Published online Aug 14, 2024. doi: 10.3748/wjg.v30.i30.3574
Revised: July 5, 2024
Accepted: July 24, 2024
Published online: August 14, 2024
Processing time: 70 Days and 0.2 Hours
The incidence of rectal cancer is increasing worldwide, and surgery remains the primary treatment modality. With the advent of total mesorectal excision (TME) technique, the probability of tumor recurrence post-surgery has significantly decreased. Surgeons' focus has gradually shifted towards minimizing the impact of surgery on urinary and sexual functions. Among these concerns, the optimal dissection of the rectal lateral ligaments and preservation of the pelvic floor neuro
To compare the differences in surgical specimen integrity and postoperative quality of life satisfaction between the traditional pelvic floor dissection strategy and the innovative eight-zone dissection strategy.
We analyzed the perioperative data of patients who underwent laparoscopic radical resection of rectal cancer at Qilu Hospital of Shandong University between January 1, 2021 and December 1, 2023. This study included a total of 218 patients undergoing laparoscopic radical surgery for rectal cancer, among whom 109 patients underwent traditional pelvic floor dissection strategy, and 109 patients received the eight-zone dissection strategy.
There were no significant differences in general characteristics between the two groups. Patients in the eight-zone dissection group had higher postoperative specimen integrity (88.1% vs 78.0%, P = 0.047). At the 3-month follow-up, patients in the eight-zone surgery group had better scores in urinary issues (6.8 ± 3.3 vs 5.3 ± 2.5, P = 0.045) and male sexual desire (2.2 ± 0.6 vs 2.5 ± 0.5, P = 0.047) compared to the traditional surgery strategy group.
This study demonstrates that the eight-zone dissection strategy for laparoscopic lateral ligament dissection of rectal cancer is safe and effective. Compared with the traditional pelvic floor dissection strategy, this approach can reduce the risk of nerve injury and minimize the impact on urinary and sexual functions. Therefore, we recommend the clinical application of this strategy to better serve patients with rectal cancer.
Core Tip: Our study addresses the imperative to minimize the adverse impact of surgery on urinary and reproductive functions, thereby enhancing the overall quality of life for patients. Specifically, we introduce the innovative eight-zone dissection strategy for pelvic floor anatomy within total mesorectal excision procedures, aimed at reducing postoperative complications in these domains. By comparing outcomes between traditional pelvic floor dissection strategies and our novel approach, we demonstrate a significant reduction in postoperative complications related to urinary and reproductive functions. These findings underscore the potential of the eight-zone dissection strategy to mitigate the negative effects of surgery in these critical areas.
- Citation: Chen C, Zhang X, Li X, Wang YL. Clinical application of eight-zone laparoscopic dissection strategy for rectal cancer: Experience and discussion. World J Gastroenterol 2024; 30(30): 3574-3583
- URL: https://www.wjgnet.com/1007-9327/full/v30/i30/3574.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i30.3574
Surgical treatment remains the main treatment for rectal cancer. In 1982, Heald et al[1] introduced the concept of total mesorectal excision (TME), which significantly improved the treatment outcomes of patients with rectal cancer, reduced local recurrence rates, and extended long-term survival rates, Gradually, TME has become the standard surgical approach for rectal cancer[1-4]. The main principles of TME include: (1) TME (for low rectal cancer) or resection of the distal mesorectum ≥ 5 cm from the tumor (for mid-high rectal cancer); and (2) A distal resection margin ≥ 2 cm[5].
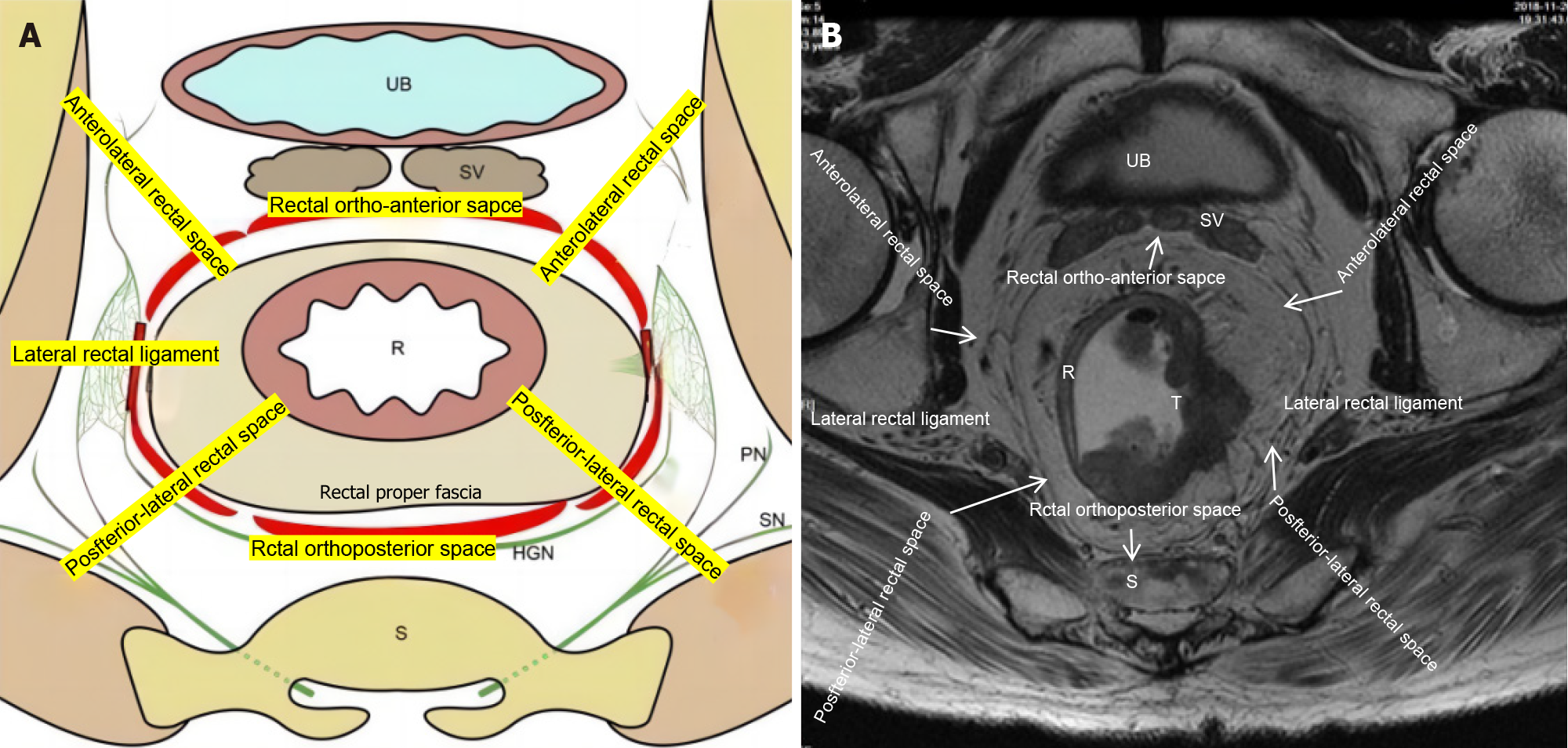
In the surgical management of mid-to-low rectal malignancies, the intricate dissection of the perirectal spaces, particularly the lateral rectal ligaments, emerges as a paramount surgical hurdle. This delicate procedure is not only pivotal for ensuring the meticulous execution of TME but also instrumental in averting neurological impairments. The mere existence of the lateral rectal ligament has been a subject of intense scholarly debate[6-9]. A closer examination of its nomenclature reveals that the term "ligament," conventionally denoting a distinct, band-like structure with a suspensory function, might not accurately encapsulate the true nature of this anatomical entity. Instead, the "lateral rectal ligament" is more aptly described as a dense connective tissue complex located at the 3 and 9 o'clock meridians of the mid-rectum, requiring meticulous surgical dissection for complete exposure. This tissue is essentially an extension of the mesorectum.
Furthermore, the composition of this ligament remains elusive, with various studies suggesting the presence of vital structures such as the middle rectal artery, pelvic plexus nerves, and the surrounding perirectal fascia[10-13]. The preservation of these delicate structures is paramount for maintaining optimal surgical outcomes and preserving the urogenital functionality of the patient[14]. Consequently, a cautious and methodical approach is imperative when addressing the lateral rectal ligament during surgical interventions.
Conventionally, the dissection of the perirectal spaces in TME follows a sequential order, commencing with the posterior aspect, proceeding to the anterior, and culminating with the lateral dissection. However, this generalized approach lacks precision, making it difficult to expose critical structures like lateral rectal ligaments and inferior hypogastric nerves, affecting surgical outcomes. In our pursuit of surgical excellence, we have introduced a novel strategy, termed the "eight-zone method," which segments the perirectal spaces into distinct anatomical zones. This meticulous dissection technique has significantly enhanced the quality and reproducibility of TME surgeries, yielding superior surgical outcomes. We believe that this innovative approach serves as a valuable adjunct to the existing anatomical understanding of rectal cancer surgery and may pave the way for future advancements in this field.
This study analyzed 218 patients with rectal cancer who underwent treatment at the Department of Colorectal Surgery, Qilu Hospital of Shandong University, between January 1, 2021, and December 1, 2023. Among these patients, 109 underwent the traditional pelvic floor dissection strategy, while 109 patients underwent the dissection strategy using the eight-zone dissection method. Inclusion criteria: (1) Pathologically diagnosed with rectal cancer; and (2) No history of severe sexual or urinary dysfunction. Exclusion criteria: (1) Presence of severe other systemic diseases, such as heart disease, diabetes, etc.; (2) Suffering from other diseases that may cause sexual or urinary dysfunction, such as nervous system diseases, urogenital system diseases, etc.; or (3) History of pelvic surgery or radiotherapy.
All patients underwent fiberoptic colonoscopy and pathological examination for definitive diagnosis. Routine abdominal enhanced computed tomography, pelvic magnetic resonance imaging, and transrectal ultrasonography were performed to assess preoperative staging. Following the administration of general anesthesia, patients were positioned in the lithotomy position. A 10 mm incision was made at the superior margin of the umbilicus, and a pneumoperitoneum needle was inserted to establish a pneumoperitoneum. The five-port technique was employed to complete the dissection of the upper rectum, as well as the anatomical dissection and ligation of the inferior mesenteric arteriovenous roots.
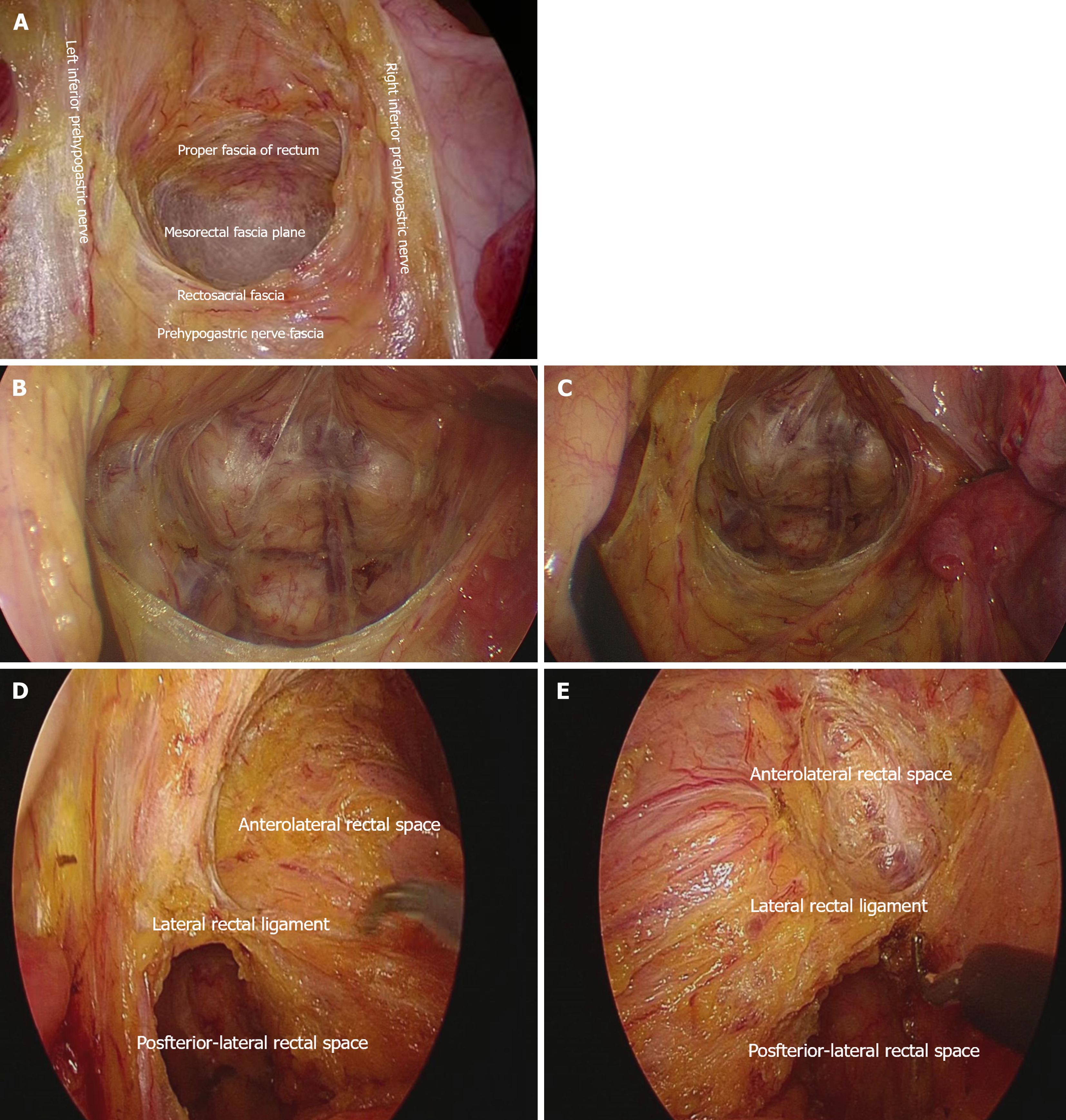
For the pelvic floor fascia, the eight-zone dissection strategy was utilized. The perirectal spaces were first divided into eight zones (Figure 1): The posterior rectal space, the left and right posterolateral rectal spaces, the anterior rectal space, the left and right anterolateral rectal spaces, and the left and right lateral rectal ligaments. Dissection was performed in the following sequence: Posterior, left and right posterolateral, anterior, left and right anterolateral, and left and right lateral. The specific surgical steps are described below:
Dissection of the posterior rectal space: With the assistant lifting the sigmoid colon and upper rectum towards the ventral and cephalic directions to maintain adequate tension, the surgeon performs a blunt and sharp dissection along the posterior rectal space between the anterior fascia of the inferior hypogastric nerve and the proper fascia of the rectum, directing towards the pelvic cavity. The surgeon's left hand may hold a small gauze roll to increase tension, aiding in the exposure of the posterior rectal space. The dissection proceeds to the level of the fourth sacral vertebra (S4), where the Waldeyer's fascia is sharply incised to enter the supralevator space. Care is taken to dissect along the proper fascia of the rectum, avoiding injury to the presacral veins. The assistant adjusts the traction position in a timely manner to maintain adequate tension, extending the dissection of the supralevator space to the level of the coccygeal tip. At this point, the dissection of the posterior rectal space is complete (Figure 2A).
Dissection of the posterolateral rectal spaces: During the dissection of the posterior rectal space, attention is paid to expanding laterally, namely towards the left and right posterolateral rectal spaces. The anatomical landmark is the piriformis muscle on both sides (Figure 2B and C). As the surgeon operates from the patient's right side, the left space is relatively easier to dissect compared to the right. If the posterolateral space cannot be fully dissected (especially on the right side), the procedure can be temporarily paused and resumed before dealing with the lateral ligaments. Some male patients have a narrow pelvic space, making the dissection more challenging compared to females. Close cooperation between the surgeon and the assistant is required. Attention is paid to the use of suction to clear pelvic smoke in a timely manner, helping to maintain a clear field of view.
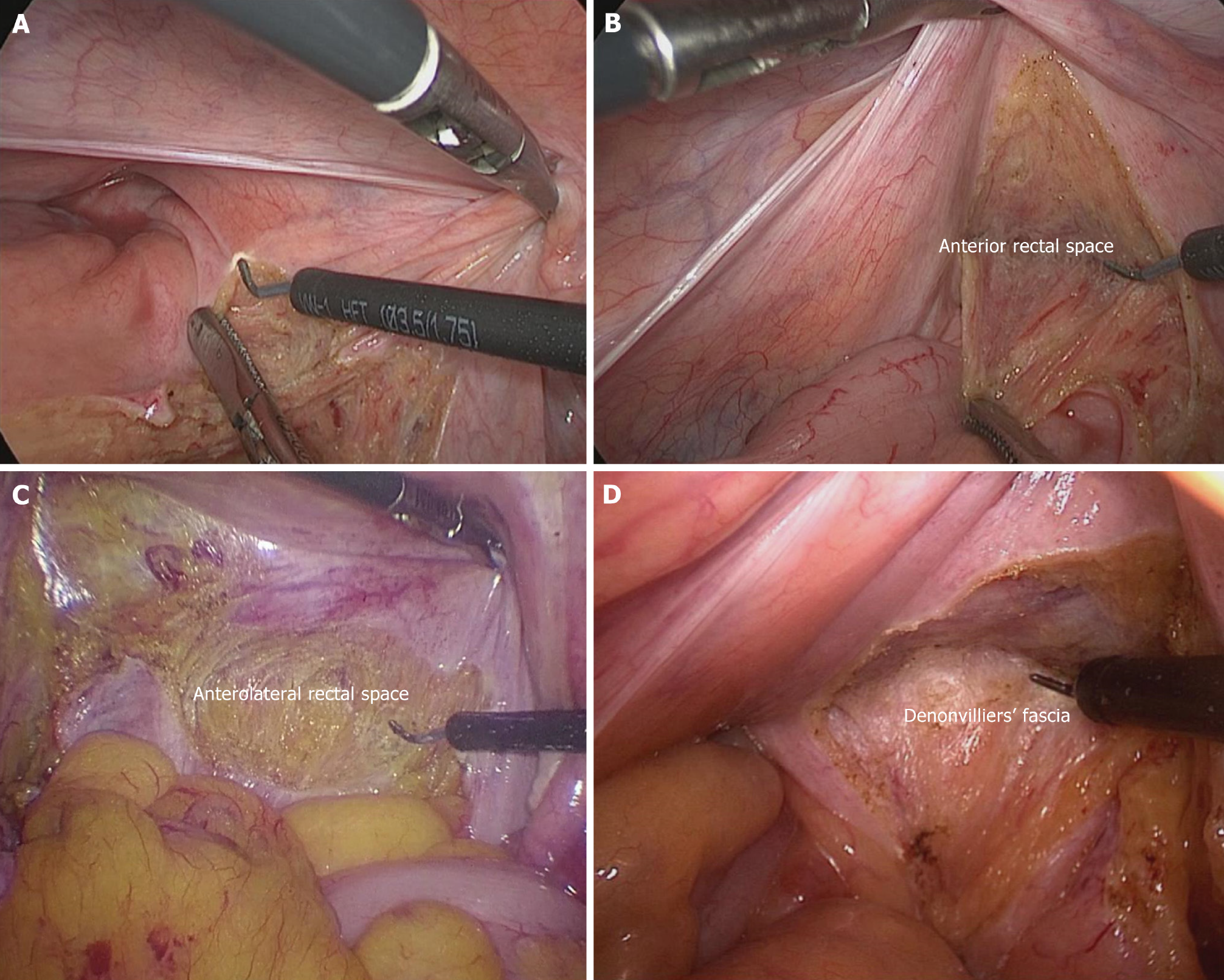
Dissection of the anterior rectal space: The peritoneum overlying the upper rectum is incised bilaterally to reach the pelvic floor. The incision into the anterior rectal space is generally made from the right side towards the middle, incising the pelvic floor peritoneum (Figure 2D), and then from the left side to meet in the middle. However, if the lateral incision line is not clearly exposed, a direct midline incision can be made, extending laterally to meet on both sides (Figure 2E). For male patients, it is generally recommended to incise 1-1.5 cm above the peritoneal reflection, entering the anterior leaf of Denonvillier's fascia, and then transecting 0.5 cm from the tail of the seminal vesicle. This not only helps to expand the pelvic space but also avoids nerve injury (Figure 2D and E). For female patients, due to the adequate pelvic space and the absence of a distinct Denonvillier's fascia in most cases, the incision can be made directly at the peritoneal reflection, effectively avoiding vaginal injury (Figure 2D and E). There is no clear boundary between the anterior rectal space and the left and right anterolateral spaces (a three-part division can be used), and dissection is often performed simultaneously, generally alternating between right and left. Suspension of the uterus and bladder facilitates the dissection process.
Dissection of the anterolateral rectal spaces: Dissection of the left and right anterolateral spaces is an important step before dealing with the lateral ligaments. In male patients, the anterior structure is the seminal vesicle, while in female patients, it is the vaginal wall. The lateral boundary is the lateral ligament. In male patients, the Denonvillier's fascia should be promptly transected to fully expand the anterolateral space. As the left surgical field is easier to expose, it can be prioritized (Figure 3A). After the completion of the left dissection and transection of the lateral ligament, the right space will be further enlarged, making the operation easier (Figure 3B). Care is taken to perform precise dissection within the plane to avoid injuring the vascular and neural bundles on both sides.
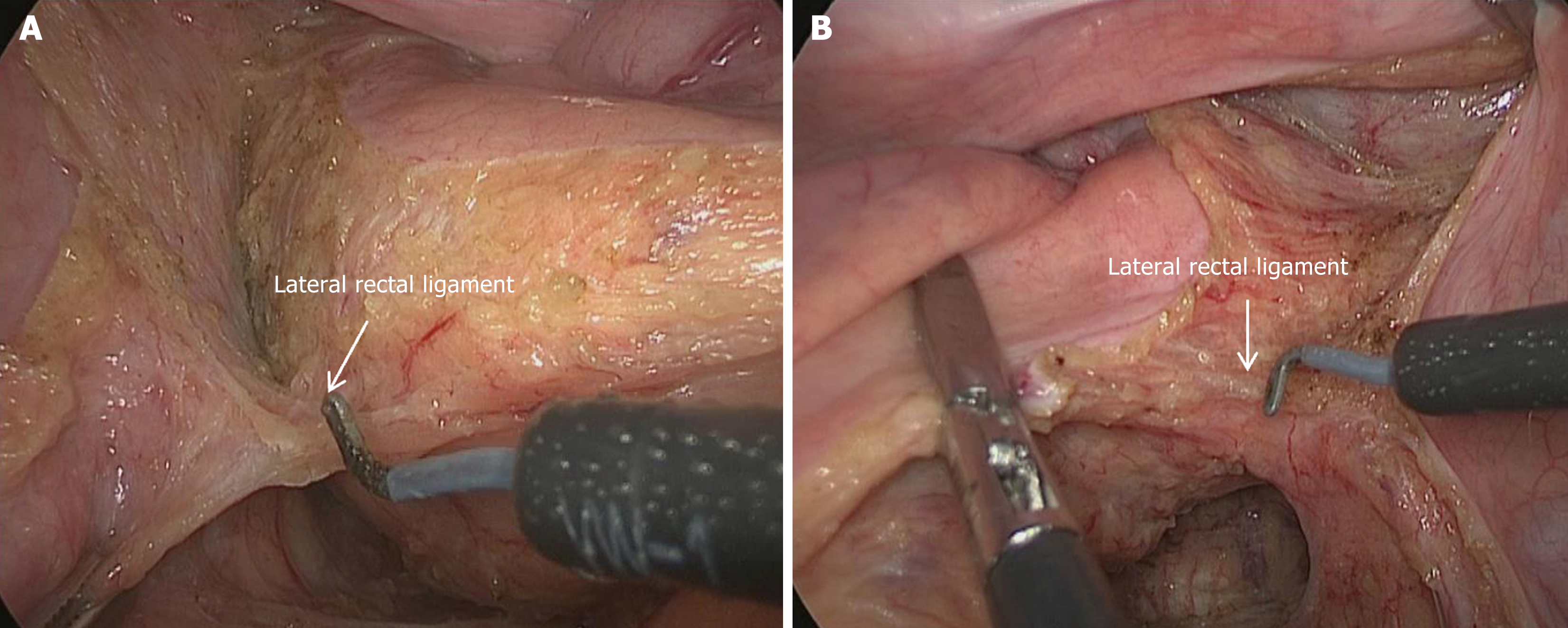
Dissection of the lateral rectal ligaments: After adequate expansion of the posterolateral and anterolateral spaces, the transection of the lateral ligaments is performed last (Figure 4). It is recommended to start with the left side followed by the right side. The anatomical landmarks are the inferior hypogastric nerve and the pelvic plexus. In most patients, the vessels within the lateral ligaments can be visualized (Figure 3). If the posterolateral and anterolateral spaces are not adequately expanded, further expansion should be performed before transecting the lateral ligaments to minimize the volume of the lateral ligaments and maximize the avoidance of nerve injury. After transection of the lateral ligaments on both sides, the dissection proceeds to the distal rectum. At this point, the pelvic space has been fully expanded, and the rectal spaces have been accurately dissected, providing a guarantee for a high-quality TME procedure.
Integrity of the mesentery of the postoperative specimen: According to the criteria proposed by the Dutch Colorectal Cancer Group (DCCG), the macroscopic evaluation of the TME specimen for rectal cancer is divided into three levels, as detailed in the Table 1.
| Integrity level | Description | Characteristics |
| Complete | Intact mesorectal tissue with a smooth surface | Defect depth ≤ 5 mm, distal margin without a cone shape, circumferential margin smooth and regular |
| More Complete | Mesorectal tissue of moderate size with an irregular surface | Defect depth > 5 mm, not reaching the muscularis propria, distal margin with a moderate cone shape, circumferential margin irregular |
| Incomplete | Small piece of mesorectal tissue with an irregular surface | Defect depth > 5 mm, reaching the muscularis propria, circumferential margin irregular |
At the 3-month postoperative outpatient review, a comprehensive follow-up using the QLQ-CR29 scoring scale was conducted to assess patients' postoperative quality of life. The European Organization for Research and Treatment of Cancer (EORTC) QLQ-CR29 questionnaire, developed by the EORTC, was utilized for this purpose. It has been previously validated in various countries including China, Spain, Poland, and others[15-19].
The QLQ-CR29 comprises several core scales (urinary problems, abdominal and pelvic pain, bowel problems, anxiety, body image) and several standalone items. The standalone items include four sexual-related items (sexual enjoyment in males, sexual enjoyment in females, impotence, sexual intercourse difficulties) and several other standalone items (abdominal swelling, dry mouth, hair loss, taste problems, skin problems, stoma embarrassment, and rectal stoma problems).
This study utilized SPSS 26.0 software for statistical analysis. Quantitative data are expressed as mean ± SD and compared using independent sample t-tests. Count data are processed using χ2 tests. A value of P < 0.05 is considered statistically significant.
This study compared the effects of two different TME lateral ligament mobilization strategies in rectal cancer surgery. The traditional pelvic floor free group comprised 109 patients, while the anatomical free group with eight-zone dissection included 109 patients. There were no statistically significant differences in gender, age, and positive lymph node ratio between the two groups. Detailed data are presented in the Table 2.
| Surgical approach | Traditional pelvic floor dissection (n = 109) | Eight-zone anatomical dissection (n = 109) | P value |
| Number of patients | 109 | 109 | - |
| Sex distribution (male/female) | 73/36 | 74/35 | 0.885 |
| Age | 60.95 ± 10.88 | 63.06 ± 9.63 | 0.281 |
| Positive lymph node ratio | 70 | 61 | 0.213 |
| Negative | 39 | 48 | |
| Stage I | 32 | 27 | 0.677 |
| Stage II | 37 | 36 | |
| Stage III | 40 | 46 |
Using the DCCG criteria, rectal cancer radical surgery specimens were categorized into three levels. Compared to the traditional pelvic floor dissection method, the eight-zone pelvic floor dissection method resulted in higher postoperative mesenteric integrity, with a statistically significant difference observed between the two groups (χ2 = 3.939, P = 0.047; Table 3).
During the outpatient follow-up at the 3rd month post-surgery, alongside routine hematological and radiological examinations, we utilized the QLQ-CR29 scale to assess patients' postoperative quality of life. Eighty patients in the traditional pelvic floor dissection strategy group and eighty-three patients in the eight-zone pelvic floor dissection strategy group completed the scale. The scale encompasses several functional dimensions, such as Urinary problems, Abdominal or pelvic pain, Defecation problems, Body image, Anxiety, among others. Scoring for each dimension involves the sum of scores for each item within the respective dimension, as well as several standalone items including Sexual interest (men), Impotence, Sexual interest (women), Dyspareunia, etc. The presence of a stoma was analyzed using the χ2 test, while scores for other dimensions were analyzed using independent sample t-tests. It was found that patients in the eight-zone group had lower scores for urinary problems at 3 months postoperatively compared to those in the traditional mobilization strategy group (5.3 ± 2.5 vs 6.8 ± 3.3, P = 0.045). Additionally, postoperative Sexual interest (men) scores were also lower in the eight-zone group compared to the traditional mobilization strategy group (2.2 ± 0.6 vs 2.5 ± 0.5, P = 0.047). And there were no statistically significant differences in quality of life scores between the two groups in other aspects, including sexual interest (women), Dyspareunia, etc. (Table 4).
| Surgical method | Traditional pelvic floor dissection (n = 80) | Eight-zone anatomical dissection (n = 83) | P value |
| Urinary problem | 6.8 ± 3.3 | 5.3 ± 2.5 | 0.045a |
| Abdominal or pelvic pain | 2.9 ± 1.5 | 3.0 ± 1.3 | |
| Defecation problem | 4.5 ± 1.3 | 4.4 ± 1.1 | |
| Body image | 9.8 ± 3.9 | 9.6 ± 4.5 | |
| Anxiety | 6.3 ± 2.2 | 6.2 ± 1.9 | |
| Flatulence | 2.8 ± 1.3 | 2.9 ± 1.0 | |
| Hair loss | 1.3 ± 0.6 | 1.4 ± 0.4 | |
| Taste | 1.5 ± 0.6 | 1.6 ± 0.4 | |
| Dry mouth | 1.6 ± 0.6 | 1.7 ± 0.3 | |
| Sore skin | 2.3 ± 0.7 | 2.4 ± 0.9 | |
| Embarrassment | 2.8 ± 1.0 | 2.7 ± 0.8 | |
| Stoma care problems | 34 | 33 | |
| Sexual interest (men) | 2.2 ± 0.6 | 2.5 ± 0.5 | 0.047a |
| Impotence | 1.8 ± 0.4 | 1.7 ± 0.3 | |
| Sexual interest (women) | 2.6 ± 1.0 | 2.5 ± 0.8 | |
| Dyspareunia | 1.5 ± 0.6 | 1.6 ± 0.5 |
This study conducted a comprehensive analysis of perioperative data from 218 patients who underwent laparoscopic radical resection for rectal cancer at Qilu Hospital of Shandong University between January 1, 2021, and December 1, 2023. The surgical outcomes and postoperative quality of life were compared between traditional pelvic floor dissection and the eight-zone dissection strategy.
The results indicated that patients treated with the eight-zone dissection strategy exhibited higher specimen integrity post-surgery. Specifically, the "Eight-zone Anatomical Dissection" group had more samples classified as "complete" (96 vs 85) and fewer as "more complete" (13 vs 24) compared to the "Traditional Pelvic Floor Dissection" group, with a statistically significant difference (χ2 = 3.939, P = 0.047). Additionally, during the three-month postoperative follow-up, the Eight-zone Anatomical Dissection group demonstrated superior outcomes in urinary and sexual function (5.3 ± 2.5 vs 6.8 ± 3.3, P = 0.045), potentially due to the clearer anatomical strategy and reduced damage to pelvic floor nerves and blood vessels.
The intricate anatomy surrounding the rectum has long posed challenges in rectal cancer radical surgery. Inevitably, surgical procedures may cause damage to surrounding structures, particularly the vascular and neural complexes within the rectal lateral ligaments. The eight-zone dissection strategy offers a standardized approach to pelvic floor dissection, facilitating better exposure and protection of critical anatomical structures, thereby minimizing unnecessary injury and yielding improved surgical outcomes with reduced damage to the genitourinary system.
The evolution of surgical treatments for rectal cancer has been a gradual process, with notable advancements made through the contributions of scholars like Miles[20], who in 1908, recognized the high recurrence and mortality rates associated with local resection of rectal cancer. His subsequent design of the abdominoperineal resection marked a significant milestone in reducing the postoperative recurrence rate of rectal cancer[20,21].
The advent of TME further revolutionized rectal cancer surgery, introducing a more refined approach that emphasized the preservation of critical anatomical structures and autonomic nerves essential for maintaining urogenital and defecation functions[22]. The eight-zone dissection strategy, building upon the principles of TME, aims to standardize and homogenize the surgical process by providing a structured framework for the dissection of lateral ligaments during laparoscopic low and mid-rectal cancer surgery. This strategy not only serves as a complement to TME but also holds promise in reducing surgical complications and improving patient outcomes.
Moreover, the eight-zone dissection strategy emphasizes the adoption of a more optimized surgical approach, focusing on the exposure and protection of critical anatomical structures to reduce injury to the pelvic floor vascular and neural plexuses. This strategy provides practical guidance for surgeons navigating the complex anatomical landscape of rectal cancer surgery, ultimately contributing to safer and more effective surgical interventions.
This study, focusing on optimizing lateral ligament dissection in laparoscopic rectal cancer surgery, shows promise but has key limitations. Notably, the small, single-center sample may restrict generalizability. Additionally, the study lacked specialized assessments for urinary (e.g., international prostate symptom score, female urological function) and sexual functions (e.g., international index of erectile function, female sexual function index), limiting the precision of these outcomes. Future work should aim for larger, more diverse samples and incorporate these standardized measures for a more comprehensive understanding of surgical impacts.
| 1. | Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg. 1982;69:613-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1985] [Cited by in RCA: 1937] [Article Influence: 45.0] [Reference Citation Analysis (1)] |
| 2. | Guren MG, Kørner H, Pfeffer F, Myklebust TÅ, Eriksen MT, Edna TH, Larsen SG, Knudsen KO, Nesbakken A, Wasmuth HH, Vonen B, Hofsli E, Færden AE, Brændengen M, Dahl O, Steigen SE, Johansen MJ, Lindsetmo RO, Drolsum A, Tollåli G, Dørum LM, Møller B, Wibe A. Nationwide improvement of rectal cancer treatment outcomes in Norway, 1993-2010. Acta Oncol. 2015;54:1714-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 3. | Kapiteijn E, Putter H, van de Velde CJ; Cooperative investigators of the Dutch ColoRectal Cancer Group. Impact of the introduction and training of total mesorectal excision on recurrence and survival in rectal cancer in The Netherlands. Br J Surg. 2002;89:1142-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 377] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 4. | Wexner SD. Total mesorectal excision and low rectal anastomosis for the treatment of rectal cancer and prevention of pelvic recurrences. Tech Coloproctol. 2001;5:177. [PubMed] |
| 5. | Chua TC, Chong CH, Liauw W, Morris DL. Approach to rectal cancer surgery. Int J Surg Oncol. 2012;2012:247107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Liang JT, Chang KJ, Wang SM. Anatomical basis of autonomic nerve-preserving total mesorectal excision for rectal cancer. Br J Surg. 1997;84:586-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Smedh K, Khani MH, Kraaz W, Raab Y, Strand E. Abdominoperineal excision with partial anterior en bloc resection in multimodal management of low rectal cancer: a strategy to reduce local recurrence. Dis Colon Rectum. 2006;49:833-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Boereboom CL, Watson NF, Sivakumar R, Hurst NG, Speake WJ. Biological tissue graft for pelvic floor reconstruction after cylindrical abdominoperineal excision of the rectum and anal canal. Tech Coloproctol. 2009;13:257-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Ishii M, Shimizu A, Lefor AK, Kokado Y, Nishigori H, Noda Y. Reappraisal of the lateral rectal ligament: an anatomical study of total mesorectal excision with autonomic nerve preservation. Int J Colorectal Dis. 2018;33:763-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Giglia MD, Stein SL. Overlooked Long-Term Complications of Colorectal Surgery. Clin Colon Rectal Surg. 2019;32:204-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Runkel N, Reiser H. Nerve-oriented mesorectal excision (NOME): autonomic nerves as landmarks for laparoscopic rectal resection. Int J Colorectal Dis. 2013;28:1367-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Zhang C, Ding ZH, Li GX, Yu J, Wang YN, Hu YF. Perirectal fascia and spaces: annular distribution pattern around the mesorectum. Dis Colon Rectum. 2010;53:1315-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Kiyomatsu T, Ishihara S, Murono K, Otani K, Yasuda K, Nishikawa T, Tanaka T, Hata K, Kawai K, Nozawa H, Yamaguchi H, Watanabe T. Anatomy of the middle rectal artery: a review of the historical literature. Surg Today. 2017;47:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Chatwin NA, Ribordy M, Givel JC. Clinical outcomes and quality of life after low anterior resection for rectal cancer. Eur J Surg. 2002;168:297-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Stiggelbout AM, Kunneman M, Baas-Thijssen MC, Neijenhuis PA, Loor AK, Jägers S, Vree R, Marijnen CA, Pieterse AH. The EORTC QLQ-CR29 quality of life questionnaire for colorectal cancer: validation of the Dutch version. Qual Life Res. 2016;25:1853-1858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 16. | Arraras JI, Suárez J, Arias de la Vega F, Vera R, Asín G, Arrazubi V, Rico M, Teijeira L, Azparren J. The EORTC Quality of Life questionnaire for patients with colorectal cancer: EORTC QLQ-CR29 validation study for Spanish patients. Clin Transl Oncol. 2011;13:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 17. | Magaji BA, Moy FM, Roslani AC, Sagap I, Zakaria J, Blazeby JM, Law CW. Health-related quality of life among colorectal cancer patients in Malaysia: a study protocol. BMC Cancer. 2012;12:384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 18. | Nowak W, Tobiasz-Adamczyk B, Brzyski P, Sałówka J, Kuliś D, Richter P. Adaptation of quality of life module EORTC QLQ-CR29 for Polish patients with rectal cancer: initial assessment of validity and reliability. Pol Przegl Chir. 2011;83:502-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Lin JB, Zhang L, Wu DW, Xi ZH, Wang XJ, Lin YS, Fujiwara W, Tian JR, Wang M, Peng P, Guo A, Yang Z, Luo L, Jiang LY, Li QQ, Zhang XY, Zhang YF, Xu HW, Yang B, Li XL, Lei YX. Validation of the chinese version of the EORTC QLQ-CR29 in patients with colorectal cancer. World J Gastroenterol. 2017;23:1891-1898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 20. | Miles WE. A method of performing abdomino-perineal excision for carcinoma of the rectum and of the terminal portion of the pelvic colon (1908). CA Cancer J Clin. 1971;21:361-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 193] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Miles WE. The Present Position of the Radical Abdomino-Perineal Operation for Cancer of the Rectum in Regard to Mortality and Post-operative Recurrence. Proc R Soc Med. 1931;24:989-991. [PubMed] |
| 22. | Morgan E, Arnold M, Gini A, Lorenzoni V, Cabasag CJ, Laversanne M, Vignat J, Ferlay J, Murphy N, Bray F. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2023;72:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 923] [Article Influence: 461.5] [Reference Citation Analysis (1)] |