Published online Aug 7, 2024. doi: 10.3748/wjg.v30.i29.3479
Revised: June 24, 2024
Accepted: July 11, 2024
Published online: August 7, 2024
Processing time: 95 Days and 15.3 Hours
Helicobacter pylori (H. pylori) is a widespread microorganism related to gastric adenocarcinoma (AC). In contrast, it has been reported that an inverse association exists between H. pylori infection and esophageal carcinoma. The mechanisms underlying this supposedly protective effect remain controversial.
To determine the prevalence of H. pylori infection in esophageal carcinoma patients, we performed a retrospective observational study of esophageal tumors diagnosed in our hospital.
We retrospectively reviewed the prevalence of H. pylori infection in a cohort of patients diagnosed with esophageal carcinoma. Concomitant or previous proton pump inhibitor (PPI) usage was also recorded.
A total of 89 patients with esophageal carcinoma (69 males, 77.5%), with a mean age of 66 years (range, 26-93 years) were included. AC was the most frequent pathological variant (n = 47, 52.8%), followed by squamous cell carcinoma (n = 37, 41.6%). Fourteen ACs (29.8%) originated in the gastroesophageal junction and 33 (70.2%) in the esophageal body. Overall, 54 patients (60.7%) presented at stages III and IV. Previous H. pylori infection occurred only in 4 patients (4.5%), 3 with AC (6.3% of all ACs) and 1 with squamous cell carcinoma (2.7% of all squamous cell tumors). All patients with previous H. pylori infection had stage III-IV. Only one patient had received prior H. pylori eradication therapy, whereas 86 (96.6%) had received previous or concomitant PPI treatment.
In our cohort of patients, and after histologic evaluation of paraffin-embedded primary tumors, we found a very low prevalence of previous H. pylori infection. We also reviewed the medical history of the patients, concluding that the majority had received or were on PPI treatment. The minimal prevalence of H. pylori infection found in this cohort of patients with esophageal carcinoma suggests a protective role.
Core Tip:Helicobacter pylori (H. pylori) is involved in gastric carcinogenesis and its eradication has become widely accepted. However, recent studies suggest that it might have a role in maintaining homeostasis in the gastroesophageal junction cells and may have a protective role in esophageal carcinogenesis. The absence of this microorganism might contribute to dysbiosis and alterations in the esophageal microenvironment which might finally be involved in the onset of esophageal tumor. We are very much concerned that the prevalence of esophageal cancer increases after the universalization of H. pylori eradication.
- Citation: López-Gómez M, Morales M, Fuerte R, Muñoz M, Delgado-López PD, Gómez-Cerezo JF, Casado E. Prevalence of Helicobacter pylori infection among patients with esophageal carcinoma. World J Gastroenterol 2024; 30(29): 3479-3487
- URL: https://www.wjgnet.com/1007-9327/full/v30/i29/3479.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i29.3479
Esophageal cancer constitutes a relevant health problem, being the sixth cause of death attributable to cancer worldwide[1]. There are two major histological subtypes: Esophageal squamous cell carcinoma (SCC) and adenocarcinoma (AC). The incidence of AC has increased in the recent decades, currently accounting for almost half of all esophageal neoplasms[2]. Well-established risk factors for AC include Barrett’s esophagus (BE), gastroesophageal reflux (GER), male sex, central obesity, older age, and tobacco smoking[3]. Interestingly, Helicobacter pylori (H. pylori) eradication with antibiotics and acid suppression therapies seem to be protective in gastric cancer[4]. H. pylori is a helical-shaped Gram-negative (GN) bacterium that generally colonizes the stomach early in life[5].
The estimated global prevalence of H. pylori infection has decreased from 58.2% (95%CI: 50.7-65.8) in the 1980-1990 decade to 43.1% (40.3%-45.9%) in the 2011-2022 period[6]. In Spain, studies report a population prevalence around 55%[7].
The prevalence of H. pylori infection in gastric cancer patients seems to vary among regions, with the highest and lowest figures in America and Africa, respectively (18.1%, 95%CI: 16.5-19.6 vs 9.5%, 95%CI: 5.9-13.1)[8].
However, a higher prevalence has been reported in other gastrointestinal malignancies. In a Finnish study, prevalence ranged from 100% for gallbladder cancer to 94% for ampulla of Vater cancer. Similarly, the prevalence of H. pylori infection in hepatocellular carcinoma has been reported to be up to 94%[9]. H. pylori has also been found in 86% patients with advanced colon neoplasia[10]. Proton pump inhibitors (PPIs) are classically prescribed for the treatment of acid-related gastrointestinal disorders and are part of the multidrug treatment for H. pylori eradication[11]. However, long-term administration of PPI can change the microbial composition in the esophagus[12] which might contribute to the development of BE and esophageal cancer. The role of H. pylori in the origin of gastric ACs has been thoroughly studied and its eradication has become one of the greatest challenges worldwide[13].
Interestingly, the presence of H. pylori infection has been associated with a reduced risk of the development of esophagus ACs[14]. The underlying mechanisms responsible for this protective effect remain unclear. Several hypotheses have been suggested: H. pylori induced atrophy and loss of the acid parietal cells in the antrum[15]; secondary alteration of esophageal microbiota[16]; induction of apoptosis of AC cells progressing from BE via the Fas apoptotic pathway[17], and ghrelin synthesis reduction, with a secondary impact on central obesity and GER[18].
Considering that H. pylori eradication has become a widely accepted healthcare policy in Spain, concerns about a plausible increase in esophageal cancer have grown. In this study we reviewed the prevalence of pre-existent H. pylori infection among patients with esophageal carcinoma and recorded which of them were on previous PPI treatment, either as part of the eradication therapy or for other reasons.
We performed a retrospective observational study that included all patients with a previous diagnosis of esophageal or gastroesophageal junction (GEJ) cancer between February 2008 and December 2023 and were managed at our center. Local Institutional Review Board approval was obtained on June 1, 2023. All patients or relatives were informed and accepted participation by signing a written informed consent form. Patients’ data were anonymized according to national regulations (RD 1720/2007, Organic Law 15/1999 on Personal Data Protection).
All patients over 18 years of age with a diagnosis of esophageal or GEJ cancer were included. Patients with gastric or other gastrointestinal neoplasms were excluded from the study. The incidence of H. pylori in gastric cancer patients diagnosed throughout the same years (2008 and 2022) were also included in the analysis.
In situ tumors or premalignant lesions were also excluded. All patients included agreed to participate in the study.
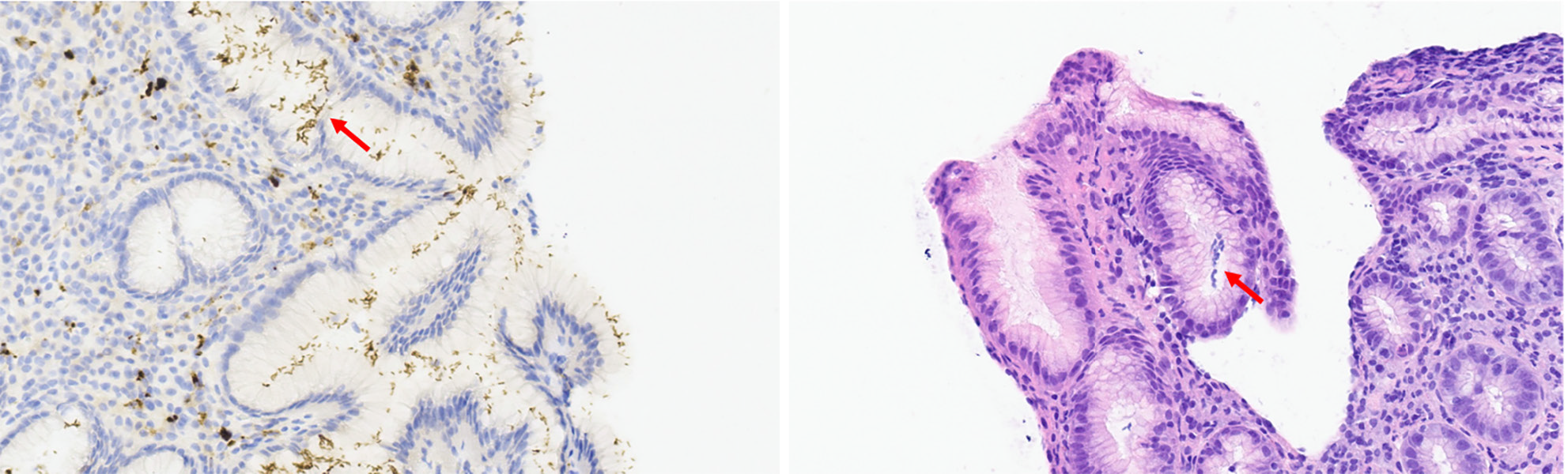
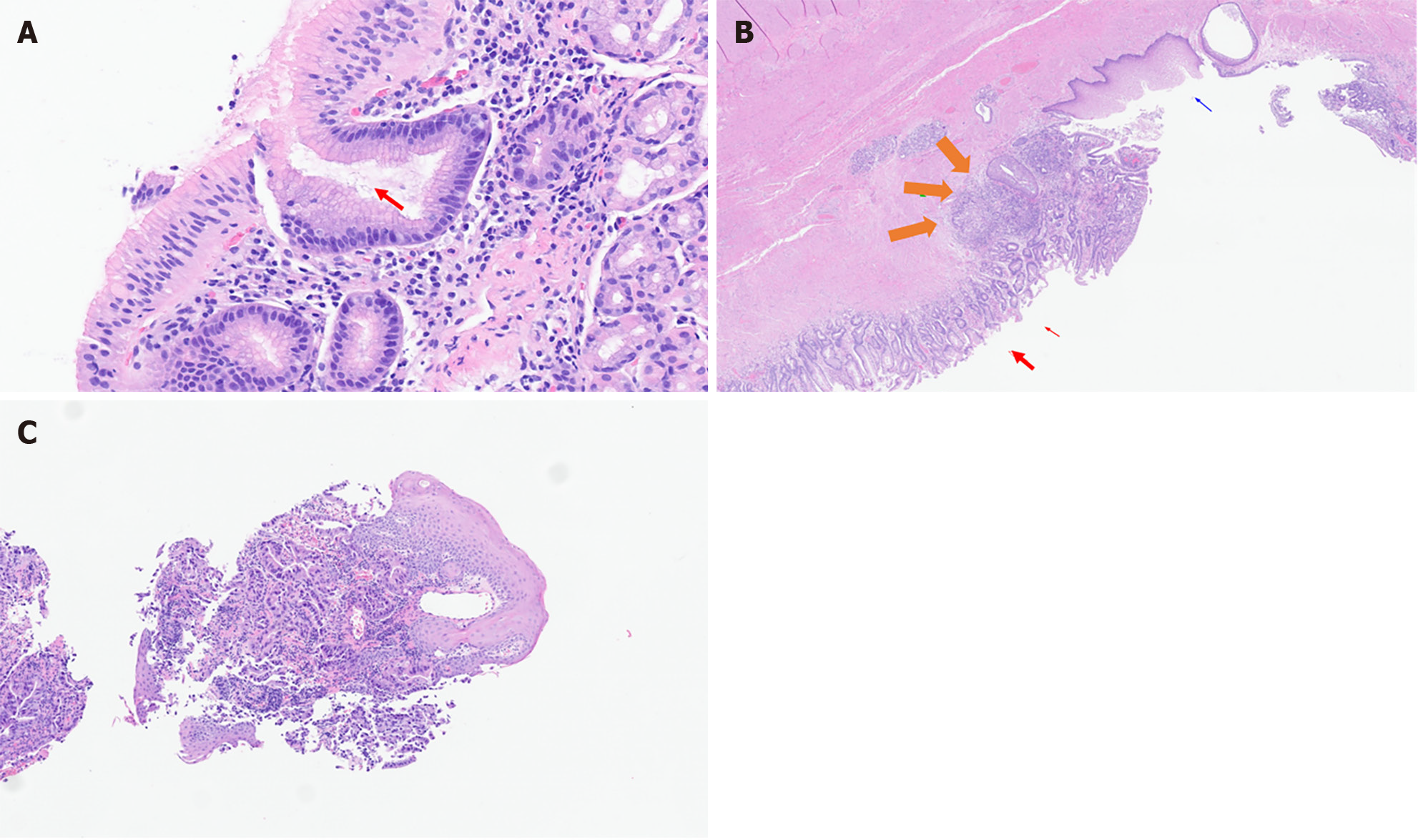
All tumors (esophageal or GEJ invasive tumors) were histologically confirmed by trained pathologists of the center. Paraffin-embedded primary tumor specimens and metastatic tumor specimens containing at least 70% of tumoral cells were selected for each patient. Specimens were reviewed and classified into three subtypes: AC, SCC, and others. The presence or absence of H. pylori was also confirmed by histologic examination. Definitive diagnosis was made by microscopic visualization of H. pylori on hematoxylin and eosin (H&E)-stained slides. Positive cases of H. pylori included patients with obvious H. pylori gastritis with characteristic inflammation and heavy bacterial load, and those with subtle H. pylori gastritis with less inflammation and fewer bacteria. Two examples of H. pylori identification are shown in Figure 1 (gastric cancer) and Figure 2A (GEJ cancer). Figure 2B and C show H&E staining of GEJ and esophageal tumors.
In addition to the presence or absence of H. pylori in biopsy specimens, the following variables were recorded: Age, sex, tumor stage at diagnosis, and previous treatment with anti-acid drugs (PPIs or others, as part of H. pylori eradication therapy or as independent treatment). The endpoint of the study was the identification of H. pylori infection in patients diagnosed with esophageal cancer. Results were expressed as mean ± SD for numerical variables, and as ratios and proportions for categorical variables, both with 95%CI.
A total of 89 patients (77.5% males, mean age 66 years) were included in our study. Demographic and clinical data are shown in Table 1.
| Number of patients | n = 89 |
| Age (median, yr) | 66 (26-93) |
| Sex | |
| Male | 69 (77.52) |
| Female | 20 (22.47) |
| Histology | |
| Adenocarcinoma | 47 (52.80) |
| Squamous cell carcinoma | 37 (41.57) |
| Others | 4 (5.5) |
| Tumor location | |
| Gastroesophageal junction | 33 (70.21) |
| Esophageal | 14 (29.78) |
| Stage | |
| Stage I-II | 25 (28.08) |
| Stage III-IV | 54 (60.67) |
| Presence of Helicobacter pylori | 4 (4.5) |
| Adenocarcinoma | 3 (6.3) |
| Squamous cell carcinoma | 1 (2.7) |
| Previous PPI treatments | 86 (96.82) |
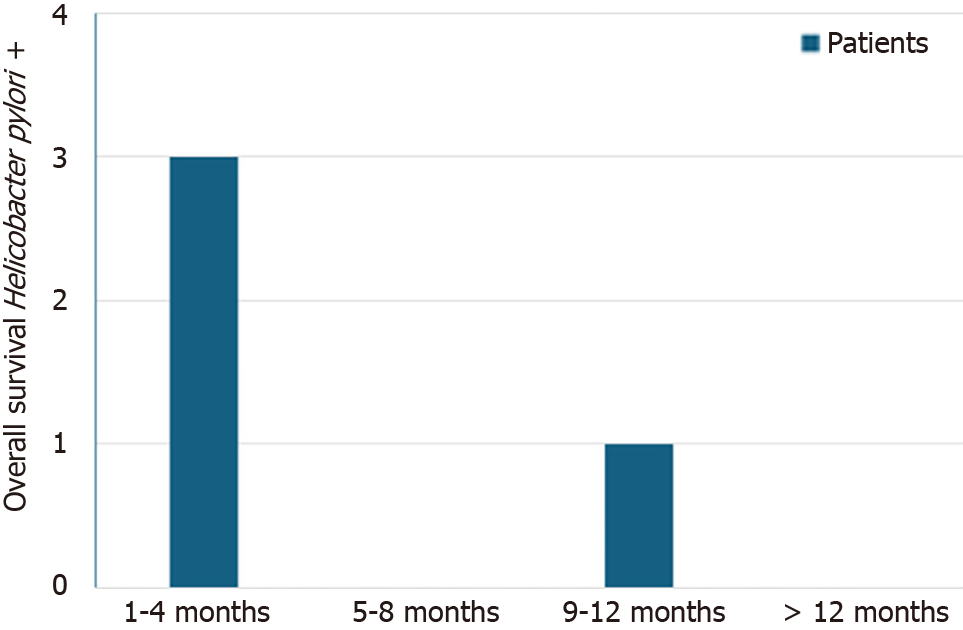
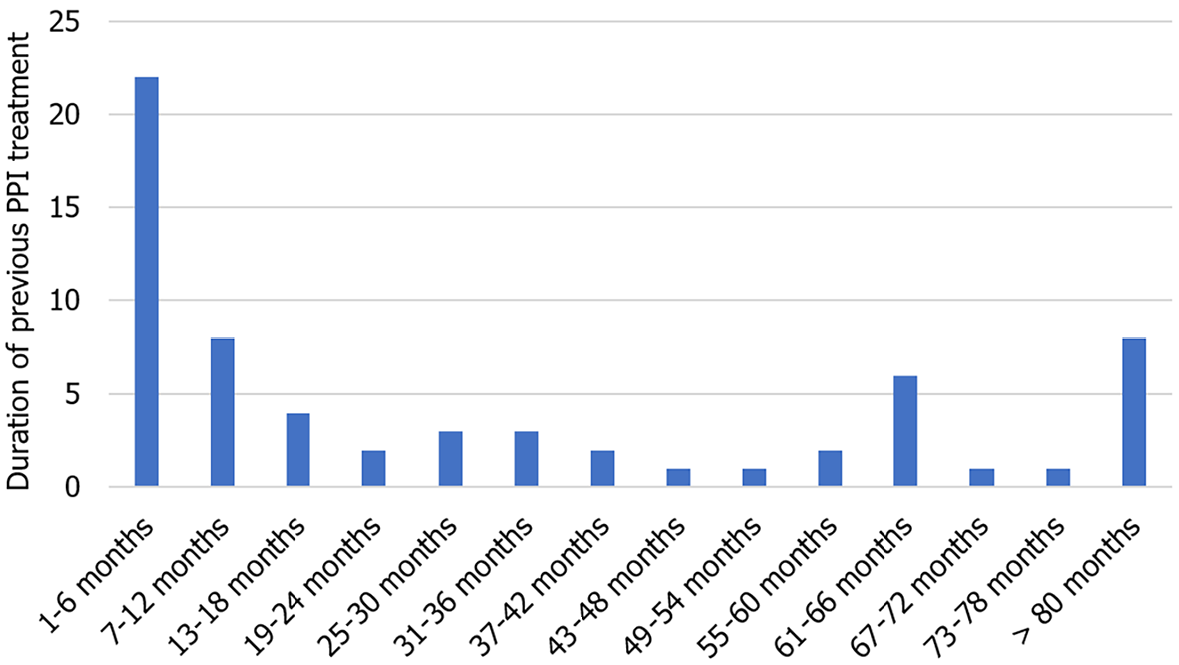
In this cohort, AC was the most frequent histological subtype (52.8%) followed by SCC (41.6%). Neuroendocrine tumors were infrequent (5.6%). As expected, most tumors were stage III-IV at the time of diagnosis (60.7%). H. pylori infection was confirmed in only 4 patients (4.5%), 3 with AC and 1 with SCC. Survival among H. pylori positive patients did not exceed 9 months after diagnosis (Figure 3). Although only one patient had undergone previous H. pylori eradication therapy, 96.6% of patients had received prior PPI treatment and 35.9% (n = 32) had received both PPIs and other antiacid treatment (such as anti-H2 or sucralfate). The median time from initiation of PPIs to the diagnosis of esophageal cancer was 15 months, ranging from 3 to 60 months (Figure 4). Total gastric cancer diagnoses were 431, with a rate in men/women of 269/162 (62.41% vs 37.58%). The mean age was 66 years. H. pylori prevalence among them was 66%.
H. pylori infection and esophageal cancer are conditions with a high geographical variability and prevalence. The purpose of this study was to analyze the prevalence of H. pylori in esophageal tumors in a sample of patients from a tertiary hospital in Madrid, Spain. In this cohort, less than 5% of patients with esophageal cancer tested positive for H. pylori, which is approximately 10 times less than the general population. Interestingly, most of them had received previous antiacid treatment, either with PPIs or with anti-H2 drugs.
The burden due to the diagnosis of esophageal cancer is expected to rise dramatically across high-income countries, with increasing incidence rates predicted for the next decades, according to some statistical models[19].
Previous epidemiologic studies provide inconclusive data on a positive, inverse or neutral association between H. pylori infection and esophageal carcinoma. Although meta-analyses of observational studies favor an inverse association, these may be biased by confounders present in older studies (Table 2). Our findings are in line with this supposedly protective role of H. pylori infection in the genesis of esophageal carcinoma.
| Paper characteristics | Sample characteristics | ||||||
| Ref. | Country | Year | Design | Age (mean, yr) | Helicobacter pylori prevalence | Tumor | Location |
| Holleczek et al[23] | Germany | 2020 | Cohort | 62.2 | 47.80% | EA | Gastric cardia; esophagus esophagogastric junction |
| Wu et al[27] | Taiwan | 2009 | Case-control | 58.3 | 35.30% | ESCC | Upper, middle or lower third of the esophagus |
| Khoshbaten et al[28] | Iran | 2011 | Case-control | 63.9 cases; 61.3 controls | 41.2% ± 36.95% cases; 56.2% ± 29.5% controls | ESCC | Esophagus |
| Hu et al[29] | Taiwan | 2009 | Case-control | 50-70 | 37% cases; 53% controls | ESCC | Upper, middle or lower third esophagus |
| Cook et al[30] | Finland | 2010 | Case-control | 57.7 cases; 58.1 controls | 80.28% cases; 78.16% controls | ESCC | Upper, middle, and lower third of the esophagus |
| Murphy et al[18] | Finland | 2012 | Case-control | 57.9 cases; 57.9 controls | 78.04% cases; 76.82% controls | ESCC | Esophagus |
To date, four meta-analyses have shown an inverse association between H. pylori infection and esophageal cancer. Islami and Kamangar[20] reviewed 19 studies (Table 2) and found an inverse association between cytotoxin-associated gene A (CagA)-positive strains of H. pylori and the risk of esophageal carcinoma [odds ratio (OR) 0.41, 95%CI: 0.28-0.62]. A similar conclusion was stated by Zhuo et al[21], in a study that included 195 articles, and found a risk of developing esophageal AC among H. pylori infected patients of 0.58 (95%CI: 0.48-0.70) as compared with controls. Xie et al[22], also confirmed this inverse association in the general population (0.59, 95%CI: 0.51-0.68, and an OR of 0.56, 95%CI: 0.45-0.70 in Cag A+ strains). However, results from these meta-analyses were based on retrospective observational studies. Only one population-based prospective study[23] conducted in Germany, which included 9949 patients followed for a mean period of 13.8 years, found a 0.65-fold increase risk of developing esophageal carcinoma among H. pylori infected individuals.
These findings support the need for further research on the inner mechanisms behind this association. Several plausible pathways have been suggested. First, H. pylori infection-related gastritis induces atrophy and loss of parietal cells in the stomach, resulting in a reduced reflux which decreases related-esophagitis and BE; second, H. pylori infection might induce apoptosis in Barrett's cells through the Fas-Caspase cascade; third, H. pylori could promote inflammatory responses by activating nuclear factor kappa B, that induces the production of certain cytokines and tumor necrosis factor-alpha, directly damaging the epithelial DNA by dysregulating DNA transcription factors such as the caudal type homeobox 2 (Cdx2); fourth, H. pylori infected patients have a significantly lower number of ghrelin producing cells, which has been shown to be involved in cancer development and metastasis[24].
Additionally, an interesting and promising relation between H. pylori infection and the esophageal microbiome has been suggested. In the normal esophageal mucosa, Streptococcus spp., together with six other major phyla (Firmicutes, Bacteroides, Actinobacteria, Proteobacteria, Fusobacteria and TM7) are the most commonly found microorganisms belonging to the local microbiota. Type I microbiota, which is mainly composed of gram-positive (GP) bacteria, is typically found in the normal esophagus mucosa. In contrast, type II microbiota, enriched in GN bacteria, is associated with an abnormal esophagus. H. pylori infection might play a role in the shift from GP to GN-enriched environment. Previous studies have reported that H. pylori seems to influence gastric microbiome diversity and composition and affects species prevalence and phylogenetic diversity. In fact, esophageal tumors colonized by H. pylori CagA positive strains were inversely associated with the risk of developing esophageal AC. These findings suggest that the absence of H. pylori in the gastroesophageal mucosa might contribute to an unbalanced esophageal microbial composition that may promote carcinogenesis[25].
Similarly, PPI treatment has been suggested to alter the esophageal microbiota, by increasing species like Firmicutes and decreasing Bacterioides and Proteobacteria. A recent study has suggested that the long-term use of PPIs is associated with an increased risk of esophageal cancer[26], likely attributable to the colonization of non-gastric microorganisms capable of producing nitrosamines, which are known to promote both esophageal AC and SCC. In our cohort, almost 95% of patients were under PPI treatment, in line with this hypothesis. PPIs-induced reduction of esophageal gastric acid reflux might avoid the death of acid sensitive bacteria involved in the maintenance of type I microbiota. This hypothesis might be in conflict with recommending PPIs in non-dysplastic BE, aimed to decrease the risk of progression to high grade dysplasia and AC. Considering the widespread use of PPIs, we believe our findings maintain a reasonable doubt on the possible deleterious effect of this medication in the development of esophageal cancer.
This study has some limitations. Most importantly, given the observational and retrospective nature of the study, a causal relation between the lack of H. pylori infection and esophageal cancer cannot be established. Second, as we did not include a non-PPI treatment control group, we cannot conclude on the relation between PPI therapy and esophageal carcinogenesis. Finally, although the results of a single center study may not be extrapolated to other populations, it highlights the importance of further research on the role of H. pylori, and other microorganisms belonging to the local microbiota, in esophageal carcinogenesis.
The very low prevalence of H. pylori infection among esophageal cancer patients found in our study is consistent with previous reports suggesting that the presence of H. pylori might have a protective role in esophageal carcinogenesis. Several mechanisms have been proposed for this inverse association, in which esophageal mucosa dysbiosis seems to play a primary role. Future research should determine to what extent H. pylori infection interacts with the esophageal microbiota, establish whether this interaction is involved in the protective role of H. pylori, and whether PPI treatment contributes to the alteration of esophageal microbiome and eventually promotes esophageal cancer.
We acknowledge and thank the patients that participated in this study.
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20478] [Article Influence: 2047.8] [Reference Citation Analysis (19)] |
| 2. | Bird-Lieberman EL, Fitzgerald RC. Early diagnosis of oesophageal cancer. Br J Cancer. 2009;101:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 3. | Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900-20; quiz 1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2368] [Cited by in RCA: 2445] [Article Influence: 128.7] [Reference Citation Analysis (2)] |
| 4. | Quante M, Abrams JA, Wang TC. The rapid rise in gastroesophageal junction tumors: is inflammation of the gastric cardia the underwater iceberg? Gastroenterology. 2013;145:708-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Piscione M, Mazzone M, Di Marcantonio MC, Muraro R, Mincione G. Eradication of Helicobacter pylori and Gastric Cancer: A Controversial Relationship. Front Microbiol. 2021;12:630852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 6. | Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, Chan FKL, Sung JJY, Kaplan GG, Ng SC. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 2028] [Article Influence: 253.5] [Reference Citation Analysis (0)] |
| 7. | Li Y, Choi H, Leung K, Jiang F, Graham DY, Leung WK. Global prevalence of Helicobacter pylori infection between 1980 and 2022: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2023;8:553-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 183] [Reference Citation Analysis (0)] |
| 8. | Shirani M, Pakzad R, Haddadi MH, Akrami S, Asadi A, Kazemian H, Moradi M, Kaviar VH, Zomorodi AR, Khoshnood S, Shafieian M, Tavasolian R, Heidary M, Saki M. The global prevalence of gastric cancer in Helicobacter pylori-infected individuals: a systematic review and meta-analysis. BMC Infect Dis. 2023;23:543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 9. | Murphy G, Michel A, Taylor PR, Albanes D, Weinstein SJ, Virtamo J, Parisi D, Snyder K, Butt J, McGlynn KA, Koshiol J, Pawlita M, Lai GY, Abnet CC, Dawsey SM, Freedman ND. Association of seropositivity to Helicobacter species and biliary tract cancer in the ATBC study. Hepatology. 2014;60:1963-1971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Shmuely H, Melzer E, Braverman M, Domniz N, Yahav J. Helicobacter pylori infection is associated with advanced colorectal neoplasia. Scand J Gastroenterol. 2014;49:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Amir I, Konikoff FM, Oppenheim M, Gophna U, Half EE. Gastric microbiota is altered in oesophagitis and Barrett's oesophagus and further modified by proton pump inhibitors. Environ Microbiol. 2014;16:2905-2914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 12. | Pei Z, Yang L, Peek RM, Jr Levine SM, Pride DT, Blaser MJ. Bacterial biota in reflux esophagitis and Barrett's esophagus. World J Gastroenterol. 2005;11:7277-7283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 96] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (2)] |
| 13. | Polyzos SA, Zeglinas C, Artemaki F, Doulberis M, Kazakos E, Katsinelos P, Kountouras J. Helicobacter pylori infection and esophageal adenocarcinoma: a review and a personal view. Ann Gastroenterol. 2018;31:8-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Thrift AP. The epidemic of oesophageal carcinoma: Where are we now? Cancer Epidemiol. 2016;41:88-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 192] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 15. | Nie S, Chen T, Yang X, Huai P, Lu M. Association of Helicobacter pylori infection with esophageal adenocarcinoma and squamous cell carcinoma: a meta-analysis. Dis Esophagus. 2014;27:645-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 16. | Castaño-Rodríguez N, Goh KL, Fock KM, Mitchell HM, Kaakoush NO. Dysbiosis of the microbiome in gastric carcinogenesis. Sci Rep. 2017;7:15957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 180] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 17. | Jones AD, Bacon KD, Jobe BA, Sheppard BC, Deveney CW, Rutten MJ. Helicobacter pylori induces apoptosis in Barrett's-derived esophageal adenocarcinoma cells. J Gastrointest Surg. 2003;7:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Murphy G, Kamangar F, Albanes D, Stanczyk FZ, Weinstein SJ, Taylor PR, Virtamo J, Abnet CC, Dawsey SM, Freedman ND. Serum ghrelin is inversely associated with risk of subsequent oesophageal squamous cell carcinoma. Gut. 2012;61:1533-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Arnold M, Laversanne M, Brown LM, Devesa SS, Bray F. Predicting the Future Burden of Esophageal Cancer by Histological Subtype: International Trends in Incidence up to 2030. Am J Gastroenterol. 2017;112:1247-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 301] [Article Influence: 37.6] [Reference Citation Analysis (2)] |
| 20. | Islami F, Kamangar F. Helicobacter pylori and esophageal cancer risk: a meta-analysis. Cancer Prev Res (Phila). 2008;1:329-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 250] [Article Influence: 14.7] [Reference Citation Analysis (1)] |
| 21. | Zhuo X, Zhang Y, Wang Y, Zhuo W, Zhu Y, Zhang X. Helicobacter pylori infection and oesophageal cancer risk: association studies via evidence-based meta-analyses. Clin Oncol (R Coll Radiol). 2008;20:757-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Xie FJ, Zhang YP, Zheng QQ, Jin HC, Wang FL, Chen M, Shao L, Zou DH, Yu XM, Mao WM. Helicobacter pylori infection and esophageal cancer risk: an updated meta-analysis. World J Gastroenterol. 2013;19:6098-6107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 108] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 23. | Holleczek B, Schöttker B, Brenner H. Helicobacter pylori infection, chronic atrophic gastritis and risk of stomach and esophagus cancer: Results from the prospective population-based ESTHER cohort study. Int J Cancer. 2020;146:2773-2783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 24. | Anderson LA, Murphy SJ, Johnston BT, Watson RG, Ferguson HR, Bamford KB, Ghazy A, McCarron P, McGuigan J, Reynolds JV, Comber H, Murray LJ. Relationship between Helicobacter pylori infection and gastric atrophy and the stages of the oesophageal inflammation, metaplasia, adenocarcinoma sequence: results from the FINBAR case-control study. Gut. 2008;57:734-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Yamamura K, Baba Y, Nakagawa S, Mima K, Miyake K, Nakamura K, Sawayama H, Kinoshita K, Ishimoto T, Iwatsuki M, Sakamoto Y, Yamashita Y, Yoshida N, Watanabe M, Baba H. Human Microbiome Fusobacterium Nucleatum in Esophageal Cancer Tissue Is Associated with Prognosis. Clin Cancer Res. 2016;22:5574-5581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 317] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 26. | Holmberg D, Mattsson F, Xie S, Ness-Jensen E, El-Serag H, Lagergren J. Risk of gastric and oesophageal adenocarcinoma following discontinuation of long-term proton-pump inhibitor therapy. J Gastroenterol. 2022;57:942-951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Wu IC, Wu DC, Yu FJ, Wang JY, Kuo CH, Yang SF, Wang CL, Wu MT. Association between Helicobacter pylori seropositivity and digestive tract cancers. World J Gastroenterol. 2009;15:5465-5471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Khoshbaten M, Zadimani A, Bonyadi MR, Mohammadzadeh M, Gachkar L, Pourhoseingholi MA. Helicobacter pylori infection reduces the risk of esophageal squamous cell carcinoma: a case-control study in iran. Asian Pac J Cancer Prev. 2011;12:149-151. [PubMed] |
| 29. | Hu HM, Kuo CH, Lee CH, Wu IC, Lee KW, Lee JM, Goan YG, Chou SH, Kao EL, Wu MT, Wu DC. Polymorphism in COX-2 modifies the inverse association between Helicobacter pylori seropositivity and esophageal squamous cell carcinoma risk in Taiwan: a case control study. BMC Gastroenterol. 2009;9:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Cook MB, Dawsey SM, Diaw L, Blaser MJ, Perez-Perez GI, Abnet CC, Taylor PR, Albanes D, Virtamo J, Kamangar F. Serum pepsinogens and Helicobacter pylori in relation to the risk of esophageal squamous cell carcinoma in the alpha-tocopherol, beta-carotene cancer prevention study. Cancer Epidemiol Biomarkers Prev. 2010;19:1966-1975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |