Published online Jun 21, 2024. doi: 10.3748/wjg.v30.i23.3005
Revised: April 24, 2024
Accepted: May 13, 2024
Published online: June 21, 2024
Processing time: 173 Days and 15.6 Hours
Gastric cancer (GC) is the most common malignant tumor and ranks third for cancer-related deaths among the worldwide. The disease poses a serious public health problem in China, ranking fifth for incidence and third for mortality. Knowledge of the invasive depth of the tumor is vital to treatment decisions.
To evaluate the diagnostic performance of double contrast-enhanced ultrasonography (DCEUS) for preoperative T staging in patients with GC by comparing with multi-detector computed tomography (MDCT).
This single prospective study enrolled patients with GC confirmed by preoperative gastroscopy from July 2021 to March 2023. Patients underwent DCEUS, including ultrasonography (US) and intravenous contrast-enhanced ultrasonography (CEUS), and MDCT examinations for the assessment of preoperative T staging. Features of GC were identified on DCEUS and criteria developed to evaluate T staging according to the 8th edition of AJCC cancer staging manual. The diagnostic performance of DCEUS was evaluated by comparing it with that of MDCT and surgical-pathological findings were considered as the gold standard.
A total of 229 patients with GC (80 T1, 33 T2, 59 T3 and 57 T4) were included. Overall accuracies were 86.9% for DCEUS and 61.1% for MDCT (P < 0.001). DCEUS was superior to MDCT for T1 (92.5% vs 70.0%, P < 0.001), T2 (72.7% vs 51.5%, P = 0.041), T3 (86.4% vs 45.8%, P < 0.001) and T4 (87.7% vs 70.2%, P = 0.022) staging of GC.
DCEUS improved the diagnostic accuracy of preoperative T staging in patients with GC compared with MDCT, and constitutes a promising imaging modality for preoperative evaluation of GC to aid individualized treatment decision-making.
Core Tip: This current prospective study identified double contrast-enhanced ultrasonography (DCEUS) findings in preoperative T staging, developed DCEUS criteria based on the 5-layer gastric wall structure and perfusion characteristics of CEUS, and evaluated the diagnostic performance of DCEUS in gastric cancer (GC) T staging using DCEUS criteria, a method that may overcome limitations by detailing hemodynamic changes of GCs. DCEUS showed superior performance in GC T staging to multi-detector computed tomography and constitutes a promising imaging modality for preoperative evaluation of GC to aid individualized treatment decision-making.
- Citation: Xu YF, Ma HY, Huang GL, Zhang YT, Wang XY, Wei MJ, Pei XQ. Double contrast-enhanced ultrasonography improves diagnostic accuracy of T staging compared with multi-detector computed tomography in gastric cancer patients. World J Gastroenterol 2024; 30(23): 3005-3015
- URL: https://www.wjgnet.com/1007-9327/full/v30/i23/3005.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i23.3005
Gastric cancer (GC) is the most common malignant tumor and ranks third for cancer-related deaths among the worldwide[1]. The disease poses a serious public health problem in China, ranking fifth for incidence and third for mortality[2]. It is important to stage GC as accurately as possible due to the increased availability of minimally invasive surgery, such as endoscopic mucosal resection (EMR)/endoscopic submucosal dissection (ESD) or laparoscopic surgery[1]. Knowledge of the invasive depth of the tumor is vital to treatment decisions[1,3].
Endoscopic ultrasonography (EUS) and computed tomography (CT) are commonly used methods for baseline clinical GC staging[4-6]. However, the precision of these preoperative diagnostic tools has not kept pace with advances in GC treatment. EUS is operator dependent and lesions are difficult to identify when located in gastroesophageal junctions, subcardiac regions and lesser curvatures. The diagnostic accuracy of EUS ranges from 57% to 88% for GC T staging[7] and it differentiates T3 and T4 tumors poorly[3,7]. EUS is not routinely performed in clinical practice except for EMR/ESD indications. CT is the principal imaging modality used for staging but has limitations in terms of T staging accuracy, particularly for early GC (EGC)[1,8-12].
The limitations of available imaging methods illustrate the need for a noninvasive, reliable approach to improve GC T staging accuracy. Double contrast-enhanced ultrasonography (DCEUS) examination combines ultrasonography (US) with an ultrasonic oral contrast agent and intravenous contrast-enhanced ultrasonography (CEUS) with an intravenous contrast agent[12-14]. DCEUS has been explored in China for screening gastrointestinal diseases, including gastric and rectal tumors. Studies[12-14] have reported that DCEUS could be used for preoperative T staging based on the 5-layer structure of gastric wall, while ignoring vascularity of the gastric wall and tumors.
The current prospective study aimed to identify DCEUS findings on GC, develop DCEUS criteria based on the concept of the 5-layer gastric wall structure of US and perfusion characteristics of CEUS and evaluate preoperative GC T staging using DCEUS criteria. Diagnostic performance of DCEUS for GC T staging was compared with multi-detector CT (MDCT).
This prospective study was approved by our hospital ethics committee and all patients provided written informed consent.
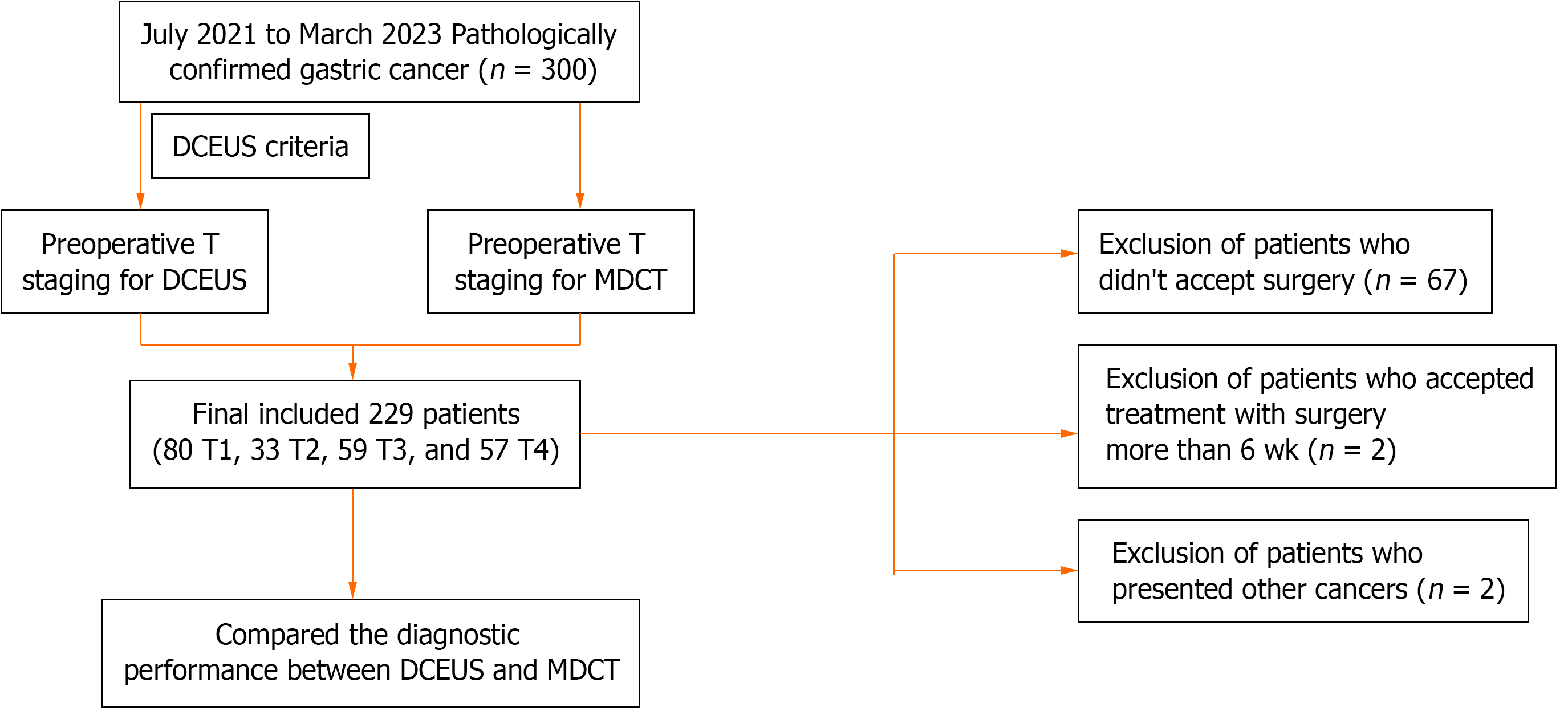
A total of 300 patients with pathological confirmation of GC were recruited from July 2021 to March 2023 and all underwent DCEUS and MDCT examinations before radical gastrectomy. Inclusion criteria were: (1) Adult; (2) no other treatment received before operation; (3) no allergy or contraindication for oral or intravenous contrast media or iodine contrast media; and (4) no contraindications for surgical resection. Exclusion criteria were: (1) The presence of other cancers; (2) no the treatment of surgery; and (3) surgical treatment accepted more than 6 wk after DCEUS and MDCT examinations. A final total of 229 patients were enrolled. The study protocol flowchart is presented in Figure 1.
Patients were fasted for > 8 h. The powdered oral contrast agent (50 g; G.F. Acoustic contrast, Yanbian, China) made from lotus root, rice, corn and soybeans was dissolved in 500 mL boiling water to form a homogenous suspension, cooled and given orally.
DCEUS examination was performed with Siemens Healthineers, equipped with a 5C1 abdominal probe and a 10 L high-frequency probe. The US examination was conducted using a 5C1 probe from the distal esophagus to the duodenal bulb in both the supine and decubitus positions. Additionally, a 10 L high-frequency probe was employed to provide a more detailed evaluation when necessary. The imaging features include the presence, location, morphology and estimated invasive depth of the lesion. CEUS examination was performed to evaluate the enhancement patterns of the lesion. The proper contrast mode, including gain, depth, acoustic window, mechanical index, and focal zone were adjusted. An injection of 1.5 mL contrast agent (SonoVue®; Bracco, Milan, Italy) was given, followed by a 5.0-mL saline flush. The timer started simultaneously when the contrast agent was being injected and the probe was kept in a stable state for 180 s to detect the gastric lesion, the surrounding normal gastric wall and the possibility of a late phase liver examination metastasis. Arterial and venous phases of the lesion were recorded for 0 s to 120 s. DCEUS images and videos were recorded in Dicom format.
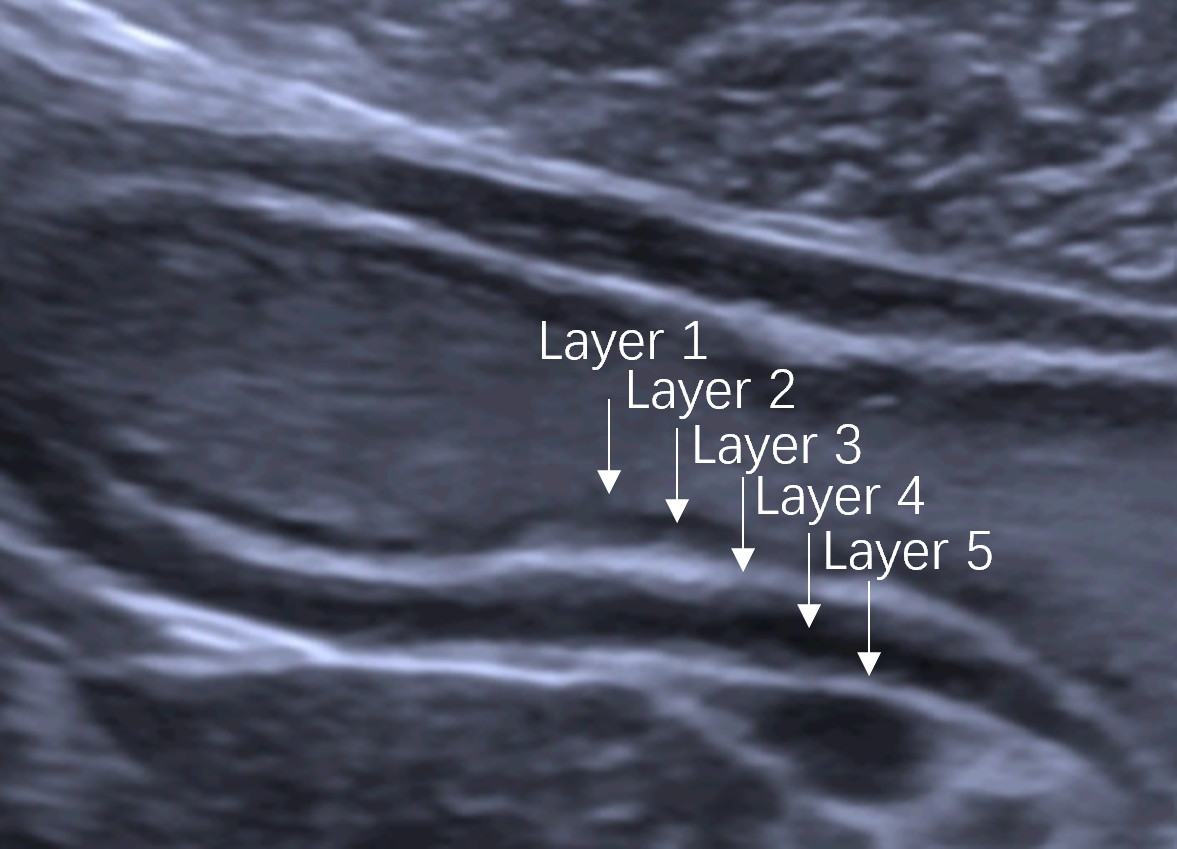
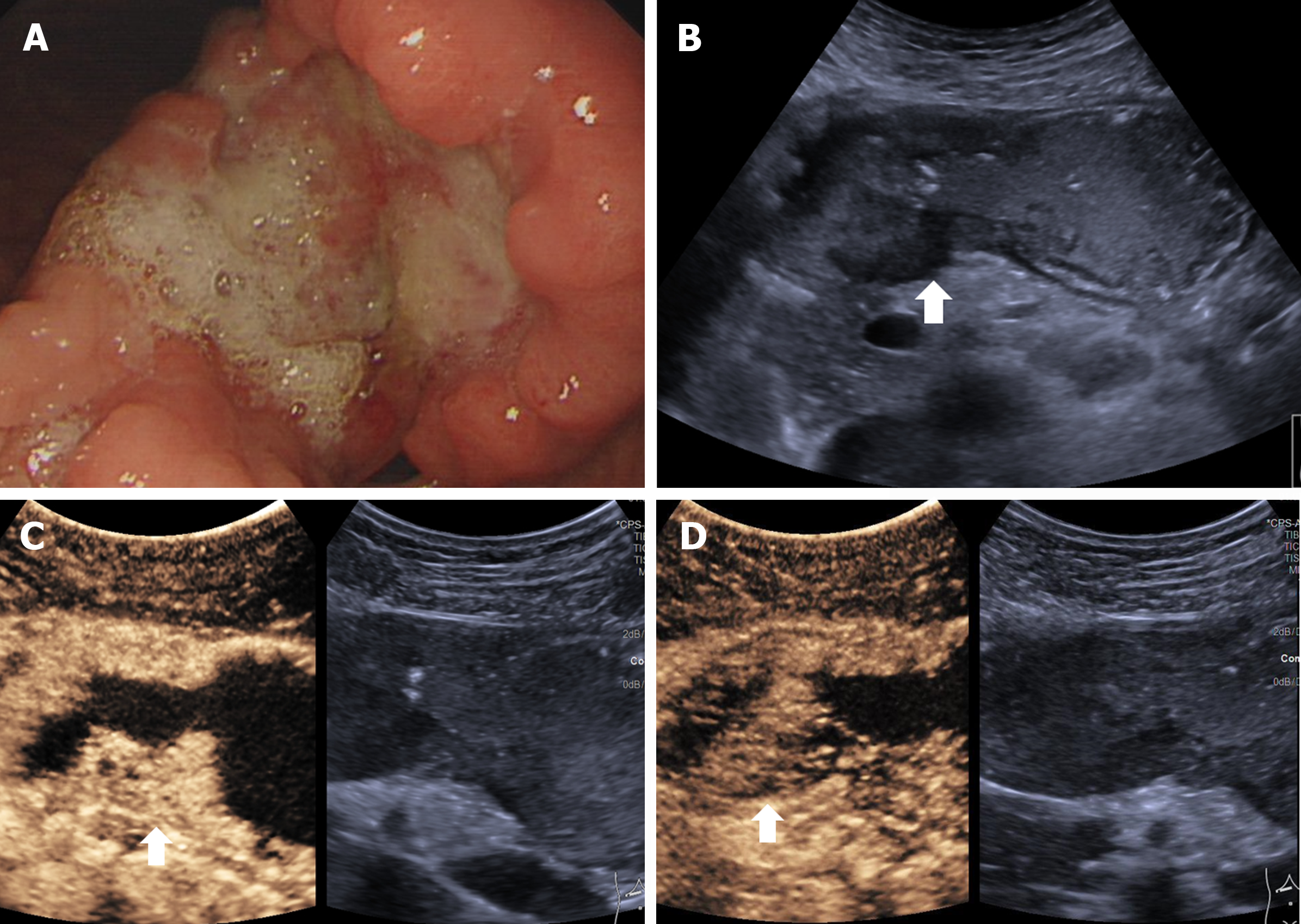
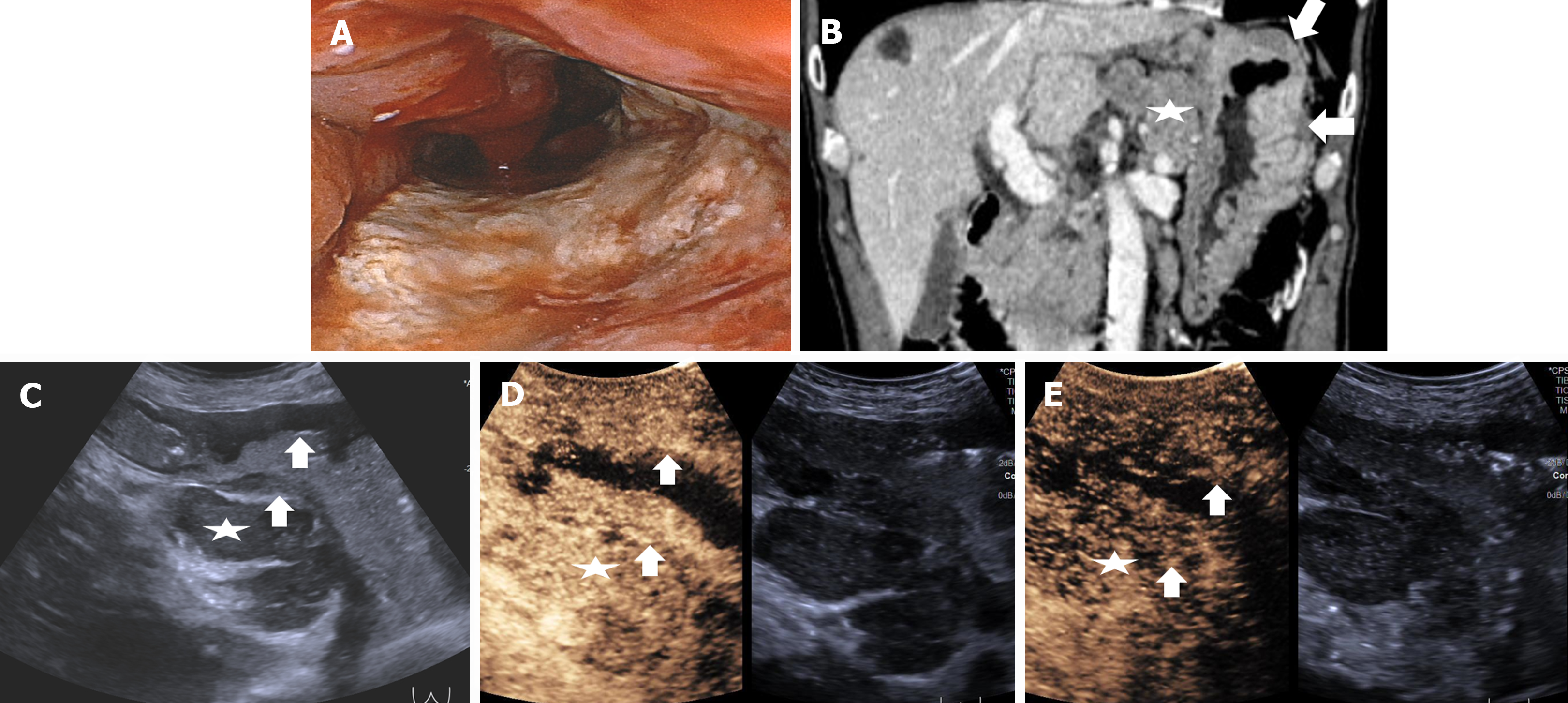
Normal gastric wall consists of five layers, mucosa, submucosa, muscularis propria, subserosa and serosa. The five layers, including the interface echo between the gastric lumen and the mucosa, the rest of the mucosa, submucosa, muscularis propria, and serosa[15] , can be visualized by US (Figure 2). The outer serosa enhances first in the CEUS examination, followed rapidly by the submucosa and mucosa, completing the enhancement of the entire gastric wall. The layers of the mucosa and particularly the submucosa, exhibit early and intense enhancement whereas muscularis propria layer shows lesser and delayed enhancement[16] (Video 1). GCs manifest as the thickening and disruption of normal gastric wall structures on US, “hyper-enhancement” in the arterial phase and “hypo-enhancement” in the venous phase on CEUS. Therefore, the 5-layer gastric wall structure of US and perfusion characteristics of CEUS give detailed information for T staging.
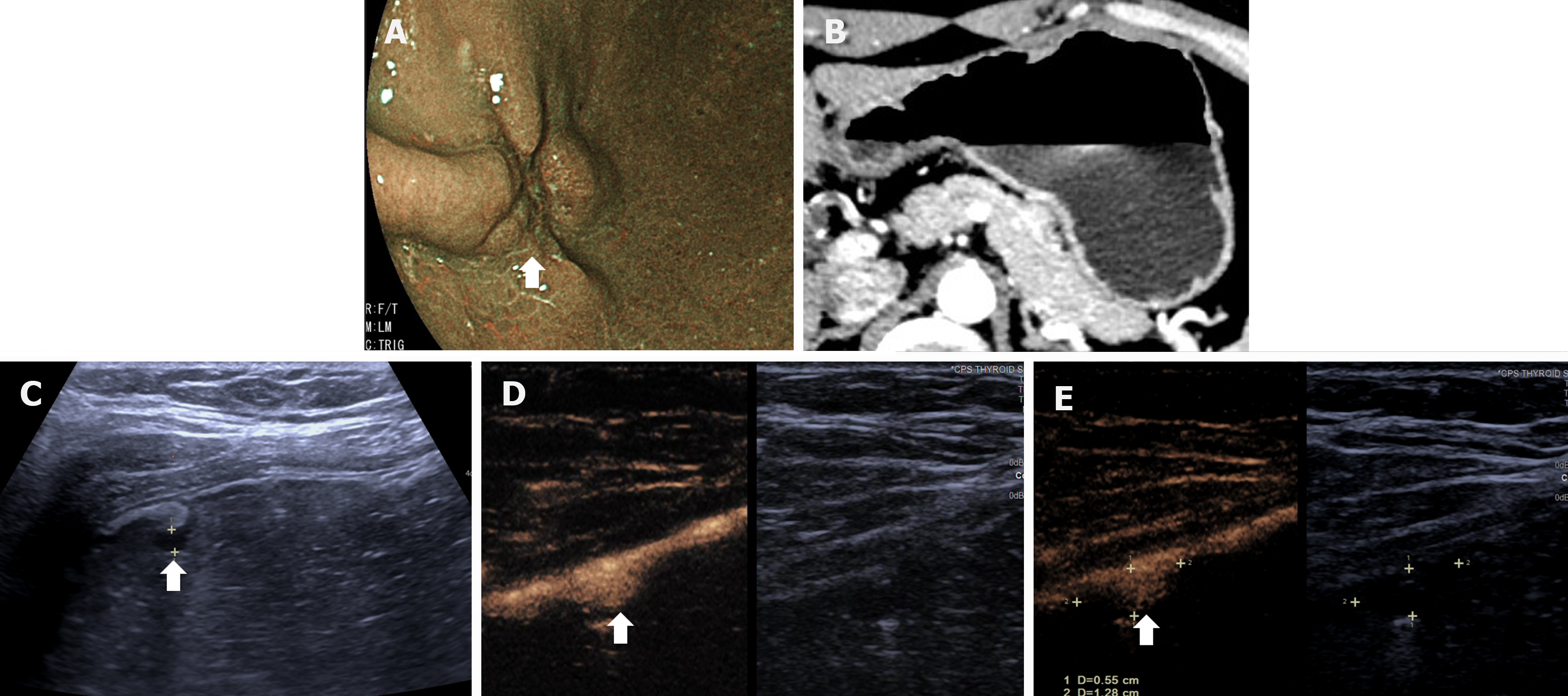
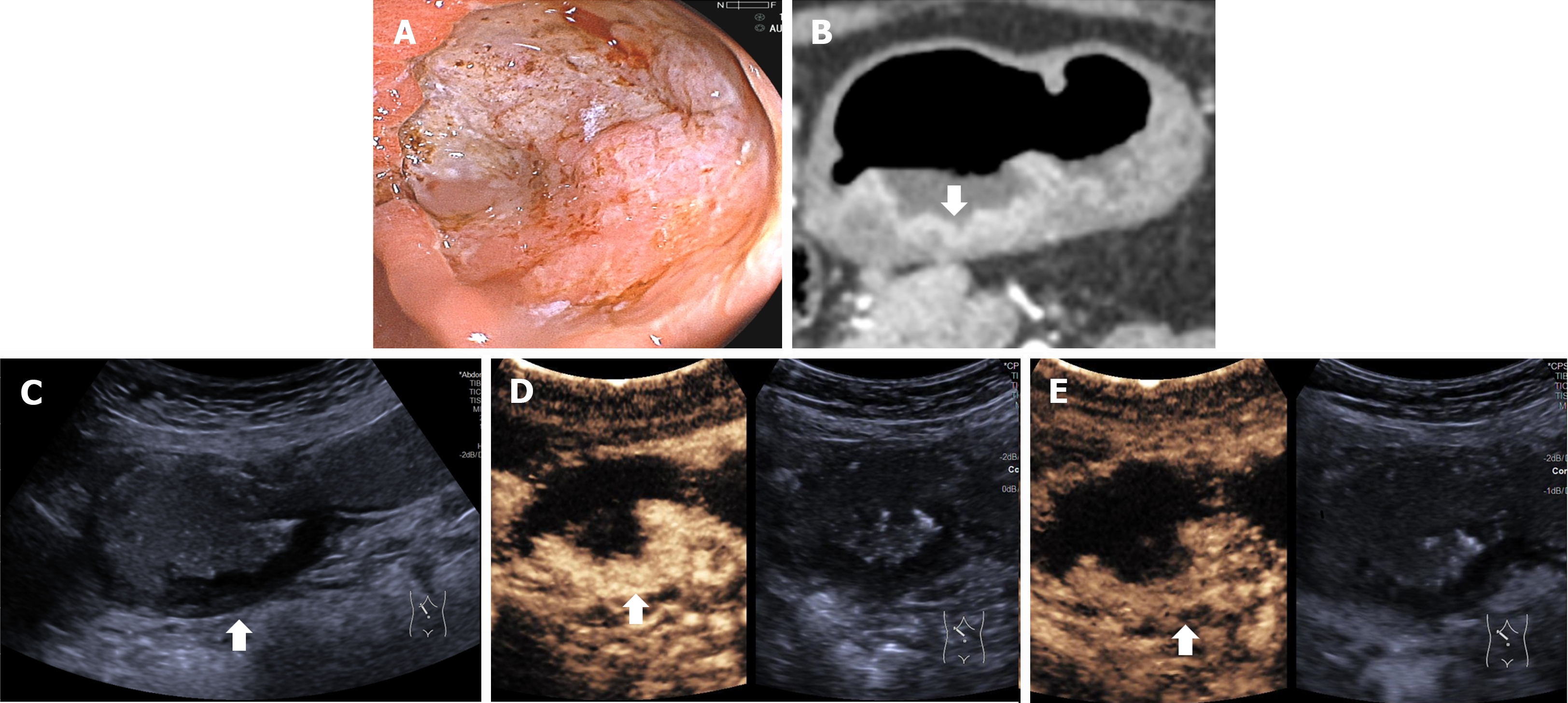
The DCEUS criteria for T staging were developed according to the 8th edition of AJCC cancer staging manual, shown in Table 1. Figures 3-6 showed the typical T1a, T2, T3, T4a GCs.
| T stage | Pathological definition | DCEUS criteria |
| T1 | Invasion of the mucosa or submucosa | T1a: In the arterial phase, focal thickening of the mucosa is visualized. The lesion shows slightly delayed hyper-enhancement, similar to the submucosal layer. In the venous phase, the lesion shows hypo-enhancement compared to the submucosal layer. The submucosal layer consistently shows hyper-enhancement and is continuous and intact. The muscular layer shows linear hypo-enhancement and is continuous and intact; T1b: In the arterial phase, focal thickening of the mucosa and submucosa are visualized. The lesion shows homogenous hyper-enhancement, similar to the normal submucosal layer. In the venous phase, the lesion shows hypo-enhancement. The enhancing submucosal layer is continuous. The muscular layer shows linear hypo-enhancement and is continuous and intact |
| T2 | Invasion of the muscularis propria | In the arterial phase, disruption of the mucosa, submucosa and partly muscularis propria are visualized. The lesion shows homogenous hyper-enhancement, similar to the normal submucosal layer. In the venous phase, the lesion shows homogenous hypo-enhancement. The hyper-enhancement strip of submucosal layer and partly hypo-enhancement strip of the muscularis propria are disruptive |
| T3 | Invasion of the subserosal connective tissue without invading the visceral peritoneum | In the arterial phase, disruption of the mucosa, submucosa and muscularis propria are visualized. The lesion shows homogenous hyper-enhancement, similar to the normal submucosal layer. In the venous phase, the lesion shows homogenous hypo-enhancement. The hyper-enhancement strip of submucosal layer and hypo-enhancement strip of the muscularis propria are disruptive. A smooth outer margin of the serosa or a few small linear stranding within the serosa are observed. The enhancing serosa is continuous |
| T4 | Invasion of the serosa (visceral peritoneum) or adjacent structures/organs | In the arterial phase, disruption of the mucosa, submucosa, muscularis propria and serosa are visualized. The lesion shows homogenous hyper-enhancement, similar to the normal submucosal layer. In the venous phase, the lesion shows homogenous hypo-enhancement. The hyper-enhancement strip of submucosal and serosal layers and hypo-enhancement strip of the muscularis propria are disruptive; T4a: An irregular nodular margin of the serosa and densely burred or banded infiltration of the adjacent fat plane are visualized; T4b: The adjacent fat plane between the tumor and the adjacent organ is obliterated or the tumor directly infiltrates the adjacent organ |
The All preoperative DCEUS images and videos of 229 cancers were analyzed by two experienced radiologists before surgery who were aware that the patients had GC based on findings of preoperative endoscopy before performing the DCEUS examination. Two radiologists evaluated the T staging for DCEUS based on the DCEUS criteria. If there was disagreement, both radiologists re-evaluated DCEUS images and videos and reached a consensus.
Patients were fasted for > 8 h and given 600-1000 mL of tap water to dilate the stomach 5 min before the CT examination. A dose of 1.5 mL/kg of a contrast agent (Ultravist, Guangzhou, China) was administered intravenously at a rate of 3.5 mL/s using an automatic power injector (MEDRAD Vistron CT, PA, United States) during the entire contrast-enhanced CT examination (Philips Brilliance 128 row 256 slice spiral CT). MDCT scans were obtained at 30 s (arterial phase) and 70 s (portal-venous phase) after administration of an intravenous contrast agent. The subject was positioned prone on the scanning table to avoid artifacts caused by air in the stomach. Two radiologists evaluated the T staging for MDCT according to the 8th edition of the AJCC cancer staging manual. They were aware of the results of preoperative endoscopy before performing the MDCT examination. In case of disagreement, a consensus was reached by re-evaluation with the two radiologists.
Surgical resection was performed on all 229 patients and specimens subjected to histopathological evaluation as the gold standard for T staging.
All statistical analyses were performed using SPSS 25.0 software. Continuous data were expressed as means ± SD and categorical data as percentages. The χ2 test and Fisher exact test were used to compare categorical variables. A value of P < 0.05 was considered statistically significant.
Two hundred and twenty-nine patients with GC, 92 (40.2%) females and 137 (59.8%) males of mean age 54.9 ± 13.0 years, were included. Two hundred and twenty-nine lesions were classified 47 (20.5%) as T1a, 33 (14.4%) as T1b, 33 (14.4%) as T2, 59 (25.8 %) as T3, and 53 (23.1%) as T4a, and 4 (1.8%) as T4b (Table 2).
| Features | Total |
| Sex | |
| Male | 137 (59.8) |
| Female | 92 (40.2) |
| Age (yr; mean ± SD) | 54.9 ± 13.0 |
| Pathological T staging | |
| T1a | 47 (20.5) |
| T1b | 33 (14.4) |
| T2 | 33 (14.4) |
| T3 | 59 (25.8) |
| T4a | 53 (23.1) |
| T4b | 4 (1.8) |
| Location | |
| Upper | 17 (7.4) |
| Middle | 70 (30.6) |
| Lower | 122 (53.3) |
| Entire | 20 (8.7) |
| Histopathological type | |
| Well differentiation | 11 (4.8) |
| Moderately differentiation | 45 (19.7) |
| Poorly differentiation | 173 (75.5) |
| Bormann classification | |
| I | 7 (4.7) |
| II | 56 (37.6) |
| III | 70 (47.0) |
| IV | 16 (10.7) |
| Ulceration | |
| Yes | 198 (86.5) |
| No | 31 (13.5) |
| Tumor size (cm, mean ± SD) | 3.5 ± 2.4 |
A comparison of the accuracy for T staging between DCEUS and MDCT is shown in Table 3. DCEUS correctly staged 199 cases (74 T1, 24 T2, 51 T3, and 50 T4), giving an overall accuracy of 86.9% with 92.5% for T1, 72.7% for T2, 86.4% for T3, and 87.7% for T4. Overestimation of T staging occurred in 16 (7.0%) cases and underestimation in 14 (6.1%). MDCT correctly staged 140 cases (56 T1, 17 T2, 27 T3, and 40 T4), giving an overall accuracy of 61.1%, 70.0% for T1, 51.5% for T2, 45.8% for T3 and 70.2% for T4. Overestimation occurred in 59 (25.8%) cases and underestimation in 30 (13.1%). DCEUS was superior to MDCT for overall accuracy (86.9% vs 61.1%, P < 0.001), T1 (92.5% vs 70.0%, P < 0.001), T2 (72.7% vs 51.5%, P = 0.041), T3 (86.4% vs 45.8%, P < 0.001), and T4 (87.7% vs 70.2%, P = 0.022) staging.
| T staging | DCEUS (%) | MDCT (%) | P value |
| T1 (n = 80) | 74 (92.5) | 56 (70.0) | < 0.001 |
| T2 (n = 33) | 24 (72.7) | 17 (51.5) | 0.041 |
| T3 (n = 59) | 51 (86.4) | 27 (45.8) | < 0.001 |
| T4 (n = 57) | 50 (87.7) | 40 (70.2) | 0.022 |
| T total (n = 229) | 199 (86.9) | 140 (61.1) | < 0.001 |
Diagnostic accuracy of DCEUS and MDCT for GC T staging based on clinicopathological features is shown in Table 4. DCEUS showed superior accuracy in T staging for lesions located in the middle, lower and entire parts of the stomach and higher accuracy in T staging for Borrmann types II and III and histopathological types of moderately- and poorly-differentiated GC. The superiority can be seen when comparing the middle (DCEUS: 82.9% vs MDCT: 57.1%, P = 0.001), lower (DCEUS: 90.2% vs MDCT: 65.6%, P < 0.001) and entire parts of the stomach (DCEUS: 85.0% vs MDCT: 55.0%, P = 0.038). DCEUS showed higher accuracy in T staging for Borrmann types II (DCEUS: 82.1% vs MDCT: 48.2%, P < 0.001) and III (DCEUS: 82.9% vs MDCT: 61.4%, P < 0.001) and histopathological types of moderately- differentiated (DCEUS: 86.7% vs MDCT: 44.4%, P < 0.001), and poorly-differentiated (DCEUS: 86.1% vs MDCT: 63.6%, P < 0.001) GC. DCEUS accuracy in T staging was consistently superior to MDCT, stratified across lesions of varied size and the presence of ulceration.
| Features | Total | Accuracy of DCEUS (%) | Accuracy of MDCT (%) | P value |
| Location | ||||
| Upper | 17 | 14 (82.4) | 9 (52.9) | 0.067 |
| Middle | 70 | 58 (82.9) | 40 (57.1) | 0.001 |
| Lower | 122 | 110 (90.2) | 80 (65.6) | < 0.001 |
| Entire | 20 | 17 (85.0) | 11 (55.0) | 0.038 |
| Tumor size | ||||
| < 2.0 cm | 78 | 67 (85.9) | 48 (61.5) | < 0.001 |
| ≥ 2.0 cm | 151 | 139 (92.1) | 97 (64.2) | < 0.001 |
| Ulceration | ||||
| Yes | 198 | 168 (84.8) | 117 (59.1) | < 0.001 |
| No | 31 | 31 (100) | 23 (74.2) | 0.0051 |
| Borrmann classification | ||||
| I | 7 | 7 (100) | 5 (71.4) | 0.4621 |
| II | 56 | 46 (82.1) | 27 (48.2) | < 0.001 |
| III | 70 | 58 (82.9) | 43 (61.4) | < 0.001 |
| IV | 16 | 14 (87.5) | 9 (56.3) | 0.1131 |
| Histopathological type | ||||
| Well differentiation | 11 | 11 (100) | 10 (90.9) | 0.3061 |
| Moderately differentiation | 45 | 39 (86.7) | 20 (44.4) | < 0.001 |
| Poorly differentiation | 173 | 149 (86.1) | 110 (63.6) | < 0.001 |
Treatment options for GC patients depend on the tumor stage, including EMR/ESD for EGC, total/distal gastrectomy for locally advanced GC (AGC) and chemotherapy for unresectable/metastatic AGC[1]. Therefore, correct staging is vital for selection of the optimal therapeutic regimen. The current prospective study identified DCEUS findings in preoperative T staging, developed DCEUS criteria based on the 5-layer gastric wall structure and perfusion characteristics of CEUS, and evaluated the diagnostic performance of DCEUS in GC T staging using DCEUS criteria, a method that may overcome limitations by detailing hemodynamic changes of GCs. DCEUS showed superior performance in GC T staging to MDCT.
DCEUS is an emerging modality by combining oral and intravenous contrast agents to elucidate the morphological and perfusion characteristics of gastric lesions. The oral contrast agent allows discharge the intra-gastric air and forms a homogeneous ultrasonic transmission surface to expose gastric wall layers and location, extension and morphology of gastric lesions[15] on US. It is helpful in detecting mucosal lesions for EGC and local or diffuse lesions for AGC when the distended stomach allows visualization of thickening lesions on the gastric wall. Tumors are regarded as enhancing lesions on dynamic CEUS images due to their hypervascularity[16]. The intravenous contrast agent allows elucidation of tumor blood perfusion and enhances visualization through “hyper-enhancement” in the arterial phase and “hypo-enhancement” in the venous phase to delineate tumor location and extension[17]. The tumor contour and invasive depth may be gauged in real-time and in a dynamic manner to evaluate the invasion of adjacent structures and identify the enhancement patterns of GC for DCEUS to distinguish T1 from T2 and T3 from T4. In our study, DCEUS showed an overall accuracy of 86.9% for T staging, 92.5% for T1, 72.7% for T2, 86.4% for T3, and 87.7% for T4. Overestimation was found in 7.0% of cases and underestimation in 6.1%. Previous studies[12,14,17] have found that overall accuracies of DCEUS for T staging of GC range from 77.2% to 84.0% and accuracies from 62.5% to 90.9% for T1, 84.4% to 88.9% for T2, 78.9% to 87.9% for T3, and 82.9% to 91.3% for T4 with overestimation in 12.0% of cases and underestimation in 5.7%.
CT is the most commonly used imaging method for staging GC and allows visualization of primary tumor invasive depth, estimation of the lymph node involvement and distant metastasis. A diagnostic meta-analysis[18] comparing CT and EUS for staging GC showed that EUS was superior to CT for T1 staging, but no significant differences were found for T2-T4 lesions. Thus, it is suggested that CT might replace EUS for preoperative staging, as its accuracy in T staging is almost equivalent to that of EUS[19]. However, CT does not give clear visualization of the 5-layer gastric wall or indicate the invasive depth of the lesion. Recent studies[1,8-12] have reported relatively low accuracies of CT for T staging, ranging from 43% to 86% for overall accuracy of T staging, and 27% to 46% for T1, 53% to 56% for T2, 42% to 86% for T3, 59% to 86% for T4, respectively. MDCT had an overall accuracy of 61.1% in current work, 70.0% for T1, 51.5% for T2, 45.8% for T3, and 70.2% for T4. A CT scan is usually a diagnostic tool for preoperative staging but is not a primary screening tool for GC. The current study classified lesions that were not depicted on MDCT images as T1a[20], perhaps causing MDCT to perform better in the determination of EGC compared with previous studies. DCEUS was superior to MDCT for diagnosis of EGC in the current study, consistent with the previous studies[13,21]. MDCT gave a higher rate of overestimation and underestimation for T staging due to difficulties in observing the multilayered pattern of the gastric wall especially in thinner regions and partial volume averaging effects in areas scanned obliquely. By contrast, DCEUS showed the 5-layer structure of the gastric wall with clearer visualization of lesion invasion on CEUS. Thus, DCEUS is a more promising candidate for T staging of GC than CT.
By comparing diagnostic accuracy of T staging for GC between DCEUS and MDCT stratified across the tumor location, tumor size, status of ulceration, histopathological type and Borrmann type for AGC, our study further highlights the advantages of DCEUS in preoperative T staging. DCEUS had higher accuracy of T staging than MDCT for lesions located in the middle, lower and entire parts of the stomach. Previous reports have identified the cardia of stomach as producing the least accurate results due to inadequate filling with water[19]. However, DCEUS showed good diagnostic per
AGCs are classified into four Borrmann types, depending on gross appearance and are usually accompanied by different degrees of inflammation. Determination of invasive depth by MDCT is difficult, especially when diffuse inflammation is present[9,10]. Dynamic DCEUS images delineate tumors more precisely due to real-time observation of the 5-layer structure of the gastric wall and perfusion characteristics on CEUS. Tumor invasion is difficult to distinguish from inflammatory effect for Borrmann type II and III tumors with ulceration. DCEUS had better diagnostic performance than MDCT with significantly higher accuracy of T staging in Borrmann type II and III tumors. However, no significant differences were found in diagnostic accuracy of T staging for Borrmann type I and IV tumors between DCEUS and MDCT. Both DCEUS and MDCT showed good delineation for Borrmann type I due to the morphologic characteristics of nodular polypoid tumors. Borrmann type IV AGC is characterized by diffuse infiltration of the gastric wall without ulceration or distinct elevation and is difficult to recognize by endoscopy. CT has been shown superior for lesion detection and characterization of Borrmann type IV[24]. And our study additionally shows that DCEUS exhibits superior diagnostic performance to CT for T staging of Borrmann type IV AGC.
Histopathological type may affect the accuracy of T staging between DCEUS and MDCT. In our study, DCEUS showed higher accuracies of T staging for moderately- and poorly-differentiated GCs than MDCT. Compared with the differentiated type, undifferentiated types are hypovascular with diffuse infiltration[22,23]. Tumor vascularity could be evaluated by CEUS and contrast enhancement has been shown to correlate with histological vessel density in rectal cancer, gastrointestinal neuroendocrine tumors and stromal tumors[25,26]. And our study confirms that the enhancement characteristics of the lesion contributes to higher accurate diagnosis of T staging for moderately- and poorly-differentiated GC.
The study has several limitations. First, this was a single-center study which may result in potential bias. Second, radiologists who reviewed the DCEUS and MDCT data had prior knowledge of the endoscopic results which may lead to overestimation of accuracies for T staging of GC.
In conclusion, DCEUS shows superior accuracy for T staging of GC compared with MDCT and may facilitate optimal treatment decision in GC patients.
We thank all the authors’ contributions for this study.
| 1. | Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, Farjah F, Gerdes H, Gibson MK, Hochwald S, Hofstetter WL, Ilson DH, Keswani RN, Kim S, Kleinberg LR, Klempner SJ, Lacy J, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Outlaw D, Park H, Perry KA, Pimiento J, Poultsides GA, Reznik S, Roses RE, Strong VE, Su S, Wang HL, Wiesner G, Willett CG, Yakoub D, Yoon H, McMillian N, Pluchino LA. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:167-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 928] [Article Influence: 309.3] [Reference Citation Analysis (0)] |
| 2. | Zheng RS, Chen R, Han BF, Wang SM, Li L, Sun KX, Zeng HM, Wei WW, He J. [Cancer incidence and mortality in China, 2022]. Zhonghua Zhong Liu Za Zhi. 2024;46:221-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 130] [Reference Citation Analysis (0)] |
| 3. | Spolverato G, Ejaz A, Kim Y, Squires MH, Poultsides GA, Fields RC, Schmidt C, Weber SM, Votanopoulos K, Maithel SK, Pawlik TM. Use of endoscopic ultrasound in the preoperative staging of gastric cancer: a multi-institutional study of the US gastric cancer collaborative. J Am Coll Surg. 2015;220:48-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Abdalla EK, Pisters PW. Staging and preoperative evaluation of upper gastrointestinal malignancies. Semin Oncol. 2004;31:513-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Kwee RM, Kwee TC. Imaging in local staging of gastric cancer: a systematic review. J Clin Oncol. 2007;25:2107-2116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 210] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 6. | Weber WA, Ott K. Imaging of esophageal and gastric cancer. Semin Oncol. 2004;31:530-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Cardoso R, Coburn N, Seevaratnam R, Sutradhar R, Lourenco LG, Mahar A, Law C, Yong E, Tinmouth J. A systematic review and meta-analysis of the utility of EUS for preoperative staging for gastric cancer. Gastric Cancer. 2012;15 Suppl 1:S19-S26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 8. | Hwang SW, Lee DH, Lee SH, Park YS, Hwang JH, Kim JW, Jung SH, Kim NY, Kim YH, Lee KH, Kim HH, Park DJ, Lee HS, Jung HC, Song IS. Preoperative staging of gastric cancer by endoscopic ultrasonography and multidetector-row computed tomography. J Gastroenterol Hepatol. 2010;25:512-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Kim AY, Kim HJ, Ha HK. Gastric cancer by multidetector row CT: preoperative staging. Abdom Imaging. 2005;30:465-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Kim HJ, Kim AY, Oh ST, Kim JS, Kim KW, Kim PN, Lee MG, Ha HK. Gastric cancer staging at multi-detector row CT gastrography: comparison of transverse and volumetric CT scanning. Radiology. 2005;236:879-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 189] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 11. | Lim JS, Yun MJ, Kim MJ, Hyung WJ, Park MS, Choi JY, Kim TS, Lee JD, Noh SH, Kim KW. CT and PET in stomach cancer: preoperative staging and monitoring of response to therapy. Radiographics. 2006;26:143-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Wang J, Li X, Zhang Z, Jing C, Li J. Clinical Research of Combined Application of DCEUS and Dynamic Contrast-Enhanced MSCT in Preoperative cT Staging of Gastric Cancer. J Oncol. 2021;2021:9868585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Zhang X, Yao J, Zhang Y, Huang X, Wang W, Huang H. Updated Evaluation of the Diagnostic Performance of Double Contrast-Enhanced Ultrasonography in the Preoperative T Staging of Gastric Cancer: A Meta-Analysis and Systematic Review. Front Oncol. 2022;12:844390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 14. | Wang L, Liu Z, Kou H, He H, Zheng B, Zhou L, Yang Y. Double Contrast-Enhanced Ultrasonography in Preoperative T Staging of Gastric Cancer: A Comparison With Endoscopic Ultrasonography. Front Oncol. 2019;9:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Nylund K, Hausken T, Gilja OH. Ultrasound and inflammatory bowel disease. Ultrasound Q. 2010;26:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Sidhu PS, Cantisani V, Dietrich CF, Gilja OH, Saftoiu A, Bartels E, Bertolotto M, Calliada F, Clevert DA, Cosgrove D, Deganello A, D'Onofrio M, Drudi FM, Freeman S, Harvey C, Jenssen C, Jung EM, Klauser AS, Lassau N, Meloni MF, Leen E, Nicolau C, Nolsoe C, Piscaglia F, Prada F, Prosch H, Radzina M, Savelli L, Weskott HP, Wijkstra H. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Long Version). Ultraschall Med. 2018;39:e2-e44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 579] [Article Influence: 82.7] [Reference Citation Analysis (1)] |
| 17. | Yan C, Bao X, Shentu W, Chen J, Liu C, Ye Q, Wang L, Tan Y, Huang P. Preoperative Gross Classification of Gastric Adenocarcinoma: Comparison of Double Contrast-Enhanced Ultrasound and Multi-Detector Row CT. Ultrasound Med Biol. 2016;42:1431-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Ungureanu BS, Sacerdotianu VM, Turcu-Stiolica A, Cazacu IM, Saftoiu A. Endoscopic Ultrasound vs Computed Tomography for Gastric Cancer Staging: A Network Meta-Analysis. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Giandola T, Maino C, Marrapodi G, Ratti M, Ragusi M, Bigiogera V, Talei Franzesi C, Corso R, Ippolito D. Imaging in Gastric Cancer: Current Practice and Future Perspectives. Diagnostics (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 20. | Lee IJ, Lee JM, Kim SH, Shin CI, Lee JY, Kim SH, Han JK, Choi BI. Diagnostic performance of 64-channel multidetector CT in the evaluation of gastric cancer: differentiation of mucosal cancer (T1a) from submucosal involvement (T1b and T2). Radiology. 2010;255:805-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | He P, Miao LY, Ge HY, Wang TL, Ye JX, Meng LM, Xue H, Zhang F, Zhao B. Preoperative Tumor Staging of Gastric Cancer: Comparison of Double Contrast-Enhanced Ultrasound and Multidetector Computed Tomography. J Ultrasound Med. 2019;38:3203-3209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Lee KG, Shin CI, Kim SG, Choi J, Oh SY, Son YG, Suh YS, Kong SH, Lee HJ, Kim SH, Lee KU, Kim WH, Yang HK. Can endoscopic ultrasonography (EUS) improve the accuracy of clinical T staging by computed tomography (CT) for gastric cancer? Eur J Surg Oncol. 2021;47:1969-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Kuroki K, Oka S, Tanaka S, Yorita N, Hata K, Kotachi T, Boda T, Arihiro K, Chayama K. Clinical significance of endoscopic ultrasonography in diagnosing invasion depth of early gastric cancer prior to endoscopic submucosal dissection. Gastric Cancer. 2021;24:145-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Kim JI, Kim YH, Lee KH, Kim SY, Lee YJ, Park YS, Kim N, Lee DH, Kim HH, Park DJ, Lee HS. Type-specific diagnosis and evaluation of longitudinal tumor extent of borrmann type IV gastric cancer: CT versus gastroscopy. Korean J Radiol. 2013;14:597-606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Wang Y, Li L, Wang YX, Cui NY, Zou SM, Zhou CW, Jiang YX. Time-intensity curve parameters in rectal cancer measured using endorectal ultrasonography with sterile coupling gels filling the rectum: correlations with tumor angiogenesis and clinicopathological features. Biomed Res Int. 2014;2014:587806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Stock K, Hann von Weyhern C, Slotta-Huspenina J, Burian M, Clevert DA, Meining A, Prinz C, Pachmann C, Holzapfel K, Schmid RM, Lersch C. Microcirculation of subepithelial gastric tumors using contrast-enhanced ultrasound. Clin Hemorheol Microcirc. 2010;45:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |