Published online Jan 21, 2024. doi: 10.3748/wjg.v30.i3.268
Peer-review started: October 2, 2023
First decision: November 12, 2023
Revised: December 1, 2023
Accepted: January 2, 2024
Article in press: January 2, 2024
Published online: January 21, 2024
Processing time: 107 Days and 16.8 Hours
Enterotoxins produce diarrhea through direct epithelial action and indirectly by activating the enteric nervous system. Calcium-sensing receptor (CaSR) inhibits both actions. The latter has been well documented in vitro but not in vivo. The hypothesis to be tested was that activating CaSR inhibits diarrhea in vivo.
To determine whether CaSR agonists ameliorate secretory diarrhea evoked by cholera toxin (CTX) in mice.
CTX was given orally to C57BL/6 mice to induce diarrhea. Calcium and calci
CTX induced secretory diarrhea, as evidenced by increases in fecal Cl-, stool consistency, and weight loss following CTX exposure, but did not alter CaSR, neither in content nor in function. Accordingly, calcium and R568 were each able to ameliorate diarrhea when applied to diseased intestines. Intestinal CaSR involvement is suggested by gene knockout experiments where the anti-diarrheal actions of R568 were lost in intestinal epithelial CaSR knockouts (villinCre/Casrflox/flox) and neuronal CaSR knockouts (nestinCre/Casrflox/flox).
Treatment of acute secretory diarrheas remains a global challenge. Despite advances in diarrhea research, few have been made in the realm of diarrhea therapeutics. ORS therapy has remained the standard of care, although it does not halt the losses of intestinal fluid and ions caused by pathogens. There is no cost-effective therapeutic for diarrhea. This and other studies suggest that adding calcium to ORS or using calcimimetics to activate intestinal CaSR might represent a novel approach for treating secretory diarrheal diseases.
Core Tip: Treatment of diarrhea remains a global challenge. Enterotoxins induce diarrhea through direct epithelial action and indirectly by activating the enteric nervous system. Using in vitro models in isolated tissues, we have previously shown that calcium-sensing receptor (CaSR) inhibits both actions. In the present study, we use a mouse model of secretory diarrhea in conjunction with a tissue-specific knockout approach and demonstrate that calcium or calcimimetic via CaSR ameliorates cholera toxin-induced secretory diarrhea in vivo. This study suggests that adding calcium to oral rehydration solution or using calcimimetic to activate intestinal CaSR might represent a new approach for treating secretory diarrheal diseases.
- Citation: Tang LQ, Fraebel J, Jin S, Winesett SP, Harrell J, Chang WH, Cheng SX. Calcium/calcimimetic via calcium-sensing receptor ameliorates cholera toxin-induced secretory diarrhea in mice. World J Gastroenterol 2024; 30(3): 268-279
- URL: https://www.wjgnet.com/1007-9327/full/v30/i3/268.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i3.268
Acute infectious diarrhea remains among the top causes of morbidity and deaths in children throughout the world[1,2]. According to the United Nations Children’s Fund/World Health Organization[3], approximately 9 million children (about half the population of New York) under 5 years died in 2008. 40% of these deaths were due to two diseases: Pneumonia and diarrhea. Diarrhea remains the second leading cause of death in children younger than 5 years globally, accounting for one in every five child deaths - around 1.5 million a year - more than acquired immune deficiency syndrome, malaria, and measles combined. Importantly, most of the morbidity and mortality is not due to infection but dehydration. Accordingly, reducing the fluid loss from acute diarrhea offers a major opportunity for improving child health globally.
Enterotoxins induce diarrhea through direct epithelial action and indirectly by activating the enteric nervous system (ENS)[4]. For example, cholera induces diarrhea through the generation of cholera toxin (CTX) from V. cholera. CTX binds to the enterocyte, leading to ADP-ribosylation of the Gs α-subunit. This constitutively activates membrane-bound adenylyl cyclase in enterocytes and elevates intracellular cyclic adenosine monophosphate (cAMP). Increased cAMP stimulates protein kinase A and phosphorylation of the cystic fibrosis transmembrane conductance regulator (CFTR) channel, as well as the Na+/K+/2Cl- cotransporter (NKCC1), causing secretion of Cl- and water. Elevated cAMP levels also inhibit Cl- and water absorption mediated by Cl-/HCO3- exchange and Na+/H+ exchange[4,5]. In addition to direct epithelial action, CTX elicits neuronal secretory reflexes by binding to mucosal enterochromaffin cells, leading to the production of 5-hydroxytryptamine, activation of ENS and release of neurotransmitters (e.g., vasoactive intestinal peptide and acetylcholine) that stimulate and amplify fluid secretion, leading to dehydration and rapid body weight loss[4,6,7].
Importantly, the extracellular calcium-sensing receptor (CaSR)[8] is present on cells in both pathways[5,9,10] and, when activated in vitro, blocks both diarrhea-causing pathways evoked by CTX and other diarrhea-causing enterotoxins/secretagogues. For example, using a microperfused colonic crypt technique, it has been shown that calcium, calcimimetics or polyamines that activate CaSR can act on intestinal epithelium and reverse CTX/forskolin-induced fluid secretion using a signal transduction pathway that promotes cyclic nucleotide destruction[11-13]. Using Ussing chambers, it has been shown that the effects of CTX/forskolin and CaSR agonists on electrolyte secretion by the intestine can also be attributed to opposing actions of enterotoxins/secretagogues and CaSR on ENS activity[14,15]. These results suggest that targeting intestinal CaSR might represent a previously undescribed novel approach for treating secretory diarrheal diseases[5,9,10,16-18]. However, all of the experiments that suggest CaSR modulates dual-pathway secretion by the intestine have been performed in vitro in isolated tissues. Neither the functionality of the CaSR receptors in vivo nor the anti-diarrheal potential of CaSR agonists in live animals have been documented, although the latter is necessary before clinical trials in humans are performed.
In this study, we tested the hypothesis that calcium/calcimimetic via CaSR ameliorates secretory diarrhea in vivo in mice. A CTX mouse model of secretory diarrhea was employed and the effects of CaSR agonists on biochemical (i.e., changes in fecal Cl-) and clinical outcomes (i.e., changes in stool consistency and body weight loss) of secretory diarrheal disease were assessed. We selected the CTX mouse model because we had employed it as a model in previous in vitro studies. In addition, it has been widely used to provide proof of concept of whether an anti-diarrheal agent is therapeutic or not[19-22]. In addition to testing calcium, we also examined the effects of the calcimimetic R568, a pharmacological allosteric CaSR agonist[23]. To maximize their local intestinal actions, we delivered agonists as follows: Calcium was administered orally by adding it to oral rehydration solution (ORS) and R568 was applied serosally using an intraperitoneal route, as previously described[15]. To verify that their actions resulted from intestinal tissues and not a non-specific off-target action, the effects were also measured on intestine-specific CaSR knockouts. We show for the first time that targeting intestinal CaSR with calcium or calcimimetic is efficacious in reducing CTX-evoked secretory diarrhea in vivo in live animals and that this occurs through receptor-mediated reduction of both the neurally and non-neuronally mediated secretory responses. A portion of this work was presented in an abstract in the Global Health Forum of 5th World Congress of Pediatric Gastroenterology, Hepatology and Nutrition[24].
Experiments were performed using male/female C57BL/6 mice (wild type and Casr mutants). Mice lacking CaSR expression in intestinal epithelial cells (villinCre/Casrflox/flox mice) and mice lacking CaSR expression in intestinal neurons (nestinCre/Casrflox/flox mice) and their wild type littermates were bred and maintained in-house at the University of Florida Communicore Animal Facility. Mutant mice were generated as previously described[25,26]. Briefly, CaSR flox/flox mice were bred with transgenic mice expressing Cre recombinase under the control of the Villin 1 or Nestin promoter and genotyped prior to all experiments after approximately 20-30 generations. Mice were used at 5-10 wk of age and weight of 17-23 g in accordance with the Animal Welfare Act and the Public Health Policy on Humane Care. Animals were fed and maintained on regular chow (Harlan) with free access to water before the experiment. To maximally protect animal welfare, we used numbers of animals in each experiment group as minimal and small as scientifically or statistically allowed. Thus, depending on variation of the data obtained and/or availability of the animals tested, 5-11 animals were employed, although 2-3 animals were used in some dose-dependence studies. This was because these were the minimal numbers required for statistical significance using one-way ANOVA and P < 0.05 as determined in a pilot experiment. To minimize the effects of subjective bias in allocating animals, we treated controls, interventions, wild type, and mutants in the same manner on the same days by the same investigators. The animal protocols were designed to minimize pain or discomfort to the animals. After completion of the experiment, animals were sacrificed with standard CO2 inhalation and by cervical dislocation. The use of animals as well as the protocols for CTX treatment and colon tissue isolation was approved by the Institutional Animal Care and Use Committee (IACUC# 201807567) at the University of Florida.
Two protocols were used to induce diarrhea: Protocol 1 is long and was used to compare the effects of with vs without oral calcium, a poorly absorbed mineral agonist of CaSR[27-29]; whereas protocol 2 is short and was used to compare the effects of with vs without R568, a quickly absorbable small-molecule agonist of CaSR[30].
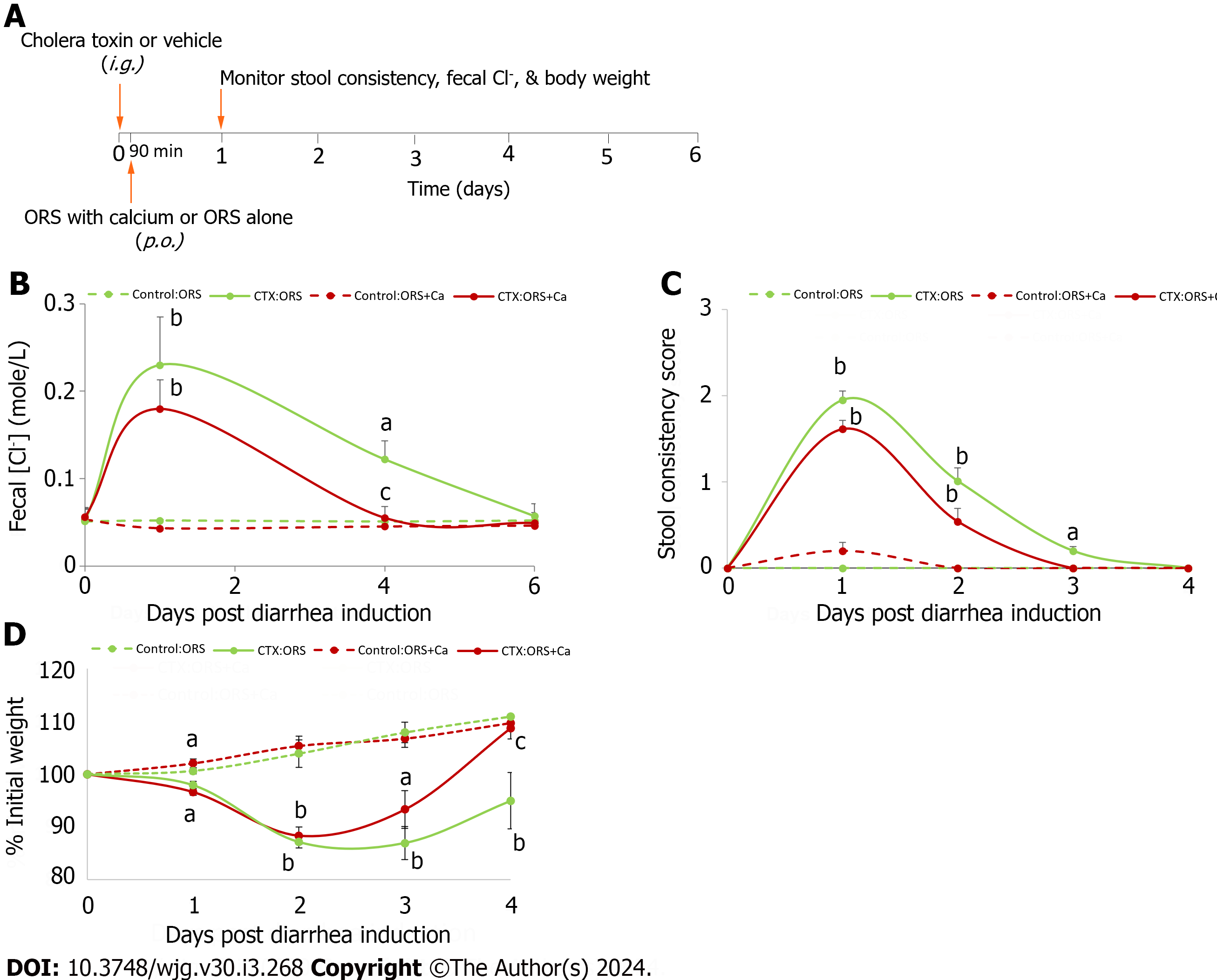
Protocol 1: Animals were first fasted overnight for 16 h before they were gavaged intragastrically (i.g.) with 200 μL 7% NaHCO3 buffer containing 20 μg CTX or vehicle per mouse to induce diarrhea. After CTX gavage, animals were fasted for an additional 90 min before they were allowed access to regular chow to avoid food interference on toxin binding and action. Afterwards, animals were divided into two groups: Group 1 received drinking bottles containing ORS only whereas Group 2 received drinking bottles containing ORS supplemented with 5 mmol/L calcium. This calcium concentration was selected because it is the lowest concentration of calcium that generated maximal CaSR-activation effects[11,12]. Diarrhea was monitored and was either semi-quantitated clinically according to stool consistency [0, normal feces (solid); 1, moist feces (semi-solid); 2, mild diarrhea (loose); and 3, severe diarrhea (watery)[31]] or quantitated biochemically according to fecal Cl- content. Degree of dehydration was measured by diarrhea-associated body weight loss[32]. In this study, the onset of diarrhea is defined as the appearance of the first diarrheic stool with stool consistency scored one or higher, as described[31].
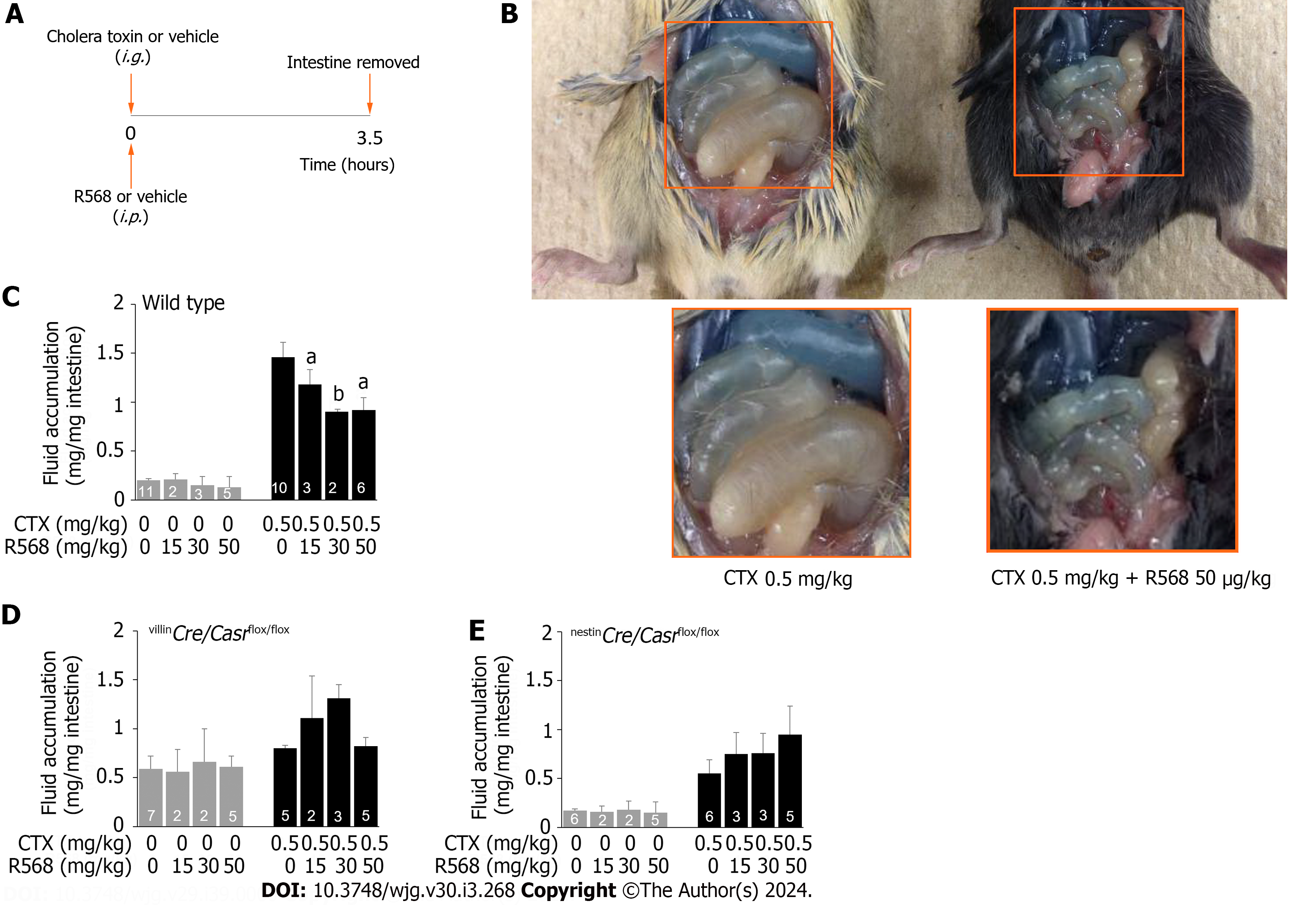
Protocol 2: Animals were pretreated and treated as in protocol 1 except for the following: (1) Calcimimetic R568 (diluted in 100 μL normal saline) was administered serosally at the time when diarrhea was induced. R568 was administered serosally using an intraperitoneally (i.p.) rather than per os route to minimize the unwanted systemic effects while maximizing the desired local intestinal action as described[15]. Neither anesthesia nor analgesia were used; (2) 1.5 h post CTX treatment, animals were allowed to drink ORS without calcium; and (3) Animals were sacrificed 3.5 h after CTX treatment before watery stool was seen. Pilot studies showed that diarrhea started to occur about 0.5 h post CTX gavage and reached a peak plateau about 1.5 h later[33], but no diarrheic stool was seen until 3.5 h post CTX treatment. Three and half-hours later, animals were killed, fluid accumulated in the intestine was removed and weighed, changes compared, and is expressed as mg/mg intestine.
Feces were collected in pre-weighed Eppendorf tubes. To avoid variations from freeze-thaw cycle and bacterial overgrowth from storage, all fecal samples were collected, gently processed, and promptly measured as described[34]. In brief, following collection, samples were weighed and diluted appropriately in deionized water so that the Cl- content in each sample fell within the linear range of the standard curve. Diluted samples were gently but thoroughly homogenized through pipetting before centrifugation at 14000 g for 10 min. Sonication was not used to minimize release of intracellular contents. The resulting supernatants were collected, and Cl- contents measured with an ion-selective electrode by potentiometric titration (Model LIS-146CLCM-XS system; JENCO Electronics, Ltd, Taipei City, Taiwan). The results were calculated according to the standard curve, and are expressed as mole/L, where 1 L of feces ≈ 1 kg of feces. Previous studies have shown that these methods caused no or minimal variations in fecal Cl-[34-36].
Isolation and preparation of intestinal homogenates and lysates and western blot analysis of CaSR protein were performed as previously described[12] with an affinity-purified mouse monoclonal antibody (5C10, ADD) raised against a 22-amino acid peptide corresponding to amino acid residues 214-235 of human CaSR (Abcam, Cambridge, MA, United States). CaSR protein signals were normalized against heat shock protein 90 (HSP90) as a loading control and are expressed as CaSR/HSP90 protein signal ratios[37].
CTX was obtained from Sigma (St Louis, MO, United States), and 5 mg/mL stock solutions were prepared in water. R568 was purchased from Tocris Bioscience (Ellisville, MI, United States), and 20 mg/mL stock solutions were prepared in 15% 2-hydroxypropyl-β-cyclodextrin (Research Biochemicals International, Natick, MA, United States). Calcium chloride was from Sigma, and rabbit polyclonal antibody against HSP90 was from Santa Cruz Biotechnology (Dallas, TX, United States). ORS was prepared fresh containing (in mmol/L) 75 Na+, 20 K+, 65 Cl-, 10 citrate and 75 glucose with total osmolarity of 245 mOsm/kg H2O.
The statistical methods of this study were reviewed by Dr. Han-Zhi Gao, PhD, member of the Biostatistics Service from the Clinical and Translational Science Institute of the University of Florida. Data from all animals were included in the analysis. Values are given as means ± standard error of the mean. The normality of variables was checked; the data for intestinal fluid accumulations exhibited a skewed distribution and were therefore log transformed. After log transformation, the data became normally distributed. Statistical comparisons between two means were performed by Student’s t-test, whereas comparisons among multiple means were by one-way ANOVA with Tukey’s post hoc tests. Both tests were performed using Microsoft Excel 2016 for Windows or using GraphPad Prism version 6.07 for Windows (GraphPad Software, San Diego, CA, United States). P < 0.05 was considered significant.
Our first set of experiments was performed with the calcimimetic R568 used in conjunction with intestinal CaSR-specific knockouts to demonstrate the functionality of intestinal CaSR in vivo and to demonstrate the intestinal specificity of the agent. In these experiments, diarrhea-provoking CTX or vehicle was given i.g. whereas the anti-diarrheal R568 or vehicle was administered i.p. to avoid interference between the two agents. For accurate quantification, diarrhea was induced such that all the secreted fluid was contained inside the intestinal lumen without loss outside of the body. Before they were sacrificed, animals did not exhibit any noticeable adverse effects. Data from all animals were included in the analysis.
In unstimulated vehicle-gavaged wild type mice, intestinal fluid accumulation was 0.20 ± 0.02 mg/mg intestine. Intragastric gavage of CTX caused diarrhea, as evidenced by increased intestinal fluid accumulation in a dose-dependent fashion (Figure 1). At the EC50 of approximately 0.5 mg/kg, the amount of intestinal fluid accumulation was 1.46 ± 0.15 mg/mg intestine, which is about a 7-fold increase in intestinal fluid accumulation compared to non-CTX controls (Table 1).
| Group | Intestinal fluid accumulation in mg fluid/mg intestine | ||
| Wild type | villinCre/Casrflox/flox | nestinCre/Casrflox/flox | |
| Control | 0.20 ± 0.02 (11) | 0.59 ± 0.13 (7)a | 0.17 ± 0.02 (6) |
| R568 (50 mg/kg) | 0.15 ± 0.09 (5) | 0.61 ± 0.11 (5) | 0.15 ± 0.11 (5) |
| CTX (0.5 mg/kg) | 1.46 ± 0.15 (10)c | 0.80 ± 0.03 (5)b | 0.55 ± 0.14 (6)b |
| CTX (0.5 mg/kg) + R568 (50 mg/kg) | 0.92 ± 0.16 (6)d | 0.82 ± 0.09 (5) | 0.95 ± 0.29 (5) |
Many conditions like carcinogenesis reduce CaSR expression in the intestine[38]. To assess if this occurred to CTX-treated intestines, we examined CaSR protein expression by western blots (Figure 1). Although CTX caused diarrhea, the toxin did not alter intestinal CaSR expression. The CaSR/HSP90 protein signal ratios were control 0.50 ± 0.02 (5) vs CTX 0.53 ± 0.03 (5), P > 0.05.
Having known that CaSR protein expression was unaltered, we then studied CaSR function by assessing the ability of the calcimimetic R568 to reverse CTX-induced diarrhea in vivo (Figure 2). For this, the EC50 dose 0.5 mg/kg of CTX was i.g. gavaged to induce moderate diarrhea, while different doses of R568 were administered i.p. Three and half hours later, animals were sacrificed, intestines were removed, and intestinal fluid was measured (Figure 2A). Indeed, CTX induced diarrhea (Figure 2B). When R568 was applied to the CTX-induced diseased intestine, it ameliorated diarrhea and reduced CTX-induced fluid accumulation (Figure 2B) in a dose-dependent manner. Fluid accumulation was reduced by approximately 50% when the near maximal effective doses of 30-50 mg/kg of R568 were applied (Figure 2C) (ANOVA test; P < 0.05). In non-CTX vehicle gavaged mice, R568 generated no or only minimal inhibitory effects (Figure 2C) (ANOVA test; P > 0.05). These results indicate that CaSR function remains unaltered in diseased intestines, consistent with the in vitro findings[13,15].
To show that intestinal CaSR was indeed targeted and was not simply due to a non-specific off-target action, additional studies on intestinal tissue-specific CaSR knockout mice (i.e., villinCre/Casrflox/flox and nestinCre/Casrflox/flox) were performed and effects compared (Table 1, Figure 2D and E). Under non-CTX vehicle-stimulated basal conditions, intestinal fluid accumulation was increased in villinCre/Casrflox/flox mice (0.59 ± 0.13 mg/mg intestine, P < 0.05 vs wild type mice) (Table 1), and was unchanged in nestinCre/Casrflox/flox mice (0.17 ± 0.02 mg/mg intestine, P > 0.05 vs wild type mice) (Table 1). Addition of CTX (0.5 mg/kg) caused diarrhea, as evidenced by significantly increased intestinal fluid accumulation of these mice (Table 1), although the diarrhea was less severe in knockouts than in wild type (Table 1) due to activation of compensatory mechanisms. Importantly, R568 administration at all tested concentrations did not inhibit CTX-induced diarrhea in neither villinCre/Casrflox/flox mice (ANOVA test; P > 0.05) (Figure 2D) nor nestinCre/Casrflox/flox mice (ANOVA test; P > 0.05) (Figure 2E), confirming that the anti-diarrheic action was indeed exerted largely, if not exclusively, via CaSR in the intestinal tissues. R568 alone had no effect in neither villinCre/Casrflox/flox mice (ANOVA test; P > 0.05) (Figure 2D) nor nestinCre/Casrflox/flox mice (ANOVA test; P > 0.05) (Figure 2E) despite the presence of diarrhea in villinCre/Casrflox/flox mice, further confirming that the CaSR is required for the calcimimetic to exert its anti-diarrheic action.
After performing the proof-of-concept studies using the calcimimetic R568 and verifying the functionality of intestinal CaSR, we tested whether targeting CaSR with calcium, an inexpensive widely available child-friendly mineral, was an anti-diarrheal in vivo. We added calcium to ORS and investigated whether it helped reduce the severity of diarrhea and enhance the rate of rehydration by ORS. In these experiments, animals were first i.g. gavaged with CTX or vehicle. Ninety minutes later, they were allowed to drink ORS with calcium or ORS alone, and development and progression of diarrhea was monitored both biochemically through changes in fecal Cl- content and clinically by assessing changes in stool consistency and diarrhea-associated body weight loss (Figure 3A).
In secretory diarrhea, active Cl- secretion and decreased Cl- absorption is the primary driving force for water moving from the blood to the intestinal lumen[39]. Thus, to assess whether calcium supplemented ORS (ORS + Ca) is better than ORS alone in reducing intestinal Cl- loss from diarrhea, we first measured and compared changes in fecal Cl- concentration (Cl-). Figure 3B shows the changes in fecal Cl- at day 1, day 4, and day 6 post-CTX gavage. Day 1 represents the acute stage of diarrhea, day 4 the recovery stage, and day 6 post-recovery. Consistent with enterotoxin-induced intestinal Cl- loss, mice in both groups displayed a significantly higher mean fecal Cl- upon CTX exposure (P < 0.01, day 1 post CTX vs day 1 Control). However, compared to the high fecal Cl- in CTX:ORS-treated mice, a lower fecal Cl- was noted in mice receiving CTX:(ORS + Ca) treatment (ANOVA test P < 0.05; Student’s t test P values at day 1, 4 and 6 = 0.56, 0.07 and 0.08 vs respective non-Ca controls). Moreover, relative to mice on CTX:ORS treatment, mice on CTX:(ORS + Ca) recovered from diarrhea-associated Cl- losses significantly faster. Thus, while the CTX:ORS group fecal Cl- losses had remained significantly elevated above baseline until day 6 post-CTX gavage, in CTX:(ORS + Ca) group, a close to normal fecal Cl- had been observed at day 4 post-CTX treatment (Figure 3B). Calcium had no or minimal effects on non-CTX vehicle-treated mice (one-way ANOVA test; P > 0.05). These results suggest that ORS + Ca is better than ORS alone in reducing diarrhea-associated intestinal Cl- losses.
Reducing intestinal Cl- loss suggests the possibility of reducing diarrhea and dehydration. Thus, we compared the onset, severity and recovery of diarrhea/dehydration induced by CTX in ORS + Ca vs ORS groups. First comparison was made regarding the onset of diarrhea (i.e., the time from CTX gavage to the appearance of the first diarrheic stool). Since it would take some time for calcium to produce a clinically visible anti-diarrheal action, calcium may or may not influence the onset of diarrhea. Before CTX gavage, all mice displayed normal solid stool. Following CTX gavage, mice receiving ORS had the first diarrheic stool at 4.5 ± 1.7 h, whereas mice receiving ORS + Ca developed diarrhea at 4.3 ± 1.8 h. No statistically significant difference was noted (P = 0.69).
We then compared the changes in stool consistency over time. Similarly, adding calcium reduced the stool consistency score in CTX-treated (ANOVA test; P < 0.05) but not in the non-CTX control (ANOVA test; P > 0.05). The result is summarized in Figure 3C. Specifically, in ORS group, CTX significantly increased stool consistency scores on day 1, day 2 and day 3 but not on day 4 compared to the non-CTX control, whereas in ORS + Ca group, CTX significantly increased stool consistency scores only on day 1 and day 2 but not on day 3 and day 4. Thus, while the ORS group stool consistency score remained significantly elevated above baseline until day 4 post-CTX gavage, in ORS + Ca group, a normal stool consistency score had been observed 1 d earlier at day 3 post-CTX treatment. These results suggest that ORS + Ca is better than ORS alone in reducing diarrhea.
Considering that the stool consistency scoring is only semi-quantitative and has large performance-dependent variations, we compared body weight changes before and after disease induction, a quantitative way of measuring diarrhea severity and degree of dehydration[32]. We chose to monitor body weight instead of monitoring 24-h stool volume because we had technical difficulties in accurately collecting stool and quantifying 24-h stool volume. Figure 3D shows body weight changes over time in mice in ORS + Ca group vs ORS only group along with their non-CTX controls. In response to CTX challenge, mice on both groups lost significant weight, particularly in day 1 and day 2. However, mice receiving ORS + Ca lost significantly less weight and recovered significantly sooner than mice receiving ORS only (ANOVA test; P < 0.01). Thus, while mice in the ORS group continued to lose weight to a statistically significant degree until after day 4 post-CTX, mice in ORS + Ca group had achieved a close to normal body weight at day 3 pos-CTX treatment (Figure 3D). The estimated time at which mice returned to their initial weight was 3.5 d in Ca-ORS group and 4.5 d in ORS only group, which is 22% faster in ORS + Ca group. These results suggest that adding calcium to ORS reduces the severity of dehydration, hastens its recovery, and accelerates the rate of rehydration by ORS.
This first in vivo study proves that targeting intestinal CaSR with calcium or calcimimetic is efficacious in reducing CTX-evoked secretory diarrhea and that this occurs through receptor-mediated reduction of both the neurally (i.e., Nestin-expressing enteric neuron) and non-neuronally (i.e., Villin-expressing epithelial cell) mediated Cl- secretory responses. A schematic diagram illustrating how CTX induces and calcium/calcimimetic inhibits these two Cl- secretory responses is depicted in Figure 4.
We showed that CTX induced secretory diarrhea in mice as previously reported[19-22]. This was evidenced by increased fecal Cl- and water content/stool consistency and weight loss following CTX induction. Importantly, although it altered intestinal fluid balance and caused diarrhea, CTX did not seem to alter CaSR content or function. Accordingly, when applied to diseased intestines, calcium and calcimimetic were each able to ameliorate diarrhea. Intestinal CaSR involvement is further supported by gene knockout experiments in which the anti-diarrheal activity of CaSR agonists observed in wild type mice was not noted in knockouts. Neither the villinCre/Casrflox/flox mice that lack epithelial CaSR nor the nestinCre/Casrflox/flox mice that lack neuronal CaSR experienced amelioration of diarrhea with CaSR agonists.
Interestingly, while both CaSR knockouts responded to CTX stimulation, their responses were less prominent than their wild types. The reason is unknown and is related to the downregulation of NKCC1 and CFTR in these animals[32]. NKCC1 and CFTR are two ion transporters required for the intestine to generate an effective diarrheal response to secretagogues[40,41].
Additionally, differences in the phenotype of two intestinal CaSR knockouts under basal conditions are noted. While villinCre/Casrflox/flox mice developed spontaneous diarrhea, as evidenced by mild but significant increased fluid accumulation in unstimulated intestines, nestinCre/Casrflox/flox mice did not, as there was no increase in fluid accumulation in unstimulated intestines (Table 1). The reason is unknown but may be related to the fasting condition used and differences in roles and functions these epithelial and neuronal CaSR receptors play in intestinal function. The primary function of the gastrointestinal (GI) tract is to digest food and absorb nutrients. To aid digestion, the GI tract secretes a large amount of fluid to mix the food components and lubricate the luminal surface. It is estimated that following the ingestion of a meal, intestinal secretion can be increased eightfold[42]. Upon completion of digestion and extraction of nutrients, intestinal secretion stops. While studies suggest that mechanical sensors in the ENS have a significant role in triggering the meal-evoked secretion[6], there is evidence that chemical sensors (e.g., CaSR) on the epithelium and enteric neurons have a key role in terminating this process. The latter do so through their ability to sense nutrients released during digestion[25] (also, a recent review by Tang et al[10]). Consistent with active regulation of intestinal secretion by CaSR, under the no-food-no-nutrient fasting condition used in the present study, neuronal CaSR would have been silent and, as a result, no intestinal phenotype would be expected in nestinCre/Casrflox/flox mice (Table 1).
The finding of spontaneous diarrhea in villinCre/Casrflox/flox mice is notable (Table 1). This indicates that unlike neuronal CaSR, epithelial CaSR does remain active, at least to some degree, under a no-food-no-nutrient fasting condition. This is not surprising given the multiple roles and functions that CaSR plays in GI biology[43]. In addition to its established function as a nutrient sensor regulating fluid secretion and absorption during food digestion, epithelial CaSR is a fundamental mechanism for sensing and regulating the ionic and nutrient compositions of extracellular milieu surrounding the epithelium of the entire GI tract[10]. Thus, at basal no-food no-nutrient fasting state, this epithelial CaSR may perform other tasks, for example, monitoring the Ca2+ surrounding the epithelium.
According to the present model of intestinal Ca2+ transport[44], the Ca2+ ion cycles between the leaking crypt, which secretes Ca2+via a passive paracellular pathway, and the electrically tight villous/surface epithelium, which absorbs back Ca2+via an active transcellular transport mechanism. Interestingly, under basal conditions, fluid also cycles in a similar fashion between the crypt, which secretes, and the villous/surface epithelium, which absorbs[45]. The purpose of this fluid cycling is to lubricate the luminal surface to prevent the crypt lumen from obstructing and to defend the crypt from invasion by lumen bacteria. It is likely that CaSR located on enterocyte apical and basolateral membranes constitutively sense Ca2+, thereby monitoring and controlling these processes.
The most notable observation of the present study is that calcium and calcimimetic both significantly ameliorated CTX-induced secretory diarrhea. The latter has important therapeutic value. Treatment of acute secretory diarrheas remains a global challenge. Despite advances in diarrhea research, few advancements have been made in the realm of diarrhea therapeutics, and ORS therapy has remained the standard of care even though it does not stop the loss of intestinal fluid and ions caused by pathogens. There is no cost-effective therapeutic for diarrhea. This study suggests that adding calcium to ORS or using calcimimetic to activate intestinal CaSR might represent a novel approach for treating secretory diarrheal diseases. Limitations of this study include: (1) The present study was an animal but not human study; (2) No data on oral calcimimetic was obtained; and (3) Neither local nor systemic adverse effects were documented, despite the fact that some animals in study protocol 1 appeared to be sick, particularly at day 2 following the exposure of disease-causing CTX. Better designed animal studies and randomized clinical trials in humans are warranted.
Treatment of diarrhea such as cholera remains a global challenge. Cholera toxin (CTX) produces diarrhea through direct epithelial action and indirectly by activating the enteric nervous system. Calcium-sensing receptor (CaSR) is present in both tissues and, when activated, inhibits both actions. The latter has been well documented in vitro but not in vivo. Thus, the present study tested whether activating intestinal epithelial or neuronal CaSR inhibits diarrhea in vivo.
Acute infectious diarrhea remains among the top causes of morbidity and death in the world. Most of the morbidity and mortality is not due to infection but dehydration. Accordingly, how to effectively reduce the fluid loss from acute diarrhea offers a major opportunity for improving global health.
The objective of the present study was to determine whether CaSR agonists ameliorate secretory diarrhea evoked by CTX in wild type mice, epithelial-specific CaSR knockout mice (villinCre/Casrflox/flox) and neuronal-specific CaSR knockout mice (nestinCre/Casrflox/flox).
To realize the objectives, CTX was administered orally to C57BL/6 mice to induce secretory diarrhea while calcium and calcimimetic R568 were employed to activate CaSR. To maximize their local intestinal actions, calcium was administered luminally via oral rehydration solution (ORS) whereas R568 was applied serosally using an intraperitoneal route. To verify that their actions resulted from the intestinal epithelium and enteric neurons, effects were also examined on two Cre-lox intestine-specific CaSR knockouts. Diarrhea outcome was measured biochemically by monitoring changes in fecal Cl- or clinically by assessing stool consistency and weight loss.
CTX induced secretory diarrhea, as evidenced by increases in fecal Cl-, stool consistency, and weight loss following CTX exposure. Calcium and R568 each ameliorated CTX-induced secretory diarrhea in wild type mice but not in either knockout mouse model.
Based on the present study, we propose that activating intestinal epithelial or neuronal CaSR can inhibit secretory diarrhea in vivo. Adding calcium to ORS or using calcimimetic to activate intestinal CaSR might represent a novel approach for treating secretory diarrheal diseases in humans.
Future research should be directed to conduct randomized clinical trials utilizing calcium or calcimimetics to treat cholera and other secretory diarrheal diseases in humans.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yang J, China; Yin CH, China S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Cai YX
| 1. | Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD; WHO-coordinated Global Rotavirus Surveillance Network. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1037] [Cited by in RCA: 945] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 2. | Moore SR, Lima AA, Guerrant RL. Infection: Preventing 5 million child deaths from diarrhea in the next 5 years. Nat Rev Gastroenterol Hepatol. 2011;8:363-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Wardlaw T, Salama P, Brocklehurst C, Chopra M, Mason E. Diarrhoea: why children are still dying and what can be done. Lancet. 2010;375:870-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 288] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 4. | Field M. Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest. 2003;111:931-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 181] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 5. | Cheng SX. Calcium-sensing receptor: A new target for therapy of diarrhea. World J Gastroenterol. 2016;22:2711-2724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (2)] |
| 6. | Cooke HJ. Neurotransmitters in neuronal reflexes regulating intestinal secretion. Ann N Y Acad Sci. 2000;915:77-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 102] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Lundgren O. Enteric nerves and diarrhoea. Pharmacol Toxicol. 2002;90:109-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993;366:575-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1993] [Cited by in RCA: 1764] [Article Influence: 55.1] [Reference Citation Analysis (0)] |
| 9. | Harrell JE, Cheng SX. Inability to reduce morbidity of diarrhea by ORS: can we design a better therapy? Pediatr Res. 2018;83:559-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Tang L, Cheng CY, Sun X, Pedicone AJ, Mohamadzadeh M, Cheng SX. The Extracellular Calcium-Sensing Receptor in the Intestine: Evidence for Regulation of Colonic Absorption, Secretion, Motility, and Immunity. Front Physiol. 2016;7:245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Cheng SX, Geibel JP, Hebert SC. Extracellular polyamines regulate fluid secretion in rat colonic crypts via the extracellular calcium-sensing receptor. Gastroenterology. 2004;126:148-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Cheng SX, Okuda M, Hall AE, Geibel JP, Hebert SC. Expression of calcium-sensing receptor in rat colonic epithelium: evidence for modulation of fluid secretion. Am J Physiol Gastrointest Liver Physiol. 2002;283:G240-G250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Geibel J, Sritharan K, Geibel R, Geibel P, Persing JS, Seeger A, Roepke TK, Deichstetter M, Prinz C, Cheng SX, Martin D, Hebert SC. Calcium-sensing receptor abrogates secretagogue- induced increases in intestinal net fluid secretion by enhancing cyclic nucleotide destruction. Proc Natl Acad Sci U S A. 2006;103:9390-9397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 14. | Cheng SX. Calcium-sensing receptor inhibits secretagogue-induced electrolyte secretion by intestine via the enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2012;303:G60-G70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Tang L, Jiang L, McIntyre ME, Petrova E, Cheng SX. Calcimimetic acts on enteric neuronal CaSR to reverse cholera toxin-induced intestinal electrolyte secretion. Sci Rep. 2018;8:7851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Chu T, Yottasan P, Goncalves LS, Oak AA, Lin R, Tse M, Donowitz M, Cil O. Calcium-sensing receptor activator cinacalcet for treatment of cyclic nucleotide-mediated secretory diarrheas. Transl Res. 2024;263:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Oak AA, Chhetri PD, Rivera AA, Verkman AS, Cil O. Repurposing calcium-sensing receptor agonist cinacalcet for treatment of CFTR-mediated secretory diarrheas. JCI Insight. 2021;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | Barahona MJ, Maina RM, Lysyy T, Finotti M, Caturegli G, Baratta V, D'Amico F, Mulligan D, Geibel JP. Activation of the Calcium Sensing Receptor Decreases Secretagogue-Induced Fluid Secretion in the Rat Small Intestine. Front Physiol. 2019;10:439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Li C, Dandridge KS, Di A, Marrs KL, Harris EL, Roy K, Jackson JS, Makarova NV, Fujiwara Y, Farrar PL, Nelson DJ, Tigyi GJ, Naren AP. Lysophosphatidic acid inhibits cholera toxin-induced secretory diarrhea through CFTR-dependent protein interactions. J Exp Med. 2005;202:975-986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | Subramanya S, Ramakrishna BS, Binder HJ, Farthing MJ, Young GP. Evaluation of oral rehydration solution by whole-gut perfusion in rats: effect of osmolarity, sodium concentration and resistant starch. J Pediatr Gastroenterol Nutr. 2006;43:568-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Thiagarajah JR, Broadbent T, Hsieh E, Verkman AS. Prevention of toxin-induced intestinal ion and fluid secretion by a small-molecule CFTR inhibitor. Gastroenterology. 2004;126:511-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Ma T, Thiagarajah JR, Yang H, Sonawane ND, Folli C, Galietta LJ, Verkman AS. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest. 2002;110:1651-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 310] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 23. | Nemeth EF, Steffey ME, Hammerland LG, Hung BC, Van Wagenen BC, DelMar EG, Balandrin MF. Calcimimetics with potent and selective activity on the parathyroid calcium receptor. Proc Natl Acad Sci U S A. 1998;95:4040-4045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 446] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 24. | Cheng SX, Tang L, Shi J, Winesett S, Binder HJ. Activation of calcium-sensing receptor inhibits enterotoxin-induced secretion and bacteria-induced inflammation in rodents and reduces infectious diarrhea in children. J Pediatr Gastroenterol Nutr. 2016;63:S2. |
| 25. | Sun X, Tang L, Winesett S, Chang W, Cheng SX. Calcimimetic R568 inhibits tetrodotoxin-sensitive colonic electrolyte secretion and reduces c-fos expression in myenteric neurons. Life Sci. 2018;194:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Tang L, Peng M, Liu L, Chang W, Binder HJ, Cheng SX. Calcium-sensing receptor stimulates Cl(-)- and SCFA-dependent but inhibits cAMP-dependent HCO3(-) secretion in colon. Am J Physiol Gastrointest Liver Physiol. 2015;308:G874-G883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Bovee-Oudenhoven IM, Lettink-Wissink ML, Van Doesburg W, Witteman BJ, Van Der Meer R. Diarrhea caused by enterotoxigenic Escherichia coli infection of humans is inhibited by dietary calcium. Gastroenterology. 2003;125:469-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Sheikh MS, Santa Ana CA, Nicar MJ, Schiller LR, Fordtran JS. Gastrointestinal absorption of calcium from milk and calcium salts. N Engl J Med. 1987;317:532-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 152] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Lupton JR, Steinbach G, Chang WC, O'Brien BC, Wiese S, Stoltzfus CL, Glober GA, Wargovich MJ, McPherson RS, Winn RJ. Calcium supplementation modifies the relative amounts of bile acids in bile and affects key aspects of human colon physiology. J Nutr. 1996;126:1421-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Fox J, Lowe SH, Petty BA, Nemeth EF. NPS R-568: a type II calcimimetic compound that acts on parathyroid cell calcium receptor of rats to reduce plasma levels of parathyroid hormone and calcium. J Pharmacol Exp Ther. 1999;290:473-479. [PubMed] |
| 31. | Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, Kim S, Fritz JV, Wilmes P, Ueha S, Matsushima K, Ohno H, Olle B, Sakaguchi S, Taniguchi T, Morita H, Hattori M, Honda K. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1875] [Cited by in RCA: 2140] [Article Influence: 178.3] [Reference Citation Analysis (2)] |
| 32. | Cheng SX, Lightfoot YL, Yang T, Zadeh M, Tang L, Sahay B, Wang GP, Owen JL, Mohamadzadeh M. Epithelial CaSR deficiency alters intestinal integrity and promotes proinflammatory immune responses. FEBS Lett. 2014;588:4158-4166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 33. | Sharp GW. Action of cholera toxin on fluid and electrolyte movement in the small intestine. Annu Rev Med. 1973;24:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Owens CW, Padovan W. Limitations of ultracentrifugation and in vivo dialysis as methods of stool analysis. Gut. 1976;17:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 35. | Schilli R, Breuer RI, Klein F, Dunn K, Gnaedinger A, Bernstein J, Paige M, Kaufman M. Comparison of the composition of faecal fluid in Crohn's disease and ulcerative colitis. Gut. 1982;23:326-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Wrong O, Metcalfe-Gibson A, Morrison RB, Ng ST, Howard AV. In vivo dialysis of faeces as a method of stool analysis. I. technique and results in normal subjects. Clin Sci. 1965;28:357-375. [PubMed] |
| 37. | Greer S, Honeywell R, Geletu M, Arulanandam R, Raptis L. Housekeeping genes; expression levels may change with density of cultured cells. J Immunol Methods. 2010;355:76-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 38. | Yang W, Liu L, Masugi Y, Qian ZR, Nishihara R, Keum N, Wu K, Smith-Warner S, Ma Y, Nowak JA, Momen-Heravi F, Zhang L, Bowden M, Morikawa T, Silva AD, Wang M, Chan AT, Fuchs CS, Meyerhardt JA, Ng K, Giovannucci E, Ogino S, Zhang X. Calcium intake and risk of colorectal cancer according to expression status of calcium-sensing receptor (CASR). Gut. 2018;67:1475-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | Barrett KE, Keely SJ. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol. 2000;62:535-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 379] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 40. | Flagella M, Clarke LL, Miller ML, Erway LC, Giannella RA, Andringa A, Gawenis LR, Kramer J, Duffy JJ, Doetschman T, Lorenz JN, Yamoah EN, Cardell EL, Shull GE. Mice lacking the basolateral Na-K-2Cl cotransporter have impaired epithelial chloride secretion and are profoundly deaf. J Biol Chem. 1999;274:26946-26955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 303] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 41. | Gabriel SE, Brigman KN, Koller BH, Boucher RC, Stutts MJ. Cystic fibrosis heterozygote resistance to cholera toxin in the cystic fibrosis mouse model. Science. 1994;266:107-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 308] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 42. | Chang EB, Rao MC. Intestinal water and electrolyte transport. In: Johnson LR. Physiolog of the Gastrointestinal Tract. New York: Raven, 1994: 2027-2081. |
| 43. | Hebert SC, Cheng S, Geibel J. Functions and roles of the extracellular Ca2+-sensing receptor in the gastrointestinal tract. Cell Calcium. 2004;35:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 44. | Hoenderop JG, Nilius B, Bindels RJ. Calcium absorption across epithelia. Physiol Rev. 2005;85:373-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 594] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 45. | Welsh MJ, Smith PL, Fromm M, Frizzell RA. Crypts are the site of intestinal fluid and electrolyte secretion. Science. 1982;218:1219-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 255] [Article Influence: 5.9] [Reference Citation Analysis (0)] |