Published online Aug 28, 2024. doi: 10.3748/wjg.v30.i32.3748
Revised: July 19, 2024
Accepted: August 9, 2024
Published online: August 28, 2024
Processing time: 183 Days and 19.1 Hours
The majority of esophageal subepithelial lesions originating from the muscularis propria (SEL-MPs) are benign in nature, although a subset may exhibit malignant characteristics. Conventional endoscopic resection techniques are time-consuming and lack efficacy for small SEL-MPs.
To evaluate the efficacy and safety of ligation-assisted endoscopic submucosal resection (ESMR-L) following unroofing technique for small esophageal SEL-MPs.
From January 2021 to September 2023, 17 patients diagnosed with esophageal SEL-MPs underwent ESMR-L following unroofing technique at the endoscopy center of Shenzhen People’s Hospital. Details of clinicopathological characteristics and clinical outcomes were collected and analyzed.
The mean age of the patients was 50.12 ± 12.65 years. The mean size of the tumors was 7.47 ± 2.83 mm and all cases achieved en bloc resection successfully. The average operation time was 12.2 minutes without any complications. Histopathology identified 2 Lesions (11.8%) as gastrointestinal stromal tumors at very low risk, 12 Lesions (70.6%) as leiomyoma and 3 Lesions (17.6%) as smooth muscle proliferation. No recurrence was found during the mean follow-up duration of 14.18 ± 9.62 months.
ESMR-L following roofing technique is an effective and safe technique for management of esophageal SEL-MPs smaller than 20 mm, but it cannot ensure en bloc resection and may require further treatment.
Core Tip: This is a retrospective study to evaluate the efficacy and safety of ligation-assisted endoscopic submucosal resection following unroofing technique for management of esophageal subepithelial lesions originating from the muscularis propria (SEL-MPs) smaller than 20 mm. The technique has numerous advantages, such as simple operation, complete tumor removal, short operation time, less complications, cost effectiveness, etc. It is an effective and safe technique for management of esophageal SEL-MPs smaller than 20 mm, but it cannot ensure en bloc resection and may require further treatment.
- Citation: Lu Q, Peng QZ, Yao J, Wang LS, Li DF. Ligation-assisted endoscopic submucosal resection following unroofing technique for small esophageal subepithelial lesions originating from the muscularis propria. World J Gastroenterol 2024; 30(32): 3748-3754
- URL: https://www.wjgnet.com/1007-9327/full/v30/i32/3748.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i32.3748
Subepithelial lesions (SELs), also known as submucosal tumors within the gastrointestinal (GI) tract, are defined as the tumors originating from the muscularis mucosa, submucosa or muscularis propria (MP). SELs are predominantly located in the stomach, followed by the esophagus, duodenum, and large intestine[1]. They are characterized as rounded protrusions or masses covered with normal overlying mucosa and are commonly detected incidentally during endoscopic examination[2]. The incidence of malignancy is proved to be greater in gastric and esophageal lesions, compared to that in small intestinal and large intestinal lesions. And lesions exceeding 20 mm in diameter exhibit a higher propensity for malignancy compared to the smaller ones[3]. Esophageal SELs constitute less than 1% of all esophageal neoplasms and demonstrate a benign nature in over 90% of cases. While esophageal leiomyomas are typically benign and represent the most common subtype, certain uncommon cases such as GI stromal tumors (GISTs), lymphoepithelioma-like carcinoma, and leiomyosarcoma exhibit malignant features and possess potential for distant metastasis[4-6].
According to American Gastroenterological Association clinical practice, utilization of endoscopic ultrasound (EUS) surveillance is recommended for SELs originating from the MP (SEL-MPs) smaller than 2 cm[2]. However, differentiating potentially malignant esophageal SELs like GISTs from benign lesions through EUS poses a considerable challenge. Although EUS-guided fine needle aspiration serves as a valuable technique to obtain pathological specimens of SELs, its diagnostic accuracy fluctuates according to the size of the target lesion and a range of other variables[7,8]. Therefore, continuous surveillance without resection may pose significant risks and substantial psychological and financial burdens on patients, necessitating the removal of small SEL-MPs in specific circumstances[9].
Various conventional endoscopic resection techniques such as endoscopic submucosal excavation (ESE), submucosal tunneling endoscopic resection (STER), endoscopic full-thickness resection, have been demonstrated to be both safe and feasible for the management of esophageal SEL-MPs[1,2,10]. However, these techniques are time-consuming and lack efficacy for small esophageal SEL-MPs. To optimize the technique of endoscopic resection, we present an innovative ligation-assisted endoscopic submucosal resection (ESMR-L) following unroofing technique developed in our center. In this retrospective study, we evaluated the efficacy and safety of ESMR-L for the management of esophageal SEL-MPs smaller than 20 mm.
The study was conducted on consecutive patients with esophageal SELs treated by ESMR-L following unroofing technique at the endoscopy center of Shenzhen People’s Hospital from January 2021 to September 2023. The inclusion criteria were as follows: (1) Esophageal SELs confirmed by EUS originated from MP layer with intracavitary growth; and (2) The diameter of the tumor was smaller than 20 mm.
The procedure was performed using a standard single-channel endoscope (GIF-260; Olympus) equipped with a soft, straight, transparent, 14.9 mm diameter cap (D-201-11802; Olympus) attached to the tip. A ligating device (MAJ-339; Olympus) with a detachable 20 mm diameter nylon endoloop was inserted into the accessory channel of the endoscope. Injection needles were used in submucosal injection and snares were used to mark dots and remove tumors.
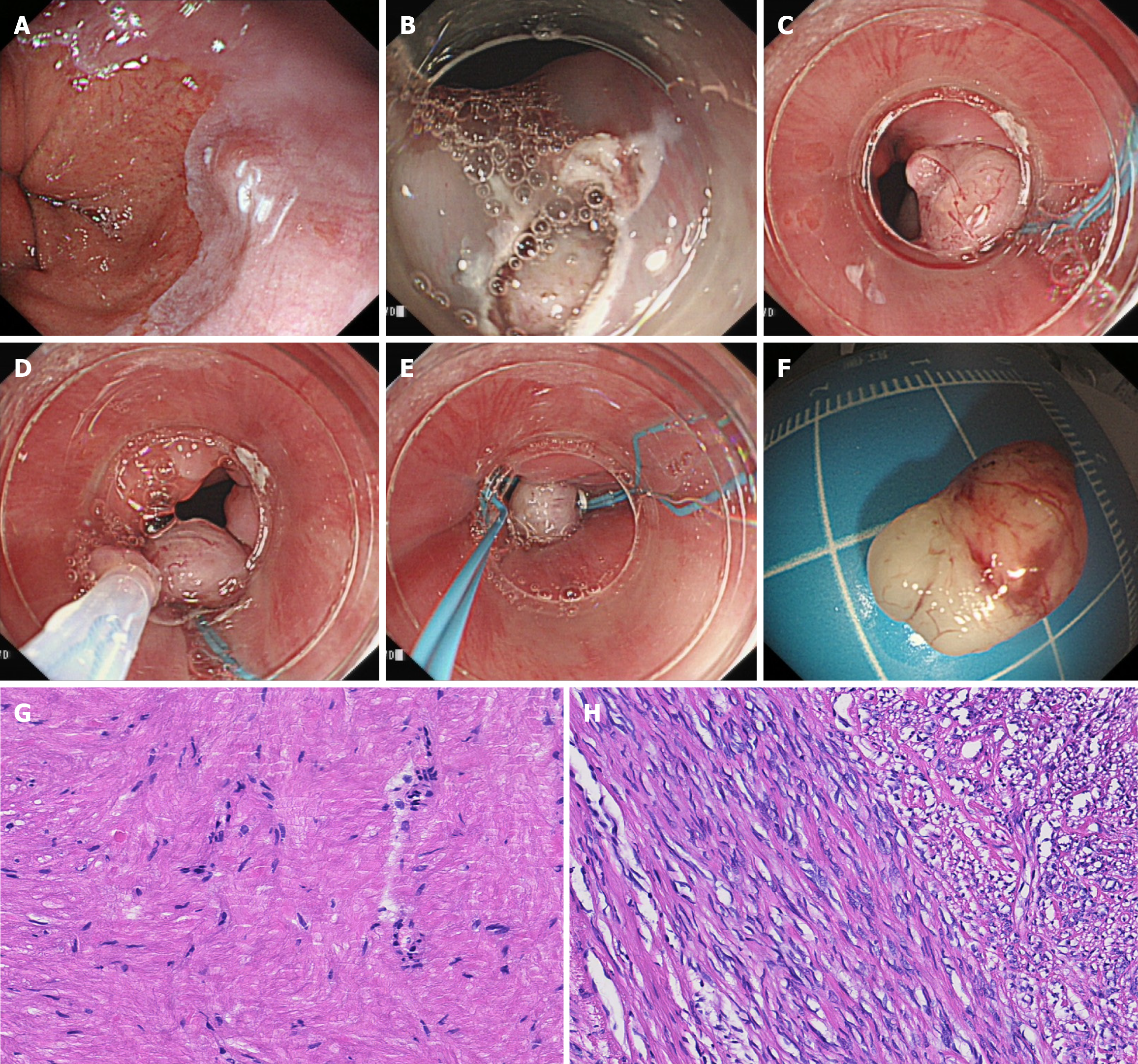
All procedures were performed by one experienced endoscopist (Dr. Li) in our center under conscious sedation. Before the procedure, all lesions confirmed by EUS were identified as hypoechoic and homogeneous masses originating from esophageal MP layer without any enlarged lymph nodes detected. The procedure of ESMR-L following unroofing technique was briefly described as follows: (1) Marked dots around the lesions by snare; (2) Injected mixture solution submucosally beneath the marking dots; (3) Unroofed the apical mucosa around the marking dots by snare; (4) Aspired the lesions and ligated the lesions fully with endoloop; (5) Resected the lesion by cold snare above the endoloop; and (6) Ligated the defect using another endoloop (Figure 1 and Video 1). After resection, the lesion sites were observed carefully to check whether there were any residual tumors, bleeding, perforations, etc. In case of complications occurred during the procedure, hemoclips or alternative devices may be used to manage the wound.
Pathological evaluation of the resected specimens included histopathologic type, depth of invasion, resection margins, etc. The pathological diagnosis was confirmed by experienced pathologists. En bloc resection was defined as endoscopic resection of the entire lesions in one piece with tumor-free margins. Complete removal was defined as the gross absence of any tumor remnant after resection.
All patients were recommended to undergo routine endoscopic follow-up 2 months after the procedure. If the pathological diagnosis revealed leiomyomas or other benign tumors, no further surveillance was deemed necessary. Patients diagnosed with GISTs were recommended to undergo annual computed tomography and endoscopic evaluations thereafter. In case of residual tumors and recurrence, further endoscopic or surgical interventions would be performed.
All statistical analyses were performed using statistical product and service solutions software version 25.0 (International Business Machines, Armonk, NY, United States). Continuous variables were expressed as mean ± SD and categorical data were displayed as numbers and percentages.
A total of 17 patients diagnosed with esophageal SEL-MPs underwent ESMR-L following unroofing technique during the study period. The clinicopathological characteristics and clinical outcomes of the patients are presented in Table 1, while Table 2 provides a summary of the information. The mean age of the patients was 50.12 ± 12.65 years (range 26-68 years). The majority of tumors were predominantly located in the distal segment of the esophagus, with an average tumor size measuring 7.47 ± 2.83 mm. En bloc resection was achieved in all patients without any complications and the mean operation time was 12.2 minutes (range 7-22 minutes). Pathologically, 2 Lesions (11.8%) were identified as GISTs at very low risk and 12 Lesions were diagnosed as leiomyoma. The remaining 3 Lesions exhibited smooth muscle proliferation. The mean follow-up duration was 14.18 ± 9.62 months (range 2-33 months) with no recurrence.
| Case | Sex/age (years) | Location | Size (mm) | Operation time (min) | En bloc resection | Complications | Pathological diagnosis | Follow-up (months) | Recurrence |
| 1 | M/54 | Lower | 12 | 8 | Yes | None | GIST | 9 | No |
| 2 | M/73 | Lower | 3 | 14 | Yes | None | Leiomyoma | 8 | No |
| 3 | F/66 | Lower | 5 | 14 | Yes | None | Leiomyoma | 8 | No |
| 4 | F/44 | Upper | 6 | 15 | Yes | None | Leiomyoma | 5 | No |
| 5 | M/58 | Middle | 8 | 22 | Yes | None | Leiomyoma | 18 | No |
| 6 | F/57 | Lower | 6 | 10 | Yes | None | Leiomyoma | 3 | No |
| 7 | M/68 | Lower | 7 | 9 | Yes | None | Leiomyoma | 5 | No |
| 8 | M/39 | Lower | 5 | 12 | Yes | None | Smooth muscle proliferation | 9 | No |
| 9 | F/35 | Lower | 9 | 9 | Yes | None | Leiomyoma | 13 | No |
| 10 | M/26 | Lower | 13 | 18 | Yes | None | Leiomyoma | 15 | No |
| 11 | F/46 | Lower | 8 | 13 | Yes | None | Smooth muscle proliferation | 17 | No |
| 12 | M/41 | Lower | 5 | 11 | Yes | None | Leiomyoma | 18 | No |
| 13 | M/51 | Lower | 8 | 13 | Yes | None | Leiomyoma | 22 | No |
| 14 | F/42 | Lower | 10 | 10 | Yes | None | Leiomyoma | 33 | No |
| 15 | M/41 | Lower | 4 | 7 | Yes | None | Smooth muscle proliferation | 34 | No |
| 16 | F/50 | Lower | 7 | 10 | Yes | None | Leiomyoma | 22 | No |
| 17 | F/61 | Lower | 11 | 13 | Yes | None | GIST | 2 | No |
| Summary of clinicopathological characteristics and clinical outcomes | |
| Age/year (mean ± SD) | 50.12 ± 12.65 |
| Gender | |
| Male | 9 (52.9) |
| Female | 8 (47.1) |
| Tumor size/mm | |
| > 10 | 4 (23.5) |
| < 10 | 13 (76.5) |
| Mean size/mm (mean ± SD) | 7.47 ± 2.83 |
| Mean operation time/min | 12.2 |
| En bloc resection | 17 (100) |
| Complications | |
| Bleeding | 0 |
| Perforation | 0 |
| Others | 0 |
| Pathological diagnosis | |
| GIST (very low risk) | 2 (11.8) |
| Leiomyoma | 12 (70.6) |
| Others | 3 (17.6) |
| Follow-up duration/month (mean ± SD) | 14.18 ± 9.62 |
| Recurrence | 0 |
The incidence of esophageal SELs remains uncommonly rare. Although the patients are typically asymptomatic, a recent study revealed that esophageal SELs exhibit pathological consequences on esophageal motility, primarily manifesting as ineffective esophageal motility disorder[11]. A considerable proportion of patients in China have requested the removal of small esophageal SEL-MPs because of the follow-up anxiety, fear, cost, uncertain malignant potential, etc. However, long procedure time and increased adverse events limited the application of conventional endoscopic resection techniques on small esophageal SEL-MPs[1,2,10].
In recent years, more and more innovative endoscopic techniques are springing up with the rapid development of GI endoscopy. Ko et al[12] reported a novel 3D-printed tailored cap for removal of small esophageal SELs. Although the 3D-printed tailored caps demonstrated high en bloc resection rates and efficient procedure time, their individual customization for each patient resulted in an augmented treatment cost. Liu et al[13] introduced a novel technique, known as endoscopic muscularis dissection, derived from endoscopic submucosal dissection, for removal of upper GI SEL-MPs. Although it was proven to be feasible and minimally invasive, the blunt dissection in the MP layer posed challenges and necessitated skilled endoscopists, ultimately resulting in a heightened risk of perforation. Guo et al[14] developed the ligation-assisted endoscopic enucleation technique for treating small esophageal SEL-MPs (less than 12 mm) with a mean procedure time of 12.5 ± 4.6 minutes. However, complete dissection of the spherical tumors posed a challenge and entailed a certain risk of intraoperative hemorrhage.
Inspired by ESMR-L of gastric SEL-MPs with a detached ligation device after apical mucosal incision[15] reported in our center, we had developed a similar ESMR-L following unroofing technique for esophageal SEL-MPs smaller than 20 mm. Our study demonstrated that ESMR-L following unroofing technique had following advantages: (1) Simple operation. Irrespective of the position of the SELs, the ligation device effectively generated negative pressure to aspirate the lesion into the transparent cap and subsequently released the endoloop, inducing a polypoid configuration with a pseudo-stalk that facilitated resection by cold snare; (2) Complete removal. Although there was a possibility of incomplete resection, the utilization of double-ligation ensured complete removal of the tumors. This was further confirmed by pathological examination, and even in cases where residual tumor existed, it would eventually undergo necrosis and be shed over time due to the “loop-and-let-go” effect[16,17]; (3) Short operation time. Lu et al[18] reported the mean operation time of ESE and STER for esophageal or cardial SEL-MPs was 65.9 minutes and 84.4 minutes respectively. The mean operation time in our study was found to be 12.2 minutes, significantly shorter than that achieved using conventional techniques; (4) Less complications. The utilizaiton of double ligation and cold snare in the ESMR-L technique significantly mitigated the risk of bleeding and perforation. The patients included in our study did not experience any postoperative complications. Relevant studies have previously indicated that conventional techniques were associated with elevated rates of adverse events, whereas in Ye et al’s study on the treatment of small esophageal SEL-MPs using endoscopic excavation technique, there were still 4 cases (8.9%) that experienced perforation[19]; and (5) Cost effectiveness. Endoscopic unroofing technique by snaring had been proved safe and effective for partial mucosal resection[20,21]. Additionally, it eliminated the need for a hook knife and reduced patients’ cost.
There were also several limitations in our study. For instance, achieving en bloc resection of all tumors using this technique seemed unattainable. Instead, complete removal, defined as “gross” absence of any remnant tumor in our study, could theoretically be achieved in all cases. Therefore, long-term follow-up and further treatments should always be considered in case of incomplete resection and malignant SELs. Additionally, it is important to note that our study was conducted at a single center with a small sample size. Hence, multi-center prospective randomized controlled trials with larger sample sizes are necessary to assess the clinical feasibility and value of this technique.
ESMR-L following unroofing technique is an effective and safe technique for management of esophageal SEL-MPs smaller than 20 mm. However, it cannot ensure en bloc resection and may require further treatment.
We acknowledge Feng X and Hu ZC for helping collect the clinical data.
| 1. | Deprez PH, Moons LMG, OʼToole D, Gincul R, Seicean A, Pimentel-Nunes P, Fernández-Esparrach G, Polkowski M, Vieth M, Borbath I, Moreels TG, Nieveen van Dijkum E, Blay JY, van Hooft JE. Endoscopic management of subepithelial lesions including neuroendocrine neoplasms: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022;54:412-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 192] [Article Influence: 64.0] [Reference Citation Analysis (1)] |
| 2. | Sharzehi K, Sethi A, Savides T. AGA Clinical Practice Update on Management of Subepithelial Lesions Encountered During Routine Endoscopy: Expert Review. Clin Gastroenterol Hepatol. 2022;20:2435-2443.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 3. | Sadeghi A, Zali MR, Tayefeh Norooz M, Pishgahi M, Ketabi Moghadam P. Management of gastrointestinal subepithelial lesions: an answer to the conflicting opinions. Gastroenterol Hepatol Bed Bench. 2023;16:378-385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 4. | Ko WJ, Song GW, Cho JY. Evaluation and Endoscopic Management of Esophageal Submucosal Tumor. Clin Endosc. 2017;50:250-253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Mutrie CJ, Donahue DM, Wain JC, Wright CD, Gaissert HA, Grillo HC, Mathisen DJ, Allan JS. Esophageal leiomyoma: a 40-year experience. Ann Thorac Surg. 2005;79:1122-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Jin Y, Chengbo L, Qiuyuan W, Yuyong T, Deliang L. Danger hidden underneath: a rare esophageal subepithelial lesion. Endoscopy. 2019;51:E225-E226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 7. | Lian J, Ji Y, Chen T, Wang G, Wang M, Li S, Cao J, Shen L, Lu W, Xu M. Endoscopic resection for esophageal gastrointestinal stromal tumors: a multi-center feasibility study. Therap Adv Gastroenterol. 2024;17:17562848241255304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 8. | Verloop CA, Goos JAC, Bruno MJ, Quispel R, van Driel LMJW, Hol L. Diagnostic yield of endoscopic and EUS-guided biopsy techniques in subepithelial lesions of the upper GI tract: a systematic review. Gastrointest Endosc. 2024;99:895-911.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 9. | Chen H, Li B, Li L, Vachaparambil CT, Lamm V, Chu Y, Xu M, Cai Q. Current Status of Endoscopic Resection of Gastric Subepithelial Tumors. Am J Gastroenterol. 2019;114:718-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Ponte Neto FL, de Moura DTH, Sagae VMT, Ribeiro IB, Mancini FC, Boghossian MB, McCarty TR, Miyajima NT, Ide E, Bernardo WM, de Moura EGH. Endoscopic resection of esophageal and gastric submucosal tumors from the muscularis propria layer: submucosal tunneling endoscopic resection versus endoscopic submucosal excavation: A systematic review and meta-analysis. Surg Endosc. 2021;35:6413-6426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Çifcibaşi Örmeci A, Çavuş B, Akas R, Istemihan Z, Imanov Z, Şenkal V, Nuriyev K, Bayraktar A, Külle CB, Keskin M, Demir K, Beşişik F, Kaymakoğlu S, Akyüz F. What is the effect of subepithelial lesions of the esophagus on esophageal motility? Eur Rev Med Pharmacol Sci. 2022;26:6300-6309. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Ko WJ, Song GW, Hong SP, Kwon CI, Hahm KB, Cho JY. Novel 3D-printing technique for caps to enable tailored therapeutic endoscopy. Dig Endosc. 2016;28:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Liu BR, Song JT, Qu B, Wen JF, Yin JB, Liu W. Endoscopic muscularis dissection for upper gastrointestinal subepithelial tumors originating from the muscularis propria. Surg Endosc. 2012;26:3141-3148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Guo J, Liu Z, Sun S, Liu X, Wang S, Ge N. Ligation-assisted endoscopic enucleation for treatment of esophageal subepithelial lesions originating from the muscularis propria: a preliminary study. Dis Esophagus. 2015;28:312-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Zhang D, Lin Q, Shi R, Wang L, Yao J, Tian Y. Ligation-assisted endoscopic submucosal resection with apical mucosal incision to treat gastric subepithelial tumors originating from the muscularis propria. Endoscopy. 2018;50:1180-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Arezzo A, Verra M, Miegge A, Morino M. Loop-and-let-go technique for a bleeding, large sessile gastric gastrointestinal stromal tumor (GIST). Endoscopy. 2011;43 Suppl 2 UCTN:E18-E19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Veloso R, Pinho R, Rodrigues A, Pais T, Fernandes C, Carvalho J, Fraga J. Endoloop ligation ("loop-and-let-go") of a large ileal lipoma by balloon-assisted enteroscopy. Endoscopy. 2012;44 Suppl 2 UCTN:E176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Lu J, Jiao T, Zheng M, Lu X. Endoscopic resection of submucosal tumors in muscularis propria: the choice between direct excavation and tunneling resection. Surg Endosc. 2014;28:3401-3407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Ye LP, Zhu LH, Zhou XB, Mao XL, Zhang Y. Endoscopic excavation for the treatment of small esophageal subepithelial tumors originating from the muscularis propria. Hepatogastroenterology. 2015;62:65-68. [PubMed] |
| 20. | Mimura T, Kuramoto S, Hashimoto M, Yamasaki K, Kobayashi K, Kobayashi M, Oohara T. Unroofing for lymphangioma of the large intestine: a new approach to endoscopic treatment. Gastrointest Endosc. 1997;46:259-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Lee CK, Chung IK, Lee SH, Lee SH, Lee TH, Park SH, Kim HS, Kim SJ, Cho HD. Endoscopic partial resection with the unroofing technique for reliable tissue diagnosis of upper GI subepithelial tumors originating from the muscularis propria on EUS (with video). Gastrointest Endosc. 2010;71:188-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |