Published online Jul 28, 2024. doi: 10.3748/wjg.v30.i28.3373
Revised: June 17, 2024
Accepted: July 8, 2024
Published online: July 28, 2024
Processing time: 119 Days and 8.5 Hours
The perianal disease affects up to one-third of individuals with Crohn's disease (CD), causing disabling symptoms and significant impairment in quality of life, particularly for those with perianal fistulising CD (PFCD). The collaborative effort between gastroenterologists and surgeons is essential for addressing PFCD to achieve fistula closure and promote luminal healing. Limited fistula healing rates with conventional therapies have prompted the emergence of new biological agents, endoscopic procedures and surgical techniques that show promising results. Among these, mesenchymal stem cells injection is a particularly hopeful therapy. In addition to the burden of fistulas, individuals with perianal CD may face an increased risk of developing anal cancer. This underscores the importance of surveillance programmes and timely interventions to prevent late diagnoses and poor outcomes. Currently, there is no established formal anal screening programme. In this review, we provide an overview of the current state of the art in managing PFCD, including novel medical, endoscopic and surgical approaches. The discussion also focuses on the relevance of establishing an anal cancer screening programme in CD, intending to propose a risk-based surveillance algorithm. The validation of this surveillance programme would be a significant step forward in improving patient care and outcomes.
Core Tip: Perianal fistulising Crohn's disease remains one of the most complex phenotypes of inflammatory bowel disease. Effective management involves a multidisciplinary approach. This review seeks to assess the existing evidence and emerging literature to provide clinicians with objective guidance for clinical practice concerning the optimal medical, endoscopic and surgical treatment of perianal fistulas. Future directions in management are also being reviewed. Additionally, the discussion underscores the significance of implementing an anal cancer screening programme, given the heightened risk faced by these patients. An algorithm for anal cancer screening is proposed with the ultimate goal of enhancing patient outcomes.
- Citation: Pacheco T, Monteiro S, Barros L, Silva J. Perianal disease in inflammatory bowel disease: Broadening treatment and surveillance strategies for anal cancer. World J Gastroenterol 2024; 30(28): 3373-3385
- URL: https://www.wjgnet.com/1007-9327/full/v30/i28/3373.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i28.3373
Inflammatory bowel disease (IBD) is a chronic and progressive inflammatory condition affecting the gastrointestinal tract, categorised into two major subtypes: Crohn's disease (CD) and ulcerative colitis (UC). These conditions are highly disabling and can present with a broad spectrum of clinical manifestations, encompassing both intestinal and extra-intestinal symptoms[1-3]. The perianal CD is one of the many phenotypes of IBD, presenting with skin tags, fissures and anorectal stenosis, as well as fistulas and abscesses, affecting up to one-third of patients[4-6]. Individuals with perianal fistulising CD (PFCD) experience a more challenging condition characterised by a substantial decrease in quality of life due to incapacitating symptoms and limited treatment effectiveness. With the development of novel therapeutics, fistula healing rates seem to be promising[7-9].
Rarely, PFCD can complicate malignancy, as patients might be at increased risk for anal canal-located and fistula-related cancer development[3,10-12]. This risk increases with disease duration[3,11,12]. The patient prognosis is generally poor due to a late diagnosis, and the lack of specificity of symptoms may explain why cancer diagnosis is often delayed[11,13]. For this reason, surveillance programmes aimed at early detection of neoplastic lesions are urgent.
In this review, we discuss the management of PFCD with a focus on emerging therapies. We also propose an anal cancer screening programme for individuals with IBD.
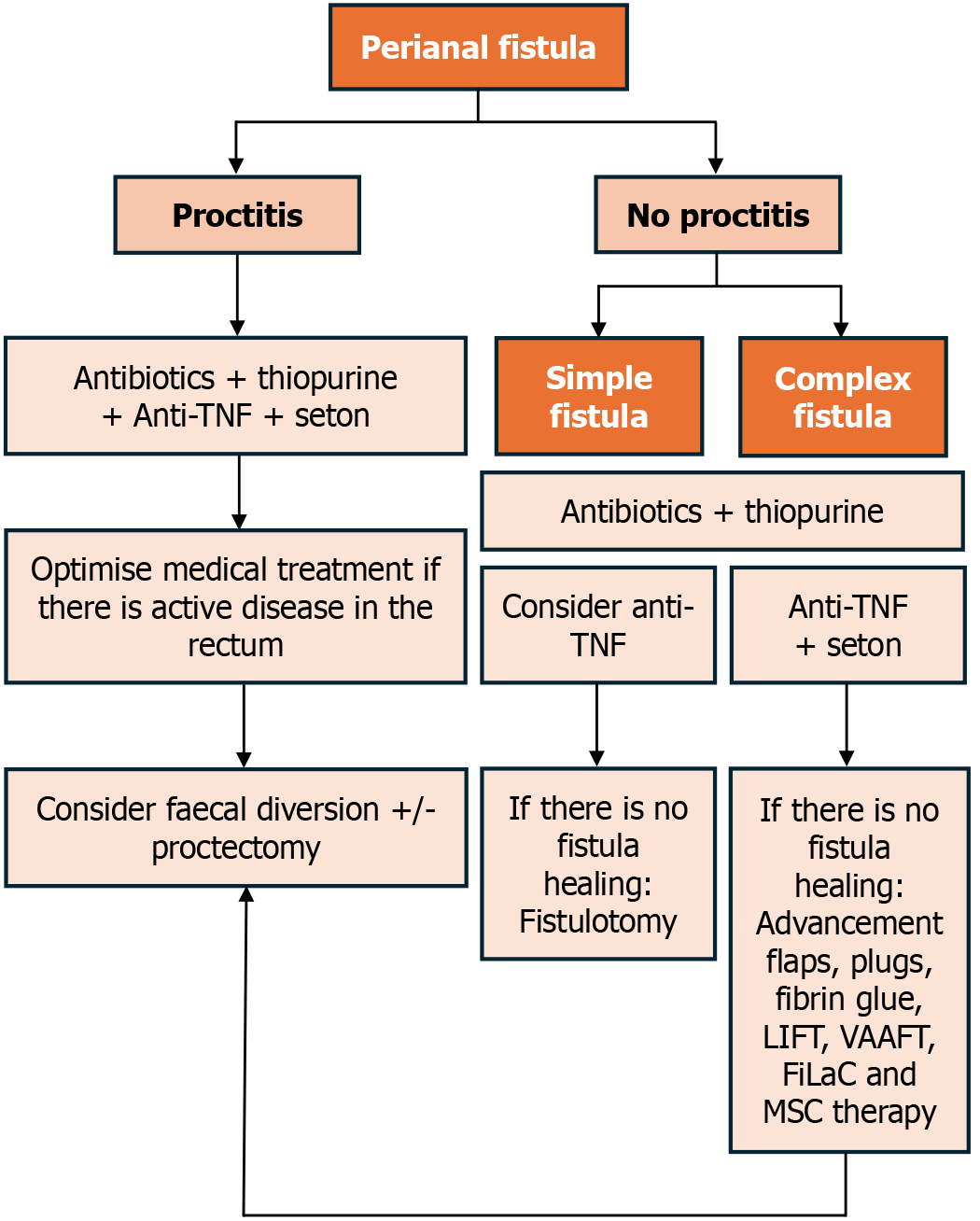
An adequate evaluation, characterisation and classification of perianal lesions are essential for determining the appropriate therapeutic approach.
One commonly used classification system for perianal fistulas is the Parks classification, which categorises fistulas into five anatomical categories based on their relation to the sphincter complex (superficial, intersphincteric, transsphincteric, suprasphincteric and extrasphincteric)[14]. Another major classification system is the American Gastroenterological Association classification, which divides perianal fistulas into two subtypes: Simple and complex. A simple fistula is characterised by a low location, a single external opening, and no association with a perianal abscess, rectovaginal fistula or anorectal stricture. A complex fistula has a high origin, may have multiple external openings, and is often associated with a perianal abscess, a rectovaginal fistula or anorectal stricture[15]. Complex fistulas are more commonly found in CD patients[8].
Diagnostic modalities for PFCD include examination under anaesthesia (EUA), fistulography, computed tomography[16], magnetic resonance imaging (MRI) and endoscopic ultrasound (EUS).
Imaging with fistulography or pelvic computed tomography has limited accuracy. In contrast, pelvic MRI, rectal EUS and EUA are reasonably accurate methods for classifying PFCD. Combining either pelvic MRI or rectal EUS with surgical evaluation may represent the optimal approach for clinical practice. The choice of imaging modality should depend on local expertise[17].
The management of PFCD constitutes a difficult challenge in treating patients with IBD. In addition to unsatisfactory fistula healing rates, these patients have an increased risk of more severe luminal disease[18]. The treatment goal for fistulas is their closure, which requires addressing and preventing infection and abscesses, as well as promoting luminal healing[19]. The most recent advances in perianal fistula treatment in CD have shown promising efficacy and underscored the importance of a multidisciplinary approach involving gastroenterologists and surgeons[7,20,21].
Our proposed algorithm for guiding management is presented in Figure 1.
Antibiotics, especially ciprofloxacin and metronidazole, are commonly used as first-line treatments for PFCD. They are often used as adjuncts to surgery, immunomodulatory or biological treatment to manage perianal sepsis effectively[22].
Several observational studies with small sample sizes have demonstrated the efficacy of metronidazole (at doses of 10-20 mg/kg/day) and ciprofloxacin (at doses of 1000 mg/day) alone or in combination, achieving fistula closure in up to 50% of cases after six to eight weeks of treatment[23,24].
Adding antibiotics to thiopurines, infliximab (IFX) or adalimumab (ADA), has been shown to be significantly more effective than these agents in monotherapy[25,26]. However, side effects such as digestive intolerance with high doses of metronidazole or neuropathy with low but sustained doses, as well as disease recurrence upon discontinuation or dose reduction of antibiotics, limit their long-term use[24].
Evidence regarding the use of azathioprine or 6-mercaptopurine (6-MP) in monotherapy is indirect, as none of the studies were specifically designed to evaluate the response of perianal disease to the medications. Therefore, the results should be interpreted with caution.
A meta-analysis published in 1995 analysed five studies on azathioprine or 6-MP in PFCD as a secondary outcome. It was found that thiopurines improved fistula symptoms and healing compared with a placebo (odds ratio 4.44, 95%CI: 1.5-13)[27].
A Cochrane review from 2016 reported no differences in fistula response between patients who received azathioprine and those who were given a placebo (3 studies, 18 patients; relative risk: 2.0; 95%CI: 0.67-5.93). The overall quality of the evidence was found to be low[28].
A randomised, double-blind, placebo-controlled trial of azathioprine or 6-MP therapy in patients with PFCD would be of significant interest. Future research should also assess the efficacy and safety of the use of thiopurines with biologics in PFCD. Currently, the most recent guidelines do not recommend the use of thiopurines as monotherapy but rather as an adjuvant to anti-tumour necrosis factor (anti-TNF) agents[19,22].
The efficacy of tacrolimus in the treatment of PFCD was evaluated in a multicentre trial where 48 patients were randomised to receive either placebo or tacrolimus orally at a dose of 0.2 mg/kg/day for 10 weeks[29]. Although there was a significantly higher improvement in fistulas (defined as the closure of more than 50% of active fistulous orifices) in the tacrolimus group (43% vs 8%), there were no differences in complete healing, defined as the closure of all fistulas for at least four weeks (10% vs 8%). Furthermore, significant side effects were observed. However, some studies have shown tacrolimus to be effective in inducing remission and serving as a bridge to treatment with azathioprine or 6-MP[30].
In 2011, a systematic review was published on the effect of tacrolimus on IBD[31]. It also analysed studies related to PFCD and concluded that when administered orally long-term (six months), tacrolimus achieves improvement and remission of perianal fistulas in 57% and 29% of cases, respectively. All studies reported side effects such as tremor, paraesthesia and nephrotoxicity, which usually decrease with a dose reduction or discontinuation of tacrolimus.
A subsequent systematic review and meta-analysis showed similar results, indicating only a modest effect on fistula response and remission rates for systemic administration of tacrolimus in patients with perianal CD, with a tolerable incidence of adverse events[32].
Regarding topical tacrolimus, a recently published systematic review demonstrated no differences in fistula outcomes when compared with placebo[33].
Anti-TNF drugs revolutionised the treatment of PFCD. Both IFX and ADA have shown their usefulness in the induction and maintenance of remission in PFCD; data on certolizumab pegol (CZP) are scarce and inconclusive. No studies have evaluated the resolution of perianal disease as a primary outcome with ADA or CZP.
In 1999, Present et al[34] published the first randomised trial, which included 94 patients with at least one active fistula. Patients were treated with IFX at doses of 5 or 10 mg/kg, compared to a control group treated with a placebo. The 5 mg/kg dose of IFX, administered via intravenous infusion and repeated at two and six weeks, was significantly superior to placebo. It resulted in both complete healing of all fistulas (55% vs 13%) and a decrease of 50% or more in the number of active fistulas (68% vs 26%).
Subsequently, the ACCENT II study demonstrated that IFX (5 mg/kg every eight weeks) was superior to placebo in preventing recurrence at 12 months, with complete fistulous closure rates of 36% and 19%, respectively (P = 0.009)[35].
Recent interest has emerged in evaluating and optimising the therapeutic dosing of anti-TNF agents to enhance outcomes. While optimal trough levels have been proposed for treating luminal disease, their adequacy for perianal disease remains incompletely assessed. Several studies have shown a clear exposure-response relationship, with higher serum therapeutic levels of IFX associated with improved outcomes in fistula closure[36-40]. Yarur et al[39] demonstrated that achieving IFX levels of ≥ 10.1 mcg/mL during maintenance therapy in PFCD may improve fistula healing rates. Moreover, in the post hoc analysis of the ACCENT-II study, it was also observed that higher post-induction IFX concentrations are associated with early fistula response and complete fistula response[40].
Regarding ADA, evidence of its effectiveness in PFCD comes from several post hoc analyses. In the CHARM study, which included 113 patients with PFCD, it was concluded that 30% achieved fistula closure after 26 weeks of treatment, compared to 13% in the placebo group. This effect was maintained throughout the 56 weeks of the study, with 33% achieving fistula closure compared to 13%[41].
The CHOICE study demonstrated the effectiveness of ADA for PFCD following IFX failure, with fistula closure associated with an improvement in quality of life observed in 39% of patients[42].
ADA studies have also found an association between higher serum levels and improved fistula outcomes, with levels above 9.0 mg/mL consistently demonstrating increased rates of fistula closure[36,38,39,43].
The efficacy of CZP in achieving fistula closure has been analysed in the PRECISE 1 and 2 studies, with no significant differences observed compared to placebo in the induction of remission[44,45]. In a subgroup analysis of 58 patients from PRECISE 2 (55 of whom had PFCD) who responded to induction therapy with CZP and were randomised to placebo vs anti-TNF, fistula closure was observed at week 26 in 36% of those who received certolizumab vs 17% of those who received placebo (P = 0.038). However, this difference disappeared during the follow-up[46]. Based on the available evidence, the recommendation for the use of CZP in the treatment of PFCD is limited.
The local injection of anti-TNF agents into perianal fistulas has been described as an effective therapeutic approach. However, studies on this method have several limitations that should be taken into consideration, including small sample sizes, short follow-up periods, a lack of controls and variability in the technique of injections and outcome measures[7,8].
Exploratory analyses from the GEMINI 2 trial suggested that vedolizumab might be effective in PFCD. In this study, data from 57 patients with a draining fistula showed a non-significant trend towards improved fistula healing among patients randomised to vedolizumab compared with those receiving a placebo[47]. A small sample size leads to imprecise estimates of efficacy, which limits the extrapolation of results.
A large multicentre French study on vedolizumab in fistulising CD refractory to anti-TNF agents identified a low success rate of vedolizumab. Less than a quarter of the 102 patients with active perianal CD achieved fistula closure. Furthermore, one-third of patients with inactive perianal lesions at the initiation of vedolizumab treatment experienced perianal CD recurrence[48].
The ENTERPRISE study was the only randomised controlled trial (RCT) that compared two different induction schedules of vedolizumab (300 mg intravenously at weeks 0, 2, 6, 14 and 22 vs the same regimen plus an additional dose at week 10). Sustained improvements in fistulising CD were seen with both vedolizumab regimens (42.9% of patients achieved fistula closure at week 30), and an additional dose at week 10 does not appear to affect treatment outcomes[49]. However, the lack of a placebo control group and the small number of participants do not allow the study to provide a definitive answer on the impact of vedolizumab on fistula closure.
Limited evidence supports fistula healing with ustekinumab therapy.
No prospective study has specifically evaluated the efficacy of ustekinumab in PFCD.
The Spanish experience in a large multicentre study demonstrated clinical improvement in most of the patients with active PFCD. Specifically, 11 (61%) patients showed clinical improvement in their perianal fistula. However, these promising findings should be considered with caution because of the small number of patients assessed[50].
In the Dutch Initiative on Crohn and Colitis[51] registry, a nationwide prospective observational study, 36% of patients achieved clinical remission of perianal fistulas after 24 weeks of treatment[52].
Results for fistula healing from the pivotal trials of ustekinumab UNITI-1, UNITI-2 and CERTIFI were published as an abstract[53-55]. This post hoc analysis of 238 patients with PFCD revealed a non-significant trend towards improved fistula healing in patients randomised to ustekinumab compared with placebo[55].
Further confirmation through RCTs is required to determine the beneficial role of ustekinumab in PFCD.
Hyperbaric oxygen therapy (HBOT) involves the inhalation of 100% oxygen inside a pressurised chamber. While it is a well-established treatment for certain conditions, its role in treating IBD remains somewhat controversial. In the context of PFCD, HBOT has the potential to enhance tissue oxygen levels, creating an inhospitable environment for anaerobic organisms. Additionally, it may diminish active inflammation by suppressing proinflammatory cytokines, enhancing the host antibacterial response, promoting the synthesis of growth factors and stimulating angiogenesis, ultimately aiding in the healing process of fistulas[51]. In a meta-analysis, complete fistula healing in PFCD was reported at 47.64% (22.05%-74.54%), while partial healing was observed at 34.29% (17.33%-56.50%). The majority of adverse events were minor, including intolerance, anxiety, difficulty normalising middle ear pressure, abdominal pain, vomiting, fatigue and visual changes. Severe adverse events primarily involved middle ear barotrauma[56].
HBOT has also been investigated as a potential adjunctive therapy for perianal disease. Feitosa et al[57] showed that adjunctive HBOT was associated with significant healing rates (80%) for PFCD.
In the HOT-TOPIC trial, Lansdorp et al[58] reported the long-term (week 60) follow-up of 20 patients with therapy-refractory perianal fistulas in CD who underwent 40 sessions of HBOT over a period of eight weeks. Four patients (20%) achieved a fibrotic fistula complex with no other signs of activity on MRI, indicating deep healing. Furthermore, 60% of patients had inactive perianal disease, as measured by the perianal disease activity index at week 60. The absence of a control group is a limitation of this study.
The overall scientific evidence suggests that HBOT is linked with beneficial effects in patients with IBD. However, RCTs are needed to make a definitive recommendation.
The role of novel endoscopic therapies in PFCD management is now being studied. This growing interest and need are likely related to the lower invasiveness of these procedures compared to surgical ones and the possibility of them being performed on an outpatient basis.
In a case series involving 29 patients with fistulas and IBD who underwent endoscopic fistulotomy, the technique was found to be both safe and effective. Successful treatment was observed in 26 patients (89.6%)[59]. To date, no single therapy has shown such high success rates. The main limitation of endoscopic fistulotomy is that it cannot be used for long and complex fistulas[59-63].
The use of endoscopic seton placement has been described in treating perianal fistula-associated abscesses, irrespective of the presence of underlying IBD. While it proves to be effective in managing simple, single-tract perianal fistulas, it is not feasible when dealing with complex or branched fistulas[62].
Limited information exists in the literature regarding the use of over-the-scope clips in PFCD.
In a small retrospective study conducted at a single centre on refractory anal fistulas involving 10 patients, including six individuals with perianal CD, the procedure showed technical success in all patients. Permanent fistula closure was achieved in seven out of 10 patients (70%) within a median time of 72 days. Notably, among the six patients with CD, five experienced successful closure of their anal fistulas[64]. The long-term efficacy of it is unknown.
The epithelialisation of the fistula track, along with the presence of inflammation and fibrosis at and around the fistula opening, complicates the implementation of this technique in clinical practice[7,62].
Managing PFCD remains a complex challenge that frequently requires a multidisciplinary approach[65]. Before initiating surgical treatment, it is crucial to evaluate the presence of luminal inflammation, with special attention to proctitis. It is recommended to adopt a conservative approach in such cases because the presence of inflammation is associated with fistula persistence and a higher complication rate, including the need for proctectomy.
Conventional surgical therapies carry the risk of faecal incontinence or permanent stoma, in addition to disappointing fistula closure rates. Consequently, research has been dedicated to finding effective treatments with fewer side effects. Recently, the local injection of mesenchymal stem cells (MSCs) has emerged as the most promising minimally invasive treatment for fistulas, demonstrating higher efficacy in PFCD management[66,67].
There is a consensus that managing sepsis and preventing perianal infections is essential prior to initiating any treatment that impacts the immune system response. Therefore, loose setons should be positioned to prevent abscess formation[9,20,21,68]. It is crucial to avoid treatments that disturb the sphincter, such as a cutting seton[20,69]. The optimal timing of seton removal is uncertain. The removal of setons is necessary to allow for the complete healing of fistula tracks. However, in some cases, patients may necessitate long-term setons to prevent or delay proctectomy[9,21].
In cases of simple fistulas without proctitis, fistulotomy is the most commonly employed technique, yielding favourable outcomes. In patients with complex perianal fistulas, the use of fistulotomy may be avoided due to the risks of impairment of continence, recurrence and poor wound healing, making this treatment rarely appropriate in the context of CD[8,9,20].
Surgical options for PFCD also include ligation of the intersphincteric fistula tract (LIFT), advancement flaps (AF), fistula plugs and fibrin glue injection. A surgical attempt at fistula closure is recommended only after achieving endoscopic remission of the proctitis[20].
For PFCD, no significant differences were observed between AF and LIFT for the overall success rate (61% vs 53%, respectively), but continence was better preserved after LIFT[70]. Systematic reviews have demonstrated the effectiveness of anal fistula plugs in approximately 50%-60% of fistulas related to CD[71,72]. This procedure is considered safe, with reasonable success rates, low morbidity and a minimal risk of incontinence. However, the evidence supporting its efficacy is not robust due to factors such as small cohort sizes, heterogeneity and a lack of standardisation. Fibrin glue is a conse
Prospective studies with a large number of CD patients are further awaited.
Newer sphincter-preserving therapies, such as fistula laser closure (FiLaC) and video-assisted anal fistula treatment (VAAFT), have been described for PFCD treatment[75-78]. Both techniques share the fundamental principle of destroying the epithelium of the fistula tract. FiLaC achieves this using laser energy, while VAAFT accomplishes it through caute
A notable increase in clinical trials has been focused on investigating the safety and effectiveness of MSCs in treating PFCD.
In the first RCT of adipose-derived MSCs (Cx601) for the treatment of 202 patients with complex treatment-refractory PFCD, the results revealed that 50% of patients treated with Cx601 (darvadstrocel) achieved complete remission 24 weeks after treatment, compared to 34% of the placebo group. The administration of stem cell treatment was well tolerated[79].
A subsequent randomised placebo-controlled trial aimed to assess the long-term efficacy and safety of a single local administration of allogeneic expanded adipose-derived stem cells in patients with CD and perianal fistulas. One-year outcome data demonstrated a significantly higher proportion of patients who received Cx601 (darvadstrocel) achieved combined remission (56.3%) compared to controls (38.6%), showing a difference of 17.7% (P = 0.010). Combined remission was defined as the closure of all treated external openings that were draining at baseline and the absence of collections > 2 cm on MRI.
Despite the heterogeneity in protocols using allogeneic or autologous MSCs derived from bone marrow or adipose tissue, different dosages of MSCs, variability in the number of times patients were treated, and use or non-use of scaffolding in delivery, additional studies have demonstrated higher efficacy and a lower incidence of adverse events with MSCs compared to control subjects for the treatment of PFCD[66,67].
The findings from the mentioned studies provide positive indications of MSCs for treating PFCD, demonstrating favourable outcomes in terms of both safety and effectiveness. However, the optimal dosage and the required number of MSC injections for achieving the highest healing rates remain undetermined. Consequently, there is an opportunity to refine treatment protocols in the field of cell-based therapy. The authors believe that this promising area holds the potential to revolutionise the management of PFCD in the foreseeable future.
Guidelines recommend that in cases of refractory complex perianal CD, diverting the faecal stream may be necessary to relieve clinical symptoms when medical and local surgical management strategies have failed. Patients should becounselled about the low rates of successful reversal and the likelihood of progression to proctectomy[21,68]. Proctectomy is effective but should be considered a last resort[80].
After faecal diversion, most patients experience initial clinical improvement; however, the probability of restoring bowel continuity is low (around 20%). Furthermore, nearly half of the patients ultimately require proctectomy with a permanent stoma for severe perianal disease[81,82]. Rectal involvement in CD was linked to a reduced likelihood of restoring bowel continuity, and biological therapy did not appear to enhance the outcomes of faecal diversion[81].
IBD of the colon is associated with a higher risk of certain complications, including the onset of colorectal cancer (CRC)[83]. CRC significantly contributes to mortality rates among patients with CD[84]. Although the primary focus in IBD is usually on CRC, emerging literature suggests that individuals with IBD, especially those with anal or perianal CD, have an increased risk of anal cancer[10,83,85]. In an analysis of data collected in the French CESAME study from 19486 IBD patients, Beaugerie et al[85] found an incidence rate ratio of 9.36 [95%CI: 2.61-33.54] for anorectal cancer in perianal CD patients compared to CD patients without anal and/or perianal involvement. Patients who develop anal cancer are typically those with long-standing anorectal inflammation[11,85]. In fact, early disease onset, disease duration exceeding 10 years, chronic colitis with high inflammatory activity and chronic active fistulas and stenosis appear to be associated with malignant transformation[86]. These risk factors seem to validate the role of chronic inflammation in tumorigenesis, providing yet another reason why we should be increasingly ambitious with our therapeutic targets in perianal CD. In addition to systemic and local inflammatory processes, the pathophysiological mechanisms that lead to anal cancer in IBD are also related to human papilloma virus (HPV) infection, decreased local gut immunity and drug-induced immunosuppression[13,87].
Squamous-cell carcinoma (SCC) is the most frequent histological subtype of anal canal cancer, followed by adenocarcinoma[88]. SCC and adenocarcinoma can also develop from the fistula-lining epithelium in patients with perianal CD. However, in comparison to the overall incidence of anal cancers in CD, the occurrence of cancer in perineal fistulas seems to be relatively low[89].
Symptoms attributed to cancer are non-specific and often similar to those associated with anal and perianal diseases. Bleeding, pain and discharge may be noted[90]. In the series reported by Matsui et al[91], among 29 CD patients with rectal and anal cancer, 20 were diagnosed because of cancer-related symptoms (persistent pain in 15 patients, mucus discharge in four patients, and bleeding in four patients). All patients had advanced cancer despite the average duration from symptom onset to diagnosis being only 4.2 months. In case of any changes in anal symptoms, patients with chronic perianal CD should undergo appropriate studies to exclude the development of cancer. This may include a biopsy of any suspicious lesion and a biopsy under anaesthesia or curettage of fistula tracts when needed[83]. Given that the non-specificity of symptoms can lead to a delay in diagnosis and, consequently, a poor prognosis, preventive measures and surveillance programmes aimed at early detection of asymptomatic lesions in high-risk CD patients seem justified.
Anal SCC and high-grade squamous intraepithelial lesion (HSIL), its precursor lesion, are attributable to HPV infection in 80%-85% of cases[90]. Prophylactic vaccination against oncogenic HPV is recommended by the European Crohn's and Colitis Organisation for both young female and young male patients with IBD, similar to what is recommended in most local guidelines for the general population. This recommendation is based on its efficacy in preventing cervical cancer[92]. As an inactivated vaccine, it can be administered to immunocompromised IBD patients.
Routine screening for anal cancer in the general population is not justified due to its low prevalence. Due to the increased risk of SCC in certain populations, such as HIV-infected patients, men who have sex with men, solid organ transplant recipients or women with genital HPV-related cancer, screening programmes using anal cytology with HPV testing have been recommended for them by several societies, such as the Infectious Diseases Society of America[93]. However, there is no consensus on national and international guidelines regarding screening. Recommendations often vary and may be conflicting, with a low strength of evidence, and largely based on achievements obtained in cervical cytology screening. Recently, the benefits of diagnosing and treating anal HSIL were demonstrated. In an RCT involving 4459 individuals living with HIV, aged 35 years or older and with a biopsy-proven anal HSIL, it was demonstrated that the risk of anal cancer was significantly lower with the treatment of HSIL than with active monitoring. This finding provides support for the use of screening with cytology and treatment for precursor lesions in this high-risk group[94]. This finding may also be relevant for other groups at increased risk for anal cancer. Perhaps an anal cancer screening programme could also be justified for CD patients with high-risk factors for malignancy, namely those with anal and/or perianal involvement.
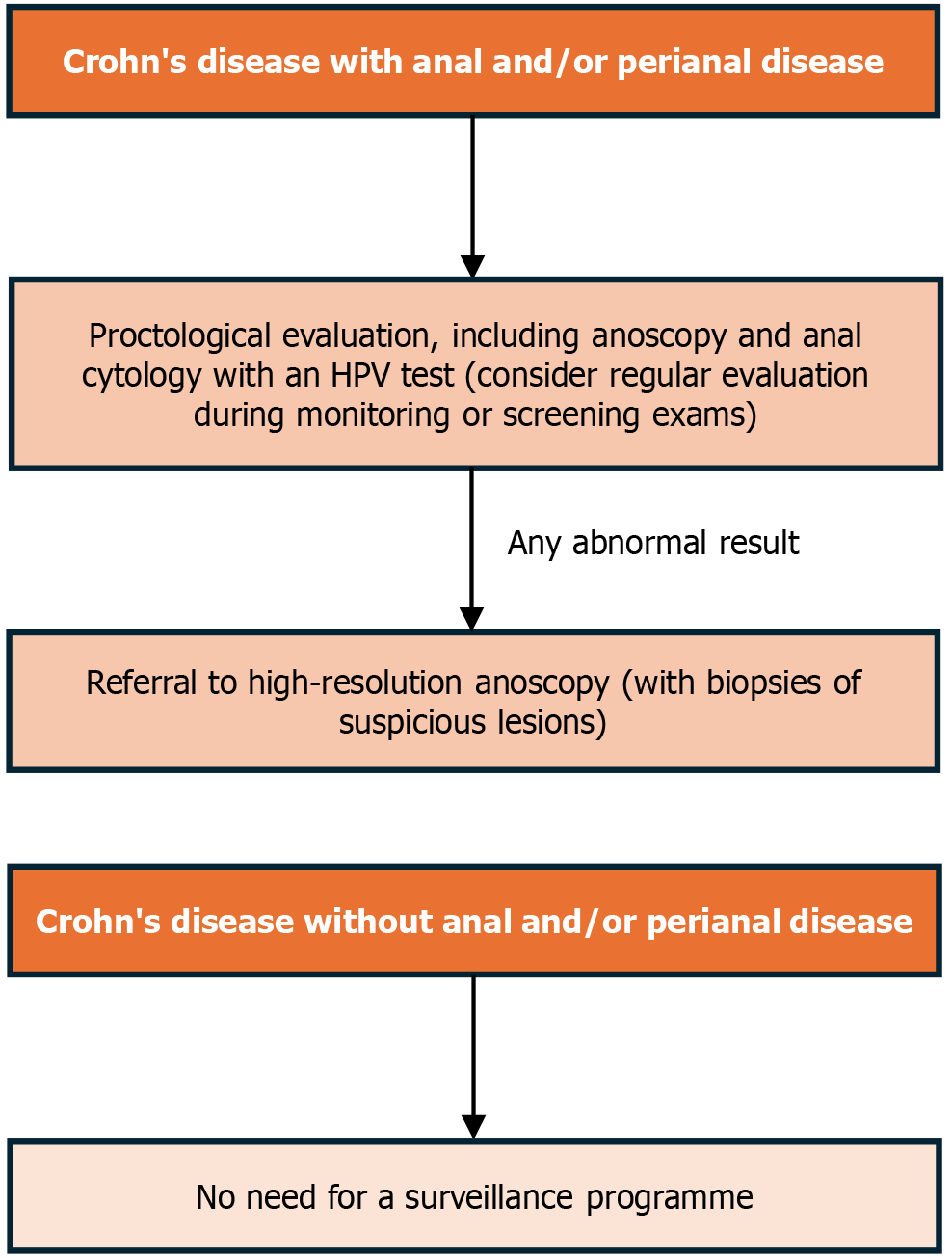
The incidence rates for anorectal cancer in the Beaugerie study do not distinguish between the different manifestations of perianal CD, including patients with any anal and/or perianal lesion, and not only fistulising disease. Furthermore, the risk analysis does not specify whether these manifestations occurred in the present or the past. As a result, the algorithm proposed in this article, as illustrated in Figure 1, includes all phenotypes of anal and perianal CD, whether active or not. Modalities of surveillance could be similar to those used in other high-risk groups, which include anal cytology with HPV testing followed by high-resolution anoscopy and biopsy. The significance of randomised transanal biopsy under anaesthesia for cancer surveillance in CD has been previously investigated, and its effectiveness was demonstrated in a Japanese population, with a detection rate of neoplastic lesions reported at 5.8% (6 patients in 103 patients undergoing surveillance)[95]. However, further investigated is warranted.
There is also a lack of evidence and, consequently, recommendations regarding time intervals for screening. We suggest that the evaluation for anorectal cancer should be opportunistically incorporated into monitoring exams or as part of CRC screening programmes. Perhaps screening every 24 to 36 months could be justified, as the risk, although higher than in the general population and in the total IBD population, does not approach that of HIV-positive patients over 35 years of age[93,96]. The optimal frequency of surveillance algorithms should be validated in future studies. Cases with abnormal results should be referred to high-resolution anoscopy and treated accordingly. For patients experiencing pain during a perianal examination or those with anal strictures, alternative surveillance algorithms should be considered.
The diagnostic work-up for anal cancer when malignancy is clinically suspected is not included in our algorithm as it is beyond the scope of the article. However, a heightened suspicion for malignancy should be upheld, and persistent or new symptoms warrant thorough investigation.
The validation of our surveillance programme (Figure 2), which aims to detect asymptomatic lesions early in patients with chronic perianal CD, is urgent to prevent late diagnoses and poor outcomes.
PFCD is one of the most feared phenotypes of IBD due to its complexity and the challenges associated with its treatment. A multidisciplinary approach involving gastroenterologists, colorectal surgeons and other healthcare providers is often necessary to optimise outcomes for affected patients. We still need to establish the best management strategies, and future prospective controlled studies can aid in determining the optimal treatment algorithm for IBD. Nevertheless, stem cell injection appears to be the most promising therapy. Patients with PFCD also face an increased risk of anal cancer. To prevent adverse outcomes, an algorithm for anal cancer screening in IBD is proposed. The optimal management of PFCD should include not only the best medical and surgical therapies but also preventive measures to avoid negative outcomes.
| 1. | Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, Calabrese E, Baumgart DC, Bettenworth D, Borralho Nunes P, Burisch J, Castiglione F, Eliakim R, Ellul P, González-Lama Y, Gordon H, Halligan S, Katsanos K, Kopylov U, Kotze PG, Krustinš E, Laghi A, Limdi JK, Rieder F, Rimola J, Taylor SA, Tolan D, van Rheenen P, Verstockt B, Stoker J; European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR]. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1242] [Cited by in RCA: 1161] [Article Influence: 193.5] [Reference Citation Analysis (0)] |
| 2. | Roda G, Chien Ng S, Kotze PG, Argollo M, Panaccione R, Spinelli A, Kaser A, Peyrin-Biroulet L, Danese S. Crohn's disease. Nat Rev Dis Primers. 2020;6:22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 601] [Article Influence: 120.2] [Reference Citation Analysis (0)] |
| 3. | Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet. 2017;389:1741-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1797] [Article Influence: 224.6] [Reference Citation Analysis (111)] |
| 4. | Eglinton TW, Barclay ML, Gearry RB, Frizelle FA. The spectrum of perianal Crohn's disease in a population-based cohort. Dis Colon Rectum. 2012;55:773-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 180] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 5. | Park SH, Aniwan S, Scott Harmsen W, Tremaine WJ, Lightner AL, Faubion WA, Loftus EV. Update on the Natural Course of Fistulizing Perianal Crohn's Disease in a Population-Based Cohort. Inflamm Bowel Dis. 2019;25:1054-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 6. | Wewer MD, Zhao M, Nordholm-Carstensen A, Weimers P, Seidelin JB, Burisch J. The Incidence and Disease Course of Perianal Crohn's Disease: A Danish Nationwide Cohort Study, 1997-2015. J Crohns Colitis. 2021;15:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (1)] |
| 7. | Kotze PG, Shen B, Lightner A, Yamamoto T, Spinelli A, Ghosh S, Panaccione R. Modern management of perianal fistulas in Crohn's disease: future directions. Gut. 2018;67:1181-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 8. | Wang X, Shen B. Advances in Perianal Disease Associated with Crohn's Disease-Evolving Approaches. Gastrointest Endosc Clin N Am. 2019;29:515-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Adegbola SO, Sahnan K, Twum-Barima C, Iqbal N, Reza L, Lung P, Warusavitarne J, Tozer P, Hart A. Current review of the management of fistulising perianal Crohn's disease. Frontline Gastroenterol. 2021;12:515-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Johansen MP, Wewer MD, Nordholm-Carstensen A, Burisch J. Perianal Crohn's Disease and the Development of Colorectal and Anal Cancer: A Systematic Review and Meta-analysis. J Crohns Colitis. 2023;17:361-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 11. | Kotsafti A, Scarpa M, Angriman I, Castagliuolo I, Caruso A. Fistula-Related Cancer in Crohn's Disease: A Systematic Review. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Iesalnieks I, Gaertner WB, Glass H, Strauch U, Hipp M, Agha A, Schlitt HJ. Fistula-associated anal adenocarcinoma in Crohn's disease. Inflamm Bowel Dis. 2010;16:1643-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Wisniewski A, Fléjou JF, Siproudhis L, Abramowitz L, Svrcek M, Beaugerie L. Anal Neoplasia in Inflammatory Bowel Disease: Classification Proposal, Epidemiology, Carcinogenesis, and Risk Management Perspectives. J Crohns Colitis. 2017;11:1011-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Parks AG, Gordon PH, Hardcastle JD. A classification of fistula-in-ano. Br J Surg. 1976;63:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1072] [Cited by in RCA: 928] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 15. | Sandborn WJ, Fazio VW, Feagan BG, Hanauer SB; American Gastroenterological Association Clinical Practice Committee. AGA technical review on perianal Crohn's disease. Gastroenterology. 2003;125:1508-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 410] [Article Influence: 18.6] [Reference Citation Analysis (1)] |
| 16. | Singh S, Proctor D, Scott FI, Falck-Ytter Y, Feuerstein JD. AGA Technical Review on the Medical Management of Moderate to Severe Luminal and Perianal Fistulizing Crohn's Disease. Gastroenterology. 2021;160:2512-2556.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 17. | Schwartz DA, Wiersema MJ, Dudiak KM, Fletcher JG, Clain JE, Tremaine WJ, Zinsmeister AR, Norton ID; Boardman LA; Devine RM, Wolff BG, Young-Fadok TM, Diehl NN, Pemberton JH, Sandborn WJ. A comparison of endoscopic ultrasound, magnetic resonance imaging, and exam under anesthesia for evaluation of Crohn's perianal fistulas. Gastroenterology. 2001;121:1064-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 341] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 18. | Eglinton TW, Gearry RB. Clinical factors predicting disease course in Crohn's disease. Expert Rev Clin Immunol. 2010;6:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Parian AM, Obi M, Fleshner P, Schwartz DA. Management of Perianal Crohn's Disease. Am J Gastroenterol. 2023;118:1323-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 20. | Gecse KB, Bemelman W, Kamm MA, Stoker J, Khanna R, Ng SC, Panés J, van Assche G, Liu Z, Hart A, Levesque BG, D'Haens G; World Gastroenterology Organization, International Organisation for Inflammatory Bowel Diseases IOIBD, European Society of Coloproctology and Robarts Clinical Trials; World Gastroenterology Organization International Organisation for Inflammatory Bowel Diseases IOIBD European Society of Coloproctology and Robarts Clinical Trials. A global consensus on the classification, diagnosis and multidisciplinary treatment of perianal fistulising Crohn's disease. Gut. 2014;63:1381-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 277] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 21. | Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, Hayee B, Lomer MCE, Parkes GC, Selinger C, Barrett KJ, Davies RJ, Bennett C, Gittens S, Dunlop MG, Faiz O, Fraser A, Garrick V, Johnston PD, Parkes M, Sanderson J, Terry H; IBD guidelines eDelphi consensus group, Gaya DR, Iqbal TH, Taylor SA, Smith M, Brookes M, Hansen R, Hawthorne AB. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1-s106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1402] [Cited by in RCA: 1565] [Article Influence: 260.8] [Reference Citation Analysis (0)] |
| 22. | Torres J, Bonovas S, Doherty G, Kucharzik T, Gisbert JP, Raine T, Adamina M, Armuzzi A, Bachmann O, Bager P, Biancone L, Bokemeyer B, Bossuyt P, Burisch J, Collins P, El-Hussuna A, Ellul P, Frei-Lanter C, Furfaro F, Gingert C, Gionchetti P, Gomollon F, González-Lorenzo M, Gordon H, Hlavaty T, Juillerat P, Katsanos K, Kopylov U, Krustins E, Lytras T, Maaser C, Magro F, Marshall JK, Myrelid P, Pellino G, Rosa I, Sabino J, Savarino E, Spinelli A, Stassen L, Uzzan M, Vavricka S, Verstockt B, Warusavitarne J, Zmora O, Fiorino G. ECCO Guidelines on Therapeutics in Crohn's Disease: Medical Treatment. J Crohns Colitis. 2020;14:4-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 991] [Cited by in RCA: 903] [Article Influence: 180.6] [Reference Citation Analysis (2)] |
| 23. | Bernstein LH, Frank MS, Brandt LJ, Boley SJ. Healing of perineal Crohn's disease with metronidazole. Gastroenterology. 1980;79:357-365. [PubMed] [DOI] [Full Text] |
| 24. | Jakobovits J, Schuster MM. Metronidazole therapy for Crohn's disease and associated fistulae. Am J Gastroenterol. 1984;79:533-540. [PubMed] |
| 25. | Dewint P, Hansen BE, Verhey E, Oldenburg B, Hommes DW, Pierik M, Ponsioen CI, van Dullemen HM, Russel M, van Bodegraven AA, van der Woude CJ. Adalimumab combined with ciprofloxacin is superior to adalimumab monotherapy in perianal fistula closure in Crohn's disease: a randomised, double-blind, placebo controlled trial (ADAFI). Gut. 2014;63:292-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 173] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 26. | West RL, van der Woude CJ, Hansen BE, Felt-Bersma RJ, van Tilburg AJ, Drapers JA, Kuipers EJ. Clinical and endosonographic effect of ciprofloxacin on the treatment of perianal fistulae in Crohn's disease with infliximab: a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2004;20:1329-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 153] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 27. | Pearson DC, May GR, Fick GH, Sutherland LR. Azathioprine and 6-mercaptopurine in Crohn disease. A meta-analysis. Ann Intern Med. 1995;123:132-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 628] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 28. | Chande N, Townsend CM, Parker CE, MacDonald JK. Azathioprine or 6-mercaptopurine for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2016;10:CD000545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | Sandborn WJ, Present DH, Isaacs KL, Wolf DC, Greenberg E, Hanauer SB, Feagan BG, Mayer L, Johnson T, Galanko J, Martin C, Sandler RS. Tacrolimus for the treatment of fistulas in patients with Crohn's disease: a randomized, placebo-controlled trial. Gastroenterology. 2003;125:380-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 208] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 30. | Lowry PW, Weaver AL, Tremaine WJ, Sandborn WJ. Combination therapy with oral tacrolimus (FK506) and azathioprine or 6-mercaptopurine for treatment-refractory Crohn's disease perianal fistulae. Inflamm Bowel Dis. 1999;5:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 72] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | McSharry K, Dalzell AM, Leiper K, El-Matary W. Systematic review: the role of tacrolimus in the management of Crohn's disease. Aliment Pharmacol Ther. 2011;34:1282-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Iida T, Nojima M, Nakase H. Therapeutic Efficacy and Adverse Events of Tacrolimus in Patients with Crohn's Disease: Systematic Review and Meta-Analysis. Dig Dis Sci. 2019;64:2945-2954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Salem G, Ding K, Sakuraba A, Cohen R. Role of topical tacrolimus in the management of proctitis, perianal manifestations in Crohn's disease, and chronic pouchitis: a systematic review. J Investig Med. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Present DH, Rutgeerts P, Targan S, Hanauer SB, Mayer L, van Hogezand RA, Podolsky DK, Sands BE, Braakman T, DeWoody KL, Schaible TF, van Deventer SJ. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999;340:1398-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1969] [Cited by in RCA: 1839] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 35. | Sands BE, Anderson FH, Bernstein CN, Chey WY, Feagan BG, Fedorak RN, Kamm MA, Korzenik JR, Lashner BA, Onken JE, Rachmilewitz D, Rutgeerts P, Wild G, Wolf DC, Marsters PA, Travers SB, Blank MA, van Deventer SJ. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med. 2004;350:876-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1581] [Cited by in RCA: 1553] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 36. | Gu B, Venkatesh K, Williams AJ, Ng W, Corte C, Gholamrezaei A, Ghaly S, Xuan W, Paramsothy S, Connor S. Higher infliximab and adalimumab trough levels are associated with fistula healing in patients with fistulising perianal Crohn's disease. World J Gastroenterol. 2022;28:2597-2608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 37. | Plevris N, Jenkinson PW, Arnott ID, Jones GR, Lees CW. Higher anti-tumor necrosis factor levels are associated with perianal fistula healing and fistula closure in Crohn's disease. Eur J Gastroenterol Hepatol. 2020;32:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 38. | Strik AS, Löwenberg M, Buskens CJ, B Gecse K, I Ponsioen C, Bemelman WA, D'Haens GR. Higher anti-TNF serum levels are associated with perianal fistula closure in Crohn's disease patients. Scand J Gastroenterol. 2019;54:453-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 39. | Yarur AJ, Kanagala V, Stein DJ, Czul F, Quintero MA, Agrawal D, Patel A, Best K, Fox C, Idstein K, Abreu MT. Higher infliximab trough levels are associated with perianal fistula healing in patients with Crohn's disease. Aliment Pharmacol Ther. 2017;45:933-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 232] [Article Influence: 29.0] [Reference Citation Analysis (1)] |
| 40. | Papamichael K, Vande Casteele N, Jeyarajah J, Jairath V, Osterman MT, Cheifetz AS. Higher Postinduction Infliximab Concentrations Are Associated With Improved Clinical Outcomes in Fistulizing Crohn's Disease: An ACCENT-II Post Hoc Analysis. Am J Gastroenterol. 2021;116:1007-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 41. | Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, Schreiber S, Byczkowski D, Li J, Kent JD, Pollack PF. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007;132:52-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1598] [Cited by in RCA: 1620] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 42. | Lichtiger S, Binion DG, Wolf DC, Present DH, Bensimon AG, Wu E, Yu AP, Cardoso AT, Chao J, Mulani PM, Lomax KG, Kent JD. The CHOICE trial: adalimumab demonstrates safety, fistula healing, improved quality of life and increased work productivity in patients with Crohn's disease who failed prior infliximab therapy. Aliment Pharmacol Ther. 2010;32:1228-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (1)] |
| 43. | Sirmai L, Pelletier AL, Gault N, Zallot C, Bouguen G, Bouchard D, Roland Nicaise P, Peyneau M, Sironneau S, Bittencourt MC, Petitcollin A, Fernandez P, Roblin X, Siproudhis L, Abramowitz L. Relationship between clinical remission of perianal fistulas in Crohn's disease and serum adalimumab concentrations: A multi-center cross-sectional study. World J Gastroenterol. 2022;28:961-972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 44. | Sandborn WJ, Feagan BG, Stoinov S, Honiball PJ, Rutgeerts P, Mason D, Bloomfield R, Schreiber S; PRECISE 1 Study Investigators. Certolizumab pegol for the treatment of Crohn's disease. N Engl J Med. 2007;357:228-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 871] [Cited by in RCA: 807] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 45. | Schreiber S, Khaliq-Kareemi M, Lawrance IC, Thomsen OØ, Hanauer SB, McColm J, Bloomfield R, Sandborn WJ; PRECISE 2 Study Investigators. Maintenance therapy with certolizumab pegol for Crohn's disease. N Engl J Med. 2007;357:239-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 730] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 46. | Schreiber S, Lawrance IC, Thomsen OØ, Hanauer SB, Bloomfield R, Sandborn WJ. Randomised clinical trial: certolizumab pegol for fistulas in Crohn's disease - subgroup results from a placebo-controlled study. Aliment Pharmacol Ther. 2011;33:185-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 47. | Feagan BG, Schwartz D, Danese S, Rubin DT, Lissoos TW, Xu J, Lasch K. Efficacy of Vedolizumab in Fistulising Crohn's Disease: Exploratory Analyses of Data from GEMINI 2. J Crohns Colitis. 2018;12:621-626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 48. | Chapuis-Biron C, Bourrier A, Nachury M, Nancey S, Bouhnik Y, Serrero M, Armengol-Debeir L, Buisson A, Tran-Minh ML, Zallot C, Fumery M, Bouguen G, Abitbol V, Viennot S, Chanteloup E, Rajca S, Dib N, Parmentier AL, Peyrin-Biroulet L, Vuitton L; GETAID BioLAP Study Group. Vedolizumab for perianal Crohn's disease: a multicentre cohort study in 151 patients. Aliment Pharmacol Ther. 2020;51:719-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 49. | Schwartz DA, Peyrin-Biroulet L, Lasch K, Adsul S, Danese S. Efficacy and Safety of 2 Vedolizumab Intravenous Regimens for Perianal Fistulizing Crohn's Disease: ENTERPRISE Study. Clin Gastroenterol Hepatol. 2022;20:1059-1067.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 50. | Khorrami S, Ginard D, Marín-Jiménez I, Chaparro M, Sierra M, Aguas M, Sicilia B, García-Sánchez V, Suarez C, Villoria A, Taxonera C, Velasco-Guardado A, Martínez-González J, Gisbert JP. Ustekinumab for the Treatment of Refractory Crohn's Disease: The Spanish Experience in a Large Multicentre Open-label Cohort. Inflamm Bowel Dis. 2016;22:1662-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 138] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 51. | Kirby JP, Snyder J, Schuerer DJE, Peters JS, Bochicchio GV. Essentials of Hyperbaric Oxygen Therapy: 2019 Review. Mo Med. 2019;116:176-179. [PubMed] |
| 52. | Biemans VBC, van der Meulen-de Jong AE, van der Woude CJ, Löwenberg M, Dijkstra G, Oldenburg B, de Boer NKH, van der Marel S, Bodelier AGL, Jansen JM, Haans JJL, Theeuwen R, de Jong D, Pierik MJ, Hoentjen F. Ustekinumab for Crohn's Disease: Results of the ICC Registry, a Nationwide Prospective Observational Cohort Study. J Crohns Colitis. 2020;14:33-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 142] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 53. | Sandborn WJ, Gasink C, Gao LL, Blank MA, Johanns J, Guzzo C, Sands BE, Hanauer SB, Targan S, Rutgeerts P, Ghosh S, de Villiers WJ, Panaccione R, Greenberg G, Schreiber S, Lichtiger S, Feagan BG; CERTIFI Study Group. Ustekinumab induction and maintenance therapy in refractory Crohn's disease. N Engl J Med. 2012;367:1519-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 810] [Cited by in RCA: 872] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 54. | Feagan BG, Sandborn WJ, Gasink C, Jacobstein D, Lang Y, Friedman JR, Blank MA, Johanns J, Gao LL, Miao Y, Adedokun OJ, Sands BE, Hanauer SB, Vermeire S, Targan S, Ghosh S, de Villiers WJ, Colombel JF, Tulassay Z, Seidler U, Salzberg BA, Desreumaux P, Lee SD, Loftus EV Jr, Dieleman LA, Katz S, Rutgeerts P; UNITI-IM-UNITI Study Group. Ustekinumab as Induction and Maintenance Therapy for Crohn's Disease. N Engl J Med. 2016;375:1946-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1467] [Cited by in RCA: 1342] [Article Influence: 149.1] [Reference Citation Analysis (0)] |
| 55. | Sands BE, Gasink C, Jacobstein D, Gao L, Johanns J, Colombel JF, de Villiers WJ, Sandborn WJ. Fistula Healing in Pivotal Studies of Ustekinumab in Crohn's Disease. Gastroenterology. 2017;152:S185. [RCA] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 56. | Singh AK, Jha DK, Jena A, Kumar-M P, Sebastian S, Sharma V. Hyperbaric oxygen therapy in inflammatory bowel disease: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2021;33:e564-e573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 57. | Feitosa MR, Parra RS, Machado VF, Vilar GN, Aquino JC, Rocha JJR, Kotze PG, Féres O. Adjunctive Hyperbaric Oxygen Therapy in Refractory Crohn's Disease: An Observational Study. Gastroenterol Res Pract. 2021;2021:6628142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 58. | Lansdorp CA, Buskens CJ, Gecse KB, Löwenberg M, Stoker J, Bemelman WA, D'Haens GRAM, van Hulst RA. Hyperbaric oxygen therapy for the treatment of perianal fistulas in 20 patients with Crohn's disease: Results of the HOT-TOPIC trial after 1-year follow-up. United European Gastroenterol J. 2022;10:160-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 59. | Kochhar G, Shen B. Endoscopic fistulotomy in inflammatory bowel disease (with video). Gastrointest Endosc. 2018;88:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 60. | Pal P, Kanaganti S, Banerjee R, Ramchandani M, Nabi Z, Reddy DN, Tandan M. Systematic Review of Endoscopic Management of Stricture, Fistula and Abscess in Inflammatory Bowel Disease. Gastroenterol Insights. 2023;14:45-63. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 61. | Gold SL, Cohen-Mekelburg S, Schneider Y, Steinlauf A. Perianal Fistulas in Patients With Crohn's Disease, Part 2: Surgical, Endoscopic, and Future Therapies. Gastroenterol Hepatol (NY). 2018;14:521-528. [PubMed] |
| 62. | Shen B. Exploring endoscopic therapy for the treatment of Crohn's disease-related fistula and abscess. Gastrointest Endosc. 2017;85:1133-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 63. | Shen B, Kochhar G, Navaneethan U, Liu X, Farraye FA, Gonzalez-Lama Y, Bruining D, Pardi DS, Lukas M, Bortlik M, Wu K, Sood A, Schwartz DA, Sandborn WJ; Global Interventional Inflammatory Bowel Disease Group. Role of interventional inflammatory bowel disease in the era of biologic therapy: a position statement from the Global Interventional IBD Group. Gastrointest Endosc. 2019;89:215-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 64. | Mennigen R, Laukötter M, Senninger N, Rijcken E. The OTSC(®) proctology clip system for the closure of refractory anal fistulas. Tech Coloproctol. 2015;19:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 65. | Gecse KB, Sebastian S, Hertogh Gd, Yassin NA, Kotze PG, Reinisch W, Spinelli A, Koutroubakis IE, Katsanos KH, Hart A, van den Brink GR, Rogler G, Bemelman WA. Results of the Fifth Scientific Workshop of the ECCO [II]: Clinical Aspects of Perianal Fistulising Crohn's Disease-the Unmet Needs. J Crohns Colitis. 2016;10:758-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 66. | Cao Y, Su Q, Zhang B, Shen F, Li S. Efficacy of stem cells therapy for Crohn's fistula: a meta-analysis and systematic review. Stem Cell Res Ther. 2021;12:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 67. | Lightner AL, Wang Z, Zubair AC, Dozois EJ. A Systematic Review and Meta-analysis of Mesenchymal Stem Cell Injections for the Treatment of Perianal Crohn's Disease: Progress Made and Future Directions. Dis Colon Rectum. 2018;61:629-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 68. | Adamina M, Bonovas S, Raine T, Spinelli A, Warusavitarne J, Armuzzi A, Bachmann O, Bager P, Biancone L, Bokemeyer B, Bossuyt P, Burisch J, Collins P, Doherty G, El-Hussuna A, Ellul P, Fiorino G, Frei-Lanter C, Furfaro F, Gingert C, Gionchetti P, Gisbert JP, Gomollon F, González Lorenzo M, Gordon H, Hlavaty T, Juillerat P, Katsanos K, Kopylov U, Krustins E, Kucharzik T, Lytras T, Maaser C, Magro F, Marshall JK, Myrelid P, Pellino G, Rosa I, Sabino J, Savarino E, Stassen L, Torres J, Uzzan M, Vavricka S, Verstockt B, Zmora O. ECCO Guidelines on Therapeutics in Crohn's Disease: Surgical Treatment. J Crohns Colitis. 2020;14:155-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 367] [Article Influence: 73.4] [Reference Citation Analysis (0)] |
| 69. | Lee MJ, Heywood N, Sagar PM, Brown SR, Fearnhead NS; pCD Collaborators. Surgical management of fistulating perianal Crohn's disease: a UK survey. Colorectal Dis. 2017;19:266-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 70. | Stellingwerf ME, van Praag EM, Tozer PJ, Bemelman WA, Buskens CJ. Systematic review and meta-analysis of endorectal advancement flap and ligation of the intersphincteric fistula tract for cryptoglandular and Crohn's high perianal fistulas. BJS Open. 2019;3:231-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 71. | O'Riordan JM, Datta I, Johnston C, Baxter NN. A systematic review of the anal fistula plug for patients with Crohn's and non-Crohn's related fistula-in-ano. Dis Colon Rectum. 2012;55:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 72. | Nasseri Y, Cassella L, Berns M, Zaghiyan K, Cohen J. The anal fistula plug in Crohn's disease patients with fistula-in-ano: a systematic review. Colorectal Dis. 2016;18:351-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 73. | Grimaud JC, Munoz-Bongrand N, Siproudhis L, Abramowitz L, Sénéjoux A, Vitton V, Gambiez L, Flourié B, Hébuterne X, Louis E, Coffin B, De Parades V, Savoye G, Soulé JC, Bouhnik Y, Colombel JF, Contou JF, François Y, Mary JY, Lémann M; Groupe d'Etude Thérapeutique des Affections Inflammatoires du Tube Digestif. Fibrin glue is effective healing perianal fistulas in patients with Crohn's disease. Gastroenterology. 2010;138:2275-2281, 2281.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 74. | Cirocchi R, Santoro A, Trastulli S, Farinella E, Di Rocco G, Vendettuali D, Giannotti D, Redler A, Coccetta M, Gullà N, Boselli C, Avenia N, Sciannameo F, Basoli A. Meta-analysis of fibrin glue versus surgery for treatment of fistula-in-ano. Ann Ital Chir. 2010;81:349-356. [PubMed] |
| 75. | Adegbola SO, Sahnan K, Tozer P, Warusavitarne J. Emerging Data on Fistula Laser Closure (FiLaC) for the Treatment of Perianal Fistulas; Patient Selection and Outcomes. Clin Exp Gastroenterol. 2021;14:467-475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 76. | Adegbola SO, Sahnan K, Tozer PJ, Strouhal R, Hart AL, Lung PFC, Phillips RKS, Faiz O, Warusavitarne J. Symptom Amelioration in Crohn's Perianal Fistulas Using Video-Assisted Anal Fistula Treatment (VAAFT). J Crohns Colitis. 2018;12:1067-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 77. | Cao D, Li W, Ji Y, Wang X, Cui Z. Efficacy and safety of FiLaC™ for perianal fistulizing Crohn's disease: a systematic review and meta-analysis. Tech Coloproctol. 2022;26:775-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 78. | Schwandner O. Video-assisted anal fistula treatment (VAAFT) combined with advancement flap repair in Crohn's disease. Tech Coloproctol. 2013;17:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 79. | Panés J, García-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, Dignass A, Nachury M, Ferrante M, Kazemi-Shirazi L, Grimaud JC, de la Portilla F, Goldin E, Richard MP, Leselbaum A, Danese S; ADMIRE CD Study Group Collaborators. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn's disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016;388:1281-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 730] [Article Influence: 81.1] [Reference Citation Analysis (1)] |
| 80. | de Groof EJ, Cabral VN, Buskens CJ, Morton DG, Hahnloser D, Bemelman WA; research committee of the European Society of Coloproctology. Systematic review of evidence and consensus on perianal fistula: an analysis of national and international guidelines. Colorectal Dis. 2016;18:O119-O134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 81. | Yamamoto T, Shimoyama T. Fecal Diversion in Complex Perianal Fistulizing Crohn's Disease. Clin Colon Rectal Surg. 2022;35:5-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 82. | Singh S, Ding NS, Mathis KL, Dulai PS, Farrell AM, Pemberton JH, Hart AL, Sandborn WJ, Loftus EV Jr. Systematic review with meta-analysis: faecal diversion for management of perianal Crohn's disease. Aliment Pharmacol Ther. 2015;42:783-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 139] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 83. | Gordon H, Biancone L, Fiorino G, Katsanos KH, Kopylov U, Al Sulais E, Axelrad JE, Balendran K, Burisch J, de Ridder L, Derikx L, Ellul P, Greuter T, Iacucci M, Di Jiang C, Kapizioni C, Karmiris K, Kirchgesner J, Laharie D, Lobatón T, Molnár T, Noor NM, Rao R, Saibeni S, Scharl M, Vavricka SR, Raine T. ECCO Guidelines on Inflammatory Bowel Disease and Malignancies. J Crohns Colitis. 2023;17:827-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 152] [Reference Citation Analysis (0)] |
| 84. | Yasukawa S, Matsui T, Yano Y, Sato Y, Takada Y, Kishi M, Ono Y, Takatsu N, Nagahama T, Hisabe T, Hirai F, Yao K, Ueki T, Higashi D, Futami K, Sou S, Sakurai T, Yao T, Tanabe H, Iwashita A, Washio M. Crohn's disease-specific mortality: a 30-year cohort study at a tertiary referral center in Japan. J Gastroenterol. 2019;54:42-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 85. | Beaugerie L, Carrat F, Nahon S, Zeitoun JD, Sabaté JM, Peyrin-Biroulet L, Colombel JF, Allez M, Fléjou JF, Kirchgesner J, Svrcek M; Cancers et Surrisque Associé aux Maladies Inflammatoires Intestinales En France Study Group. High Risk of Anal and Rectal Cancer in Patients With Anal and/or Perianal Crohn's Disease. Clin Gastroenterol Hepatol. 2018;16:892-899.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 86. | Annese V, Beaugerie L, Egan L, Biancone L, Bolling C, Brandts C, Dierickx D, Dummer R, Fiorino G, Gornet JM, Higgins P, Katsanos KH, Nissen L, Pellino G, Rogler G, Scaldaferri F, Szymanska E, Eliakim R; ECCO. European Evidence-based Consensus: Inflammatory Bowel Disease and Malignancies. J Crohns Colitis. 2015;9:945-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 323] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 87. | Selimagic A, Dozic A, Husic-Selimovic A, Tucakovic N, Cehajic A, Subo A, Spahic A, Vanis N. The Role of Inflammation in Anal Cancer. Diseases. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 88. | Islami F, Ferlay J, Lortet-Tieulent J, Bray F, Jemal A. International trends in anal cancer incidence rates. Int J Epidemiol. 2017;46:924-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 89. | Thomas M, Bienkowski R, Vandermeer TJ, Trostle D, Cagir B. Malignant transformation in perianal fistulas of Crohn's disease: a systematic review of literature. J Gastrointest Surg. 2010;14:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 90. | Rao S, Guren MG, Khan K, Brown G, Renehan AG, Steigen SE, Deutsch E, Martinelli E, Arnold D; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Anal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up(☆). Ann Oncol. 2021;32:1087-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 138] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 91. | Matsui T, Iwashita A, Matsumoto T, Hisabe T, Futami K, Tanabe H. Atlas of Inflammatory Bowel Disease-Associated Intestinal Cancer. Singapore: Springer, 2022. [DOI] [Full Text] |
| 92. | Kucharzik T, Ellul P, Greuter T, Rahier JF, Verstockt B, Abreu C, Albuquerque A, Allocca M, Esteve M, Farraye FA, Gordon H, Karmiris K, Kopylov U, Kirchgesner J, MacMahon E, Magro F, Maaser C, de Ridder L, Taxonera C, Toruner M, Tremblay L, Scharl M, Viget N, Zabana Y, Vavricka S. ECCO Guidelines on the Prevention, Diagnosis, and Management of Infections in Inflammatory Bowel Disease. J Crohns Colitis. 2021;15:879-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 264] [Article Influence: 66.0] [Reference Citation Analysis (32)] |
| 93. | Barroso LF, Stier EA, Hillman R, Palefsky J. Anal Cancer Screening and Prevention: Summary of Evidence Reviewed for the 2021 Centers for Disease Control and Prevention Sexually Transmitted Infection Guidelines. Clin Infect Dis. 2022;74:S179-S192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 94. | Palefsky JM, Lee JY, Jay N, Goldstone SE, Darragh TM, Dunlevy HA, Rosa-Cunha I, Arons A, Pugliese JC, Vena D, Sparano JA, Wilkin TJ, Bucher G, Stier EA, Tirado Gomez M, Flowers L, Barroso LF, Mitsuyasu RT, Lensing SY, Logan J, Aboulafia DM, Schouten JT, de la Ossa J, Levine R, Korman JD, Hagensee M, Atkinson TM, Einstein MH, Cracchiolo BM, Wiley D, Ellsworth GB, Brickman C, Berry-Lawhorn JM; ANCHOR Investigators Group. Treatment of Anal High-Grade Squamous Intraepithelial Lesions to Prevent Anal Cancer. N Engl J Med. 2022;386:2273-2282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 263] [Article Influence: 87.7] [Reference Citation Analysis (0)] |
| 95. | Hirano Y, Futami K, Higashi D, Mikami K, Maekawa T. Anorectal cancer surveillance in Crohn's disease. J Anus Rectum Colon. 2018;2:145-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 96. | Albuquerque A. Cytology in Anal Cancer Screening: Practical Review for Clinicians. Acta Cytol. 2020;64:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |