Published online Jun 21, 2024. doi: 10.3748/wjg.v30.i23.2991
Revised: May 7, 2024
Accepted: May 20, 2024
Published online: June 21, 2024
Processing time: 87 Days and 14.5 Hours
Colorectal cancer significantly impacts global health, with unplanned reoperations post-surgery being key determinants of patient outcomes. Existing pre
To develop and validate a machine learning model for predicting unplanned re
Data of patients treated for colorectal cancer (n = 2044) at the First Affiliated Hospital of Wenzhou Medical University and Wenzhou Central Hospital from March 2020 to March 2022 were retrospectively collected. Patients were divided into an experimental group (n = 60) and a control group (n = 1984) according to unplanned reoperation occurrence. Patients were also divided into a training group and a validation group (7:3 ratio). We used three different machine lear
More patients in the experimental group were ≥ 60 years old, male, and had a history of hypertension, laparotomy, and hypoproteinemia, compared to the control group. Multiple logistic regression analysis confirmed the following as independent risk factors for unplanned reoperation (P < 0.05): Prognostic Nutritional Index value, history of laparotomy, hypertension, or stroke, hypoproteinemia, age, tumor-node-metastasis staging, surgical time, gender, and American Society of Anesthesiologists classification. Receiver operating characteristic curve analysis showed that the model had good discrimination and clinical utility.
This study used a machine learning approach to build a model that accurately predicts the risk of postoperative unplanned reoperation in patients with colorectal cancer, which can improve treatment decisions and prognosis.
Core Tip: This study developed a machine learning model to predict unplanned reoperations in colorectal cancer patients, using data from two hospitals over two years. It employed support vector machine, least absolute shrinkage and selection operator, and extreme gradient boosting for feature selection and logistic regression to identify key risk factors. The model showed good predictive accuracy, validated by receiver operating characteristic curves, calibration curves, and decision curve analysis. Key predictors included age, gender, prior surgeries, and nutritional status. This predictive tool aims to enhance clinical decision-making, reduce reoperation rates, and improve patient outcomes in colorectal cancer care.
- Citation: Cai LQ, Yang DQ, Wang RJ, Huang H, Shi YX. Establishing and clinically validating a machine learning model for predicting unplanned reoperation risk in colorectal cancer. World J Gastroenterol 2024; 30(23): 2991-3004
- URL: https://www.wjgnet.com/1007-9327/full/v30/i23/2991.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i23.2991
According to the World Health Organization, colorectal cancer is one of the most common malignant tumors of the digestive tract[1]. In 2018, there were more than 1.8 million cases of colorectal cancer globally, with a total of 881000 deaths — an average of 1 death out of every 10 cases[2]. Colorectal cancer is one of the top three cancer contributors to morbidity and mortality rates in the world[3]. Colorectal cancer poses a significant threat to the physical and mental health of the Chinese population. Early diagnosis of colorectal cancer in China is generally poor, and the majority of patients are in the middle-to-late stage of disease at the time of diagnosis[4]. Postoperative recurrence and metastasis of colorectal cancer are influenced by multiple factors such as lymph node metastasis, tumor type, growth location, and degree of infiltration. These factors are also key in determining the prognosis of patients with colorectal cancer[5].
Colorectal cancer is a serious malignant tumor and its treatment can include surgery, radiation therapy, chemotherapy, molecular-targeted therapy, immunotherapy, endocrinotherapy, and traditional Chinese medicine[6]. Currently, a combination approach based on surgery is the preferred strategy for the treatment of colorectal cancer[7]. Common surgical methods include radical surgery. However, in recent years, laparoscopy has been widely adopted due to its rapid recovery time, minimal trauma, and significant short-term efficacy[8].
Postoperative reoperation, particularly the rate of unplanned reoperation within 30 d, is an important indicator of surgical quality and has been adopted by the United States Centers for Medicare and Medicaid Services in its Physician Quality Reporting System[9]. Due to the high morbidity and mortality of colorectal cancer, patients undergoing surgery are at risk of later reoperation. The percentage of postoperative unplanned reoperation in patients with colorectal cancer ranges from 3% to 11%[10,11]. The causes of reoperation include complications such as anastomotic leakage, bowel obstruction, and postoperative bleeding. Understanding the causes of reoperation helps improve patient prognosis. Despite improvements in surgical techniques and perioperative management, postoperative unplanned reoperation is still closely associated with complications[12]. These complications not only affect the short-term prognosis of the patient but may also apply surgical stress on the immune system, affecting postoperative outcomes. Unplanned reoperation is an independent predictor of a patient's mortality within one year of surgery[13].
Machine learning has great potential for disease risk prediction and diagnosis. In colorectal cancer, machine learning models can accurately predict the risk of undesired postoperative return to surgery by comprehensively analyzing multidimensional data on surgical approaches, and a patient's clinical characteristics and comorbidities[14]. The ability of such techniques to learn and adapt to new data means that their predictive accuracy continues to improve over time and data accumulation, reducing unnecessary reoperations, optimizing patient prognosis, and improving quality of life.
The purpose of this study is to establish and validate a model of colorectal cancer postoperative unplanned re
Clinical data of patients with colorectal cancer admitted to the First Hospital of Wenzhou Medical University and Wenzhou Central Hospital from March 2020 to March 2022 were retrospectively collected. This study was approved by the Clinical Research Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University and the Medical Ethics Committee of Wenzhou Municipal Central Hospital, No. KY2024-R016.
Inclusion criteria: (1) Preoperative pathological findings confirmed the diagnosis of colorectal cancer[15]; (2) laparoscopic radical resection of the primary lesion; and (3) combined with distant metastases only radical resection of the primary lesion.
Exclusion criteria: (1) Open surgery and intermediate open surgery; (2) intraoperative exploration found extensive metastases that could not be resected and only palliative surgery was performed; (3) resection of multiple bowel segments of both primary tumors or total or subtotal colectomy; (4) combined distant metastases were performed with simultaneous resection of the lesions; and (5) missing or incomplete clinical data.
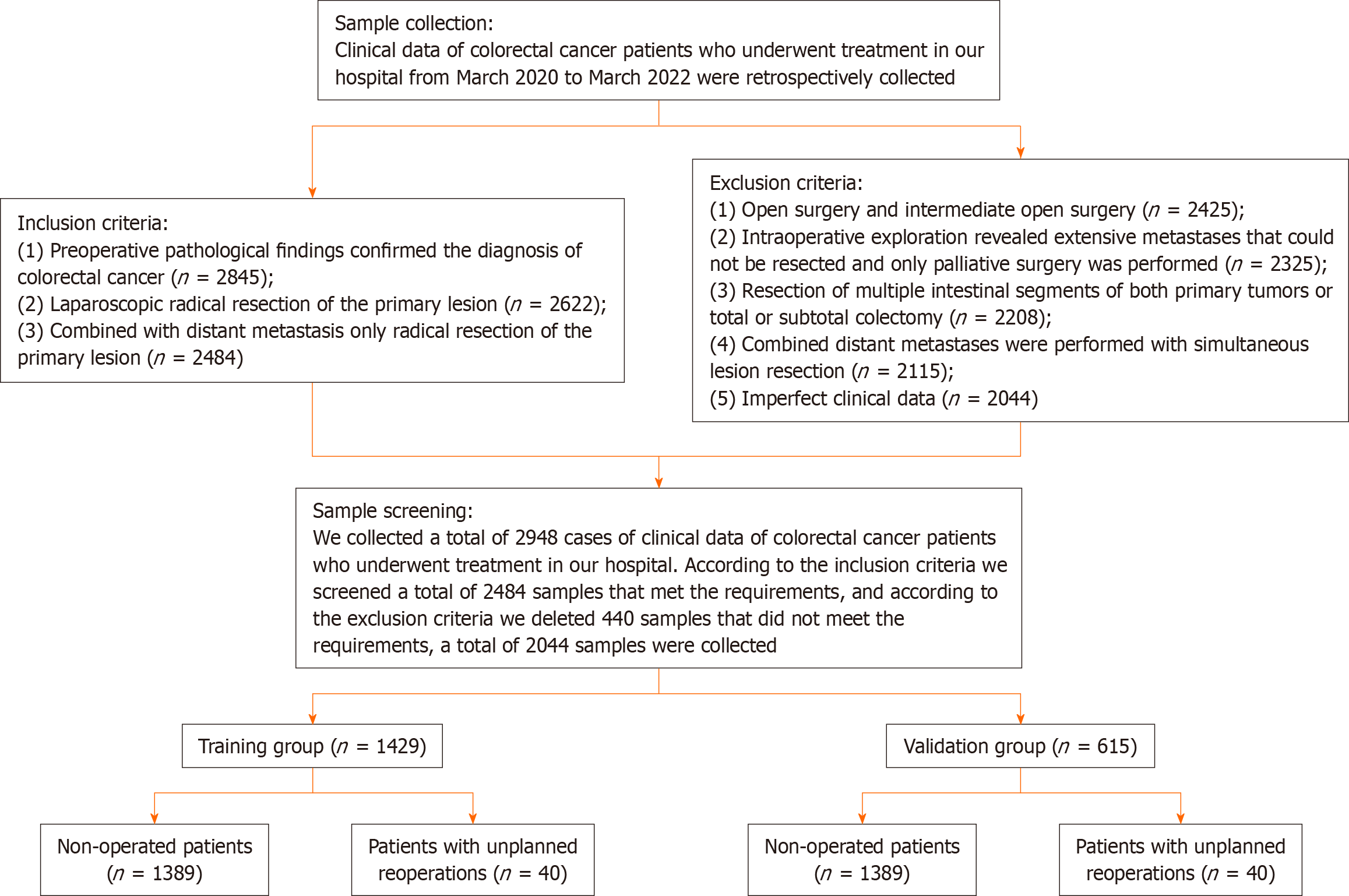
We collected a total of 2948 patient records treated at the First Affiliated Hospital of Wenzhou Medical University and Wenzhou Central Hospital. According to the inclusion criteria, a total of 2484 samples met the requirements, and we excluded 440 samples. A total of 2044 samples were included. The patients with unplanned reoperation presenting at 30 d were assigned to the experimental group (n = 60). Among the 60 patients, 34 patients had anastomotic leakage, 21 patients had bowel obstruction, and 5 patients had abdominal cavity infection. The remaining patients were placed into the control group (n = 1984). To validate our model, we divided the patients into a training group (n = 1429) and a validation group (n = 615) based on a ratio of 7:3. Figure 1 depicts a flow chart of the process.
From the electronic medical records, we collected the clinical data of all patients, including age, gender, body mass index, history of hypertension, history of diabetes, history of stroke, history of laparotomy, preoperative hypoproteinemia, tumor site, history of preoperative radiotherapy, history of preoperative chemotherapy, tumor-node-metastasis (TNM) stage, American Society of Anesthesiologists (ASA) classification, surgical time, intraoperative bleeding, and preoperative prognostic and preoperative prognostic nutritional index (PNI).
To efficiently screen feature variables associated with colorectal cancer postoperative unplanned reoperation, we used three different machine learning methods: support vector machine (SVM)[16] least absolute shrinkage and selection operator (LASSO) regression[17], and extreme gradient boosting (XGBoost)[18].
The SVM method effectively distinguishes between two classes of data points (i.e., patients with or without unplanned reoperation) by finding an optimal hyperplane in a high-dimensional space. SVM is particularly effective when dealing with large datasets because it can work with high-dimensional feature spaces and nonlinear classification problems.
LASSO regression is particularly useful for feature selection as it reduces the coefficients of unimportant features to zero. This method limits the complexity of the model by adding a regularization term to avoid overfitting, while still identifying the most relevant features.
XGBoost is an integrated learning method based on decision trees, which improves prediction accuracy by constructing multiple models and combining them. It is an effective feature selection method as it optimizes the performance of the model through a gradient-boosting framework.
To fully evaluate our unplanned reoperation, we used the following key statistical tools. The receiver operating characteristic curve (ROC) was used to assess the model's ability to discriminate between two types of outcomes (e.g., occurrence and non-occurrence of unplanned reoperation). The more diagnostic the model is, the closer the area under the curve (AUC) is to 1. We also used calibration curves to test the accuracy of the model's predicted outcomes. Ideally, the calibration curve should be close to 45 degrees, showing a high degree of agreement between predicted and actual values. The Hosmer-Lemeshow test (H-L test) was used to assess the fit of the model. A high P value implies a good agreement between model predictions and actual observations. Decision curve analysis (DCA) was used to assess the utility of the model in clinical decision-making, as it identifies the thresholds at which the use of the model best improves patient care.
Measurement of results: (1) The differences in clinical data between the control and experimental groups were compared; (2) SVM, LASSO, and XGBoost were used to screen for unplanned reoperation feature variables, and a Venn diagram was used to identify common feature variables; (3) Independent risk factors for postoperative unplanned reoperation were screened using logistic regression; (4) A nomogram was created based on the multifactorial logistic regression; (5) ROC curve, calibration curve, H-L test, and DCA were used to evaluate the differentiation, calibration, and clinical utility of the nomogram; and (6) Based on the risk coefficients, the risk scores of patients in the training and the validation groups were calculated. The differences in the risk scores of the patients were compared, and the predictive effect of the model was verified using the ROC.
Statistical analysis was carried out using SPSS 26.0 software. For normally distributed continuous data, used mean ± SD. Comparisons between groups were made using t-tests. The χ2 test was used for count data. We screened all variables using SVM, LASSO, and XGBoost, and the common variables were screened using a Venn diagram. Multiple logistic regression analysis of the common variables was used to identify the independent risk factors. Then, we constructed a nomogram prediction model based on the selected independent risk factors using R software and the rms package. We obtained the calibration curve using Bootstrap and calculated the C-index. We also plotted the independent risk factors using ROC and calculated the AUC to validate the performance of the nomogram prediction model.
Comparison of the clinical data of the two groups showed that the number of patients in the experimental group aged ≥ 60 years, male, with a history of hypertension, a history of laparotomy and hypoproteinemia, and surgical time ≥ 240 mins was significantly higher than that of patients in the control group. The PNI of patients in the experimental group was also significantly higher than that of patients in the control group (P < 0.05; Table 1). The remaining variables were not statistically different (P > 0.05).
| Control group (n = 1984) | Experiment group (n = 60) | χ2/t | P value | |
| Age (yr) | ||||
| ≥ 60 | 1151 | 48 | 11.609 | < 0.001 |
| < 60 | 833 | 12 | ||
| Gender | ||||
| Male | 1131 | 43 | 5.120 | 0.024 |
| Female | 853 | 17 | ||
| BMI (kg/m2) | ||||
| ≥ 24 | 1250 | 36 | 0.225 | 0.635 |
| < 24 | 734 | 24 | ||
| History of hypertension | ||||
| Yes | 615 | 27 | 5.300 | 0.021 |
| No | 1369 | 33 | ||
| History of diabetes | ||||
| Yes | 238 | 6 | 0.221 | 0.639 |
| No | 1746 | 54 | ||
| History of stroke | ||||
| Yes | 238 | 9 | 0.495 | 0.482 |
| No | 1746 | 51 | ||
| History of laparotomy | ||||
| Yes | 337 | 20 | 10.797 | 0.001 |
| No | 1647 | 40 | ||
| Hypoproteinemia | ||||
| Yes | 181 | 12 | 8.058 | 0.005 |
| No | 1803 | 48 | ||
| Location of the tumor | ||||
| Colon | 1250 | 36 | 0.225 | 0.635 |
| Rectum | 734 | 24 | ||
| History of preoperative radiation therapy | ||||
| Present | 198 | 7 | 0.184 | 0.668 |
| No present | 1786 | 53 | ||
| History of preoperative chemotherapy | ||||
| Present | 238 | 8 | 0.098 | 0.753 |
| No present | 1746 | 52 | ||
| TNM staging | ||||
| I + II | 1171 | 32 | 0.778 | 0.378 |
| III + IV | 813 | 28 | ||
| ASA classification | ||||
| I + II | 1786 | 51 | 1.613 | 0.204 |
| III + IV | 198 | 9 | ||
| Surgical time (min) | ||||
| ≥ 240 | 675 | 30 | 6.580 | 0.010 |
| < 240 | 1309 | 30 | ||
| Intraoperative bleeding (mL) | ||||
| ≥ 120 | 218 | 4 | 1.123 | 0.289 |
| < 120 | 1766 | 56 | ||
| PNI | 46.21 ± 5.15 | 38.97 ± 4.75 | 10.749 | < 0.001 |
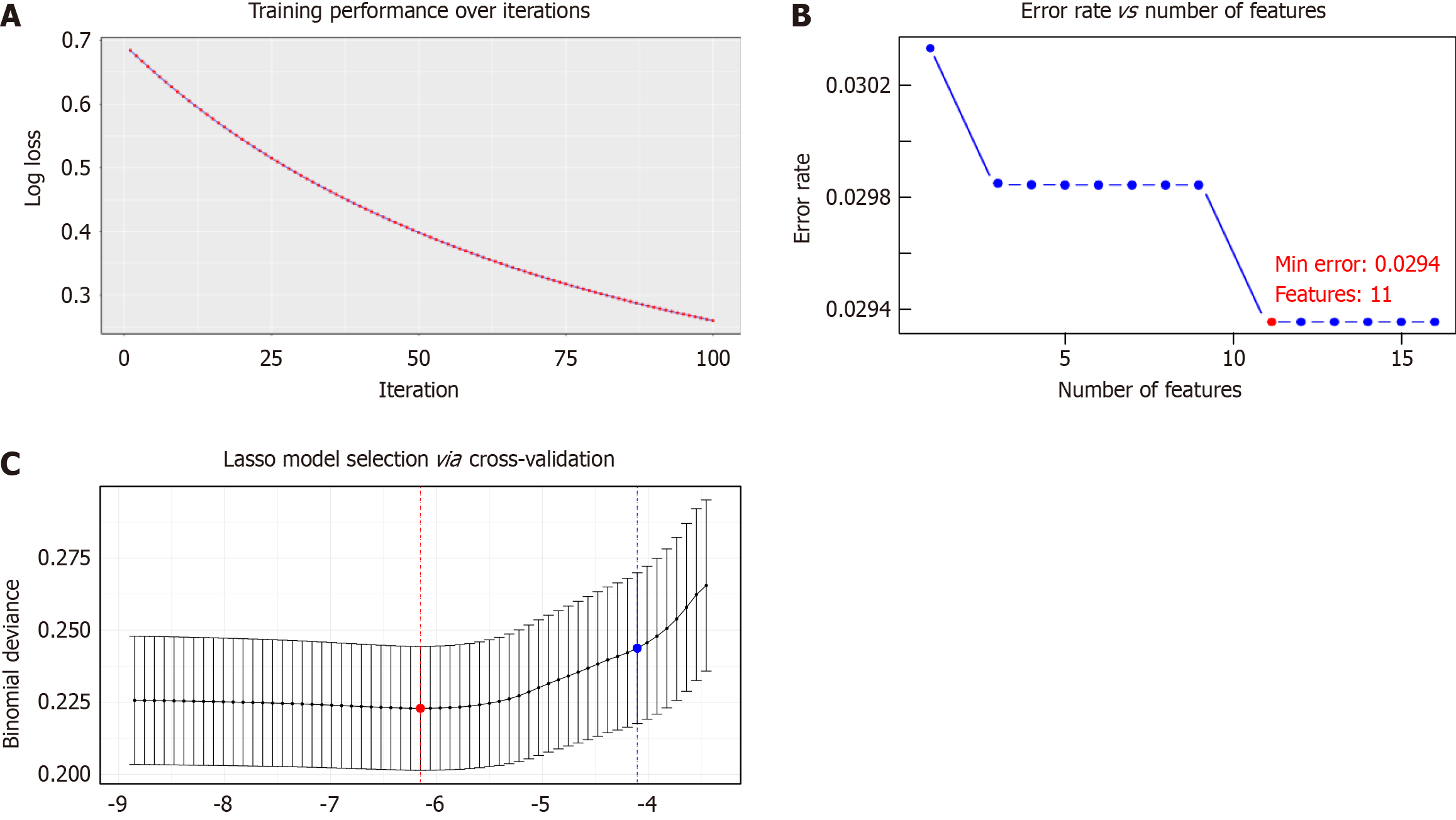
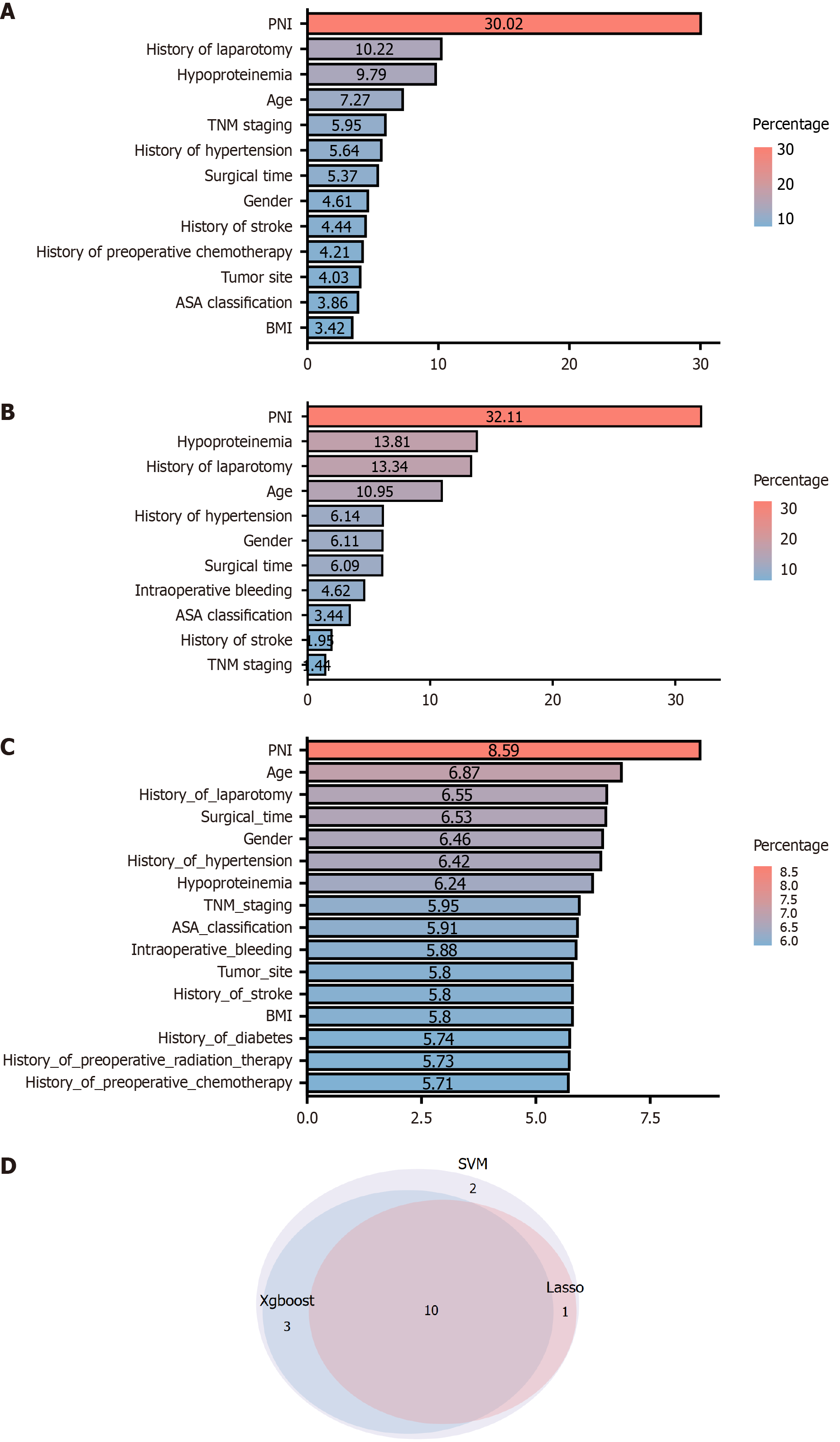
We screened the unplanned reoperation feature variables using XGBoost, SVM, and Lasso methods (Figure 2). We found that XGBoost identified a total of 13 feature variables (Figure 3A), SVM identified 16 feature variables (Figure 3B), and Lasso identified 11 feature variables (Figure 3C). Using a Venn diagram (Figure 3D), we found that the 3 methods screened 10 common characteristic variables: PNI, history of laparotomy, hypoproteinemia, age, TNM staging, history of hypertension, surgical time, gender, history of stroke, and ASA classification.
We analyzed the 10 identified signature variables using multifactor logistic regression. The 10 signature variables were first assigned values (Supplementary Table 1). The resulting analysis revealed that age, gender, history of hypertension, history of laparotomy, hypoproteinemia, and PNI were independent risk factors impacting the likelihood of unplanned reoperation (P < 0.05; Table 2).
| β | SE | χ2 | P value | OR | 95%CI | ||
| Lower | Upper | ||||||
| Age | 1.072 | 0.335 | 10.268 | 0.001 | 2.922 | 1.516 | 5.63 |
| Gender | -0.717 | 0.301 | 5.674 | 0.017 | 0.488 | 0.27 | 0.881 |
| History of hypertension | 0.592 | 0.278 | 4.545 | 0.033 | 1.807 | 1.049 | 3.115 |
| History of stroke | 0.306 | 0.392 | 0.61 | 0.435 | 1.358 | 0.63 | 2.926 |
| History of laparotomy | 0.939 | 0.297 | 9.986 | 0.002 | 2.557 | 1.428 | 4.576 |
| Hypoproteinemia | 0.918 | 0.357 | 6.59 | 0.010 | 2.504 | 1.242 | 5.045 |
| TNM staging | -0.286 | 0.275 | 1.081 | 0.299 | 0.751 | 0.438 | 1.288 |
| ASA classification | -0.308 | 0.396 | 0.605 | 0.437 | 0.735 | 0.339 | 1.597 |
| Surgical time | 0.509 | 0.276 | 3.387 | 0.066 | 1.663 | 0.967 | 2.859 |
| PNI | 2.476 | 0.369 | 45.073 | < 0.001 | 11.894 | 5.773 | 24.505 |
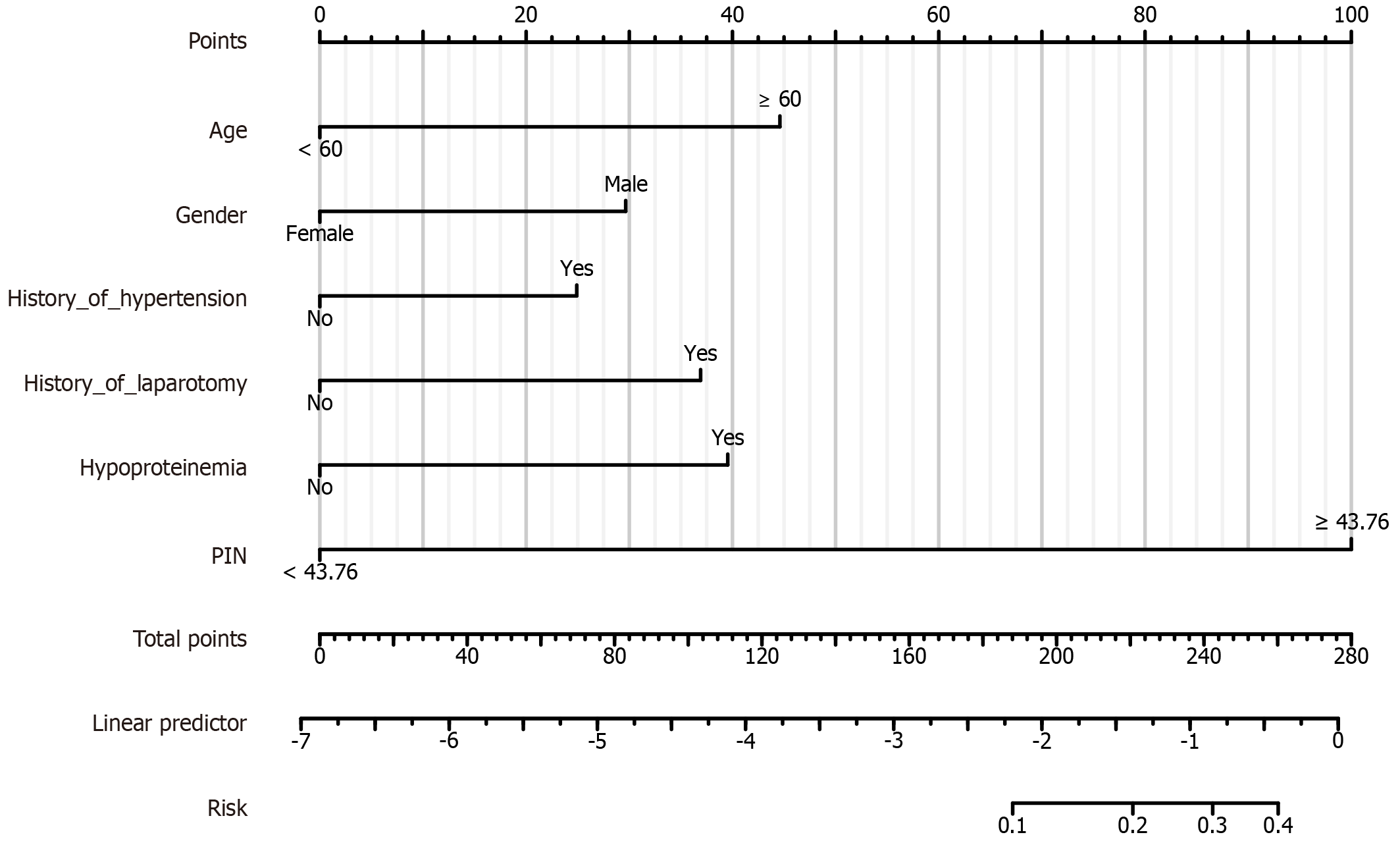
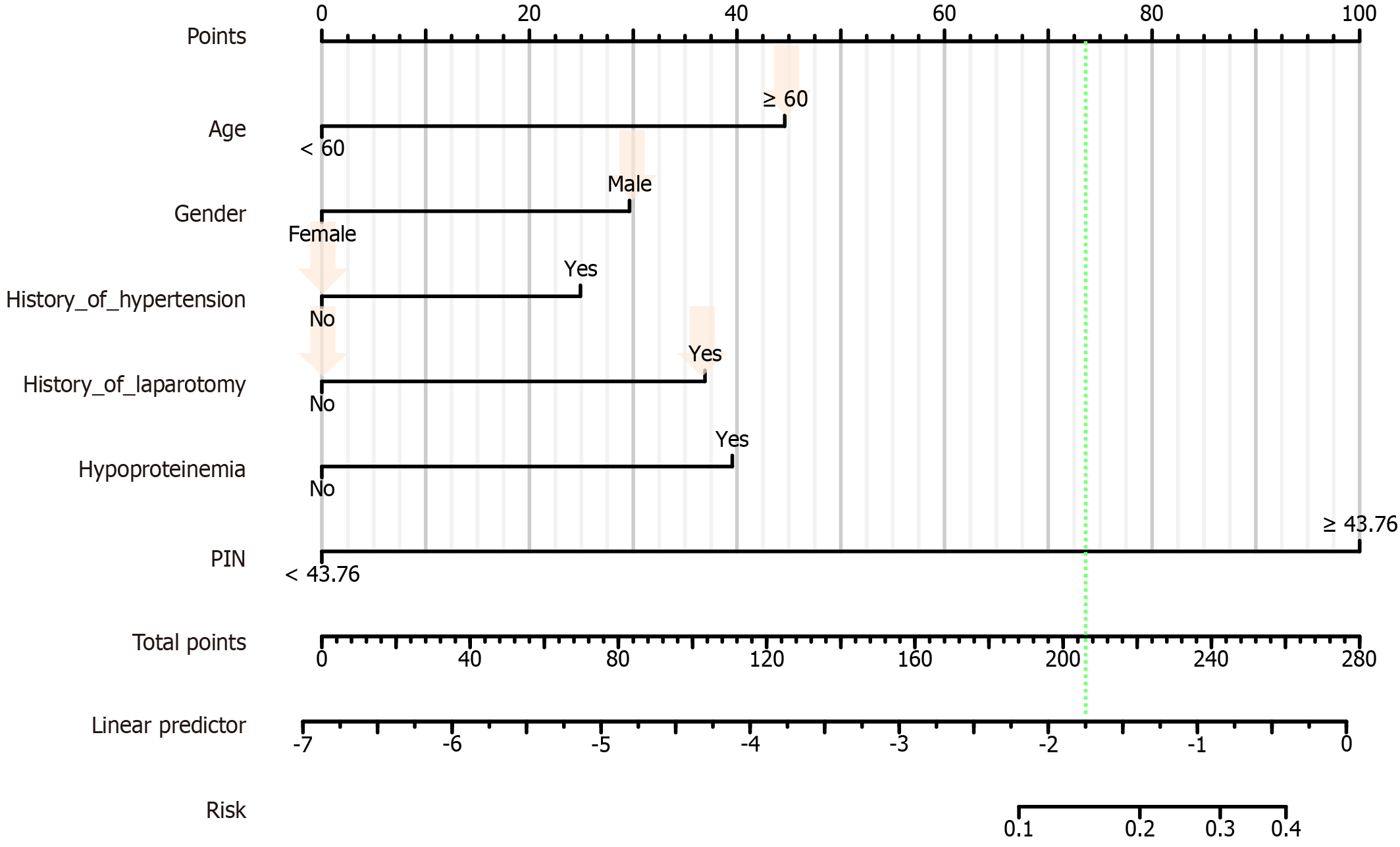
A nomogram prediction model was created based on the 10 predictors (age, gender, history of hypertension, history of laparotomy, hypoproteinemia, and PNI). The final prediction model equation was: Logit (P) = -6.8730575 + Age × 1.108872309 + Gender × 0.737188569 + History_of_hypertension × 0.619231168 + History_of_ History_of_hypertension × 0.619231168 + History_of_hypertension × 0.917723145 + Hypoproteinemia × 0.983183577 + PNI × 2.48620524.
The total score was obtained by summing the scores of each variable and finding the corresponding value on the "Total Score Axis". The value of the "Total Score Axis" was compared with the probability prediction line at the bottom of the nomogram to find the risk of postoperative unplanned reoperation (Figure 4).
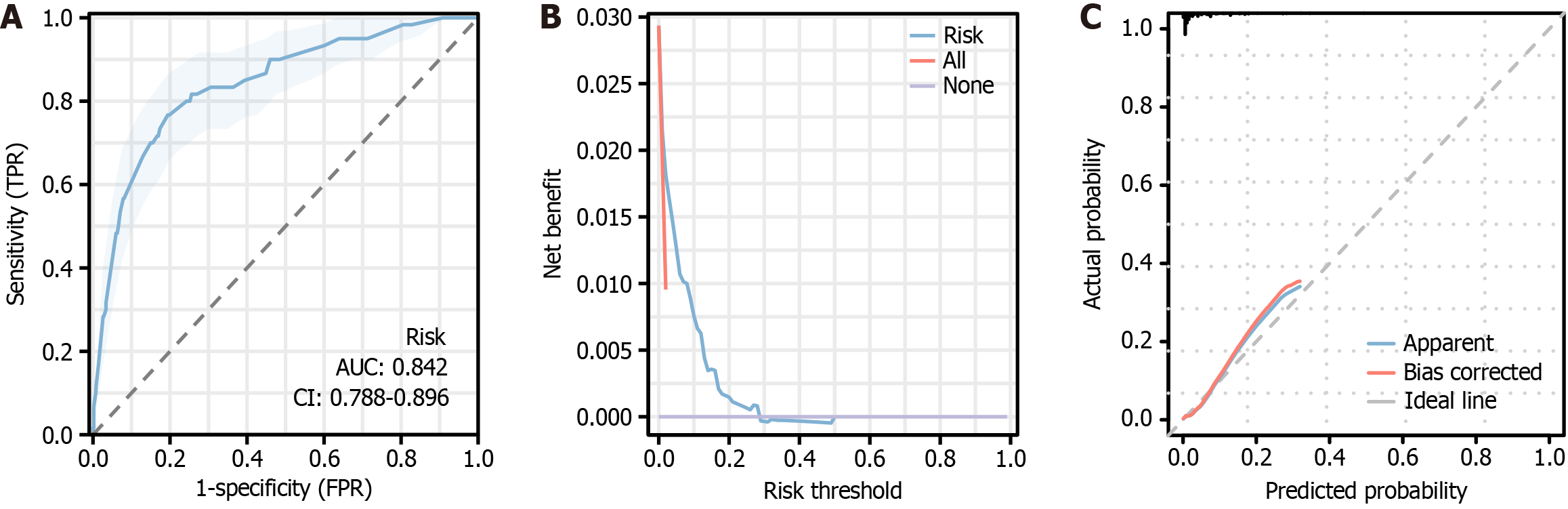
The differentiation, calibration, and clinical utility of the model were evaluated by four methods: ROC, calibration curve, H-L test, and DCA. The ROC analysis revealed that the AUC of the nomogram was 0.842, with 80.59% specificity, 76.67% sensitivity, and 57.26% Youden index (Figure 5A). This indicates that the model has a good degree of discrimination and can correctly distinguish the ending event from the non-ending event. Calibration curve analysis found that the nomogram’s calibration curve had a slightly poorer overlap, but generally went in the same direction (Figure 5B). The H-L test value was 8.588 (P = 0.378). The DCA curve indicated that the unplanned reoperation net benefit rate was higher than other, i.e., the blue line corresponding to the threshold probability was located to the upper right of the All line (red line), indicating that the model had some clinical utility (Figure 5C).
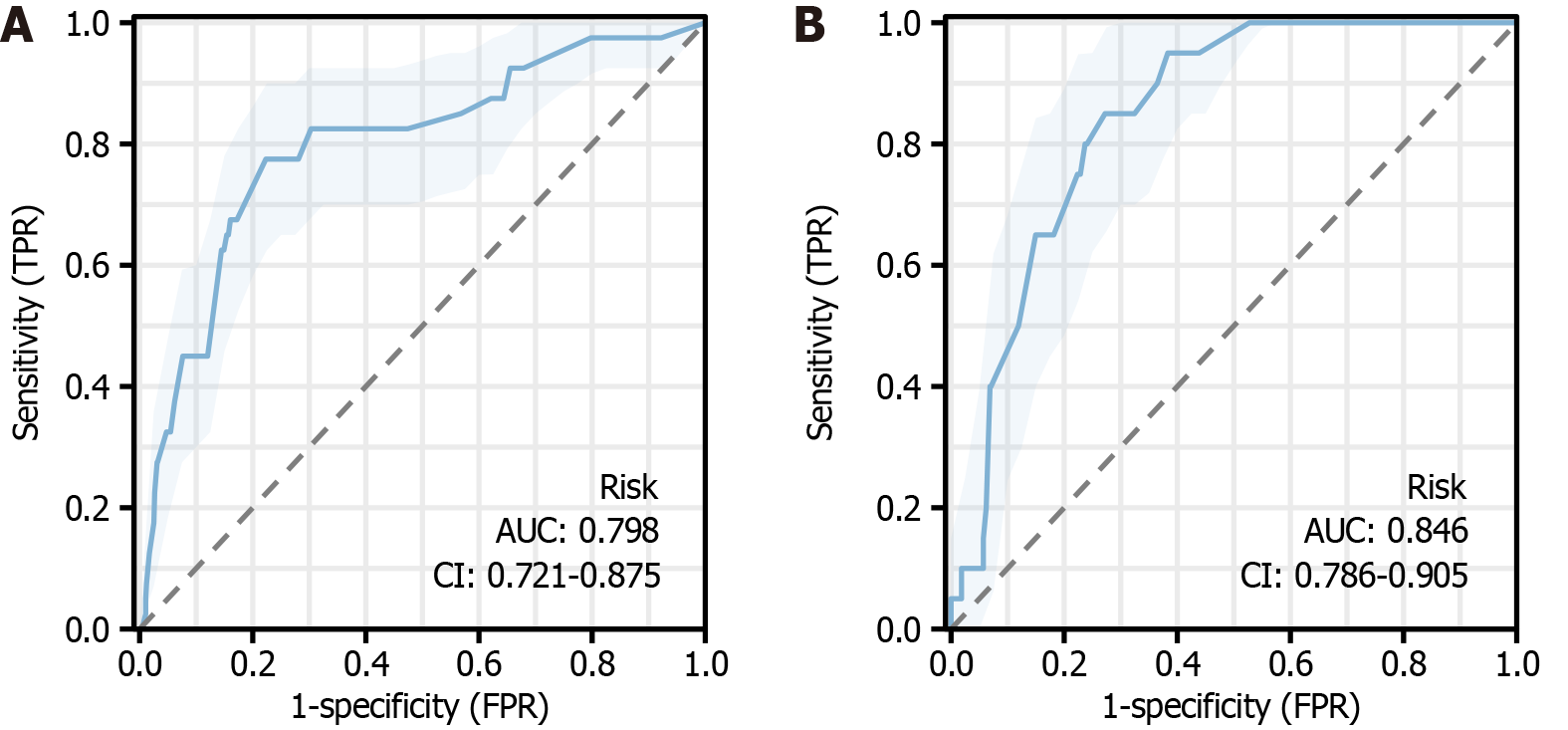
We divided the data into a training group and a validation group. The risk scores were calculated separately for both groups and then validated using ROC, calibration curve, H-L test, and DCA. As before, we compared the baseline in
| Training group (n = 1429) | Validation group (n = 615) | χ2 | P value | |
| Reoperation | ||||
| Yes | 40 | 20 | 0.309 | 0.578 |
| No | 1389 | 595 | ||
| Age (yr) | ||||
| ≥ 60 | 853 | 346 | 2.088 | 0.148 |
| < 60 | 576 | 269 | ||
| Gender | ||||
| Male | 817 | 357 | 0.135 | 0.713 |
| Female | 612 | 258 | ||
| BMI (kg/m2) | ||||
| ≥ 24 | 891 | 395 | 0.649 | 0.421 |
| < 24 | 538 | 220 | ||
| History of hypertension | ||||
| Yes | 451 | 191 | 0.051 | 0.822 |
| No | 978 | 424 | ||
| History of diabetes | ||||
| Yes | 164 | 80 | 0.959 | 0.327 |
| No | 1265 | 535 | ||
| History of stroke | ||||
| Yes | 174 | 73 | 0.038 | 0.845 |
| No | 1255 | 542 | ||
| History of laparotomy | ||||
| Yes | 248 | 109 | 0.041 | 0.840 |
| No | 1181 | 506 | ||
| Hypoproteinemia | ||||
| Yes | 144 | 49 | 2.238 | 0.135 |
| No | 1285 | 566 | ||
| Location of the tumor | ||||
| Colon | 894 | 392 | 0.256 | 0.613 |
| Rectum | 535 | 223 | ||
| History of preoperative radiation therapy | ||||
| Present | 144 | 61 | 0.012 | 0.913 |
| No present | 1285 | 554 | ||
| History of preoperative chemotherapy | ||||
| Present | 173 | 73 | 0.023 | 0.880 |
| No present | 1256 | 542 | ||
| TNM staging | ||||
| I + II | 825 | 378 | 2.471 | 0.116 |
| III + IV | 604 | 237 | ||
| ASA classification | ||||
| I + II | 1281 | 556 | 0.275 | 0.600 |
| III + IV | 148 | 59 | ||
| Surgical time (min) | ||||
| ≥ 240 | 476 | 229 | 2.933 | 0.087 |
| < 240 | 953 | 386 | ||
| Intraoperative bleeding (mL) | ||||
| ≥ 120 | 145 | 77 | 2.502 | 0.114 |
| < 120 | 1284 | 538 | ||
| PNI | ||||
| ≥ 43.76 | 487 | 207 | 0.034 | 0.854 |
| < 43.76 | 942 | 408 |
To validate our model, we randomized the clinical data of 1 patient with unplanned reoperation. This patient was aged ≥ 60 years, male, had no history of hypertension, no history of laparotomy, hypoproteinemia, and his PNI was ≥ 43.76. The probability of occurrence was calculated for this patient (45 + 30 + 0 + 0 + 39 + 100 = 216). The results showed that the probability of the patient having unplanned reoperation was about 73% (Figure 8).
Treatment of colorectal cancer through laparoscopy allows comprehensive observation; clear peeling and resection of the lesion, as well as procedures such as hemostasis and lymph node dissection[19]. Laparoscopy has a low impact on the patient's abdominal cavity, reduces postoperative pain, and promotes recovery of gastrointestinal function[20]. However, despite the improved precision and safety of laparoscopy, unplanned reoperation remains a challenge for colorectal cancer outcomes[21]. Reoperation not only prolongs the hospital stay and increases the financial burden of the disease, but also it affects the subsequent treatment plan and significantly increases the perioperative morbidity and mortality rate[22]. Therefore, investigation of the causes and risk factors of postoperative reoperation in colorectal cancer has important clinical applications in reducing the rate of reoperation.
The absence of a standardized definition for unplanned reoperation has resulted in notable variations in the reported endpoint indicators for postoperative colorectal cancer across different medical centers. In a study by Feo and colleagues, covering 92 hospitals in China, the average reoperation rate for colorectal cancer surgeries was 9.7%[23]. Unplanned reoperation’s discrepancies were primarily attributed to disparities in medical resources and treatment approaches, which influence the risk of postoperative unplanned reoperations across various levels and regions of healthcare institutions. In contrast, the incidence of unplanned reoperations following laparoscopic surgery for patients with colorectal cancer was 2.94%. Our results generally align with the laparoscopic reoperation rate for bowel cancer (approximately 3.8%) reported by Speicher et al[24]. These observations reinforce the efficacy and safety of laparoscopic surgery as a preferred treatment option for colorectal surgical interventions.
Patients with colorectal cancer undergoing abdominal surgery have a higher incidence of unplanned postoperative reoperation compared to other general surgical procedures[25] due to their susceptibility to incisional and abdominal infections, venous thromboembolism, and perioperative complications[26,27]. In addition, the inherent necessity of reconstructing abdominal organs during colorectal surgery increases the likelihood of postoperative complications, thereby increasing the likelihood of subsequent reoperations.
This study aimed to develop a predictive nomogram model. To construct this predictive model, we first employed three advanced computational techniques: SVM[28], LASSO[29], and XGBoost[30]. These methods are known for their efficacy in managing high-dimensional datasets and their ability to identify critical variables in such datasets[18]. Specifically, SVM excels at handling a wide range of datasets, LASSO mitigates overfitting through a penalty-based approach, and XGBoost is particularly effective at dealing with nonlinear relationships between data points. This multifaceted methodological framework facilitates a robust assessment of the significance of variables from multiple analytical perspectives[31]. After identifying essential variables through these preliminary methods, we applied logistic regression analysis to investigate these identified variables. This analysis allowed us to identify independent risk factors that significantly impacted the probability of unplanned reoperation. Our findings suggest that age, gender, prior hypertension, history of laparotomy, hypoproteinemia, and PNI are key independent risk factors. These insights provide an understanding of the patient-specific risks associated with unplanned reoperation after colorectal cancer surgery and contribute to the clinical decision-making process.
Recent studies have identified the male gender as an independent risk factor for unplanned reoperation[32,33]. This correlation is likely attributable to the male physiology, lifestyle habits, and adherence to postoperative rehabilitation protocols. Li et al[34] also highlighted age as a determinant, positing that elderly patients are at an elevated risk of undergoing unplanned reoperations, a conclusion that aligns with our observations. While not directly causing complications, the presence of comorbidities significantly influences surgical outcomes. Therefore, a comprehensive preoperative assessment and management of comorbid conditions are imperative to mitigating the likelihood of reoperation[35].
Numerous studies have substantiated the association between preoperative hypoproteinemia and the risk of unplanned reoperation. Saadat et al[36] recognized preoperative hypoalbuminemia as an independent risk factor in patients with rectal cancer, a finding corroborated by Michaels et al[37], who linked malnutrition to increased risk of unplanned reoperation. Our study further confirms that patients with diminished preoperative albumin levels are at a heightened risk for such interventions.
The PNI is a crucial marker for evaluating a patient’s preoperative nutritional and immunological status. Lower PNI values often indicate suboptimal nutritional health, which can potentially compromise wound healing through impaired collagen synthesis and fibroblast proliferation[38,39]. Improving patients' nutrition by enhancing albumin concentrations and optimizing PNI scores may significantly curtail the risk of unplanned reoperations following rectal cancer surgeries. Moreover, a history of prior abdominal surgeries is an independent risk factor for postoperative bowel obstruction following rectal resections[40]. This suggests that such historical surgical interventions may lead to extensive abdominal adhesions, thereby complicating subsequent procedures and elevating the risk of complications.
Screening patients with high reoperation risk helps clinicians target perioperative observations and interventions, thus reducing unplanned reoperation and improving patient prognosis. In this study, we successfully predicted the incidence of unplanned reoperation through a constructed nomogram. The internal validation showed that the model was highly accurate and had good predictive efficacy.
However, there are some limitations to this study. First, the retrospective design of this study may lead to information and selection bias. Second, the lack of an external independent dataset for validation limits the generalizability and reproducibility of the model. Finally, the lack of long-term follow-up data in this study prevented assessment of the long-term outcomes of surgery and patient quality of life. In the future, we hope to use a prospective design to reduce bias, conduct external validation to enhance the generalizability of the model, and include long-term follow-up to assess the long-term impact of surgery. These improvements may more accurately predict colorectal cancer postoperative risk and improve patient outcomes and quality of life.
This study successfully established and validated a postoperative unplanned reoperation risk model for colorectal cancer. Through comprehensive analysis, we accurately identified independent risk factors affecting the risk of unplanned reoperation: age; gender; history of hypertension; history of dissection; history of hypoproteinemia, and PNI. The application of the model in clinical practice can help to more accurately assess the postoperative risk of patients, thus optimizing treatment decisions, reducing the occurrence of unplanned reoperation, and improving patient prognosis and quality of life.
| 1. | Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2268] [Cited by in RCA: 3270] [Article Influence: 654.0] [Reference Citation Analysis (2)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55825] [Article Influence: 7975.0] [Reference Citation Analysis (132)] |
| 3. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13213] [Article Influence: 1468.1] [Reference Citation Analysis (3)] |
| 4. | Wang Z, Dan W, Zhang N, Fang J, Yang Y. Colorectal cancer and gut microbiota studies in China. Gut Microbes. 2023;15:2236364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 50] [Reference Citation Analysis (0)] |
| 5. | Wang R, Su Q, Yan ZP. Reconsideration of recurrence and metastasis in colorectal cancer. World J Clin Cases. 2021;9:6964-6968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 6. | Morris VK, Kennedy EB, Baxter NN, Benson AB 3rd, Cercek A, Cho M, Ciombor KK, Cremolini C, Davis A, Deming DA, Fakih MG, Gholami S, Hong TS, Jaiyesimi I, Klute K, Lieu C, Sanoff H, Strickler JH, White S, Willis JA, Eng C. Treatment of Metastatic Colorectal Cancer: ASCO Guideline. J Clin Oncol. 2023;41:678-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 312] [Article Influence: 156.0] [Reference Citation Analysis (0)] |
| 7. | Ustuner MA, Deniz A, Simsek A. Laparoscopic versus Open Surgery in Colorectal Cancer: Is Laparoscopy Safe Enough? J Coll Physicians Surg Pak. 2022;32:1170-1174. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Hardy NP, Mac Aonghusa P, Neary PM, Cahill RA. Intraprocedural Artificial Intelligence for Colorectal Cancer Detection and Characterisation in Endoscopy and Laparoscopy. Surg Innov. 2021;28:768-775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Huntington CR, Blair LJ, Cox TC, Prasad T, Kercher KW, Augenstein VA, Heniford BT. The Centers for Medicare and Medicaid Services (CMS) two midnight rule: policy at odds with reality. Surg Endosc. 2016;30:751-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Numata M, Yamaguchi T, Kinugasa Y, Shiomi A, Kagawa H, Yamakawa Y, Furuatni A, Manabe S, Yamaoka Y, Torii K, Kato S. Safety and feasibility of laparoscopic reoperation for treatment of anastomotic leakage after laparoscopic colorectal cancer surgery. Asian J Endosc Surg. 2018;11:227-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Mege D, Sabbagh C, Deleuze A, Gugenheim J, Millat B, Fabre JM, Borie F. Unplanned surgery after colorectal resection: laparoscopy at the index surgery is a protective factor against mortality. Surg Endosc. 2023;37:7100-7105. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Zawadzki M, Krzystek-Korpacka M, Rząca M, Czarnecki R, Obuszko Z, Sitarska M, Witkiewicz W. Risk factors in reoperations in colorectal surgery. Pol Przegl Chir. 2019;91:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Yin SQ, Liang X, Wu XH, Yang J, Wang A. [Comprehensive analysis of unplanned reoperations in colorectal cancer surgery]. Zhonghua Zhongliu Zazhi. 2018;40:837-840. |
| 14. | Chen L, Wang Y, Cai C, Ding Y, Kim RS, Lipchik C, Gavin PG, Yothers G, Allegra CJ, Petrelli NJ, Suga JM, Hopkins JO, Saito NG, Evans T, Jujjavarapu S, Wolmark N, Lucas PC, Paik S, Sun M, Pogue-Geile KL, Lu X. Machine Learning Predicts Oxaliplatin Benefit in Early Colon Cancer. J Clin Oncol. 2024;42:1520-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Yao H, Wu H, Liu Y. [Improvement of prognostic and predictive network of colorectal cancer based upon the 8th edition of AJCC colorectal cancer staging system]. Zhonghua Weichang Waike Zazhi. 2017;20:24-27. |
| 16. | Chen D, Liu J, Zang L, Xiao T, Zhang X, Li Z, Zhu H, Gao W, Yu X. Integrated Machine Learning and Bioinformatic Analyses Constructed a Novel Stemness-Related Classifier to Predict Prognosis and Immunotherapy Responses for Hepatocellular Carcinoma Patients. Int J Biol Sci. 2022;18:360-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 100] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 17. | Zhai T, Dou M, Ma Y, Wang H, Liu F, Zhang L, Chong T, Wang Z, Xue L. Lipid metabolism-related miRNAs with potential diagnostic roles in prostate cancer. Lipids Health Dis. 2023;22:39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 18. | Lai Y, Lin H, Chen M, Lin X, Wu L, Zhao Y, Lin F, Lin C. Integration of bulk RNA sequencing and single-cell analysis reveals a global landscape of DNA damage response in the immune environment of Alzheimer's disease. Front Immunol. 2023;14:1115202. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MH, de Lange-de Klerk ES, Lacy AM, Bemelman WA, Andersson J, Angenete E, Rosenberg J, Fuerst A, Haglind E; COLOR II Study Group. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med. 2015;372:1324-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 864] [Cited by in RCA: 931] [Article Influence: 93.1] [Reference Citation Analysis (0)] |
| 20. | Tavernier C, Flaris AN, Passot G, Glehen O, Kepenekian V, Cotte E. Assessing Criteria for a Safe Early Discharge After Laparoscopic Colorectal Surgery. JAMA Surg. 2022;157:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Koedam TWA, Bootsma BT, Deijen CL, van de Brug T, Kazemier G, Cuesta MA, Fürst A, Lacy AM, Haglind E, Tuynman JB, Daams F, Bonjer HJ; on behalf of the COLOR COLOR II study group. Oncological Outcomes After Anastomotic Leakage After Surgery for Colon or Rectal Cancer: Increased Risk of Local Recurrence. Ann Surg. 2022;275:e420-e427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 94] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 22. | Zouari A, Masmoudi A, Khanfir F, Ketata S, Rejab H, Bouzid A, Loukil I, Zribi I, Talbi S, Abdelhedi A, Abid B, Boujelben S. [Predictive factors for anastomotic leakage after colon cancer surgery]. Pan Afr Med J. 2022;42:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (1)] |
| 23. | Feo LJ, Jrebi N, Asgeirsson T, Dujovny N, Figg R, Hoedema R, Slay H, Kim D, Luchtefeld M. Anastomotic leaks: technique and timing of detection. Am J Surg. 2014;207:371-4; discussion 374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Speicher PJ, Englum BR, Jiang B, Pietrobon R, Mantyh CR, Migaly J. The impact of laparoscopic versus open approach on reoperation rate after segmental colectomy: a propensity analysis. J Gastrointest Surg. 2014;18:378-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Burns EM, Bottle A, Aylin P, Darzi A, Nicholls RJ, Faiz O. Variation in reoperation after colorectal surgery in England as an indicator of surgical performance: retrospective analysis of Hospital Episode Statistics. BMJ. 2011;343:d4836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 26. | Caroff DA, Chan C, Kleinman K, Calderwood MS, Wolf R, Wick EC, Platt R, Huang S. Association of Open Approach vs Laparoscopic Approach With Risk of Surgical Site Infection After Colon Surgery. JAMA Netw Open. 2019;2:e1913570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 27. | Zhang X, Yang Y, Liu P, Wang P, Li X, Zhu J, Mai W, Jin W, Liu W, Zhou Z, Wang J, Wu M, Ma R, Chi J, Wu X, Ren J. Identification of Risk Factors and Phenotypes of Surgical Site Infection in Patients After Abdominal Surgery. Ann Surg. 2023;278:e988-e994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Naqvi AAT, Rizvi SAM, Hassan MI. Pan-cancer analysis of Chromobox (CBX) genes for prognostic significance and cancer classification. Biochim Biophys Acta Mol Basis Dis. 2023;1869:166561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 29. | Zhou B, Yu J, Cai X, Wu S. Constructing a molecular subtype model of colon cancer using machine learning. Front Pharmacol. 2022;13:1008207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 30. | Chen X, Wang W, Chen J, Xu L, He X, Lan P, Hu J, Lian L. Predicting pathologic complete response in locally advanced rectal cancer patients after neoadjuvant therapy: a machine learning model using XGBoost. Int J Colorectal Dis. 2022;37:1621-1634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Wang X, Meng L, Zhang J, Zhao Z, Zou L, Jia Z, Han X, Zhao L, Song M, Zong J, Wang S, Qu X, Lu M. Identification of ferroptosis-related molecular clusters and genes for diabetic osteoporosis based on the machine learning. Front Endocrinol (Lausanne). 2023;14:1189513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 32. | van Westreenen HL, Ijpma FF, Wevers KP, Afzali H, Patijn GA. Reoperation after colorectal surgery is an independent predictor of the 1-year mortality rate. Dis Colon Rectum. 2011;54:1438-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Wu Z, Gao H, Zhang W, Zhu B. [Risk factors of unplanned reoperation after colorectal cancer surgery: a case-control study]. Zhonghua Weichang Waike Zazhi. 2015;18:483-486. |
| 34. | Li P, Huang CM, Tu RH, Lin JX, Lu J, Zheng CH, Xie JW, Wang JB, Chen QY, Cao LL, Lin M. Risk factors affecting unplanned reoperation after laparoscopic gastrectomy for gastric cancer: experience from a high-volume center. Surg Endosc. 2017;31:3922-3931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Qiu H, Shan RF, Ai JH, Ye SP, Shi J. Risk factors for 30-day unplanned reoperation after pancreatoduodenectomy: A single-center experience. J Cancer Res Ther. 2019;15:1530-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Saadat LV, Fields AC, Lyu H, Urman RD, Whang EE, Goldberg J, Bleday R, Melnitchouk N. National Surgical Quality Improvement Program analysis of unplanned reoperation in patients undergoing low anterior resection or abdominoperineal resection for rectal cancer. Surgery. 2019;165:602-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Michaels AD, Mullen MG, Guidry CA, Krebs ED, Turrentine FE, Hedrick TL, Friel CM. Unplanned Reoperation Following Colorectal Surgery: Indications and Operations. J Gastrointest Surg. 2017;21:1480-1485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Fife CE, Horn SD. The Wound Healing Index for Predicting Venous Leg Ulcer Outcome. Adv Wound Care (New Rochelle). 2020;9:68-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Sakurai K, Ohira M, Tamura T, Toyokawa T, Amano R, Kubo N, Tanaka H, Muguruma K, Yashiro M, Maeda K, Hirakawa K. Predictive Potential of Preoperative Nutritional Status in Long-Term Outcome Projections for Patients with Gastric Cancer. Ann Surg Oncol. 2016;23:525-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 40. | Shin JY. Risk factors of early postoperative small bowel obstruction following a proctectomy for rectal cancer. J Korean Soc Coloproctol. 2011;27:315-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |