Res. 2017;66:497-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 72. | Zhou D, Yang F, Lin L, Tang L, Li L, Yang Y, Liu D, Zhang C, Wu T, Wei H, Zhang X, Zhang L. The sirtuin 1 activator SRT1720 alleviated endotoxin-induced fulminant hepatitis in mice. Exp Anim. 2021;70:302-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 73. | Kayalı A, Bora ES, Acar H, Erbaş O. Evaluation of the Reparative Effect of Sinomenine in an Acetaminophen-Induced Liver Injury Model. Curr Issues Mol Biol. 2024;46:923-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 74. | Zhang J, Hu C, Li X, Liang L, Zhang M, Chen B, Liu X, Yang D. Protective Effect of Dihydrokaempferol on Acetaminophen-Induced Liver Injury by Activating the SIRT1 Pathway. Am J Chin Med. 2021;49:705-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 75. | Cao P, Chen Q, Shi C, Pei M, Wang L, Gong Z. Pinocembrin ameliorates acute liver failure via activating the Sirt1/PPARα pathway in vitro and in vivo. Eur J Pharmacol. 2022;915:174610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 76. | Nakamura K, Zhang M, Kageyama S, Ke B, Fujii T, Sosa RA, Reed EF, Datta N, Zarrinpar A, Busuttil RW, Araujo JA, Kupiec-Weglinski JW. Macrophage heme oxygenase-1-SIRT1-p53 axis regulates sterile inflammation in liver ischemia-reperfusion injury. J Hepatol. 2017;67:1232-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 172] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 77. | Chang N, Li J, Lin S, Zhang J, Zeng W, Ma G, Wang Y. Emerging roles of SIRT1 activator, SRT2104, in disease treatment. Sci Rep. 2024;14:5521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 78. | Moussa C, Hebron M, Huang X, Ahn J, Rissman RA, Aisen PS, Turner RS. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer's disease. J Neuroinflammation. 2017;14:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 503] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 79. | Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, Moonen-Kornips E, Hesselink MKC, Kunz I, Schrauwen-Hinderling VB, Blaak E, Auwerx J, Schrauwen P. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 901] [Cited by in RCA: 986] [Article Influence: 70.4] [Reference Citation Analysis (0)] |
| 80. | Ardehjani NA, Agha-Hosseini M, Nashtaei MS, Khodarahmian M, Shabani M, Jabarpour M, Fereidouni F, Rastegar T, Amidi F. Resveratrol ameliorates mitochondrial biogenesis and reproductive outcomes in women with polycystic ovary syndrome undergoing assisted reproduction: a randomized, triple-blind, placebo-controlled clinical trial. J Ovarian Res. 2024;17:143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 81. | van der Meer AJ, Scicluna BP, Moerland PD, Lin J, Jacobson EW, Vlasuk GP, van der Poll T. The Selective Sirtuin 1 Activator SRT2104 Reduces Endotoxin-Induced Cytokine Release and Coagulation Activation in Humans. Crit Care Med. 2015;43:e199-e202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 82. | Krueger JG, Suárez-Fariñas M, Cueto I, Khacherian A, Matheson R, Parish LC, Leonardi C, Shortino D, Gupta A, Haddad J, Vlasuk GP, Jacobson EW. A Randomized, Placebo-Controlled Study of SRT2104, a SIRT1 Activator, in Patients with Moderate to Severe Psoriasis. PLoS One. 2015;10:e0142081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 83. | Libri V, Brown AP, Gambarota G, Haddad J, Shields GS, Dawes H, Pinato DJ, Hoffman E, Elliot PJ, Vlasuk GP, Jacobson E, Wilkins MR, Matthews PM. A pilot randomized, placebo controlled, double blind phase I trial of the novel SIRT1 activator SRT2104 in elderly volunteers. PLoS One. 2012;7:e51395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |