Published online Aug 21, 2024. doi: 10.3748/wjg.v30.i31.3680
Revised: July 11, 2024
Accepted: August 1, 2024
Published online: August 21, 2024
Processing time: 141 Days and 22 Hours
Commonly used cleaning brushes in the reprocessing of flexible endoscopes often cause damage within the working channels.
To develop a spray flushing system to achieving effective cleaning of the working channels while minimizing damage.
This prospective study included 60 used endoscopes and 60 Teflon tubes ran
The ATP levels (RLU) in the two groups were 32.5 (13-66) and 26 (16-40), respectively (P > 0.05). Cleanliness scores were 1.5 (1-2) and 1 (1-2), respectively (P > 0.05). Debris was found in 73.3% of the control group, which was significantly higher than 46.7% in the experimental group (P < 0.05). Microbiological tests for both groups yielded negative results. Teflon tube damage in the control group was rated at 4 (4-5.25), which was significantly higher than in the experimental group 4 (3-4) (P < 0.01).
The spray flushing system demonstrated superior efficacy in removing debris and resulted in less damage to the endoscope working channels compared with traditional cleaning brushes.
Core Tip: Commonly used cleaning brushes in the reprocessing of flexible endoscopes often cause damage within the working channels. Our team has developed a spray flushing brush to replace traditional bristle brushes in the cleaning process. Compared with traditional cleaning brushes, the spray flushing system in reprocessing of flexible endoscopes did not compromise the cleaning quality and microbial cultures. However, the spray flushing system demonstrated better debris removal from the working channels and reduced working channel damage.
- Citation: Du J, Zhang M, Tao SY, Ye LS, Gong H, Hu B, Zhang QY, Qiao F. Efficacy of spray flushing in the reprocessing of flexible endoscopes: A randomized controlled trial. World J Gastroenterol 2024; 30(31): 3680-3688
- URL: https://www.wjgnet.com/1007-9327/full/v30/i31/3680.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i31.3680
With the continuous advancement of endoscopic technology, minimally invasive endoscopic therapy has become a vital method for diagnosing and treating gastrointestinal diseases[1,2]. Techniques such as endoscopic submucosal dissection and endoscopic retrograde cholangiopancreatography have largely replaced surgical procedures in certain diagnoses and treatments. Ensuring the safe implementation of endoscopic surgery relies on the quality of endoscope reprocessing; a key focus of infection control. As minimally invasive endoscopic diagnosis and treatment techniques rapidly develop, our demands for the quality of endoscope reprocessing have heightened. The growth of biofilms in endoscope working channels has emerged as a significant factor affecting reprocessing quality. Studies have shown a positive correlation between the formation of scratches in endoscopic working channels and biofilm development[3-5]. Bacterial adhesion and biofilm formation are particularly concentrated in areas with dense scratches. Repeated passage of various instruments through these channels is a major cause of scratch formation and increased roughness, with cleaning brushes being the primary contributor[6].
Currently, the most commonly used tool for cleaning endoscopic working channels is a brush, with its hard bristles being crucial for thorough cleaning but also posing a risk of channel damage. Therefore, developing cleaning brushes that minimize damage to the working channel is crucial for reducing endoscope damage, preventing bacterial adhesion and biofilm formation, and enhancing the quality of endoscope reprocessing.
Our team has developed a spray flushing brush to replace traditional bristle brushes in the cleaning process, aiming to clean endoscopic working channels while minimizing damage. In this study, we investigate commonly used digestive endoscopes and simulated endoscopic working channels to assess the effectiveness of the spray cleaning brush in cleaning the channel and reducing damage to it.
This study was conducted at the Digestive Endoscopy Center of West China Hospital, Sichuan University, China. The center, which receives over 500 endoscopic treatments per day, serves as a prominent facility for gastrointestinal procedures.
The study utilized electronic gastroscopes (GIF-H290; Olympus, Japan), a common choice in clinical practice. And Teflon tubes (RBF4; Oupli, China) with a length of 100 cm and an inner diameter of 3 mm, consistent with the study by Santos et al[7]. The material of Teflon tubes was the same as that of the endoscope working channel. All electronic gastroscopes have not undergone repairs on working channels, and had been in use for > 6 months. Prior to the study, a borescope (Test-H; Xinhua, China) was used to confirm that all Teflon tubes had no initial damage within the pipes.
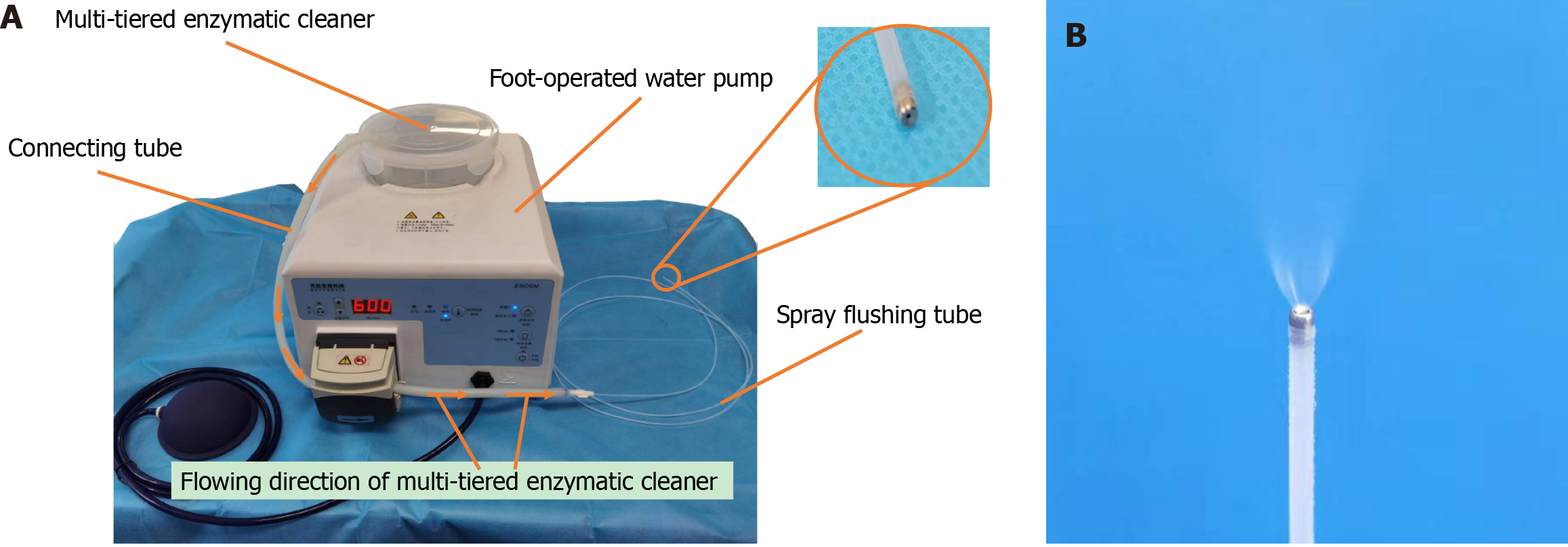
An excel table was utilized to generate 60 random numbers, which were then used to randomly assign the electronic gastroscopes and Teflon tubes into a control group (n = 30) and an experimental group (n = 30). In the control group, manual cleaning was performed using a traditional cleaning brush (BW-20T; Olympus), whereas the experimental group underwent manual cleaning with a spray flushing system. The spray flushing system consisted of a spray flushing tube (VDK-ST-23-180-B; Vedkang, China), a connecting tube (MAJ-1608; Olympus), and a foot-operated water pump (ESCGV; Skyforbio, China). The spray flushing tube was connected to the foot-operated water pump via the connecting tube, which contained 1.8 L of multi-tiered enzymatic cleaner (SCOPEZIME; Ruhof, Mineola, NY, United States) and sprayed at a rate of 600 mL/minute. Figure 1 provides detailed information on the spraying system and the effect of the spray flushing tube.
After completion of manual cleaning of the electronic gastroscopes, the ATP value of the endoscopic working channel was measured using an ATP fluorescence detector (Clean Trace TM NGi handheld fluorescence detector; 3M, United States). Subsequently, the cleanliness of the working channel was assessed by observing it with a borescope (Test-H; Xinhua), and scored accordingly. High-level disinfection (HLD) was performed using an automated endoscope repro
Microbial cultures were conducted following HLD to detect any microbial residue in the endoscopic working channel. Eluent (Chongqing Pangtong Medical Equipment Co. Ltd., China) was injected into the endoscopic forceps and collected. Under sterile conditions, 1 mL eluent was inoculated into nutrient agar medium and spread onto a culture dish. The process was repeated with another 1 mL eluent. The remaining 48 mL eluent was filtered and concentrated using a 0.45-μm filter membrane. The filter membrane was inoculated onto a culture dish and incubated at 36 ± 1 °C for 48 hours, after which the number of colonies was counted. Additionally, two sets of Teflon tubes were simulated to undergo manual cleaning 500 times, once with traditional cleaning brushes and once with spray-type flushing tubes. Subsequently, the tubes were examined for damage using the aforementioned borescope.
The evaluation indicators of this study primarily included ATP concentration, cleanliness score, microbial cultures, and Teflon tube damage score. ATP is a widely used clinical method for assessing the quality of endoscopic cleaning, providing a quick reflection of the cleaning effectiveness. Cleanliness score and damage score of Teflon tubes were measured as described by Barakat et al[8] (Tables 1 and 2). However, the specifics regarding the evaluators of these research indicators and the grouping of electronic endoscopes and Teflon tubes are not provided.
| Working channel findings | Endoscope inspection ratings |
| Dark colored debris | 0 (none): No dark colored debris |
| 1 (mild): Small dark particles or linear debris in up to 3 locations | |
| 2 (moderate): Small dark particles or debris in > 3 locations or larger debris in 1 location | |
| 3 (severe): Larger dark particles or debris in > 1 location | |
| Light colored debris | 0 (none): No light colored/reflective debris |
| 1 (mild): Small light-colored/reflective particles or residue in up to 3 locations | |
| 2 (moderate): Small light-colored/reflective particles or residue in > 3 locations, or larger debris/residue in 1 | |
| 3 (severe): Larger light-colored/reflective particles or debris in > 1 location or present in continuous large | |
| Opaque fluid | 0 (none): No opaque fluid |
| 1 (mild): Up to 5 individual drops of opaque fluid within channel | |
| 2 (moderate): 6 to 10 individual drops of opaque fluid within channel | |
| 3 (severe): Greater than 10 individual drops of opaque fluid or pooling of fluid within channel | |
| Clear fluid | 0 (none): No clear fluid |
| 1 (mild): Up to 5 individual drops of clear fluid within channel | |
| 2 (moderate): 6 to 10 individual drops of clear fluid within channel | |
| 3 (severe): Greater than 10 individual drops of clear fluid or pooling of fluid within channel |
| Damage | Endoscope inspection ratings |
| Scratch characteristics | 0 (none): No scratches visualized |
| 1 (mild): Superficial, with regular margins, < 1 image field in length | |
| 2 (moderate): Deeper, with irregular margins or > 1 image field in length | |
| 3 (severe): Deep with irregular margins | |
| Scratch severity | 0 (none): No scratches visualized |
| 1 (mild): < 10 scratches visualized | |
| 2 (moderate): 10-20 scratches visualized | |
| 3 (severe): > 20 scratches visualized | |
| Adherent peel | 0 (none): No adherent peel visualized |
| 1 (mild): Localized to 1 small (< 1 cm) site | |
| 2 (moderate): Present in 2–3 locations or 1-2 cm site | |
| 3 (severe): Present in > 3 locations or single > 2 cm site |
Variations in damage to endoscopic working channels occur when different instruments are passed through them 500 times. In this study, 20 brand-new Teflon tubes were randomly assigned into a control group and an experimental group, each containing 10 tubes. The control group underwent manual cleaning using traditional cleaning brushes, with each Teflon tube being passed through 500 times. Conversely, the experimental group utilized a spray flushing tube for manual cleaning. Subsequently, the damage to the Teflon tubes was assessed using a Teflon tube damage scale. Results revealed that the control group had a damage score of 4.70 ± 0.95 points, whereas the experimental group scored 3.90 ± 0.57 points, indicating a significant difference (P < 0.05). The sample size calculation was performed with a desired power of 90%, a significance level (α) of 0.05 on both sides, and an expected dropout rate of no more than 5%, using PASS 15.0 software. Each group required 28 cases, and considering an expected dropout rate, 30 cases were included in each group, resulting in a total of 60 cases.
SPSS 23.0 software was used for statistical analysis. Categorical variables were presented as frequencies or percentages (%). For intergroup comparisons, the χ² test or Fisher’s exact probability method was selected. Continuous variables were represented as mean ± SD if they followed a normal distribution. If not, they were represented by the median and upper and lower quartiles (M, P25, P75). Depending on the data distribution, either t tests or rank sum tests were chosen for intergroup comparisons. All statistical analyses were conducted using a two-sided test, with P < 0.05 indicating statistically significant differences.
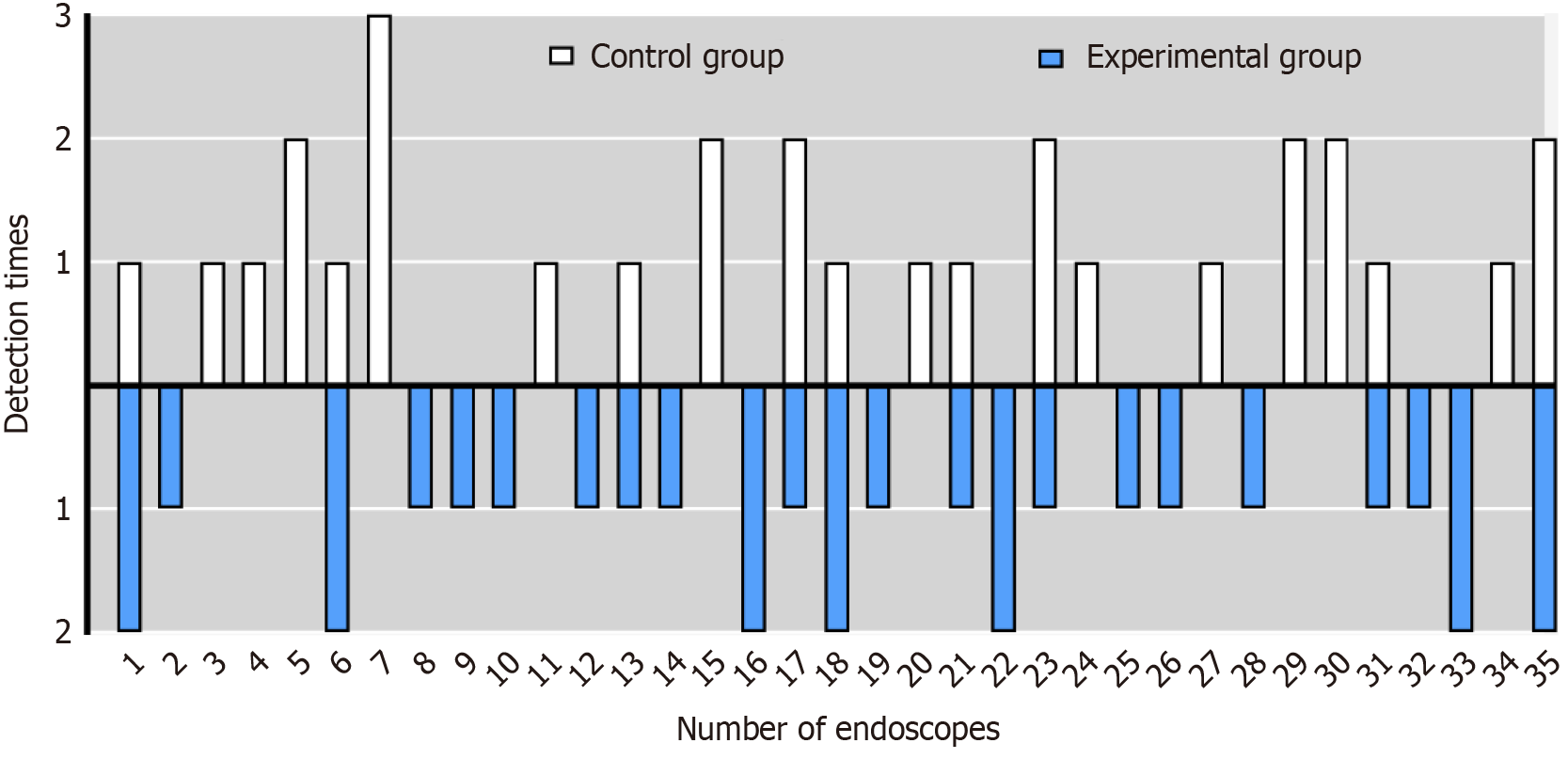
Sixty inspections were conducted on 35 electronic gastroscopes (GIF-H290) currently in use at our endoscopy unit, which were sequentially numbered from 1 to 35. Following gastroscopy examinations, manual cleaning was randomly performed using traditional cleaning brushes (30 cases) and a spray flushing system (30 cases). The specific utilization of gastroscopes is illustrated in Figure 2. The results indicated no significant difference in the utilization of endoscopes between the two groups (P > 0.05).
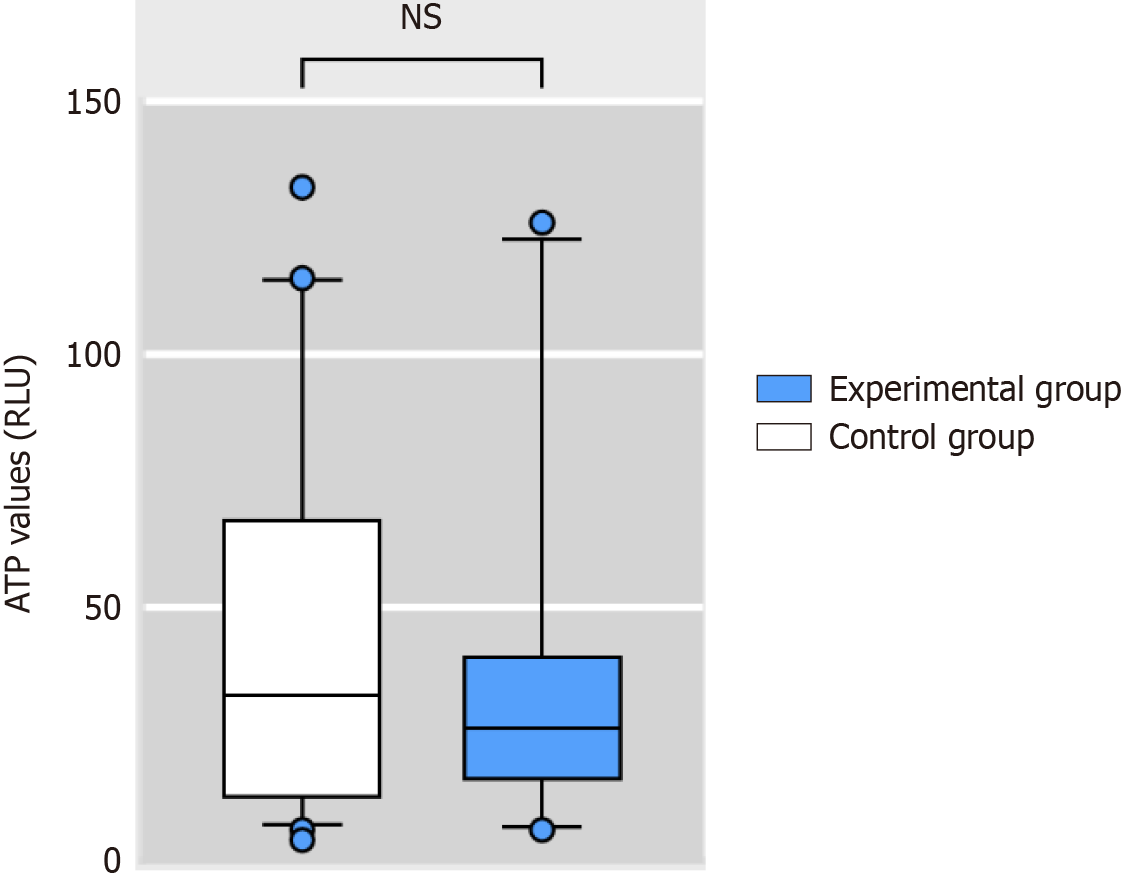
The median ATP concentration of the endoscopic working channel after manual cleaning was 29.5 RLU [interquartile range (IQR) 13.5-59.25]; all of which met the requirement of < 200 RLU. In the control and experimental groups, the ATP concentrations (RLU) were 32.5 (13-66) and 26 (16-40), respectively. There was no significant difference between the two groups (Z = -0.266, P > 0.05) (Figure 3).
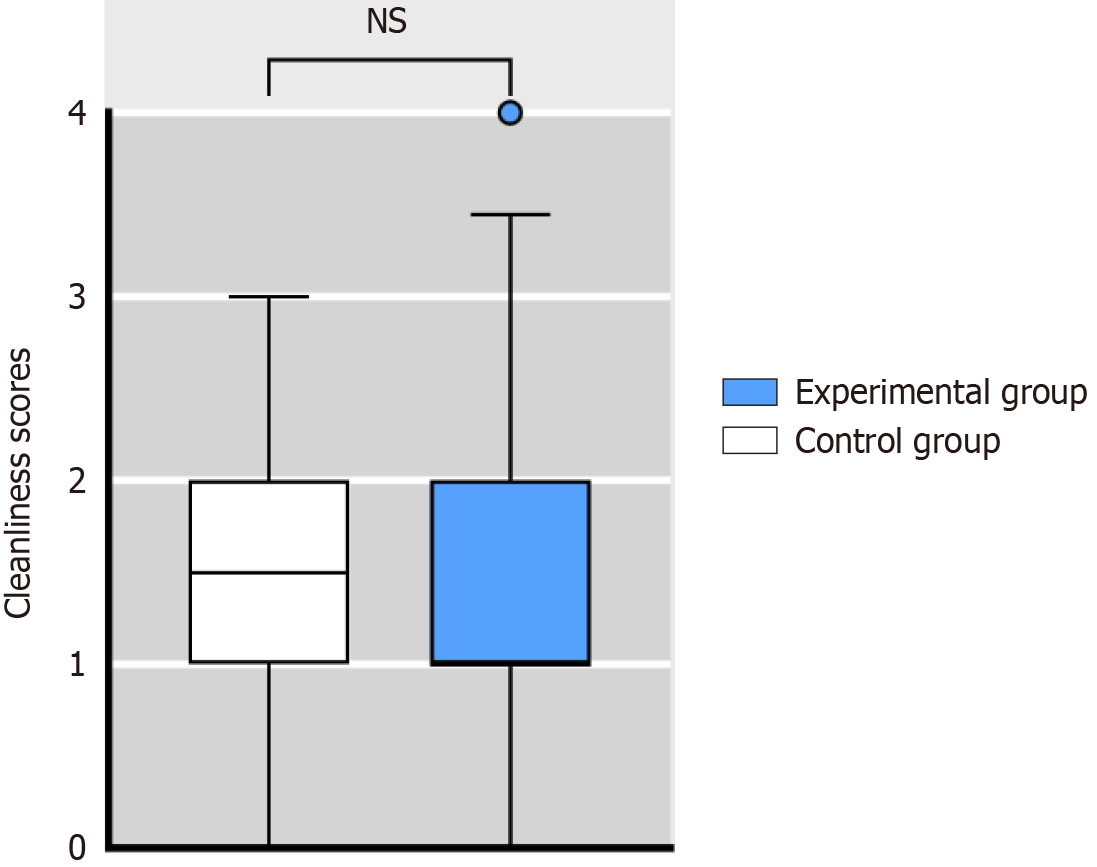
The median cleanliness score of the endoscopic working channel after manual cleaning was 1 (IQR 1-2). In the control and experimental groups, the cleanliness scores were 1.5 (IQR 1-2) and 1 (IQR 1-2), respectively. There was no significant difference between the two groups (Z = -0.96, P > 0.05) (Figure 4).
To further compare the cleanliness of the two groups of electronic endoscopic working channels, we assessed four factors: Dark colored debris, light colored debris, opaque fluid, and clear fluid (Table 3). The results indicated no significant difference (P > 0.05) between the two groups in terms of dark colored debris, light colored debris, opaque fluid, and clear fluid. However, it was observed that 73.3% of the endoscopic working channels in the control group had residual debris, which was higher than 46.7% in the experimental group, which was significant (χ² = 4.44, P < 0.05).
| Working channel findings | Control group (n = 30) | Experimental group (n = 30) | χ2 | P value |
| Dark colored debris | 4 (13.3) | 2 (6.7) | 0.74 | 0.671 |
| Light colored debris | 20 (66.7) | 13 (43.3) | 3.30 | 0.069 |
| Residual debris | 22 (73.3) | 14 (46.7) | 4.44 | 0.035 |
| Opaque fluid | 2 (6.7) | 3 (10.0) | 0.22 | 1.00 |
| Clear fluid | 14 (46.7) | 18 (60.0) | 1.07 | 0.438 |
A reprocessed endoscope is permitted to carry 20 CFU of viable bacteria[9]. Both sets of endoscopic working channels underwent microbiological culture and were deemed to be within the acceptable limits.
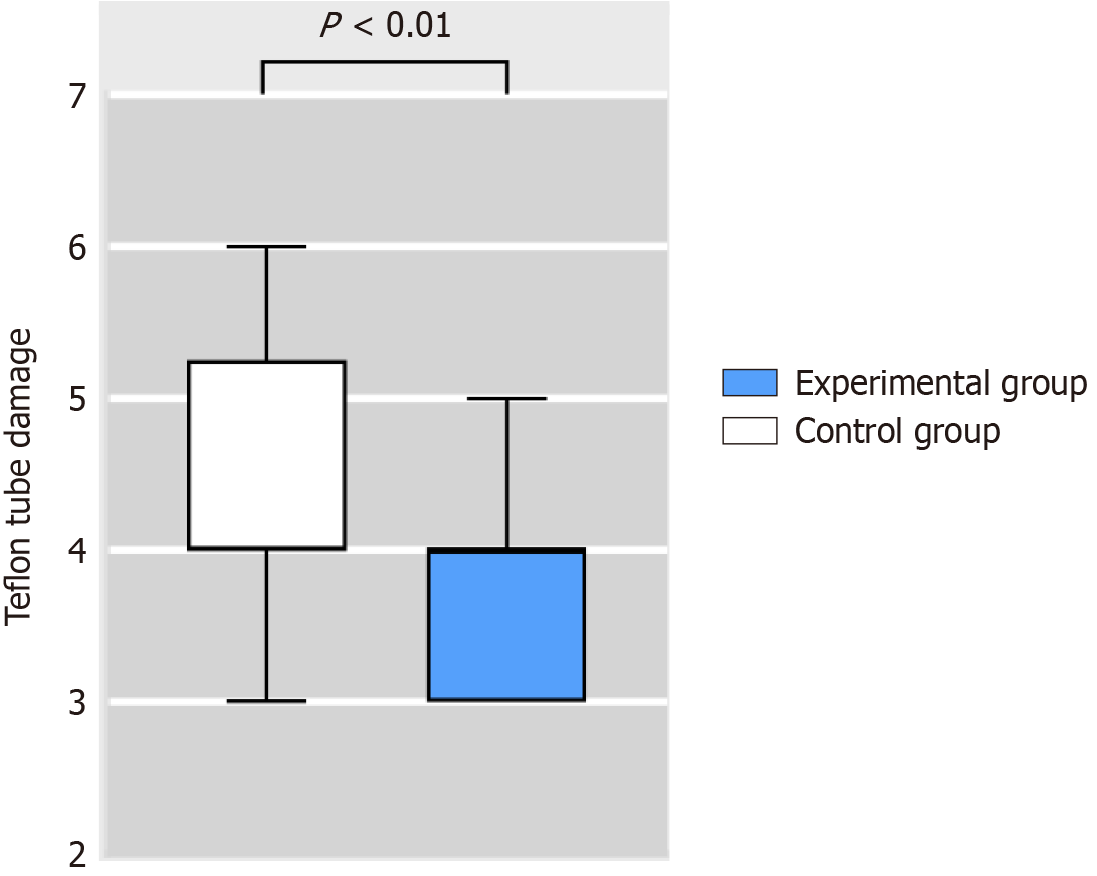
The damage score of all 60 Teflon tubes was 4 (IQR 3-5), indicating consistent damage across the board. In the control group, the damage score was 4 (IQR 4-5.25), which was higher than 4 (IQR 3-4) in the experimental group. This difference was significant (Z = -2.775, P < 0.01) (Figure 5). To further compare the damage of the two groups of Teflon tubes, we evaluated scratch characteristics, scratch severity, and adherent peel (Table 4). The results revealed no significant difference (P > 0.05) between the control and experimental groups in terms of scratch characteristics, scratch severity, and adherent peel (Table 4).
| Working channel findings | Control group (n = 30) | Experimental group (n = 30) | Z | P value |
| Scratch characteristics | 1 (1, 2) | 1 (1, 2) | -1.473 | 0.141 |
| Scratch severity | 1 (1, 2) | 1 (1, 1) | -1.629 | 0.103 |
| Adherent peel | 1.5 (1, 2) | 1 (1, 2) | -1.777 | 0.076 |
This study confirmed that our self-developed spray flushing system achieved cleaning quality comparable to traditional cleaning brushes. Additionally, microbial cultures demonstrate that this system did not compromise the HLD efficacy of endoscopes. Notably, the spray flushing system showed superior performance in debris removal from the tube, with 73.3% effectiveness compared with 46.7% for traditional brushes. Furthermore, the system demonstrated significant advantages in reducing damage to the working channel.
ATP biofluorescence detection has become widely used for assessing the efficacy of soft endoscopic cleaning. Both the Chinese “Technical Specification for Reprocessing of Soft Endoscopes (2016 Edition)” and several studies recommend its use for evaluating the quality of soft endoscopic cleaning[9-12]. In this study, all ATP biofluorescence detection procedures were conducted by the same team member, who was blinded, to minimize bias resulting from human factors. The results revealed that the ATP concentration (RLU) in the endoscopes in the experimental group was 26 (IQR 16-40), which was lower than in the control group (32.5; IQR 13-66). However, the difference between the two groups was not significant (P > 0.05). These findings indicate that the cleaning quality achieved with the spray flushing system is comparable to that achieved with traditional brushes.
The borescope plays a crucial role in directly observing the working channel of the endoscope, facilitating the detection of endoscopic damage and residual dirt. Widely utilized in various endoscopic examinations, the borescope has been instrumental in observation and detection[13-16]. In this study, we used a borescope to assess and score the cleanliness of the endoscopic working channel, using the scoring system outlined in the study of Barakat et al[8]. Our scoring system comprised two categories: Debris and liquid. Debris included both dark-colored and light-colored debris, while liquid encompassed opaque fluid and clear fluid. Each category had four subitems, with scores ranging from 0 to 3 points, resulting in a total score range of 0 to 12 points. A lower score indicated a cleaner endoscope. The results indicated that the cleanliness score of the endoscopic working channel in the control group was 1.5 (IQR 1-2), while that of the experimental group was 1 (IQR 1-2). This finding closely aligns with the results reported by Barakat et al[8], suggesting that both the traditional cleaning brush and spray flushing system effectively ensure the cleanliness of the working channel.
The gold standard for assessing the adequacy of flexible endoscope reprocessing is microbial culture. Following reprocessing, our study conducted microbial culture on all 60 endoscopes involved. The results indicated negative microbial culture outcomes for both the control and experimental groups, demonstrating the absence of microbial contamination. These findings highlight that the spray flushing system achieves satisfactory results in ATP biofluorescence detection and tube cleanliness but also performs comparably to traditional brush cleaning in microbial cultivation. This underscores the effectiveness of the spray flushing system in ensuring the microbiological safety of reprocessed endoscopes.
Studies have highlighted that repeated passage of various instruments through endoscopic working channels, particularly using cleaning brushes, is a significant factor contributing to the formation of scratches and increased roughness[6]. Following the approach outlined by Santos et al[7], our study compared and scored the effects of traditional brush cleaning and the spray flushing system on endoscopic working channel damage. Drawing on the scoring system proposed by Barakat et al[8], we established a damage scoring scale for endoscopic working channels, consisting of three items: Scratch characteristics, scratch severity, and adherent peel. Each item was scored on a scale of 0-3 points, resulting in a total score of 0-9 points. A higher score indicated more severe damage. The results revealed that the Teflon tube damage score in the control group was 4 (IQR 4-5.25), which was significantly higher than 4 (IQR 3-4) in the experimental group (P < 0.01). This suggests that the spray flushing system causes less damage to the endoscopic working channel. Previous studies have demonstrated that traditional cleaning brushes may experience bristle shedding or damage after repeated use[17-19]. This not only diminishes the cleaning efficacy of the endoscopic working channel but also exacerbates channel damage. Consequently, multiple domestic guidelines recommend using a cleaning brush only once[20-26], which may increase costs. In contrast, the spray flushing tube does not exhibit bristle shedding, eliminating the need for repeated use and reducing associated costs. However, this study has some limitations. This study has a limited sample size. It is hoped that future research can increase the sample size.
The spray flushing system in the manual cleaning of flexible endoscopes demonstrates comparable effectiveness to traditional brush cleaning in terms of ATP concentration, cleanliness, and microbial culture of the endoscope working channel after manual cleaning. The spray flushing system offers significant advantages in removing debris from the endoscopic working channel and reducing damage to it. These findings have clinical relevance, suggesting that the spray flushing system could be beneficial in endoscopic reprocessing protocols.
| 1. | Ning B, Linghu EQ. [New progress in the field of digestive endoscopy in 2021]. Zhonghua Yixue Xinxi Daobao. 2022;37:8-8. [DOI] [Full Text] |
| 2. | Jiang YX, Ying Chen. [Quality control of digestive endoscopy in China: Current status and future direction]. Tongji Daxue Xuebao. 2020;41:805-810. [DOI] [Full Text] |
| 3. | Bajolet O, Ciocan D, Vallet C, de Champs C, Vernet-Garnier V, Guillard T, Brasme L, Thiefin G, Cadiot G, Bureau-Chalot F. Gastroscopy-associated transmission of extended-spectrum beta-lactamase-producing Pseudomonas aeruginosa. J Hosp Infect. 2013;83:341-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Aumeran C, Poincloux L, Souweine B, Robin F, Laurichesse H, Baud O, Bommelaer G, Traoré O. Multidrug-resistant Klebsiella pneumoniae outbreak after endoscopic retrograde cholangiopancreatography. Endoscopy. 2010;42:895-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 5. | Epstein L, Hunter JC, Arwady MA, Tsai V, Stein L, Gribogiannis M, Frias M, Guh AY, Laufer AS, Black S, Pacilli M, Moulton-Meissner H, Rasheed JK, Avillan JJ, Kitchel B, Limbago BM, MacCannell D, Lonsway D, Noble-Wang J, Conway J, Conover C, Vernon M, Kallen AJ. New Delhi metallo-β-lactamase-producing carbapenem-resistant Escherichia coli associated with exposure to duodenoscopes. JAMA. 2014;312:1447-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 318] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 6. | Bi ZQ. [Study on the correlation between soft endoscope forceps pipescratches and biofilm formation]. Nanchang University, 2022. |
| 7. | Santos LCS, Parvin F, Huizer-Pajkos A, Wang J, Inglis DW, Andrade D, Hu H, Vickery K. Contribution of usage to endoscope working channel damage and bacterial contamination. J Hosp Infect. 2020;105:176-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Barakat MT, Girotra M, Huang RJ, Banerjee S. Scoping the scope: endoscopic evaluation of endoscope working channels with a new high-resolution inspection endoscope (with video). Gastrointest Endosc. 2018;88:601-611.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 9. | Liu YX, Xing YB, Gong YX, Tian XL, Li ZS, Wang LH, Zhang LB, Li LY, Fang Y, Liu F, Suo JJ, Kong JY, Deng M, Zhang Y, Zhang JL, Yang YS, Chen CM, Ren X, Zhang ST, Jiang B, Guo XG. [Regulation for cleaning and disinfection technique of flexible endoscope]. Zhongguo Ganran Kongzhi Zazhi. 2017;587-592. [DOI] [Full Text] |
| 10. | Barakat MT, Huang RJ, Banerjee S. Simethicone is retained in endoscopes despite reprocessing: impact of its use on working channel fluid retention and adenosine triphosphate bioluminescence values (with video). Gastrointest Endosc. 2019;89:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Shi LK, Wang Y, Liu Y, Jia Y, Wang LY, Ren L, Chen YL. [Research on feasibility of ATP bioluminescence method in monitoring microbial contamination]. Zhongguo Xiaoduxue Zazhi. 2014;. |
| 12. | Wang HF, Lai RP, Liao HL, Wang XH. [The Application of ATP Biofluorescence Detection in Improving the Quality of Digestive Endoscopy Disinfection]. Zhongguo Xiaoduxue Zazhi. 2015;32. |
| 13. | Zhou MJ, Huang X, Liu LL, He RP, Hu L, Xun-Zhang, Zhang YX, Ma JH. Investigation of the Internal Conditions of 213 Reprocessed Endoscopic Channels. Surg Laparosc Endosc Percutan Tech. 2023;33:4-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 14. | Barakat MT, Huang RJ, Banerjee S. Comparison of automated and manual drying in the elimination of residual endoscope working channel fluid after reprocessing (with video). Gastrointest Endosc. 2019;89:124-132.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Alfa MJ, Ribeiro MM, da Costa Luciano C, Franca R, Olson N, DeGagne P, Singh H. A novel polytetrafluoroethylene-channel model, which simulates low levels of culturable bacteria in buildup biofilm after repeated endoscope reprocessing. Gastrointest Endosc. 2017;86:442-451.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Ofstead CL, Wetzler HP, Eiland JE, Heymann OL, Held SB, Shaw MJ. Assessing residual contamination and damage inside flexible endoscopes over time. Am J Infect Control. 2016;44:1675-1677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Thaker AM, Kim S, Sedarat A, Watson RR, Muthusamy VR. Inspection of endoscope instrument channels after reprocessing using a prototype borescope. Gastrointest Endosc. 2018;88:612-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 18. | Huang Q. Study on the removal efficacy of flexible endoscope cleaning brushes on endoscopic biofilm. Southern Medical University, 2012. |
| 19. | Su B, Lou LH, Yang SM, Lu XJ, Xve RG, Kong YJ, Ji YF, Chu XY, Cui B, Wu HY, Zhang JL, Li JH. [Effect of cleaning brush material and brushing speed on the quality of suction pipe cleaning in endoscopy light hose]. Zhongguo Neijing Zazhi. 2019;25:12-16. [DOI] [Full Text] |
| 20. | Beilenhoff U, Biering H, Blum R, Brljak J, Cimbro M, Dumonceau JM, Hassan C, Jung M, Kampf B, Neumann C, Pietsch M, Pineau L, Ponchon T, Rejchrt S, Rey JF, Schmidt V, Tillett J, van Hooft JE. Reprocessing of flexible endoscopes and endoscopic accessories used in gastrointestinal endoscopy: Position Statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology Nurses and Associates (ESGENA) - Update 2018. Endoscopy. 2018;50:1205-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 21. | ASGE Quality Assurance in Endoscopy Committee; Calderwood AH, Day LW, Muthusamy VR, Collins J, Hambrick RD 3rd, Brock AS, Guda NM, Buscaglia JM, Petersen BT, Buttar NS, Khanna LG, Kushnir VM, Repaka A, Villa NA, Eisen GM. ASGE guideline for infection control during GI endoscopy. Gastrointest Endosc. 2018;87:1167-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 134] [Article Influence: 19.1] [Reference Citation Analysis (1)] |
| 22. | Son BK, Kim BW, Kim WH, Myung DS, Cho YS, Jang BI; Disinfection Management and Conscious Sedation Committee of Korean Society of Gastrointestinal Endoscopy. Korean Society of Gastrointestinal Endoscopy Guidelines for Endoscope Reprocessing. Clin Endosc. 2017;50:143-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Loyola M, Babb E, Bocian S, Diskey A, Friis CM, Granato A, Schmit A, Selking S; SGNA Practice Committee 2017-18. STANDARDS OF INFECTION PREVENTION IN REPROCESSING FLEXIBLE GASTROINTESTINAL ENDOSCOPES. Gastroenterol Nurs. 2020;43:E142-E158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 24. | Aorn. Guideline Summary: Processing Flexible Endoscopes. AORN J. 2016;104:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Aorn. Guideline at a Glance: Processing Flexible Endoscopes. AORN J. 2016;104:610-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Day LW, Muthusamy VR, Collins J, Kushnir VM, Sawhney MS, Thosani NC, Wani S. Multisociety guideline on reprocessing flexible GI endoscopes and accessories. Gastrointest Endosc. 2021;93:11-33.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |