Published online Jul 28, 2024. doi: 10.3748/wjg.v30.i28.3393
Revised: May 18, 2024
Accepted: June 21, 2024
Published online: July 28, 2024
Processing time: 182 Days and 4 Hours
Biliary stone disease is a highly prevalent condition and a leading cause of hospitalization worldwide. Hepatolithiasis with associated strictures has high residual and recurrence rates after traditional multisession percutaneous transhepatic cholangioscopic lithotripsy (PTCSL).
To study one-step PTCSL using the percutaneous transhepatic one-step biliary fistulation (PTOBF) technique guided by three-dimensional (3D) visualization.
This was a retrospective, single-center study analyzing, 140 patients who, between October 2016 and October 2023, underwent one-step PTCSL for hepatolithiasis. The patients were divided into two groups: The 3D-PTOBF group and the PTOBF group. Stone clearance on choledochoscopy, complications, and long-term clearance and recurrence rates were assessed.
Age, total bilirubin, direct bilirubin, Child-Pugh class, and stone location were similar between the 2 groups, but there was a significant difference in bile duct strictures, with biliary strictures more common in the 3D-PTOBF group (P = 0.001). The median follow-up time was 55.0 (55.0, 512.0) days. The immediate stone clearance ratio (88.6% vs 27.1%, P = 0.000) and stricture resolution ratio (97.1% vs 78.6%, P = 0.001) in the 3D-PTOBF group were significantly greater than those in the PTOBF group. Postoperative complication (8.6% vs 41.4%, P = 0.000) and stone recurrence rates (7.1% vs 38.6%, P = 0.000) were significantly lower in the 3D-PTOBF group.
Three-dimensional visualization helps make one-step PTCSL a safe, effective, and promising treatment for patients with complicated primary hepatolithiasis. The perioperative and long-term outcomes are satisfactory for patients with complicated primary hepatolithiasis. This minimally invasive method has the potential to be used as a substitute for hepatobiliary surgery.
Core Tip: Hepatolithiasis is a clinical benign biliary tract disease with a high incidence and a leading cause of hospitalization, seriously affecting the quality of life of patients. However, current treatment modalities have not achieved good curative effects, with high rates of stone and stenosis retention and recurrence. In the present study we introduce a new technology that one-step percutaneous transhepatic cholangioscopic lithotripsy using the percutaneous transhepatic one-step biliary fistulation (PTOBF) technique guided by three-dimensional (3D) visualization technology. And we performed a randomized trial to assess the efficacy and safety of 3D-PTOBF in the treatment of patients with hepatolithiasis. We found that 3D-PTOBF offered significant improvement of immediate stone clearance ratio and stricture resolution ratio. 3D-PTOBF as a safe, effective, and promising treatment for patients with complicated primary hepatolithiasis.
- Citation: Ye YQ, Cao YW, Li RQ, Li EZ, Yan L, Ding ZW, Fan JM, Wang P, Wu YX. Three-dimensional visualization technology for guiding one-step percutaneous transhepatic cholangioscopic lithotripsy for the treatment of complex hepatolithiasis. World J Gastroenterol 2024; 30(28): 3393-3402
- URL: https://www.wjgnet.com/1007-9327/full/v30/i28/3393.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i28.3393
Hepatolithiasis, a common benign biliary tract disease in East Asia, is the presence of gallstones (calculi) in the biliary ducts of the liver. According to statistics, hepatolithiasis accounts for 20% to 45% of patients who undergo surgery for gallstones[1-4]. Women have a lifetime risk of developing gallstones approaching 25%[5]. Gallstones can cause several clinical symptoms, such as biliary colic and jaundice, and some may be lethal. If left untreated, this causes bile flow stagnation, recurrent cholangitis, liver parenchymal destruction, and, eventually, secondary biliary cirrhosis or cholangiocarcinoma[6].
Stone removal is the primary method for treating clinical symptoms. Current treatments for hepatolithiasis include surgical resection of affected liver segments, laparoscopic surgery, endoscopic retrograde cholangiopancreatography, and percutaneous transhepatic cholangioscopy lithotripsy (PTCSL). Surgical intervention is preferred when possible, but many patients are ineligible due to multiple recurring surgeries, comorbidities, or anatomical factors[7]. In traditional PTCSL surgery, an external biliary fistula must be gradually dilated over 2-3 weeks prior to lithotripsy using serial fascial dilators[8]. This method is associated with longer hospitalization, a high recurrence rate, and a high rate of calculus reoperation[9].
Recent technological advances have enabled modifications, such as percutaneous transhepatic one-step biliary fistulation (PTOBF) combined with the rigid choledochoscopy technique, for optimizing the PTCSL procedure[10,11]. One-step PTCSL using the PTOBF technique enables the clearing of intrahepatic stones and the resolution of strictures. Ultrasound (US) images cannot directly reveal the three-dimensional spatial relationships of calculi, bile ducts and blood vessels. Thus, it is difficult to obtain their exact anatomical locations, which will influence the precision of the operation.Furthermore, the choledochoscope can only acquire the local abdominal information of the patient while the corresponding global abdominal information is a little different from the preoperative two-dimensional (2D) computed tomography (CT) image.
In recent years, 3D visualization technology has been employed to assist in making surgical decisions involving liver resection[12-15]. With the assistance of 3D visualization, the liver anatomy, the morphological structure of the intrahe-patic duct system, the location of liver lesions, and the spatial relationship with adjacent liver vessels can be displayed visually and clearly from any angle[14]; this can prevent the excessive resection of liver tissues, maximally maintain functional liver tissues, and aid in the development of individualized surgical plans[16].
The purpose of this study was to introduce a detailed protocol and assess the long-term outcomes of the application of 3D visualization technology in one-step PTCSL for the treatment of complex hepatolithiasis patients with biliary strictures.
This retrospective study included 140 patients with complex hepatolithiasis from October 2016 to October 2023 at The First Affiliated Hospital of Guangzhou Medical University. A total of 140 patients underwent one-step PTCSL with or without 3D visualization. All patients provided written informed consent for participation in these procedures. Data were gathered for all patients. For further evaluation, the patients were divided into two groups, the 3D-PTCSL group (n = 70) and the PTCSL group (n = 70), who were subsequently compared. This study was approved by the Ethics Committee of The First Affiliated Hospital of Guangzhou Medical University, No. 09, 2017.
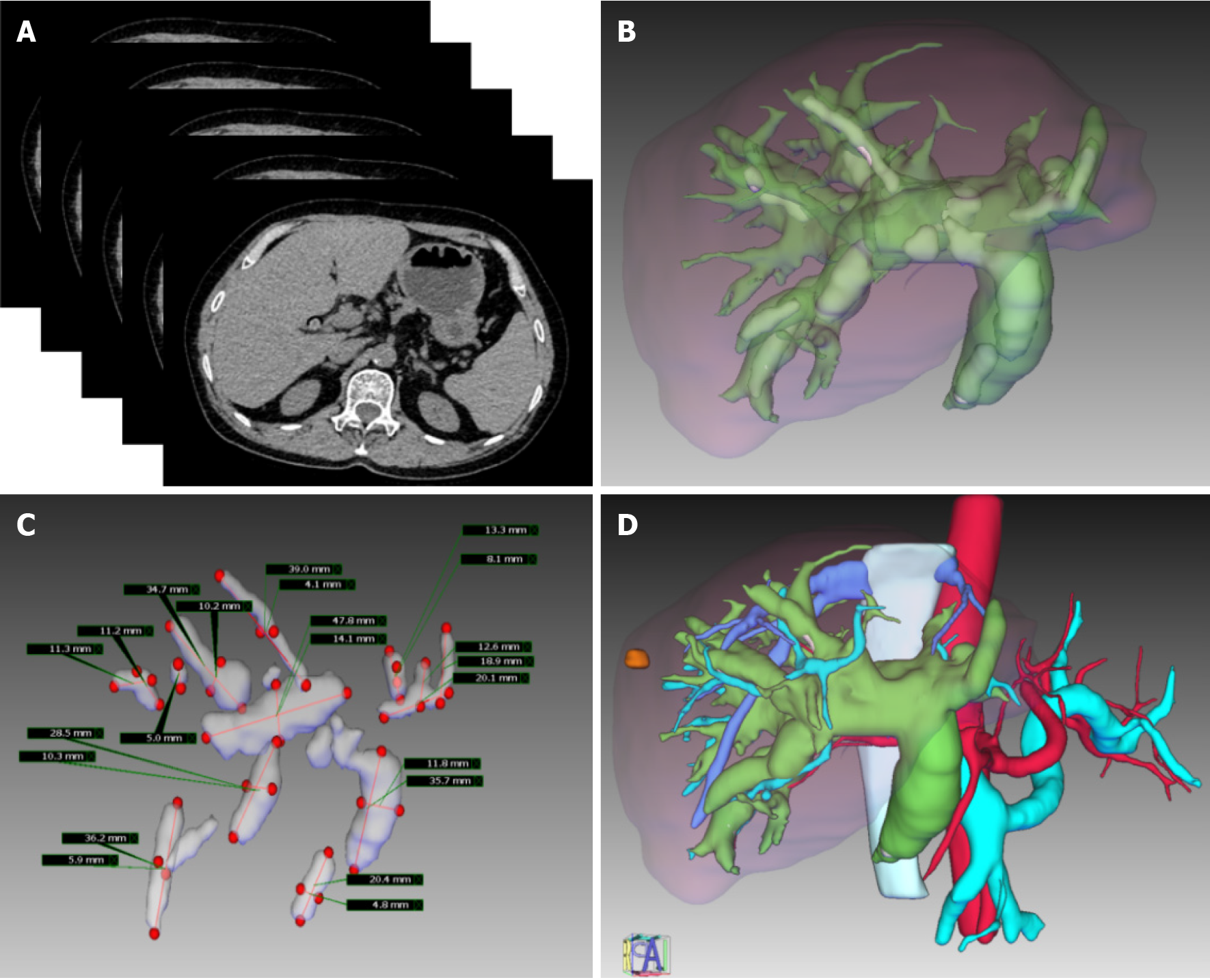
CT data were reconstructed in sections with the IQQA-3D Digital Medicine Central Server. Important anatomical structures on the CT images, such as intrahepatic biliary ducts, calculi, and intrahepatic vessels, were extracted via segmentation, and 3D models were generated by the surface rendering algorithm. The spatial distribution of the intrahepatic duct system and the relationship between lesions and surrounding tissues from different perspectives could be observed in the reconstructed model, which can be exported as a standard template library. In the 3D reconstruction model, the anatomical features of the tissue structure, stone distribution, bile duct stricture, malformation, and vascular arrangement were identified by magnifying, deleting, rotating, and transparentizing the patient’s organs (Figure 1).
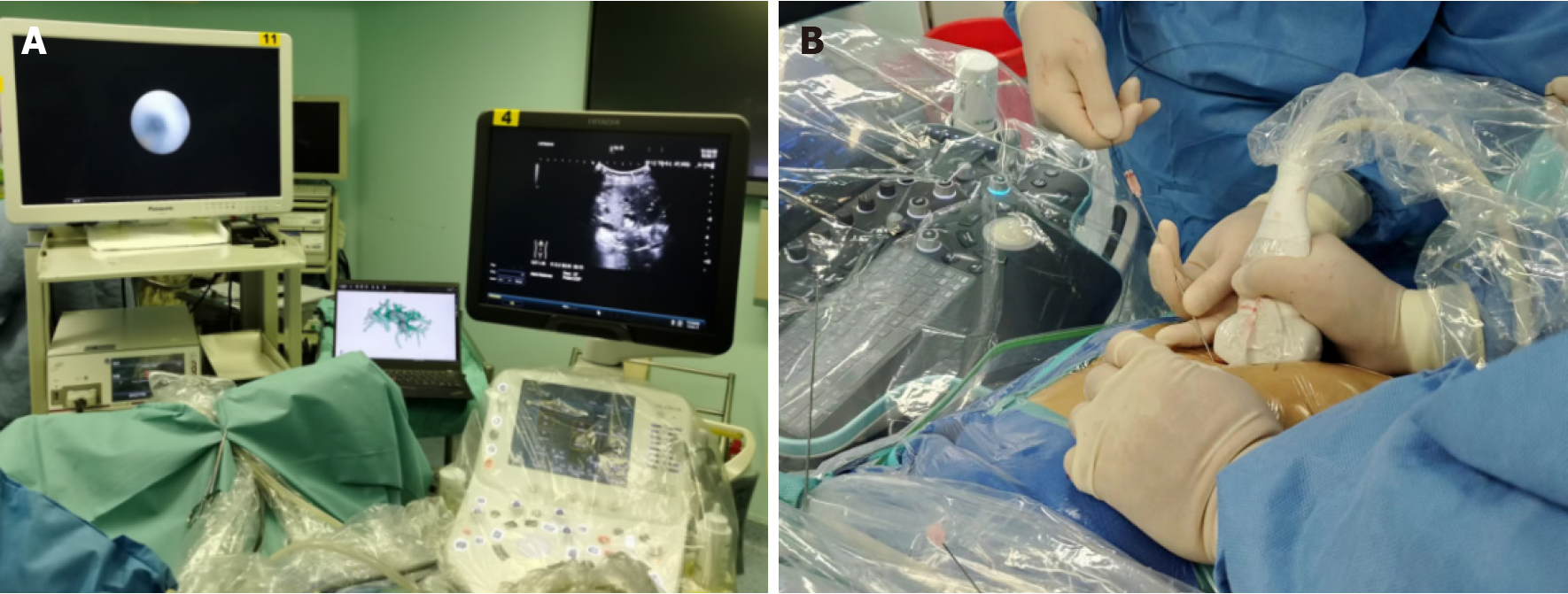
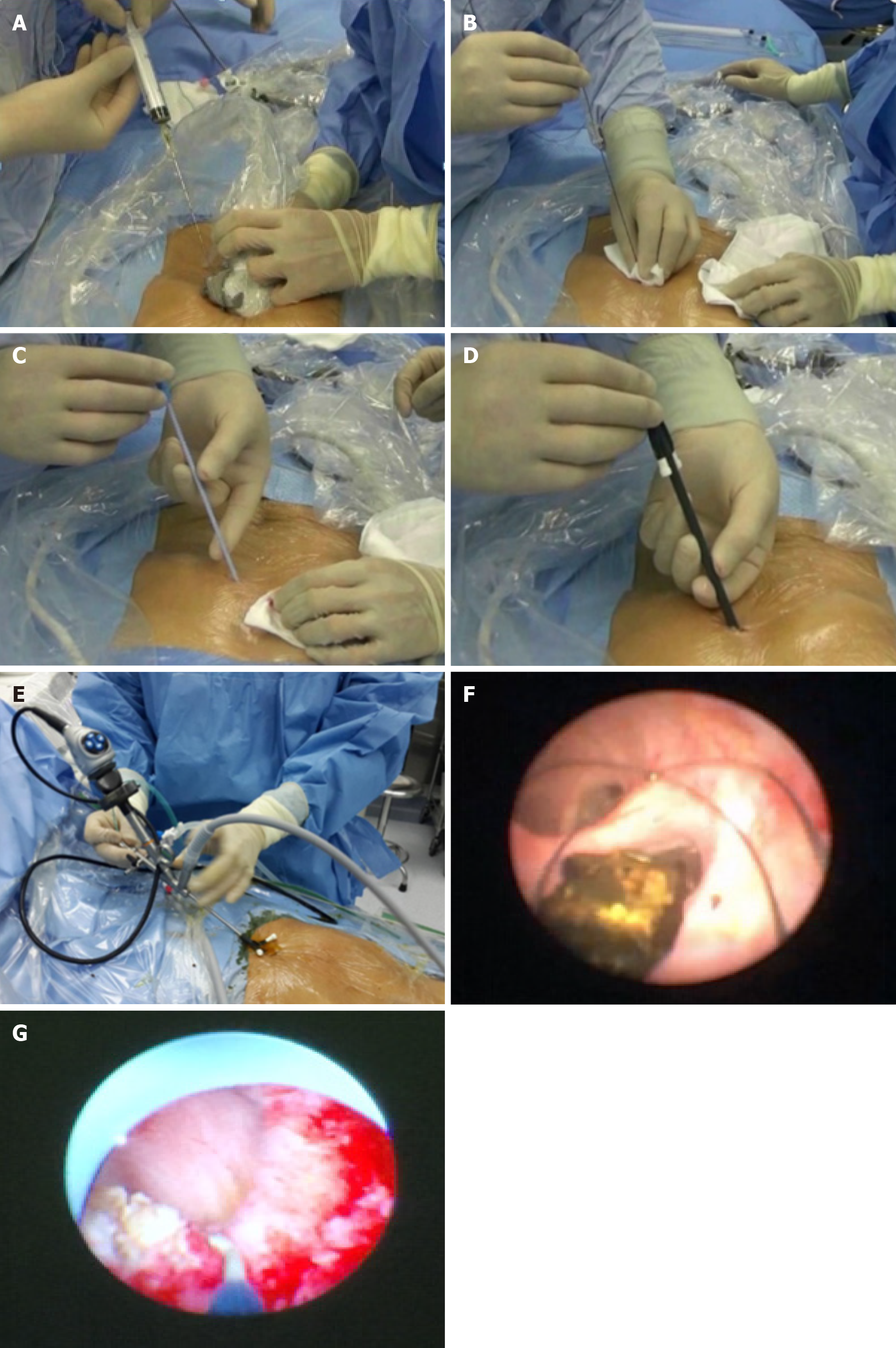
All procedures were performed under general anesthesia by an experienced biliary surgeon. The one-step PTOBF technique was utilized as follows (Figures 2 and 3, Video).
Preoperative planning: Important preoperative evaluation data, including the location of intrahepatic stones and strictures, the degree of stricture, the biliary anatomy, and the relationship between the vasculature and biliary system, were collected. The preoperative assessment tools include US, CT, magnetic resonance imaging (MRI) or magnetic resonance cholangiopancreatography, and 3D visualization.
Three-dimensional visualization and US-guided puncture: According to preoperative planning, target biliary tract puncture was performed using an 18G needle and a 0.035-inch hydrophilic guidewire under real-time intraoperative US and 3D-model guidance. The patients were encouraged to hold their breath for 2 minutes during puncture to minimize interference.
Establishing channel: After successful biliary puncture, the sinuses were expanded with serial fascial dilators from 8 Fr to 14 Fr. A 14 Fr sheath was passed over the guidewire to establish the channel for the rigid choledochoscope.
Choledochoscopic stone removal: Small stones were flushed out with a “wash and suction” procedure. Larger stones were removed by using a basket, a clamp, or electrohydraulic shock wave lithotripsy.
Managing anastomotic strictures: Choledochoscopy and cholangiography were used to confirm the degree and location of the anastomotic stricture. A membranous stricture with a thin fibrous layer was designated as a mildly anastomotic stricture and could be expanded with a 16 Fr or 18 Fr dilator. A scar-like stricture of the bile duct was designated a severe anastomotic stricture. These could be dilated by flushing with mannitol solution, inserting a 40-W electric knife to cut the open stricture, and/or employing a 4, 6 or 8 mm balloon dilatation catheter.
Supporting drainage tube insertion: A 14 Fr drainage tube was placed into each hepatolithiasis patient at the end of the procedure. If the patient had strictures, a 16- or 18-Fr supporting drainage tube had to be placed across them. The drainage tubes were exchanged every 2-3 months and removed 6 to 9 months after percutaneous transhepatic cholangioscopy, when the strictures were resolved on the last endoscopic intervention.
Statistical analysis was performed using R version 3.4.1. Categorical variables are expressed as frequencies and percentages. Continuous variables are expressed as the mean ± SD or median (range). P values < 0.05 were considered to indicate statistical significance.
From October 2016 to October 2022, 140 patients who underwent one-step PTCSL for hepatolithiasis with biliary stricture were eligible and included in the study. There were 54 male and 86 female patients, and the mean age was 19.1 ± 11.4 years. The operation-related data, including age, sex, total bilirubin, direct bilirubin, alanine aminotransferase, Child-Pugh class, stone, and stricture location, were similar between the 2 groups. There was a significant difference in bile duct strictures, with biliary strictures more common in the 3D-PTOBF group (35.7% vs 7.1%, P = 0.001). A comparison of the specific characteristics of the three groups is listed in Table 1.
| Variables | 3D-PTOBF (n = 70) | PTOBF (n = 70) | P value |
| Age (year) | 50.5 ± 17.3 | 49.0 ± 17.2 | 0.608 |
| Sex | |||
| Male | 24 (34.3) | 30 (42.9) | 0.298 |
| Female | 46 (65.7) | 40 (57.1) | |
| TBIL (mmol/L) | 33.9 ± 32.9 | 30.8 ± 33.6 | 0.581 |
| DBIL (mmol/L) | 13.3 ± 18.1 | 13.4 ± 20.3 | 0.990 |
| ALT (U/L) | 59.9 ± 61.7 | 56.5 ± 57.0 | 0.791 |
| γ-GGT (U/L) | 210.6 ± 262.2 | 238.0 ± 236.3 | 0.517 |
| ALB (g/L) | 39.3 ± 6.4 | 37.6 ± 5.8 | 0.105 |
| PT (second) | 14.2 ± 4.5 | 13.5 ± 1.0 | 0.174 |
| Child-Pugh score | |||
| Grade A | 67 (95.7) | 58 (82.9) | 0.014 |
| Grade B | 3 (4.3) | 12 (17.1) | |
| Stone location | |||
| S1 | 1 (1.4) | 2 (2.9) | 0.559 |
| S2 | 9 (12.9) | 12 (17.1) | 0.478 |
| S3 | 11 (15.7) | 13 (18.6) | 0.654 |
| S4 | 17 (24.3) | 23 (32.9) | 0.262 |
| S5 | 4 (5.7) | 5 (7.1) | 0.730 |
| S6 | 12 (17.1) | 12 (17.1) | 1.0 |
| S7 | 8 (11.4) | 16 (22.9) | 0.137 |
| S8 | 12 (17.1) | 17 (24.3) | 0.297 |
| Common bile duct | 23 (32.9) | 13 (18.6) | 0.053 |
| Biliary stricture Location | |||
| S1 | 0 | 0 | 1.0 |
| S2 | 1 (1.4) | 8 (11.4) | 0.008 |
| S3 | 8 (11.4) | 12 (17.1) | 0.334 |
| S4 | 14 (20.0) | 14 (20.0) | 1.0 |
| S5 | 4 (5.7) | 6 (8.5) | 0.512 |
| S6 | 11 (15.7) | 6 (8.5) | 0.196 |
| S7 | 6 (8.6) | 3 (4.2) | 0.301 |
| S8 | 7 (10.0) | 8 (11.4) | 0.785 |
| Bilio-enteric anastomosis | 12 (17.1) | 21 (30.0) | 0.073 |
| Common bile duct | 25 (35.7) | 5 (7.1) | 0.001 |
The intraoperative data of the 2 groups are shown in Table 2. The immediate stone clearance ratio in the 3D-PTOBF group was significantly greater than that in the PTOBF group after analysis (88.6% vs 27.1%, P = 0.000). The postoperative complication rate were significantly lower in the 3D-PTOBF group (8.6% vs 41.4%, P = 0.000). No significant differences were found between the groups in terms of the operation time and intraoperative blood loss.
| Variables | 3D-PTOBF (n = 70) | PTOBF (n = 70) | P value |
| Operation time (minute) | 55.2 ± 34.0 | 59.8 ± 37.8 | 0.448 |
| Intraoperative blood loss (mL) | 26.5 ± 52.1 | 16.4 ± 13.4 | 0.117 |
| Immediate stone clearance ratio | 62 (88.6) | 19 (27.1) | 0.000a |
| Complications | 6 (8.6) | 29 (41.4) | 0.000a |
| Hemobilia | 1 (1.4) | 2 (2.9) | 0.559 |
| Pulmonary infection | 2 (2.9) | 4 (5.7) | 0.384 |
| Cholangitis | 2 (2.9) | 14 (20.0) | 0.001 |
| Pleural effusion | 1 (1.4) | 9 (12.9) | 0.009 |
The patients were followed up for a period that ranged between 55 and 512 days (mean 55 days). The stricture resolution ratio (97.1% vs 78.6%, P = 0.001) in the 3D-PTOBF group was significantly better than that in the PTOBF group after analysis. The stone recurrence rate (7.1% vs 38.6%, P = 0.000) was significantly lower in the 3D-PTOBF group. The final total stone clearance rates were 77.1% (3D-PTOBF) and 78.6% (PTOBF). The reoperation rates were 1.4% (3D-PTOBF) and 12.9% (PTOBF) (Table 3).
| Variables | 3D-PTOBF (n = 70) | PTOBF (n = 70) | P value |
| Follow-up time | 498.0 ± 373.5 | 1031.3 ± 573.0 | 0.000a |
| Final stone clearance ratio | 54 (77.1) | 55 (78.6) | 0.839 |
| Stricture resolution ratio | 68 (97.1) | 55 (78.6) | 0.001 |
| Number of operations | 3.0 ± 1.5 | 2.9 ± 1.8 | 0.535 |
| Stone recurrence | 5 (7.1) | 27 (38.6) | 0.000a |
| Reoperation rate | 1 (1.4) | 9 (12.9) | 0.009 |
| Late complications | 9 (12.9) | 9 (12.9) | 1.0 |
| Cholangitis | 8 (11.4) | 7 (10.0) | 0.785 |
| Liver failure | 1 (1.4) | 2 (2.9) | 0.559 |
This study demonstrated that 3D-guided one-step PTCSL using PTOBF is highly effective and safe for the treatment of hepatolithiasis patients with associated biliary strictures. The procedure achieved an immediate stone clearance ratio of 88.6%, a final total stone clearance rate of 77.1%, a stricture resolution rate of 97.1%, a postoperative complication rate of 8.6%, and a stone recurrence rate of 7.1% over a median follow-up period of 55.0 (55.0, 512.0) days.
In one-step PTCSL, 3D visualization of the hepatobiliary tract is carried out beforehand to design an appropriate preoperative plan via preoperative CT imaging, in which the calculi and bile duct stricture can be visually located[17]. According to the preoperative plan, percutaneous transhepatic cholangiography is performed intraoperatively under real-time US navigation, and the sinus tract is expanded to an appropriate diameter (16-18 Fr). Then, a protective sheath is used to ensure that biliary endoscopic surgery can be continued immediately without waiting for full recovery of the sinus tissue. This approach significantly shortens the operation and treatment times. Due to the convenience of the operation, multiple punctures can be made in a single operation to create multiple stone-extracting channels, which significantly enhances the success rate of stone extraction[18,19]. This approach is especially suitable when primary intrahepatic bile duct stones are distributed in a diffuse manner. In this case, one stone-extracting channel is not sufficient to clear all the stones.
Previous 2D plane structure generated based on B-US, CT or MRI medical images is very different from the 3D bile duct tree structure. Limited spatial information of 2D structure makes it difficult for determining the spatial locations of the stones, therefore leading to high surgical risk and low efficiency of stone removal. The results of this study are comparable to those of prior studies showing immediate clearance rates of 41%-46% with traditional multisession PTCSL[20]. This reflects the technical complexity of clearing all stones in a single setting from the intrahepatic biliary tree. The limited spatial information available in 2D makes it difficult to determine the spatial locations of the stones, leading to high surgical risk and low stone removal efficiency. However, with 3D visualization technology and adjunctive techniques such as biliary stenting and saline irrigation, the immediate stone clearance ratio in the 3D-PTOBF group was 88.6% (62/70). Three-dimensional visualization can promote one-step PTCSL with a preoperative simulated operation plan, intraoperative digitization of the patient’s anatomy, and a predetermined diagnosis.
The long-term recurrence rate of 7.1% (5/70) in our study is also lower than the 15%-40% reported in the literature[21-23]. This may be attributed to the complete initial stone clearance and prolonged biliary stenting performed to prevent stricture recurrence in our patients. Our surgeons employ a rigid choledochoscope to treat stenosed bile ducts and leave an 18 Fr drainage tube in the distal end of the stenosed bile ducts for 6-9 months[11]. Bacterial biofilms and bile sludge cause blockage and restenosis of the bile ducts, so the drainage tubes need to be replaced every 3 months.
The postoperative complication rates were significantly lower in the 3D-PTOBF group than in the PTOBF group (8.6% vs 41.4%, P = 0.000). The perioperative complication rate of traditional multisession PTCSL is between 15% and 23%. Complex and dangerous situations that may occur in the actual operation can be simulated and estimated by 3D visualization technology prior to the procedure. It is highly important to compare the advantages and disadvantages of different surgical plans through simulation, as this can aid in developing reasonable individual surgical plans and preoperative demonstrations for the patients.
Our study adds to the limited data on the long-term efficacy of one-step PTCSL. Fang et al[24] described 3D visualization technology for treating hepatolithiasis patients in 2013. Yang et al[20] first described the PTOBF technique in 2013[20]. However, these studies focused only on immediate outcomes. Our long-term follow-up provides evidence that 3D-guided one-step PTCSL is beneficial and effective for treating complex hepatolithiasis.
Clinically, choledochoscopy can only be performed until maturation of the sinus tract at least 1 week following percutaneous biliary drainage[25]. The one-step approach avoids incremental fistula dilation and reduces overall hospitalization times. We introduced 3D visualization into the one-step PTCSL technique to further show that the reconstructed 3D model can be used to localize lesions with submillimeter accuracy and can therefore contribute to planning an accurate puncture route before surgery and guide the puncture and stone extraction operations during surgery.
There were still some limitations in this study. This was a single-center study with a limited number of patients; hence, there was some inevitable selection bias between the groups that may have impacted the results.
This study confirmed that one-step PTCSL guided by 3D visualization can be used to safely puncture the biliary tract and effectively remove stones, improving the treatment of patients with complicated primary hepatolithiasis. The perioperative and long-term outcomes for these complicated primary hepatolithiasis patients were satisfactory.
| 1. | Mori T, Sugiyama M, Atomi Y. Gallstone disease: Management of intrahepatic stones. Best Pract Res Clin Gastroenterol. 2006;20:1117-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 2. | Otani K, Shimizu S, Chijiiwa K, Ogawa T, Morisaki T, Sugitani A, Yamaguchi K, Tanaka M. Comparison of treatments for hepatolithiasis: hepatic resection versus cholangioscopic lithotomy. J Am Coll Surg. 1999;189:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Li F, Zhou Y, Cheng N, Mao H, Jiang L, Li N, Li Q, de Jong MC, Pawlik TM. Epidermal growth factor receptor as a target for anti-proliferative treatment of proliferative cholangitis in hepatolithiasis. J Surg Res. 2011;166:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Lee JY, Kim JS, Moon JM, Lim SA, Chung W, Lim EH, Lee BJ, Park JJ, Bak YT. Incidence of Cholangiocarcinoma with or without Previous Resection of Liver for Hepatolithiasis. Gut Liver. 2013;7:475-479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Portincasa P, Moschetta A, Palasciano G. Cholesterol gallstone disease. Lancet. 2006;368:230-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 490] [Article Influence: 25.8] [Reference Citation Analysis (2)] |
| 6. | Lin CC, Lin PY, Chen YL. Comparison of concomitant and subsequent cholangiocarcinomas associated with hepatolithiasis: Clinical implications. World J Gastroenterol. 2013;19:375-380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Yoon YI, Kim KH, Cho HD, Kwon JH, Jung DH, Park GC, Song GW, Ha TY, Lee SG. Long-term perioperative outcomes of pure laparoscopic liver resection versus open liver resection for hepatocellular carcinoma: a retrospective study. Surg Endosc. 2020;34:796-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Ma S, Hu S, Gao F, Liang R. Endoscopy Lithotomy for Intrahepatic Gallstones: A Meta-Analysis. Surg Laparosc Endosc Percutan Tech. 2015;25:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Buddingh KT, Weersma RK, Savenije RA, van Dam GM, Nieuwenhuijs VB. Lower rate of major bile duct injury and increased intraoperative management of common bile duct stones after implementation of routine intraoperative cholangiography. J Am Coll Surg. 2011;213:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Tao H, Wang P, Sun B, Zhou X, Xie J. One-step Percutaneous Transhepatic Cholangioscopy Combined With High-frequency Needle-knife Electrotomy in Biliary Strictures After Liver Transplantation. Surg Laparosc Endosc Percutan Tech. 2021;31:787-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Huang MH, Chen CH, Yang JC, Yang CC, Yeh YH, Chou DA, Mo LR, Yueh SK, Nien CK. Long-term outcome of percutaneous transhepatic cholangioscopic lithotomy for hepatolithiasis. Am J Gastroenterol. 2003;98:2655-2662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 116] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Liu F, Cheng Z, Han Z, Yu X, Yu M, Liang P. A three-dimensional visualization preoperative treatment planning system for microwave ablation in liver cancer: a simulated experimental study. Abdom Radiol (NY). 2017;42:1788-1793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Huang Z, Zeng S, Zeng X, Wen S, Zhou Y, Cai P, Zhong H, Liu Z, Xiang N, Zhou C, Fang C, Zeng N. Efficacy of hepatectomy for hepatolithiasis using 3D visualization combined with ICG fluorescence imaging: A retrospective cohort study. World J Surg. 2024;48:1242-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Panwar A, Das P, Tan LP. 3D Hepatic Organoid-Based Advancements in LIVER Tissue Engineering. Bioengineering (Basel). 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Gong Y, Tang Y, Geng Y, Zhou Y, Yu M, Huang B, Sun Z, Tang H, Jian Z, Hou B. Comparative safety and effectiveness of ultrasound-guided radiofrequency ablation combined with preoperative three-dimensional reconstruction versus surgical resection for solitary hepatocellular carcinoma of 3-5 cm. J Cancer. 2019;10:5568-5574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Yamanaka J, Saito S, Fujimoto J. Impact of preoperative planning using virtual segmental volumetry on liver resection for hepatocellular carcinoma. World J Surg. 2007;31:1249-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Ni ZK, Lin D, Wang ZQ, Jin HM, Li XW, Li Y, Huang H. Precision Liver Resection: Three-Dimensional Reconstruction Combined with Fluorescence Laparoscopic Imaging. Surg Innov. 2021;28:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Ozden I, Kamiya J, Nagino M, Uesaka K, Sano T, Nimura Y. Clinicoanatomical study on the infraportal bile ducts of segment 3. World J Surg. 2002;26:1441-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Kamiya J, Nagino M, Uesaka K, Sano T, Nimura Y. Clinicoanatomical studies on the dorsal subsegmental bile duct of the right anterior superior segment of the human liver. Langenbecks Arch Surg. 2003;388:107-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Yang YL, Zhang C, Zhao G, Wu P, Ma YF, Zhang HW, Shi LJ, Li JY, Lin MJ, Yang SM, Lv Y. Choledochoscopic high-frequency needle-knife electrotomy as an effective treatment for intrahepatic biliary strictures. J Gastroenterol Hepatol. 2015;30:1438-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Chen C, Huang M, Yang J, Yang C, Yeh Y, Wu H, Chou D, Yueh S, Nien C. Reappraisal of percutaneous transhepatic cholangioscopic lithotomy for primary hepatolithiasis. Surg Endosc. 2005;19:505-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Uenishi T, Hamba H, Takemura S, Oba K, Ogawa M, Yamamoto T, Tanaka S, Kubo S. Outcomes of hepatic resection for hepatolithiasis. Am J Surg. 2009;198:199-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Cha SW. [Management of Intrahepatic Duct Stone]. Korean J Gastroenterol. 2018;71:247-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Fang CH, Liu J, Fan YF, Yang J, Xiang N, Zeng N. Outcomes of hepatectomy for hepatolithiasis based on 3-dimensional reconstruction technique. J Am Coll Surg. 2013;217:280-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Cheung MT, Wai SH, Kwok PC. Percutaneous transhepatic choledochoscopic removal of intrahepatic stones. Br J Surg. 2003;90:1409-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |